Introduction
Although inflammation is required for a healthy
immune response, it is also responsible for causing a number of
diseases, including arthritis, diabetes, cardiovascular disease and
cancer. The balance between pro- and anti-inflammatory cytokines is
involved in the inflammatory response (1). Acute inflammation is a short-term
response characterized by extravasation of plasma, erythrocytes and
leukocytes into the injured tissue. On the other hand, chronic
inflammation is characterized by tissue infiltration by macrophages
and lymphocytes.
During inflammatory processes, macrophages are
critical mediators of immune and inflammatory responses that are
activated by binding with various stimuli (2). One of the most potent activators of
macrophages is lipopolysaccharide (LPS), which binds with Toll-like
receptor 4, the activation of which results in the production of
proinflammatory cytokines, such as interleukin (IL)-6, tumor
necrosis factor-α (TNF-α) and IL-1β, and inflammatory mediators,
such as prostaglandin (PGE2) and nitric oxide (NO) (3–5).
Uncontrolled production of these mediators and cytokines can lead
to chronic inflammatory-derived diseases (6). Accordingly, substances that reduce
the production of these mediators have attracted significant
attention in the development of anti-inflammatory drugs.
The key targets of anti-inflammatory agents include
transcription factors, cytokines and enzymes (7). NF-κB plays an important role in the
expression of inflammatory mediators containing NF-κB binding
motifs (8). It is involved in the
regulation of numerous genes, such as inducible nitric oxide
synthase (iNOS) and cyclooxygenase-2 (COX-2), that are mediators of
acute-phase immune and inflammatory responses (9). Additionally, activation of the
mitogen-activated protein kinase (MAPK) pathway also plays an
essential role in the initiation and development of inflammatory
processes that are transmitted by sequential phosphorylation events
(10). The three major groups of
MAPK cascades are ERK, p38 and c-Jun N-terminal kinase (JNK). Each
MAPK is activated by the upstream activation of MAPK kinase (MKK)
and MAPK kinase kinase. Once activated by kinases, MAPK can
phosphorylate transcription factors or other downstream kinases
that promote the expression of proinflammatory mediators of
extracellular stimuli (10,11).
Flavonoids are natural compounds with various
biological and pharmacological properties, including anticancer,
antimicrobial, antiviral, anti-inflammatory and antithrombotic
effects (10,11). Specifically, the anti-inflammatory
activities of flavonoids have long been known (12). Some flavonoids have been reported
to attenuate chronic inflammation in animal models (13). Thus, continued evaluation of the
anti-inflammatory activity of flavonoids may be valuable. Notably,
Citrus-derived flavonoids and their metabolites have been
reported to have important biological activities, including
anti-inflammatory, antiviral, anticancer and anti-atherogenic
properties (14–16). The highest concentration of
polymethoxyflavones (PMFs;
6,7,4′,5′-tetramethoxy-5-monohydroxyflavone,
5,6,8,3′,6′-pentamethoxyflavone, 5,6,7,3′,4′,5′-hexamethoxyflavone)
is found in the Citrus peel (17,18).
Tangeretin (4′,5,6,7,8-pentamethoxyflavone, TAN) and nobiletin
(5,6,7,8,3′,4′-hexamethoxyflavone, NOB) are two PMFs that are
relatively common in Citrus peel (19). Previous research demonstrated that
PMFs suppress degranulation in antigen-stimulated basophilic
leukemia cells (20). Likewise,
NOB had inhibitory effects on H2O2-induced
apoptosis in SH-SY5Y cells, while a binary mixture of
5-demethylnobiletin and TAN attenuated cell growth (21). Furthermore, the PMF-rich fractions
from the peel of Citrus sunki showed stronger
antiproliferative activity than single PMFs in human leukemia cells
(22). Previously, synergistic
anti-inflammatory effects have been reported on RAW264.7 cells
treated with a binary mixture of sulforaphane and NOB (23,24).
However, pharmacological studies of the biological effects of PMF
compounds have been limited.
In the present study,
3,5,6,7,3′,4′-hexamethoxyflavone (quercetogetin, QUE) from extracts
of Citrus unshiu peel (CUP) were isolated and identified. As
the anti-inflammatory effects of QUE have not been studied, the
anti-inflammatory effects and molecular mechanisms regulated by QUE
in LPS-induced RAW264.7 cells were examined. These findings
suggested that QUE may be beneficial in preventing LPS-induced
inflammation and is a promising agent against various inflammatory
diseases.
Materials and methods
Cell culture
Murine macrophage, RAW264.7 cells were purchased
from the American Type Culture Collection. This cell line was
cultured in Dulbecco's modified Eagle's medium (DMEM; WeGene) with
100 µg/ml streptomycin (Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin (Gibco; Thermo Fisher Scientific, Inc.) and fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.).
Chemicals and reagents
LPS, Griess reagent, MTT and dimethyl sulfoxide
(DMSO) were purchased from Sigma-Aldrich; Merck KGaA. The
antibodies against COX-2 (cat. no. sc-376861), iNOS (cat. no.
sc-7271), β-actin (cat. no. sc-8432) and horseradish peroxidase
(HRP)-conjugated secondary antibodies [anti-mouse (cat. no.
sc-2005), anti-goat (cat. no. sc-2020) and anti-rabbit (cat. no.
sc-2004)] were purchased from Santa Cruz Biotechnology, Inc. The
antibodies against histone H3 (cat. no. 9715), NF-κB (p65; cat. no.
8242), NF-κB inhibitor α (IκB-α; cat. no. 4812), phosphorylated
(p)-IκB-α (cat. no. 2859), p38 (cat. no. 9212), p-p38 (cat. no.
9211), ERK (cat. no. 4695), p-ERK (cat. no. 4377), JNK (cat. no.
9258) and p-JNK (cat. no. 4671) were purchased from Cell Signaling
Technology, Inc. The antibody against GAPDH (cat. no. LF-PA0018)
was purchased from AbFrontier Co., Ltd.
Isolation of QUE
CUP was harvested along the coast of Jeju Island,
Korea in 2010. The dried CUP (3 kg) was extracted three times in
95% (v/v) ethyl alcohol overnight at room temperature. The ethyl
alcohol (838 g) extract was evaporated and suspended in distilled
water, and then divided into two fractions using chloroform as the
non-aqueous phase. The chloroform fraction (155.9 g) was separated
on a liquid chromatographic column (15×40 cm) packed with silica
gel column (230–400 mesh) using gradient mixtures of
CHCl3-MeOH (from CHCl3:MeOH=100:1 to 1:1,
v/v) as the mobile phase to obtain nine fractions, F01 to F09,
through thin-layer chromatography. F04 (50.2 g) was applied to a
liquid chromatographic column (15×40 cm) packed with silica gel
column (230–400 mesh) using gradient mixtures of Hexan-EtOAc (from
Hexan:EtOAc=80:1 to 1:3, v/v) to obtain 14 sub-fractions (F04-1 to
14). The active fraction F04-11 (271 mg) was fractionated through
solid-phase extraction using 100% MeOH as the solvent system to
obtain the active compound 3 (3,5,6,7,3′,4′-hexamethoxyhomoflavone,
9.48 mg, QUE). The structure of the isolated QUE was elucidated
through spectroscopic analysis [proton nuclear magnetic resonance
(1H-NMR) and carbon-13 (13C)-NMR] and comparison with published
data (19).
QUE showed the following characteristics: C21H22O8,
yellow amorphous powder, electrospray ionization mass spectrometry
(ESI-MS) m/z 402.1187 (M-H)+, 1H-NMR (300 MHz,
CDCl3) δ (ppm): 7.56 (1H, dd, J=8.5, 2.0 Hz, H-6′), 7.41
(1H, d, J=2.0 Hz, H-2′), 7.00 (1H, d, J=8.5 Hz, H-5′), 6.61 (1H, s,
H-3), 4.10, 4.02, 3.98, 3.96, 3.95 X2 (each 3H, s, 6 OMe at C-3,
−5, −6, −7, −3′, −4′); 13C NMR (75 MHz, CDCl3) δ (ppm):
177.5 (s, C-4), 161.2 (s, C-2), 152.1 (s, C-4′), 151.6 (s, C-7),
149.5 (s, C-3′), 148.6 (s, C-8), 147.9 (s, C-9), 144.3 (s, C-5),
138.2 (s, C-6), 124.2 (s, C-1′), 119.8 (d, C-6′), 114.6 (s, C-10),
111.4 (d, C-5′), 108.7 (d, C-2′), 107.1 (d, C-3), 62.4, 62.1, 62.0,
61.8, 56.3, 56.2 (6 q, OMe at C-5, −6, −7, −3, −3′, −4′).
Cell viability assay
Cells were seeded in 96-well plates at
5ⅹ103 cells/well and treated with QUE (0, 1, 10 or 100
µM) at 37°C for 24 h. Then, 0.5 mg/ml MTT solution was added to
each well and cells were incubated 37°C for 4 h, 100 µl DMSO was
added. The absorbance was measured at a wavelength of 570 nm (Tecan
Group, Ltd.).
Nitric oxide measurements
Cells were seeded in 48-well plates at
1ⅹ105 cells/well and induced with 1 µg/ml LPS and
treated with QUE (0, 1, 10 or 100 µM) at 37°C for 24 h. In brief,
100 µl of culture medium was mixed with the same volume of Griess
reagent (Sigma-Aldrich; Merck KGaA) and incubated for 10 min at
room temperature. The absorbance was measured at 550 nm and the
concentration of nitrite was determined using sodium nitrite as a
standard.
Measurement of PGE2, IL-1β, IL-6 and
TNF-α
RAW264.7 cells were seeded in 24-well plates at
2ⅹ105 cells/well and then treated with QUE with or
without LPS (1 µg/ml) at 37°C for 24 h. The concentrations of IL-6
(cat. no. R600B), PGE2 (cat. no. KGE004B), IL-1β (cat. no. RLB00)
and TNF-α (cat. no. RTA00) in the cell culture supernatant were
determined using ELISA kits (R&D Systems, Inc.).
Preparation of cytosolic and nuclear
extracts
Macrophage cells were plated in 60 mm cell culture
plates, pretreated with QUE or DMSO for 1 h, and then stimulated
with LPS for 30 min at 37°C. Cells were washed with PBS, scraped
from the plates into PBS, and centrifuged at 500 × g for 3 min at
room temperature. The pellets were suspended in Cytoplasmic
Extraction Reagent (CER) I (with protease inhibitor), and then, the
samples were added to cold CER II. The mixture was centrifuged at
16,000 × g for 5 min at 4°C, and the supernatant (cytosolic
extract) was collected. The pellets were resuspended in Nuclear
Extraction Reagent (with protease inhibitor) for 40 min on ice, and
then centrifuged at 16,000 × g for 10 min at 4°C. The supernatant
(nuclear extract) was collected.
Western blot analysis
Cells were washed with Dulbecco's PBS (WeGene), and
then scraped into 500 µl of 2X RIPA (50 mM Tris-HCl, pH 7.5, 2 mM
EDTA, 0.5% deoxycholate, 150 mM NaCl, 1% NP-40, 0.1% SDS and 1X
protease inhibitor) buffer. The protein concentration was
determined using BSA reagent (Bio-Rad Laboratories, Inc.). Then,
proteins (20–40 µg) from each sample were resolved via SDS-PAGE on
10% gel, and the separated proteins were transferred to PVDF
membranes (EMD Millipore). The membranes were blocked with 5% (w/v)
non-fat milk powder in TBS with Tween-20 (TBST; 50 mM Trish-HCl,
150 mM NaCl and 0.1% Tween-20) for 1 h at room temperature,
following which they were incubated with primary antibodies
(1:1,000) in blocking solution overnight at 4°C. After three washes
in TBST, the membranes were incubated with HRP-conjugated secondary
antibodies (1:5,000) for 1 h at room temperature. Each protein was
detected using the Western BLoT Hyper substrate kit (Takara Bio,
Inc.). Band intensity was semi-quantified by densitometry analysis
using ImageJ software (version 1.52a; National Institutes of
Health).
Reverse transcription
(RT)-semi-quantitative PCR
After isolation of total RNA using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), the reverse
transcription of RNA (1 µg) was performed using oligo-dT18 and the
ImProm-II™ reverse transcription kit (Promega Corporation).
Reactions were incubated at 25°C for 5 min, at 42°C for 60 min, and
then for 10 min at 70°C to inactivate the reverse transcriptase.
The primers used for PCR were as follows: COX-2 forward,
5′-CACTACATCCTGACCCACTT-3′ and reverse, 5′-ATGCTCCTGCTTGAGTATGT-3′;
iNOS forward, 5′-CCCTTCCGAAGTTTCTGGCAGCAGC-3′ and reverse,
5′-GGCTGTCAGAGCCTCGTGGCTTTGG-5′; IL-6 forward,
5′-GTACTCCAGAAGACCAGAGG-3′ and reverse, 5′-TGCTGGTGACAACCACGGCC-3′;
IL-1β forward, 5′-CAGGATGAGGACATGAGCACC-3′ and reverse,
5′-CTCTGCAGACTCAAACTCCAC-3′; TNF-α forward,
5′-TTGACCTCAGCGCTGAGTTG-3′ and reverse, 5′-CCTGTAGCCCACGTCGTAGC-3′;
GAPDH forward, 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse,
5′-GAAGATGGTGATGGGATTTC-3′. The thermocycling conditions were as
follows: Initial denaturation at 95°C for 5 min; 30 cycles of 95°C
for 30 sec, 60°C for 30 sec and 72°C for 30 sec; and a final
extension at 72°C for 10 min. The amplification was performed on a
thermal cycler (Takara PCR Thermal Cycler model TP600; Takara Bio,
Inc.). The products were visualized using 1.0% agarose gels stained
with EtBr. GAPDH was used as a housekeeping gene when indicated.
Band intensity was semi-quantified by densitometry analysis using
ImageJ software (National Institutes of Health).
Statistical analysis
All data represent the mean ± SD of at least three
independent experiments. One-way ANOVA followed by Tukey's test was
used for data comparison among groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Chemical structure and cell viability
effects of QUE on LPS-induced RAW264.7 cells
The activity of the extracts and fractions obtained
from CUP was assessed using the NO assay in LPS-induced RAW 264.7
cells. Three pure compounds were isolated and identified as the
main components driving the inhibition of NO production. The
chemical structures of these compounds were determined through
spectroscopic methods, including mass and NMR spectroscopic
analyses (Figs. S1–S3) (19). To examine the effect of QUE on
macrophage cell viability, Raw264.7 cells were treated with various
concentrations of QUE (Fig. 1A).
The survival curve shown in Fig.
1B indicates that QUE at 1, 10 and 100 µM did not exhibit
cytotoxicity on Raw264.7 cells.
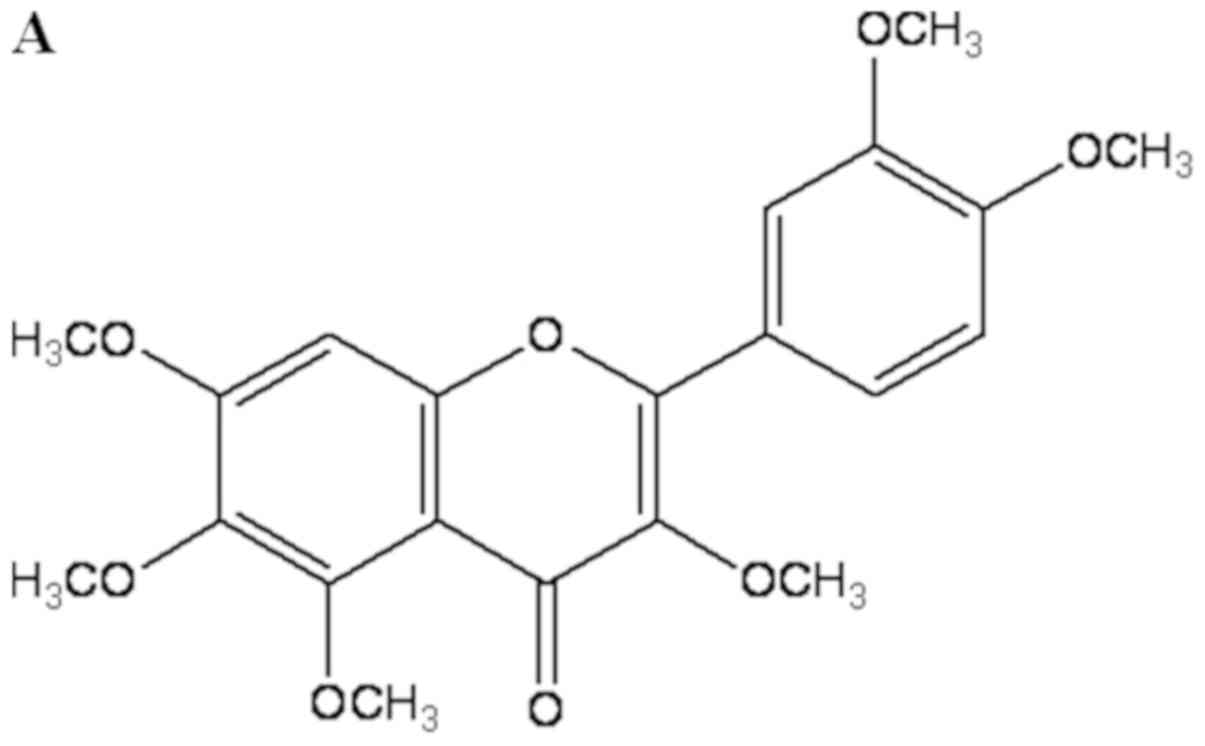 | Figure 1.Effect of QUE on the viability of
LPS-induced RAW264.7 cells. (A) Chemical structure of QUE. (B)
Cells were treated with QUE (1, 10 and 100 µM) and 1 µg/ml LPS for
24 h. The data shown represent the mean ± standard deviation of
three experiments. #P<0.05 vs. non-treated group;
**P<0.01 vs. LPS-treated group. QUE, quercetogetin; LPS,
lipopolysaccharide. (D) The protein expression levels of iNOS and
COX-2 were examined using reverse transcription-semi-quantitative
PCR and western blotting, respectively. β-actin was used as a
loading control. ###P<0.001 vs. non-treated group;
*P<0.05, **P<0.01 and ***P<0.001 vs. LPS-treated group.
NS, not significant; QUE, quercetogetin; NO, nitric oxide; PGE2,
prostaglandin E2; LPS, lipopolysaccharide; iNOS, inducible nitric
oxide synthase; COX-2, cyclooxygenase-2. |
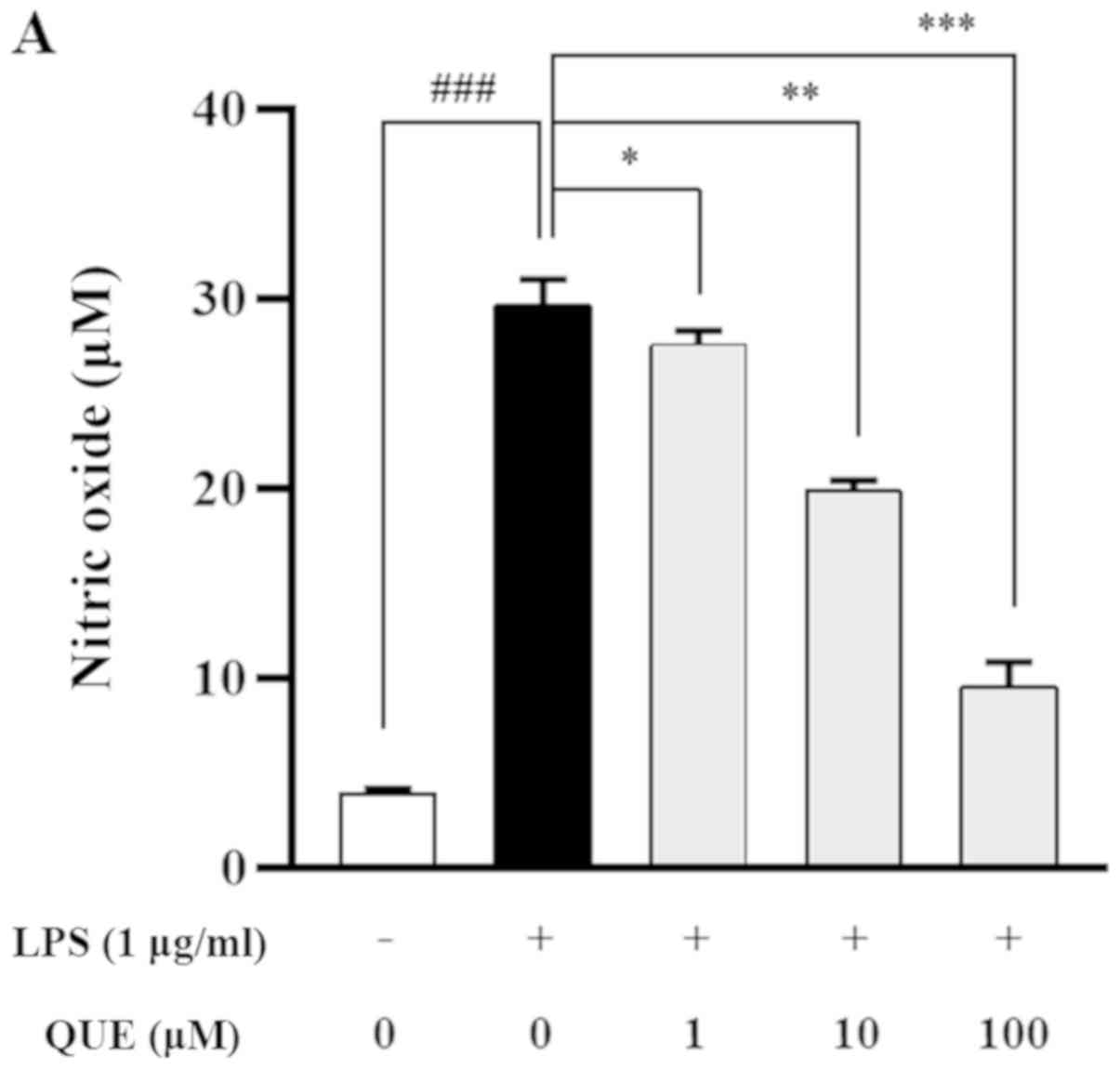
QUE inhibits production of NO and PGE2
by suppressing iNOS and COX-2 expression in LPS-induced RAW264.7
cells
As NO and PGE2 are important inflammatory mediators,
the effects of QUE on NO and PGE2 production in LPS-induced
RAW264.7 cells were examined. QUE (1, 10 or 100 µM) inhibited NO
and PGE2 production in LPS-induced cells in a dose-dependent manner
(Fig. 2A and B). To identify the
molecular mechanism through which QUE inhibited NO and PGE2
production in response to LPS, expression levels of COX-2 and iNOS
were examined using RT-PCR and western blot analyses. The results
showed that mRNA and protein expression of iNOS and COX-2 were
increased in LPS-induced RAW264.7 cells. Conversely, QUE inhibited
LPS-induced mRNA and protein expression in a dose-dependent manner
(Fig. 2C and D). These results
suggested that decreased NO and PGE2 production is related to
inhibition of iNOS and COX-2 expression in LPS-induced RAW264.7
cells.
QUE inhibits production and mRNA
expression of proinflammatory cytokines in LPS-induced RAW264.7
cells
LPS increases the production of proinflammatory
cytokines, such as IL-1β, IL-6, and TNF-α, which play important
roles in pathogen-induced inflammatory responses. ELISA and RT-PCR
analyses were performed to characterize the effects of QUE on the
production of proinflammatory cytokines. As presented in Fig. 3A-C, treatment with 100 µM QUE
significantly inhibited LPS-induced IL-1β, IL-6 and TNF-α cytokine
levels. To determine whether QUE modulated the production of
cytokines at the level of transcription RT-PCR was performed. The
mRNA expression levels of cytokines were elevated in LPS-induced
cells, but treatment with 100 µM QUE decreased this induction
(Fig. 3D). Accordingly, these
results suggested that QUE prevents the secretion of
proinflammatory cytokines by suppressing gene expression.
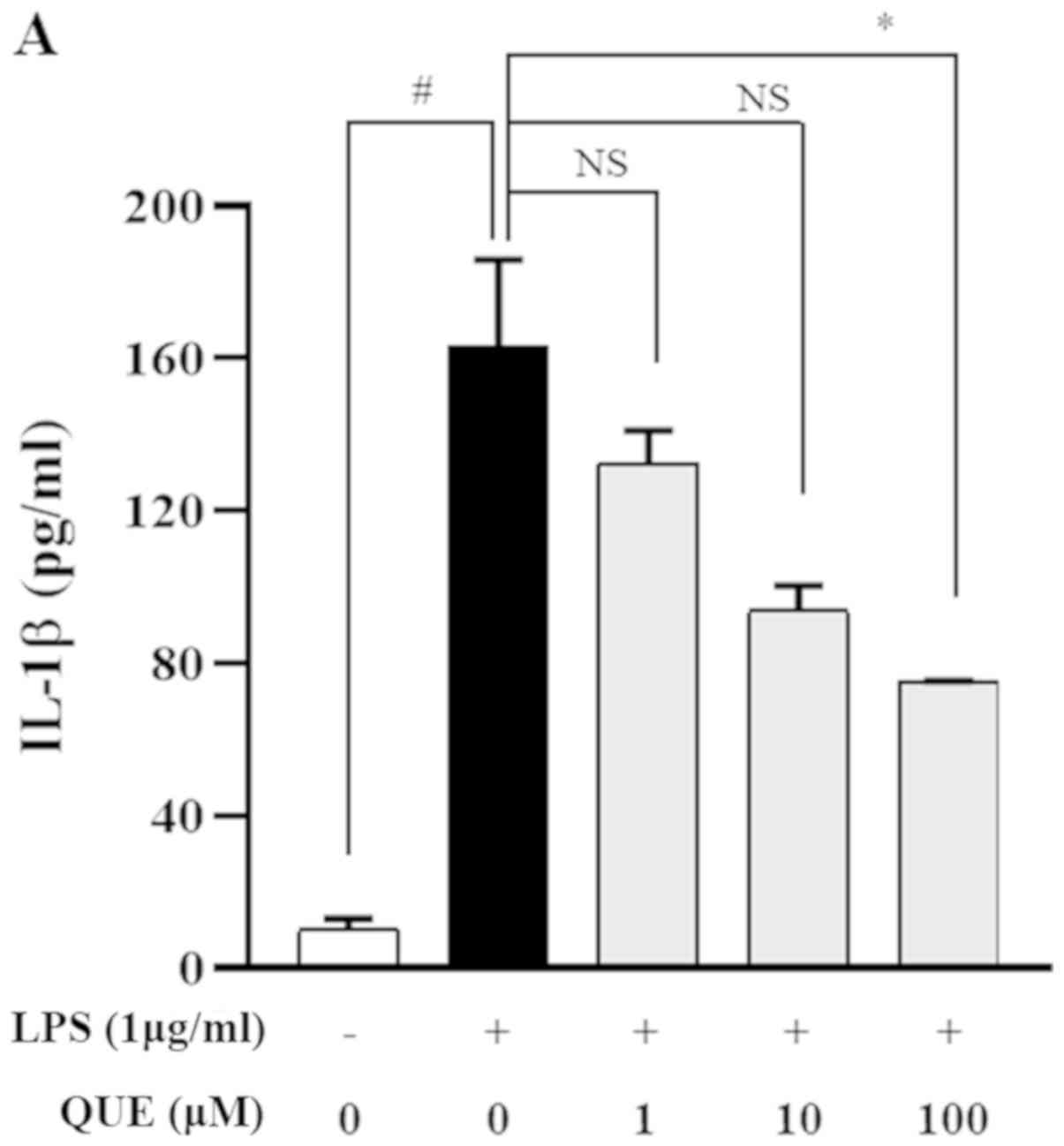 | Figure 3.Effect of QUE on IL-1β, IL-6 and
TNF-α mRNA expression levels in LPS-induced RAW264.7 macrophages.
Cells were induced with 1 µg/ml LPS and treated with various
concentrations (1, 10 or 100 µM) of QUE for 24 h. The production of
(A) IL-1β, (B) IL-6 and (C) TNF-α in the cell culture supernatants
were measured using an ELISA assay. (D) The mRNA expression levels
of IL-1β, IL-6 and TNF-α were determined through reverse
transcription-semi-quantitative PCR. GAPDH was used as a loading
control. The data shown represent the mean ± SD of three
experiments. #P<0.05 and ###P<0.001 vs.
non-treated group; *P<0.05, **P<0.01 and ***P<0.001 vs.
LPS-treated group. NS, not significant; QUE, quercetogetin; LPS,
lipopolysaccharide; TNF-α, tumor necrosis factor-α; IL-6,
interleukin-6; IL-1β, interleukin-1β. |
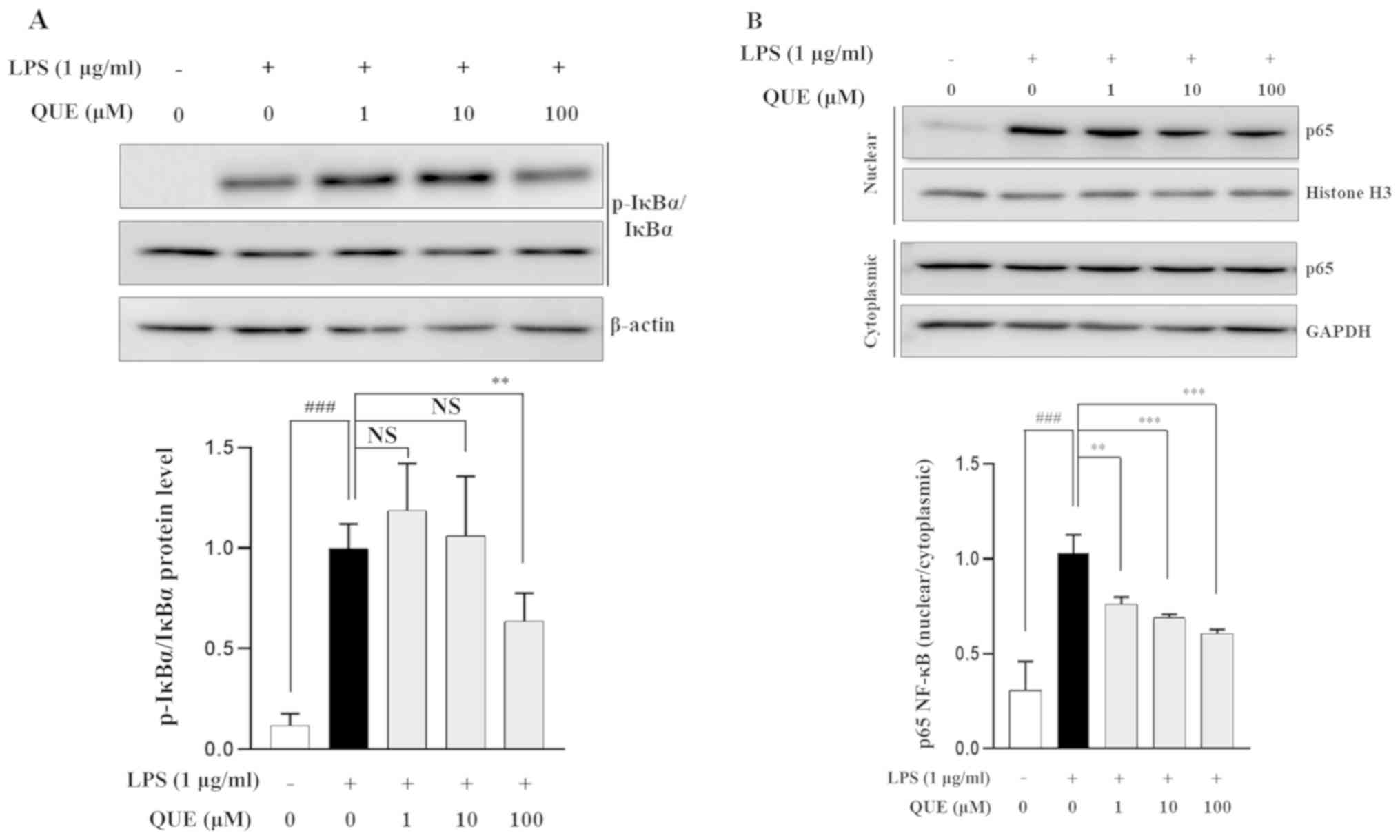
QUE inhibits phosphorylation of IκB-α
and NF-κB binding activity in LPS-induced RAW264.7 cells
NF-κB is an important transcription factor complex
that controls the expression of proinflammatory mediators (25). To determine whether QUE regulates
the NF-κB pathway, the phosphorylation level of IκB-α was
determined using western blot analysis. The results showed that 100
µM QUE inhibited the phosphorylation of IκB-α in LPS-induced
RAW264.7 cells (Fig. 4A). As p65
is the major subunit of the NF-κB complex, the nuclear
translocation of p65 after its release from IκB-a was investigated.
As shown in Fig. 4B, QUE
significantly decreased the LPS-induced translocation of p65 to the
nucleus. Overall, these results suggested that the NF-κB signaling
pathway might be involved in the regulation of inflammatory factors
by QUE in RAW264.7 cells.
QUE inhibits phosphorylation of ERK in
LPS-induced RAW264.7 cells
MAPK regulates inflammatory mediators, including
proinflammatory cytokines and NO (26). LPS activates the MAPK signaling
pathway in RAW264.7 cells (27).
To determine whether the inhibition of inflammation by QUE was
mediated via MAPK signaling, the effects of QUE on the
phosphorylation of ERK, p38, and JNK were observed in LPS-induced
cells. Fig. 5 shows that LPS
significantly increased the activation of all three MAPK molecules.
However, the phosphorylation of ERK was decreased by QUE in a
dose-dependent manner, whereas the phosphorylation levels of p38
and JNK were not changed. Collectively, these results indicated
that QUE has inhibitory effects on the ERK pathway in LPS-induced
macrophage cells.
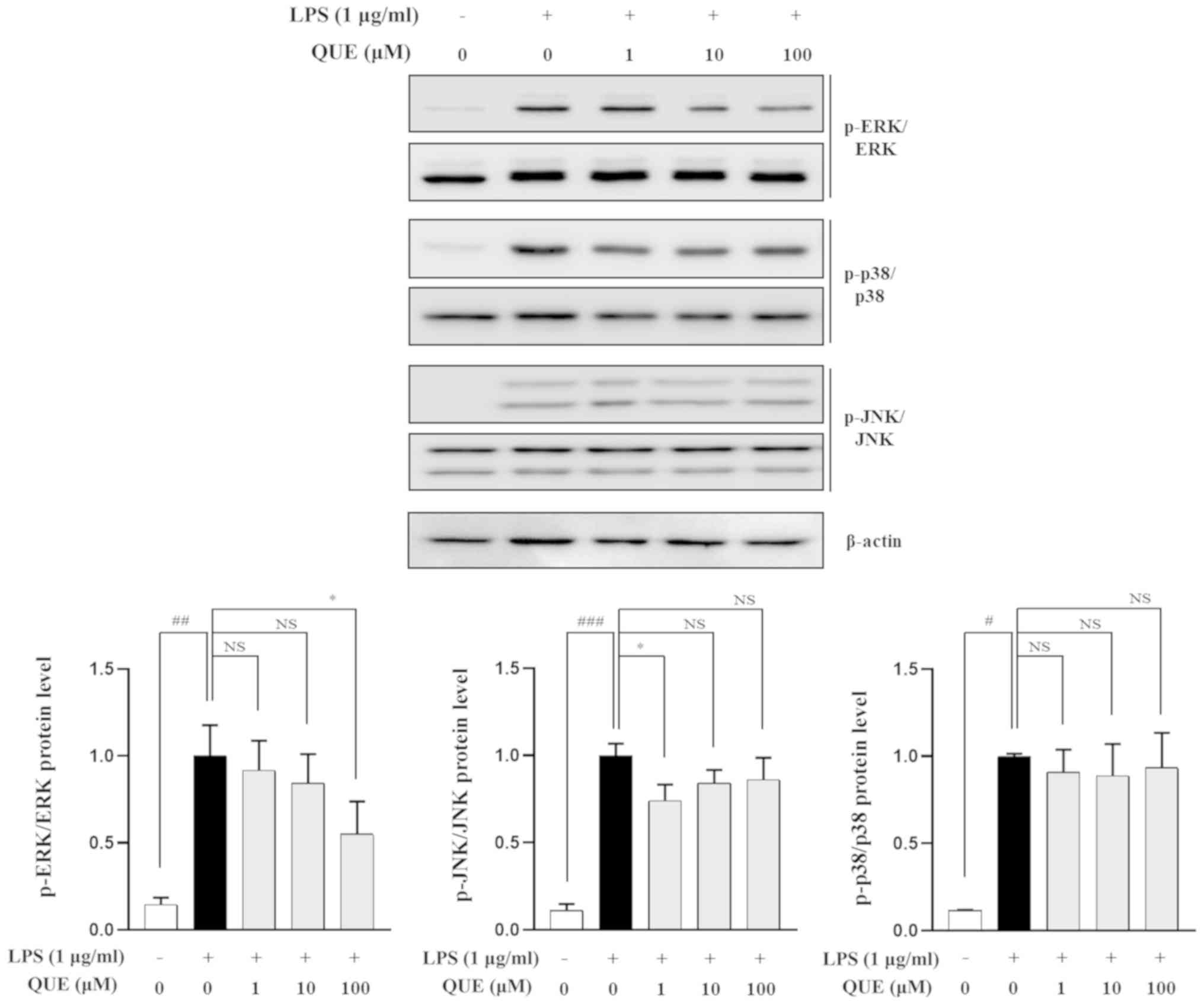 | Figure 5.Effect of QUE on the phosphorylation
of MAPK in LPS-induced RAW264.7 macrophages. Cells were pre-treated
for 2 h with various concentrations (1, 10 or 100 µM) of QUE and
then induced for 30 min with 1 µg/ml of LPS. Total proteins from
the cells were subjected to detection of the phosphorylated and
total forms of three MAPK molecules, ERK, p38 and JNK.
#P<0.05, ##P<0.01 and
###P<0.001 vs. non-treated group; *P<0.05 vs.
LPS-treated group. NS, not significant; QUE, quercetogetin; LPS,
lipopolysaccharide; MAPK, mitogen-activated protein kinase; JNK,
Jun N-terminalkinase; p-, phosphorylated. |
Discussion
CUP is used as a traditional medicine in Asia to
treat coughs, asthma and bronchial disorders. It is a rich source
of a number of flavanones and PMFs, which are also found in smaller
quantities in other plants (28).
PMFs are usually flavone aglycones with ≥4 methoxy substituents.
NOB and TAN belong to this class of flavonoids and are commonly
found in CUP (19). Also, these
compounds are of commercial interest due to their diverse
applications in the pharmaceutical and food industries. Previously,
several studies have reported that some PMFs possess
anti-inflammatory and anticancer properties (29–31).
However, the human health-related activities of the QUE, including
its anti-inflammatory effects, are relatively unknown. Therefore,
the anti-inflammatory mechanism of this compound was
investigated.
In the present study, compounds from extracts of CUP
that inhibited NO production and had anti-inflammatory effects were
isolated and characterized, and the molecular mechanisms underlying
these effects in LPS-stimulated RAW 264.7 cells were identified.
Based on the screening of NO production from CUP extracts, PMF
compounds were isolated from the chloroform phase, including NOB,
TAN and QUE. These compounds inhibited NO production in LPS-induced
cells in a dose-dependent manner. Recently, we reported that QUE
suppresses cigarette smoke extract-induced mitochondrial
dysfunction and mitophagy in two human bronchial epithelial cell
lines, Beas-2B and NHBE (32).
Inflammation is regulated by numerous inflammatory mediators, such
as NO, PGE2 and cytokines. Excessive production of iNOS-derived NO
and COX-2-derived PGE2 can cause chronic diseases involving
inflammatory and autoimmune disorders (33). These mediators regulate a variety
of pathological and physiological processes related to immune
responses and inflammation (34).
The regulation of inflammatory mediators is therefore important for
the development of novel anti-inflammatory drugs. In the present
study, it was observed that QUE (10–100 µM) inhibited iNOS and
COX-2 expression, thereby suppressing iNOS-induced NO production
and COX-2-induced PGE2 production. Moreover, QUE reduced TNF-α,
IL-6 and IL-1β expression in LPS-induced macrophage cells. These
results suggested that QUE has anti-inflammatory effects that are
mediated via the suppression of proinflammatory cytokines and
inflammatory mediators.
In the NF-κB signaling pathway, the p50/p65
heterodimer is the most common dimer found (35). In general, NF-κB is inactivated by
an interaction with IκB, which sequesters it in the cytoplasm.
However, when IκB is phosphorylated, NF-κB is released and
translocated to the nucleus (36).
The present results indicated that the nuclear translocation of p65
and LPS-induced phosphorylation of IκB-α are significantly reduced
after pretreatment with QUE. These results suggested that QUE
regulates inflammatory events via suppression of the NF-κB
pathway.
MAPKs play a pivotal role in the regulation of
cellular stress responses and activation of NF-κB (37). They regulate cellular response to
extracellular stimulation and various cellular activities, such as
apoptosis, differentiation, inflammation and gene expression
(38,39). The MAPK signaling pathway is known
to be involved in the regulation of iNOS and COX-2, as well as the
production of proinflammatory cytokines by LPS (40). The inhibition of the ERK, p38 and
JNK pathways is able to suppress the induction of proinflammatory
mediators by LPS (41). The ERK
pathway plays a critical role in mediating pain and inflammation in
joints (42). Goodrige et
al (43) suggested that ERK
might be associated with the production of proinflammatory
cytokines by macrophages. p38 is a potential therapeutic target
among MAPKs for inflammatory diseases because it is involved in the
regulation of a number of inflammatory processes (44). JNK is activated by proinflammatory
cytokines and mediators, and plays a crucial role in immune system
signaling (45). To examine
whether the inhibition of inflammation by QUE was regulated via
MAPK signaling, the effect of QUE on the phosphorylation of ERK,
p38 and JNK in LPS-stimulated RAW264.7 cells was investigated. It
significantly suppressed the phosphorylation of ERK in a
dose-dependent manner, but did not affect the phosphorylation of
p38 and JNK.
In the present study, QUE was isolated from CUP and
its anti-inflammatory effects via the inhibition of NF-κB and ERK
signaling pathways were demonstrated in RAW264.7 macrophage cells.
These findings suggested that QUE may be a potential
anti-inflammatory agent for the treatment of inflammatory
diseases.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by Gachon University Gil
Medical Center (grant no. FRD2017-10-02) and the Korea Research
Foundation (grant no. NRF-2019R1A2C2089867).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ESS made substantial contributions to conception and
design, analysis and interpretation of data, and drafting of the
manuscript. JWP contributed to data acquisition and interpretation,
drafted the manuscript and revised it critically for important
intellectual input. WH, OCK and JYN made substantial contributions
to data acquisition and interpretation, and the extraction and
isolation of quercetogetin. HRP and SHK made substantial
contributions to design, and data analysis and interpretation, and
revised the manuscript critically for important intellectual input.
SHJ interpreted the data, drafted the manuscript and revised it
critically for important intellectual input. CSL supervised the
manuscript, and made substantial contributions to conception and
design, and data analysis and interpretation. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gori AM, Cesari F, Marcucci R, Giusti B,
Paniccia R, Antonucci E, Gensini GF and Abbate R: The balance
between pro- and anti-inflammatory cytokines is associated with
platelet aggregability in acute coronary syndrome patients.
Atherosclerosis. 202:255–262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujiwara N and Kobayashi K: Macrophages in
inflammation. Curr Drug Targets Inflamm Allergy. 4:281–286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aderem A and Ulevitch RJ: Toll-like
receptors in the induction of the innate immune response. Nature.
406:782–787. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujihara M, Muroi M, Tanamoto K, Suzuki T,
Azuma H and Ikeda H: Molecular mechanisms of macrophage activation
and deactivation by lipopolysaccharide: Roles of the receptor
complex. Pharmacol Ther. 100:171–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim YH, Lee SH, Lee JY, Choi SW, Park JW
and Kwon TK: Triptolide inhibits murine-inducible nitric oxide
synthase expression by down-regulating lipopolysaccharide-induced
activity of nuclear factor-kappa B and c-Jun NH2-terminal kinase.
Eur J Pharmacol. 494:1–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Charo IF and Ransohoff RM: The many roles
of chemokines and chemokine receptors in inflammation. N Engl J
Med. 354:610–621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kiemer AK, Hartung T, Huber C and Vollmar
AM: Phyllanthus amarus has anti-inflammatory potential by
inhibition of iNOS, COX-2, and cytokines via the NF-kappaB pathway.
J Hepatol. 38:289–297. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhai XT, Zhang ZY, Jiang CH, Chen JQ, Ye
JQ, Jia XB, Yang Y, Ni Q, Wang SX, Song J, et al: Nauclea
officinalis inhibits inflammation in LPS-mediated RAW 264.7
macrophages by suppressing the NF-κB signaling pathway. J
Ethnopharmacol. 183:159–165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He J, Lu X, Wei T, Dong Y, Cai Z, Tang L
and Liu M: Asperuloside and asperulosidic acid exert an
anti-inflammatory effect via suppression of the NF-κB and MAPK
signaling pathways in LPS-induced RAW 264.7 Macrophages Int J Mol
Sci. 19:2027. 2018.
|
|
10
|
Havsteen B: Flavonoids, a class of natural
products of high pharmacological potency. Biochem Pharmacol.
32:1141–1148. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Middleton E Jr, Kandaswami C and
Theoharides TC: The effects of plant flavonoids on mammalian cells:
Implications for inflammation, heart disease, and cancer. Pharmacol
Rev. 52:673–751. 2000.PubMed/NCBI
|
|
12
|
Kim HP, Son KH, Chang HW and Kang SS:
Anti-inflammatory plant flavonoids and cellular action mechanisms.
J Pharmacol Sci. 96:229–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gil B, Sanz MJ, Terencio MC, Ferrándiz ML,
Bustos G, Payá M, Gunasegaran R and Alcaraz MJ: Effects of
flavonoids on Naja naja and human recombinant synovial
phospholipases A2 and inflammatory responses in mice. Life Sci.
54:PL333–PL338. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Manthey DE and Teichman J:
Nephrolithiasis. Emerg Med Clin North Am. 19633–654. (viii)2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu YQ, Zhou CH, Tao J and Li SN:
Antagonistic effects of nobiletin, a polymethoxyflavonoid, on
eosinophilic airway inflammation of asthmatic rats and relevant
mechanisms. Life Sci. 78:2689–2696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu JJ, Liu Z, Tang W, Wang GC, Chung HY,
Liu QY, Zhuang L, Li MM and Li YL: Tangeretin from citrus
reticulate inhibits respiratory syncytial virus replication and
associated inflammation in vivo. J Agric Food Chem. 63:9520–9527.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanes K, Tisserat B, Berhow M and
Vandercook C: Phenolic composition of various tissues of Rutaceae
species. Phytochemistry. 32:967–974. 1993. View Article : Google Scholar
|
|
18
|
Rouseff RL and Ting SV: Quantitation of
polymethoxylated flavones in orange juice by high-performance
liquid chromatography. J Chromatogr A. 176:75–87. 1979. View Article : Google Scholar
|
|
19
|
Li S, Lo CY and Ho CT: Hydroxylated
polymethoxyflavones and methylated flavonoids in sweet orange
(Citrus sinensis) peel. J Agric Food Chem. 54:4176–4185.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Itoh T, Ohguchi K, Iinuma M, Nozawa Y and
Akao Y: Inhibitory effects of polymethoxy flavones isolated from
Citrus reticulate on degranulation in rat basophilic
leukemia RBL-2H3: Enhanced inhibition by their combination. Bioorg
Med Chem. 16:7592–7598. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akao Y, Itoh T, Ohguchi K, Iinuma M and
Nozawa Y: Interactive effects of polymethoxy flavones from Citrus
on cell growth inhibition in human neuroblastoma SH-SY5Y cells.
Bioorg Med Chem. 16:2803–2810. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ko HC, Jang MG, Kang CH, Lee NH, Kang SI,
Lee SR, Park DB and Kim SJ: Preparation of a polymethoxyflavone
rich fraction (PRF) of Citrus sunki Hort. ex Tanaka and its
antiproliferative effects. Food Chem. 123:484–488. 2010. View Article : Google Scholar
|
|
23
|
Guo S, Qiu P, Xu G, Wu X, Dong P, Yang G,
Zheng J, McClements DJ and Xiao H: Synergistic anti-inflammatory
effects of nobiletin and sulforaphane in
lipopolysaccharide-stimulated RAW 264.7 cells. J Agric Food Chem.
60:2157–2164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harasstani OA, Moin S, Tham CL, Liew CY,
Ismail N, Rajajendram R, Harith HH, Zakaria ZA, Mohamad AS,
Sulaiman MR, et al: Flavonoid combinations cause synergistic
inhibition of proinflammatory mediator secretion from
lipopolysaccharide-induced RAW 264.7 cells. Inflamm Res.
59:711–721. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan GW, Gao XM, Wang H, Zhu Y, Zhang J, Hu
LM, Su YF, Kang LY and Zhang BL: The anti-inflammatory activities
of Tanshinone IIA, an active component of TCM, are mediated by
estrogen receptor activation and inhibition of iNOS. J Steroid
Biochem Mol Biol. 113:275–280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng CG, Kullberg MC, Jankovic D, Cheever
AW, Caspar P, Coffman RL and Sher A: Transgenic mice expressing
human interleukin-10 in the antigen-presenting cell compartment
show increased susceptibility to infection with Mycobacterium avium
associated with decreased macrophage effector function and
apoptosis. Infect Immun. 70:6672–6679. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rao KM, Meighan T and Bowman L: Role of
mitogen-activated protein kinase activation in the production of
inflammatory mediators: Differences between primary rat alveolar
macrophages and macrophage cell lines. J Toxicol Environ Health A.
65:757–768. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nogata Y, Sakamoto K, Shiratsuchi H, Ishii
T, Yano M and Ohta H: Flavonoid composition of fruit tissues of
citrus species. Biosci Biotechnol Biochem. 70:178–192. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lai CS, Li S, Chai CY, Lo CY, Ho CT, Wang
YJ and Pan MH: Inhibitory effect of citrus
5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone on
12-O-tetradecanoylphorbol 13-acetate-induced skin inflammation and
tumor promotion in mice. Carcinogenesis. 28:2581–2588. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li S, Sang S, Pan MH, Lai CS, Lo CY, Yang
CS and Ho CT: Anti-inflammatory property of the urinary metabolites
of nobiletin in mouse. Bioorg Med Chem Lett. 17:5177–5181. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pan MH, Lai YS, Lai CS, Wang YJ, Li S, Lo
CY, Dushenkov S and Ho CT:
5-Hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone induces apoptosis
through reactive oxygen species production, growth arrest and DNA
damage-inducible gene 153 expression, and caspase activation in
human leukemia cells. J Agric Food Chem. 55:5081–5091. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Son ES, Kim SH, Ryter SW, Yeo EJ, Kyung
SY, Kim YJ, Jeong SH, Lee CS and Park JW: Quercetogetin protects
against cigarette smoke extract-induced apoptosis in epithelial
cells by inhibiting mitophagy. Toxicol In Vitro. 48:170–178. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Palmer RM, Ashton DS and Moncada S:
Vascular endothelial cells synthesize nitric oxide from L-arginine.
Nature. 333:664–666. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Griswold DE and Adams JL: Constitutive
cyclooxygenase (COX-1) and inducible cyclooxygenase (COX-2):
Rationale for selective inhibition and progress to date. Med Res
Rev. 16:181–206. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karin M and Greten FR: NF-κB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Viatour P, Merville MP, Bours V and
Chariot A: Phosphorylation of NF-κB and IκB proteins: Implications
in cancer and inflammation. Trends Biochem Sci. 30:43–52. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Y, Gong Q, Guo W, Kan X, Xu D, Ma H, Fu
S and Liu J: Farrerol relieve lipopolysaccharide (LPS)-induced
mastitis by inhibiting AKT/NF-κB p65, ERK1/2 and P38 signaling
pathway. Int J Mol Sci. 19:17702018. View Article : Google Scholar
|
|
38
|
Arthur JSC and Ley SC: Mitogen-activated
protein kinases in innate immunity. Nat Rev Immunol. 13:679–692.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Uto T, Fujii M and Hou DX:
6-(Methylsulfinyl)hexyl isothiocyanate suppresses inducible nitric
oxide synthase expression through the inhibition of Janus kinase
2-mediated JNK pathway in lipopolysaccharide-activated murine
macrophages. Biochem Pharmacol. 70:1211–1221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yoon WJ, Moon JY, Song G, Lee YK, Han MS,
Lee JS, Ihm BS, Lee WJ, Lee NH and Hyun CG: Artemisia fukudo
essential oil attenuates LPS-induced inflammation by suppressing
NF-κB and MAPK activation in RAW 264.7 macrophages. Food Chem
Toxicol. 48:1222–1229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cruz CD, Neto FL, Castro-Lopes J, McMahon
SB and Cruz F: Inhibition of ERK phosphorylation decreases
nociceptive behaviour in monoarthritic rats. Pain. 116:411–419.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Goodridge HS, Harnett W, Liew FY and
Harnett MM: Differential regulation of interleukin-12 p40 and p35
induction via Erk mitogen-activated protein kinase-dependent and
-independent mechanisms and the implications for bioactive IL-12
and IL-23 responses. Immunology. 109:415–425. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Korb A, Tohidast-Akrad M, Cetin E, Axmann
R, Smolen J and Schett G: Differential tissue expression and
activation of p38 MAPK alpha, beta, gamma, and delta isoforms in
rheumatoid arthritis. Arthritis Rheum. 54:2745–2756. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dong C, Davis RJ and Flavell RA: MAP
kinases in the immune response. Annu Rev Immunol. 20:55–72. 2002.
View Article : Google Scholar : PubMed/NCBI
|