Introduction
Head and neck cancer is historically common and
lethal in adults worldwide (1). As
a frequently occurring malignancy, hypopharyngeal squamous cell
carcinoma (HSCC) is a major focus of research due to its high
morbidity and mortality rates. The incidence of HSCC, regardless of
age and sex, has increased, particularly in the developing world
(2). Risk factors for HSCC include
alcohol use, tobacco exposure, betel quid chewing, HPV infection
and personal history, such as genetics, toxin exposure, diet and
environmental factors (3,4). Due to advances in diagnostic and
therapeutic modalities, the survival rate for hypopharyngeal cancer
has improved since 1990 (5).
However, ~3,000 new cases are diagnosed annually in the USA
(6). Thus, a better understanding
of the molecular mechanisms underlying HSCC progression will likely
provide insight regarding the treatment of HSCC on an individual
basis.
Tumors have large bio-energetic requirements for the
maintenance of cell growth and proliferation. The hypoxic and
glucose-poor tumor micro-environment requires tumor cells to alter
their metabolic patterns to adapt to these conditions (7). A high rate of glycolysis, with
production of lactate and decreasing mitochondrial oxidative
phosphorylation (OXPHOS) metabolism is a universal phenomenon in
carcinoma cells (8,9), which results in the accumulation of
lactate within cells. Lactate in the tumor micro-environment is
catalyzed by lactate dehydrogenase to synthesize pyruvate, and
provide metabolic fuel for OXPHOS.
Lactate also takes part in the production of ATP, as
part of the tricarboxylic acid cycle (10,11),
and can also be used as a substrate for gluconeogenesis (12). In the process of energy metabolism,
the accumulation of lactate tends to rely on the cell membrane
receptor G-protein coupled receptor 81 (GPR81) and monocarboxylase
transporters (MCTs). GPR81 is mainly expressed in adipocytes,
skeletal muscle and brain, and is involved in energy metabolism
(13–15). Previously, GPR81 was observed to be
important in various types of cancer, participating in cell
survival, metastasis, tumor growth and chemoresistance (16–18).
GPR81 is activated by lactate, which inhibits the conversion of ATP
to cAMP (19). MCT1 has a high
affinity binding ability for lactate and is mainly responsible for
lactate uptake (20,21). Roland et al (18) demonstrated that GPR81 is important
for cancer cell regulation of lactate transport mechanisms, and
alters the expression of MCT1 and MCT4 in the presence of lactate
and glucose.
Phosphofructokinase 1 (PFK-1) is a primary control
enzyme in the glycolytic pathway, which catalyzes the
phosphorylation of fructose 6-phosphate to fructose
1,6-bisphosphate, accompanied by ATP conversion to ADP. In tumors,
PFK-1 levels are increased compared with non-tumor cells (18,22),
suggesting that PFK-1 might be a functional biomarker of abnormal
energy metabolism. As a key rate-limiting enzyme, the modification
and alteration of PFK-1 in glycolysis can disturb the glycolytic
pathway and result in metabolic disorders.
Tumor cells consume glucose through anaerobic
glycolysis and generate lactate and ATP even in the presence of
oxygen, which is known as the Warburg effect (23). Glycolysis and OXPHOS co-exist in
cancer cells and facilitate tumorigenesis and metastasis (24).
Translocase of the outer mitochondrial membrane 20
(TOMM20) is a key subunit of the TOM complex and a vital
mitochondrial transport protein. TOMM20 is regarded as a positive
marker of OXPHOS (25) and is
associated with many malignant tumors (25,26).
Lactate, which is mainly generated by glycolysis in
tumors, was recently identified as a major fuel for OXPHOS and an
activator of energy conversion signaling pathways (27). To the best of the authors'
knowledge, only a few previous publications have studied the effect
of GPR81 silencing and cisplatin treatment on cell survival and
energy metabolism in HSCC. Therefore, in the present study, several
factors associated with glycolysis and OSPHOS were analyzed. The
molecular role of GPR81 in the HSCC cell line FaDu was
investigated. Furthermore, the influence of silencing GPR81
combined with cisplatin on the expression of PFK-1 and TOMM20 was
studied, in order to identify the role played by GPR81 in
glycolysis and OXPHOS, in the context of HSCC. The effect of GPR81
knockdown combined with cisplatin treatment on cell survival was
also examined.
Materials and methods
Cell lines and cell culture
The human FaDu cell line is derived from
hypopharyngeal carcinoma. FaDu cells obtained from China Center For
Type Culture Collection were cultured in RPMI-1640 (Gibco, Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Hyclone; GE
Healthcare Life Sciences), 100 U/ml penicillin, 100 mg/ml
streptomycin and 0.1 M HEPES in a humidified atmosphere containing
5% CO2 at 37°C.
Plasmid construction
The interference plasmids containing human short
hairpin shRNA (shRNA)-GPR81 (also known as hHCAR1) and
shRNA-scramble were obtained from Cyagen Biosciences, Inc. The
shRNA-GPR81 plasmid effectively inhibited the expression of GPR81,
and the shRNA-scramble plasmid acted as a control.
GPR81 knockdown and cell
challenge
In order to inhibit the expression of GPR81, FaDu
cells were transfected with 2.5 µg shRNA-GPR81 plasmid and 2.5 µg
shRNA-scramble plasmid was used as a control. Transfections were
carried out using Lipofectamine® 3000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Transfection efficiency was determined in
the experimental group and the control group by western blot
analysis. Following transfection for 48 h, the transfected cells
were then challenged for 24 h with cisplatin at concentrations of
0, 0.5, 1, 2 and 5 µg/ml. Each group was plated in triplicate.
Cells were collected for RNA and protein extraction.
Cell proliferation assay
Following transfection with shRNA-GPR81 and
shRNA-scramble plasmid for 48 h, cells were seeded into a 96-well
plate at a density of 5×103 cells/well and stimulated
with 5 µg/ml cisplatin. Cell proliferation was evaluated using Cell
Counting Kit-8 reagent (CCK-8; Beyotime Institute of Biotechnology)
according to the manufacturer's protocol. The proliferation of FaDu
cells was measured by the absorbance at a wavelength of 450 nm
(OD450; where OD indicates optical density).
Transwell Matrigel™
invasion assay
The invasive capacity of FaDu cells transfected with
shRNA-GPR81 was assessed with Transwell Matrigel invasion assays. A
24-well chamber covering Matrigel™-coated (diluted 1:3 in
serum-free medium on ice; 100 µl/cm2) membranes with a
pore size of 8 µm (Corning, Inc.) was used to assess cell invasion.
Transfected cells were diluted in serum-free medium to a
concentration of 1×105 cells/ml. Cell suspensions (100
µl) were added to the upper chamber, and the lower chamber was
injected with 500 µl cell culture medium containing 20% FBS. Cells
were incubated for 24 h at 37°C with 5% CO2.
The upper chamber was washed with PBS twice and
cells under the chamber membrane were fixed with 4%
paraformaldehyde for 5–10 min at 4°C, then stained with 1% crystal
violet for 10 min at room temperature. The stained cells were
washed with PBS twice to wipe the staining fluid off of the
membrane. Cells were passed through the filter, and cotton tips
were used to remove cells remaining on the upper membrane. The
invasion of cells was evaluated with an inverted fluorescent
microscope (magnification, ×200, Olympus). Each group was set
triplicate wells.
Semi-quantitative-PCR
Total RNA derived from challenged FaDu cell lines
was isolated using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The quality of total RNA was detected by electrophoresis on a 1.2%
agarose gel. The concentration of RNA was measured using a NanoDrop
2000c Spectrophotometer (Thermo Fisher Scientific, Inc.). First
strand cDNA was synthesized from total RNA using Fast Reverse
Transcriptase (Tiangen Biotech Co., Ltd.), following the
manufacturer's instructions. Semi-quantitative PCR was used to
detect mRNA expression levels of related genes. The PCR product was
visualized by agarose gel containing 0.5 mg/ml ethidium bromide
(EB). And the grey scale value of PCR band was analyzed with ImageJ
software v1.8.0 (National Institutes of Health). The primers were
designed to amplify specific fragments. The primer sequences of
detected genes are presented in Table
I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Name | Sequence,
5′-3′ | Length,
nucleotides | Tm, °C |
|---|
| GPR81-F1 |
TGGCTGCGGACAGGTATT | 18 | 58.8 |
| GPR81-R1 |
CCAAACAATCTTGAAGGAGC | 20 | 57.8 |
| TOMM20-F1 |
GACCGCAAAAGACGAAGTG | 19 | 57.3 |
| TOMM20-R1 |
GGAACACTGGTGGTGGAAG | 19 | 58.2 |
| PFK-1-F1 |
TCAGAAGAGGGCAAAGGC | 18 | 58.6 |
| PFK-1-R1 |
GCTTGAGCCACCACTGTTCT | 19 | 58.4 |
| MCT1-F1 |
TCAAAATAGTCCGATGCC | 18 | 51.0 |
| MCT1-R1 |
TGCTGTTTTCCTTCTGCC | 18 | 53.9 |
| Caspase-3-F1 |
GAATGACATCTCGGTCTGGT | 20 | 54.1 |
| Caspase-3-R1 |
TGTCTCAATGCCACAGTCC | 19 | 53.8 |
| Bcl-2-F2 |
TCCAGGATAACGGAGGCT | 18 | 54.9 |
| Bcl-2-R2 |
CACTTGTGGCTCAGATAGGC | 20 | 55.5 |
| β-actin-F1 |
TTGGCAATGAGCGGTTCC | 18 | 58.3 |
| β-actin-R1 |
GAAGGTGGACAGCGAGGC | 19 | 57.2 |
The amplification was performed in a total volume of
20 µl, containing 10 µl 2X Taq PCR Master Mix (Tiangen Biotech Co.,
Ltd.), 1 µl diluted cDNA (1 µg/µl), 0.5 µl each primer (10 µM) and
8 µl nuclease-free water. Thermocycling conditions for GPR81,
TOMM20, PFK-1 and actin genes were as follows: 94°C for 3 min as
initial denaturation, 35 cycles of 94°C for 30 sec, 56°C for 30
sec, 72°C for 40 sec, then 72°C extension for 10 min. For caspase-3
and Bcl-2, the same thermocycling conditions were used, except for
annealing, which was carried out at 53°C for 30 sec. The annealing
temperature of MCT1 was 51°C, and the other steps were identical to
other genes. All PCR products were held at 16°C at the final stage
of PCR before detection on an agarose gel.
Western blotting
Cells were lysed with cold RIPA protein lysis buffer
supplemented with protease inhibitor (Beijing Solarbio Science
& Technology Co., Ltd.), then placed on a horizontal shaking
table for 40 min at 4°C. Split samples were centrifuged at 12,000 ×
g for 20 min at 4°C. The supernatant was collected to determine
protein density using a BCA Protein Assay kit (Beijing Solarbio
Science & Technology Co., Ltd.). The isolated protein was added
to 6X SDS-PAGE loading buffer and boiled for 10 min at 99°C. The
denatured protein (~40 µg) was first separated by SDS-PAGE on
pre-cast polyacrylamide gels (12% separating gel; 5% stacking gel),
then transferred to a wet polyvinylidene fluoride membrane. The
transferred membrane was dipped in 5% bovine serum albumin at 37°C
for 1 h, then incubated with primary antibodies (GPR81, Abcam, cat.
no. ab106942, 1:1,000; PFK-1, Santa Cruz Biotechnology, Inc., cat.
no. sc-166722, 1:1,000; TOMM20, Abcam, cat. no. ab186734, 1:1,000;
ß-actin, Santa Cruz Biotechnology, Inc., cat. no. sc-47778,
1:2,000) at 4°C overnight. Antibody dilutions were made according
to the manufacturer's protocol. The membrane was washed with TBS
with Tween-20 for 5 min three times. The membrane was then
incubated with HRP-conjugated goat anti-rabbit IgG antibody (Abcam,
cat. no. ab181662, 1:2,000) at room temperature for 2 h. The blots
were visualized using an enhanced chemiluminescence detection kit
(Thermo Fisher Scientific, Inc.). Grayscale blots were analyzed
with ImageJ v1.8.0 (National Institutes of Health).
Apoptosis detection
FaDu cells (~1×105-per well) were seeded
in 6-well plates were transfected with shRNA-GPR81 and
shRNA-scramble plasmids. Then, the transfected cells were
challenged for 24 h with cisplatin at the concentration of 5 µg/ml.
Cells were digested with 0.25% EDTA-trypsin and washed twice with
cold PBS. Apoptosis was assessed in each group according to the
annexin V-allophycocyanin/7-aminoatinomycin D apoptosis kit
protocol (MultiSciences Biotech Co,. Ltd.). The apoptotic cell rate
was calculated by the sum of Q2 (apoptosis at late phase or
necrotic cells) and Q3 (apoptosis at early stage).
Statistical analysis
SPSS 21.0 statistical software (IBM Corp.) was used
to analyze the data, using one-way ANOVA followed by Tukey's
multiple comparison test. The differences between two groups were
analyzed by Student's t-test. All data are presented as the mean ±
SD. P-values were two-sided and P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of GPR81 on cell proliferation
and invasion
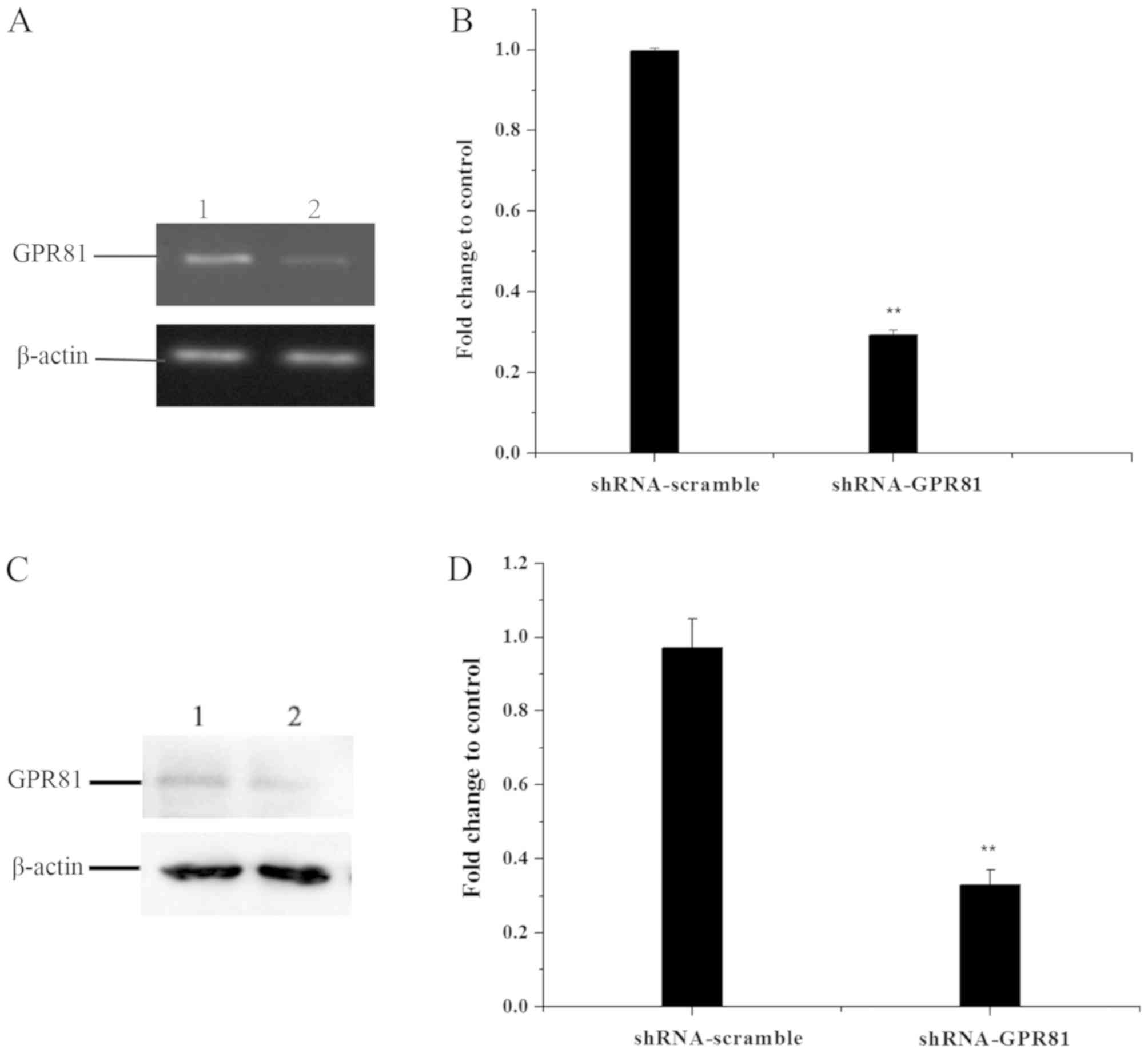
The efficiency of GPR81 gene deletion was detected
by semi-quantitative PCR and western blotting in FaDu cells
transfected with shRNA-GPR81 and shRNA-scramble plasmid vector
(Fig. 1). To examine the effect of
GPR81 on cell proliferation and invasion, a CCK-8 assay and a
Transwell Matrigel™ assay were performed, respectively.
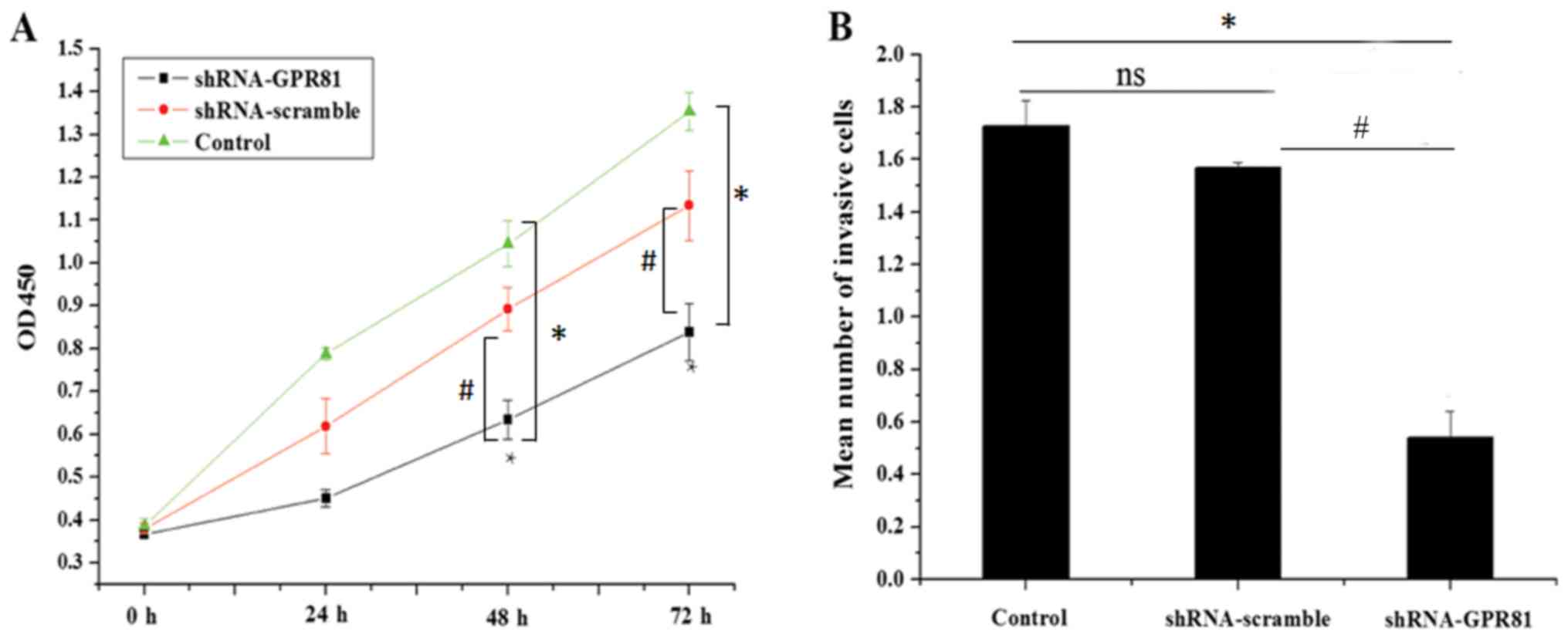
For the CCK-8 assay, the mean OD450 value in the
control, shRNA-scramble and shRNA-GPR81 groups increased to
different extents, suggesting a difference in proliferative
capacity between theshRNA-GPR81, shRNA-scramble and untreated cell
groups. The mean values of OD450 in the control group were
0.386±0.017, 0.788±0.013, 1.045±0.053 and 1.354±0.035 at time
points 0, 24, 48 and 72 h, respectively. The mean values of OD450
in the shRNA-scramble group were 0.38±0.012, 0.689±0.065,
0.932±0.051 and 1.194±0.08 at 0, 24, 48 and 72 h, respectively.
This was not statistically significant, compared with the control
group (P>0.05). The mean OD450 values in the shRNA-GPR81
group at 0, 24, 48 and 72 h, were 0.367±0.01, 0.451±0.02,
0.634±0.046 and 0.838±0.066, respectively. At the time points of 48
and 72 h, the OD450 values in shRNA-GPR81 group were significantly
lower compared with the control group and shRNA-scramble group
(with *P<0.05 vs. control and #P<0.05 vs.
shRNA-scramble), indicating that silencing GPR81 could inhibit the
proliferation of FaDu cells (Fig.
2A). These results suggested that GPR81 affected cell
proliferation.
In the Transwell Matrigel™ assay, the mean number of
cells passing through the membrane in the control, shRNA-scramble
and shRNA-GPR81 groups were 1.726±0.098×104,
1.5677±0.082×104 and 0.514±0.102×104,
respectively. The invading cells in the GPR81 silencing group was
significantly lower than the shRNA-scramble group (with *P<0.05
vs. control and #P<0.05 vs. shRNA-scramble; Fig. 2B). These results indicated that
silencing GPR81 in FaDu cells inhibited cell invasion.
GPR81 combined with cisplatin affects
the expression of genes involved in glycolysis and OXPHOS
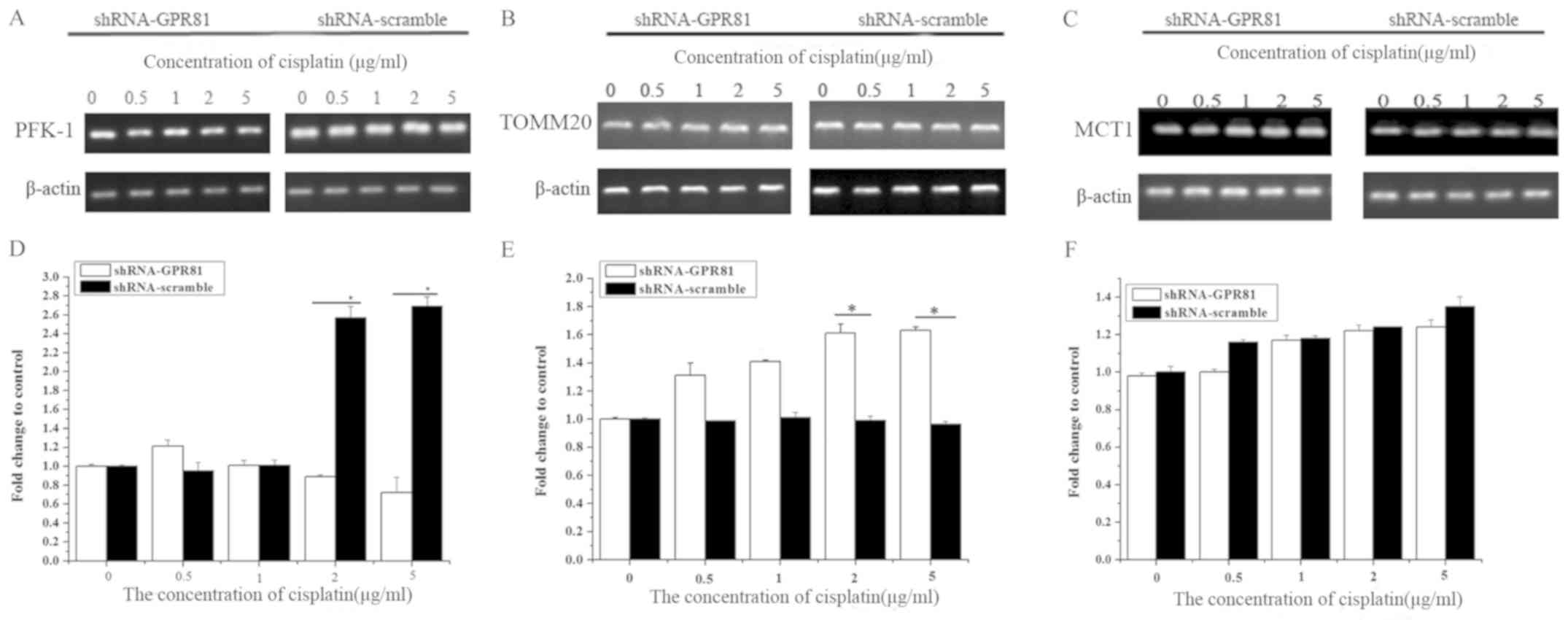
RT-semi quantitative PCR and western blotting were
used to detect the expression of PFK-1, TOMM20 and MCT1 in FaDu
cells at the nucleic acid and protein levels. The influence of
cisplatin challenge on PFK-1, TOMM20 and MCT-1 is shown in Fig. S1.
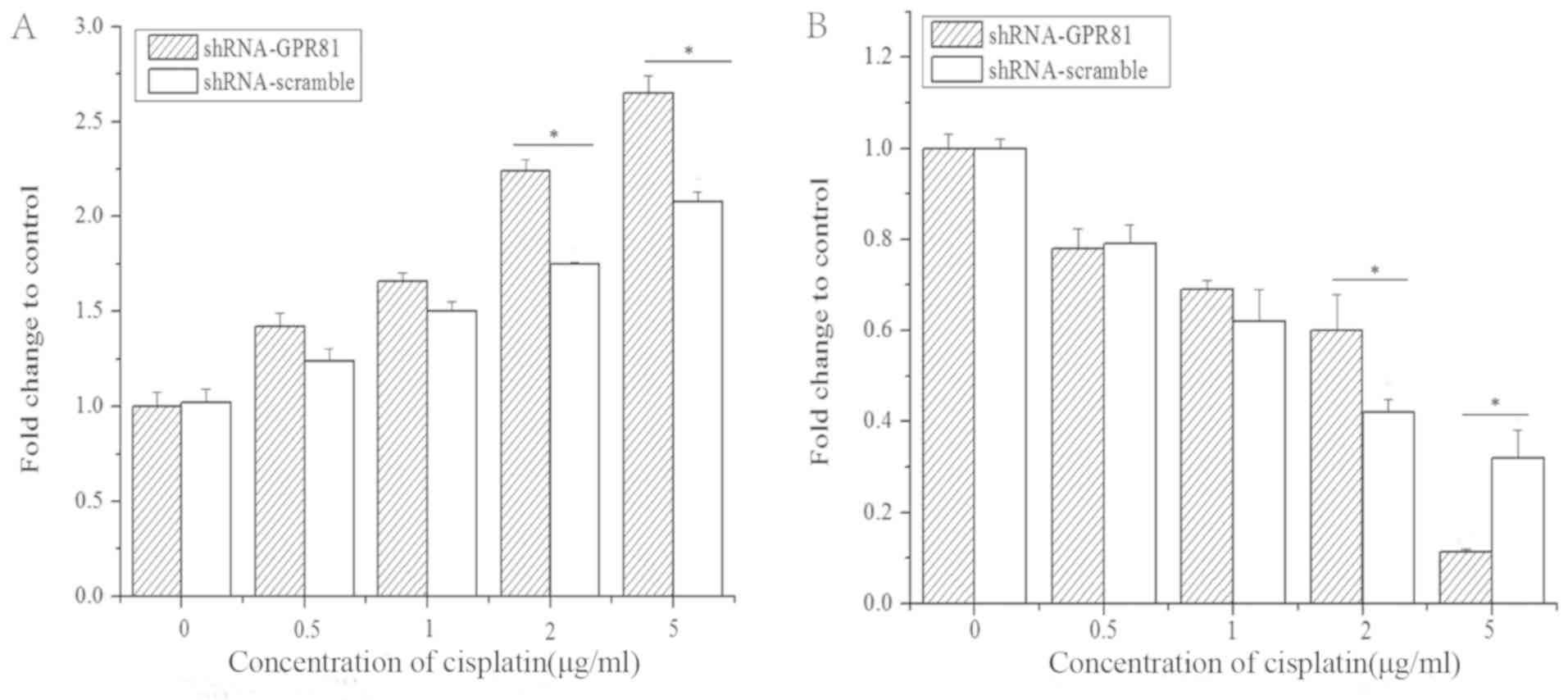
The mRNA expression of PFK-1 in GPR81 knockdown
cells combined with cisplatin stimulation slightly decreased, but
there was no significant difference compared with untreated cells
(P>0.05). PFK-1 in cells transfected with shRNA-scramble
significantly increased rapidly at higher concentrations of
cisplatin, which were significantly higher than shRNA-GPR81 cells
(P<0.05). At 2 and 5 µg/ml cisplatin, the difference between two
groups was statistically significant (P<0.05). These results
indicated that GPR81 combined with cisplatin might have some
influence on the expression of PFK-1 compared with shRNA-scramble
group, but no significant effect vs. untransfected cells (Fig. 3A and D).
For the TOMM20 gene, the mRNA expression increased
significantly after challenge with cisplatin in GPR81 silenced
cells, and peaked at the highest concentration of cisplatin, which
was 1.63-fold higher than the control (Fig. 3B and E). The expression of TOMM20
in the shRNA-scramble group increased slightly after being
stimulated with cisplatin, with no significant difference compared
with untreated cells. However, the expression of TOMM20 increased
significantly at 2 and 5 µg/ml cisplatin (P<0.05; Fig. 3E). This suggested that OXPHOS might
be affected by GPR81 and cisplatin treatment, through changes in
the expression of TOMM20.
The expression of MCT1 gradually increased in both
groups stimulated by cisplatin. In addition, there was no
significant difference compared with untreated cells, indicating
that MCT1 was not affected by GPR81 after challenge with cisplatin
(Fig. 3C and F).
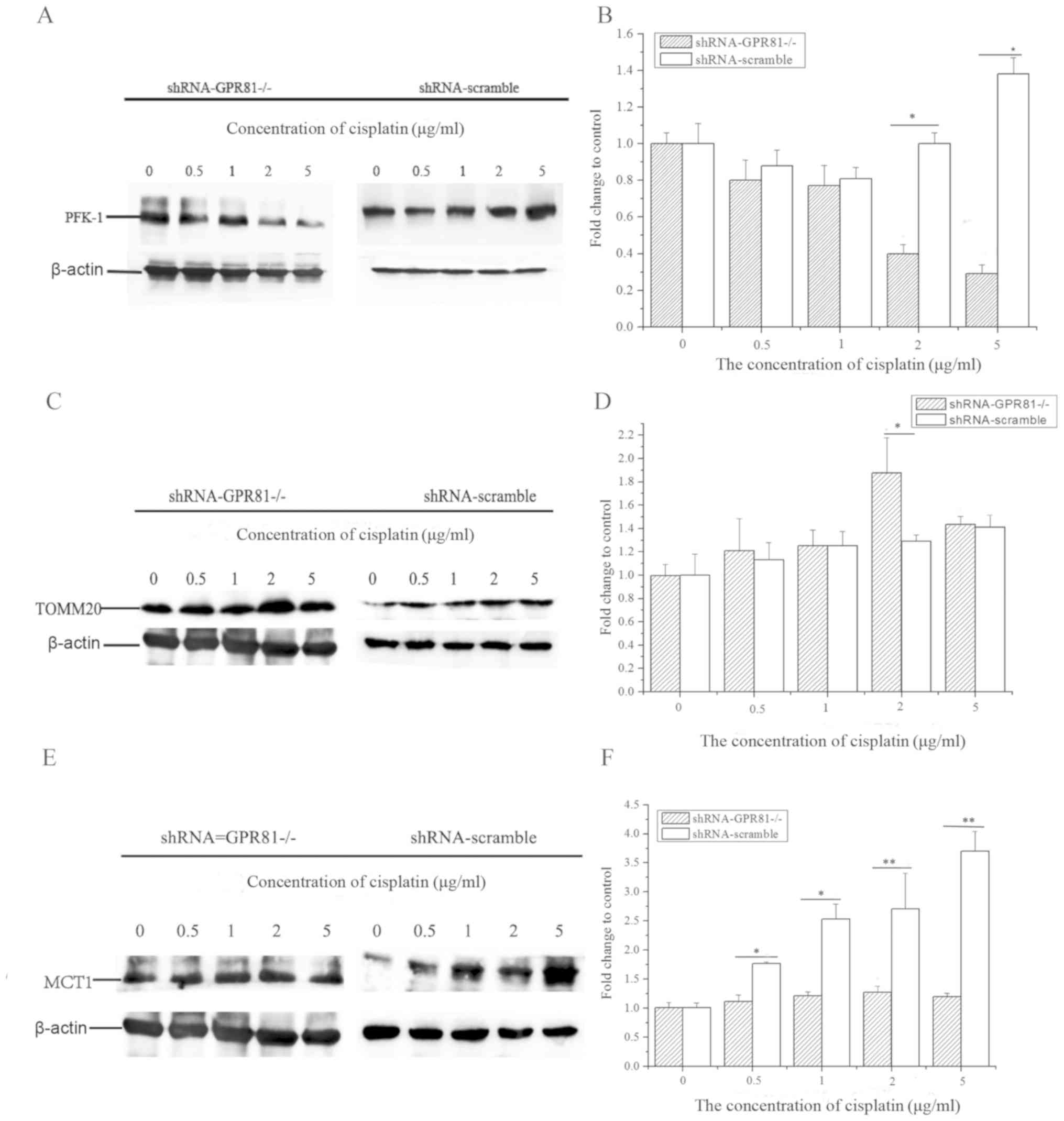
At the protein level, PFK-1 expression in GPR81
knockdown cells markedly decreased after cisplatin treatment
(lowest value ~0.29-fold of control at the highest concentration of
cisplatin). However, the expression of PFK-1 in the shRNA-scramble
group was increased after cisplatin challenge (Fig. 4A and B). The expression of PFK-1
with 2 and 5 µg/ml cisplatin incubation in shRNA-GPR81 and
shRNA-scramble groups exhibited a significant difference
(P<0.05). These results indicated that GPR81 affected the levels
of PFK-1, which in turn might inhibit glycolysis following
challenge with cisplatin.
For TOMM20, the total expression trend of the
protein level was similar to that of the mRNA level, with a slight
upregulation in both shRNA-GPR81 and shRNA-scramble groups. TOMM20
protein expression was the highest at a concentration of 2 µg/ml
cisplatin. The expression of TOMM20 in shRNA-GPR81 was
significantly higher compared with the shRNA-scramble group
(P<0.05) after challenge with 22 µg/ml cisplatin. These results
suggested that cisplatin stimulation might enhance the process of
OXPHOS in cancer cells (Fig. 4C and
D).
The expression of MCT1 in two groups manifested
upregulation following cisplatin incubation. However, the
expression of MCT1 in shRNA-scramble group was significantly higher
than that in shRNA-GPR81 group after stimulation by 0.5 and 1 µg/ml
cisplatin (P<0.05), and significant differences were found after
stimulation by 2 and 5 µg/ml cisplatin (P<0.01; Fig. 4E and F).
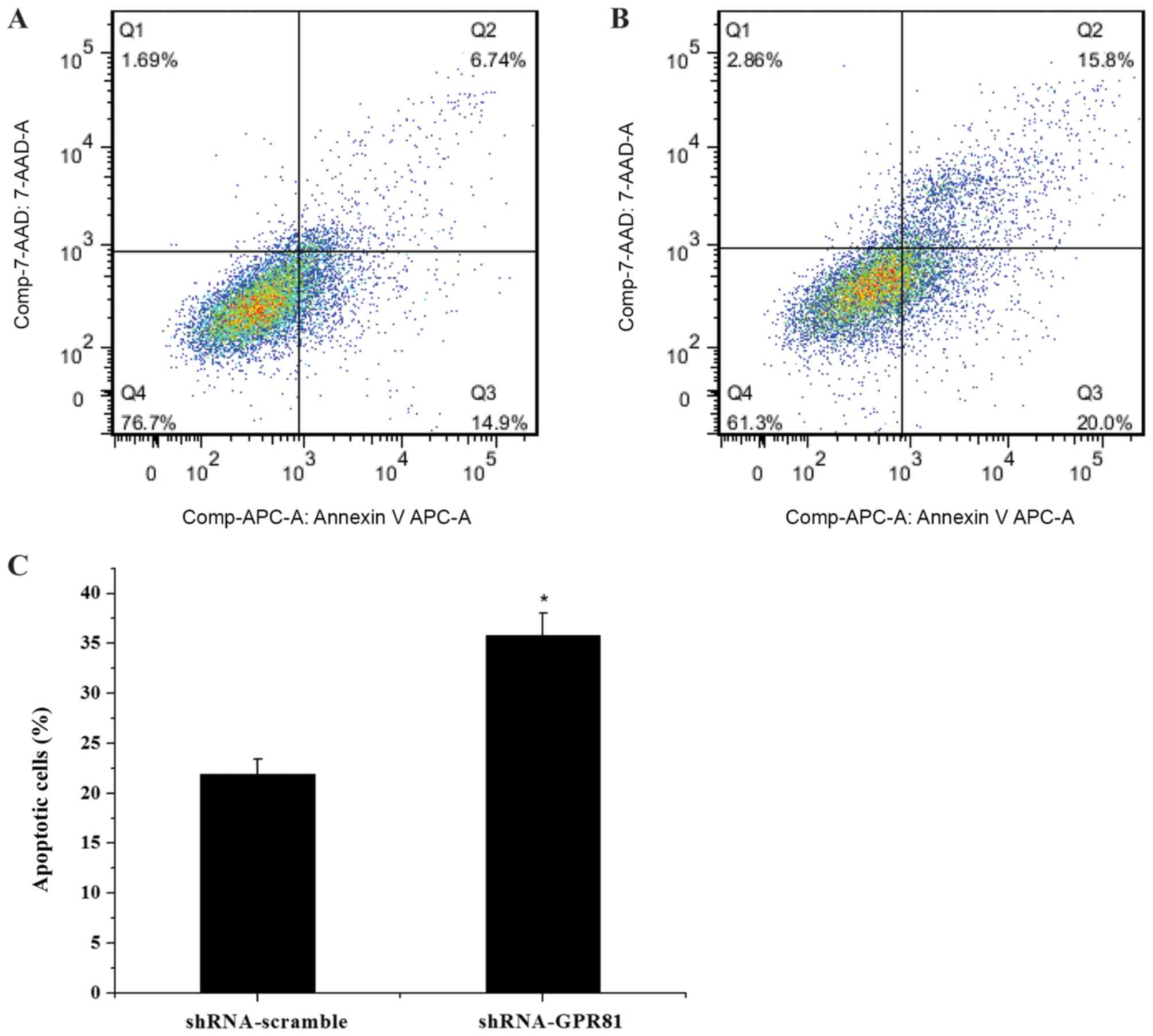
GPR81 silencing combined with
cisplatin promotes apoptosis in FaDu cells
The mRNA expression profile of caspase-3 and Bcl-2
in GPR81-silenced cells after cisplatin stimulation was also
assessed (Fig. 5). In both the
shRNA-GPR81 silencing group and shRNA-scramble group, the
expression of caspase-3 was gradually elevated, to a peak at 5
µg/ml of cisplatin. Following incubation with 2 and 5 µg/ml
cisplatin, the expression of caspase-3 in shRNA-GPR81 and
shRNA-scramble groups showed a significant difference (P<0.05).
For the Bcl-2 gene, mRNA expression decreased in shRNA-GPR81 and
shRNA-scramble groups, to ~0.1 and 0.3-fold of the untreated cells
group. A significant difference in the expression of Bcl-2 was
observed in the shRNA-GPR81 and shRNA-scramble groups at 2 and 5
µg/ml cisplatin (P<0.05). These results suggested that GPR81
affected apoptosis following cisplatin treatment. Flow cytometry
analyses showed that the apoptotic cell rate of the shRNA-GPR81
group was higher than that of the shRNA-scramble group (P<0.05;
Fig. 6). The results indicated
that GPR81 combined with cisplatin enhanced apoptosis in cancer
cells.
Discussion
The present study demonstrated the influence of
GPR81 on energy metabolism and apoptosis in hypopharyngeal
carcinoma in vitro, using an shRNA interference technique
combined with the chemotherapeutic agent cisplatin. The results of
the present study suggested that GPR81 gene knockdown inhibited
cell proliferation and invasion. Moreover, GPR81 silencing combined
with cisplatin affects the expression of PFK-1, TOMM20 and MCT1,
which are involved in energy metabolism and lactate transport.
Furthermore, cisplatin treatment increased apoptosis in
GPR81-silenced cells, as indicated by altered levels ofcaspase-3.
Altogether, the findings of the present study suggested that GPR81
knockdown might increase the efficacy of chemotherapy agents, such
as cisplatin, enhance apoptosis and influence the pathways related
to energy metabolism.
As a crucial lactate receptor, GPR81 directly
affects metabolites involved in tumor cell survival and energy
metabolism, altering the uptake of glucose and modulating the
normal process in mammalian organisms by activating various
receptors (28). A similar
observation regarding the role of GPR81 was made in breast cancer
and pancreatic carcinoma cell lines (29,30).
These previous studies indicated that the absence of GPR81 in
breast cancer cells and pancreatic carcinoma cell lines markedly
decreased cell proliferation and invasion, leading to cell death
in vitro.
GPR81 also functions as a vital regulator of cell
survival by inhibiting apoptosis and promoting cell survival
(19,18). In the present study, it was
hypothesized that the inhibition of GPR81 on cell proliferation
would depend on apoptosis. Cisplatin is a conventional
chemotherapeutic reagent used in the treatment of various tumor
types (30), and blocks malignant
cell growth through various mechanisms, such as DNA damage, cell
cycle deregulation, apoptosis and autophagy (31–33).
Our previous study demonstrated that cisplatin treatment enhanced
apoptosis in FaDu cells (34).
GPR81 knockdown in FaDu cells led to an increase in apoptosis in
the presence of cisplatin. In the present study, the proportion of
apoptotic cells was also significantly increased in shRNA-GPR81
FaDu cells. In addition, the expression of caspase-3 increased,
while the expression of Bcl-2 decreased in the presence of
cisplatin. A similar conclusion regardingGPR81 was also previously
suggested in HeLa cells, demonstrating that silencing GPR81
accelerated apoptosis in another in vitro system (16). Therefore, GPR81 regulates survival
and apoptosis in hypopharyngeal carcinoma cells.
The coexistence of glycolysis with the generation of
lactate and mitochondrial OXPHOS is a hallmark in carcinoma cells
(35,36). Lactate is a vital bridge in energy
metabolism. The lack of the lactate receptor GPR81 would affect the
content of lactate and ultimately influence biological metabolism.
The present study examined whether the absence of GPR81 and
cisplatin stimulation would have an impact on glycolysis and OXPHOS
by detecting key markers at the mRNA and protein levels. It was
demonstrated that shRNA-GPR81 combined with cisplatin decreased
PFK-1 mRNA and protein levels, but increased TOMM20 and MCT1 mRNA
and protein levels to varying degrees. PFK-1 is essential for
glycolysis, as it catalyzes rate-limiting steps of fructose
6-phosphate and ATP conversion into fructose 1,6-bisphosphate and
ADP (37). A previous study
identified that cisplatin treatment decreased glycolysis by
inhibiting PFK-1 expression (38).
In the present study, the importance of GPR81 in triggering
cisplatin efficacy and weakening the glycolysis pathway was
demonstrated in HSCC. Thus, reduced expression of PFK-1 could
contribute to the alteration of lactate, allowing tumor cells to
alter glycolysis to survive in a complex micro-environment.
TOMM20, a pivotal translocase located in the outer
mitochondrial membrane, has been reported as susceptible to
cisplatin. In addition, the protein level of TOMM20 was increased
when incubated with cisplatin in cholangiocarcinoma cells (39). However, the relationship between
GPR81 and TOMM20 is still ambiguous. In our previous study,
cisplatin induced a slight downregulation of TOMM20 in FaDu cells
(34). In the present study, the
elevated mRNA and protein levels of TOMM20 could be caused by
changes in GPR81levels in the presence of cisplatin.
MCT1 is involved in cellular uptake of lactate. The
expression of MCT1 is induced by lactate, and accumulation of
lactate also increases the expression of MCT1 (40). The absence of GPR81 affects lactate
binding, leading to lactate accumulation in cells. Moreover,
expression of MCT1 was correlated with cisplatin-resistance in
epithelial ovarian tumors (41).
In epithelial ovarian tumors, the expression of MCT1 was enhanced
after treatment with cisplatin. Hu et al (42) demonstrated that both GPR81 and MCT1
were highly expressed in squamous carcinoma; however, the
expression of GPR81 and MCT1 had no association in squamous
carcinoma. In the present study, MCT1 showed a marginal increase in
GPR81-silenced cells and after cisplatin treatment. Thus, depletion
of GPR81could change the concentration of lactate, which would
indirectly influence the process of lactate uptake. Finally,
changes in the expression of MCT1 were due to a combined effect of
GPR81 and cisplatin.
In summary, the present study suggested a critical
role for GPR81 in hypopharyngeal carcinoma following cisplatin
treatment. Moreover, the present study provided an insight into the
mechanism underlying the cellular metabolism and resistance to
cisplatin through PFK-1, TOMM20 and MCT1. Notably, GPR81 was
crucial for cell proliferation and invasion, which may account for
the changes in energy metabolism alteration seen in hypopharyngeal
cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The research was supported by Excellent Talents Plan
Foundation of Hebei province (grant no. 361004) and Hebei province
Natural Science Foundation (grant no. H2016206535).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW performed the of cell culture and cell challenge.
OX performed the of semi-quantitative PCR and analyzed the data. JD
designed the sequence of primers used in the present study. XJ
performed the RNA extraction and protein lysis. QJ performed cell
transfection, cell proliferation, invasion, western blotting and
apoptosis assays, and was a major contributor in writing the
manuscript. XR analyzed the western blotting data and revised the
manuscript. CS designed the present study and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cohen N, Fedewa S and Chen AY:
Epidemiology and demographics of the head and neck cancer
population. Oral Maxillofac Surg Clin North Am. 30:381–395. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bradley PJ: Epidemiologyz of
hypopharyngeal cancer. Adv Otorhinolaryngol. 83:1–14.
2019.PubMed/NCBI
|
|
3
|
Znaor A, Brennan P, Gajalakshmi V, Mathew
A, Shanta V, Varghese C and Boffetta P: Independent and combined
effects of tobacco smoking, chewing and alcohol drinking on the
risk of oral, pharyngeal and esophageal cancers in Indian men. Int
J Cancer. 105:681–686. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mehanna H, Beech T, Nicholson T, El-Hariry
I, McConkey C, Paleri V and Roberts S: Prevalence of human
papillomavirus in oropharyngeal and nonoropharyngeal head and neck
cancer-systematic review and meta-analysis of trends by time and
region. Head Neck. 35:747–755. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Newman JR, Connolly TM, Illing EA, Kilgore
ML, Locher J and Carroll WR: Survival trends in hypopharyngeal
cancer: A population-based review. Laryngoscope. 125:624–629. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
American Cancer Society: Laryngeal and
hypopharyngeal cancer. January
4–2015
|
|
7
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moreno-Sánchez R, Rodríguez-Enríquez S,
Marín-Hernández A and Saavedra E: Energy metabolism in tumor cells.
FEBS J. 274:1393–1418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fantin VR, St-Pierre J and Leder P:
Attenuation of LDH-A expression uncovers a link between glycolysis,
mitochondrial physiology, and tumor maintenance. Cancer Cell.
9:425–434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Díaz-Muñiz I, Banavara DS, Budinich MF,
Rankin SA, Dudley EG and Steele JL: Lactobacillus casei metabolic
potential to utilize citrate as an energy source in ripening
cheese: A bioinformatics approach. J Appl Microbiol. 101:872–882.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brodsky AN, Odenwelder DC and Harcum SW:
High extracellular lactate causes reductive carboxylation in breast
tissue cell lines grown under normoxic conditions. PLoS One.
14:e02134192019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grasmann G, Smolle E, Olschewski H and
Leithner K: Gluconeogenesis in cancer cells-Repurposing of a
starvation-induced metabolic pathway? Biochim Biophys Acta Rev
Cancer. 1872:24–36. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu C, Wu J, Zhu J, Kuei C, Yu J, Shelton
J, Sutton SW, Li X, Yun SJ, Mirzadegan T, et al: Lactate inhibits
lipolysis in fat cells through activation of an orphan
G-protein-coupled receptor, GPR81. J Biol Chem. 284:2811–2822.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rooney K and Trayhurn P: Lactate and the
GPR81 receptor in metabolic regulation: Implications for adipose
tissue function and fatty acid utilisation by muscle during
exercise. Br J Nutr. 106:1310–1316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morland C, Lauritzen KH, Puchades M,
Holm-Hansen S, Andersson K, Gjedde A, Attramadal H, Storm-Mathisen
J and Bergersen LH: The lactate receptor, G-protein-coupled
receptor 81/hydroxycarboxylic acid receptor 1: Expression and
action in brain. J Neurosci Res. 93:1045–1055. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wagner W, Kania KD, Blauz A and Ciszewski
WM: The lactate receptor (HCAR1/GPR81) contributes to doxorubicin
chemoresistance via ABCB1 transporter up-regulation in human
cervical cancer HeLa cells. J Physiol Pharmacol. 68:555–564.
2017.PubMed/NCBI
|
|
17
|
Feng J, Yang H, Zhang Y, Wei H, Zhu Z, Zhu
B, Yang M, Cao W, Wang L and Wu Z: Tumor cell-derived lactate
induces TAZ-dependent upregulation of PD-L1 through GPR81 in human
lung cancer cells. Oncogene. 36:5829–5839. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roland CL, Arumugam T, Deng T, Liu SH,
Philip B, Gomez S, Burns WR, Ramachandran V, Wang H,
Cruz-Monserrate Z and Logsdon CD: Cell surface lactate receptor
GPR81 is crucial for cancer cell survival. Cancer Res.
74:5301–5310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Caslin HL, Abebayehu D, Abdul Qayum A,
Haque TT, Taruselli MT, Paez PA, Pondicherry N, Barnstein BO,
Hoeferlin LA, Chalfant CE and Ryan JJ: Lactic acid inhibits
lipopolysaccharide-induced mast cell function by limiting
glycolysis and ATP availability. J Immunol. 203:453–464. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ideno M, Kobayashi M, Sasaki S, Futagi Y,
Narumi K, Furugen A and Iseki K: Involvement of monocarboxylate
transporter 1 (SLC16A1) in the uptake of l-lactate in human
astrocytes. Life Sci. 192:110–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Curry JM, Tuluc M, Whitaker-Menezes D,
Ames JA, Anantharaman A, Butera A, Leiby B, Cognetti DM, Sotgia F,
Lisanti MP, et al: Cancer metabolism, stemness and tumor
recurrence: MCT1 and MCT4 are functional biomarkers of metabolic
symbiosis in head and neck cancer. Cell Cycle. 12:1371–1384. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan Y, Guo-Qing P, Yan T, Hong-Lin Y,
Gong-Hua H and Cai-Gao Z: A study of PKM2, PFK-1, and ANT1
expressions in cervical biopsy tissues in China. Med Oncol.
29:2904–2910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gooptu M, Whitaker-Menezes D, Sprandio J,
Domingo-Vidal M, Lin Z, Uppal G, Gong J, Fratamico R, Leiby B,
Dulau-Florea A, et al: Mitochondrial and glycolytic metabolic
compartmentalization in diffuse large B-cell lymphoma. Semin Oncol.
44:204–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sotgia F, Whitaker-Menezes D,
Martinez-Outschoorn UE, Flomenberg N, Birbe RC, Witkiewicz AK,
Howell A, Philp NJ, Pestell RG and Lisanti MP: Mitochondrial
metabolism in cancer metastasis: Visualizing tumor cell
mitochondria and the ‘reverse Warburg effect’ in positive lymph
node tissue. Cell Cycle. 11:1445–1454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao Z, Han F, He Y, Yang S, Hua L, Wu J
and Zhan W: Stromal-epithelial metabolic coupling in gastric
cancer: Stromal MCT4 and mitochondrial TOMM20 as poor prognostic
factors. Eur J Surg Oncol. 40:1361–1368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park SH, Lee AR, Choi K, Joung S, Yoon JB
and Kim S: TOMM20 as a potential therapeutic target of colorectal
cancer. BMB Rep. 52:712–717. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duan K, Liu ZJ, Hu SQ, Huo HY, Xu ZR, Ruan
JF, Sun Y, Dai LP, Yan CB, Xiong W, et al: Lactic acid induces
lactate transport and glycolysis/OXPHOS interconversion in
glioblastoma. Biochem Biophys Res Commun. 503:888–894. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brown TP and Ganapathy V: Lactate/GPR81
signaling and proton motive force in cancer: Role in angiogenesis,
immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther.
206:1074512020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee YJ, Shin KJ, Park SA, Park KS, Park S,
Heo K, Seo YK, Noh DY, Ryu SH and Suh PG: G-protein-coupled
receptor 81 promotes a malignant phenotype in breast cancer through
angiogenic factor secretion. Oncotarget. 7:70898–70911. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rancoule C, Guy JB, Vallard A, Ben Mrad M,
Rehailia A and Magné N: 50th anniversary of cisplatin. Bull Cancer.
104:167–176. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang K, Zhang B, Bai Y and Dai L: E2F1
promotes cancer cell sensitivity to cisplatin by regulating the
cellular DNA damage response through miR-26b in esophageal squamous
cell carcinoma. J Cancer. 11:301–310. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi S, Tan P, Yan B, Gao R, Zhao J, Wang
J, Guo J, Li N and Ma Z: ER stress and autophagy are involved in
the apoptosis induced by cisplatin in human lung cancer cells.
Oncol Rep. 35:2606–2614. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zheng AW, Chen YQ, Fang J, Zhang YL and
Jia DD: Xiaoaiping combined with cisplatin can inhibit
proliferation and invasion and induce cell cycle arrest and
apoptosis in human ovarian cancer cell lines. Biomed Pharmacother.
89:1172–1177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu O, Jia QJ, Wang JX and Shan C: A
preliminary study on the function of GPR81 and TOMM20 in
hypopharyngeal carcinoma. Chin J Otorhinolaryngol Skull Base Surg.
25:498–503. 2019.
|
|
35
|
Ashton TM, McKenna WG, Kunz-Schughart LA
and Higgins GS: Oxidative phosphorylation as an emerging target in
cancer therapy. Clin Cancer Res. 24:2482–2490. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bottoni P and Scatena R: Mitochondrial
metabolism in cancer. A tangled topic. Which role for proteomics?
Adv Exp Med Biol. 1158:1–16. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mor I, Cheung EC and Vousden KH: Control
of glycolysis through regulation of PFK-1: Old friends and recent
additions. Cold Spring Harb Symp Quant Biol. 76:211–216. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marín-Hernández A, Gallardo-Pérez JC,
López-Ramírez SY, García-García JD, Rodríguez-Zavala JS,
Ruiz-Ramírez L, Gracia-Mora I, Zentella-Dehesa A, Sosa-Garrocho M,
Macías-Silva M, et al: Casiopeina II-gly and bromo-pyruvate
inhibition of tumor hexokinase, glycolysis, and oxidative
phosphorylation. Arch Toxicol. 86:753–766. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fan Z, Yu H, Cui N, Kong X, Liu X, Chang
Y, Wu Y, Sun L and Wang G: ABT737 enhances cholangiocarcinoma
sensitivity to cisplatin through regulation of mitochondrial
dynamics. Exp Cell Res. 335:68–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lottes RG, Newton DA, Spyropoulos DD and
Baatz JE: Lactate as substrate for mitochondrial respiration in
alveolar epithelial type II cells. Am J Physiol Lung Cell Mol
Physiol. 308:L953–L961. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yan C, Yang F, Zhou C, Chen X, Han X, Liu
X, Ma H and Zheng W: MCT1 promotes the cisplatin-resistance by
antagonizing Fas in epithelial ovarian cancer. Int J Clin Exp
Pathol. 8:2710–2718. 2015.PubMed/NCBI
|
|
42
|
Hu Y and Zeng F: Expressions of GPR81,
MCT1 and MCT4 in squamous carcinoma and their clinical
significance. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 43:950–956.
2018.(In Chinese). PubMed/NCBI
|