Introduction
Necrotising enterocolitis (NEC) is one of the main
causes of neonatal gastrointestinal disease-associated death, which
primarily occurs in premature and low birth weight infants
(1). The main clinical
manifestations of NEC are abdominal distention, diarrhoea and blood
in stool, and patients with severe forms of the disease may have
intestinal perforation or stenosis, peritonitis, sepsis and
intestinal fistula (2). With the
development of perinatal medicine and the establishment of neonatal
intensive care units, the survival rate of premature infants and
infants with a very low birth weight has increased significantly,
and the incidence rate of NEC has increased rapidly (3). A large number of in vitro
studies and clinical studies have investigated NEC (4,5).
However, the pathogenesis of the disease remains unclear.
Therefore, it is necessary to explore the mechanisms underlying
pathogenesis to inform effective strategies for the treatment of
NEC.
Vitamin D participates in cell differentiation,
proliferation and inflammation, and it acts as a key component in
bone metabolism and calcium homeostasis by interacting with the
vitamin D receptor (VDR) (6,7).
Clinical research has shown that deficiency of serum vitamin D is a
high-risk factor for inflammatory bowel disease (IBD) and is
associated with IBD duration and prognosis (8). Vitamin D deficiency is common in
preterm labour, especially in premature infants born before 32
weeks of gestation (9). Du et
al (10) confirmed that
1,25-dihydroxyvitamin D protects the intestinal epithelial barrier
by regulating the myosin light-chain kinase signalling pathway in a
mouse model of 2,4,6-trinitrobenzene sulfonic acid-induced colitis.
However, the possible protective mechanism of vitamin D against NEC
remains unclear.
ERK1/2 is a member of the mitogen-activated protein
kinase family of proteins, the members of which transmit regulatory
signals involved in cell proliferation, apoptosis or
differentiation (11–14). Bai et al (15) reported that sesamin enhances
NADH-dependent flavin reductase subunit 2-mediated defence against
oxidative stress and inflammation in ulcerative colitis by
activating AKT/ERK. Dai et al (16) revealed that VSL#3 probiotics
protect the intestinal epithelial barrier in acute colitis rats by
activating ERK signalling pathways. Bai et al (17) demonstrated that
serine/threonine-protein kinase Sgk1 protects intestinal epithelial
cells against tumour necrosis factor (TNF)-α-induced apoptosis in
colitis partly by activating the dual-specificity mitogen-activated
protein kinase kinase (MEK)/ERK signalling pathway. These studies
demonstrate that the ERK signalling pathway plays an important
regulatory role in colitis; however, the specific regulatory role
of this pathway in NEC remains unclear. In addition, Yuan et
al (18) revealed that the VDR
protects against neurological deficits and neuronal death in rats
with global cerebral ischemia by activating the ERK signalling
pathway. Therefore, it was hypothesised that vitamin D may protect
against NEC by regulating the ERK signalling pathway.
The present study aimed to establish in vitro
and in vivo models of NEC. The effects of vitamin D on NEC
were then investigated, including pathological changes in
intestinal tissue, oxidative stress, inflammation, proliferation
and apoptosis of intestinal cells, and the ERK signalling pathway.
The findings of the present research may provide a useful
theoretical basis for the treatment of NEC.
Materials and methods
Establishment of an animal model of
NEC
In total, 32 C57BL/6J mice (16 female and 16 male
mice; age, 5–6 weeks; weight, 18–25 g) were used for the
establishment of the NEC animal model in vivo based on
previous studies (19–21). The mice were fostered in a cage and
maintained on a 12:12 light/dark cycle at room temperature under
dry atmosphere. C57BL/6J mice were purchased from Changzhou Cavens
Laboratory Animal Co., Ltd (license no. SCXK(Su)2016-0010; Jiangsu,
China). The mice were divided into four groups of 8 animals each:
i) Control; ii) NEC; iii) vitamin D + control and; iv) vitamin D +
NEC. Control mice were fed artificial formula (Abbott
Pharmaceutical Co., Ltd.) and given normal saline by gavage. NEC
mice were fed artificial formula and subjected to hypoxia-cold
stimulation. Vitamin D + control mice were fed artificial formula
and administered vitamin D (0.5 g/kg/day; Sigma-Aldrich; Merck
KGaA) by gavage. Vitamin D + NEC mice were fed artificial formula,
subjected to hypoxia-cold stimulation, and given 0.5 g/kg/day
vitamin D by gavage. Artificial feeding and hypoxia-cold
stimulation were used to establish the NEC model, as previously
described (16). Briefly, mice
were exposed to hypoxia with 5% O2 and 95% N2
for 1 min; then, they were immediately placed in a 4°C cold chamber
for 10 min. The hypoxia-cold stimulation was controlled within 1 h
after the meal and performed twice a day for 4 days. The body
weight of the mice was measured every day. Upon appearance of
clinical signs (abdominal distension, diarrhea and vomiting) of NEC
distress (~96 h after modelling), the mice were anesthetised by an
intraperitoneal injection of 50 mg/kg pentobarbital sodium.
Anesthesia was confirmed when mice presented with symptoms
including excitement, convulsion and fainting. They were then
sacrificed by cervical dislocation. Intestinal tissues of the mice
were collected for follow-up tests. All animal experiments were
approved by the Institutional Animal Care and Use Ethics Committee
(approval no. L2019-4; Foshan, China).
Histological injury score
The terminal ileum was fixed in 4% neutral buffered
formalin solution for 24 h at room temperature, embedded in
paraffin and cut into sections with a thickness of 3 µm.
Pathological changes were detected using haematoxylin and eosin
(H&E) staining. Briefly, after dewaxing and hydration, sections
were stained with hematoxylin (Sigma-Aldrich; Merck KGaA) for 20
min at room temperature, and then stained with 0.5% eosin
(Sigma-Aldrich; Merck KGaA) for 90 sec at room temperature.
Sections were washed with 70% ethl alcohol to remove the eosin, and
were dehydrated with anhydrous alcohol for 1 min. Finally, these
sections were treated with xylene I or II (Sigma-Aldrich; Merck
KGaA) for 10 min at room temperature. After blocking the sections
with neutral balsam at room temperature for 24 h, images were
captured via an Olympus light microscope (magnification, ×200;
Nikon Corporation).
Histopathological changes were scored by two blinded
evaluators based on the following criteria (22): 0, normal, no damage; 1, slight
submucosal and/or lamina propria separation; 2, moderate separation
of submucosa and/or lamina propria and/or oedema in submucosal and
muscular layers; 3, severe separation of the submucosa and/or
lamina propria and/or severe oedema in the submucosa and muscular
layers, region villous sloughing; and 4, loss of villi, with
necrosis. NEC was defined as a histological score ≥2.
ELISA
Intestinal tissues were placed in cold physiological
saline (4°C) to create a tissue homogenate. Next, the tissue
homogenate was centrifuged at 10,000 × g for 15 min at 4°C and the
supernatant was collected to determine the levels of inflammatory
cytokines, including interleukin (IL)-6, IL-1β and TNF-α, using
IL-6, IL-1β and TNF-α ELISA kits (cat. nos. ab100713, ab100705 and
ab208348, respectively; all Abcam) according to the manufacturer's
instructions.
Malondialdehyde (MDA) and glutathione
peroxidase (GPx) assays
The supernatant of the intestinal homogenate was
collected to detect MDA and GPx activity. The Lipid Peroxidation
(MDA) assay kit (Sigma-Aldrich; Merck KGaA) was used to detect MDA
activity. GPx activity was detected using the Glutathione
Peroxidase assay kit (Cayman Chemical Company). The procedures were
conducted according to the manufacturers' instructions.
Cell culture and grouping
Rat epithelial IEC-6 cells were used for the
establishment of the NEC cell model in vitro based on
previous studies (23–25). IEC-6 cells were purchased from
Shanghai Kanglang Biotechnology Co., Ltd. (Shanghai, China) and
cultured on 96-well plates in DMEM (Sigma-Aldrich; Merck KGaA)
supplemented with 10% FBS (Sigma-Aldrich; Merck KGaA). IEC-6 cells
were divided into four groups: i) Control; ii) lipopolysaccharide
(LPS); iii) vitamin D + LPS; and iv) vitamin D + PD98059 + LPS.
Control cells underwent no treatment. LPS cells were treated with
LPS at a concentration of 50 µg/ml for 6 h at room temperature.
Vitamin D + LPS cells were first treated with LPS at a
concentration of 50 µg/ml for 6 h and then with vitamin D at
a concentration of 50 nM for 48 h at room temperature. Vitamin D +
PD98059 + LPS cells were treated first with LPS at a concentration
of 50 µg/m for 6 h, then with vitamin D at a concentration
of 50 nM and the MEK inhibitor PD98059 (Shanghai BeiZhuo Biotech
Co., Ltd.) at a concentration of 5 µM for 48 h at room
temperature.
Reverse transcription-quantitative
(RT-q)PCR
TRIzol® reagent (Thermo Fisher
Scientific, Inc.) was used to extract total RNA from intestinal
tissues and cells. cDNA synthesis was conducted at 55°C using the
PrimeScript RT reagent kit (Takara Bio, Inc.) according to the
manufacturer's protocols. qPCR was performed with an ABI 7500
quantitative PCR instrument (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using SYBR Green Realtime PCR Master mix (Toyobo
Life Science). The qPCR thermocycling conditions were as follows:
Pre-denaturation at 95°C for 10 min, followed by 40 cycles at 95°C
for 1 min and 55°C for 30 sec. The qPCR primers (Table I) were synthesized by Sangon
Biotech Co., Ltd. Relative mRNA expression was calculated using the
2−ΔΔCq method and GAPDH served as the internal control
(26).
 | Table I.Sequences of primers used for reverse
transcription quantitative-PCR. |
Table I.
Sequences of primers used for reverse
transcription quantitative-PCR.
|
| Sequence
(5′-3′) |
|---|
|
|
|
|---|
| Primers | Forward | Reverse |
|---|
| IL-6 |
GATACCACTCCCAACAGAC |
CTTTTCTCATTTCCACGAT |
| IL-1β |
GTGGTGTGTGACGTTCCCATTA |
CCGACAGCACGAGGCTTT |
| TNF-α |
TGCAGCAGGACATCAAGTTC |
TACGCCTCAGCAGTCTCCTT |
| GAPDH |
GCTTCGGCAGCACATATACTAAAAT |
CGCTTCACGAATTTGCGTGTCAT |
Western blotting
Intestinal tissue was homogenized in RIPA protein
lysis buffer (Beyotime Institute of Biotechnology) and centrifuged
at 4°C for 15 min at 16,000 × g. A BCA Protein assay kit (Thermo
Fisher Scientific, Inc.) was used to detect protein concentrations.
In total, 50 μg protein was loaded on a 10% gel, resolved
using SDS-PAGE and subsequently transferred to PVDF membranes
(Thermo Fisher Scientific, Inc.). The membranes were blocked with
5% BSA for 1 h at 37°C and were then incubated with the primary
antibodies against ERK1/2 (1:1,000; cat. no. ab17942; Abcam), Ki67
(1:1,000; cat. no. MA5-14520; Thermo Fisher Scientific, Inc.),
p-ERK1/2 (1:1,000; cat. no. 4376; Cell Signalling Technology,
Inc.), cleaved caspase-3 (1:1,000; cat. no. 9661; Cell Signalling
Technology, Inc.), Bcl-2 (1:1,000; cat. no. 3498; Cell Signalling
Technology, Inc.) and GAPDH (1:1,000; cat. no. 5174, Cell
Signalling Technology, Inc.) at 4°C overnight. The next day, the
membranes were washed three times with 0.2% TBS-Tween-20 solution
and incubated with HRP-conjugated goat anti-rabbit immunoglobulin G
(1:2,000; cat. no. 31460; Thermo Fisher Scientific, Inc.) for 2 h
at room temperature. An enhanced chemiluminescence detection kit
(Beijing Solarbio Science & Technology Co., Ltd) was used to
visualize the protein bands. The intensity of these bands was
quantified via Image Lab™ 3.0 software (version 3.0; Bio-Rad
Laboratories, Inc.)
MTT assay
An MTT assay was used to determine cell
proliferation. Briefly, cells were cultured in an incubator with 5%
CO2 at 37°C for 48 h and then the cells were incubated
with 20 µl MTT solution (Shanghai Jianglai Biotechnology Co., Ltd.)
for 4 h at 37°C. Next, medium was replaced with 150 µl DMSO for 10
min to promote crystal dissolution. The absorbance of cell
suspensions was measured at 570 nm using a spectrophotometer
(Shanghai Onlab Instrument Co., Ltd.). Cell viability was
normalized to that of the control group.
Apoptosis assay
An Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis kit (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to determine apoptosis of the
aforementioned cell groups. Briefly, cells were resuspended in
binding buffer and incubated with Annexin V-FITC (5 µl) and PI (5
µl) for 10 min at 25°C. A flow cytometer (DLK0002095; BD
Biosciences) was used to determine the apoptosis rate. CellQuest
software (version 3.1; BD Biosciences) was used to analyze the
data.
Statistical analysis
SPSS version 22.0 statistical software (IBM, Corp.)
was used to analyse the experimental data. The data are expressed
as mean ± standard deviation of three independent repeats. One-way
ANOVA followed by Tukey's post hoc test was used for comparisons
among multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
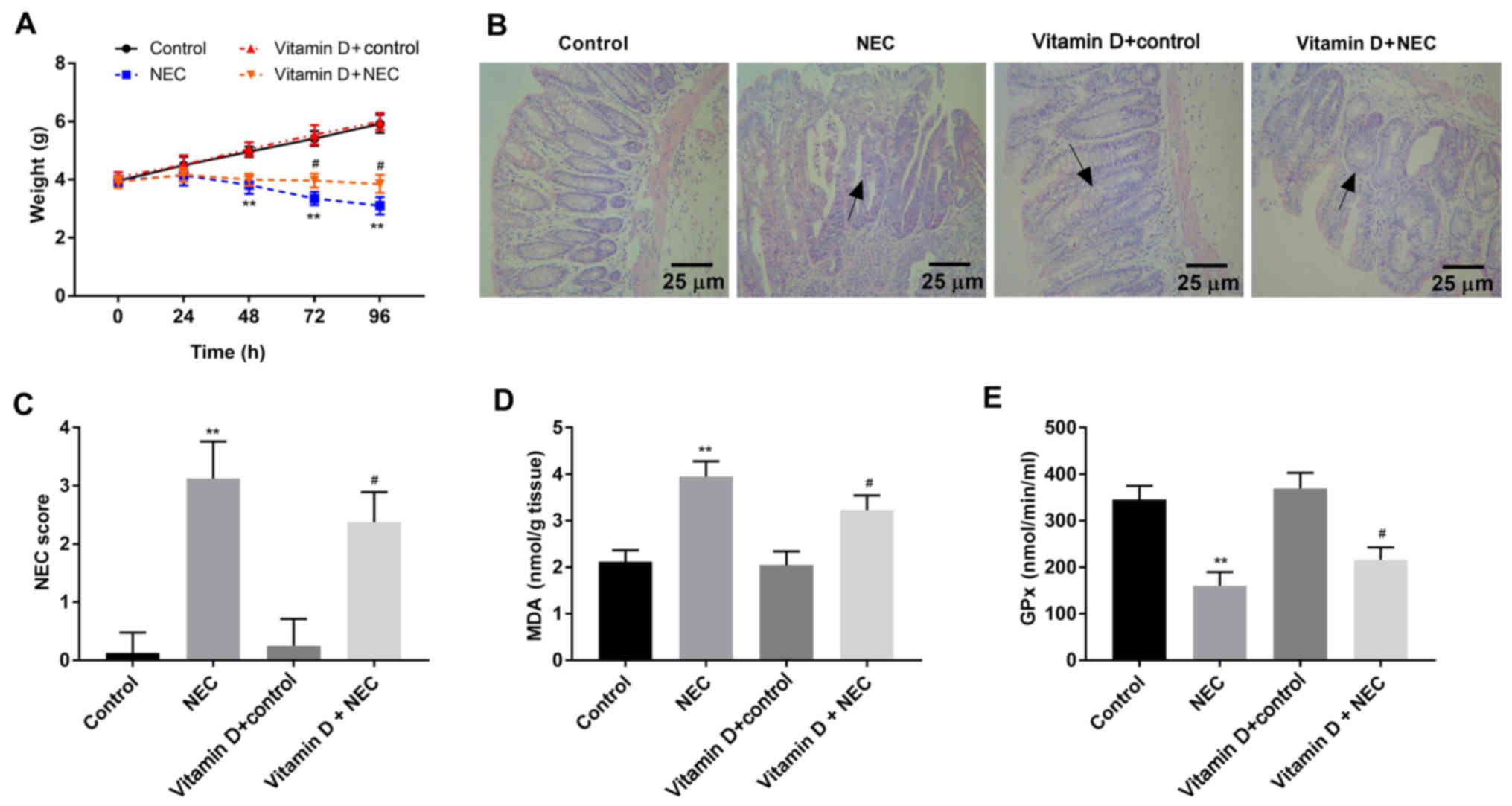
Vitamin D reduces damage and oxidative
stress in intestinal tissues of NEC mice
To determine whether vitamin D has a protective
effect on NEC, the body weight of newborn mice was monitored. No
significant difference in body weight was found between the control
and vitamin D + control group (Fig.
1A). The body weight of mice in the NEC group was lower
compared with that of control mice 48 h after establishing the NEC
model (P<0.01). Notably, compared with the NEC group, the body
weight of mice in the vitamin D + NEC group was higher after 72 and
96 h of modelling (P<0.05). H&E staining (Fig. 1B) demonstrated that the intestinal
tissue structure of mice was normal in the control and vitamin D +
control group. Mice in the NEC group exhibited severe necrosis and
the infiltration of a number of inflammatory cells in intestinal
tissues. Notably, necrosis and inflammation in the vitamin D + NEC
group were less compared with the NEC group. In NEC group,
separation and necrosis of the villous center, submucosal edema and
epithelial cell beating down were observed; in the vitamin D + NEC
group, this phenomenon was significantly improved. Furthermore, the
vitamin D + NEC group had a significantly lower NEC score compared
with the NEC group (P<0.05; Fig.
1C). As presented in Fig. 1D and
E, the levels of MDA were significantly higher, while the GPx
levels were significantly lower in the NEC group compared with the
control group (both P<0.01). Furthermore, vitamin D intervention
in the vitamin D + NEC group significantly decreased the levels of
MDA and significantly increased the levels of GPx compared the NEC
group (both P<0.05).
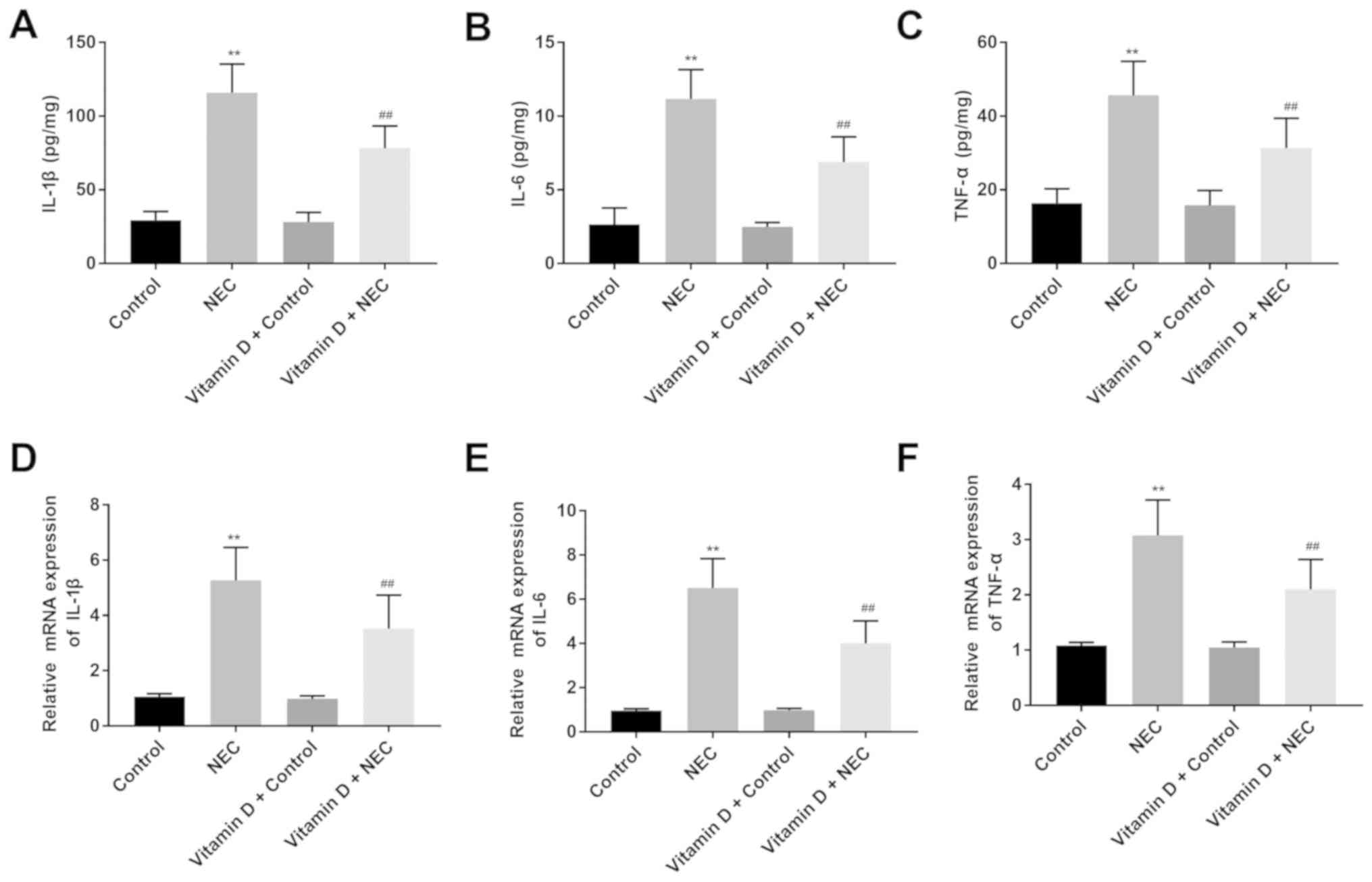
Vitamin D reduces intestinal
inflammatory cytokine levels in NEC mice
Proinflammatory cytokines play important roles in
NEC-induced intestinal damage (27). Inflammatory cytokines (IL-6, IL-1β
and TNF-α) in the intestinal tissues of mice were analysed using
ELISA and RT-qPCR assays. According to ELISA, no significant
differences were observed in the levels of IL-6, IL-1β and TNF-α
between the control and vitamin D + control groups (Fig. 2A-C). Notably, the levels of IL-6,
IL-1β and TNF-α in the NEC group were significantly higher compared
with those in the control group (all P<0.01). Furthermore, the
levels of IL-6, IL-1β and TNF-α were significantly lower in the
vitamin D + NEC group compared with the NEC group (all P<0.01).
The results of RT-qPCR were consistent with those of ELISA
(Fig. 2D-F).
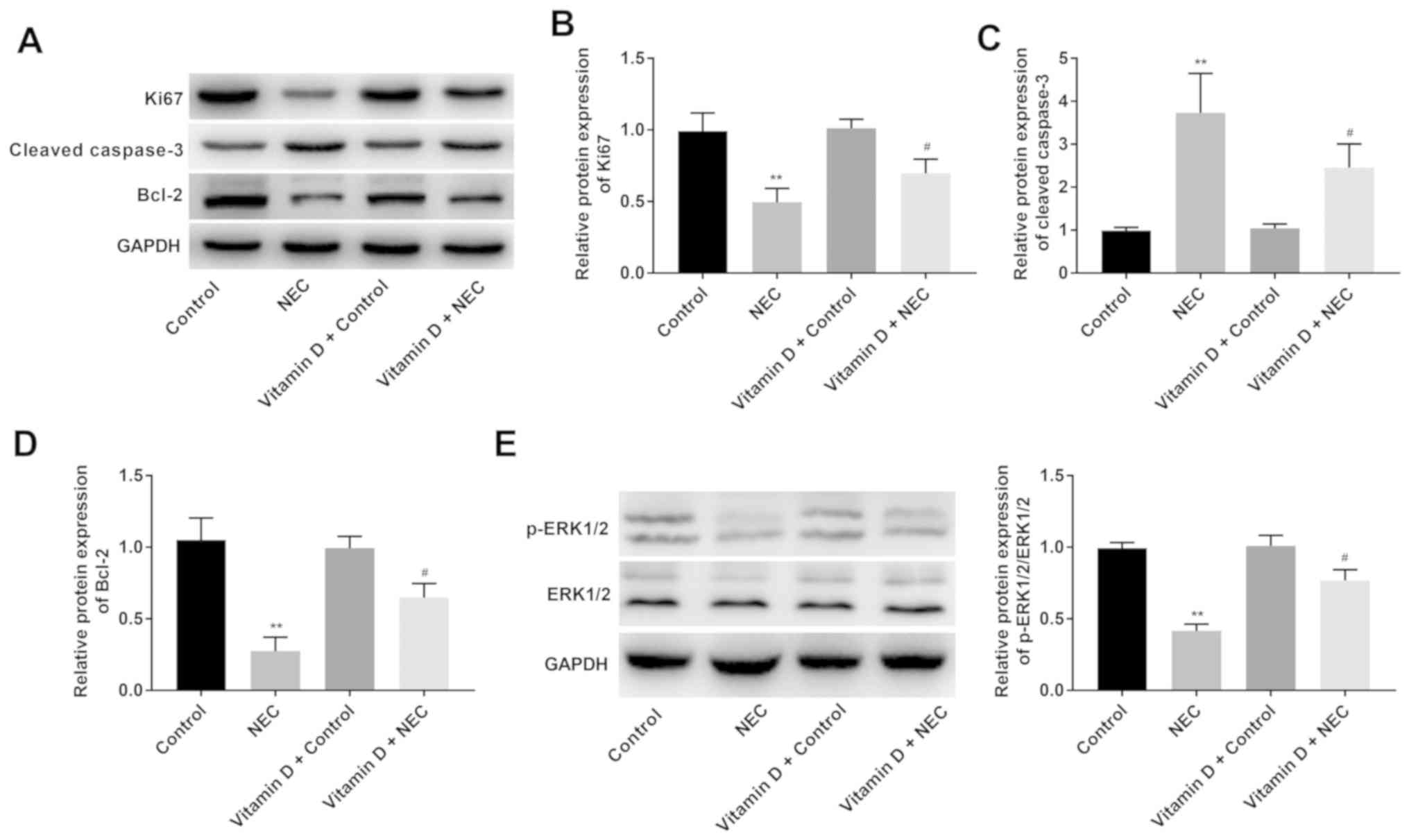
Vitamin D increases the proliferation
and decreases the apoptosis of intestinal cells and activates the
ERK signalling pathway in NEC mice
The expression levels of ERK signalling pathway-
(p-ERK1/2 and ERK1/2), proliferation- (Ki67) and
apoptosis-associated proteins (cleaved caspase-3 and Bcl-2) in the
intestinal tissues of mice were analysed using western blotting. As
presented in Fig. 3A-E, there were
no significant differences in the protein expression levels of
p-ERK1/2/ERK1/2, Ki67, cleaved caspase-3 and Bcl-2 between the
control and vitamin D + control groups. Notably, the protein
expression levels of Ki67, Bcl-2 and p-ERK1/2/ERK1/2 were
significantly lower (P<0.01), and the protein expression of
cleaved caspase-3 was significantly higher (P<0.01) in the NEC
group compared with the control group. Furthermore, the protein
expression levels of Ki67, Bcl-2 and p-ERK1/2/ERK1/2 in the vitamin
D + NEC group were significantly higher, but the protein expression
of cleaved caspase-3 was significantly lower compared with that in
the NEC group (all P<0.05).
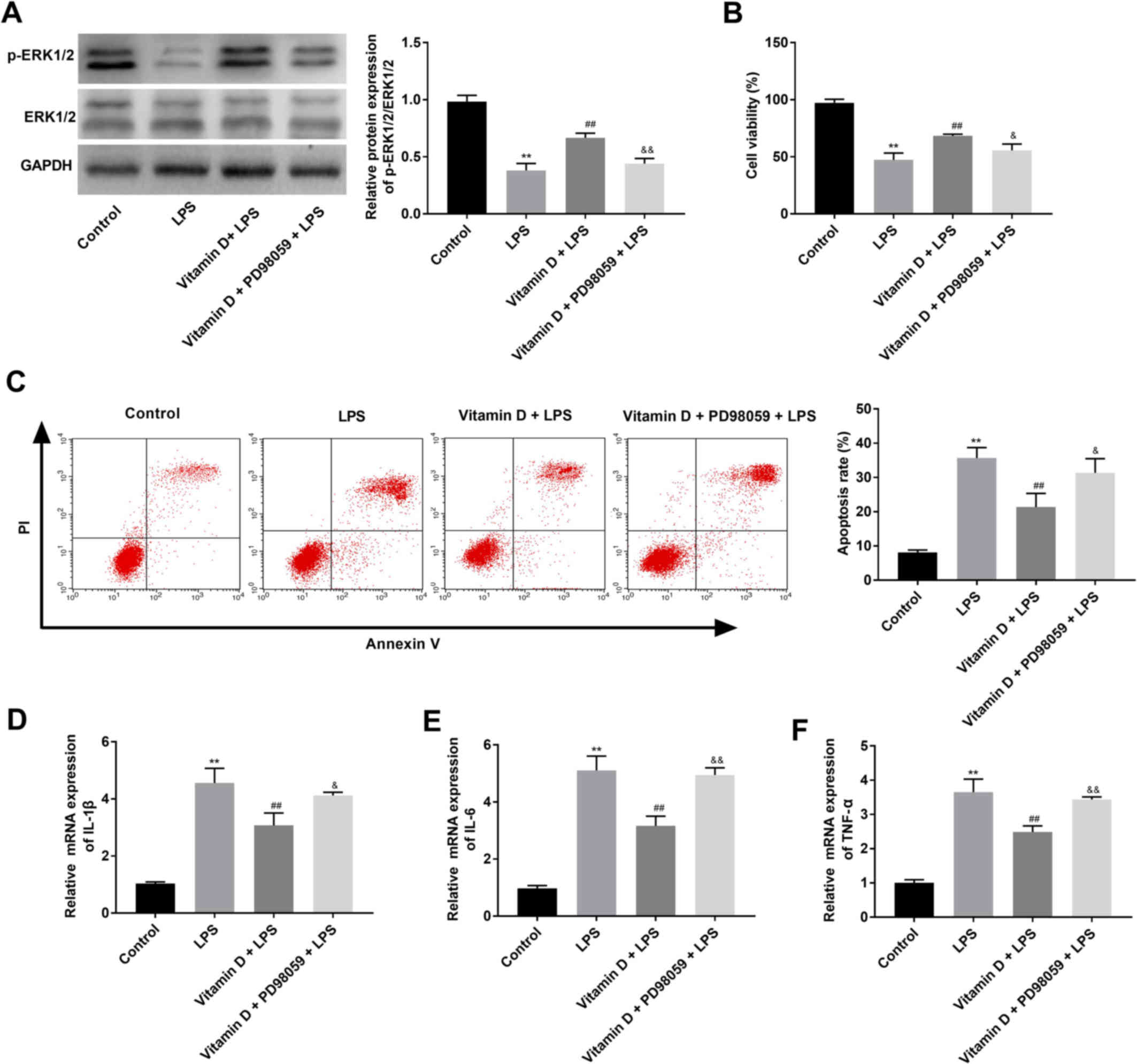
Blocking the ERK signalling pathway
reverses the protective effect of vitamin D on LPS-treated IEC-6
cells
IEC-6 cells were treated with LPS to establish an
in vitro NEC model. As presented in Fig. 4A, the ratio of p-ERK1/2/ERK1/2 was
significantly lower in the LPS group compared with the control
group (P<0.01), however the ratio of p-ERK1/2/ERK1/2 was
significantly higher in the vitamin D + LPS group compared with the
LPS group (P<0.01). Subsequently, the proliferation and
apoptosis of IEC-6 cells were determined using an MTT assay and
flow cytometry, respectively. As presented in Fig. 4B and C, cell viability was
significantly decreased and the apoptosis rate was significantly
increased in the LPS group compared with the control group (both
P<0.01). Vitamin D treatment in the vitamin D + LPS group
significantly increased the viability and significantly decreased
the rate of apoptosis of IEC-6 cells compared with the LPS group
(both P<0.01). Furthermore, RT-qPCR revealed that the mRNA
expression levels of IL-6, IL-1β and TNF-α were significantly
increased in the LPS group compared the control group, and vitamin
D treatment significantly reversed the effect of LPS (all
P<0.01; Fig. 4D-F). Notably,
the MEK inhibitor PD98059 significantly reversed the effects of
vitamin D on p-ERK1/2 level, proliferation, apoptosis and
inflammatory-associated proteins in IEC-6 cells (Fig. 4A-F).
Discussion
NEC is a serious intestinal disease that occurs in
the neonatal period and it is a common cause of early death in
neonates (28). At present, NEC is
considered to be caused by premature birth, hypoxia, enteral
feeding, bacterial infection and intestinal ischemia (29); however, its pathogenesis is still
not completely clear. The results of the present study demonstrated
that vitamin D reduced intestinal tissue damage, oxidative stress
and inflammation in NEC mice. Furthermore, vitamin D promoted cell
viability, inhibited apoptosis and inflammation, and activated the
ERK signalling pathway in LPS-treated IEC-6 cells.
Reactive oxygen species produced by oxidative stress
can directly or indirectly damage the physiological functions of
proteins, lipids, nucleic acids and other biological macromolecules
in cells, which is the pathophysiological basis for the occurrence
and development of NEC (30). MDA
is a marker of lipid oxidation and an indicator of the degree of
membrane damage (31). GPx is a
marker of anti-oxidative capacity (32). A prior study revealed that vitamin
D3 reduces serum MDA levels in patients with irritable bowel
syndrome (33). In an animal
model, Farhangi et al (34)
reported that vitamin D treatment leads to a significant reduction
in GPx concentrations in the high-fat diet + vitamin D group. The
present study demonstrated that vitamin D treatment decreased MDA
and increased GPx levels in the intestinal tissues of NEC mice.
These results suggest that vitamin D reduced oxidative stress in
the intestinal tissue of NEC mice.
Vitamin D plays a key regulatory role in calcium and
phosphorus metabolism and anti-inflammation (35–37).
For example, Golan et al (38) conducted experiments using VDR
transgenic mice and revealed that overexpression of VDR in the
intestinal epithelium inhibits the occurrence of spontaneous
enteritis caused by IL-10 deficiency. Liu et al (39) reported that
1,25(OH)2D3 (an active form of vitamin
D)-deficient mice exhibit notable colon inflammation phenotypes,
and high expression of inflammatory cytokines. Mousavi et al
(40) confirmed that
25-OH-cholecalciferol (a circulating form of vitamin D)-treated
mice display low compromised colonic epithelial barrier function
and low levels of pro-inflammatory mediators including IL-6, MCP1
and IFN-γ. Consistent with previous results, the present study
demonstrated that vitamin D treatment reduced the levels of
inflammatory cytokines (IL-6, IL-1β and TNF-α) in the intestinal
tissue of mice and LPS-treated IEC-6 cells. In brief, the present
results suggest that vitamin D reduces inflammation in NEC.
Apoptosis is one of the important pathological
changes in intestinal injury during NEC. As reported previously,
VDR signalling leads to a reduction in IEC apoptosis by blocking
NF-κB activation (41). Zhu et
al (42) reported that vitamin
D deficiency is common in patients with IBD and that vitamin D
decreases the expression levels of cleaved caspase-3 in mice with
2,4,6-trinitrobenzene sulfonic acid-induced colitis. He et
al (43) found that the
absence of gut epithelial VDR promotes the apoptosis of epithelial
cells and increases the permeability of the mucosal barrier. In the
present study, vitamin D treatment increased the protein expression
levels of Bcl-2, decreased the protein expression levels of cleaved
caspase-3 in the intestinal tissue of mice and inhibited the
apoptosis of LPS-treated IEC-6 cells. The aforementioned results
suggest that vitamin D inhibits the apoptosis of intestinal cells
in NEC mice.
Previous studies have reported that the ERK1/2
signalling pathway is involved in the genesis and development of
intestinal diseases. For example, Ban et al (44) demonstrated that treatment with
U0126 (an ERK1/2 inhibitor) leads to an increase in intestinal
permeability, intestinal injury and inflammatory cytokines in mice
with intestinal ischemia-reperfusion. Deng et al (45) suggested that leptin reduces
intestinal histological alterations and MDA and IL-6 levels by
enhancing ERK1/2 phosphorylation and promoting the NO synthesis
signalling pathway. Jeong et al (46) revealed that spirulina crude protein
treatment promotes cell migration and proliferation, and
dysregulates the protein expression of H-Ras, p-Raf-1, p-MEK-1 and
p-ERK-1/2 in IEC-6 cells. The present study demonstrated that
vitamin D increased the protein expression levels of p-ERK1/2 in
NEC intestinal tissue and IEC-6 cells. Notably, the MEK inhibitor
PD98059 reversed the effects of vitamin D on the proliferation,
apoptosis and inflammation of LPS-treated IEC-6 cells. The
aforementioned findings demonstrate that vitamin D improved cell
viability and inhibited cell apoptosis and inflammation in
intestinal cells of NEC mice by activating the ERK signalling
pathway.
The present study has some limitations. First, the
histopathological change of NEC was only observed in the mucosa
layer. The observations of different layers of the intestine may
better reflect the therapeutic effect of vitamin D on NEC, as each
layer exhibits different characteristics. Second, the potential
mechanisms underlying the of action of vitamin D still need to be
further investigated. For example, the genes and signaling pathways
through which vitamin D improves NEC should be elucidated.
In conclusion, vitamin D reduced intestinal tissue
damage, oxidative stress and inflammation, and activated the ERK
signalling pathway in NEC mice in the present study. Furthermore,
vitamin D promoted the viability, and inhibited the apoptosis and
inflammation of LPS-treated IEC-6 cells by activating the ERK
signalling pathway. The present findings may provide a new
theoretical basis for the treatment of NEC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL and SJ were mainly responsible for
conceptualization, data analysis and interpretation. SJ was
responsible for drafting and revising the manuscript. MK performed
the experiments. XC and LZ were responsible for software management
and visualization. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by The
Institutional Animal Care and Use Ethics Committee (approval no.
L2019-4; Foshan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Neu J and Walker WA: Necrotizing
enterocolitis. N Engl J Med. 364:255–264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guyot D, Kuo P, Pawlotsky F, Papouin-Rauzy
M and Delbreil JP: Intestinal fistula: An unusual complication of
necrotizing enterocolitis in the preterm infant. Arch Pediatr.
16:435–438. 2009.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zangari A, Noviello C, Nobile S, Cobellis
G, Gulia C, Piergentili R, Gigli S and Carnielli V: Surgical
management of Necrotizing Enterocolitis in an Incredibly Low Birth
Weight infant and review of the Literature. Clin Ter.
168:e297–e299. 2017.PubMed/NCBI
|
|
4
|
Vieten D, Corfield A, Ramani P and Spicer
R: Proliferative response in necrotising enterocolitis is
insufficient to prevent disease progression. Pediatr Surg Int.
22:50–56. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llanos AR, Moss ME, Pinzòn MC, Dye T,
Sinkin RA and Kendig JW: Epidemiology of neonatal necrotising
enterocolitis: A population-based study. Paediatr Perinat
Epidemiol. 16:342–349. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pittas AG, Harris SS, Stark PC and
Dawson-Hughes B: The effects of calcium and vitamin D
supplementation on blood glucose and markers of inflammation in
nondiabetic adults. Diabetes Care. 30:980–986. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Veldurthy V, Wei R, Oz L, Dhawan P, Jeon
YH and Christakos S: Vitamin D, calcium homeostasis and aging. Bone
Res. 4:160412016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ardesia M, Ferlazzo G and Fries W: Vitamin
D and inflammatory bowel disease. BioMed Res Int. 2015:4708052015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu T, Liu TJ, Ge X, Kong J, Zhang LJ and
Zhao Q: High prevalence of maternal vitamin D deficiency in preterm
births in northeast China, Shenyang. Int J Clin Exp Pathol.
8:1459–1465. 2015.PubMed/NCBI
|
|
10
|
Du J, Chen Y, Shi Y, Liu T, Cao Y, Tang Y,
Ge X, Nie H, Zheng C and Li YC: 1,25-Dihydroxyvitamin D protects
intestinal epithelial barrier by regulating the myosin light chain
kinase signaling pathway. Inflamm Bowel Dis. 21:2495–2506. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Q, Chen M, Liu H, Yang L, Yang T and He
G: The dual role of ERK signaling in the apoptosis of neurons.
Front Biosci. 19:1411–1417. 2014. View
Article : Google Scholar
|
|
12
|
Wang X, Wang Y, Zhu Y, Yan L and Zhao L:
Neuroprotective effect of S-trans, trans-farnesylthiosalicylic acid
via inhibition of RAS/ERK pathway for the treatment of alzheimer's
Disease. Drug Des Devel Ther. 13:4053–4063. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dziarski R, Jin YP and Gupta D:
Differential activation of extracellular signal-regulated kinase
(ERK) 1, ERK2, p38, and c-Jun NH2-terminal kinase mitogen-activated
protein kinases by bacterial peptidoglycan. J Infect Dis.
174:777–785. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu SM, Yao XH, Hao YH, Pan AH and Zhou XW:
8 Gingerol regulates colorectal cancer cell proliferation and
migration through the EGFR/STAT/ERK pathway. Int J Oncol.
56:390–397. 2020.PubMed/NCBI
|
|
15
|
Bai X, Gou X, Cai P, Xu C, Cao L, Zhao Z,
Huang M and Jin J: Sesamin enhances Nrf2-mediated protective
defense against oxidative stress and inflammation in colitis via
AKT and ERK activation. Oxid Med Cell Longev. 2019:24324162019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai C, Zhao DH and Jiang M: VSL#3
probiotics regulate the intestinal epithelial barrier in vivo and
in vitro via the p38 and ERK signaling pathways. Int J Mol Med.
29:202–208. 2012.PubMed/NCBI
|
|
17
|
Bai JA, Xu GF, Yan LJ, Zeng WW, Ji QQ, Wu
JD and Tang QY: SGK1 inhibits cellular apoptosis and promotes
proliferation via the MEK/ERK/p53 pathway in colitis. World J
Gastroenterol. 21:6180–6193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan J, Guo X, Liu Z, Zhao X, Feng Y, Song
S, Cui C and Jiang P: Vitamin D receptor activation influences the
ERK pathway and protects against neurological deficits and neuronal
death. Int J Mol Med. 41:364–372. 2018.PubMed/NCBI
|
|
19
|
Ginzel M, Feng X, Kuebler JF, Klemann C,
Yu Y, von Wasielewski R, Park JK, Hornef MW, Vieten G, Ure BM, et
al: Dextran sodium sulfate (DSS) induces necrotizing
enterocolitis-like lesions in neonatal mice. PLoS One.
12:e01827322017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Tran DQ, Fatheree NY and Marc
Rhoads J: Lactobacillus reuteri DSM 17938 differentially modulates
effector memory T cells and Foxp3+ regulatory T cells in a mouse
model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver
Physiol. 307:G177–G186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ginzel M, Yu Y, Klemann C, Feng X, von
Wasielewski R, Park JK, Hornef MW, Torow N, Vieten G, Ure BM, et
al: The viral dsRNA analogue poly (I:C) induces necrotizing
enterocolitis in neonatal mice. Pediatr Res. 79:596–602. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu SF, Chiu HY, Chen AC, Lin HY, Lin HC
and Caplan M: Efficacy of different probiotic combinations on death
and necrotizing enterocolitis in a premature rat model. J Pediatr
Gastroenterol Nutr. 57:23–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang D, Wen J, Zhou J, Cai W and Qian L:
Milk fat globule membrane ameliorates necrotizing enterocolitis in
neonatal rats and suppresses lipopolysaccharide-induced
inflammatory response in IEC-6 enterocytes. JPEN J Parenter Enteral
Nutr. 43:863–873. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maynard AA, Dvorak K, Khailova L, Dobrenen
H, Arganbright KM, Halpern MD, Kurundkar AR, Maheshwari A and
Dvorak B: Epidermal growth factor reduces autophagy in intestinal
epithelium and in the rat model of necrotizing enterocolitis. Am J
Physiol Gastrointest Liver Physiol. 299:G614–G622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou Y, Li Y, Zhou B, Chen K, Lyv Z, Huang
D, Liu B, Xu Z, Xiang B, Jin S, et al: Inflammation and apoptosis:
dual mediator role for toll-like receptor 4 in the development of
necrotizing enterocolitis. Inflamm Bowel Dis. 23:44–56. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Δ Δ C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Korkut S, Özdemir A, Yay AH, Yalçın B,
Ceylan M, Korkmaz L, Yazıcı C, Güntürk İ and Kurtoğlu S: Obestatin
reduces intestinal damage in experimental necrotizing enterocolitis
in newborn rats. Am J Perinatol. 36:1179–1187. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rolnitsky A, Ng E, Asztalos E, Shama Y,
Karol D, Findlater C, Garsch M and Dunn M: A quality improvement
intervention to reduce necrotizing enterocolitis in premature
infants with probiotic supplementation. Pediatr Qual Saf.
4:e2012019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rose AT and Patel RM: A critical analysis
of risk factors for necrotizing enterocolitis. Semin Fetal Neonatal
Med. 23:374–379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aceti A, Beghetti I, Martini S, Faldella G
and Corvaglia L: Oxidative stress and necrotizing enterocolitis:
Pathogenetic mechanisms, opportunities for intervention, and role
of human milk. Oxid Med Cell Longev. 2018:73976592018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang D, Wen J, Zhou J, Cai W and Qian L:
Milk fat globule membrane ameliorates necrotizing enterocolitis in
neonatal rats and suppresses lipopolysaccharide-induced
inflammatory response in IEC-6 enterocytes. JPEN J Parenter Enteral
Nutr. 43:863–873. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Klinke M, Vincent D, Trochimiuk M, Appl B,
Tiemann B, Bergholz R, Reinshagen K and Boettcher M: Degradation of
extracellular DNA significantly ameliorates necrotizing
enterocolitis severity in mice. J Surg Res. 235:513–520. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Amani R, Abbasnezhad A, Hajiani E,
Cheraghian B, Abdoli Z and Choghakhori R: Vitamin D3 induced
decrease in IL-17 and malondialdehyde, and increase in IL-10 and
total antioxidant capacity levels in patients with irritable bowel
syndrome. Iran J Immunol. 15:186–196. 2018.PubMed/NCBI
|
|
34
|
Farhangi MA, Mesgari-Abbasi M, Hajiluian
G, Nameni G and Shahabi P: Adipose tissue inflammation and
oxidative stress: The ameliorative effects of vitamin D.
Inflammation. 40:1688–1697. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kühne H, Hause G, Grundmann SM,
Schutkowski A, Brandsch C and Stangl GI: Vitamin D receptor
knockout mice exhibit elongated intestinal microvilli and increased
ezrin expression. Nutr Res. 36:184–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dauletbaev N, Herscovitch K, Das M, Chen
H, Bernier J, Matouk E, Bérubé J, Rousseau S and Lands LC:
Down-regulation of IL-8 by high-dose vitamin D is specific to
hyperinflammatory macrophages and involves mechanisms beyond
up-regulation of DUSP1. Br J Pharmacol. 172:4757–4771. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Holick MF: Vitamin D deficiency. N Engl J
Med. 357:266–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Golan MA, Liu W, Shi Y, Chen L, Wang J,
Liu T and Li YC: Transgenic expression of vitamin D receptor in gut
epithelial cells ameliorates spontaneous colitis caused by
Interleukin-10 deficiency. Dig Dis Sci. 60:1941–1947. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu Y, Chen L, Zhi C, Shen M, Sun W, Miao
D and Yuan X: 1,25(OH)2D3 deficiency induces colon inflammation via
secretion of senescence-associated inflammatory cytokines. PLoS
One. 11:e01464262016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mousavi S, Lobo de Sá FD, Schulzke JD,
Bücker R, Bereswill S and Heimesaat MM: Vitamin D in Acute
Campylobacteriosis-Results From an Intervention Study Applying a
Clinical Campylobacter jejuni Induced Enterocolitis Model. Front
Immunol. 10:20942019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu W, Chen Y, Golan MA, Annunziata ML, Du
J, Dougherty U, Kong J, Musch M, Huang Y, Pekow J, et al:
Intestinal epithelial vitamin D receptor signaling inhibits
experimental colitis. J Clin Invest. 123:3983–3996. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu T, Liu TJ, Shi YY and Zhao Q: Vitamin
D/VDR signaling pathway ameliorates 2,4,6-trinitrobenzene sulfonic
acid-induced colitis by inhibiting intestinal epithelial apoptosis.
Int J Mol Med. 35:1213–1218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He L, Liu T, Shi Y, Tian F, Hu H, Deb DK,
Chen Y, Bissonnette M and Li YC: Gut epithelial vitamin D receptor
regulates microbiota-dependent mucosal inflammation by suppressing
intestinal epithelial cell apoptosis. Endocrinology. 159:967–979.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ban K, Peng Z and Kozar RA: Inhibition of
ERK1/2 worsens intestinal ischemia/reperfusion injury. PLoS One.
8:e767902013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Deng ZH Jr, Yan GT, Wang LH, Zhang JY, Xue
H and Zhang K: Leptin relieves intestinal ischemia/reperfusion
injury by promoting ERK1/2 phosphorylation and the NO signaling
pathway. J Trauma Acute Care Surg. 72:143–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jeong SJ, Choi JW, Lee MK, Choi YH and Nam
TJ: Spirulina crude protein promotes the migration and
proliferation in IEC-6 cells by activating EGFR/MAPK signaling
pathway. Mar Drugs. 17:2052019. View Article : Google Scholar
|