Introduction
Lupus nephritis is a kidney inflammation caused by
systemic lupus erythematosus (SLE) (1). SLE is characterized by persistent and
severe inflammation that damages multiple organs (2). In particular, 60–80% of patients with
SLE develop renal or urinary function abnormalities (3). It has been estimated that in North
America, the annual prevalence of SLE is 23.2/100,000 and
241/100,000 for males and female, respectively (4). In addition, >50% of patients with
SLE have lupus nephritis (5);
thus, it is necessary to find effective methods for the treatment
of lupus nephritis.
The main treatment regimen for lupus nephritis are
immunosuppressants, such as corticosteroids and cyclophosphamide
(6,7); however, the use of immunosuppressants
increases the risk of infections in patients (8). In view of the limitations of
immunosuppressants, the use of Bailing capsules, which are prepared
from the dry powder of Ophiocordyceps sinensis mycelium, has
been explored previously in lupus nephritis therapy (9). Bailing capsules possess antihypoxic,
anti-inflammatory and antitumor effects, regulate the endocrine
system and enhance the immune function (10). Bailing capsules have been widely
used in adjuvant therapy of glomerulonephritis, pyelonephritis,
nephrotic syndrome and other diseases without adverse effects
(11). Studies have reported the
treatment of lupus nephritis with Bailing capsules. For example,
Zhou et al (12) indicated
that treatment with Bailing capsules regulated cellular immunity
levels in patients with lupus nephritis. A previous study in
patients with lupus nephritis indicated that the 24-h urinary
protein, serum creatinine and urea nitrogen levels after treatment
were markedly lower in the treatment group (Bailing capsules with
cyclophosphamide) compared with those in the control group
(13). In a similar study, the
serum creatinine, 24-h urinary protein, β2 microglobulin
and SLE Disease Activity Index (SLEDAI) scores in the treatment
group (Bailing capsules + Leflunomide + Prednisone acetate) were
lower compared with those in the control group, whereas the albumin
(Alb), complement C3 and red blood cell (RBC) levels were higher
compared with those of the control group (14). Despite the existence of multiple
studies, no definitive conclusions has been drawn on the
effectiveness of Bailing capsules in the treatment of lupus
nephritis due to limited sample sizes and inconsistent results
across these studies.
The present study conducted a meta-analysis of
studies which explored the use of Bailing capsules to treat lupus
nephritis.
Materials and methods
Data source
Relevant clinical studies were obtained from PubMed
(ncbi.nlm.nih.gov/pubmed/), Embase (embase.com), Cochrane Library
(cochranelibrary.com/), China National Knowledge Infrastructure
(cnki.net/), WanFang database (wanfangdata.com.cn/) and the Chinese
Biomedical Literature Database (sinomed.ac.cn/). A literature
search was performed using the following search strategy: Sources
published between July 2004 and 2019 were searched, without
language limitations, using the keywords including ‘Bailing
capsule’, AND ‘lupus nephritis’ OR ‘lupus erythematosus
nephritis’.
Inclusion and exclusion criteria
The selected studies involved the use of Bailing
capsules for the treatment of lupus nephritis. For repeated
publications or when the same dataset was used for multiple
studies, only the latest or the most comprehensive article was
included. Reviews, reports, letters, comments and studies without
complete data which could be used for statistical analysis were
excluded.
Data extraction and quality
assessment
The following data from each study: First author,
publication year, study year, follow-up time, number of Bailing
capsule and control (non-Bailing capsule) groups, medication, age,
sex, course of the disease and outcome indicators including SLEDAI
score, Alb levels, 24-h urinary protein, serum creatinine,
anti-ds-DNAIg, C3 and the number of effective treatments and
complications were extracted by two independent researchers.
Quality assessment of the included studies was based on the
guidelines recommended by the Cochrane Collaboration (15).
Statistical analysis
Meta-analysis was performed using R-3.12 software,
and the odds ratio (OR) or standardized mean differences (SMD) and
95% confidence interval (95% CI) (16) were used for the effect index.
Heterogeneity was analysed using the Cochran's Q test (17) and the I2 test. If
P<0.05 or I2>50%, indicating that all the studies
were heterogeneous, the random effects model was applied. If not,
the fixed effect model was used (18). Sensitivity analysis was conducted
by trimming one study at a time (19). The pooled effect differences before
and after the trim were compared. A reversal of the pooled results
after the trim was indicative of unstable results. Publication bias
was evaluated using Egger's test (20).
Results
Characteristics of the included
studies
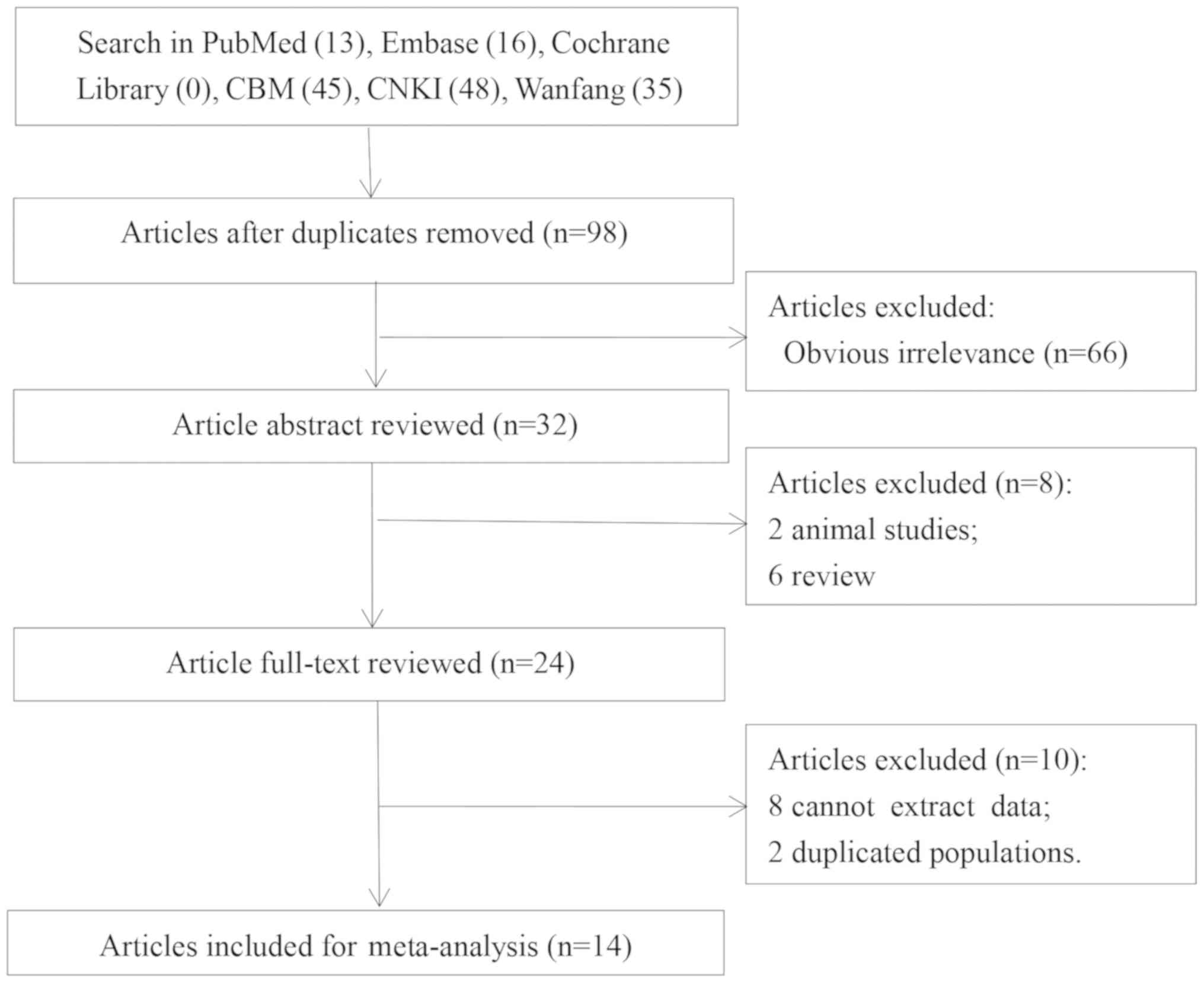
A total of 98 articles were obtained after excluding
49 duplicates. Then, 66 obvious irrelevant articles were
eliminated. Two animal studies, six reviews, eight studies without
useable data and two studies with duplicated populations were
subsequently removed after abstract and full-text review. 14
articles (11,13,14,21–31)
were included in the present meta-analysis; the flow diagram of the
search and selection process is presented in Fig. 1.
The included studies were published between 2006 and
2019 and performed between 2000 and 2018. The follow-up time was
1–12 months. The studies comprised 1,301 participants, including
654 patients with lupus nephritis and 647 controls. The Bailing
capsule group were treated with Bailing capsule combined with
cortisone, prednisone, prednisolone, low molecular weight heparin,
tacrolimus, methyprednisolone cyclophosphamide, methylprednisolone
or leflunomide. No significant differences were observed in sex,
age or course of the disease between the Bailing capsule and
control groups in each publication (Table I). The outcome indicators,
including SLEDAI score, complications (such as respiratory
infection, fatigue, drowsiness, gastrointestinal symptoms, rash and
leukocytopenia), Albumin, 24-h urinary protein, serum creatinine,
C3 and the number of effective treatments and were recorded
(Table II). The results of the
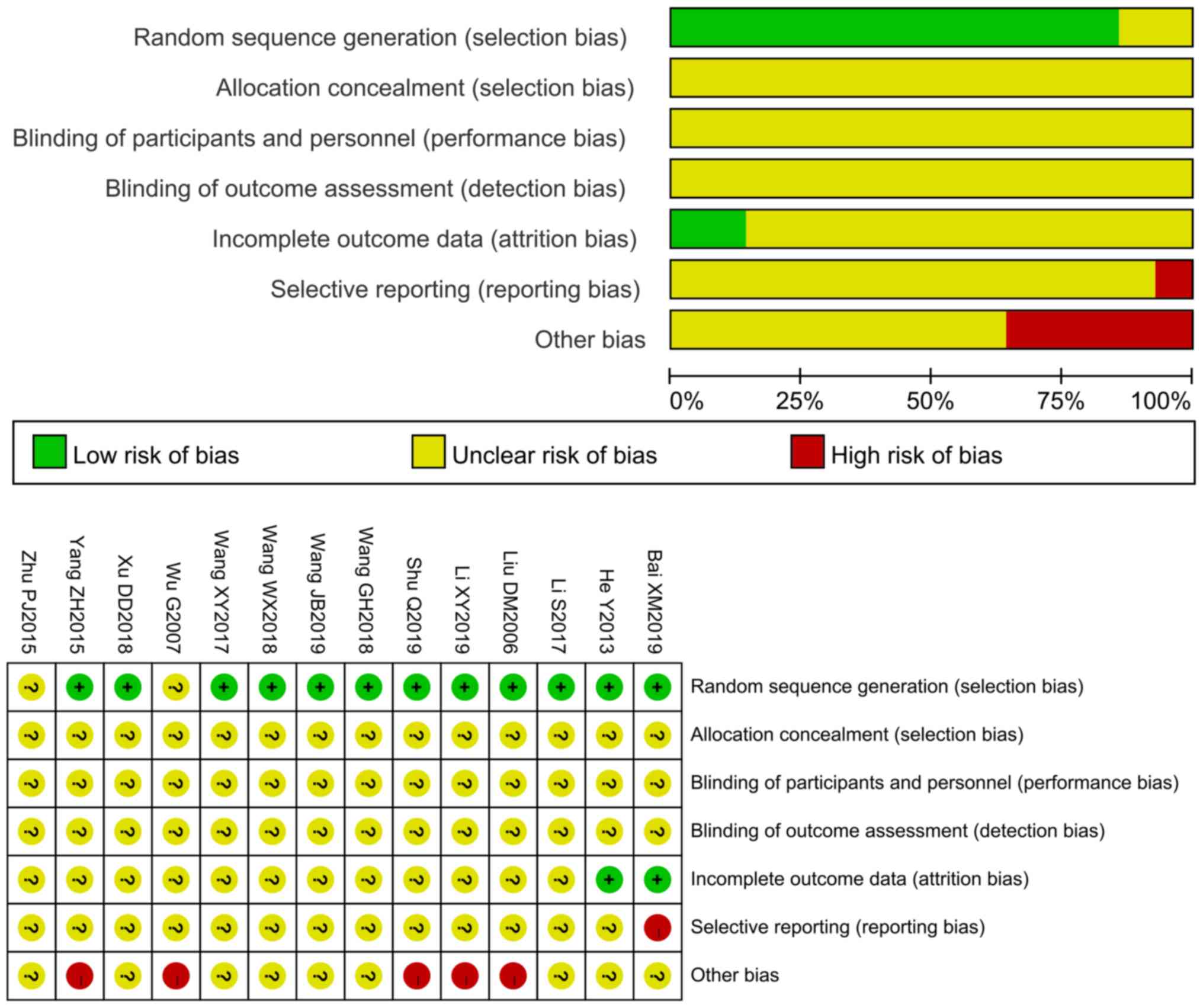
quality assessment demonstrated high or unclear risk of bias
(Fig. 2), indicating that the
literature was of average quality.
 | Table I.Characteristics of the included
studies. |
Table I.
Characteristics of the included
studies.
| Author, year | Study year | Follow-up,
months | Group | N | Drugs | Sex
(male/female) | Age, years | Course of the
disease, months | (Refs.) |
|---|
| Bai et al,
2019 | 2017.01-2018.06 | 6 | Bailing capsule | 30 | Cyclophosphamide +
Bailing capsule | 7/23 | 31.42±12.39 | 13.91±6.17 | (13) |
|
|
|
| Control | 30 | Cyclophosphamide | 15/15 | 35.17±14.21 | 13.54±7.48 |
|
| He et al,
2013 | 2010.03-2012.03 | 2 | Bailing capsule | 21 | Methylprednisolone +
heparin + Bailing capsule | 2/19 | 31.80±4.40 | 12±10.8 (year) | (21) |
|
|
|
| Control | 21 | Methylprednisolone +
heparin | 3/17 | 30.20±5.30 | 0.9±1.2 (year) |
|
| Li and
Wang, | 2010.06-2016.06 | NA | Bailing capsule | 45 | Prednisone + Bailing
capsule | 4/41 | 35.26±10.89 | 59.50±17.21 | (11) |
| 2017 |
|
| Control | 45 | Prednisone | 5/40 | 36.21±9.32 | 60.89±23.24 |
|
| Li et al,
2019 | 2012.12-2017.03 | 6 | Bailing capsule | 63 | Leflunomide +
prednisone acetate + Bailing capsule | 29/34 | 38.92±12.75 | 30.70±8.67 | (14) |
|
|
|
| Control | 63 | Leflunomide +
prednisone acetate | 28/35 | 38.20±9.16 | 31.28±9.30 |
|
| Liu et al,
2006 | 2000-2005 | 1 | Bailing capsule | 65 | Prednisone + Bailing
capsule | NA | 15-55 | 12-180 | (16) |
|
|
|
| Control | 61 | Prednisone |
|
|
|
|
| Shu et al,
2019 | 2016.01-2017.06 | 12 | Bailing capsule | 24 | Cyclophosphamide +
Bailing capsule | 7/17 | 31.80±9.40 | 12±9.6 y | (31) |
|
|
|
| Control | 23 |
Cyclophosphamide | 6/17 | 30.60±10.50 | 10.8±12 y |
|
| Wang et al,
2018 |
2012.06-2017.08 | 6 | Bailing
capsule | 56 | Prednisone acetate
+ Bailing capsule | 8/48 | 45.06±3.71 | 22.98±5.63 | (23) |
|
|
|
| Control | 56 | Prednisone
acetate | 10/46 | 46.01±3.80 | 23.58±5.71 |
|
| Wang 2019 |
2013.05-2018.03 | 6 | Bailing
capsule | 55 | Prednisone acetate
+ Bailing capsule | 32/23 | 45.18±9.46 | 20.36±12.61 | (24) |
|
|
|
| Control | 55 | Prednisone
acetate | 30/25 | 44.88±9.60 | 23.07±12.45 |
|
| Wang 2018 |
2016.05-2017.05 | 6 | Bailing
capsule | 26 | Tacrolimus +
Bailing capsule | 13/13 | 41.54±7.21 | 20.21±6.21 | (30) |
|
|
|
| Control | 26 | Tacrolimus | 14/12 | 40.21±7.21 | 20.21±6.21 |
|
| Wang et al,
2017 |
2014.09-2016.02 | 12 | Bailing
capsule | 59 | Tacrolimus +
Bailing capsule | 11/48 | 22.03±12.08 | 20.59±12.13 | (25) |
|
|
|
| Control | 59 | Tacrolimus | 9/50 | 29.96±8.02 | 22.03±12.08 |
|
| Wu 2007 |
2003.05-2005.02 | 2 | Bailing
capsule | 22 | Prednisone +
Bailing capsule | 14/28 | 34.30±12.20 | 56.4±19.2 | (26) |
|
|
|
| Control | 20 | Prednisone |
| 32.60±11.80 | 50.4±20.4 |
|
| Xu 2018 |
2016.01-2017.02 | 12 | Bailing
capsule | 60 | Tacrolimus +
Bailing capsule | 5/55 | 46.95±10.62 | 13.17±3.25 | (29) |
|
|
|
| Control | 60 | Tacrolimus | 6/54 | 47.84±11.56 | 12.87±3.43 |
|
| Zhu et al,
2015 |
2010.12-2013.12 | 12 | Bailing
capsule | 60 | Cyclophosphamide +
Bailing capsule | 6/54 | 49.87±16.34 | NA | (28) |
|
|
|
| Control | 60 |
Cyclophosphamide | 7/53 | 50.03±16.40 | NA |
|
| Yang et al,
2015 |
2013.05-2014.05 | 12 | Bailing
capsule | 68 | Cyclophosphamide +
Bailing capsule | 10/58 | 51.37±17.53 | NA | (27) |
|
|
|
| Control | 68 |
Cyclophosphamide | 12/56 | 50.89±17.48 | NA |
|
 | Table II.The outcome indicators of the
included studies. |
Table II.
The outcome indicators of the
included studies.
| Author, year | Group | N | SLEDAI | Complications | Alb, g/l | Effectiveness | 24-h urinary
protein, g/24 h | Serum creatinine,
µmol/l | Anti-ds-DNAIg,
+/- | C3, g/l | (Refs.) |
|---|
| Bai et al,
2019 | Bailing
Capsule | 30 | 5.83±2.79 | 3 | NA | 29 | 0.71±0.16 | 62.49±7.13 | NA | NA | (13) |
|
| Control | 30 | 8.64±3.84 | 11 | NA | 23 | 1.65±0.31 | 79.51±8.48 | NA | NA |
|
| He et al,
2013 | Bailing
Capsule | 21 | NA | NA | 44.5±4.2 | NA | 1.02±0.42 | 147.3±22.5 | NA | NA | (21) |
|
| Control | 21 | NA | NA | 36.1±3.6 | NA | 2.09±0.72 | 190.3±21.8 | NA | NA |
|
| Li and Wang, | Bailing
Capsule | 45 | NA | NA | NA | 43 | NA | NA | 23 | NA | (11) |
| 2017 | Control | 45 | NA | NA | NA | 35 | NA | NA | 25 | NA |
|
| Li et al,
2019 | Bailing
Capsule | 63 | 5.69±1.72 | NA | 36.27±6.80 | 58 | 0.58±0.16 | 66.52±11.80 | NA | 1.09±0.29 | (14) |
|
| Control | 63 | 9.83±2.20 | NA | 29.10±6.15 | 50 | 1.69±0.50 | 77.89±11.20 | NA | 0.77±0.20 | (16) |
| Liu et al,
2006 | Bailing
Capsule | 65 | NA | 37 | NA | 61 | NA | NA | NA | NA |
|
|
| Control | 61 | NA | 52 | NA | 47 | NA | NA | NA | NA |
|
| Shu et al,
2019 | Bailing
Capsule | 24 | NA | NA | 41.68±12.13 | 20 | 0.78±0.22 | 56.88±9.68 | NA | 0.96±0.27 | (31) |
|
| Control | 23 | NA | NA | 35.23±10.86 | 15 | 1.05±0.42 | 63.64±11.07 | NA | 0.78±0.22 |
|
| Wang et al,
2018 | Bailing
Capsule | 56 | 3.12±0.98 | 6 | NA | 51 | 0.25±0.10 | 62.33±8.53 | NA | NA | (23) |
|
| Control | 56 | 5.42±1.65 | 4 | NA | 43 | 0.75±0.23 | 85.63±10.55 | NA | NA |
|
| Wang, 2019 | Bailing
Capsule | 55 | 3.10±0.91 | 4 | NA | NA | 0.23±0.12 | 62.34±8.51 | NA | NA | (24) |
|
| Control | 55 | 5.44±1.61 | 6 | NA | NA | 0.73±0.24 | 85.66±10.51 | NA | NA |
|
| Wang, 2018 | Bailing
Capsule | 26 | 9.01±1.21 | NA | 35.21±2.54 | 25 | 0.77±0.32 | NA | NA | NA | (30) |
|
| Control | 26 | 14.21±2.32 | NA | 43.21±3.25 | 19 | 1.08±0.54 | NA | NA | NA |
|
| Wang et al,
2017 | Bailing
Capsule | 59 | 2.98±1.03 | 16 | 42.98±11.87 | 52 | 0.78±0.22 | 62.27±31.01 | 30 | 0.78±0.22 | (25) |
|
| Control | 59 | 5.11±2.15 | 28 | 36.27±10.86 | 41 | 1.07±0.92 | 64.99±33.75 | 32 | 0.72±0.23 |
|
| Wu, 2007 | Bailing
Capsule | 22 | NA | NA | NA | NA | 1.02±0.46 | 147.3±20.4 | NA | 108.8±13.7 | (26) |
|
| Control | 20 | NA | NA | NA | NA | 1.97±0.75 | 185.9±21.6 | NA | 82.6±12.8 |
|
| Xu, 2018 | Bailing
Capsule | 60 | 3.01±1.09 | 15 | 29.39±5.62 | 53 | 1.42±0.33 | 68.95±9.87 | NA | 0.76±0.24 | (29) |
|
| Control | 60 | 5.26±2.45 | 28 | 25.47±4.54 | 41 | 1.75±0.44 | 56.06±8.07 | NA | 0.61±0.16 |
|
| Zhu et al,
2015 | Bailing
Capsule | 60 | 5.12±3.21 | 23 | 39.74±9.12 | 53 | NA | 80.86±20.78 | 10 | 1.36±0.38 | (28) |
|
| Control | 60 | 6.64±3.43 | 22 | 36.28±8.23 | 43 | NA | 86.49±22.94 | 12 | 1.22±0.28 |
|
| Yang et al,
2015 | Bailing
Capsule | 68 | 5.09±3.18 | 18 | 39.95±9.21 | 61 | 1.11±0.62 | 78.97±20.56 | 13 | 1.38±0.36 | (27) |
|
| Control | 68 | 6.64±3.43 | 30 | 36.54±8.27 | 50 | 1.43±0.78 | 86.49±22.94 | 15 | 1.24±0.25 |
|
Meta-analysis
The heterogeneity test demonstrated P<0.05 and
I2>50% for SLEDAI score, Alb, 24-h urinary protein,
complement C3 and serum creatinine. The random effects model was
thus applied for these indicators. The fixed effect model was used
for the other results.
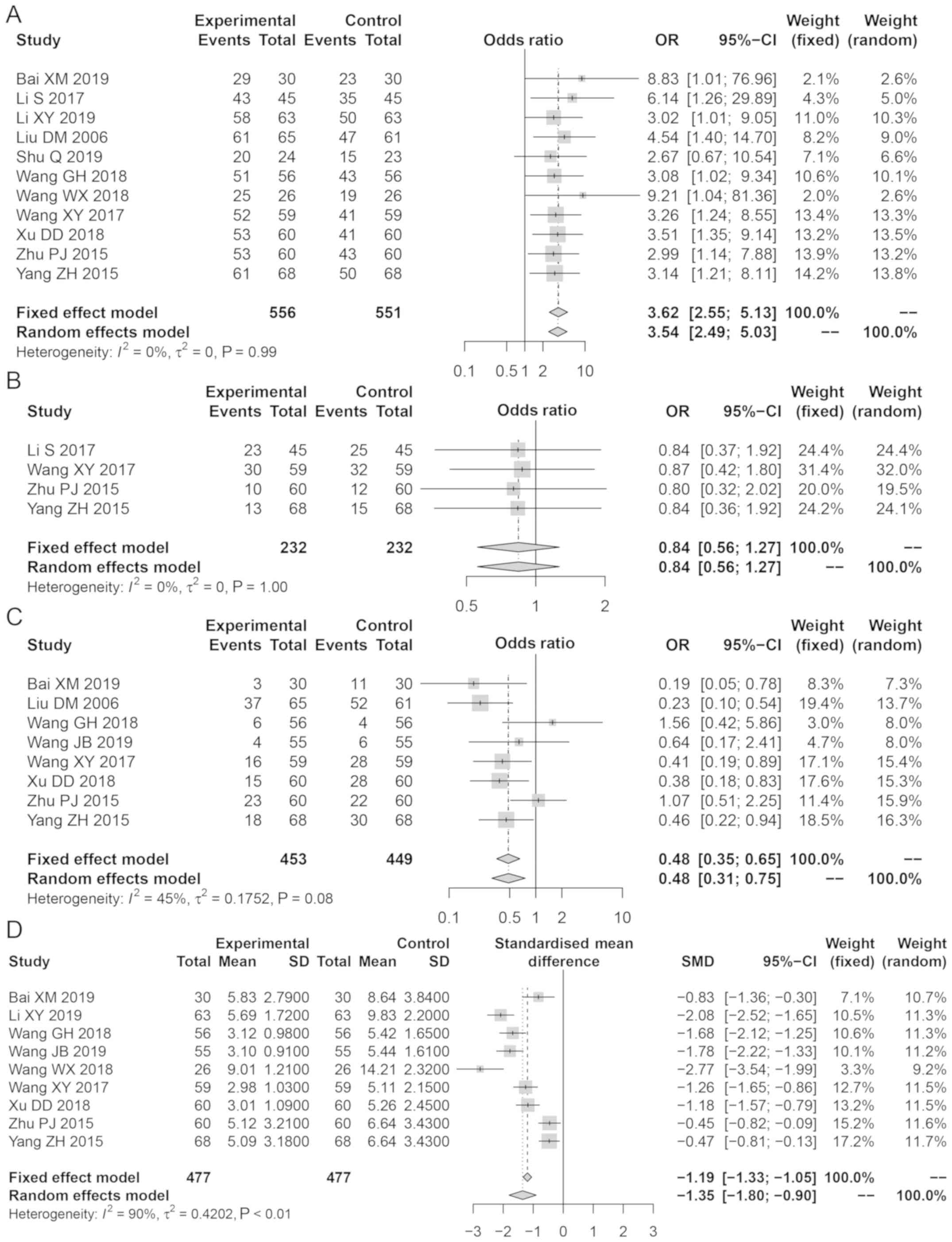
Bailing capsule application was demonstrated to be
more effective compared with treatment without the use of Bailing
capsule for lupus nephritis (OR, 3.62; 95% CI: 2.55, 5.13; Z=7.22;
P<0.001; Fig. 3A). Sensitivity
analysis demonstrated that the results were reliable; there was a
publication bias for the effectiveness of treatment Egger's test,
t=2.76; P<0.001).
No significant differences were observed in the
anti-ds-DNAIg levels between the Bailing capsule and control groups
(OR, 0.84; 95% CI: 0.56, 1.27; Z=0.83; P=0.400; Fig. 3B). Sensitivity analysis
demonstrated that the results were reliable. There was a
publication bias for anti-ds-DNAIg (t=33.40; P=0.0001).
The number of complications in the Bailing Capsule
group was lower compared with that in the control group (OR, 0.48;
95% CI: 0.31, 0.75; Z=3.22; P=0.0013; Fig. 3C). Sensitivity analysis
demonstrated that the results were reliable; there was no
publication bias for complications (t=0.45; P=0.67).
The decrease in the SLEDAI score in the Bailing
capsule group was higher compared with that in the control group
(SMD, −1.35; 95% CI: −1.80, −0.90; Z=5.89; P<0.0001; Fig. 3D). Sensitivity analysis indicated
that the results were reliable; no publication bias existed for the
SLEDAI score (t=2.38; P=0.05).
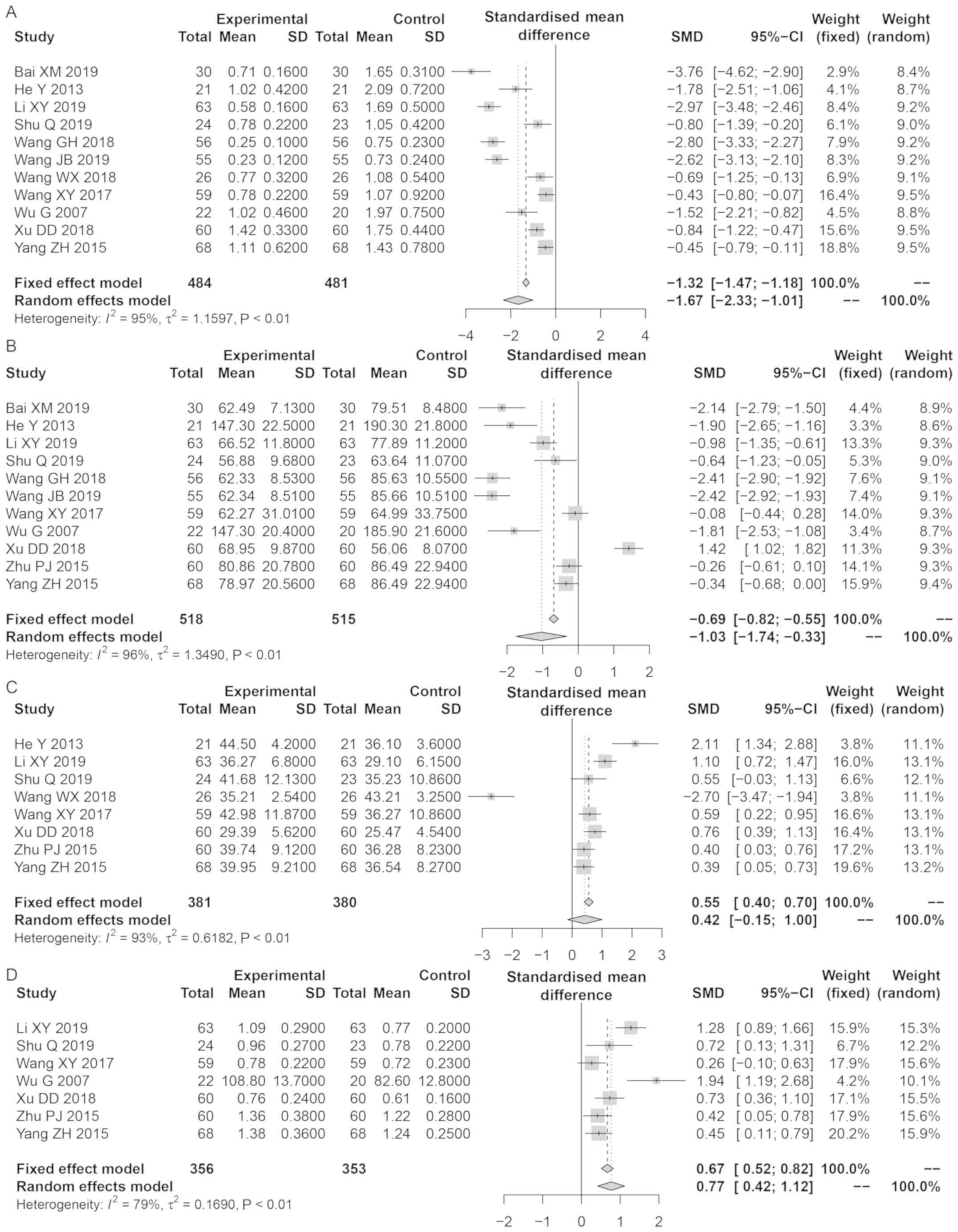
The decrease in 24-h urinary protein in the Bailing
capsule group was greater compared with those in the control group
(SMD, −1.67; 95% CI: −2.33, −1.01; Z=5.89; P<0.0001; Fig. 4A). Sensitivity analysis
demonstrated that the results were reliable and stable. Publication
bias was identified for 24-h urinary protein (t=2.42; P=0.04).
The decrease in serum creatinine in the Bailing
capsule group was greater compared with those in the control group
(SMD, −1.03; 95% CI: −1.73, −0.33; Z=2.88, P=0.004; Fig. 4B). Sensitivity analysis suggested
that the results were reliable and stable; there was no publication
bias for serum creatinine (t=2.06; P=0.07).
The increase in Alb in the Bailing capsule group was
greater compared with those in the control group (SMD, 0.77; 95%
CI: 0.42, 1.12; Z=4.32; P<0.0001; Fig. 4C). However, after eliminating the
study of Wenxin W (30), the
results of the pooled SMD were reversed. No publication bias was
identified for Alb (t=0.70; P=0.51).
No significant differences were observed in the
levels of complement C3 between the Bailing capsule and control
groups (SMD, 0.42; 95% CI: −0.15, −0.99; Z=1.45; P=0.15; Fig. 4D). Sensitivity analysis
demonstrated that the results were reliable and stable; there was
no publication bias for complement C3 (t=1.73; P=0.15).
Discussion
In this meta-analysis, a total of 14 studies
comprising 1,301 participants were included to verify the
effectiveness of Bailing capsules in the treatment of lupus
nephritis. The results demonstrated that, with the exception of
anti-ds-DNAIg and complement C3, other indicators (SLEDAI score,
Alb, 24-h urinary protein, serum creatinine, and the number of
effective treatments and complications) in the Bailing capsule
group were improved compared with those in the control group.
Quantitative analysis of the 24-h urinary protein
levels is an important diagnostic index to assess the severity and
prognosis of nephritis or kidney-related diseases (32). The SLEDAI score is an index used to
assess SLE disease activity (33).
Serum creatinine is positively associated with the degree of early
renal injury in patients with acute glomerulonephritis (33). The level of Alb, which reflects the
nutritional status of patients, is associated with lupus nephritis
development, and nephrotic syndrome is often accompanied by
hypoalbuminemia (34). A
significant reduction in complement C3, which is produced by liver
cells, is related to the occurrence of SLE and other immune
diseases, and anti-ds-DNAIg is the main immune factor involved in
the organ damage in SLE (25). In
a meta-analysis by Wang et al (35), several indicators including serum
creatinine, complement C3, 24-h urinary protein, adverse effects
and SLEDAI score were improved in the Bailing capsule group
compared with those in the non-Bailing capsule group for lupus
nephritis therapy. In the present meta-analysis, SLEDAI score, Alb,
24-h urinary protein, serum creatinine, and the number of effective
treatments and complications in the Bailing capsule group were
improved compared with those in the control group. Thus, Bailing
capsules may be used effectively in the treatment of lupus
nephritis.
However, the present study has certain limitations;
there was significant heterogeneity among the studies, likely
caused by differences in habits and customs, living conditions and
economic development level in different regions. In addition, the
effects of other confounding factors, such as sex and age, may have
contributed to this heterogeneity. In addition, due to incomplete
data, correction of covariates and subgroup analysis were not
performed. The number of eligible studies was further reduced due
to the rigorous exclusion and inclusion criteria (36). Additionally, all the included
studies were from China, which may have caused a selection bias.
Publication bias was also observed for effectiveness, anti-ds-DNAIg
and 24-h urinary protein. Furthermore, in the sensitivity analysis
of Alb, after eliminating the study of Wang (30), the results of the pooled SMD were
reversed, indicating an unreliable outcome. Lastly, since Bailing
capsule was used for SLE or lupus nephritis treatment in
combination with other medicines, these medicines were different in
different studies, and the numbers of eligible studies for each
medicine were low; thus, meta-analysis data to demonstrate the
effect to Bailing Capsule therapy with other medicines was not
added. Despite these limitations, the present study suggests that
Bailing Capsules may be used effectively in lupus nephritis
treatment. An updated meta-analysis drawing from larger scale
studies and high-quality data may validate the findings of the
present study.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81770766) and
Provincial Natural Science Foundation of Liaoning (grant no.
20170540999).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, TX and LY conceptualized and designed the study.
YL, TX, XQ, BT and CB drafted and revised the manuscript. YL and TX
performed the literature search, retrieved data and wrote the draft
for the final manuscript. XQ performed statistical analysis. BT and
CB plotted the tables and figures. YL supervised the project and
approved the manuscript to be published. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun L, Zou LX, Han YC, Wu L, Chen T, Zhu
DD and Hu P: A20 overexpression exerts protective effects on
podocyte injury in lupus nephritis by downregulating UCH-L1. J Cell
Physiol. 234:16191–16204. 2019. View Article : Google Scholar
|
|
2
|
Mu Q, Zhang H, Liao X, Lin K, Liu H,
Edwards MR, Ahmed SA, Yuan R, Li L, Cecere TE, et al: Control of
lupus nephritis by changes of gut microbiota. Microbiome. 5:732017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miranda-Hernández D, Cruz-Reyes C, Angeles
U, Jara LJ and Saavedra MA: Prognostic factors for treatment
response in patients with lupus nephritis. Reumatol Clin.
10:164–169. 2014.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rees F, Doherty M, Grainge MJ, Lanyon P
and Zhang W: The worldwide incidence and prevalence of systemic
lupus erythematosus: A systematic review of epidemiological
studies. Rheumatology (Oxford). 56:1945–1961. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hogan J and Appel GB: Update on the
treatment of lupus nephritis. Curr Opin Nephrol Hypertens.
22:224–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okpechi IG, Gcelu A and Ameh OI: Lupus
nephritis: A simplified approach to diagnosis and treatment in
South Africa. South African Medical J. 105:1071–1074. 2015.
View Article : Google Scholar
|
|
7
|
Chan TM: Treatment of severe lupus
nephritis: The new horizon. Nat Rev Nephrol. 11:46–61. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Malvar A, Pirruccio P, Alberton V, Lococo
B, Recalde C, Fazini B, Nagaraja H, Indrakanti D and Rovin BH:
Histologic versus clinical remission in proliferative lupus
nephritis. Nephrol Dial Transplant. 32:1338–1344. 2015. View Article : Google Scholar
|
|
9
|
Ren HJ, Sun YL and Yuan B: Chinese patent
medicine Bailing capsule for treating lupus nephritis: A protocol
for systematic review and meta-analysis. Medicine (Baltimore).
98:e170412019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu H and Li S: Pharmacological effects of
Bailing capsule and its application in lung disease research.
Zhongguo Zhong Yao Za Zhi. 35:2777–2781. 2010.(In Chinese).
PubMed/NCBI
|
|
11
|
Li S and Wang L: Clinical observation on
the therapeutic effect of Corbrin Capsule in the treatment of lupus
nephritis. Chin Commun Doctors. 33:61–62. 2017.(In Chinese).
|
|
12
|
Zhou L, Zhang B, Yao C and Liao A:
Clinical research of Bailing capsules on change of cellular
immunity in lupus nephritis. Chin Pharmacist. (4): 289–290.
2004.(In Chinese).
|
|
13
|
Bai XM, Li H, Li XD and Li Y: Clinical
study on corbrin capsules combined with cyclophosphamide in
treatment of lupus nephritis. Drugs Clin. 34:1181–1184. 2019.(In
Chinese).
|
|
14
|
Li XY, Li FX and Li B: Clinical study on
corbrin capsules combined with leflunomide and prednisone in
treatment of lupus nephritis. Drugs Clin. 34:154–158. 2019.(In
Chinese).
|
|
15
|
Higgins JP and Green S: Cochrane handbook
for systematic reviews of interventions: Cochrane Book Series. The
Cochrane Collaboration; 2008
|
|
16
|
Liu T, Xu QE, Zhang CH and Zhang P:
Occupational exposure to methylene chloride and risk of cancer: A
meta-analysis. Cancer Causes Control. 24:2037–2049. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lau J, Ioannidis JP and Schmid CH:
Quantitative synthesis in systematic reviews. Ann Intern Med.
127:820–826. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng RN, Zhao C, Sun CH and Li Y:
Meta-analysis of TNF 308 G/A polymorphism and type 2 diabetes
mellitus. PLoS One. 6:e184802011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma E, Wang H, Guo J, Tian R and Wei L: The
association between the rs11196218A/G polymorphism of the TCF7L2
gene and type 2 diabetes in the Chinese Han population: A
meta-analysis. Clinics (Sao Paulo). 70:593–599. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seagroatt V and Stratton I: Bias in
meta-analysis detected by a simple, graphical test. Test had 10%
false positive rate. BMJ. 316:469–471. 1997.
|
|
21
|
He Y, Huang JP, Wu RY and Tan F:
Observation of clinical effect of Bailing capsule combined with low
molecular weight heparin for the treatment of lupus of nephritis.
Asia Pacific Tradit Med. 9:207–208. 2013.(In Chinese).
|
|
22
|
Liu DM, Wang CH and Li XP: Clinical
analysis of 65 cases of lupus nephritis treated by Bailing capsule.
Chin Commun Doctors. 21. 2006.(In Chinese).
|
|
23
|
Wang GH, Su XD and Wu Y: Clinical study on
Corbrin Capsules combined with prednisone in treatment of lupus
nephritis. Drugs Clin. 33:2372–2376. 2018.(In Chinese).
|
|
24
|
Wang JB: Clinical effect of boling capsule
combined with prednisone in the treatment of lupus nephritis SLEDAI
scoring analysis. Chin J Mod Drug Appl. 121–122. 2019.(In
Chinese).
|
|
25
|
Wang XY, Wang GJ, Zhang XX, Gong YN, Li
YS, Ma S, Xiao J and Zhao ZZ: Clinical study on Bailing capsules
combined with tacrolimus in treatment of lupus nephritis. Drugs
Clin. 32:1065–1069. 2017.(In Chinese).
|
|
26
|
Wu G: Methyprednisolone with Bailing
capsule in treatment of lupus nephritis. J Medical Forum. 28:38–39.
2007.(In Chinese).
|
|
27
|
Yang ZH, Liu B, Lu J, Hou XX and Yin J: A
multicenter prospective study of the effect of Bailing capsules on
clinical efficacy and infection rate in the patients with lupus
nephritis. Heilongjiang Med J. 28:1205–1208. 2015.(In Chinese).
|
|
28
|
Zhu PJ, Ke SS and Xu F: Effect of Bailing
capsules on interleukin-2, complement and infection rate in
patients with lupus nephritis. Chin J Mod Appl Pharm. 33:364–368.
2015.(In Chinese).
|
|
29
|
Xu DD: Observation on the efficacy of
boling capsule combined with tacrolimus in the treatment of lupus
nephritis. Mod Diagn Treat. 29:2888–2890. 2018.
|
|
30
|
Wang WX: Study on the combination of
boling capsule and tacrolimus in the treatment of lupus nephritis.
Chin J Mod Drug Appl. 12:112–113. 2018.
|
|
31
|
Shu Q, Tan F, Huang LH and Peng J: Effect
of Bailing capsules on macrophage function by regulating miR- 127
expression in lupus nephritis patients. Tradit Chinese Drug Res
Clin Pharmacol. 30:733–738. 2019.(In Chinese).
|
|
32
|
Feng D: The correlation analysis of urine
microalbumin /urine creatinine and 24 h urine proteinquantification
in patients with chronic nephritis. Laborat Med Clin.
14:1942017.(In Chinese).
|
|
33
|
Zhang H and Ge X: Significance of Cys C,
BUN and sCr level detection in assessment of early renal damage of
acute glomerulonephritis. J Hainan Med Univ. 22:447–449. 2016.(In
Chinese).
|
|
34
|
Gladman DD, Ibañez D and Urowitz MB:
Systemic lupus erythematosus disease activity index 2000. J
Rheumatol. 29:288–291. 2002.PubMed/NCBI
|
|
35
|
Wang BL, Li QY and Huang WL: Assessment of
renal injury degree in patients with nephritis by β 2-m, AIb, IgG,
and α 1-m. J Radioimmunol. 14:91–93. 2001.
|
|
36
|
Szulińska M, Skrypnik D, Ratajczak M,
Karolkiewicz J, Madry E, Musialik K, Walkowiak J, Jakubowski H and
Bogdański P: Effects of endurance and endurance-strength exercise
on renal function in abdominally obese women with renal
hyperfiltration: A prospective randomized trial. Biomed Environ
Sci. 29:706–712. 2016.PubMed/NCBI
|