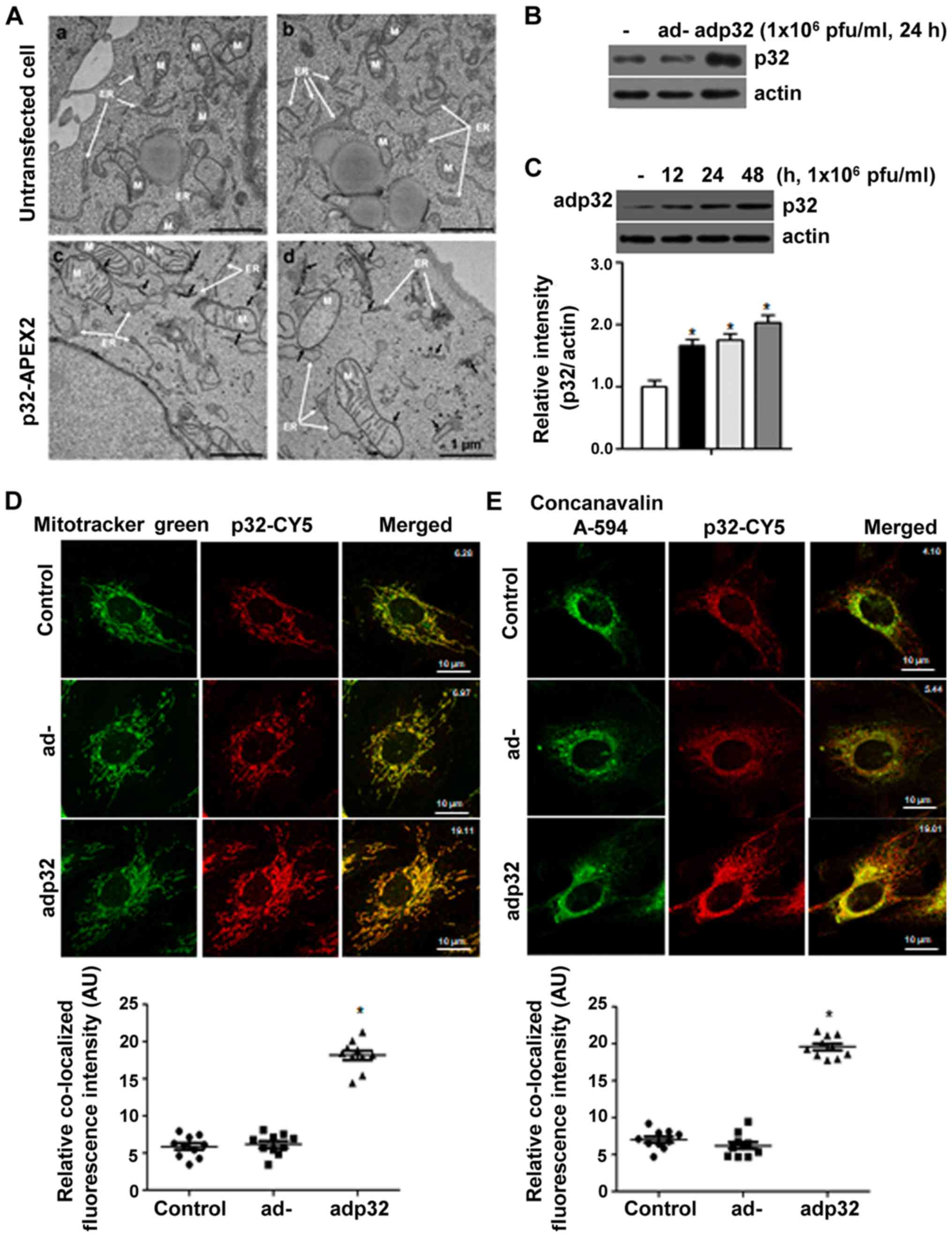
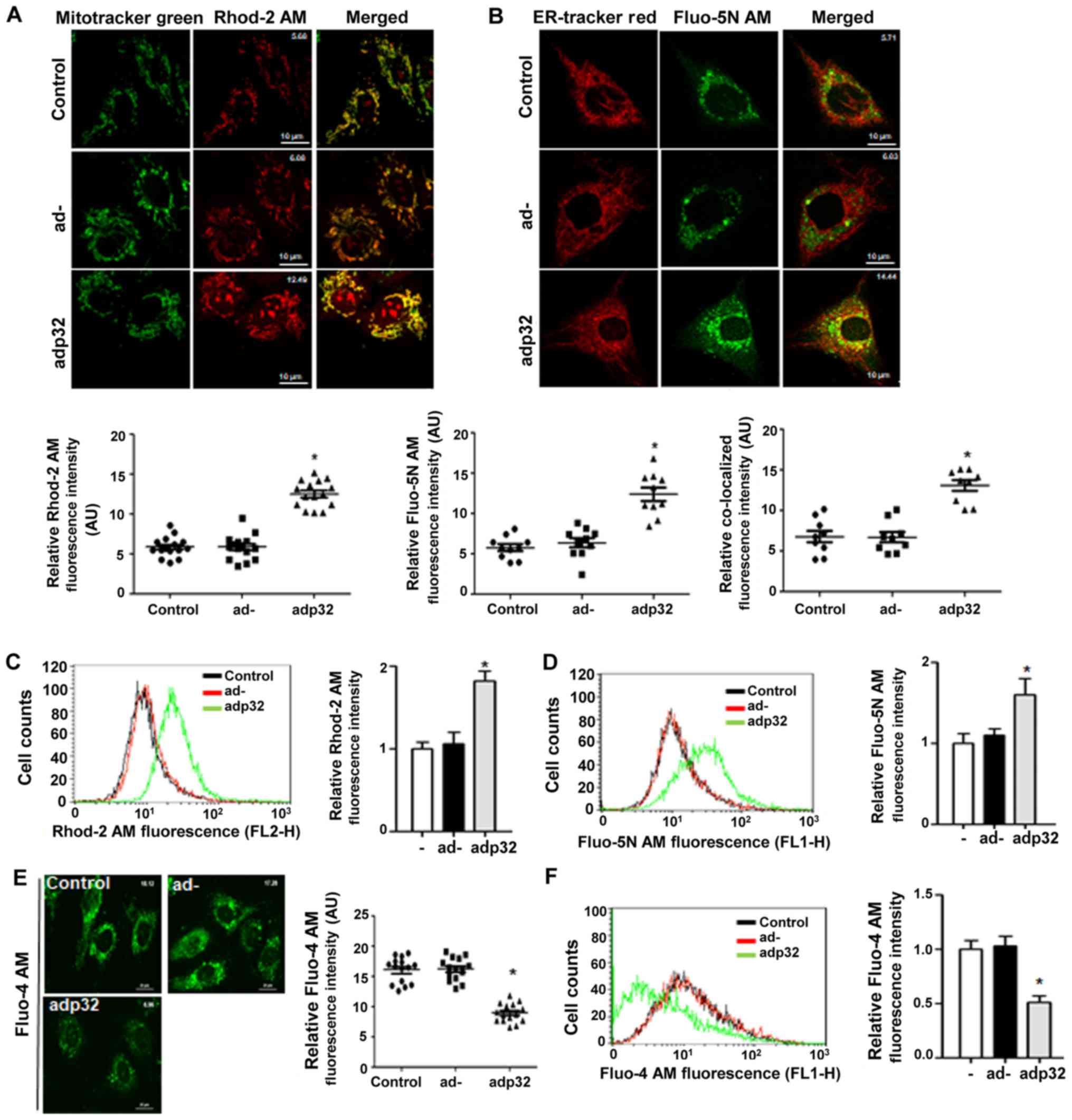
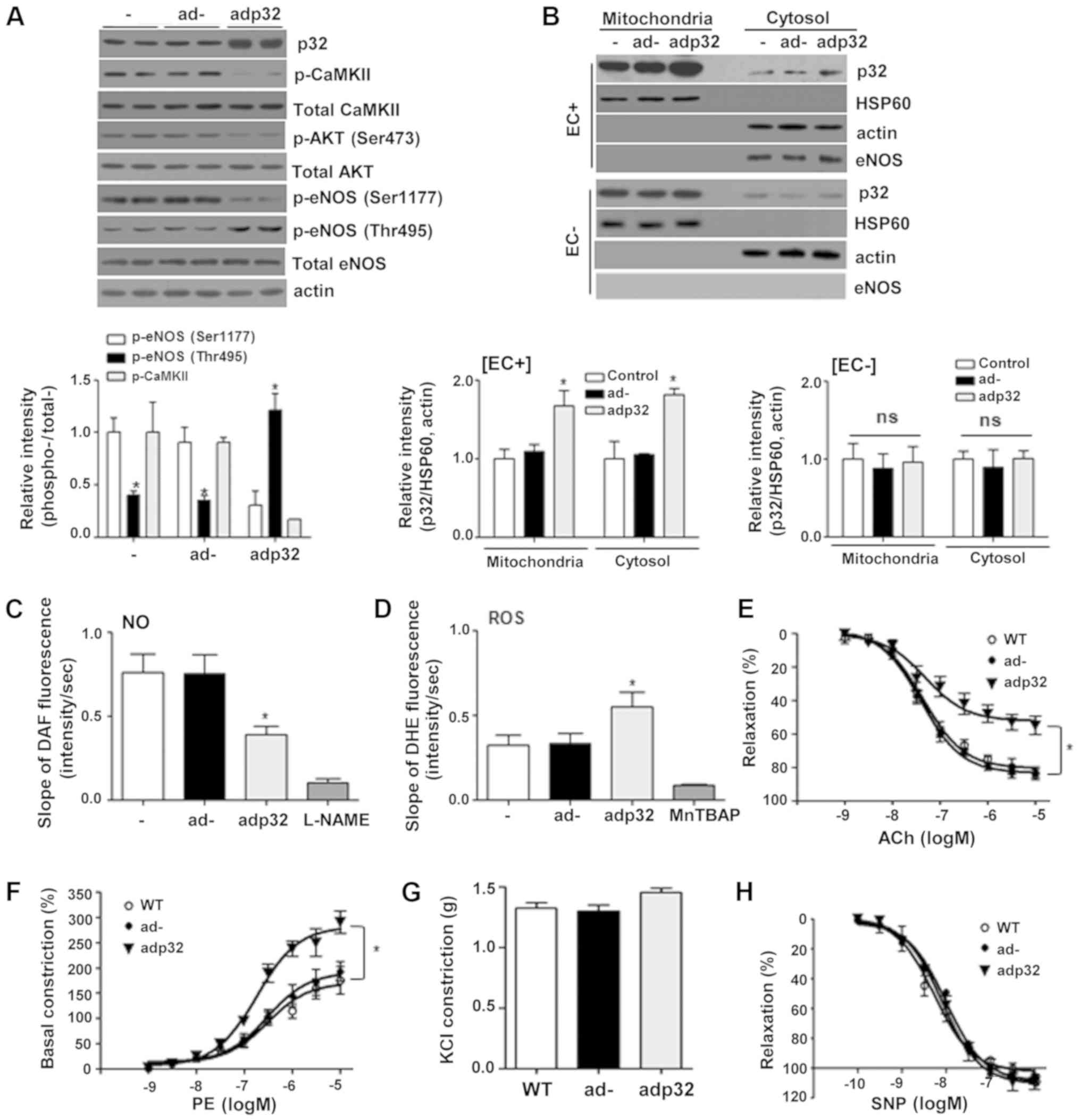
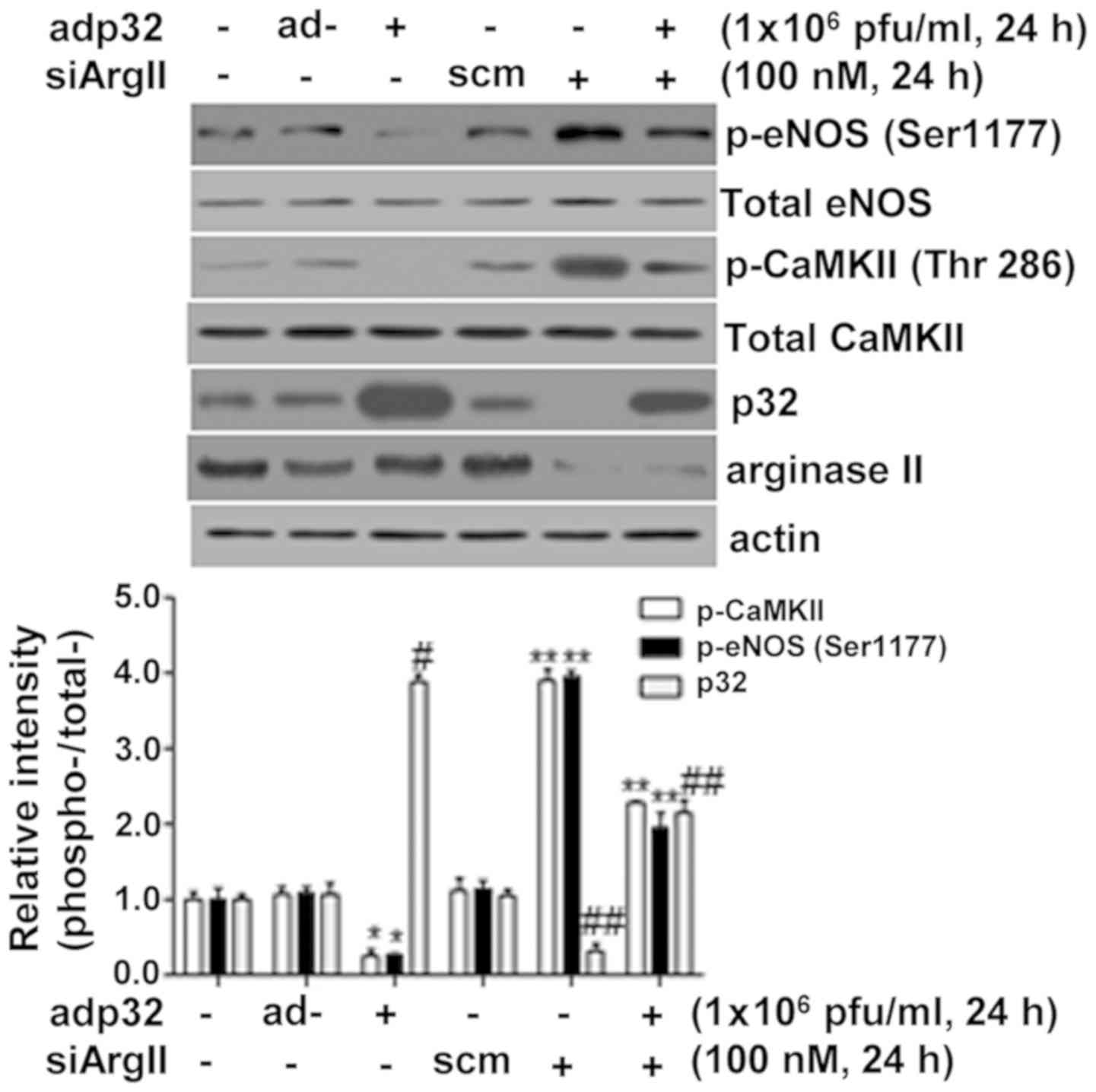
|
1
|
Ghebrehiwet B, Lim BL, Peerschke EI,
Willis AC and Reid KB: Isolation, cDNA cloning, and overexpression
of a 33-kD cell surface glycoprotein that binds to the globular
‘heads’ of C1q. J Exp Med. 179:1809–1821. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krainer AR, Mayeda A, Kozak D and Binns G:
Functional expression of cloned human splicing factor SF2: Homology
to RNA-binding proteins, U1 70K, and Drosophila splicing
regulators. Cell. 66:383–394. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muta T, Kang D, Kitajima S, Fujiwara T and
Hamasaki N: p32 protein, a splicing factor 2-associated protein, is
localized in mitochondrial matrix and is functionally important in
maintaining oxidative phosphorylation. J Biol Chem.
272:24363–24370. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sengupta A, Banerjee B, Tyagi RK and Datta
K: Golgi localization and dynamics of hyaluronan binding protein 1
(HABP1/p32/C1QBP) during the cell cycle. Cell Res. 15:183–186.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Leeuwen HC and O'Hare P: Retargeting
of the mitochondrial protein p32/gC1Qr to a cytoplasmic compartment
and the cell surface. J Cell Sci. 114:2115–2123. 2001.PubMed/NCBI
|
|
6
|
Itahana K and Zhang Y: Mitochondrial p32
is a critical mediator of ARF-induced apoptosis. Cancer Cell.
13:542–553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sunayama J, Ando Y, Itoh N, Tomiyama A,
Sakurada K, Sugiyama A, Kang D, Tashiro F, Gotoh Y, Kuchino Y, et
al: Physical and functional interaction between BH3-only protein
Hrk and mitochondrial pore-forming protein p32. Cell Death Differ.
11:771–781. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fogal V, Richardson AD, Karmali PP,
Scheffler IE, Smith JW and Ruoslahti E: Mitochondrial p32 protein
is a critical regulator of tumor metabolism via maintenance of
oxidative phosphorylation. Mol Cell Biol. 30:1303–1318. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu M, Crawford SA, Henstridge DC, Ng IH,
Boey EJ, Xu Y, Febbraio MA, Jans DA and Bogoyevitch MA: p32 protein
levels are integral to mitochondrial and endoplasmic reticulum
morphology, cell metabolism and survival. Biochem J. 453:381–391.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pupo AS and Minneman KP: Specific
interactions between gC1qR and alpha1-adrenoceptor subtypes. J
Recept Signal Transduct Res. 23:185–195. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Storz P, Hausser A, Link G, Dedio J,
Ghebrehiwet B, Pfizenmaier K and Johannes FJ: Protein kinase C
[micro] is regulated by the multifunctional chaperon protein p32. J
Biol Chem. 275:24601–24607. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simos G and Georgatos SD: The lamin B
receptor-associated protein p34 shares sequence homology and
antigenic determinants with the splicing factor 2-associated
protein p32. FEBS Lett. 346:225–228. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deb TB and Datta K: Molecular cloning of
human fibroblast hyaluronic acid-binding protein confirms its
identity with P-32, a protein co-purified with splicing factor SF2.
Hyaluronic acid-binding protein as P-32 protein, co-purified with
splicing factor SF2. J Biol Chem. 271:2206–2212. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lim BL, Reid KB, Ghebrehiwet B, Peerschke
EI, Leigh LA and Preissner KT: The binding protein for globular
heads of complement C1q, gC1qR. Functional expression and
characterization as a novel vitronectin binding factor. J Biol
Chem. 271:26739–26744. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu L, Zhang Z, Loewenstein PM, Desai K,
Tang Q, Mao D, Symington JS and Green M: Molecular cloning and
characterization of a cellular protein that interacts with the
human immunodeficiency virus type 1 Tat transactivator and encodes
a strong transcriptional activation domain. J Virol. 69:3007–3016.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Finan JE, Middeldorp JM and
Hayward SD: P32/TAP, a cellular protein that interacts with EBNA-1
of Epstein-Barr virus. Virology. 236:18–29. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Braun L, Ghebrehiwet B and Cossart P:
gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB
invasion protein of Listeria monocytogenes. EMBO J.
19:1458–1466. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fleming I, Fisslthaler B, Dimmeler S, Kemp
BE and Busse R: Phosphorylation of Thr(495) regulates
Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase
activity. Circ Res. 88:E68–E75. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salerno JC, Harris DE, Irizarry K, Patel
B, Morales AJ, Smith SM, Martasek P, Roman LJ, Masters BS, Jones
CL, et al: An autoinhibitory control element defines
calcium-regulated isoforms of nitric oxide synthase. J Biol Chem.
272:29769–29777. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lobatón CD, Vay L, Hernández-Sanmiguel E,
Santodomingo J, Moreno A, Montero M and Alvarez J: Modulation of
mitochondrial Ca(2+) uptake by estrogen receptor agonists and
antagonists. Br J Pharmacol. 145:862–871. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koo BH, Hwang HM, Yi BG, Lim HK, Jeon BH,
Hoe KL, Kwon YG, Won MH, Kim YM, Berkowitz DE, et al: Arginase II
Contributes to the Ca2+/CaMKII/eNOS Axis by Regulating
Ca2+ Concentration Between the Cytosol and Mitochondria
in a p32-Dependent Manner. J Am Heart Assoc. 7:e0095792018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ryoo S, Gupta G, Benjo A, Lim HK, Camara
A, Sikka G, Lim HK, Sohi J, Santhanam L, Soucy K, et al:
Endothelial arginase II: A novel target for the treatment of
atherosclerosis. Circ Res. 102:923–932. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peerschke EI and Ghebrehiwet B: The
contribution of gC1qR/p33 in infection and inflammation.
Immunobiology. 212:333–342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
24. Koo BH, Hong D, Hong HD, Lim HK, Hoe
KL, Won M-H, Kim YM, Berkowitz DE and Ryoo S: Arginase II activity
regulates cytosolic Ca(2+) level in a p32-dependent manner that
contributes to Ca(2+)-dependent vasoconstriction in native
low-density lipoprotein-stimulated vascular smooth muscle cells.
Exp Mol Med. 51:1–12. 2019. View Article : Google Scholar
|
|
25
|
Sessa WC: eNOS at a glance. J Cell Sci.
117:2427–2429. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ignarro LJ, Buga GM, Wei LH, Bauer PM, Wu
G and del Soldato P: Role of the arginine-nitric oxide pathway in
the regulation of vascular smooth muscle cell proliferation. Proc
Natl Acad Sci USA. 98:4202–4208. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Meininger CJ, Hawker JR Jr, Haynes
TE, Kepka-Lenhart D, Mistry SK, Morris SM Jr and Wu G: Regulatory
role of arginase I and II in nitric oxide, polyamine, and proline
syntheses in endothelial cells. Am J Physiol Endocrinol Metab.
280:E75–E82. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morris SM Jr, Kepka-Lenhart D and Chen LC:
Differential regulation of arginases and inducible nitric oxide
synthase in murine macrophage cells. Am J Physiol. 275:E740–E747.
1998.PubMed/NCBI
|
|
29
|
Louis CA, Reichner JS, Henry WL Jr,
Mastrofrancesco B, Gotoh T, Mori M and Albina JE: Distinct arginase
isoforms expressed in primary and transformed macrophages:
Regulation by oxygen tension. Am J Physiol. 274:R775–R782.
1998.PubMed/NCBI
|
|
30
|
Collado B, Sánchez-Chapado M, Prieto JC
and Carmena MJ: Hypoxia regulation of expression and angiogenic
effects of vasoactive intestinal peptide (VIP) and VIP receptors in
LNCaP prostate cancer cells. Mol Cell Endocrinol. 249:116–122.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Berkowitz DE, White R, Li D, Minhas KM,
Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, et
al: Arginase reciprocally regulates nitric oxide synthase activity
and contributes to endothelial dysfunction in aging blood vessels.
Circulation. 108:2000–2006. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hein TW, Zhang C, Wang W, Chang CI,
Thengchaisri N and Kuo L: Ischemia-reperfusion selectively impairs
nitric oxide-mediated dilation in coronary arterioles:
Counteracting role of arginase. FASEB J. 17:2328–2330. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jung C, Gonon AT, Sjöquist PO, Lundberg JO
and Pernow J: Arginase inhibition mediates cardioprotection during
ischaemia-reperfusion. Cardiovasc Res. 85:147–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang C, Hein TW, Wang W, Miller MW,
Fossum TW, McDonald MM, Humphrey JD and Kuo L: Upregulation of
vascular arginase in hypertension decreases nitric oxide-mediated
dilation of coronary arterioles. Hypertension. 44:935–943. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Johnson FK, Johnson RA, Peyton KJ and
Durante W: Arginase inhibition restores arteriolar endothelial
function in Dahl rats with salt-induced hypertension. Am J Physiol
Regul Integr Comp Physiol. 288:R1057–R1062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peyton KJ, Ensenat D, Azam MA, Keswani AN,
Kannan S, Liu XM, Wang H, Tulis DA and Durante W: Arginase promotes
neointima formation in rat injured carotid arteries. Arterioscler
Thromb Vasc Biol. 29:488–494. 2009. View Article : Google Scholar : PubMed/NCBI
|