Introduction
Acute gouty arthritis (AGA) is a common and severe
form of inflammatory arthritis (1). Epidemiological evidence demonstrates
that the prevalence of gout is rapidly increasing (2). AGA is initiated by the deposition of
monosodium urate (MSU) crystals in the articular joints and bursal
tissues (3–5). Exploring the underlying mechanisms of
MSU-induced inflammation is important in the treatment of AGA
(6).
Macrophages play an important role in the
pathogenesis of inflammation. Macrophages are usually classified
into classical subtype (M1) and alternative subtype (M2), according
to their pro-inflammatory and anti-inflammatory functions,
respectively (7). Physiologically,
the balance of M1/M2 macrophage polarization is well maintained.
However, it has been demonstrated that an imbalance in M1/M2
polarization is crucial in the development of MSU-induced
inflammation (8). Molecular
mechanisms of M1 polarization in AGA includes the sirtuin
1/PI3K/Akt/STAT6 pathway, cyclooxygenase-2 and secreted
frizzled-related protein 2 (9–11),
while M2 polarization in AGA is relatively less understood.
Recently, long non-coding RNA (lncRNA)-MM2P has been
identified as a novel modulator of M1/M2 polarization and a
promoter of tumorigenesis. A microarray-based profiling assay
demonstrated that lncRNA-MM2P is the only lncRNA upregulated during
M2 polarization and downregulated in M1 macrophages (12). Therefore, the present study aimed
to investigate whether lncRNA-MM2P is an important modulator of
MSU-induced inflammation in cell models of RAW 264.7 and THP-1
monocytes.
Materials and methods
MSU crystal synthesis
Briefly, 100 mg of uric acid (≥99%, crystalline;
cat. no. U2625; Sigma-Aldrich; Merck KGaA) was dissolved, heated at
60°C and blended in 20 ml of distilled water with 60 µl of 10
mol/L−1 NaOH, adjusted to pH 7.2–7.4 with HCl (1
mol/L−1) at 60°C. The solution was kept overnight under
constant shaking at 60°C and then kept at room temperature for 5
days. After 5 days, the mixture was transferred to a 15 ml tube and
stored at 4°C for 4 days. Needle-like crystals were recovered and
suspended using a vortex overnight. The crystals were collected
after washing twice with 100% ethanol and once with acetone,
followed by centrifugation at 990 × g for 2 min at 4°C. The MSU
crystals were suspended in sterile endotoxin-free
phosphate-buffered saline (PBS). The crystals obtained were
preserved at −20°C after evaporation by heating at 180–200°C
(13,14).
Cell culture and treatment
RAW 264.7 cells (Mus musculus, mouse,
ATCC® TIB-71™) and THP-1 cells were obtained from
American Type Culture Collection. Cells were maintained in DMEM
(Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (Sigma-Aldrich; Merck KGaA), 100 U/ml penicillin and 100
µg/ml streptomycin in a humidified atmosphere of 5% CO2
at 37°C. Cells were allowed to grow until they reached 90–95%
confluence, following which they were washed with PBS and the
culture medium was replaced. RAW 264.7 cells attaining a
concentration of 5×104 cells/ml were activated by
incubation in medium containing MSU crystals (0, 10, 50, 250 µg/ml)
for 24 h at 37°C. These concentrations of MSU were chosen according
to previous studies (15–17). Cells were also stimulated with 1
mg/ml Escherichia coli-derived lipopolysaccharide (LPS; serotype
0111:B4; cat. no. L2630; Sigma-Aldrich; Merck KGaA) for 0, 6, 12
and 24 h at 37°C. THP-1 monocytes were suspended in complete
culture medium and seeded in 24-well culture plates
(2×105 cells/ml/well). The cells were then treated with
100 ng/ml phorbol myristate acetate for 24 h at 37°C to obtain
THP-1-derived macrophages. THP-1-derived macrophages in DMEM were
added to different concentrations of MSU crystals (0, 10, 50, 250
µg/ml) for 24 h at 37°C. After transfection with lncRNA-MM2P siRNA
and scramble siRNA, cells (RAW 264.7 and THP-1-derived macrophages)
were further treated with or without MSU crystals (250 µg/ml) for
24 h at 37°C. The harvested cells were collected for RNA and
protein extraction. The supernatants were collected and stored at
−80°C for cytokine detection by ELISA (13,14).
Plasmids and cell transfection
RAW 264.7 and THP-1 cells in the logarithmic growth
phase were collected and seeded into six-well plates at a density
of 5×104 cells/well. Transfection was performed when the
cell density reached 80% the following day. The small interfering
RNA (siRNA) sequence duplexes were produced by Shanghai GenePharma
Co., Ltd. The lncRNA-MM2P siRNA sequences were as follows:
si-lncRNA-MM2P-1, 5′-CACGAAGACTGGAATGCAATT-3′; and
si-lncRNA-MM2P-2, 5′-GGACCGAAGAGATTCGGAGAA-3′. The control
(scramble siRNA) sequence was as follows:
5′-ATCCGCGCGATAGTACGTATT-3′. The transfection was performed using
50 nM siRNA and jetPRIME® (Polyplus-transfection SA),
according to the manufacturer's protocols (12). lncRNA-MM2P was cloned into a
pcDNA3.1 vector (Shanghai Integrated Biotech Solutions Co., Ltd.)
and transfected into cells (0.5 µg/ml) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). An empty pcDNA3.1 vector was transfected into
the cells as the control. The primer sequence for lncRNA-MM2P was
as follows: Forward, 5′-TAGCTCCCACGAAGACTGGAAT-3′ and reverse,
5′-CTATGCTCGTGATTTATAAAACGCAAGTC-3′. The subsequent experiments
were performed following 48 h of transfection at 37°C.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA from RAW 264.7 and THP-1-derived
macrophages was extracted from cultured cells using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. To quantitate lncRNA-MM2P, interleukin
(IL)-1β, IL-8 and tumor necrosis factor α (TNFα) mRNA, cDNA was
reverse transcribed from total RNA using the High-Capacity cDNA
Reverse Transcription kit (Thermo Fisher Scientific, Inc.) at 70°C
for 10 min. Then, 1 µg cDNA was used with the primers shown below
in a 20 µl reaction, using SYBR-Green as a marker for DNA content,
provided in the SYBR-Green Master mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The primer sequences used for RT-qPCR
were as follows: lncRNA-MM2P, forward: 5′-TAGCTCCCACGAAGACTGGAAT-3′
and reverse: 5′-CTATGCTCGTGATTTATAAAACGCAAGTC-3′; IL-1β, forward:
5′-ACGATGCACCTGTACGATCA-3′ and reverse: 5′-TCTTTCAACACGCAGGACAG-3′;
IL-8, forward: 5′-GCATAAAGACATACTCCAAACC-3′ and reverse:
5′-ACTTCTCCACAACCCTCTG-3′; TNFα, forward:
5′-CAGAGGGAAGAGTTCCCCAG-3′ and reverse: 5′-CCTTGGTCTGGTAGGAGACG-3′;
GAPDH, forward: 5′-GGAAGGTGAAGGTCGGAGTCA-3′ and reverse:
5′-GTCATTGATGGCAACAATATCCACT-3′. Amplification was performed in an
ABI-Prism 7000 Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) for a maximum of 40 cycles as follows:
94°C for 40 sec; then, 58°C for 40 sec and 72°C for 1 min. The
expression levels were quantified using the 2−∆∆Cq
method (18). Reactions were
performed in triplicate for statistical analysis, similar to our
previous studies (12–14).
ELISA to measure cytokine levels
The generation of inflammatory gene products (IL-1β,
IL-8 and TNFα) by RAW 264.7 cells was assayed using the following
ELISA kits purchased from Abcam: Human IL-1β ELISA kit (cat. no.
ab46052), human IL-8 ELISA kit (cat. no. ab46032) and human TNFα
ELISA kit (cat. no. ab181421). Generation of inflammatory gene
products (IL-1β, IL-8 and TNFα) by THP-1-derived macrophages was
assayed using the following ELISA kits, mouse IL-1β ELISA kit (cat.
no. ab197742; Abcam), mouse IL-8 ELISA kit (cat. no. MOFI01258;
ELISA Genie) and mouse TNFα ELISA kit (cat. no. ab100747; Abcam).
Cells (1.5×106 cells/well) seeded in 6-well plates were
pre-incubated with various concentrations of morin (Mingxiu
Biotechnology Company; 100–300 µM) or colchicine (Mingxiu
Biotechnology Company; 1 µM) for 24 h at 37°C, and cytokine
production was stimulated by treating cells with MSU crystals (1
mg/ml) for 24 h at 37°C. Then, the culture supernatants were
collected and used to measure levels of IL-1β, IL-8 and TNFα.
Absorbance was determined at 450 nm using a microplate reader
(BioTek Instruments, Inc.). The standard curves for respective
cytokines were used to quantify the levels of IL-1β, IL-8 and TNFα
released by the cells.
Statistical analysis
The data was presented as the mean ± SD for normally
distributed variables, and medians (interquartile range) for
non-normally distributed variables. An unpaired two-tailed
Student's t-test was used to determine significance between
controls and individual experimental groups. A one-way ANOVA
followed by a Tukey's post hoc test was used for >3 groups.
Analyses were performed using SPSS software, v22.0 (IBM Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
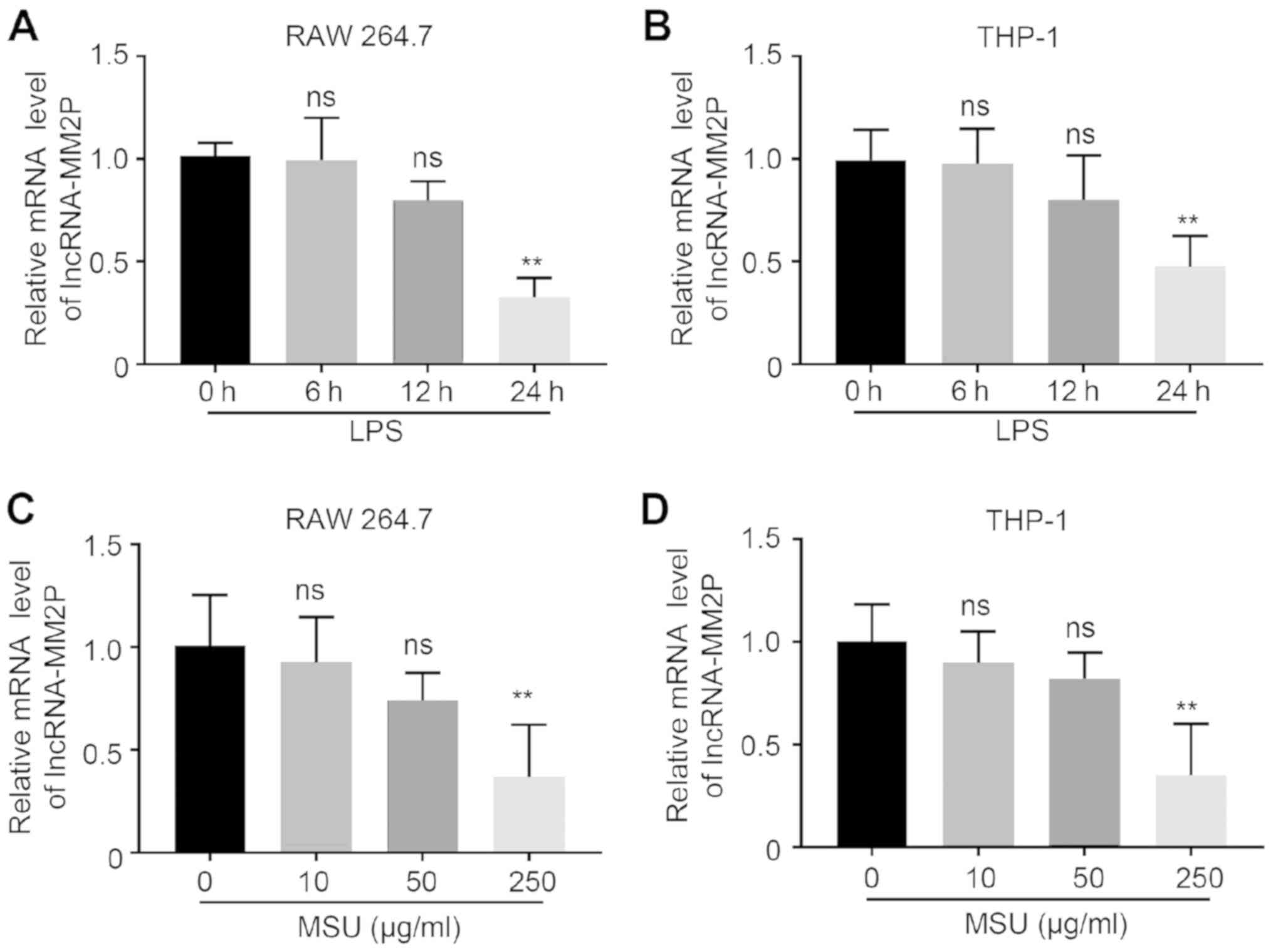
Treatment with LPS and MSU decreases
the expression of lncRNA-MM2P in macrophages
RAW 264.7 and THP-1-derived macrophages were treated
with LPS at four time points (0, 6, 12 and 24 h). Compared with
baseline (0 h) LPS stimulation, the positive expression rates of
lncRNA-MM2P were 72% at 12 h and 35% at 24 h in RAW 264.7 cells,
and 42% at 24 h in THP-1-derived macrophages. As the expression of
lncRNA-MM2P decreased significantly at 24 h in both RAW 264.7 and
THP-1-derived macrophages, this duration was used for subsequent
experiments. After treating RAW 264.7 and THP-1-derived macrophages
with different concentrations (0, 10, 50, 250 µg/ml) of MSU
crystals for 24 h, the positive expression rates of lncRNA-MM2P
were 37% in RAW 264.7 and 32% in THP-1-derived macrophages when 250
µg/ml was used, which was a significant decrease (Fig. 1).
MSU-mediated inflammatory responses in
macrophages
RAW 264.7 and THP-1-derived macrophages were treated
with different concentrations (0, 10, 50, 250 µg/ml) of MSU
crystals for 24 h. Compared with treatment with 0 µg/ml MSU
crystals, 50 µg/ml or 250 µg/ml significantly increased
inflammatory responses in RAW 264.7 cells, 50 µg/ml MSU crystals
led to a 2.8-fold increase in the mRNA expression of IL-1β and a
1.9-fold increase in the mRNA expression of IL-8; and treatment
with 250 µg/ml MSU crystals led to a 4.3-fold, 2.4-fold and
2.1-fold increase in the mRNA expression levels of IL-1β, IL-8 and
TNFα, respectively. In THP-1-derived macrophages, treatment with
250 µg/ml MSU crystals resulted in a 3.2-fold, 2.3-fold and
2.5-fold increase in the mRNA expression levels of IL-1β, IL-8 and
TNFα, respectively (Fig. 2).
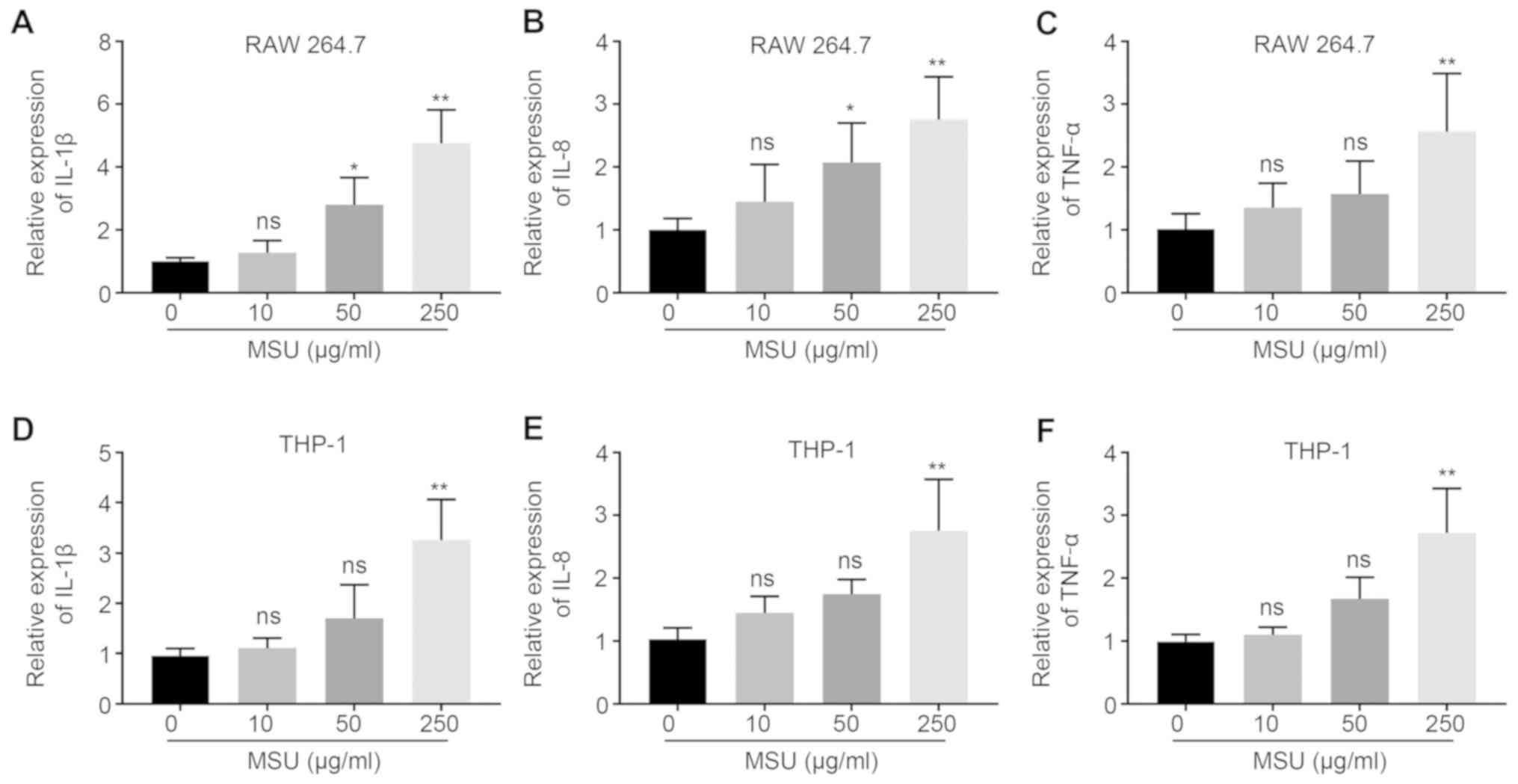 | Figure 2.Effect of MSU treatment on the
expression of pro-inflammatory cytokines. The mRNA expression
levels of inflammatory cytokines, (A) IL-1β, (B) IL-8 and (C) TNFα,
were upregulated in RAW 264.7 cells as the concentration of MSU
increased. The mRNA expression levels of inflammatory cytokines,
(D) IL-1β, (E) IL-8 and (F) TNFα, were upregulated in THP-1 cells
as the concentration of MSU increased. *P<0.05, **P<0.01 vs.
0. MSU, monosodium urate; IL, interleukin; TNF, tumor necrosis
factor; ns, not significant. |
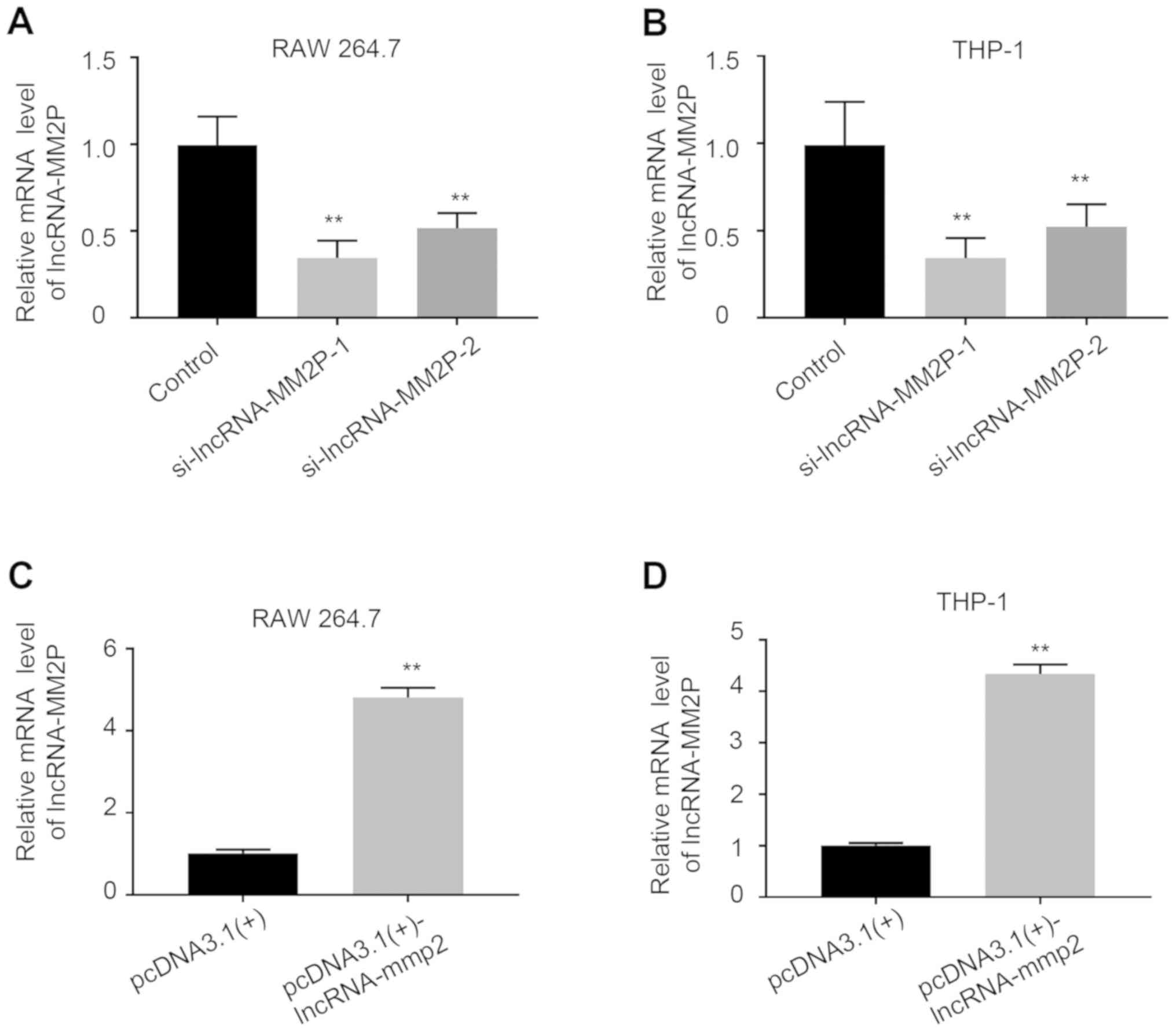
Inhibition of lncRNA-MM2P enhanced
MSU-mediated inflammatory responses
After transfection with lncRNA-MM2P siRNA-1 or −2,
the expression of lncRNA-MM2P significantly decreased in RAW 264.7
and THP-1-derived macrophages, whereas using
pcDNA3.1(+)-lncRNA-MM2P significantly increased the expression of
lncRNA-MM2P (Fig. 3). As the
knockdown efficiency of lncRNA-MM2P siRNA-2 was less than that of
lncRNA-MM2P siRNA-1, lncRNA-MM2P siRNA-1 was used in subsequent
experiments. Inhibition of lncRNA-MM2P did not influence the
inflammatory responses of macrophages without MSU treatment.
However, after treatment with 250 µg/ml MSU crystals, RAW 264.7
cells transfected with lncRNA-MM2P siRNA showed a 1.9-fold increase
in the mRNA expression of IL-1β, a 1.4-fold increase of IL-8 (not
significant) and a 0.8-fold increase of TNFα mRNA expression level
(not significant) compared with RAW 264.7 cells transfected with
scramble siRNA and pre-treated with MSU (control + MSU). Similarly,
after treatment with 250 µg/ml MSU crystals, THP-1-derived
macrophages transfected with lncRNA-MM2P siRNA showed a 3.6-fold, a
1.9-fold and a 1.4-fold increase in the mRNA expression levels of
IL-1β, IL-8 and TNFα compared with THP-1-derived macrophages
transfected with scramble siRNA and pre-treated with MSU (control +
MSU). Whereas, transfection using pcDNA3.1(+)-lncRNA-MM2P exerted
the opposite effects on these inflammatory factors (Fig. 4).
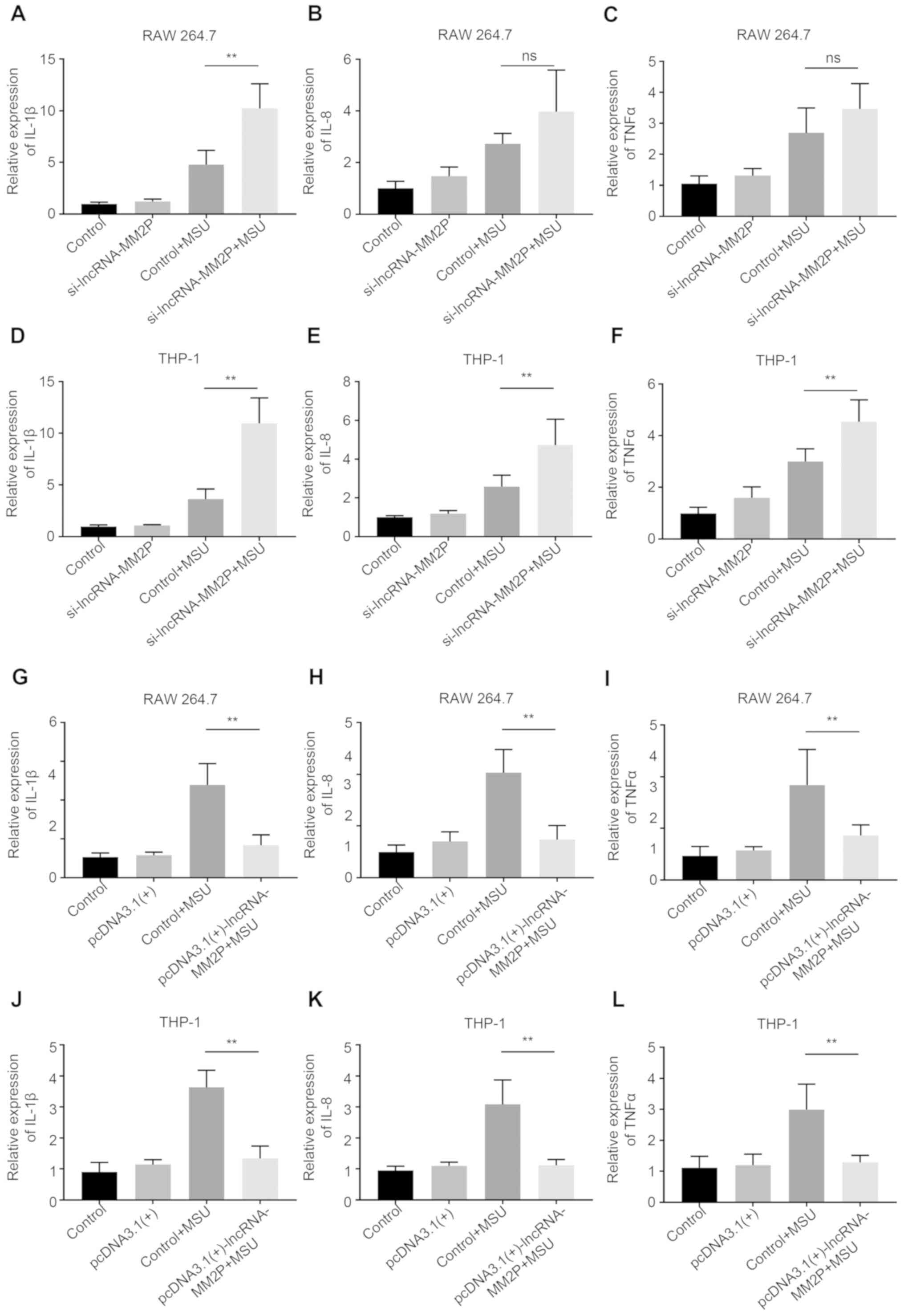 | Figure 4.Effect of lncRNA-MM2P on the
expression of pro-inflammatory cytokines in MSU-treated cells. The
mRNA expression of inflammatory cytokines, (A) IL-1β, (B) IL-8 and
(C) TNFα, increased in RAW 264.7 cells after MSU treatment, and
these effects were more notable when lncRNA-MM2P-1 was knocked down
in cells. The mRNA expression of inflammatory cytokines, (D) IL-1β,
(E) IL-8 and (F) TNFα, increased in THP-1 cells after MSU
treatment, and these effects were more notable when lncRNA-MM2P-1
was knocked down in cells. The mRNA expression of inflammatory
cytokines, (G) IL-1β, (H) IL-8 and (I) TNFα, increased in RAW 264.7
cells after MSU treatment, and these effects were reversed in
lncRNA-MM2P-1 overexpressing cells. The mRNA expression of
inflammatory cytokines, (J) IL-1β, (K) IL-8 and (L) TNFα, increased
in THP-1 cells after MSU treatment, and these effects were reversed
in lncRNA-MM2P-1-overexpressing cells. **P<0.01. lncRNA, long
non-coding RNA; MSU, monosodium urate; IL, interleukin; TNF, tumor
necrosis factor; ns, not significant. |
The supernatants were used to further verify the
effects of lncRNA-MM2P in MSU-mediated inflammatory cytokine
secretion. Compared with RAW 264.7 cells transfected with scramble
siRNA and pre-treated with MSU (control + MSU), after treatment
with 250 µg/ml MSU crystals, RAW 264.7 cells transfected with
lncRNA-MM2P siRNA showed a 2.1-fold higher IL-1β concentration, a
2.5-fold higher IL-8 concentration and a 1.2-fold higher TNFα
concentration. Compared with THP-1-derived macrophages transfected
with scramble siRNA and pre-treated with MSU (control + MSU), after
treatment with 250 µg/ml MSU crystals, THP-1 transfected with
lncRNA-MM2P siRNA exhibited a 1.8-fold higher IL-1β concentration,
a 1.5-fold higher IL-8 concentration and a 1.3-fold higher TNFα
concentration. However, transfection using pcDNA3.1(+)-lncRNA-MM2P
significantly decreased the expression of IL-1β, IL-8 and TNFα in
MSU-induced RAW 264.7 and THP-1-derived macrophages (Fig. 5).
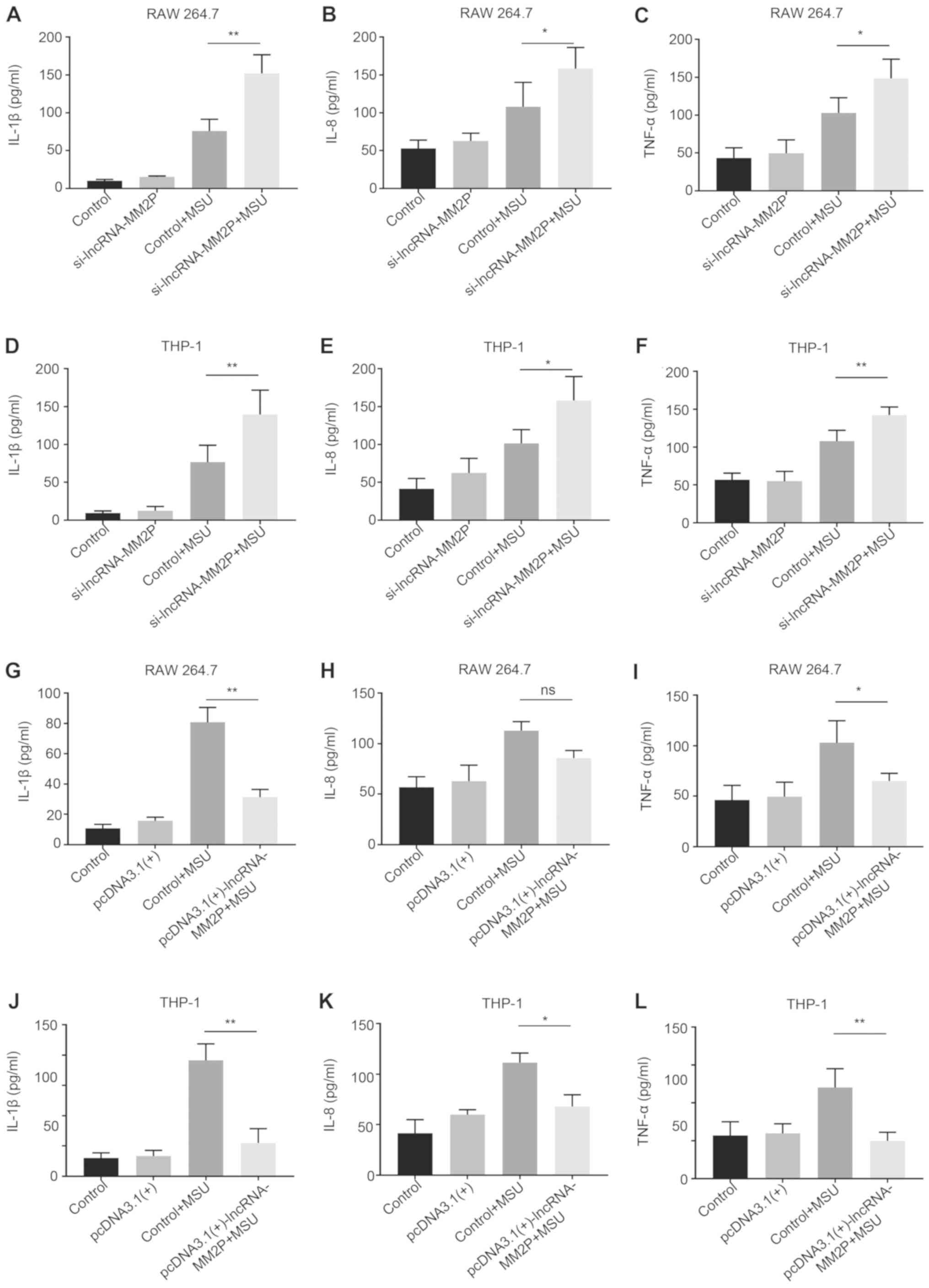 | Figure 5.Effects of lncRNA-MM2P on the
expression of pro-inflammatory cytokines in the supernatants of
MSU-induced cells. The mRNA expression of inflammatory cytokines,
(A) IL-1β, (B) IL-8 and (C) TNFα, increased in the supernatants of
RAW 264.7 cells after MSU treatment, and these effects were more
notable when lncRNA-MM2P-1 was knocked down in cells. The mRNA
expression of inflammatory cytokines, (D) IL-1β, (E) IL-8 and (F)
TNFα, increased in the supernatant of THP-1 cells after MSU
treatment, and these effects were more notable when lncRNA-MM2P-1
was knocked down in cells. The mRNA expression of inflammatory
cytokines, (G) IL-1β, (H) IL-8 and (I) TNFα, increased in the
supernatant of RAW 264.7 cells after MSU treatment, and these
effects were reversed in lncRNA-MM2P-1 overexpressing cells. The
mRNA expression of inflammatory cytokines, (J) IL-1β, (K) IL-8 and
(L) TNFα, increased in the supernatant of THP-1 cells after MSU
treatment, and these effects were reversed in lncRNA-MM2P-1
overexpressing cells. *P<0.05, **P<0.01. lncRNA, long
non-coding RNA; MSU, monosodium urate; IL, interleukin; TNF, tumor
necrosis factor; ns, not significant. |
Discussion
The pro- and anti-inflammatory functions of
macrophages are important for MSU-induced inflammation; however,
the mechanism is still unclear. In the present study, it was found
that lncRNA-MM2P, a novel modulator of M1/M2 polarization of
macrophages, was a key regulator of MSU-induced inflammation, which
can trigger the development of AGA. Inhibition of lncRNA-MM2P
enhanced MSU-mediated inflammatory responses. Therefore,
lncRNA-MM2P may be a potential target in the treatment of AGA.
It is hypothesized that the polarization of
macrophages is a dynamic process of development and heterogeneity
(19). When macrophages polarize
to M1 type, it facilitates the release of inflammatory cytokines,
these inflammatory cytokines can also induce the polarization of
macrophages to M2 type, thus leading to the inhibition of the
inflammatory response (20). It
has recently been demonstrated that lncRNAs, which have multiple
regulatory functions, are also modulators of polarization. A
previous study reported that lncRNA GAS5 has a role in the
development of childhood pneumonia by activating M1 macrophage
polarization and suppressing M2 macrophage polarization (21). Another study revealed that the
knockdown of lncRNA Malat1 leads to M2 activation, which then
decreases LPS-induced systemic and pulmonary inflammation and
injury (22). Furthermore, it has
been demonstrated that lncRNA MIR155HG is highly expressed in
granulocyte-macrophage colony-stimulating factor (GM-CSF)-induced
macrophages from patients with chronic obstructive pulmonary
disease, and overexpression of lncRNA MIR155HG promotes
GM-CSF-induced M1 macrophage polarization and inhibits M2
macrophage polarization (23).
Among a variety of lncRNAs studied, lncRNA-MM2P was reported to be
the only lncRNA upregulated during M2 polarization and
downregulated in M1 macrophages at the same time (12). In the present study, it was
demonstrated that the expression of lncRNA-MM2P significantly
decreased in MSU-treated macrophages, which was accompanied by an
increase in inflammatory responses. Knockdown of lncRNA-MM2P in
macrophages enhanced MSU-mediated inflammatory responses. The
functional characterization of lncRNA-MM2P in MSU-induced
inflammation can help generate further insight beyond the
traditional physiopathology of AGA.
The mechanism of lncRNA-MM2P-regulated macrophage
polarization remains unclear, and STAT6 activation could provide a
possible explanation. In a model of macrophage-promoted
tumorigenesis, it was revealed that knockdown of lncRNA-MM2P
suppressed M2 polarization of macrophages by reducing
phosphorylation of STAT6 (12).
Furthermore, using a model of MSU-induced inflammation, it was
demonstrated that the activation of the PI3K/Akt/STAT6 pathway is
associated with decreased M1 macrophage polarization (9). Therefore, we suggest that inhibition
of lncRNA-MM2P may lead to a decrease in the expression of
phosphorylated STAT6, which aggravates MSU-mediated inflammatory
responses and this hypothesis will be our next research
objective.
In conclusion, the present results suggest that
lncRNA-MM2P, a novel modulator of M1/M2 polarization of
macrophages, is an important regulator of MSU-induced inflammation,
and could have a role in the development AGA. Further knowledge of
the role of lncRNA-MM2P in AGA could contribute to an improved
understanding of the pathogenesis of AGA and may provide further
insight into the potential and efficacy of lncRNA-MM2P-based
prevention and treatment options.
Acknowledgements
Not applicable.
Funding
This study was supported by The Traditional Chinese
Medicine Research Project of Jiangxi Provincial Health and Family
Planning Commission (grant nos. 2014A121 and 2014A155).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
XZ, YZ and JZ designed all of the experiments and
revised the manuscript; YZ and MQ revised the manuscript; SJ, FY,
MQ and XW performed the experiments; and JLi, XL and JLe analyzed
and interpreted the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cimmino MA, Zampogna G, Parodi M, Andracco
R, Barbieri F, Paparo F, Ferrero G and Garlaschi G: MRI synovitis
and bone lesions are common in acute gouty arthritis of the wrist
even during the first attack. Ann Rheum Dis. 70:2238–2239. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
VanItallie TB: Gout: Epitome of painful
arthritis. Metabolism. 59 (Suppl 1):S32–S36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schauer C, Janko C, Munoz LE, Zhao Y,
Kienhöfer D, Frey B, Lell M, Manger B, Rech J, Naschberger E, et
al: Aggregated neutrophil extracellular traps limit inflammation by
degrading cytokines and chemokines. Nat Med. 20:511–517. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Manger B, Lell M, Wacker J, Schett G and
Rech J: Detection of periarticular urate deposits with dual energy
CT in patients with acute gouty arthritis. Ann Rheum Dis.
71:470–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen YH, Hsieh SC, Chen WY, Li KJ, Wu CH,
Wu PC, Tsai CY and Yu CL: Spontaneous resolution of acute gouty
arthritis is associated with rapid induction of the
anti-inflammatory factors TGFβ1, IL-10 and soluble TNF receptors
and the intracellular cytokine negative regulators CIS and SOCS3.
Ann Rheum Dis. 70:1655–1663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Joosten LA, Crişan TO, Azam T, Cleophas
MC, Koenders MI, van de Veerdonk FL, Netea MG, Kim S and Dinarello
CA: Alpha-1-anti-trypsin-Fc fusion protein ameliorates gouty
arthritis by reducing release and extracellular processing of IL-1β
and by the induction of endogenous IL-1Ra. Ann Rheum Dis.
75:1219–1227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jha AK, Huang SC, Sergushichev A,
Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart
KM, Ashall J, Everts B, et al: Network integration of parallel
metabolic and transcriptional data reveals metabolic modules that
regulate macrophage polarization. Immunity. 42:419–430. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J, Yang Q, Zhang Q, Yin C, Zhou L,
Zhou J, Wang Y and Mi QS: Invariant natural killer T cells
ameliorate monosodium urate crystal-induced gouty inflammation in
mice. Front Immunol. 8:17102017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu L, Zhu X, Zhao T, Yu Y, Xue Y and Zou
H: Sirt1 ameliorates monosodium urate crystal-induced inflammation
by altering macrophage polarization via the PI3K/Akt/STAT6 pathway.
Rheumatology (Oxford). 58:1674–1683. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Tang H, Liu X, Chen H, Feng N,
Zhang J, Wang C, Qiu M, Yang J and Zhou X: Frontline Science:
Reprogramming COX-2, 5-LOX, and CYP4A-mediated arachidonic acid
metabolism in macrophages by salidroside alleviates gouty
arthritis. J Leukoc Biol. 105:11–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mei J, Zhou F, Qiao H, Li H and Tang T:
Nerve modulation therapy in gouty arthritis: Targeting increased
sFRP2 expression in dorsal root ganglion regulates macrophage
polarization and alleviates endothelial damage. Theranostics.
9:3707–3722. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao J, Dong R, Jiang L, Gong Y, Yuan M,
You J, Meng W, Chen Z, Zhang N, Weng Q, et al: LncRNA-MM2P
Identified as a Modulator of Macrophage M2 Polarization. Cancer
Immunol Res. 7:292–305. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dhanasekar C, Kalaiselvanand S and Rasool
M: Morin, a bioflavonoid suppresses monosodium urate
crystal-induced inflammatory immune response in RAW 264.7
Macrophages through the inhibition of inflammatory mediators,
intracellular ROS levels and NF-kappaB activation. PLoS One.
10:e01450932015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li H, Ou G, He Y, Ren L, Yang X and Zeng
M: Resveratrol attenuates the MSU crystal-induced inflammatory
response through the inhibition of TAK1 activity. Int
Immunopharmacol. 67:62–68. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jhang JJ, Cheng YT, Ho CY and Yen GC:
Monosodium urate crystals trigger Nrf2-and heme
oxygenase-1-dependent inflammation in THP-1 cells. Cell Mol
Immunol. 12:424–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu R, Aupperle K and Terkeltaub R: Src
family protein tyrosine kinase signaling mediates monosodium urate
crystal-induced IL-8 expression by monocytic THP-1 cells. J Leukoc
Biol. 70:961–968. 2001.PubMed/NCBI
|
|
17
|
Zeng M, Dang W, Chen B, Qing Y, Xie W,
Zhao M and Zhou J: IL-37 inhibits the production of
pro-inflammatory cytokines in MSU crystal-induced inflammatory
response. Clin Rheumatol. 35:2251–2258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pisanu A, Lecca D, Mulas G, Wardas J,
Simbula G, Spiga S and Carta AR: Dynamic changes in pro- and
anti-inflammatory cytokines in microglia after PPAR-γagonist
neuroprotective treatment in the MPTPp mouse model of progressive
Parkinson's disease. Neurobiol Dis. 71:280–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oishi S, Takano R, Tamura S, Tani S,
Iwaizumi M, Hamaya Y, Takagaki K, Nagata T, et al: M2 polarization
of murine peritoneal macrophages induces regulatory cytokine
production and suppresses T-cell proliferation. Immunology.
149:320–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chi X, Ding B, Zhang L, Zhang J, Wang J
and Zhang W: lncRNA GAS5 promotes M1 macrophage polarization via
miR-455-5p/SOCS3 pathway in childhood pneumonia. J Cell Physiol.
234:13242–13251. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui H, Banerjee S, Guo S, Xie N, Ge J,
Jiang D, Zornig M, Thannickaland VJ and Liu G: Long noncoding RNA
Malat1 regulates differential activation of macrophages and
response to lung injury. JCI Insight. 4:e1245222019. View Article : Google Scholar
|
|
23
|
Li N, Liu Y and Cai J: LncRNA MIR155HG
regulates M1/M2 macrophage polarization in chronic obstructive
pulmonary disease. Biomed Pharmacother. 117:1090152019. View Article : Google Scholar : PubMed/NCBI
|