Introduction
Following improvements to living standards, the
worldwide incidence of diabetes has increased annually, with ~1/3
of all patients with diabetes developing diabetic nephropathy (DN),
a common microvascular complication of the disease (1). Previous evidence has suggested that
function and structure podocyte injuries arise in the early stages
of DN (2). For example, high
glucose (HG) induces podocyte apoptosis, reduces the number of
podocytes and damages the normal cell morphology (3), leading to glomerulosclerosis and
ultimately, the development of severe proteinuria (4,5).
Both oxidative stress and the inflammatory response are closely
associated with podocyte injury. Increased levels of reactive
oxygen species (ROS) are produced in a HG environment, resulting in
oxidative stress and damage to podocytes (6). Inflammatory cytokines, such as
interleukin (IL)-1β, IL-6, tumor necrosis factor-α (TNF-α), are
also secreted following HG stimulation, further exacerbating
podocyte injury (7,8). Podocyte injury is associated with the
majority of pathological alterations that occur in glomerular
diseases, serving as a critical factor for the progression of
chronic kidney disease and an important target for clinical
treatment (9).
Dual-specificity phosphatases (DUSPs) belong to a
large family, which contains 25 phosphatases (10) that all contain a common phosphatase
domain (11). DUSPs have been
reported to mediate cell proliferation, migration and apoptosis
(12–14). Accumulating evidence has
demonstrated that DUSP6 serves a role in multiple types of cancer,
such as glioblastoma, breast cancer and pancreatic cancer, where it
displays either an oncogenic or tumor-suppressive function
(15–18), which ultimately affects and
determines the fate of a specific cancer (19). Another previous study suggested
that DUSP6 inhibition enhanced T cell-modulated immunity in
end-stage renal disease (20). In
addition, it was also discovered that DUSP6 regulated the colon
inflammatory response and protected the intestinal epithelium from
oncogenic stress by promoting ERK1/2 activation (21). Similarly, DUSP6 overexpression
restores β-amyloid-induced oxidative stress, endoplasmic reticulum
stress and mitochondrial dysfunction via ERK1/2 activation in
neural stem cells (22). However,
the role of DUSP6 in DN and whether DUSP6 exerts a protective
effect against podocyte injury are not completely understood.
Therefore, the present study aimed to investigate the function of
DUSP6 in HG-induced podocytes and to determine the underlying
molecular mechanisms.
Materials and methods
Animals
A total of 26 C57BL/6J mice (male; weight, 20±2 g;
age, 6 weeks) were purchased from the Animal Experimental Center of
Jilin University. All experiments were approved by the Biological
and Medical Ethics Committee of Jamusi University (approval no.
SYXK 2016-014). Mice were maintained in standardized conditions at
22±2°C with 55±5% relative humidity and 12-h light/dark cycles,
with free access to food and water.
Following a week of acclimatization, mice were
randomly divided into two groups: i) Control (n=8) and ii) diabetic
(n=18). The diabetic group (age, 7 weeks) were placed on a 60%
high-fat diet for 4 weeks. Mice were intraperitoneally injected
with 120 mg/kg streptozocin (STZ; Sigma-Aldrich; Merck KGaA) in pH
4.2 citrate buffer at 11 weeks old and mice received a second dose
5 days later, as previously reported (23). The control group were placed on a
10% low fat diet for 4 weeks, and received an intraperitoneal
injection of citrate buffer without streptozocin at 11 weeks old
and a second injection 5 days later. Animal behaviors (activity,
appetite and mental status) were monitored every 2 days. At 72 h
after the second injection, 0.1 ml blood was collected through the
tail vein. Subsequently, non-fasting blood glucose levels were
assessed using an Accu-Check Compact® glucometer (Roche
Diagnostics). Mice with non-fasting blood glucose levels ≥16.7
mmol/l (300 mg/dl) were considered as diabetic model mice. The
duration of the experiment was 6 weeks, including a week of
adaptive feeding. Mice were placed in individual metabolic cages
for 24-h urine collection prior to sacrifice. Mice were sacrificed
by cervical dislocation at the end of the experiment, and death was
verified by monitoring cessation of the heartbeat and responses to
external stimuli. Subsequently, the kidneys were extracted.
Determination of urinary
microalbumin/creatinine ratio (UACR)
The urine microalbumin ELISA kit (cat. no. ml063626)
and creatinine ELISA kit (cat. no. ml037582) were purchased from
Shanghai Enzyme-linked Biotechnology Co., Ltd.; both urine
microalbumin and creatinine levels were determined according to the
manufacturers protocols. The UACR was calculated to evaluate renal
function.
Hematoxylin and eosin staining
Kidney tissue was fixed with 4% paraformaldehyde for
36 h at 4°C, embedded in paraffin and cut into 4-µm thick paraffin
sections. The sections were subsequently deparaffinized twice using
xylene for 10 min each at room temperature and rehydrated using a
descending ethanol series. Subsequently, sections were stained with
hematoxylin (nuclei) for 5 min at room temperature and eosin
(cytoplasm) for 3 min at room temperature. The sections were
dehydrated using an ascending alcohol series, sealed with neutral
balsam (Beijing Solarbio Science & Technology Co., Ltd.; cat.
no. G8590) and observed using a light microscope (Olympus
Corporation; magnification, ×200).
Masson staining
Kidney tissue samples were fixed with 4%
paraformaldehyde for 36 h at 4°C, paraffin-embedded, cut into 4-µm
thick sections, deparaffinized twice using xylene for 10 min each
at room temperature and rehydrated using a descending series of
ethanol. Subsequently, nuclear staining was performed using Regaud
hematoxylin dye for 10 min at room temperature. Following washing
with distilled water, the sections were stained with Ponceau S
staining solution for 2 min at room temperature. The sections were
soaked with 0.2% glacial acetic acid aqueous solution for 1 min, 1%
phosphomolybdate for 5 min and 0.2% glacial acetic acid for 2 min
(all at room temperature). Following washing with distilled water,
methyl green staining was conducted for 3 min at room temperature.
The sections were then treated with 95% ethanol for 10 sec to
dehydrate, then 100% ethanol was used in triplicate to dehydrate
the sections for 10 sec each time. After sealing with neutral
balsam, sections were observed using a light microscope (Olympus
Corporation; magnification, ×200).
Immunohistochemistry
Kidney tissue was fixed in 4% paraformaldehyde for
36 h at 4°C, paraffin-embedded, cut into 4-µm thick sections and
deparaffinized twice using xylene for 10 min at room temperature.
The sections were then rehydrated with a descending series of
ethanol. Antigen retrieval was performed by heating the sections in
boiling 0.01 M sodium citrate buffer solution (pH 6.0) for 10 min.
After washing with PBS, slides were blocked with 10% goat serum
(Gibco; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature. Subsequently, slides were incubated overnight at 4°C
with an anti-DUSP6 (Abcam; cat. no. ab76310; 1:50) primary
antibody. Following washing with PBS, slides were incubated with a
goat anti-rabbit IgG horseradish peroxidase (HRP)-conjugated
secondary antibody (BIOSS; cat. no. bs-0295G-HRP; 1:3,000) for 1 h
at 37°C. Stained sections were observed under a light microscope
(Olympus Corporation; magnification, ×200).
Cell culture and transfection
Conditionally immortalized MPC5 cells (Beijing Beina
Chuanglian Biotechnology Research Institute; cat. no. BNCC337685)
were cultured in RPMI-1640 medium (Hyclone; Cytiva) supplemented
with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 50 IU/ml
IFN-γ (PeproTech, Inc.) at 33°C in collagen I-coated dishes (BD
Biosciences). To induce differentiation, MPC5 cells were cultured
for 14 days at 37°C without IFN-γ and used for subsequent
experiments. To induce cell injury, 2×105 MPC5
cells/well were seeded into a six-well plate and incubated with
different concentrations of D-glucose (5, 10, 15, 20, 25 or 30 mM;
Sigma-Aldrich; Merck KGaA) for 24 h at 37°C. For the control group,
30 mM D-mannitol (MA; Sigma-Aldrich; Merck KGaA) was added into the
culture medium at 37°C for 24 h to adjust the osmotic pressure to
be consistent with the 30 mM D-glucose group (24). DUSP6 overexpression (OE) plasmids
(OE-DUSP6) and empty vector (negative control; NC) plasmids (OE-NC)
were purchased from Shanghai Qincheng Biological Technology Co.,
Ltd. MPC5 cells were seeded into a 6-well plate. At 60–70%
confluence, MPC5 cells were transfected with 4 µg OE-DUSP6 or OE-NC
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After incubation for 6 h at 37°C, the medium was
replaced with fresh complete medium and incubated for 48 h at
37°C.
RT-qPCR
Total RNA was extracted from MPC5 cells or kidney
tissues using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA was reverse transcribed into cDNA
using a PrimeScript™ RT Reagent kit (Takara Bio, Inc.). The
following RT temperature protocol was used: 37°C for 5 min and 85°C
for 5 sec, then maintained at 4°C. Subsequently, qPCR was performed
using a SYBR Green PCR Master mix (Thermo Fisher Scientific, Inc.)
on an ABI 7500 Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The following thermocycling conditions
were used for the qPCR: Initial denaturation at 95°C for 5 min;
followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec;
followed by 95°C for 15 sec, 60°C for 60 sec and 95°C for 15 sec.
The following primers were used for the qPCR: DUSP6 forward,
5-CGACTGGAATGAGAACACTGGTGG-3 and reverse,
5-TCTAGATTGGTCTCGCAGTGCAGG-3; and GAPDH forward,
5-GGCCCCTCTGGAAAGCTGTG-3 and reverse, 5-CCGCCTGCTTCACCACCTTCT-3.
The relative expression levels of each gene were quantified using
the 2−ΔΔCq method (25)
and normalized to the endogenous reference gene GAPDH.
Western blotting
Total protein was extracted from MPC5 cells using
RIPA lysis buffer (Beyotime Institute of Biotechnology) containing
phenylmethanesulfonyl fluoride and protease inhibitor. Following
quantification using a bicinchoninic acid assay kit (Beyotime
Institute of Biotechnology), 30 µg protein/lane was separated via
10% SDS-PAGE and transferred to PVDF membranes (EMD Millipore).
Following blocking with 5% non-fat milk for 2 h at room
temperature, the membranes were incubated overnight at 4°C with
primary antibodies against: DUSP6 (Abcam; cat. no. ab76310;
1:1,000), nephrin (Abcam; cat. no. ab216341; 1:1,000), Bcl-2
(Abcam; cat. no. ab182858; 1:1,000), Bax (Abcam; cat. no. ab182733;
1:1,000), cleaved caspase-3 (Abcam; cat. no. ab2302; 1:1,000),
caspase-3 (Abcam; cat. no. ab44976; 1:1,000), synaptopodin (Santa
Cruz Biotechnology, Inc.; cat. no. sc-515842; 1: 500),
phosphorylated (p)-ERK1/2 (Cell Signaling Technology, Inc.; cat.
no. 9101; 1:1,000), ERK1/2 (Cell Signaling Technology, Inc.; cat.
no. 4695; 1:1,000) and GAPDH (Cell Signaling Technology, Inc.; cat.
no. 2118; 1:1,000). Following the primary incubation, the membranes
were incubated with a goat anti-rabbit IgG HRP-conjugated
(1:10,000) or goat anti-mouse IgG HRP-conjugated (BIOSS; cat. no.
bs-0296G-HRP; 1:10,000) secondary antibody for 1 h at room
temperature. Protein bands were visualized using an ECL Substrate
kit (BioVision, Inc.) and analyzed using Image Lab software (v2.1;
Bio-Rad Laboratories, Inc.). GAPDH was used as the loading
control.
Determination of IL-1β, IL-6 and TNF-α
levels
The cell medium of MPC5 cells was centrifuged at
2,000 × g for 5 min at room temperature, and the levels of IL-1β
(cat. no. SBJ-M0027), IL-6 (cat. no. SBJ-M0044) and TNF-α (cat. no.
SBJ-M0030) were measured using ELISA kits (Nanjing Senbega
Biological Technology Co., Ltd.).
MP5 cells were lysed using RIPA lysis buffer and
centrifuged at 10,000 × g for 10 min at 4°C. The cell supernatant
was subsequently collected to detect malondialdehyde (MDA) levels
using a commercial colorimetric kit (cat. no. S0131S; Beyotime
Institute of Biotechnology).
Cell Counting Kit-8 (CCK-8)
MPC5 cells were seeded (5×103 cells/well)
in a 96-well plate and cultured with 30 mM D-glucose for 24 h at
37°C. Subsequently, 10 µl CCK-8 solution (Beyotime Institute of
Biotechnology) was added to each well and incubated for 4 h at
37°C, according to the manufacturers protocol. The absorbance of
each well was measured at a wavelength of 450 nm using a microplate
spectrophotometer.
ROS staining
Fluorescent Probe-dihydroethidium (DHE; Vigorous
Biotechnology (Beijing) Co., Ltd.) was used to determine cellular
ROS levels. Briefly, 2×104 MPC5 cells/well were seeded
into a 24-well plate and treated with DHE (10 µM) for 20 min at
37°C, then observed using a fluorescence microscope (Olympus
Corporation; magnification, ×100). The fluorescence intensity was
analyzed using ImageJ software (v1.8.0; National Institutes of
Health).
Flow cytometry assay
MPC5 cells were washed with PBS and harvested using
0.25% trypsin (Beyotime Institute of Biotechnology). Subsequently,
cells were centrifuged (1000 × g; 5 min; room temperature),
collected and re-suspended in PBS. Cells
(5×104−1×105) were centrifuged (200 × g; 5
min; room temperature) and re-suspended in 300 µl Annexin-V binding
buffer (Biomars). Subsequently, cells were stained with 5 µl
Annexin-V-FITC (Biomars) for 15 min at room temperature and 5 µl
propidium iodide (Biomars) for 10 min on ice in the dark. Apoptotic
cells were detected by flow cytometry (BD FACSCalibur; BD
Biosciences) and analyzed using Accuri C6 software (v1.0.264.21; BD
Biosciences). FITC-positive (Q2 and Q3) cells were considered as
apoptotic cells (early and late apoptosis).
Statistical analysis
Data are presented as the mean ± SD of at least
three replicates. Comparisons between two groups were analyzed
using an unpaired Students t-test. Comparisons among multiple
groups were analyzed using one-way ANOVA followed by Tukeys post
hoc test. Statistical analyses were performed using SPSS software
(v19.0; IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
DUSP6 expression levels are reduced in
diabetes-induced DN model mice
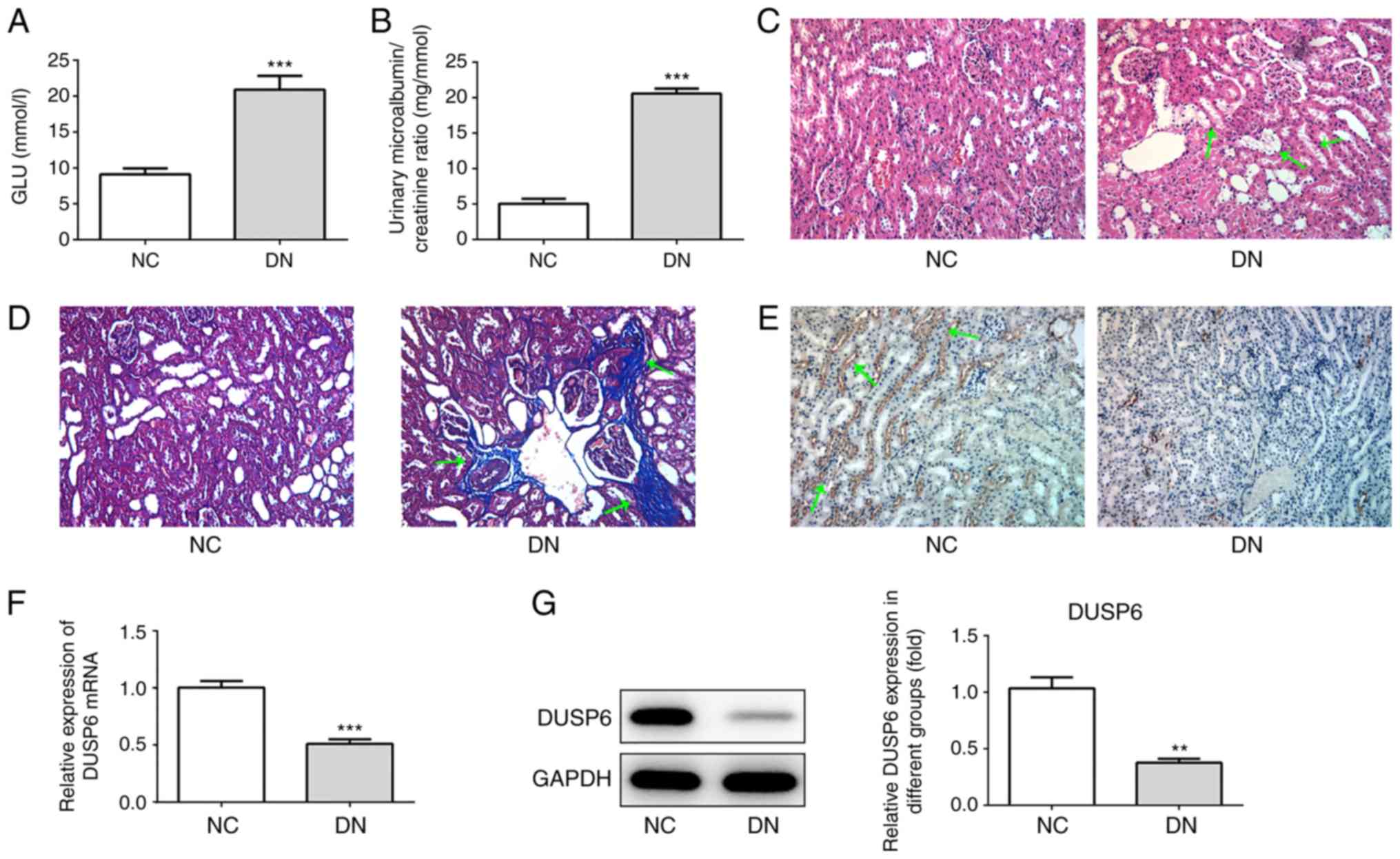
At 72 h after the final STZ injection, non-fasting
blood glucose levels were assessed. A total of 16 mice in the
diabetic group displayed a blood glucose level ≥16.7 mmol/l and
were therefore considered diabetic model mice. The blood glucose
levels of the remaining two mice in the diabetic group did not
exceed 16.7 mmol/l; therefore, these mice were excluded from the
present study. In the diabetic group, a mean blood glucose level of
20 mmol/l was reported (Fig. 1A),
and the mice displayed a significantly increased UACR compared with
the control group (Fig. 1B). The
morphological examination of the kidneys of the control group
indicated a normal histology, whereas six mice in the diabetic
group (n=16) displayed an extended glomerular area and mesangial
matrix expansion (Fig. 1C). Masson
staining also indicated collagen fiber accumulation in the diabetic
group, suggesting the occurrence of renal fibrosis (Fig. 1D). The pathological alterations to
the kidneys of the six mice in the diabetic group indicated the
successful establishment of the DN mouse model, as previously
reported (26). Subsequently, the
expression levels of DUSP6 in diabetic model mice with significant
pathological alterations in the kidneys were analyzed. The results
indicated that increased DUSP6 expression levels were observed in
the control mice compared with the DN model mice, which was
consistent with the RT-qPCR and western blotting results (Fig. 1F and G). Overall, the results
suggested that DUSP6 expression levels may be decreased in DN model
mice.
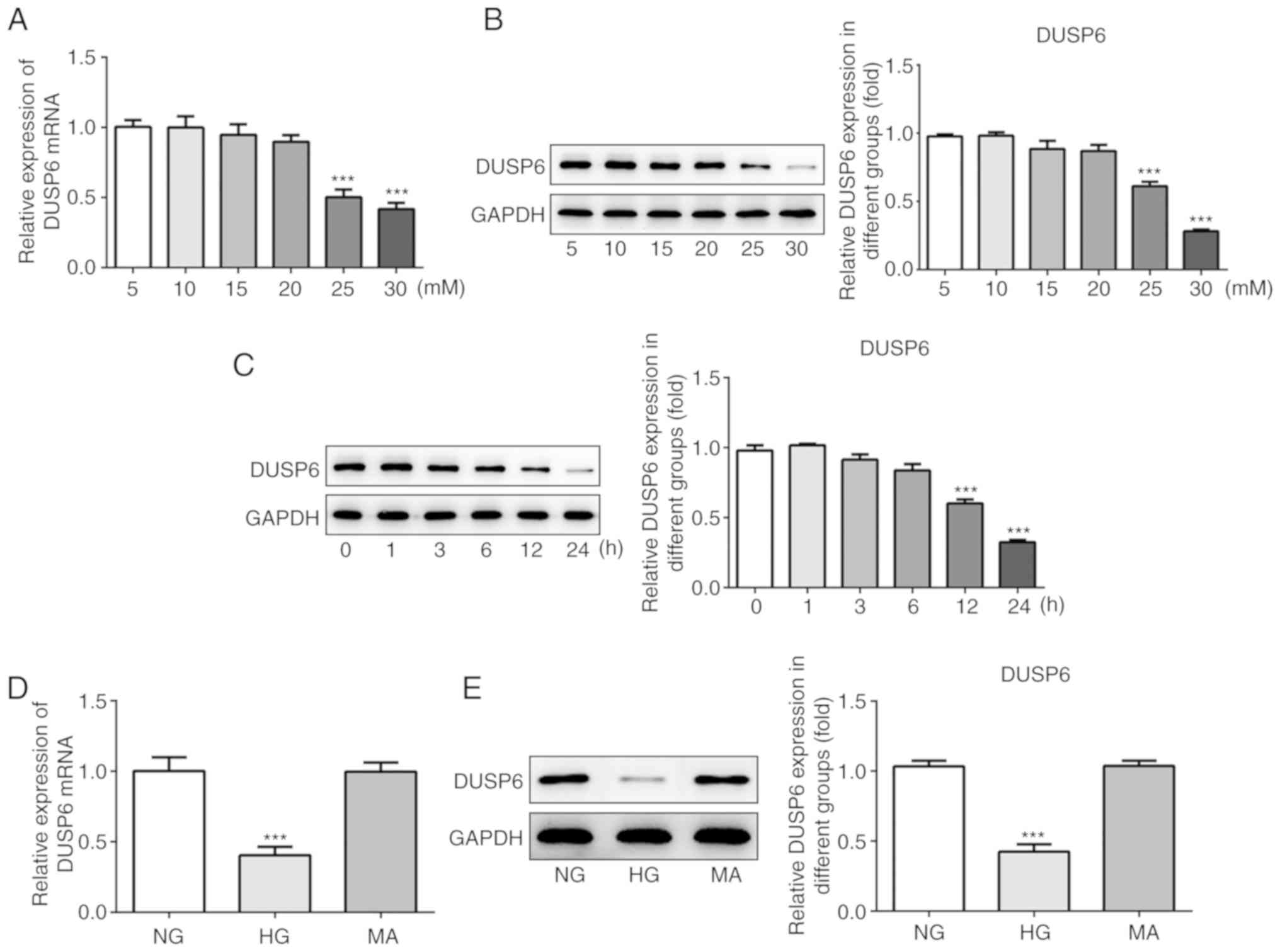
DUSP6 expression levels are decreased
in HG-induced murine podocytes
It was previously demonstrated that DUSP6 expression
levels were downregulated in the kidneys of DN model mice, which
suggested that podocyte functions may be closely linked to DN
(2). D-glucose is used to simulate
a HG environment in vivo (24,27);
therefore, the present study used a gradient of D-glucose
concentrations (5–30 mM) to treat MCP5 murine podocytes. The
results indicated that 30 mM D-glucose was the dose that most
effectively inhibited DUSP6 mRNA and protein expression levels
compared with the 5 mM D-glucose group (Fig. 2A and B). Subsequently, MPC5 cells
were induced with 30 mM D-glucose (HG) for different incubation
periods. The results indicated that the optimum incubation period
for D-glucose-mediated stimulation of MPC5 podocytes was 24 h, as
DUSP6 expression levels were reduced to the lowest levels at 24 h
compared with the 0 h group (Fig.
2C). Moreover, compared with the normal glucose (NG) group (5
mM D-glucose), MA did not significantly alter the expression levels
of DUSP6. By contrast, HG significantly decreased DUSP6 mRNA and
protein expression levels compared with the NG group (Fig. 2D and E). Altogether, the results
suggested that DUSP6 expression levels may be reduced in HG-induced
MPC5 cells.
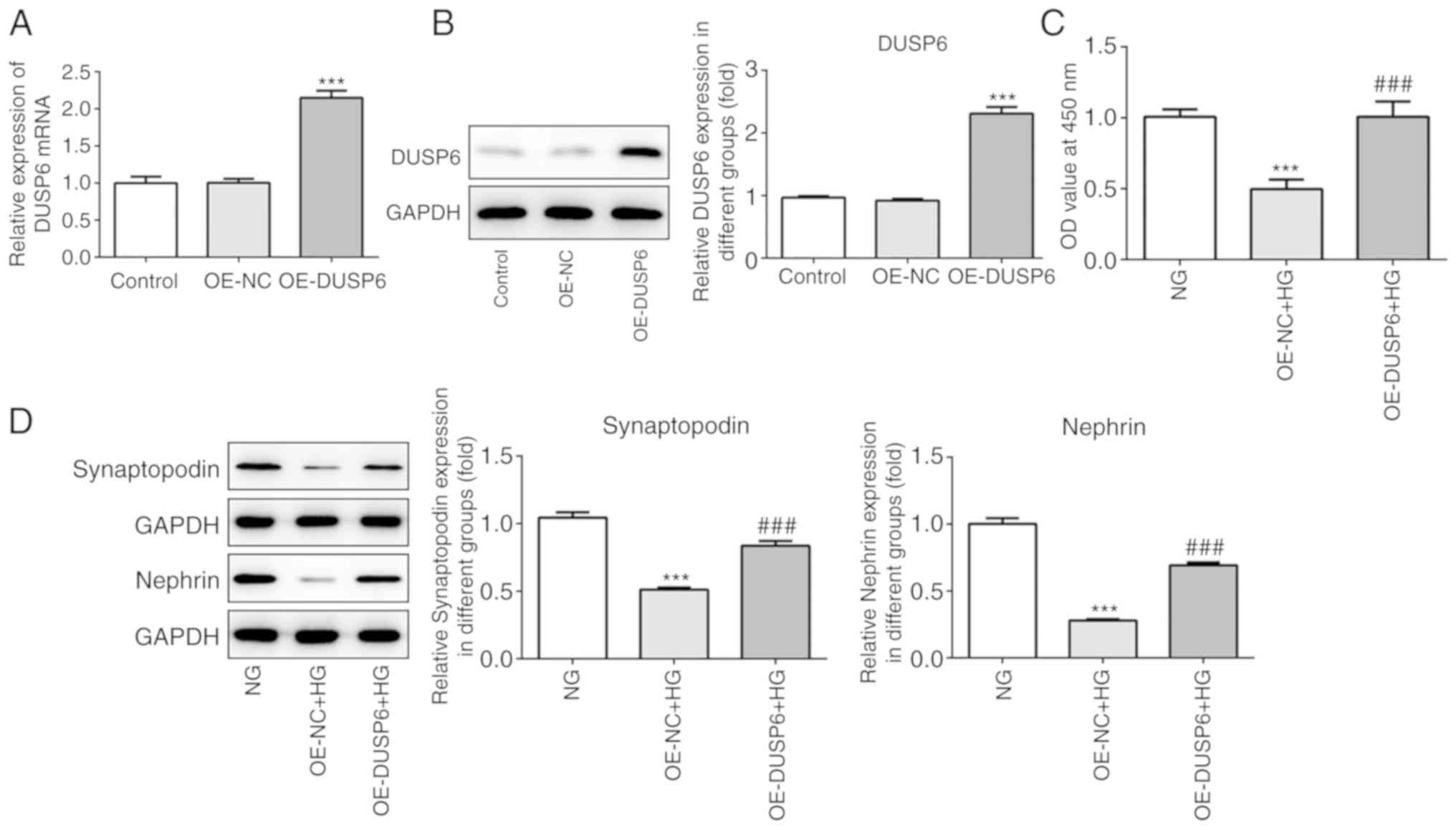
DUSP6 overexpression protects against
HG-induced podocyte injury
To further elucidate the role of DUSP6 in podocyte
injury, OE-DUSP6 and OE-NC were constructed and transfected into
MPC5 cells. RT-qPCR and western blotting were performed to assess
transfection efficiency. DUSP6 mRNA and protein expression levels
were significantly increased in the OE-DUSP6 group compared with
the OE-NC group (Fig. 3A and B).
Subsequently, MPC5 cell viability and the expression of specific
markers (synaptopodin and nephrin) were also investigated. MPC5
cell viability was significantly decreased by HG compared with the
NG group; however, OE-DUSP6 reversed HG-mediated effects on cell
viability (Fig. 3C). Similarly,
synaptopodin and nephrin protein expression levels were
significantly reduced in HG-treated MPC5 cells compared with
NG-treated cells, whereas DUSP6 overexpression reversed HG-mediated
downregulation of protein expression (Fig. 3D). In summary, the results
indicated that DUSP6 alleviated HG-induced cell injury.
DUSP6 overexpression attenuates
HG-induced oxidative stress and inflammatory responses
Oxidative stress and the inflammatory response are
implicated in podocyte injury, which is indicated by increased
levels of ROS and inflammatory cytokines (6). Compared with the NG group, the HG
group displayed significantly increased ROS production levels,
which were reversed by DUSP6 overexpression (Fig. 4A). MDA, a cellular oxidative stress
product, was also significantly reduced in DUSP6-overexpression
MPC5 cells under HG conditions compared with the OE-NC + HG group
(Fig. 4B). In addition, the levels
of TNF-α, IL-1β and IL-6 were determined using ELISA kits. DUSP6
overexpression reversed HG-induced upregulation of inflammatory
cytokine levels (Fig. 4B). The
results indicated that DUSP6 overexpression may relieve oxidative
stress and the inflammatory response in MPC5 cells.
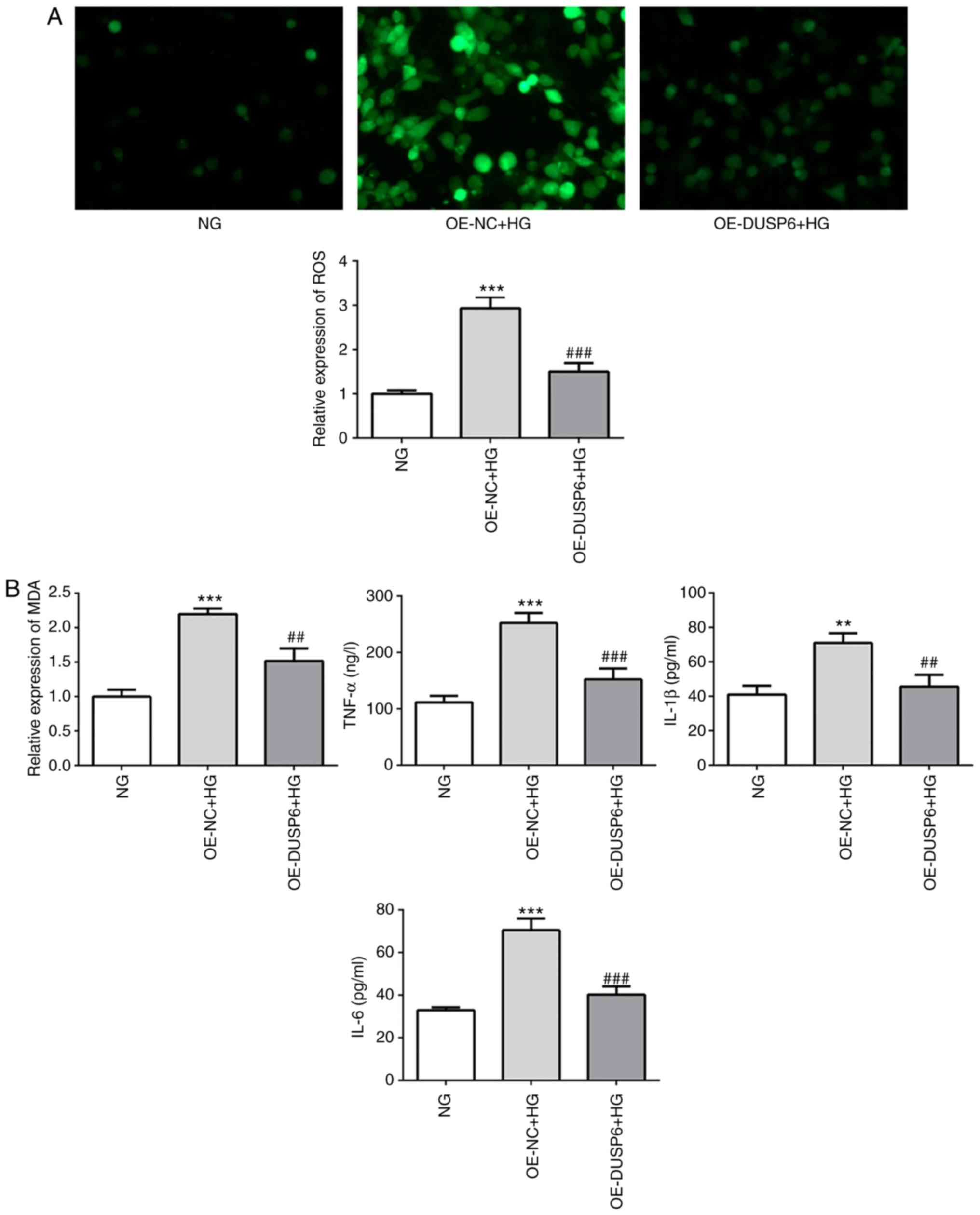 | Figure 4.DUSP6 overexpression attenuates
HG-induced oxidative stress and inflammatory responses. (A) ROS
levels were determined by ROS staining using a DHE-probe and the
relative fluorescence intensity was semi-quantified (magnification,
×100). (B) The levels of MDA, IL-1β, IL-6 and TNF-α were measured
using ELISA kits. **P<0.01 and ***P<0.001 vs. NG;
##P<0.01 and ###P<0.001 vs. OE-NC + HG.
DUSP6, dual-specificity phosphatase 6; HG, high glucose; ROS,
reactive oxygen species; MDA, malondialdehyde; IL, interleukin;
TNF, tumor necrosis factor; NG, normal glucose; OE, overexpression;
NC, negative control; DHE, dihydroethidium. |
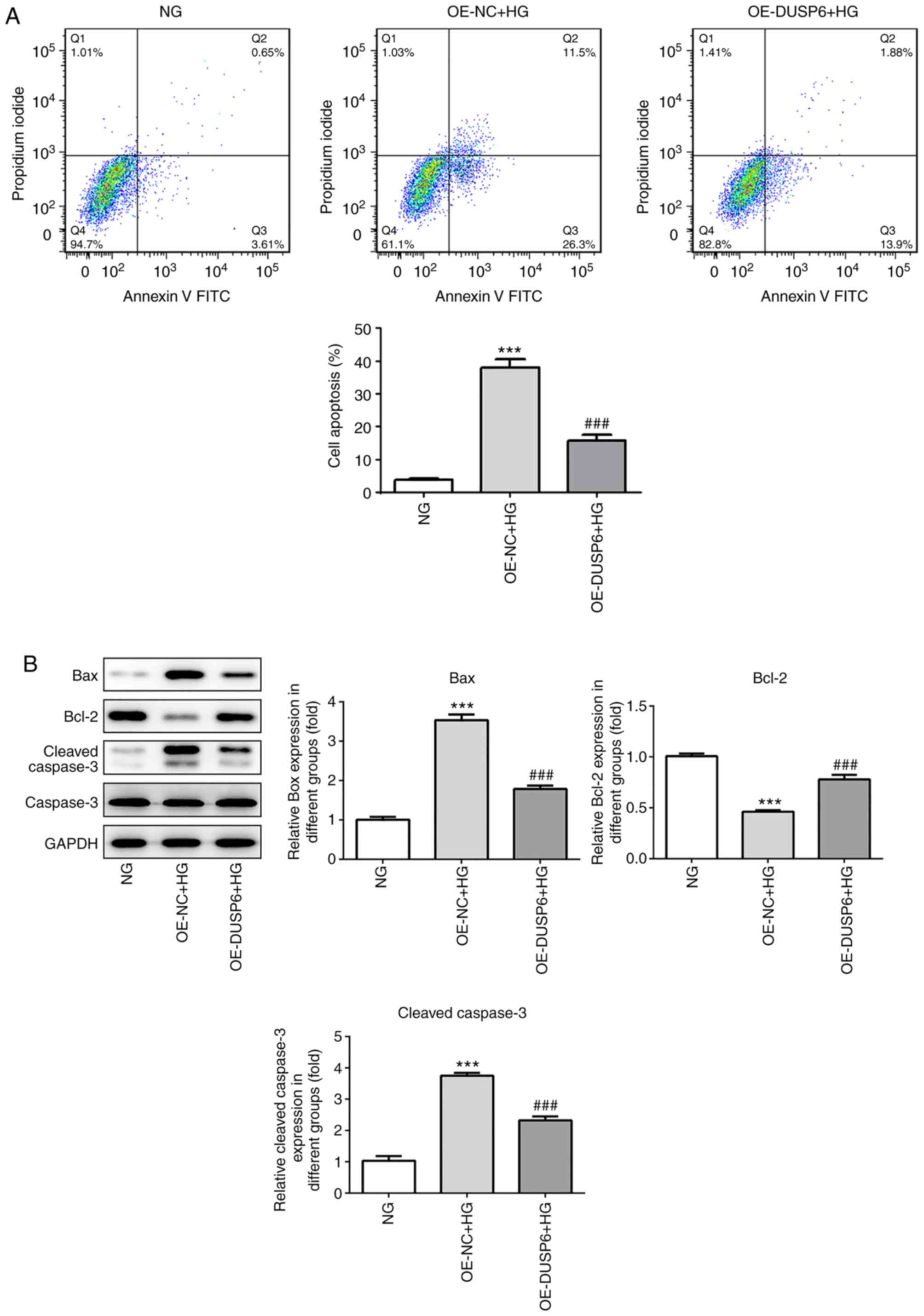
DUSP6 overexpression alleviates
HG-induced podocyte apoptosis
Subsequently, the effect of DUSP6 on podocyte
apoptosis was investigated. In a previous study, the HG environment
promoted podocyte apoptosis, leading to glomerulosclerosis and
severe proteinuria (4,5). The effective prevention of podocyte
apoptosis is crucial for the treatment of DN. HG-induced MPC5 cells
displayed an increased rate of apoptosis compared with the NG
group; however, DUSP6 overexpression significantly reduced
HG-induced apoptosis (Fig. 5A).
Consistently, HG significantly upregulated Bax protein expression
levels compared with the NG group, but DUSP6 overexpression
reversed HG-mediated alterations to Bax expression. By contrast,
Bcl-2 expression levels displayed the opposite trend. Cleaved
caspase-3 expression levels were significantly upregulated in the
HG group compared with the NG group, which was reversed by DUSP6
overexpression (Fig. 5B).
Collectively, the results suggested that DUSP6 may prevent
HG-induced podocyte apoptosis.
ERK1/2 activation is associated with
the role of DUSP6 in murine podocytes
Finally, the potential mechanism underlying the
protective effects of DUSP6 in MPC5 cells was investigated. DUSPs
tightly mediate the spatiotemporal activity and inactivation of
mitogen-activated protein kinases (MAPKs) (28). As members of the MAPK family,
ERK1/2 are highly homologous and function in the same protein
kinase cascade (29), which is
involved in a variety of pathological conditions, including
inflammation, oxidative stress and cell senescence (30–32).
Therefore, the modulatory effect of DUSP6 on ERK1/2 was
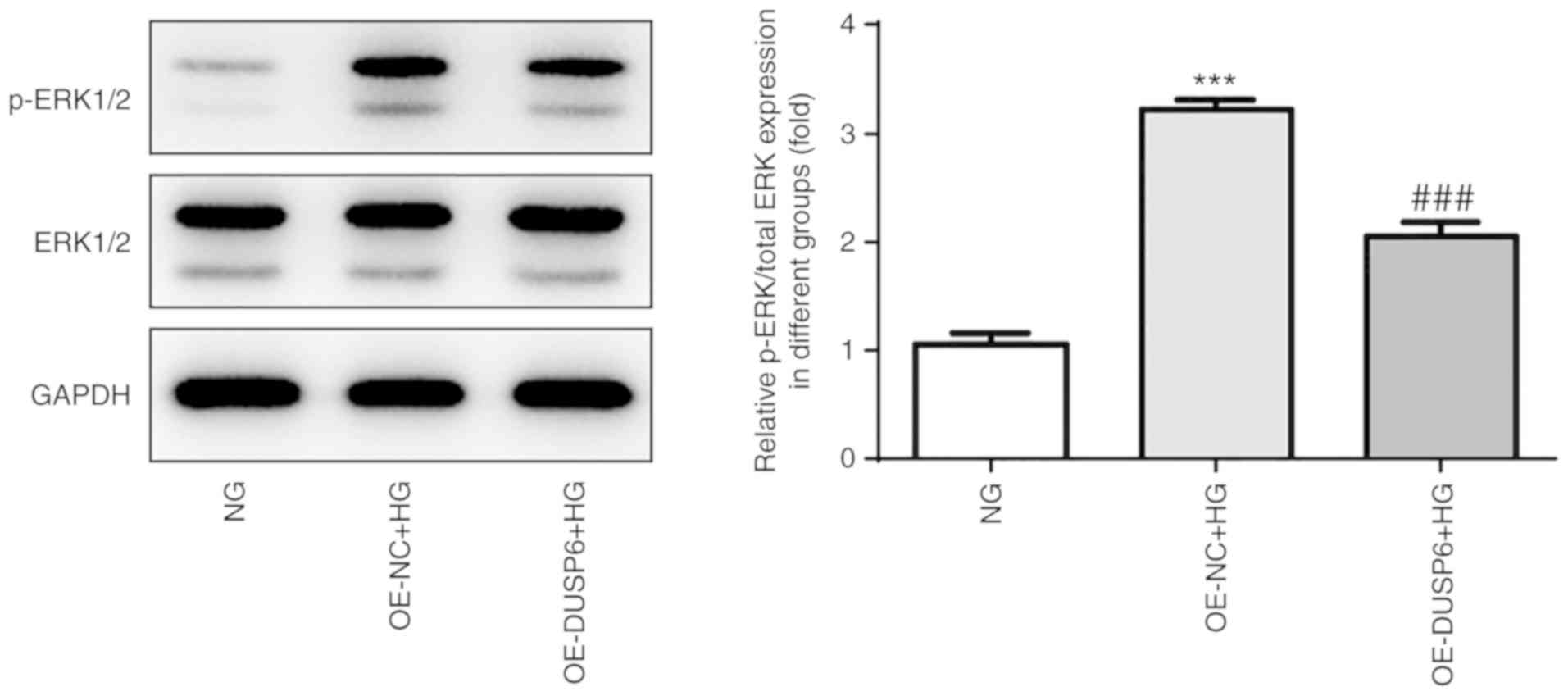
investigated. Compared with NG conditions, HG conditions increased
ERK1/2 phosphorylation, but displayed no effect on the expression
levels of total-ERK1/2. Conversely, DUSP6 overexpression suppressed
HG-induced ERK1/2 phosphorylation (Fig. 6), suggesting that DUSP6 may prevent
HG-induced activation of ERK1/2, which may further affect the role
of DUSP6 in MPC5 cells.
Discussion
Accumulating research has demonstrated that DUSPs
serve as tumor suppressors or oncogenes by regulating cell
proliferation, migration and apoptosis (12–14),
which ultimately influences and determines the fate of specific
types of cancer (19). A previous
study demonstrated that DUSP1 was tightly associated with a glucose
metabolism disorder and glomerular apoptosis via interrupting
JNK-mitochondrial fission factor-mitochondrial fission, reducing
hyperglycemia-regulated mitochondrial damage and improving renal
function (33). Huang et al
(34), reported that DUSP26
expression levels in the kidneys of patients with DN were elevated
compared with non-diabetic patients. In addition, the progression
of DUSP26-regulated DN was largely dependent on the generation of
ROS. DUSP9 downregulation in clear cell renal cell carcinoma was
also associated with a poor prognosis in a large number of clinical
samples (35).
Previous studies demonstrated that DUSP6 was
implicated in various types of cancer, including endometrial
adenocarcinoma, breast cancer and ovarian cancer (36–39).
However, to the best of our knowledge, few studies have
investigated the role of DUSP6 in kidney disease, particularly in
DN. Therefore, the present study aimed to reveal the regulatory
effects of DUSP6 in DN and to determine the pathogenesis of DN to
identify effective novel targets for clinical therapy.
Diabetic model mice were established, and it was
indicated that DUSP6 expression levels were downregulated in the
kidneys of the diabetic model mice, which was accompanied by
evident pathological alterations, compared with control mice.
Subsequently, in vitro studies using MPC5 cells were
conducted to validate the role of DUSP6 following stimulation with
D-glucose. MPC5 cells treated with 30 mM D-glucose for 24 h
displayed significantly reduced DUSP6 expression levels compared
with control cells. In addition, DUSP6 overexpression inhibited
HG-mediated inhibition of cell viability and expression levels of
MPC5 cell markers.
A previous study reported that DUSP5 might serve as
an endogenous regulator of adipose tissue inflammation (40). Ye et al (41), demonstrated that DUSP9 is a key
suppressor of high-fat diet-induced hepatic steatosis and
inflammatory responses. Furthermore, DUSP4−/− hearts and
DUSP4-knockdown cells are more susceptible to oxidant-induced death
and tissue injury, indicating a critical defensive role of DUSP4
against oxidative stress (42). In
the present study, the results suggested that DUSP6 overexpression
attenuated HG-induced oxidative stress and inflammatory responses.
In addition, DUSP6 overexpression also inhibited HG-induced
podocyte apoptosis.
DUSP6, a negative regulator of ERK1/2, regulates the
ERK1/2 signaling cascade (43).
The phosphorylation of ERK and p38MAPK is required for human DN
(44), contributing to the
pathogenesis of DN via stimulation of ROS and inflammatory factors
(45). HG rapidly enhances Ras
activation, and progressively increases ERK and nuclear c-Jun
activation (46), which may be
involved in the regulation of inflammation and fibrosis in human
renal disease (47). The present
study indicated that DUSP6 overexpression decreased ERK1/2
phosphorylation under HG conditions compared with the HG + OE-NC
group, which indicated that DUSP6 may exert protective effects
against oxidative stress, the inflammatory response and apoptosis
in MPC5 cells via altering the activity of ERK1/2. However, the
expression levels of c-Jun and p38MAPK were not investigated in the
present study; therefore, further investigation into the role of
ERK1/2 in MPC5 cell injury using inhibitors to determine the
specific mechanisms underlying DN is required.
In conclusion, the present study demonstrated that
DUSP6 expression levels were decreased in the kidneys of DN model
mice compared with control mice, which was also indicated by
decreased DUSP6 expression levels in HG-induced MPC5 cells compared
with control MPC5 cells. Collectively, the results of the present
study suggested that DUSP6 protected MPC5 cells from the
inflammatory response and oxidative stress potentially via
activation of ERK1/2.
Acknowledgements
Not applicable.
Funding
The present study was supported by the College
Students Innovative Entrepreneurial Training Program of
Heilongjiang Province (grant no. 20190222020), the Joint Guiding
Program of Natural Science Foundation of Heilongjiang Province
(grant no. LH2019H060) and the Key Program of Natural Science
Foundation of Heilongjiang Province (grant no. ZD2017020).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors contributions
LQC and YKW drafted the manuscript and performed the
experiments; HYL and GYM analyzed and interpreted the data; and HMZ
and GC contributed to the conception and design of the study. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Biological and
Medical Ethics Committee of Jamusi University (approval no. SYXK
2016-014).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Farag YM and Al Wakeel JS: Diabetic
nephropathy in the Arab Gulf countries. Nephron Clin Pract.
119:c317–c322, discussion c322-c323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pagtalunan ME, Miller PL, Jumping-Eagle S,
Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L and Meyer TW:
Podocyte loss and progressive glomerular injury in type II
diabetes. J Clin Invest. 99:342–348. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Susztak K, Raff AC, Schiffer M and
Böttinger EP: Glucose-induced reactive oxygen species cause
apoptosis of podocytes and podocyte depletion at the onset of
diabetic nephropathy. Diabetes. 55:225–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meyer TW, Bennett PH and Nelson RG:
Podocyte number predicts long-term urinary albumin excretion in
Pima Indians with Type II diabetes and microalbuminuria.
Diabetologia. 42:1341–1344. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jefferson JA, Shankland SJ and Pichler RH:
Proteinuria in diabetic kidney disease: A mechanistic viewpoint.
Kidney Int. 74:22–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu H, Li Y, Zhang T, Liu M, Chi Y, Liu S
and Shi Y: Salidroside reduces high-glucose-induced podocyte
apoptosis and oxidative stress via upregulating heme oxygenase-1
(HO-1) expression. Med Sci Monit. 23:4067–4076. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu L, Han J, Yuan R, Xue L and Pang W:
Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-κB
pathway. Biol Res. 51:92018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo J, Li J, Zhao J, Yang S, Wang L, Cheng
G, Liu D, Xiao J, Liu Z and Zhao Z: MiRNA-29c regulates the
expression of inflammatory cytokines in diabetic nephropathy by
targeting tristetraprolin. Sci Rep. 7:23142017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haraldsson B: A new era of
podocyte-targeted therapy for proteinuric kidney disease. N Engl J
Med. 369:2453–2454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen HF, Chuang HC and Tan TH: Regulation
of Dual-Specificity Phosphatase (DUSP) Ubiquitination and Protein
Stability. Int J Mol Sci. 20:26682019. View Article : Google Scholar
|
|
11
|
Farooq A and Zhou MM: Structure and
regulation of MAPK phosphatases. Cell Signal. 16:769–779. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang CY and Tan TH: DUSPs, to MAP kinases
and beyond. Cell Biosci. 2:242012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin HP, Ho HM, Chang CW, Yeh SD, Su YW,
Tan TH and Lin WJ: DUSP22 suppresses prostate cancer proliferation
by targeting the EGFR-AR axis. FASEB J. 33:14653–14667. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YR, Chou HC, Yang CH, Chen HY, Liu
YW, Lin TY, Yeh CL, Chao WT, Tsou HH, Chuang HC, et al: Deficiency
in VHR/DUSP3, a suppressor of focal adhesion kinase, reveals its
role in regulating cell adhesion and migration. Oncogene.
36:6509–6517. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Messina S, Frati L, Leonetti C, Zuchegna
C, Di Zazzo E, Calogero A and Porcellini A: Dual-specificity
phosphatase DUSP6 has tumor-promoting properties in human
glioblastomas. Oncogene. 30:3813–3820. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song H, Wu C, Wei C, Li D, Hua K, Song J,
Xu H, Chen L and Fang L: Silencing of DUSP6 gene by RNAi-mediation
inhibits proliferation and growth in MDA-MB-231 breast cancer
cells: An in vitro study. Int J Clin Exp Med. 8:10481–10490.
2015.PubMed/NCBI
|
|
17
|
Furukawa T, Sunamura M, Motoi F, Matsuno S
and Horii A: Potential tumor suppressive pathway involving
DUSP6/MKP-3 in pancreatic cancer. Am J Pathol. 162:1807–1815. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong VC, Chen H, Ko JM, Chan KW, Chan YP,
Law S, Chua D, Kwong DL, Lung HL, Srivastava G, et al: Tumor
suppressor dual-specificity phosphatase 6 (DUSP6) impairs cell
invasion and epithelial-mesenchymal transition (EMT)-associated
phenotype. Int J Cancer. 130:83–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahmad MK, Abdollah NA, Shafie NH, Yusof NM
and Razak SRA: Dual-specificity phosphatase 6 (DUSP6): A review of
its molecular characteristics and clinical relevance in cancer.
Cancer Biol Med. 15:14–28. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang L, Litjens NHR, Kannegieter NM,
Klepper M, Baan CC and Betjes MGH: pERK-dependent defective
TCR-mediated activation of CD4(+) T cells in end-stage renal
disease patients. Immun Ageing. 14:142017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beaudry K, Langlois MJ, Montagne A, Cagnol
S, Carrier JC and Rivard N: Dual-specificity phosphatase 6 deletion
protects the colonic epithelium against inflammation and promotes
both proliferation and tumorigenesis. J Cell Physiol.
234:6731–6745. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao W, Zheng Y, Fang W, Liao S, Xiong Y,
Li Y, Xiao S, Zhang X and Liu J: Dual specificity phosphatase 6
protects neural stem cells from β-amyloid-induced cytotoxicity
through ERK1/2 inactivation. Biomolecules. 8:1812018. View Article : Google Scholar
|
|
23
|
Neumann UH, Ho JSS, Chen S, Tam YYC,
Cullis PR and Kieffer TJ: Lipid nanoparticle delivery of glucagon
receptor siRNA improves glucose homeostasis in mouse models of
diabetes. Mol Metab. 6:1161–1172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang H, Lei CT, Ye C, Gao P, Wan C, Chen
S, He FF, Wang YM, Su H and Zhang C: MDM2 is implicated in
high-glucose-induced podocyte mitotic catastrophe via Notch1
signalling. J Cell Mol Med. 21:3435–3444. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brosius FC III, Alpers CE, Bottinger EP,
Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M,
Leiter EH, et al Animal Models of Diabetic Complications
Consortium, : Mouse models of diabetic nephropathy. J Am Soc
Nephrol. 20:2503–2512. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu M, Wang R, Li X, Fan M, Lin J, Zhen J,
Chen L and Lv Z: LncRNA MALAT1 is dysregulated in diabetic
nephropathy and involved in high glucose-induced podocyte injury
via its interplay with β-catenin. J Cell Mol Med. 21:2732–2747.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu R and Molkentin JD: Regulation of
cardiac hypertrophy and remodeling through the dual-specificity
MAPK phosphatases (DUSPs). J Mol Cell Cardiol. 101:44–49. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patterson KI, Brummer T, OBrien PM and
Daly RJ: Dual-specificity phosphatases: Critical regulators with
diverse cellular targets. Biochem J. 418:475–489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu N and Malemud CJ: Extracellular
signal-regulated kinase: a regulator of cell growth, inflammation,
chondrocyte and bone cell receptor-mediated gene expression. Int J
Mol Sci. 20:37922019. View Article : Google Scholar
|
|
31
|
Karimi P, Gheisari A, Gasparini SJ,
Baharvand H, Shekari F, Satarian L and Ader M: Crocetin prevents
RPE cells from oxidative stress through protection of cellular
metabolic function and activation of ERK1/2. Int J Mol Sci.
21:29492020. View Article : Google Scholar
|
|
32
|
Zou J, Lei T, Guo P, Yu J, Xu Q, Luo Y, Ke
R and Huang D: Mechanisms shaping the role of ERK1/2 in cellular
senescence (Review). Mol Med Rep. 19:759–770. 2019.PubMed/NCBI
|
|
33
|
Sheng J, Li H, Dai Q, Lu C, Xu M, Zhang J
and Feng J: DUSP1 recuses diabetic nephropathy via repressing
JNK-Mff-mitochondrial fission pathways. J Cell Physiol.
234:3043–3057. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang F, Sheng XX and Zhang HJ: DUSP26
regulates podocyte oxidative stress and fibrosis in a mouse model
with diabetic nephropathy through the mediation of ROS. Biochem
Biophys Res Commun. 515:410–416. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu S, Wang Y, Sun L, Zhang Z, Jiang Z, Qin
Z, Han H, Liu Z, Li X, Tang A, et al: Decreased expression of
dual-specificity phosphatase 9 is associated with poor prognosis in
clear cell renal cell carcinoma. BMC Cancer. 11:4132011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gu W, Yuan Y, Wang L, Yang H, Li S, Tang Z
and Li Q: Long non-coding RNA TUG1 promotes airway remodelling by
suppressing the miR-145-5p/DUSP6 axis in cigarette smoke-induced
COPD. J Cell Mol Med. 23:7200–7209. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fan MJ, Liang SM, He PJ, Zhao XB, Li MJ
and Geng F: Dusp6 inhibits epithelial-mesenchymal transition in
endometrial adenocarcinoma via ERK signaling pathway. Radiol Oncol.
53:307–315. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li J, Yang C, Yang J and Zou L:
Down-regulation of CCL17 in cancer-associated fibroblasts inhibits
cell migration and invasion of breast cancer through ERK1/2
pathway. Cancer Manag Res. 11:7439–7453. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
James NE, Beffa L, Oliver MT, Borgstadt
AD, Emerson JB, Chichester CO, Yano N, Freiman RN, DiSilvestro PA
and Ribeiro JR: Inhibition of DUSP6 sensitizes ovarian cancer cells
to chemotherapeutic agents via regulation of ERK signaling response
genes. Oncotarget. 10:3315–3327. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Habibian JS, Jefic M, Bagchi RA, Lane RH,
McKnight RA, McKinsey TA, Morrison RF and Ferguson BS: DUSP5
functions as a feedback regulator of TNFα-induced ERK1/2
dephosphorylation and inflammatory gene expression in adipocytes.
Sci Rep. 7:128792017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ye P, Xiang M, Liao H, Liu J, Luo H, Wang
Y, Huang L, Chen M and Xia J: Dual-specificity phosphatase 9
protects against nonalcoholic fatty liver disease in mice through
ASK1 suppression. Hepatology. 69:76–93. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barajas-Espinosa A, Basye A, Angelos MG
and Chen CA: Modulation of p38 kinase by DUSP4 is important in
regulating cardiovascular function under oxidative stress. Free
Radic Biol Med. 89:170–181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vo AH, Swaggart KA, Woo A, Gao QQ,
Demonbreun AR, Fallon KS, Quattrocelli M, Hadhazy M, Page PGT, Chen
Z, et al: Dusp6 is a genetic modifier of growth through enhanced
ERK activity. Hum Mol Genet. 28:279–289. 2019.PubMed/NCBI
|
|
44
|
Sakai N, Wada T, Furuichi K, Iwata Y,
Yoshimoto K, Kitagawa K, Kokubo S, Kobayashi M, Hara A, Yamahana J,
et al: Involvement of extracellular signal-regulated kinase and p38
in human diabetic nephropathy. Am J Kidney Dis. 45:54–65. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rane MJ, Song Y, Jin S, Barati MT, Wu R,
Kausar H, Tan Y, Wang Y, Zhou G, Klein JB, et al: Interplay between
Akt and p38 MAPK pathways in the regulation of renal tubular cell
apoptosis associated with diabetic nephropathy. Am J Physiol Renal
Physiol. 298:F49–F61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lin CL, Wang FS, Kuo YR, Huang YT, Huang
HC, Sun YC and Kuo YH: Ras modulation of superoxide activates
ERK-dependent fibronectin expression in diabetes-induced renal
injuries. Kidney Int. 69:1593–1600. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
De Borst MH, Prakash J, Melenhorst WB, van
den Heuvel MC, Kok RJ, Navis G and van Goor H: Glomerular and
tubular induction of the transcription factor c-Jun in human renal
disease. J Pathol. 213:219–228. 2007. View Article : Google Scholar : PubMed/NCBI
|