Introduction
Intramedullary nailing (IN) is a method of choice
for treatment of long bone fractures. This treatment has two
choices of bone preparation for fixation: un-reamed and reamed
technique. The reamed IN (RIN) method uses a long reamer instrument
in order to open the fractured bone medullar canal to insert the
nail in a more fitted way to the fractured long bone (1). The nailing procedure affects the
biological environment of the bone and thus the fracture healing
(2).
During RIN procedure several changes take place in
the medullary canal. The first parameter that is affected is the
intramedullary pressure which rises as a result of the effect of
the reamer device in the bone canal. The normal blood flow in the
long bones is centripetal. Under the increase of the intramedullary
pressure, the blood flow is reversed to centrifugal (3,4). The
reversed flow drifts medullary content into the systematic
circulation through the metaphyseal vascular systems (5). The medullary content is called
‘debris’ and several investigations were performed in order to
reveal its components. It was shown that ‘debris’ is a source of
mesenchymal stem cells (MSCs) (6),
which can be cultured in vitro. RIN releases several growth
factors which along with the cell debris promote an osteogenic
effect. MSCs are multipotent stem cells which can differentiate
into several tissues as adipocytes, chondrocytes and osteocytes.
Their ability to regenerate human body tissues directed a large
amount of research to investigate their possible role in several
diseases and most importantly resulted in their usage as treatment
in the field of medical practice of regenerative medicine (7). Mobilization and homing of MSCs from
the bone marrow (BM) pool occurs following tissue damage such as
burn trauma, muscle damage and cardiac stroke (8–12).
Several molecules are implicated in the MSC homing and migration.
Among the chemokines tested in an in vitro migration model,
stromal cell-derived factor 1 (SDF-1) showed significant
chemotactic influence. BM-MSCs express C-X-C motif chemokine
receptor 4 (CXCR4) which circulates toward SDF-1 gradients
(13). Under normal circumstances
BM-MSCs remain at the BM niche under the action of SDF-1/CXCR4
axis. The CXCR4/SDF-1 axis is considered to be the key effector of
MSC homing and migration. In case of inflammation following tissue
damage, such as RIN, several enzymes released following the release
of chemokines affect the integrity of SDF-1 and CXCR4 and promote
mobilization by breaking the ‘anchors’ of MSCs inside the BM pool
(14,15). There are data suggesting that
platelet-derived growth factors AA and BB can influence MSC
migration at remote sites from the initial injury in patients with
fractures by changing the cell surface receptors CD140a and CD140b,
which lead to MSC proliferation (16). However, there are to date no
studies addressing the effect of CXCR4/SDF-1 axis in patients with
fractures. The purpose of the present study was to evaluate the
effects of RIN on the MSC pool in the iliac crest BM (IC-BM) in
patients treated for long bone fractures and the possible role of
CXCR4/SDF-1 axis.
We report increased serum levels of SDF-1 following
RIN, inversely correlated to CXCR4 mRNA level alterations in IC-BM
MSCs. These results indicate evidence for a systemic SDF-1
up-regulation and an influence of RIN at MSCs pool, a remote site
from RIN.
Patients and methods
Selection of patients
In total, 15 patients were included. They were
diagnosed with fracture on the diaphysis of a long bone, femur or
tibia. The selected therapy was surgical and specifically IN of the
injured bone. The IN procedure included reaming (RIN) of the bone
canal in all cases. The inclusion criteria were: i) age ≤65 years;
ii) no co-morbidities; iii) no history of steroid medication; and
iv) the surgery to take place within the first 24 h following the
incident of fracture. The Ethics Committee of the University
Hospital of Heraklion approved the experimental study protocol
(Decision no. 185, Protocol no. 14880/14/1/2015). All patients
included in the study were informed and written consent was
obtained.
BM-MSCs isolation and culture
BM samples were aspirated from the anterior
posterior iliac crest and cultured in vitro according to a
previously described protocol (17,18).
For each patient two BM samples of 10 ml each were aspirated, one
before the surgical procedure and a second immediately after the
end of the surgery. Briefly, BM mononuclear cells (BM-MNCs)
isolated by Histopaque-1077 (Sigma-Aldrich; Merck KGaA)
centrifugation, were cultured in Dulbecco's modified Eagle's
medium-low glucose (DMEM-LG; Gibco; Thermo Fisher Scientific, Inc.)
in the presence of 10% fetal calf serum (FCS; HyClone; GE
Healthcare Life Sciences) and 100 IU/ml penicillin-streptomycin
(PS; Gibco; Thermo Fisher Scientific, Inc.) consisting the MSC
medium). Cells were cultured at a concentration of 2×105
cells/cm2 in 25 cm2 flasks at 37°C
temperature/5% CO2 humidified atmosphere. One to three
days after seeding, floating cells were removed and the medium was
replaced by fresh MSC medium. Cells were trypsinized (P) when the
confluence reached 70–90% by 0.25% trypsin-1 mM EDTA (Gibco; Thermo
Fisher Scientific, Inc.). Cell counts in passages P1 and P2 were
performed and doubling time (DT) was calculated according to the
formula:
DT=t/n=t × log(2)/log(cells
harvested/cells inoculated),
where t is the time between initial plating and
harvest for the respective passage.
MSCs were characterized by their morphologic (all
displayed the characteristic spindle-shape) and immunophenotypic
features (data not shown) (19).
Blood sample collection
Peripheral blood samples were obtained from all
participants at the same two time points as the BM samples. The
first was obtained before the surgery and the second immediately
after the end of the surgical procedure. All samples were processed
appropriately according to standard procedures (7) and serum was stored at −80°C.
Reverse transcription-quantitative
polymerase chain reaction assay
One million cells from all P2 BM-MSCs samples were
homogenized in TRIzol reagent (Molecular Research Center Inc.).
Total RNA from both time point samples was extracted, and cDNA was
synthesized by RT with the Thermo Scientific RevertAid™ First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). The
mRNA expression was measured using a RT-qPCR assay with SYBR-Green
I. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the
internal control, in order to normalize SDF-1 and CXCR4 expression
levels. Relative expression levels of the examined factors per
sample were calculated according to 2−ΔΔCt method
(20) followed by log2
transformation: ΔΔCT={(Ct CXCR4-Ct GAPDH) sample after}-{(Ct
CXCR4-Ct GAPDH) sample before}.
The mRNA-specific primers used are listed in
Table I.
 | Table I.Primer sequences used for
RT-qPCR. |
Table I.
Primer sequences used for
RT-qPCR.
| Gene | Primer sequence
(5′-3′) | Annealing
temperature, °C |
|---|
| CXCR4 | F:
GGTGGTCTATGTTGGCGTCT | 55 |
|
| R:
TGGAGTGTGACAGCTTGGAG |
|
| GAPDH | F:
AGCCACATCGCTCAGACA | 53 |
|
| R:
CCAATACGACCAAATCCGTT |
|
Serum SDF-1
SDF-1 levels in sera were evaluated by ELISA method
(Quantikine; R&D Systems).
Statistical analysis
The results are expressed as mean ± SD unless
otherwise stated. Pearson's rank coefficient (rho) was used to
evaluate correlations of the expression of the molecules CXCR4 and
SDF1. Wilcoxon matched-pairs signed rank test was used to compare
DT from the two time point collections. Paired t-test was used to
compare serum SDF-1 levels before and after nailing. Analysis was
performed using GraphPad Prism v.6 statistical program (GraphPad
Software, Inc). P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographics and patient data
A total of 15 patients were included to the
experimental study. All the patients were diagnosed with fracture
at the diaphysis of the femur or the tibia. The average age of
patients was 34.64±14.30 years. The maximum age was 62 years
according to the selection criteria and the minimum was 18 years.
The ratio between male/female was 10/5. The most common injury
occurred in the tibia (9 out of 15) and the most common injured
lower limb was the right side (8 out of 15).
MSC characteristics and data
IC-BM samples and blood samples were collected at
two time points. One sample was collected before the RIN (BN) and
the other immediately after RIN (AN). Initially, a possible effect
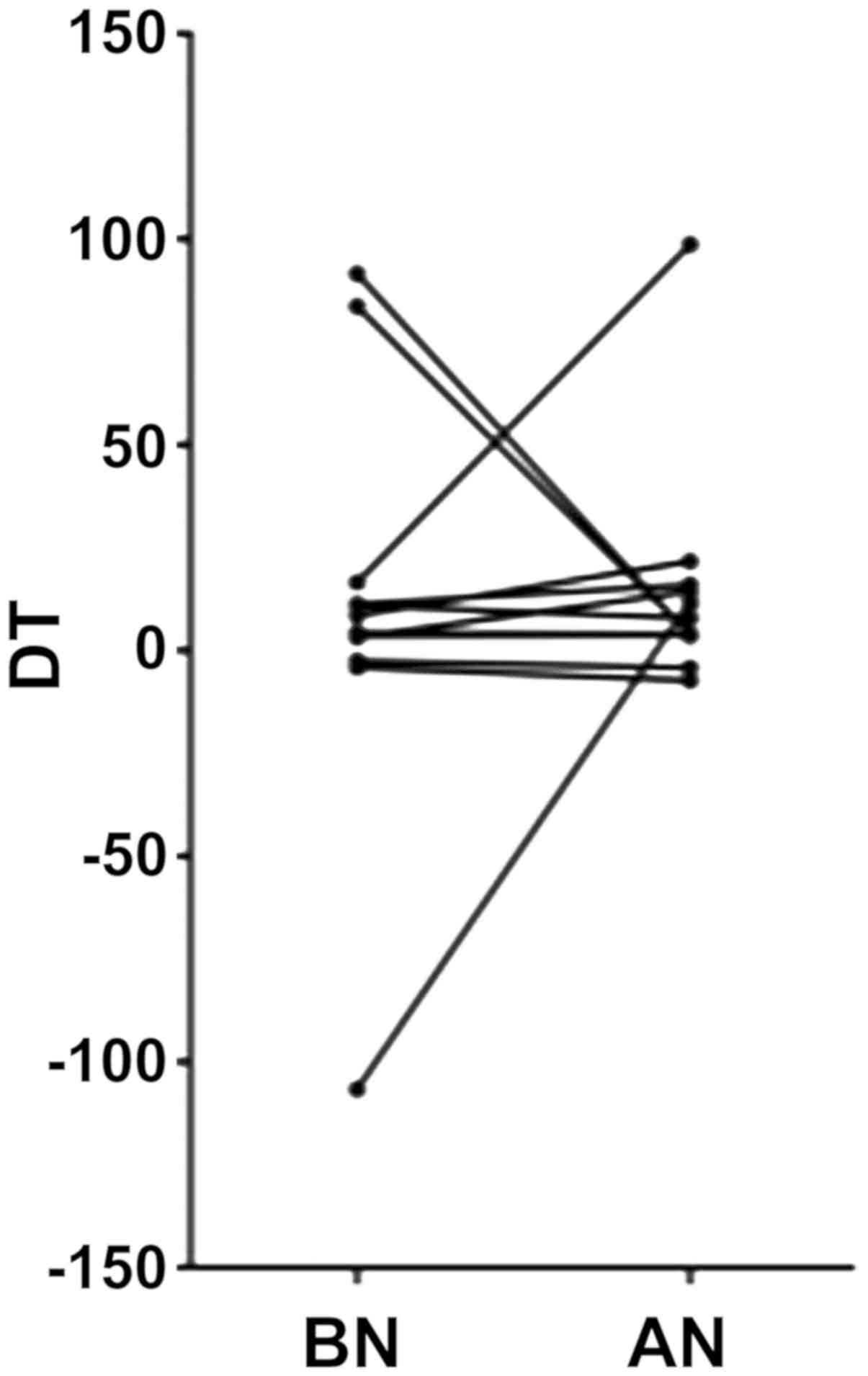
of RIN on cell DT was evaluated. DT was measured for both time
point samples of MSCs for 13 out of 15 patients (median ± SEM:
BN=4.37±12.97 and AN=7.87±6.34). There was no statistical
difference between the DT of BN and AN samples (Wilcoxon
matched-pairs signed rank test Π=0.54) (Fig. 1).
SDF-1 levels following RIN
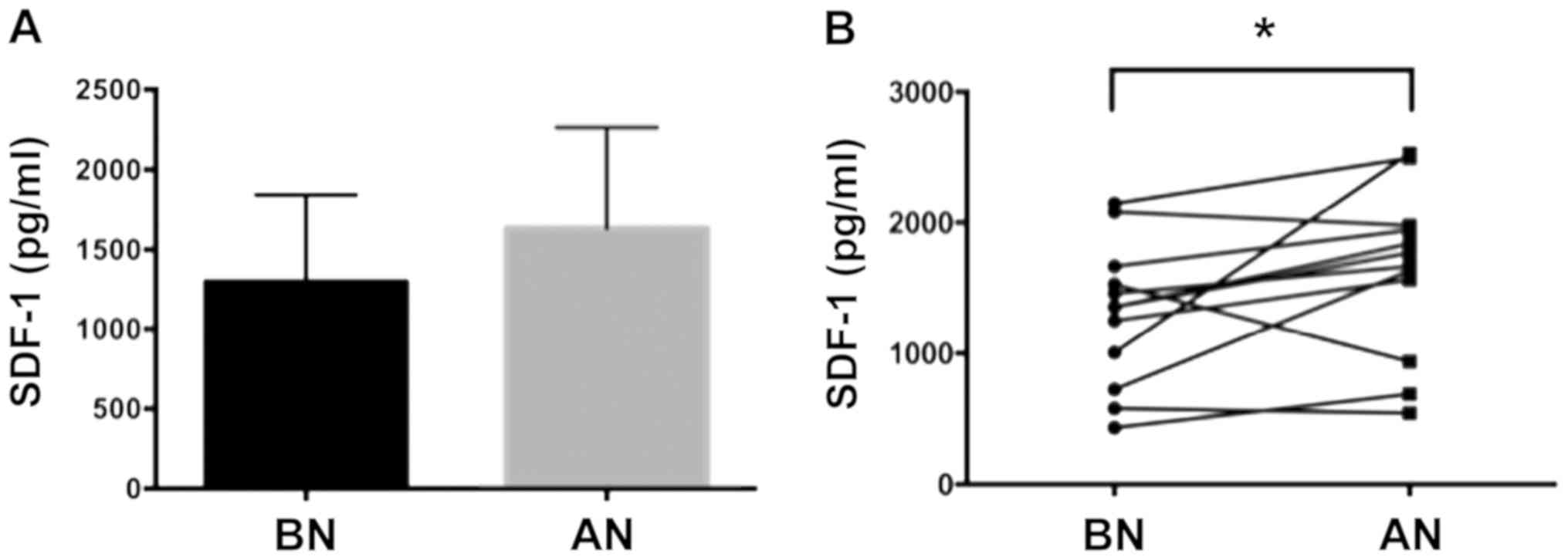
SDF-1 levels were compared in the serum between the
two time point samples (mean ± SD: BN=1,300±542.8 and
AN=1,633±632.4) (Fig. 2A). The
levels of SDF-1 showed a significant increase in the sera of
patients after nailing (paired t-test p=0.049) (Fig. 2B). The change of the levels of
SDF-1 factor was also calculated with 9 out of 12 patients showing
an increase in SDF-1 levels. This suggested that nailing influences
the levels of SDF-1 factor. There was no statistically significant
correlation between age and changes in SDF-1 serum levels
(Pearson's r=−0.27, P=0.4), neither, any correlation between
changes in SDF-1 serum levels with the patient's gender (Spearman's
r=−0.19, P=0.6) or the DT (BN Spearman's r=0.56, P=0.1, AN
Spearman's r=0.18, P=0.57).
Changes of CXCR4 in MSCs after reaming
nailing
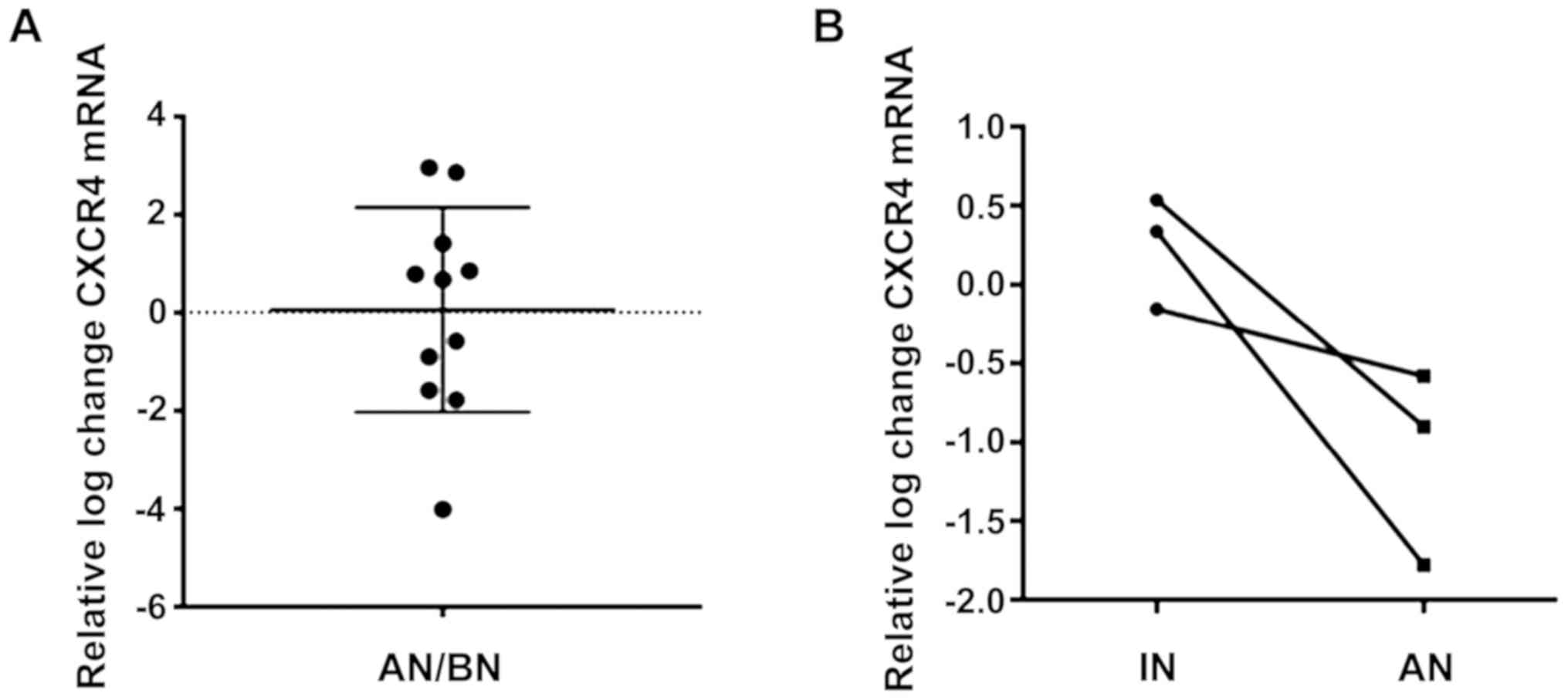
The mRNA levels of CXCR4 receptor were compared
between the two BM-MSC samples (BN and AN) obtained from the iliac
crest. There was no significant change in the relative mRNA levels
of CXCR4 receptor between the two samples (0.06±2.1) (Fig. 3A). In detail, a mixed trend was
observed with 2 out of 9 patients showing more than 3-fold increase
in CXCR4, 4 patients with moderate increase (less than 2-fold) and
3 patients with moderate decrease (less than 2-fold) in CXCR4
expression.
Furthermore, no statistically significant
correlation was seen either between age or patient's gender or
CXCR4 levels (Pearson's r=0.76, P=0.4 and Spearman's r=0.24,
P=0.53, respectively). There was no correlation between the DT and
CXCR4 mRNA expression (BN Spearman's =−0.2, P=0.58, AN Spearman's
=−0.46, P=0.15). Interestingly, comparison of the relative mRNA
CXCR4 levels of MSCs obtained from the surgical site during RIN
(IN) and the MSCs aspirated from the BM pool from the iliac crest
AN, a subset of patients showed that IN derived MSCs tended to have
higher CXCR4 expression relative to the IC-BM derived cells
following RIN (mean ± SD: IN=0.24±0.35, AN=−1.1±0.6) (Fig. 3B).
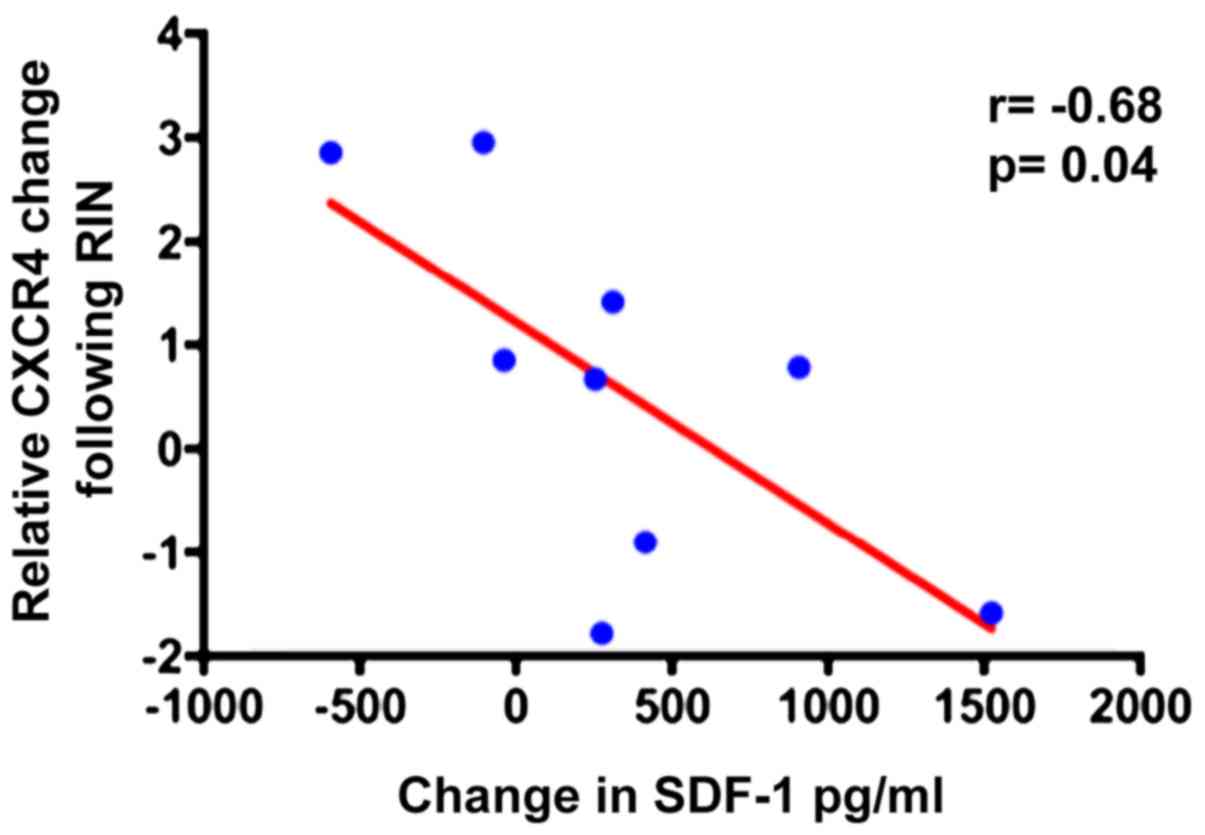
Correlation of serum SDF-1 and MSC
CXCR4 mRNA changes after reamed nailing
As shown above, SDF-1 increased in patient's sera
and hence a possible correlation between SDF-1 in the serum and
CXCR4 mRNA in MSCs was examined. An inverse correlation between
SDF-1 in peripheral blood and CXCR4 in BM-MSCs following RIN was
revealed. This observation suggested that the CXCR4 levels in MSCs
are related to SDF-1 levels in the serum and possibly indicates the
mobilization of MSCs through a decrease in the CXCR4/SDF-1
interaction in the IC-BM (Fig.
4).
Discussion
In a sudden event of a fracture, an inflammatory
cascade is activated in order to achieve bone healing which starts
from the formation of hematoma at the fractured site and ends in
callus formation. RIN procedure represents a gold standard method
for surgical treatment of diaphyseal long bone fractures for
successful fracture stabilization and healing. This surgical
treatment stabilization of the fractured bone requires the use of a
device called intramedullary nail. The preparation of the bone
canal is achieved with the aid of a sharp instrument. After the
stabilization of the fracture site, a race starts for the callus
formation. In addition, the RIN procedure, as a traumatic event
itself, participates in the inflammatory cascade. During RIN the so
called debris is produced (6). The
debris consists of MSCs which are able to promote bone healing.
Their number increases in blood during organ and tissue injuries
and other strenuous situations (21). MSCs are multipotent stromal cells
capable of differentiating and contributing to the regeneration of
mesenchymal tissues such as bone, cartilage, fat, tendon and muscle
(22). MSCs are a source of
progenitors for cell replacement and also activate or revive other
local cells, such as tissue-resident progenitor or stem cells,
endothelial cells and fibroblasts, to facilitate tissue
regeneration via paracrine stimulation (23–26).
The movement of MSCs to injured tissues is described
as endogenous stem cell homing and their subsequent participation
to tissue repair is considered a natural self-healing response. In
cases of injuries MSCs are recruited and mobilized into the damaged
bone via local membrane and the peripheral circulation. There, MSCs
enhance bone regeneration by differentiating directly into bone
formation cells and modulating the biological environment by
secreting growth factors and anti-inflammatory cytokines (26,27).
It has been demonstrated that MSCs migrate via the CXCR4 receptor
to the fracture site and improve healing by affecting the
biomechanical properties and increasing the cartilage and bone
content of the callus (28). This
migration is a multistep process which is mediated by homing
receptors, endothelial co-receptors and chemotactic cytokines. The
SDF-1/CXCR4 signaling axis has been demonstrated to be vital for
MSCs homing (29).
The stress response after a fracture is the summary
of physiologic response to the initial injury - the first hit,
followed by the response to any ongoing surgical intervention such
as RIN which represents the second hit (30). RIN provokes an inflammation as a
cellular response that occurs during tissue injury due to the
procedure. Inflammation is characterized by increased vascular
permeability, recruitment of inflammatory cells, release of
inflammatory mediators and turnover of matrices. That inflammatory
wave is an important regulator for bone regeneration which
initiates the repair cascade (31). Since MSC are implicated in tissue
regeneration by recruitment to the inflammatory sites and the
SDF-1/CXCR4 axis is vital for MSC homing and mobilization, this
axis was selected to be investigated in order to examine the
correlation, if any, to the RIN procedure and the contribution to
fracture healing and callus formation (32,33).
In order to examine the effect of RIN in the MSC BM
pool, samples before and after the surgical procedure were
aspirated from the iliac crest. In addition, blood samples were
collected in parallel for the evaluation of the SDF-1 levels. The
serum levels of SDF-1 were increased in peripheral blood following
RIN and were associated with a decrease in CXCR4 mRNA in BM-MSCs.
Taking into consideration that SDF-1 is a chemokine strongly
chemoattractant to MSCs through its unique receptor CXCR4 (33) and has also been demonstrated highly
expressed at injury sites and enhanced endochondral bone formation
by homing MSCs (34,35), it could be suggested that RIN
induces the rise of SDF-1 serum levels as long as the surgery
represents a traumatic event for bone tissue at the injury site.
Under normal circumstances MSCs and progenitor cells remain in the
BM niche, where they are anchored due to the interaction between
SDF-1 expressed in MSC niche and the CXCR4 receptor which is a
membrane lipid (29). RIN as the
second hit phenomenon promotes an inflammatory wave, provokes the
release of several proteolytic enzymes such as MMPs and neutrophil
elastase by granulocytes and monocytes and the enzymes affect the
integrity of SDF-1/CXCR4 axis connection which acts as anchor for
MSCs thus promoting their mobilization from BM niche (13). The present study showed that SDF-1
levels following RIN were increased which may be explained because
SDF-1 is showed to be stimulated by hypoxic environment such as
during RIN (36). The mobilization
of BM stem cells by interfering with the axis SDF-1/CXCR4
interactions, has been demonstrated to enhance bone regeneration.
SDF-1 secreted by BM-MSCs, bone endothelium, osteoblasts and
stimulated proliferation and growth of B-cell progenitors represent
the soluble factor for CXCR4 receptor at the surface of MSCs. This
event at the initial stage of bone healing is crucial because the
SDF-1 presence promotes osteoblast progenitors migration,
differentiation and bone remodeling (37,38).
Therefore, RIN through MSC migration under the
action of CXCR4/SDF-1 axis may promote the fracture healing
procedure through the gathering of multipotent MSCs to the
‘damaged’ and fractured bone site. The increased SDF-1 serum levels
are reversely correlated to mRNA CXCR4 on BM-MSCs after nailing.
This could possibly indicate mobilization of activated MSCs from
the IC-BM pool. Activation of SDF-1 pathway could be expected due
to inflammatory events that follow the surgical procedure and as a
consequence of tissue damage. Our findings that SDF-1 levels
increased after nailing point to this direction.
The mRNA levels of CXCR4 receptor after RIN,
relative to the levels before the procedure (baseline) were also
investigated. In contrast to the changes in SDF-1 serum levels, no
specific trend in CXCR4 mRNA levels in MSCs following RIN was found
and highly variable, patient-dependent response that included mixed
trend of CXCR4 mRNA levels following RIN was observed (12). CXCR4 expression has been reported
to be down-regulated in aged BM-MSCs, thus reducing their migration
and anti-inflammatory capacity, however, we did not observe any
correlation of patient's age or gender with CXCR4 changes (39). It could be hypothesized that the
increase of SDF-1 expression following RIN, induces a cascade of
mobilization through the decrease in expression of CXCR4 and
subsequently reduction of MSC anchorage within the BM pool
stimulating their migration. In support of this hypothesis we
determined the CXCR4 mRNA expression of MSCs obtained directly from
the site of the fractured limb. They conversely showed higher CXCR4
mRNA level expression compared to MSCs obtained from the iliac
crest following RIN suggesting MSCs which possessed enhanced homing
capacity (40).
The time interval between the incidence of the
fracture and the RIN procedure ranged from 3 to 14 h. This could be
a limitation of our study since CXCR4 change was determined using
BN sample as baseline and if there is a fluctuation in mRNA CXCR4
levels the different time windows among the patients may explain
the mixed trend of their levels compared to baseline CXCR4. It
could be hypothesized that CXCR4 mRNA changes may represent a
different phase of cell migration under the effect of the axis
CXCR4-SDF-1 that occurred prior to RIN. This is supported by
previously reported data that the signals which regulate stem cells
mobilization are often weakened or impaired because the function of
SDF-1 is short lived (41).
There was no correlation between MSC count comparing
the two time points, BN and AN. The absolute number of cells was
not correlated with changes during the RIN. Also the dynamics of
the cells for both samples (BN and AN), as represented by DT, did
not show any statistically significant difference. RIN did not
trigger MSC population to proliferate in a different way at these
specific time points. There was no difference in fracture healing
among the patients and the time to achieve callus formation was
similar. Also, no nonunion or malunion was detected among the
patients.
There are limitations in the present study. First,
the adopted inclusion criteria and the type of bone fracture
limited the number of included patients in the experimental study.
Second, the femur or tibia fractures are not common or frequent
diagnoses. However, this is a first attempt to investigate the
connection of the axis CXCR4/SDF-1 with RIN, which based on the
extracted results, should be expanded to a larger patient group. A
future study could include the characterization of more MSC
migration and homing pathways, which could further illuminate the
molecular and biochemical effects of RIN in the MSC population.
In conclusion, the present study provides evidence
of the effects of RIN on MSC population. The axis CXCR4/SDF-1 which
contributes to MSC migration was selected and the levels of serum
SDF-1 factor were shown to be elevated after the RIN. In addition,
the increased levels of SDF-1 in peripheral blood were reversely
correlated to the mRNA levels of CXCR4 on BM-MSCs after the RIN. It
could be suggested that RIN induce an inflammation cascade through
the phenomenon of second hit which increases the inflammation
response within the BM niche. The enzymes digest the integrity of
CXCR4/SDF-1 complex which is in balance and that event brakes the
anchors of MSCs and facilitate the MSC migration. MSC migration
happens through various expression of CXCR4 receptor at the surface
of stem cells in response to the gradient of SDF-1 by
chemoattaction. SDF-1 is the director of the attraction and
migration of MSCs since it is released also from the injury site.
The recruitment and homing of MSCs are essential for bone healing
since MSC mobilization stimulates angiogenesis and bone
remodeling.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IS, ET, CK, SM and THT collected the samples and
carried out the experiments. IS and ET wrote the manuscript. DAS,
KMA and GK contributed to the conception of the work and provided
critical revision.
Ethics approval and consent to
participate
Ethics committee of the University Hospital of
Heraklion approved the protocol and all patients included in the
study were informed and provided written consent.
Patient consent for publication
All patients provided written informed consent for
publication of the results.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Zelle BA and Boni G: Safe surgical
technique: Intramedullary nail fixation of tibial shaft fractures.
Patient Saf Surg. 9:402015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hansen ST and Winquist RA: Closed
intramedullary nailing of the femur. Küntscher technique with
reaming. Clin Orthop Relat Res. 138:56–61. 1979.
|
|
3
|
Brookes M: Blood supply of long bones.
BMJ. 2:1064–1065. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cuthbertson EM, Siris E and Gilfillan RS:
The femoral diaphyseal medullary venous system as a venous
collateral channel in the dog. J Bone Joint Surg Am. 47:965–974.
1965. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pape HC and Giannoudis P: The biological
and physiological effects of intramedullary reaming. J Bone Joint
Surg Br. 89:1421–1426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wenisch S, Trinkaus K, Hild A, Hose D,
Herde K, Heiss C, Kilian O, Alt V and Schnettler R: Human reaming
debris: A source of multipotent stem cells. Bone. 36:74–83. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choumerianou DM, Dimitriou H and Kalmanti
M: Stem cells: Promises versus limitations. Tissue Eng Part B Rev.
14:53–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mansilla E, Marín GH, Drago H, Sturla F,
Salas E, Gardiner C, Bossi S, Lamonega R, Guzmán A, Nuñez A, et al:
Bloodstream cells phenotypically identical to human mesenchymal
bone marrow stem cells circulate in large amounts under the
influence of acute large skin damage: New evidence for their use in
regenerative medicine. Transplant Proc. 38:967–969. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shyu WC, Lee YJ, Liu DD, Lin SZ and Li H:
Homing genes, cell therapy and stroke. Front Biosci. 11:899–907.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramírez M, Lucia A, Gómez-Gallego F,
Esteve-Lanao J, Pérez-Martínez A, Foster C, Andreu AL, Martin MA,
Madero L, Arenas J, et al: Mobilisation of mesenchymal cells into
blood in response to skeletal muscle injury. Br J Sports Med.
40:719–722. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuznetsov SA, Mankani MH, Gronthos S,
Satomura K, Bianco P and Robey PG: Circulating skeletal stem cells.
J Cell Biol. 153:1133–1140. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jones E and McGonagle D: Human bone marrow
mesenchymal stem cells in vivo. Rheumatology (Oxford). 47:126–131.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ponte AL, Marais E, Gallay N, Langonné A,
Delorme B, Hérault O, Charbord P and Domenech J: The in vitro
migration capacity of human bone marrow mesenchymal stem cells:
Comparison of chemokine and growth factor chemotactic activities.
Stem Cells. 25:1737–1745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ratajczak MZ and Kim C: Bioactive
sphingolipids and complement cascade as new emerging regulators of
stem cell mobilization and homing. J Stem Cell Res Ther. 1:12011.
View Article : Google Scholar
|
|
15
|
Ratajczak MZ: A novel view of the adult
bone marrow stem cell hierarchy and stem cell trafficking.
Leukemia. 29:776–782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan HB, Giannoudis PV, Boxall SA,
McGonagle D and Jones E: The systemic influence of platelet-derived
growth factors on bone marrow mesenchymal stem cells in fracture
patients. BMC Med. 13:62015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Antoniou KM, Papadaki HA, Soufla G,
Kastrinaki MC, Damianaki A, Koutala H, Spandidos DA and Siafakas
NM: Investigation of bone marrow mesenchymal stem cells (BM MSCs)
involvement in Idiopathic Pulmonary Fibrosis (IPF). Respir Med.
104:1535–1542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kastrinaki M-C, Sidiropoulos P, Roche S,
Ringe J, Lehmann S, Kritikos H, Vlahava VM, Delorme B, Eliopoulos
GD, Jorgensen C, et al: Functional, molecular and proteomic
characterisation of bone marrow mesenchymal stem cells in
rheumatoid arthritis. Ann Rheum Dis. 67:741–749. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karagiannis K, Proklou A, Tsitoura E,
Lasithiotaki I, Kalpadaki C, Moraitaki D, Sperelakis I, Kontakis G,
Antoniou KM and Tzanakis N: Impaired mRNA expression of the
migration related chemokine receptor CXCR4 in mesenchymal stem
cells of COPD patients. Int J Inflamm. 2017:60894252017. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pountos I, Jones E, Tzioupis C, McGonagle
D and Giannoudis PV: Growing bone and cartilage. The role of
mesenchymal stem cells. J Bone Joint Surg Br. 88:421–426. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang Y, Jahagirdar BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, et al: Pluripotency of mesenchymal stem cells
derived from adult marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Armstrong MA and Li G: Mesenchymal
stem cells in immunoregulation. Immunol Cell Biol. 84:413–421.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ren G, Zhang L, Zhao X, Xu G, Zhang Y,
Roberts AI, Zhao RC and Shi Y: Mesenchymal stem cell-mediated
immunosuppression occurs via concerted action of chemokines and
nitric oxide. Cell Stem Cell. 2:141–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Chen X, Cao W and Shi Y:
Plasticity of mesenchymal stem cells in immunomodulation:
Pathological and therapeutic implications. Nat Immunol.
15:1009–1016. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zwingenberger S, Yao Z, Jacobi A, Vater C,
Valladares RD, Li C, Nich C, Rao AJ, Christman JE, Antonios JK, et
al: Enhancement of BMP-2 induced bone regeneration by SDF-1α
mediated stem cell recruitment. Tissue Eng Part A. 20:810–818.
2014.PubMed/NCBI
|
|
27
|
Gibon E, Yao Z, Rao AJ, Zwingenberger S,
Batke B, Valladares R, Smith RL, Biswal S, Gambhir SS and Goodman
SB: Effect of a CCR1 receptor antagonist on systemic trafficking of
MSCs and polyethylene particle-associated bone loss. Biomaterials.
33:3632–3638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Granero-Moltó F, Weis JA, Miga MI, Landis
B, Myers TJ, O'Rear L, Longobardi L, Jansen ED, Mortlock DP and
Spagnoli A: Regenerative effects of transplanted mesenchymal stem
cells in fracture healing. Stem Cells. 27:1887–1898. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moll NM and Ransohoff RM: CXCL12 and CXCR4
in bone marrow physiology. Expert Rev Hematol. 3:315–322. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Giannoudis PV, Smith RM, Bellamy MC,
Morrison JF, Dickson RA and Guillou PJ: Stimulation of the
inflammatory system by reamed and unreamed nailing of femoral
fractures. An analysis of the second hit. J Bone Joint Surg Br.
81:356–361. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Claes L, Recknagel S and Ignatius A:
Fracture healing under healthy and inflammatory conditions. Nat Rev
Rheumatol. 8:133–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gerstenfeld LC, Cullinane DM, Barnes GL,
Graves DT and Einhorn TA: Fracture healing as a post-natal
developmental process: Molecular, spatial, and temporal aspects of
its regulation. J Cell Biochem. 88:873–884. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aiuti A, Webb IJ, Bleul C, Springer T and
Gutierrez-Ramos JC: The chemokine SDF-1 is a chemoattractant for
human CD34+ hematopoietic progenitor cells and provides
a new mechanism to explain the mobilization of CD34+
progenitors to peripheral blood. J Exp Med. 185:111–120. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zlotnik A and Yoshie O: The chemokine
superfamily revisited. Immunity. 36:705–716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yellowley C: CXCL12/CXCR4 signaling and
other recruitment and homing pathways in fracture repair. Bone key
Rep. 2:3002013.
|
|
36
|
Mohyeldin A, Garzón-Muvdi T and
Quiñones-Hinojosa A: Oxygen in stem cell biology: A critical
component of the stem cell niche. Cell Stem Cell. 7:150–161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xing Z, Lu C, Hu D, Yu YY, Wang X, Colnot
C, Nakamura M, Wu Y, Miclau T and Marcucio RS: Multiple roles for
CCR2 during fracture healing. Dis Model Mech. 3:451–458. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shirley D, Marsh D, Jordan G, McQuaid S
and Li G: Systemic recruitment of osteoblastic cells in fracture
healing. J Orthop Res. 23:1013–1021. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baker N, Boyette LB and Tuan RS:
Characterization of bone marrow-derived mesenchymal stem cells in
aging. Bone. 70:37–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rombouts WJC and Ploemacher RE: Primary
murine MSC show highly efficient homing to the bone marrow but lose
homing ability following culture. Leukemia. 17:160–170. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wei FY, Leung KS, Li G, Qin J, Chow SK,
Huang S, Sun MH, Qin L and Cheung WH: Low intensity pulsed
ultrasound enhanced mesenchymal stem cell recruitment through
stromal derived factor-1 signaling in fracture healing. PLoS One.
9:e1067222014. View Article : Google Scholar : PubMed/NCBI
|