Introduction
Ovarian cancer (OVCA) has a high mortality rate of
30–50%, is the fifth most common type of cancer worldwide, and is
also the cause of the majority of cancer-related mortality in women
(1,2). Chemotherapy is the standard treatment
for OVCA; however, 70% of patients with terminal cancer experience
chemoresistance or recurrence within 15 months of treatment
(3). Currently, chemotherapy is
extensively used to treat OVCA. However, certain antitumor drugs,
including cisplatin and sorafenib, can have serious side effects
that limit their clinical applications (4,5).
Thus, exploration of new drugs or active compounds for the therapy
of OVCA is urgent.
Steroid hormones are very important for the
development of OVCA, as the progesterone receptor (PR) and estrogen
receptor (ER) have been confirmed to be associated with survival
rate and prognosis of patients with cancer (6,7). The
ERs, including the two ER subtypes ERα and ERβ, have been widely
studied in OVCA (8,9). The two ER subtypes have different
specificity in ligand binding, with opposing functions in cell
growth in some types of cancer (10,11).
ERα and a gene target of estrogen signaling, PR, are prognostic
biomarkers in OVCA. It has been elucidated that 40–60% of OVCA
cases express ERα, but only a small proportion of patients respond
to ER antagonist tamoxifen therapy (12). Previous studies have suggested, in
contrast to ERα, that ERβ expression is significantly lower in
malignant tissue compared with normal tissue, and its expression is
a useful predictor for disease-free survival and overall survival
(13,14). Previous studies have indicated that
the expression level of ERβ is decreased in localized prostate
cancer suggesting that ERβ may be a suppressor gene (14). Functionally, exogenous expression
of ERβ in OVCA can inhibit cell proliferation and increase cell
apoptosis via promoting the degradation of hypoxia-inducible factor
(HIF)-1α (15). Vascular
endothelial growth factor A (VEGFA) signaling was inhibited by low
levels of HIF-1α, which is a critical component in preventing
apoptosis and motility of tumor cells. In short, the activation of
ERβ expression may be an effective target for the exploration of
compounds to treat OVCA (16,17).
Traditional Chinese medicines have been used to
treat cancer for thousands of years, using a large collection of
biologically active products (18–21).
Natural compounds from medicinal plants, including dioscin and
berberine, have potent effects against various types of cancer
(22–28); it is reported that berberine
inhibited human cervical carcinoma HeLa cells and dioscin induced
DNA damage and activated mitochondrial signal pathway in human
A549, NCI-H446 and NCI-H460 cancer cell lines (29,30).
Additionally, some natural products, including genistein and
daidzein, have been reported to inhibit migration, invasion and
proliferation of OVCA cells (31).
Thus, exploration of effective natural products from medicinal
plants is a reasonable approach to treat OVCA.
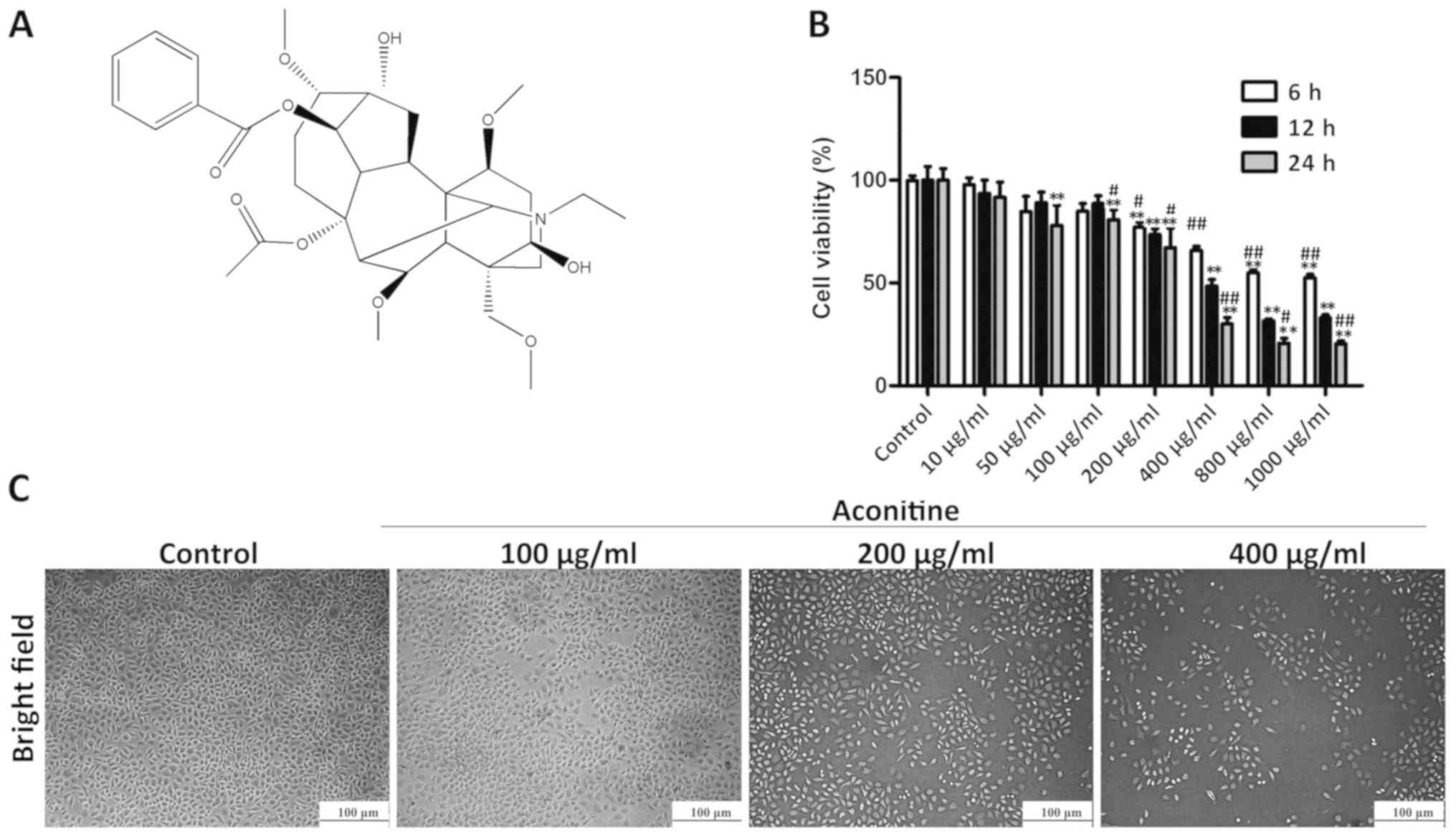
Aconitine (Fig.
1A), also known as monkshood or devil's helmet, is a type of
toxin produced by the Aconitum plant that has been used as a
traditional medicine in China due to its analgesic and
anti-inflammatory effects (32). A
previous study has indicated that aconitine can induce apoptosis in
human pancreatic cancer cells via the NF-κB signaling pathway
(33). In addition, aconitine can
induce apoptosis in human cervical carcinoma HeLa cells by
adjusting endoplasmic reticulum stress signaling (34). However, no previous studies have
reported the effects and molecular mechanisms of aconitine against
OVCA, and therefore, the aim of the current study was to
investigate this compound in OVCA cells in vitro.
Materials and methods
Chemicals and reagents
Aconitine was obtained from the Chengdu Research
Institute of Biology of the Chinese Academy of Sciences. MTT was
obtained from Roche Diagnostics. Protein Extraction kit (cat. no.
KGP250), penicillin and streptomycin combination were purchased
from Nanjing KeyGen Biotech Co., Ltd. Dulbecco's modified Eagle's
medium (DMEM) and fetal bovine serum (FBS; cat. no. 10099-141) were
purchased from Gibco; Thermo Fisher Scientific, Inc.
4′,6′-Diamidino-2-phenylindole (DAPI) was purchased from
Sigma-Aldrich; Merck KGaA. Comet assay kit (cat. no. 4250) was
purchased from Cell Biolabs, Inc. The TUNEL assay kit (cat. no.
FA201) was purchased from Beijing Transgen Biotech Co., Ltd. JC-1
mitochondrial membrane potential assay kits (cat. no. C2006) were
obtained from Beyotime Institute of Biotechnology. Primary and
secondary antibodies were purchased from ProteinTech Group or BIOSS
and are presented in Table I. BSA
blocking buffer was purchased from Beijing Solarbio Science &
Technology Co, Ltd (cat. no. SW3015).
 | Table I.Antibodies used in the present
study. |
Table I.
Antibodies used in the present
study.
| Antibody | Source | Dilution | Company | Catalog number |
|---|
| ERβ | Rabbit | 1:1,000 | ProteinTech Group,
Inc. | 21244-1-AP |
| VEGF-A | Rabbit | 1:1,000 | ProteinTech Group,
Inc. | 19003-1-AP |
| HIF-α | Rabbit | 1:500 | ProteinTech Group,
Inc. | 20960-1-AP |
| PHD2 | Rabbit | 1:500 | ProteinTech Group,
Inc. | 19886-1-AP |
| p53 | Rabbit | 1:1,000 | ProteinTech Group,
Inc. | 10442-1-AP |
| Bax | Rabbit | 1:1,000 | ProteinTech Group,
Inc. | 50599-2-Ig |
| Bcl-2 | Rabbit | 1:1,000 | ProteinTech Group,
Inc. | 60178-1-Ig |
| Bcl-xl | Rabbit | 1:1,000 | ProteinTech Group,
Inc. | 26967-1-AP |
| Apaf-1 | Rabbit | 1:1,000 | ProteinTech Group,
Inc. | 21710-1-AP |
| Cleaved
Caspase-3 | Rabbit | 1:1,000 | ProteinTech Group,
Inc. | 19677-1-AP |
| Cleaved
Caspase-9 | Rabbit | 1:1,000 | ProteinTech Group,
Inc. | 10380-1-AP |
| Cleaved PARP | Rabbit | 1:1,000 | ProteinTech Group,
Inc. | 13371-1-AP |
| Cytochrome C | Rabbit | 1:1,000 | ProteinTech Group,
Inc. | 66264-1-Ig |
| MMP2 | Rabbit | 1:1,000 | ProteinTech Group,
Inc. | 10373-2-AP |
| MMP9 | Rabbit | 1:1,000 | ProteinTech Group,
Inc. | 10375-2-AP |
| ATM | Rabbit | 1:500 | BIOSS | bs-1370R |
| p-ATM | Rabbit | 1:500 | BIOSS | bs-12545R |
| Coralite
594-conjugated AffiniPure Goat Anti-Rabbit IgG (H+L) | Rabbit | 1:2,000 | ProteinTech Group,
Inc. | SA00013-4 |
| Horse radish
peroxide-conjugated AffiniPure Goat Anti-Rabbit IgG (H+L) | Rabbit | 1:2,000 | Proteintech Group,
Inc. | SA00001-2 |
Cell lines and culture
The human OVCA cell lines, A2780 and normal ovarian
cell IOSE80, were purchased from Wuhan Boster Biological
Technology, Ltd. A2780 and IOSE80 cells were cultured in DMEM
medium in the presence of 10% FBS, supplemented with 100 U/ml
penicillin and 100 g/ml streptomycin, and cultured in a humidified
5% (v/v) atmosphere of CO2 in an incubator at 37°C.
Cell viability assay
A cell viability assay was performed using MTT.
Cells were plated into 96-well plates (5×104 cells/well)
and incubated at 37°C overnight. In the present study the
concentrations of aconitine used in the cell viability assay were
10, 50, 100, 200, 400, 800 and 1,000 µg/ml. 5×104
cells/well A2780 cells and normal ovarian IOSE80 cells were then
treated with various concentrations of aconitine (10, 50, 100, 200,
400, 800 and 1,000 µg/ml) and positive control cisplatin (1, 5, 25,
50 and 100 µg/ml) for different exposure times of 6, 12 and 24 h.
Next, 10 µl of MTT (5 mg/ml) was added to each well and incubated
for a further 4 h at 37°C. Following this, 150 µl DMSO was added to
dissolve formazan crystals, the absorbance was read at 490 nm using
a microplate reader (Thermo Fisher Scientific, Inc.), and cell
morphology was obtained with a phase-contrast microscope (Nikon
Corporation).
Plate colony-forming assay
A2780 cells in 6-well plates at a density of 500
cells per well were treated with aconitine (0, 100, 200 and 400
µg/ml) once every 3 days. The treated cells were incubated at 37°C
for 10 days, and the medium was changed every day. Finally, the
cells were stained with crystal violet at room temperature for 30
min and then, colonies containing >50 cells were counted. The
colony formation numbers were analyzed using ImageJ software 1.3
(National Institutes of Health).
Wound healing assay
A2780 cells (2×105 cells/well) were
cultured for 12 h at 37°C, then the cells were scratched with a
sterile micro-pipette tip and washed with PBS to remove cell debris
in the serum-free medium. After cells were treated with aconitine
(0, 25, 50 and 100 µg/ml) at 37°C for 24 h, the non-adherent cells
were washed away with PBS and the migration distance was taken and
analyzed using ImageJ software 1.3. The morphology of the cells was
obtained by a phase contrast microscope (Nikon Corporation) at ×200
magnification.
Transwell migration assay
The migration potential of A2780 cells was assessed
using Transwell chambers (8 µm pore; Corning Inc.). A total of
6×104 cells in serum-free medium (200 µl) were added to
the upper chamber, while the lower chamber was filled with 500 µl
medium containing 20% FBS. After incubation with aconitine (0, 25,
50 and 100 µg/ml) in the upper chamber for 12 h at 37°C, the cells
were fixed with methanol for 30 min and stained with crystal violet
for 20 min at room temperature. Finally, cells were imaged under an
inverted phase-contrast microscope and ImageJ software 1.3
(National Institutes of Health) was used for quantification.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
Early and late apoptosis apoptosis was determined
using the TransDetect® In Situ Fluorescein TUNEL
Cell Apoptosis Detection kit according to the manufacturer's
protocols. The solution of 10% green fluorescein labeled dUTP was
added to 6×104 A2780 cells that were incubated at 37°C
for 1 h. Next, the samples were washed with cell permeable fluid
and neutral gum sealer was used as the mounting medium. Images of
five random fields of view were captured using a fluorescence
microscope (Olympus Corporation) at ×200 magnification.
Mitochondrial membrane potential
assay
6×104 A2780 cells were incubated with
JC-1 dye working fluid in an incubator for 20 min at 37°C. After
washing twice with a dye buffer, the JC-1 levels were determined by
fluorescence microscope at ×200 magnification.
Comet assay
The culture and treatment of A2780 cells were the
same as TUNEL staining. After the treatment with different
concentrations of aconitine (100, 200 and 400 µg/ml) for 24 h, the
extent of DNA damage was determined by the Comet assay kit (Cell
Biolabs, Inc.), according to the manufacturer's instructions.
Images were taken using a fluorescence microscope and the Comet
Assay Software Project (CASP) 1.2.2 (CaspLab) was used to analyze
50 cells from each of the 2 replicate slides.
Immunofluorescence assay
A total of 6×104 A2780 cells were
incubated with DMEM in a 6-well plate at 37°C overnight. After
pretreatment, the cells were washed with PBS, fixed with 4%
paraformaldehyde for 15 min at 4°C, then washed with PBS and
permeabilized with 0.2% Triton-100 for 8 min at 4°C. Non-specific
binding was blocked by incubating cells in 3% BSA for 1 h at 37°C
and then cells were incubated with rabbit anti-ERβ primary antibody
(1:500) at 4°C overnight. After washing with PBS three times, the
samples were incubated with a FITC-conjugated goat anti-rabbit IgG
(1:2,000) for 1 h at room temperature, and then DAPI (5 µg/ml) was
used to stain the cell nucleus for 10 min at 4°C. Images were
captured using a fluorescence microscope at ×200 magnification. The
cells in five randomly selected high-power fields were counted
under the fluorescence microscope and relative fluorescence
intensity (total fluorescence intensity/area) represented the
fluorescence intensity of the positive cells compared with the
control group.
Western blotting
Total protein samples from A2780 cells were
extracted by cell lysis buffer containing 1% phenylmeth-anesulfonyl
fluoride (PMSF). A bicinchoninic acid assay was performed to
determine the protein content. The samples (50 ng/µl) were
separated by SDS-PAGE on 10–15% gels and transferred to a PVDF
membrane. Following this, the membranes were incubated with primary
antibodies (listed in Table I) at
4°C overnight. The membranes were then incubated with horseradish
peroxidase-conjugated secondary antibody (1:2,000) for 2 h at room
temperature. Subsequently, protein bands were detected using an
enhanced chemiluminescence system and ChemiDoc XRS (Bio-Rad
Laboratories, Inc.). Intensity values of the relative expression
levels of the proteins were normalized to GAPDH.
Molecular docking assay
The 3-dimensional (3D) structure of aconitine was
optimized using the Gaussian 09 program (35) with the level of B3LYP/6-31G
(36,37). The Protein Data Bank (PDB) files of
aconitine were produced using PRODRG Server and the crystal
structure of ERβ (PDB-code, 4GV1) was downloaded from RCSB Protein
Data Bank (38). The docking
studies on the proteins ERβ (PDB, 2YLY) and ligand aconitine were
performed with the AutoDock 4.2 software (Aka Olson Laboratory),
whose process mainly involves the removal of crystal water, ions
and non-standard amino-acid residues. The binding free energy and
the actions of hydrogen bonds, hydrophobic and electrostatic, were
analyzed. Following the requirements of the docking study, ions,
water molecules and non-standard amino acid residues were removed
from the proteins. For the docking case, the model with the lowest
energy was selected as the binding mode for analysis.
Statistical analysis
All data are presented as mean ± SD. Statistical
analysis was performed using GraphPad Prism 5.0 software (GraphPad
Software, Inc.). Comparisons between two groups were performed
using unpaired Student's t-test, and one-way or two-way ANOVA was
followed by Bonferroni's post hoc to analyze multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cytotoxicity of aconitine in A2780
cells
The MTT results presented in Fig. 1B show that aconitine treatment at
the concentrations of 100, 200 and 400 µg/ml for 24 h significantly
decreased cell viability to 85, 68 and 33%, respectively, compared
with the control group, while effects of positive control cisplatin
on the viability of A2780 cells were detected for 24 h in Fig. S1A. However, aconitine did not
significantly inhibit the growth of a normal ovarian cell line
(Fig. S1B). For subsequent
experiments, the cells were exposed to 100, 200 or 400 µg/ml
aconitine for 24 h. As shown in Fig.
1C, bright-field images suggested that cell death was induced
by aconitine.
Effects of aconitine on proliferation,
invasion and migration of A2780 cells
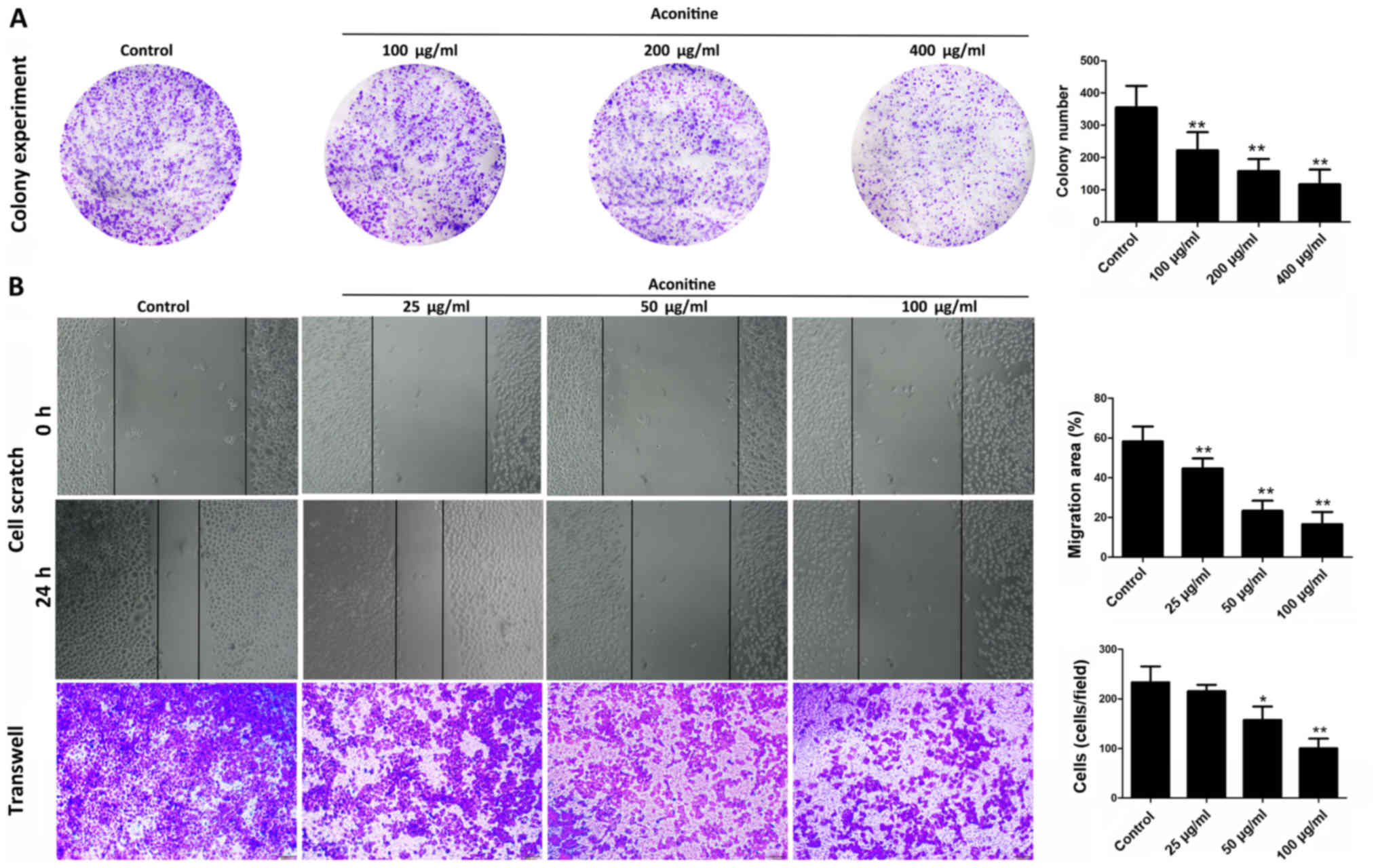
As shown in Fig.
2A, aconitine (100, 200 and 400 µg/ml) significantly decreased
colony formation in A2780 cells. In addition, only low
concentrations of aconitine were needed to suppress the invasive
and migratory capabilities of A2780 cells compared with the control
group, as demonstrated by migration and invasion assays (Fig. 2B) which excluded the influence of
aconitine-induced reduction of cell viability to the experimental
results of cell migration and invasion (29). These results suggested that
aconitine significantly inhibited the proliferation, migration and
invasion of A2780 cells (Fig.
2B).
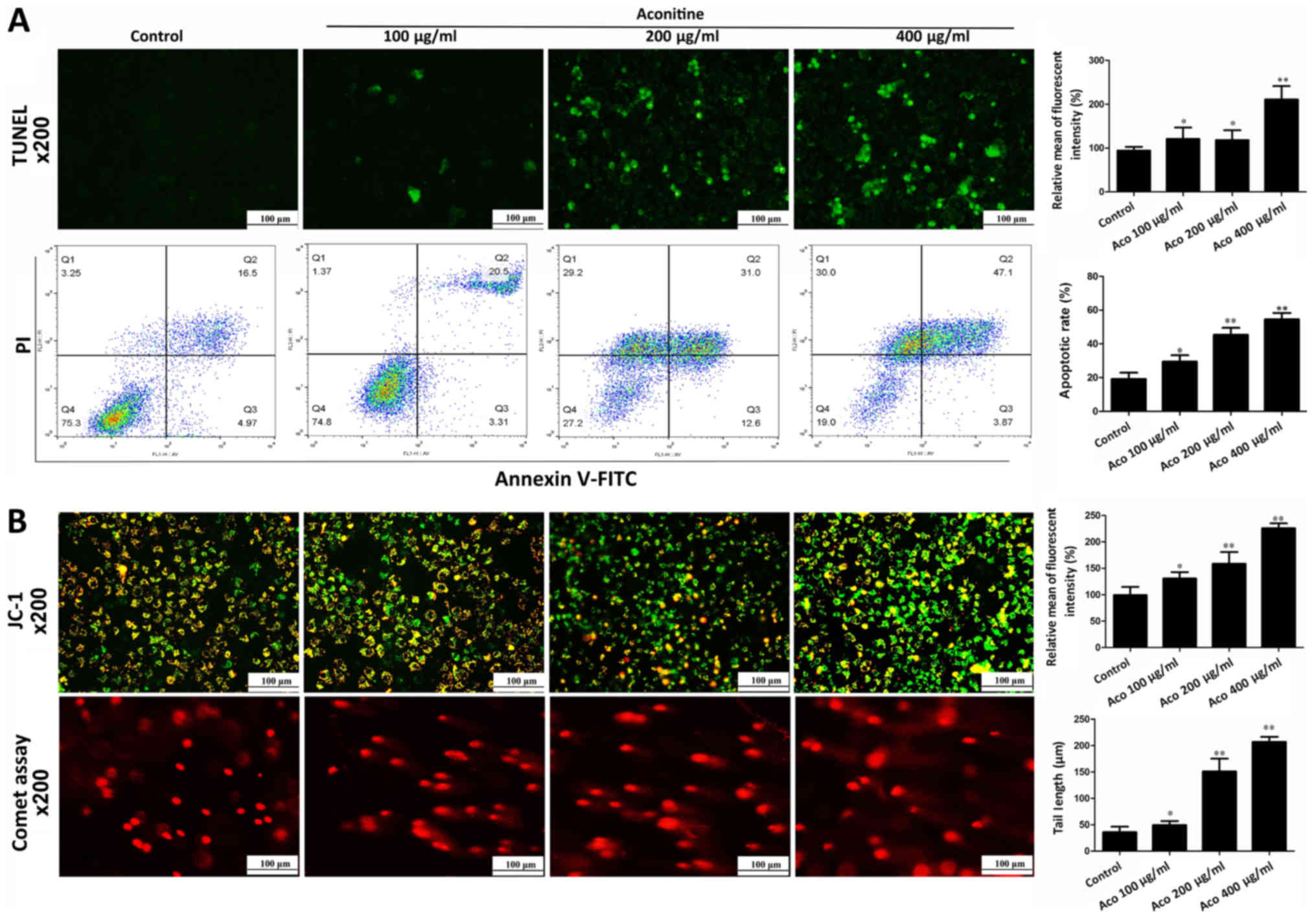
Aconitine induces apoptosis and DNA
damage in A2780 cells
To investigate the effect of aconitine on cell
apoptosis, apoptosis was measured by TUNEL assay and flow
cytometry. As presented in Fig.
3A, TUNEL-positive cells and apoptotic rates were significantly
increased by aconitine. In addition, the results of jc-1 stain
demonstrated that the intensities of red orange fluorescence in
aconitine-treated groups were significantly decreased and the
intensities of green fluorescence were increased compared with the
control group, suggesting that aconitine increased mitochondrial
membrane potential to induce mitochondria injury (Fig. 3B). In a comet assay, aconitine
decreased the contents of head DNA and increased the length of DNA
migration smear (comet tail) compared with the control group
(Fig. 3B).
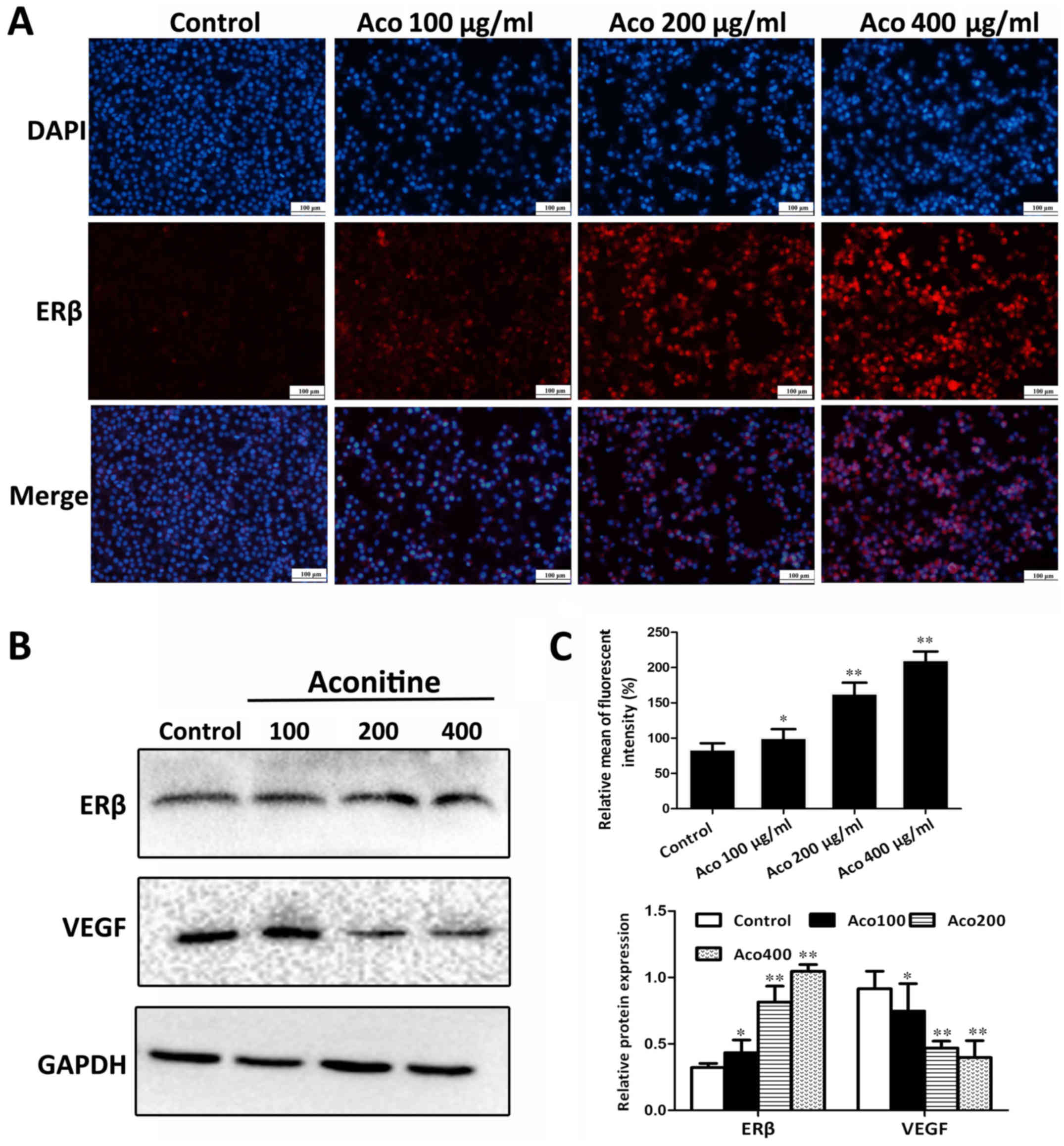
Aconitine activates the expression of
ERβ in vitro
To investigate the impact of aconitine on ERβ
signaling, A2780 cells were treated with different concentrations
of aconitine and the expression levels of ERβ were assessed by
immunofluorescence. The results presented in Fig. 4A and C indicated that, compared
with the control group, the ERβ fluorescent intensity was
significantly increased. In addition, the western blot analysis
results in Fig. 4B indicated that
aconitine significantly upregulated the expression of ERβ, and
significantly downregulated the expression of VEGF compared with
the control group.
Aconitine activates ERβ signaling and
inhibits expression of matrix metalloproteinase (MMP) 2/9 and
phosphorylated ATM serine/threonine kinase (p-ATM)
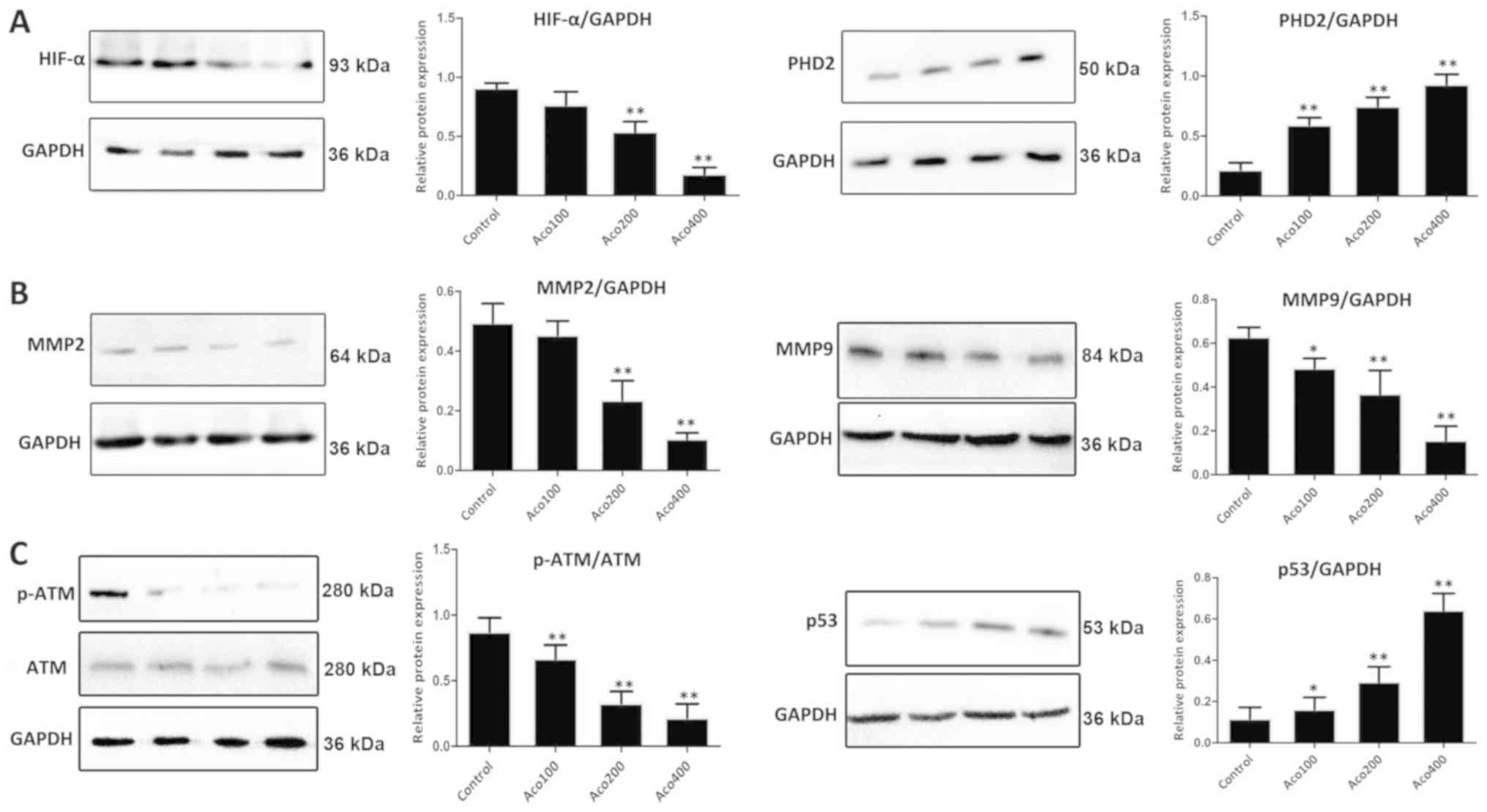
As presented in Fig.
5A, western blot analysis revealed that expression of prolyl
hydroxylase domain-containing protein 2 (PHD2) was significantly
upregulated, and that expression of HIF-1α was markedly
downregulated by aconitine compared with the control group. These
results suggested that aconitine inhibited the VEGF signaling
pathway by activating ERβ. Furthermore, compared with the control
group, aconitine significantly increased the expression of p53 and
inhibited the expression of MMP2, MMP9 and p-ATM (Fig. 5B-C).
Aconitine induces the expression of
proteins associated with the mitochondria-associated apoptosis
pathway
As shown in Fig. 6,
compared with the control group, aconitine significantly
upregulated the levels of cleaved poly (ADP-ribose) polymerase
(PARP), cleaved caspase-3/9 and Bax, and downregulated the
expression of Bcl-2 and Bcl-xl in A2780 cells. In addition, the
expression of apoptotic peptidase activating factor 1 (Apaf-1) and
cytochrome C were significantly increased by the compound.
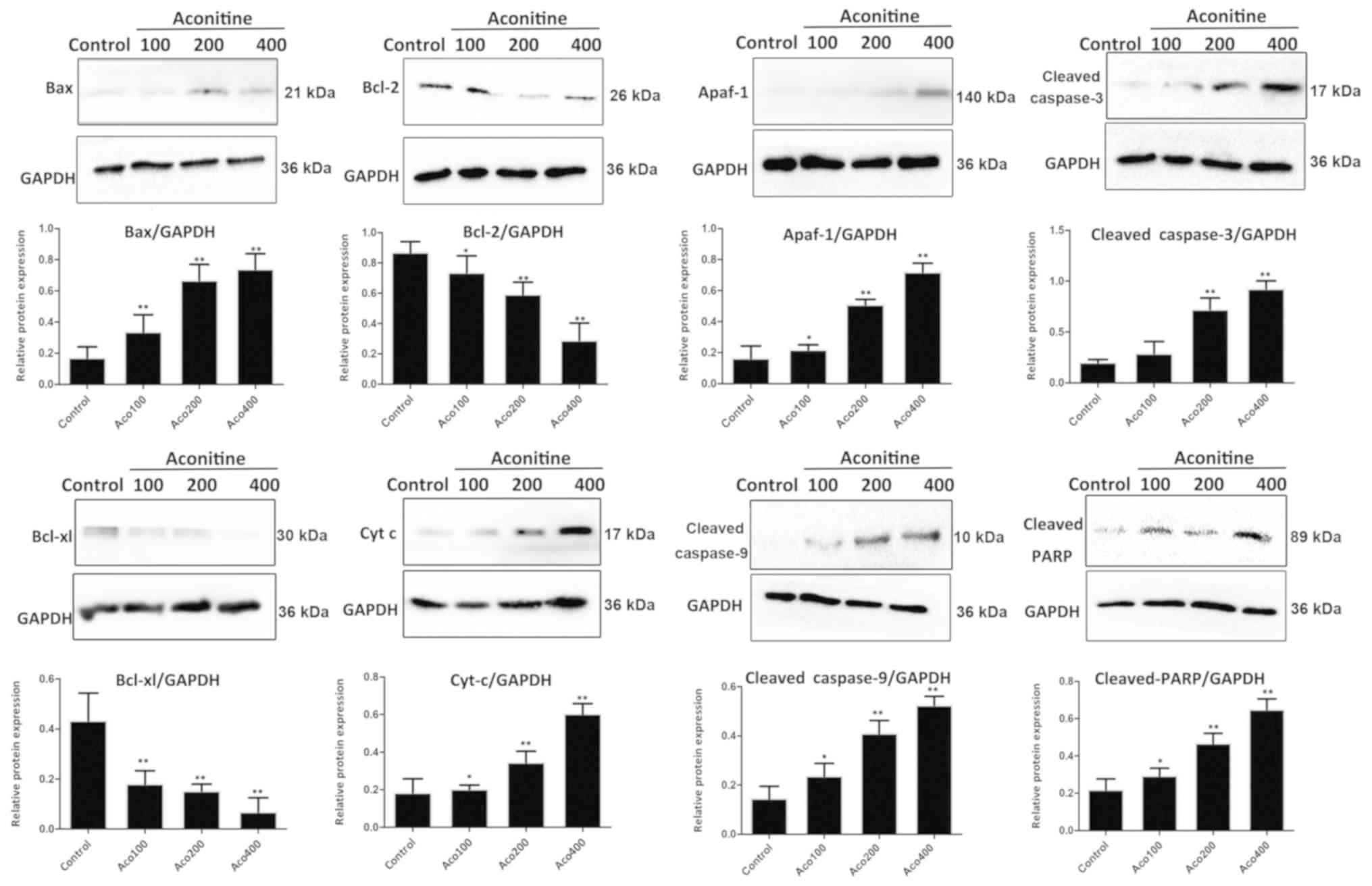 | Figure 6.Effects of aconitine on the
expression levels of proteins associated with the mitochondria
pathway, including Bax, Bcl-2, Apaf-1, cleaved caspase-3, cleaved
caspase-9, Bcl-xl, Cyt C and cleaved PARP. Data are presented as
mean ± SD of three independent experiments. *P<0.05, **P<0.01
vs. control group. Aco, aconitine; Apaf-1, apoptotic peptidase
activating factor 1; Cyt C, cytochrome C; PARP, poly (ADP-ribose)
polymerase. |
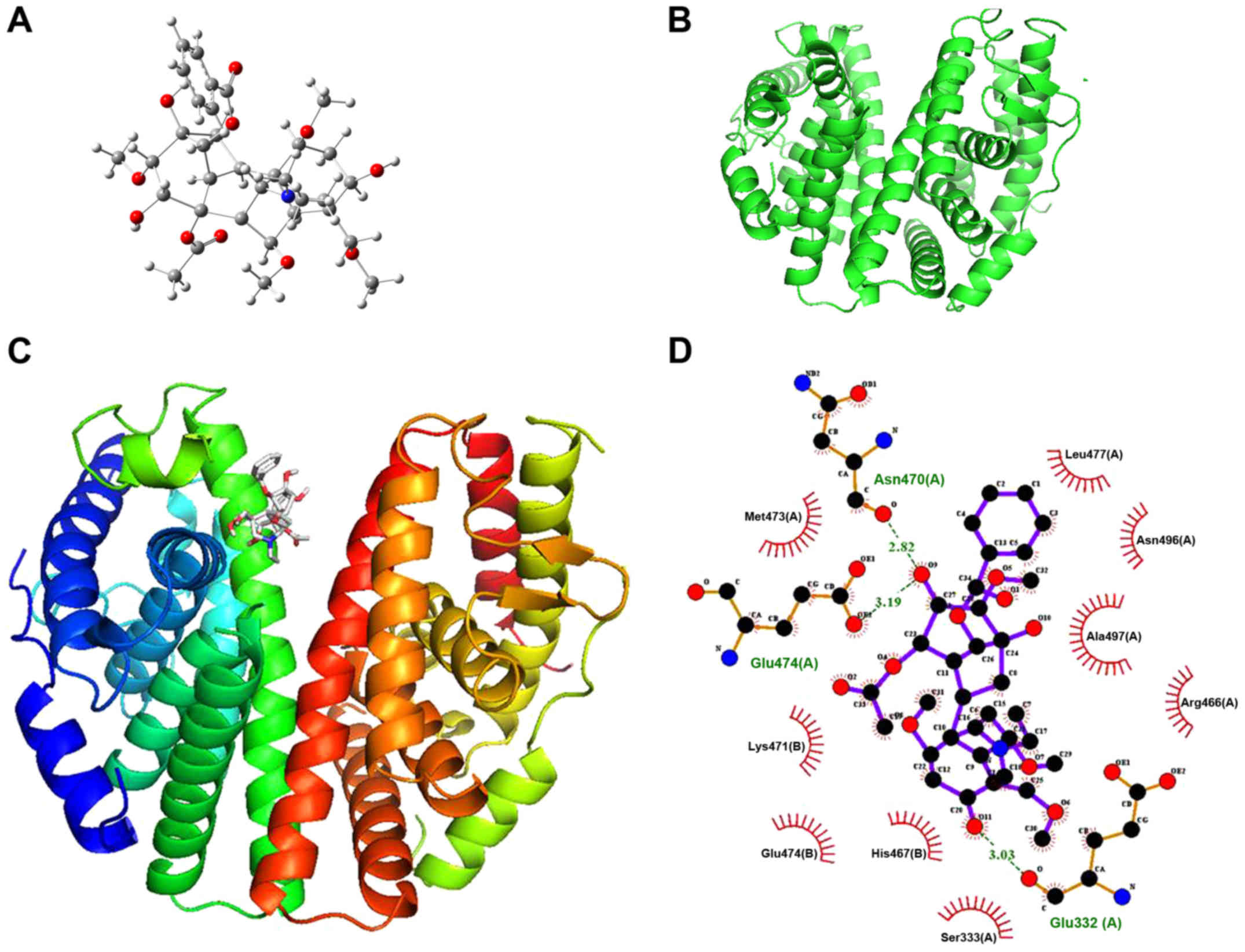
Aconitine directly targets ERβ
A molecular docking assay was performed on ERβ to
investigate the target of aconitine against OVCA. The 3D structure
of aconitine and the crystal structure of ERβ (PDB ID, 2YLY) are
shown in Fig. 7A and B, and the
binding mode of aconitine and ERβ is shown in Fig. 7C. By molecular docking, the binding
energy of aconitine towards ERβ was found to be −6.5 kcal/mol. In
addition, the hydrogen bonding model of aconitine and the ERβ
structure are presented in Fig.
7D, and the analyses of hydrogen bonding and hydrophobic
effects showed that the amino acid residues involved in the
formation of hydrogen bonds included glutamic-474 (Glu-474),
asparagine-470 (Asn-470) and Glu-332 (Glu-332).
The hydrophobic bond formation of aconitine-ERβ
bonding and the hydrophobic residues within the ERβ active site
involves methionine-473, lysine-471, Glu-474, histidine-467,
serine-333, arginine-466, alanine-497, Asn-496 and leucine-477,
which further strengthens the binding of aconitine to ERβ. From the
docking results, due to the strong hydrogen bonding, hydrophobic
effects and electrostatic interactions, aconitine with lower
binding energy possessed powerful affinity towards ERβ.
Discussion
OVCA is one of the most prevalent gynecological
malignancies, with a global fatality rate of 6.2% (39). Although >70% of patients with
OVCA can be effectively treated with surgery, novel and more
effective drug interventions are needed due to the serious side
effects of chemotherapy and low overall cure rates (40). As an active ingredient in the
Aconitum plant, aconitine has been reported to interact with
voltage-dependent sodium channels of excitable tissue cell
membranes and can be metabolized by cytochrome P450 (41). However, few studies have reported
the effects of aconitine on tumorigenesis. Therefore, the
inhibitory effect of aconitine on human ovarian A2780 cells was
investigated in the current study. The results suggested that cell
viability and colony formation of A2780 cells were significantly
suppressed by aconitine. In addition, low concentrations of
aconitine (25, 50 and 100 µg/ml) were found to significantly
suppress the invasive and migratory capabilities of A2780 cells,
although these concentrations did not induce significant rates of
cell death according to the MTT results. These results suggested
that aconitine may have potential as a treatment for OVCA.
Cytogenetic analysis showed that A2780 cells had a greater genomic
stability in comparison with SKOV3 cells (42). On the other hand, A2780 cells
undergo apoptosis and consequently cell death more easily than
SKOV3. Thus, A2780 was chosen as the cell line to confirm the
research data.
The collapse of mitochondrial function, including
changes to the mitochondrial membrane potential and permeability,
are associated with apoptosis (43). A previous study reported that the
collapse of the mitochondrial membrane potential is an early step
in apoptosis. Also, DNA fragmentation is one of the most typical
phenomena of apoptosis (44). In
the present study, aconitine was found to cause the disintegration
of the mitochondrial membrane potential and the transformation of
mitochondrial permeability. The comet assay showed that after
aconitine treatment, the DNA content was transferred to the tail of
the comet, suggesting that aconitine treatment induced DNA damage.
In addition, the number of apoptotic cells was increased by
aconitine treatment. These data showed that aconitine had potent
activity against OVCA cells in vitro by regulating DNA
damage and mitochondrial apoptosis.
A wide range of expression levels of steroid hormone
receptors has been reported in OVCA. In previous years, ERβ
targeting has attracted attention in various types of tumors due to
its anticancer effect (45). With
the decreased expression of ERβ in breast, colon and prostate
cancer, the receptor is considered to be a tumor suppressor factor
(6,7,46).
ERβ has the highest expression in normal ovarian tissues, and this
is decreased during dedifferentiation (47). A previous study has demonstrated
that the decrease of ERβ can stabilize HIF-1α, which can lead to
autocrine VEGF signaling by decreasing the enzyme activity of PHD2,
the key hydroxylase of HIF-1α which suggested that ERβ is an
important part of this process in tumorigenesis (14). As HIF-1α/VEGF signaling is an
important regulator of cell migration and apoptosis, apoptosis can
be promoted, and migration and invasion can be decreased by
activation of ERβ (29). The
results of the present study demonstrated that aconitine
significantly upregulated the expression of ERβ in A2780 cells, and
decreased the expression of HIF-1α and VEGF-A. Therefore, the
anticancer effect of aconitine in OVCA cells may result primarily
from ERβ activation.
Apoptosis serves important roles in eliminating
unwanted or damaged cells in a number of physiological and
pathological conditions such as hypoxia and inflammation (48), and the Bcl-2 family contains 2
classes, antiapoptotic members and proapoptotic members (49). Bcl-2 is an antiapoptotic member.
Bax, a proapoptotic member, is mainly distributed on the
mitochondrial membrane, and the loss of mitochondrial membrane
potential is closely related to the release of cytochrome C
(46). Increased ERβ and PHD2 can
induce apoptosis via adjusting caspase-3 and Bax/Bcl-2 signals
in vitro and in vivo (27). MMPs are a family of zinc-dependent
proteinases involved in the degradation of the extracellular matrix
and the basement membrane component, and they are associated with
the invasion and metastasis of malignant tumors (50). Recent studies have demonstrated
that ERβ gene knockdown augments cell proliferation, migration and
invasion by regulating cyclin D1 and MMP2 (51). ATM is a serine/threonine kinase
that regulates DNA damage by breaking down into active monomers
(51). Previous studies revealed
that ERβ is required for optimal chemotherapy-induced DNA damage
and apoptosis through activation of ATM during the DNA damage
response, suggesting that ERβ may function as a tumor suppressor
via ATM signaling (52,53). The results of the present study
indicated that aconitine decreased the mitochondrial membrane
potential and induced mitochondria injury in A2780 cells, and
induced apoptosis via adjusting the expression of p53, cytochrome
C, cleaved caspases-3/9, Bax/Bcl-2, Bcl-xl, Apaf-1 and cleaved
PARP. Notably, the expression levels of MMP2, MMP9 and p-ATM were
decreased by aconitine, which may inhibit the development and
progression of tumor metastasis and induce DNA damage. In addition,
the molecular docking assay results further showed that aconitine
possessed powerful affinity towards ERβ, indicating that the
compound may directly target the protein for its biological
activities. Previously, an ectopic implantation model in nude mice
was established to investigate the role of aconitine in vivo
and the trial suggested that aconitine showed an antimelanoma
effect in suppressing tumor growth in vivo (54). However, there is no report
concerning the antitumor effects of aconitine in OVCA animal
models. A previous study has demonstrated that processing (usually
boiling) of crude aconite roots decreases the number of toxic
alkaloids and increases the concentration of lipo-alkaloids,
suggesting that toxic aconite alkaloids cannot be responsible for
the anticancer activity, but lipo-alkaloids may be (55). Therefore, in the next study we will
work on chemical modification of the drug to decrease the toxicity
through lipolyzing in order to make it applicable in
vivo.
In conclusion, the current study has demonstrated
that aconitine decreased cell viability, tumor metastasis and
induced apoptosis and DNA damage in A2780 cells via ERβ-mediated
signaling (Fig. S2). Further
research is required to investigate the mechanisms, drug targets
and clinical applications of aconitine against OVCA.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW designed the experiments and wrote the
manuscript. YL performed data analysis. YZ performed the western
blotting assay. XW and YZ edited the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vang R, Shih IM and Kurman RJ: Ovarian
Low-grade and High-grade Serous Carcinoma. Pathogenesis
clinicopathologic and molecular biologic features and diagnostic
problems. Adv Anatomic Pathol. 16:267–282. 2009. View Article : Google Scholar
|
|
2
|
Liu J, Zhang Z, Guo Q, Dong Y, Zhao Q and
Ma X: Syringin prevents bone loss in ovariectomized mice via TRAF6
mediated inhibition of NF-κB and stimulation of PI3K/AKT.
Phytomedicine. 42:43–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han CY, Patten DA, Richardson RB, Harper
ME and Tsang BK: Tumor metabolism regulating chemosensitivity in
ovarian cancer. Genes Cancer. 9:155–175. 2018.PubMed/NCBI
|
|
4
|
Hainsworth JD, Thompson DS, Bismayer JA,
Gian VG, Merritt WM, Whorf RC Finney LH and Dudley BS:
Paclitaxel/carboplatin with or without sorafenib in the first-line
treatment of patients with stage III/IV epithelial ovarian cancer:
A randomized phase II study of the Sarah Cannon Research Institute.
Cancer Med. 4:673–681. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43-9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Treeck O, Lattrich C, Springwald A and
Ortmann O: Estrogen receptor beta exerts growth-inhibitory effects
on human mammary epithelial cells. Breast Cancer Res Treat.
120:557–565. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Treeck O, Juhasz-Boess I, Lattrich C, Horn
F, Goerse R and Ortmann O: Effects of exon-deleted estrogen
receptor beta transcript variants on growth, apoptosis and gene
expression of human breast cancer cell lines. Breast Cancer Res
Treat. 110:507–520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan KKL, Siu MKY, Jiang YX, Wang JJ,
Leung THY and Ngan HYS: Estrogen receptor modulators genistein,
daidzein and ERB-041 inhibit cell migration, invasion,
proliferation and sphere formation via modulation of FAK and
PI3K/AKT signaling in ovarian cancer. Cancer Cell Int. 18:652018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leung YK, Mak P, Hassan S and Ho SM:
Estrogen receptor (ER)-beta isoforms: A key to understanding
ER-beta signaling. Proc Natl Acad Sci USA. 103:13162–13167. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schuler-Toprak S, Weber F, Skrzypczak M,
Ortmann O and Treeck O: Estrogen receptor beta is associated with
expression of cancer associated genes and survival in ovarian
cancer. BMC Cancer. 18:9812018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mak P, Leav I, Pursell B, Bae D, Yang X,
Taglienti CA, Gouvin LM, Sharma VM and Mercurio AM: ERbeta impedes
prostate cancer EMT by destabilizing HIF-1alpha and inhibiting
VEGF-mediated snail nuclear localization: Implications for Gleason
grading. Cancer Cell. 17:319–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Niu XL, Guo XQ, Yang J, Li L, Qu
Y, Xiu Hu C, Mao LQ and Wang D: IL6 induces TAM resistance via
kinase-specific phosphorylation of ERα in OVCA cells. J Mol
Endocrinol. 54:351–361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Halon A, Nowak-Markwitz E, Maciejczyk A,
Pudelko M, Gansukh T, Györffy B, Donizy P, Murawa D, Matkowski R,
Spaczynski M, et al: Loss of estrogen receptor beta expression
correlates with shorter overall survival and lack of clinical
response to chemotherapy in ovarian cancer patients. Anticancer
Res. 31:711–718. 2011.PubMed/NCBI
|
|
14
|
Fixemer T, Remberger K and Bonkhoff H:
Differential expression of the estrogen receptor beta (ER beta) in
human prostate tissue, premalignant changes, and in primary,
metastatic, and recurrent prostatic adenocarcinoma. Prostate.
54:79–87. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mak P, Chang C, Pursell B and Mercurio AM:
Estrogen receptor βsustains epithelial differentiation by
regulating prolyl hydroxylase 2 transcription. Proc Natl Acad Sci
USA. 110:4708–4713. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goel HL and Mercurio AM: VEGF targets the
tumour cell. Nat Rev Cancer. 13:871–882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bhattacharya R, Ye XC, Wang R, Ling X,
McManus M, Fan F, Boulbes D and Ellis LM: Intracrine VEGF signaling
mediates the activity of prosurvival pathways in human colorectal
cancer cells. Cancer Res. 76:3014–3024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang ZY, Zarlenga D, Martin J, Abubucker S
and Mitreva M: Exploring metazoan evolution through dynamic and
holistic changes in protein families and domains. BMC Evolut Biol.
12:1382012. View Article : Google Scholar
|
|
19
|
Xu GL, Xie M, Yang XY, Song Y, Yan C, Yang
Y, Zhang X, Liu ZZ, Tian YX, Wang Y, et al: Spectrum-effect
relationships as a systematic approach to traditional chinese
medicine research: Current status and future perspectives.
Molecules. 19:17897–17925. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu J and Yang Y: Traditional Chinese
medicine in the Chinese health care system. Health Policy.
90:133–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang S, Wu X, Tan M, Gong J, Tan W, Bian
B, Chen M and Wang Y: Fighting fire with fire: Poisonous Chinese
herbal medicine for cancer therapy. J Ethnopharmacol. 140:33–45.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lv L, Zheng L, Dong D, Xu L, Yin L, Xu Y,
Qi Y, Han X and Peng J: Dioscin, a natural steroid saponin, induces
apoptosis and DNA damage through reactive oxygen species: A
potential new drug for treatment of glioblastoma multiforme. Food
Chem Toxicol. 59:657–669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao X, Xu L, Zheng L, Yin L, Qi Y, Han X,
Xu Y and Peng J: Potent effects of dioscin against gastric cancer
in vitro and in vivo. Phytomedicine. 23:274–282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Si L, Zheng L, Xu L, Yin L Han X, Qi Y, Xu
Y, Wang C and Peng J: Dioscin suppresses human laryngeal cancer
cells growth via induction of cell-cycle arrest and MAPK-mediated
mitochondrial derived apoptosis and inhibition of tumor invasion.
Eur J Pharmacol. 774:105–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Si L, Xu L, Yin L, Qi Y, Han X, Xu Y, Zhao
Y, Liu K and Peng J: Potent effects of dioscin against pancreatic
cancer via miR-149-3P-mediated inhibition of the AKT1 signaling
pathway. Br J Pharmacol. 174:553–568. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mao Z, Han X, Chen D, Xu Y, Xu L, Yin L,
Sun H, Qi Y, Fang L, Liu K and Peng J: Potent effects of dioscin
against hepatocellular carcinoma through regulating TIGAR-mediated
apoptosis, autophagy and DNA damage. Br J Pharmacol. 176:919–937.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tao X, Xu L, Yin L, Han X, Qi Y, Xu Y,
Song S, Zhao Y and Peng J: Dioscin induces prostate cancer cell
apoptosis through activation of Estrogen Receptor-β. Cell Death
Dis. 8:e29892017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen H, Xu L, Yin L, Xu Y, Han X, Qi Y and
Peng J: iTRAQ-based proteomic analysis of dioscin on human HCT-116
colon cancer cells. Proteomics. 14:51–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu B, Hu M, Liu K and Peng J: Cytotoxicity
of berberine on human cervical carcinoma HeLa cells through
mitochondria, death receptor and MAPK pathways, and in-silico
drug-target prediction. Toxicol in Vitro. 24:1482–1490. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei Y, Xu YS, Han X, Qi Y, Xu L, Xu Y, Yin
L, Sun H, Liu K and Peng J: Anti-cancer effects of dioscin on three
kinds of human lung cancer cell lines through inducing DNA damage
and activating mitochondrial signal pathway. Food Chem Toxicol.
59:118–128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ning Y, Feng W, Cao X, Ren K, Quan M, Chen
A, Xu C, Qiu Y, Cao J, Li X and Luo X: Genistein inhibits stemness
of SKOV3 cells induced by macrophages co-cultured with ovarian
cancer stem-like cells through IL-8/STAT3 axis. J Exp Clin Cancer
Res. 38:192019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang MY, Liang JW, Olounfeh KM, Sun Q,
Zhao N and Meng FH: A comprehensive in silico method to study the
QSTR of the aconitine alkaloids for designing novel drugs.
Molecules. 23(pii): E23852018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ji BL, Xia LP, Zhou FX, Mao GZ and Xu LX:
Aconitine induces cell apoptosis in human pancreatic cancer via
NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 20:4955–4964.
2016.PubMed/NCBI
|
|
34
|
Li XM, Liu J, Pan FF, Shi DD, Wen ZG and
Yang PL: Quercetin and aconitine synergistically induces the human
cervical carcinoma HeLa cell apoptosis via endoplasmic reticulum
(ER) stress pathway. PLoS One. 13:e01910622018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Frisch M, Trucks G, Schlegel H, Scuseria
G, Robb M, Heeseman J, Scalmani G, Barone V, Petersson GA,
Nakatsuji H, et al: Gaussian 09, Revision A.02. Gaussian, Inc.
(Wallingford CT). 2009.
|
|
36
|
Becke A: Density-functional
thermochemistry. III. The role of exact exchange. J Chem Phys.
98:5648–5653. 1993. View Article : Google Scholar
|
|
37
|
Lee C, Yang W and Parr R: Development of
the colic-salvetti correlation-energy formula into a functional of
the electron density. Phys Rev B Condens Matter. 37:785–789. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schüttelkopf AW and Van Aalten DM: PRODRG:
A tool for high-throughput crystallography of protein-ligand
complexes. Acta Crystallogr D Biol Crystallogr. 60:1355–1363. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang S, Leng T, Zhang Q, Zhao Q, Nie X
and Yang L: Sanguinarine inhibits epithelial ovarian cancer
development via regulating long non-coding RNA CASC2- EIF4A3 axis
and/or inhibiting NF-κB signaling or PI3K/AKT/mTOR pathway. Biomed
Pharmacother. 102:302–308. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu L, Liu X, Li D, Sun S, Wang Y and Sun
X: Autophagy is a pro-survival mechanism in ovarian cancer against
the apoptotic effects of euxanthone. Biomed Pharmacother.
103:708–718. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xia Z, Lundgren B, Bergstrand A, DePierre
JW and Nässberger L: Changes in the generation of reactive oxygen
species and in mitochon- drial membrane potential during apoptosis
induced by the antidepressant imipramine, clomipramine, and
citalopram and the effects on these changes by Bcl-2 and Bcl-X(L).
Biochem Pharmacol. 57:1199–1208. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|
|
43
|
Li Z, Fan EK, Liu J, Scott MJ, Li Y, Li S,
Xie W, Billiar TR, Wilson MA, Jiang Y, et al: Cold-inducible
RNA-binding protein through TLR4 signaling induces mitochondrial
DNA fragmentation and regulates macrophage cell death after trauma.
Cell Death Dis. 8:e27752017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Anestis A, Sarantis P, Theocharis S, Zoi
I, Tryfonopoulos D, Korogiannos A, Koumarianou A, Xingi E,
Thomaidou D, Kontos M, et al: Estrogen receptor beta increases
sensitivity to enzalutamide in androgen receptor-positive
triple-negative breast cancer. J Cancer Res Clin Oncol.
145:1221–1233. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Foley EF, Jazaeri AA, Shupnik MA, Jazaeri
O and Rice LW: Selective loss of estrogen receptor beta in
malignant human colon. Cancer Res. 60:245–248. 2000.PubMed/NCBI
|
|
46
|
Rutherford T, Brown WD, Sapi E, Aschkenazi
S, Muñoz A and Mor G: Absence of estrogen receptor-beta expression
in metastatic ovarian cancer. Obstet Gynecol. 96:417–421. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zimmermann KC and Green DR: How cells die:
Apoptosis pathways. J Allergy Clin Immunol. 108:99–103. 2001.
View Article : Google Scholar
|
|
48
|
Iakova P, Timchenko L and Timchenko NA:
Intracellular signaling and hepatocellular carcinoma. Semin Cancer
Biol. 21:28–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu J, Yin S, Reddy N, Spencer C and Sheng
S: Bax mediates the apoptosis-sensitizing effect of maspin. Cancer
Res. 64:1703–1711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sarig-Nadir O and Seliktar D: The role of
matrix metalloproteinases in regulating neuronal and nonneuronal
cell invasion into PEGylated fibrinogen hydrogels. Biomaterials.
31:6411–6416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhao Z, Yu H and Kong Q: Effect of
ERβ-regulated ERK1/2 signaling on biological behaviors of prostate
cancer cells. Am J Transl Res. 9:2775–2787. 2017.PubMed/NCBI
|
|
52
|
Peng M, Yang D, Hou Y, Liu S, Zhao M, Qin
Y, Chen R, Teng Y and Liu M: Intracellular citrate accumulation by
oxidized ATM-mediated metabolism reprogramming via PFKP and CS
enhances hypoxic breast cancer cell invasion and metastasis. Cell
Death Dis. 10:2282019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhou M, Sareddy GR, Li M, Liu J, Luo Y,
Venkata PP, Viswanadhapalli S, Tekmal RR, Brenner A and Vadlamudi
RK: Estrogen receptor beta enhances chemotherapy response of GBM
cells by down regulating DNA damage response pathways. Sci Rep.
9:61242019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Du J, Lu X, Long Z, Zhang Z, Zhu X, Yang Y
and Xu J: In vitro and in vivo anticancer activity of aconitine on
melanoma cell line B16. Molecules. 18:757–767. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Borcsa B, Widowitz U, Csupor D, Forgo P,
Bauer R and Hohmann J: Semisynthesis and pharmacological
investigation of lipo-alkaloids prepared from aconitine.
Fitoterapia. 82:365–368. 2011. View Article : Google Scholar : PubMed/NCBI
|