Introduction
Ovarian cancer is one of the most lethal types of
cancers in women worldwide. It is the fifth leading cause of
cancer-related deaths among women in the United States and causes
>140,000 deaths annually in women worldwide (1). The pathogenesis of ovarian cancer is
very complicated, making it difficult to diagnose and treat
(2). Despite continuous
improvements in treatment technology, the 5-year survival rate of
ovarian cancer remains unsatisfactory. The standard curative
treatment for ovarian cancer is anticancer treatment following
surgery (3,4). Therefore, in addition to improving
diagnostic technology, it is also important to develop new drugs
for the treatment of ovarian cancer.
Cucurbitacin I, a natural tetracyclic triterpenoid
extracted from Cucurbitaceae and Cruciferae, has been used in
traditional medicine for its antipyretic, analgesic,
anti-inflammatory and antimicrobial effects (5). According to recent findings,
cucurbitacin I, which is 1 of 12 variants of cucurbitacins, has
demonstrated a potent anticancer effect on non-small cell lung
cancer cells (6). Cucurbitacin I
has been reported to cause cancer cell death by inhibiting multiple
signaling pathways, such as the Janus kinase (JAK)/STAT3,
PI3K/AKT/p70S6K and serine/threonine-protein kinase PAK1
(PAK1)/PAK4 pathways (7–9). However, the underlying mechanism for
the anticancer effect of cucurbitacin I is not yet completely
understood, and further studies are required to clarify it.
A low level of reactive oxygen species (ROS) is
normal in cells, as it helps maintain cell function, but excessive
levels of ROS can damage cells and induce cell death. There are
regulatory mechanisms that neutralize excessive ROS and provide
protection against oxidative damage (10). The transcription factor nuclear
factor erythroid-derived 2-like 2 (Nrf2) is an important regulator
of the oxidative stress response, and plays a key role in the
expression of antioxidant proteins. Nrf2 is regulated by Kelch-like
ECH-associated protein 1 (Keap1). Under quiescent conditions, Nrf2
is retained in the cytoplasm after being combined with Keap1, and
is degraded in the proteasome with the E3 ubiquitin ligase Cullin
3. Cellular stimuli, such as oxidative stress, induce
conformational changes in Keap1, which are followed by the release
of Nrf2 from Keap1. Subsequently, Nrf2 translocates to the nucleus
and transactivates the expression of genes containing an
antioxidant response element in their promoter regions. Nrf2
thereby upregulates phase II detoxifying enzymes and antioxidant
proteins, and plays a vital role in maintaining cellular
homeostasis (11,12).
p190BRhoGAP (p190B), which is a GTPase-activating
protein, has been implicated in various pathological conditions,
including cancer, cardiovascular diseases and developmental
disorders (13,14). As reported by numerous studies,
p190B plays an important role in regulating small GTPase activity,
which influence cytoskeleton remodeling, cell adhesion, cell
polarity maintenance, proliferation and division, as well as cell
migration (14,15). A previous study demonstrated that
p190B can regulate Ras homolog family member A (RhoA) and Rac1 to
regulate cell migration (16).
Meanwhile, p190B is critical in the occurrence and development of
tumors, such as breast cancer, glioma and colorectal cancer
(17,18).
In the present study, cucurbitacin I, a botanical
extract, induced death in SKVO3 cells in a time- and
concentration-dependent manner. Cucurbitacin I promoted cell
apoptosis and induced an increase in the intracellular ROS level,
with decreased antioxidant-related gene expression by the
Keap1-Nrf2 signaling axis. The expression of p190B and Rac1 was
also found to be altered, which was associated with changes in the
cell morphology. The present study may have provided novel evidence
for the treatment of ovarian cancer.
Materials and methods
Reagents
Cucurbitacin I and dimethyl sulfoxide (DMSO) were
purchased from Merck KGaA. Cell Counting Kit-8 (CCK-8) was
purchased from Bimake. ROS assay kit was obtained from Beyotime
Institute of Biotechnology. Anti-BAX (cat. no. 2772) and
anti-cleaved-caspase-3 (cat. no. 9664) were from Cell Signaling
Technology, Inc. Anti-Bcl-2 (cat. no. ET1603-11) was from Hangzhou
HuaAn Biotechnology Co., Ltd. Anti-Keap1 (cat. no. 10503-2-AP),
anti-Nrf2 (cat. no. 16396-1-AP), anti-Heme oxygenase-1 (HO-1; cat.
no. 10701-1-AP), anti-p190B (cat. no. 55165-1-AP) and anti-Rac1
(cat. no. 24072-1-AP) were purchased from ProteinTech Group, Inc.
Anti-GAPDH (cat. no. GB11002) was from Wuhan Servicebio Technology
Co., Ltd., and all secondary antibodies (cat. nos. ZB-2301 and
ZB-2305) were from Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd. Other chemicals and reagents were analytically pure.
Cell culture
SKOV3 human ovarian carcinoma cells (ATCC HTB-77)
were obtained from American Type Culture Collection. SKVO3 cells
(70-80%) were cultured in Dulbecco's modified Eagle's medium (DMEM;
HyClone; GE Healthcare) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 mg/ml
streptomycin and 100 U/ml penicillin. SKVO3 cells were incubated in
a humidified incubator containing 95% air and 5% CO2
with the temperature stabilized at 37°C. The cell culture media was
refreshed every 3 days.
Cell viability assays
Cell viability assays were performed according to a
previous study (19). SKVO3 cells
were seeded into 96-well plates at a density of 10,000 cell per
well. The experimental groups were treated with 0.075, 0.15, 0.3
and 0.6 µM cucurbitacin I (dissolved in DMSO) for 12, 24 and 48 h,
and the control group was treated with DMSO. Finally, a CCK-8 assay
was performed, according to the manufacturer's protocol, to detect
cell viability using a microplate reader (BioTek). As 0.3 µM
cucurbitacin I treatment for 24 h could effectively reduce cell
viability by ~50%, this concentration and time was chosen for
subsequent experiments.
ROS assay
The ROS assay was performed according to a previous
study (20). ROS were measured
using a fluorescent probe DCFH-DA (Beyotime Institute of
Biotechnology). SKVO3 cells (2×105 per well) were seeded
into 6-well plates, and then when cells reached ~80% confluence
they were treated with 0.3 µM cucurbitacin I for 24 h at 37°C
(control cells were treated with DMSO). All cells were further
incubated with 10 µM DCFH-DA for 20 min, and then washed with
serum-free DMEM 3 times. The level of ROS was detected using a
fluorescence microscope.
Western blot analysis
Western blotting was performed as previously
described (21,22). SKVO3 cells were lysed on ice for 20
min, centrifuged at 10,000 × g for 10 min at 4°C and the cell
supernatant was collected. Protein concentration was measured by
the BCA method, and 35 µg protein per lane was loaded onto 10 or
12% gels and separated via SDS-PAGE. The separated proteins were
subsequently transferred onto polyvinylidene fluoride membranes.
The membranes were incubated with 5% skimmed milk diluted by TBS
with 0.1% Tween-20 (TBST) for 1 h at room temperature, and then
probed with primary antibodies (1:1,000) at 4°C overnight. The
membranes were washed with TBST 3 times and incubated with the
secondary antibodies (1:3,000) for 1 h at room temperature to
amplify the chemiluminescence. Bands were semi-quantified and
analyzed using ImageJ Software (V1.8.0.112; National Institutes of
Health).
Statistical analysis
All data are expressed as the mean ± SEM.
Comparisons were performed using an unpaired Student's t-test and
groups of ≥3 were analyzed by one-way ANOVA, followed by Tukey's
post hoc tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cucurbitacin I causes SKVO3 cell death
in a concentration- and time-dependent manner
To evaluate the cytotoxicity of cucurbitacin I on
SKVO3 cells, the cell viability of SKVO3 was detected by a CCK-8
assay. As shown in Fig. 1A,
cucurbitacin I treatment induced cell death in a concentration- and
time-dependent manner. Briefly, the cucurbitacin I concentration
used was 0.075–0.6 µM. The higher the concentration, the higher the
death rate and the lower the survival rate. Cells were treated with
cucurbitacin I for 12, 24 and 48 h. The longer the treatment time,
the higher the cell death rate and the lower the survival rate.
Cell death is always accompanied by changes in cell morphology. As
shown in Fig. 1B, following
cucurbitacin I treatment for 24 h, there was a decreased number of
SKVO3 cells and cell surface shrinkage; the higher the
concentration, the more severe this was.
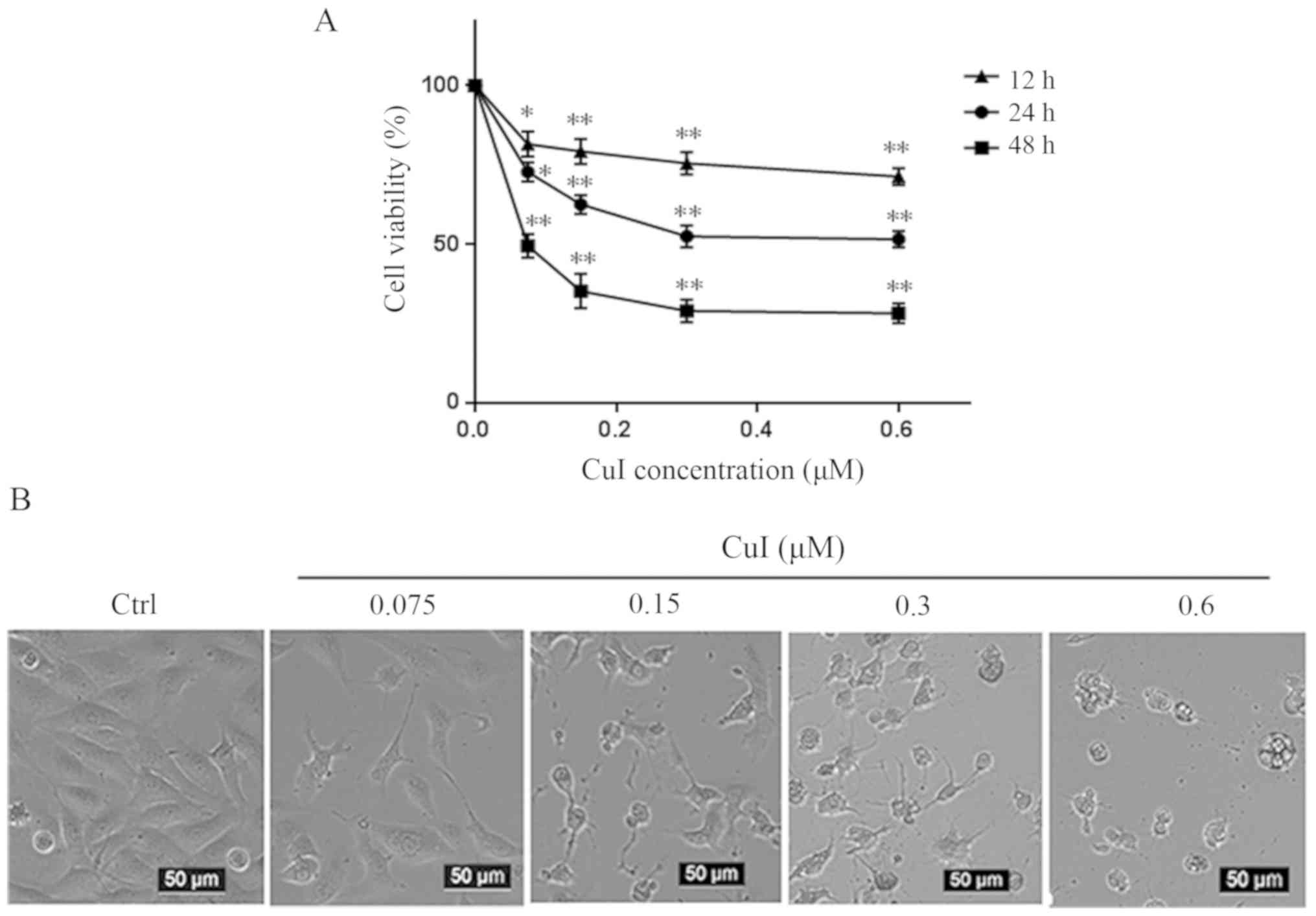 | Figure 1.CuI causes SKVO3 cell death in a
concentration- and time-dependent manner. (A) SKVO3 cells were
treated with 0.075, 0.15, 0.3 and 0.6 µM CuI for 12, 24 and 48 h,
whereas the control group was treated with DMSO alone. Cell
viability was then measured by Cell Counting Kit-8 assay
(n=3/group). (B) Cells were visualized using an inverted
microscope. Scale bars, 50 µm; magnification, ×200. Data were
analyzed with one-way ANOVA followed by Tukey's post hoc test and
are presented as the mean ± SEM. *P<0.05, **P<0.01 vs.
DMSO-treated group (control). Ctrl, DMSO-treated cells; DMSO,
dimethyl sulfoxide; CuI, cucurbitacin I. |
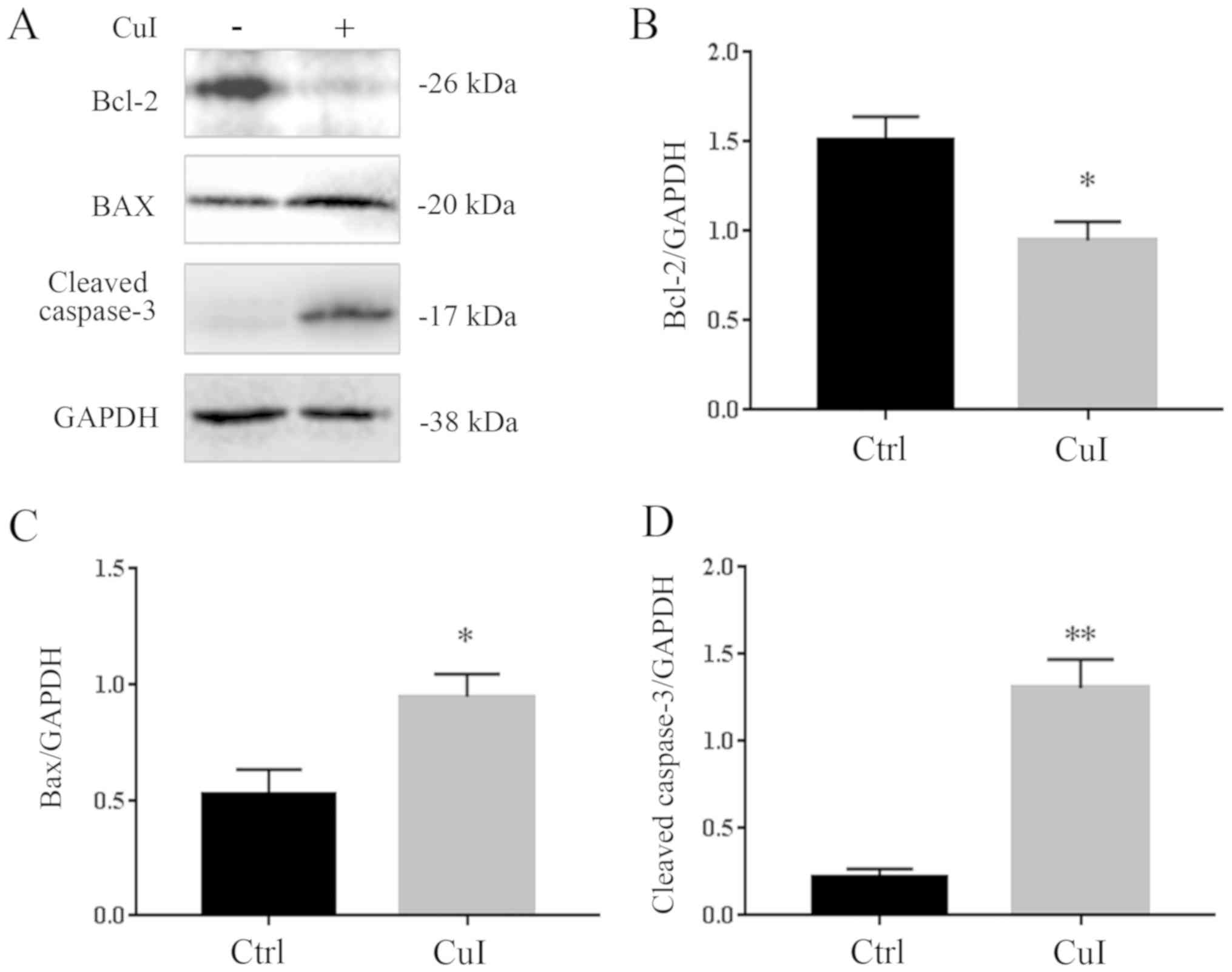
Cucurbitacin I induces apoptosis in
SKVO3 cells
To determine whether cucurbitacin I induced cell
apoptosis, a concentration of 0.3 µM was selected as it was shown
to kill ~50% of SKVO3 cells. It was observed that treatment with
0.3 µM cucurbitacin I for 24 h induced a significant decrease in
Bcl-2 (37.3%, P<0.05) and a significant increase in BAX (80.2%,
P<0.05). The expression level of cleaved-caspase-3, an index of
apoptosis, was also examined, and it was found to have increased by
380% (P<0.01) compared with the control group treated with DMSO
alone (Fig. 2).
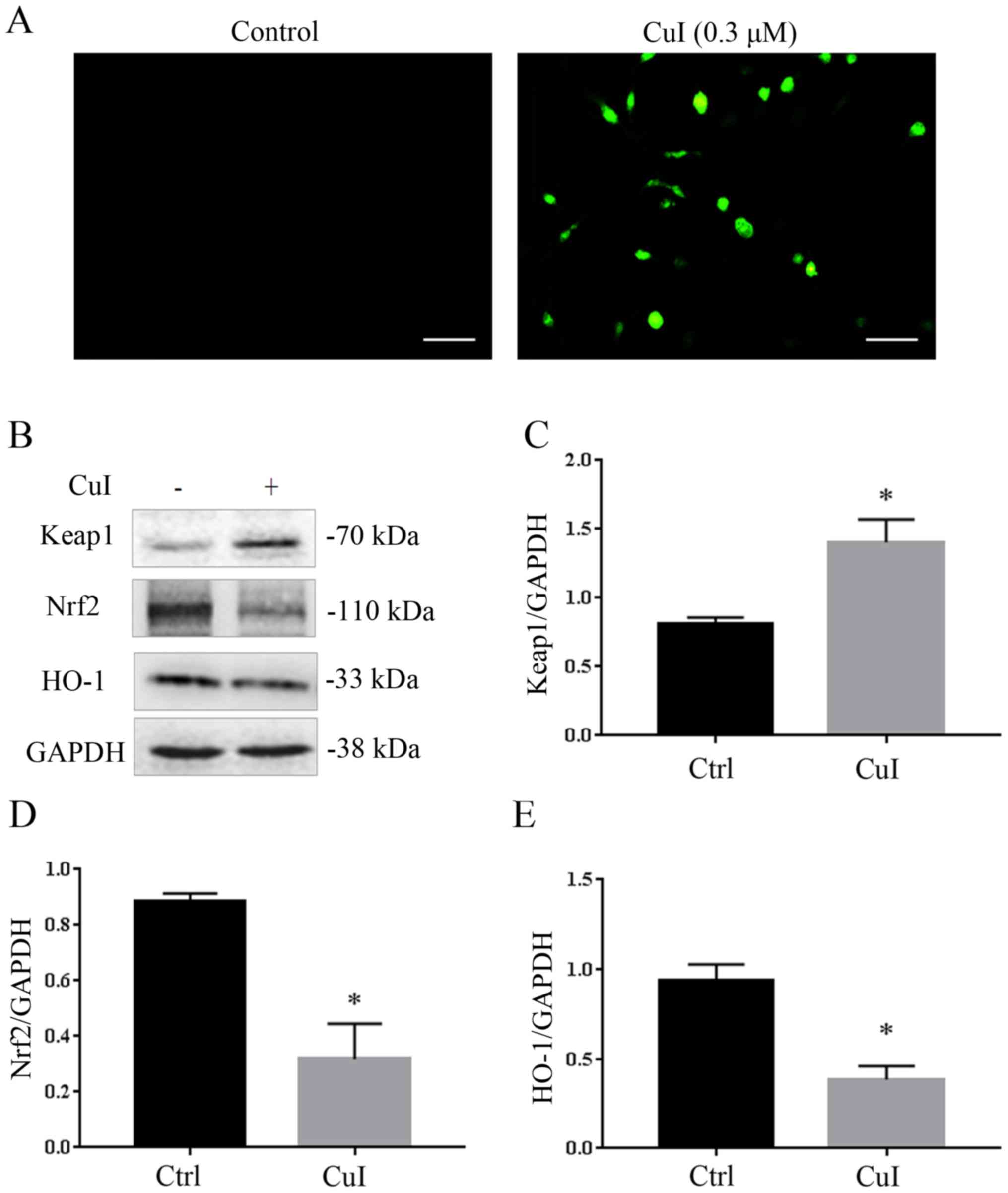
Cucurbitacin I increases cellular
oxidative stress via the Keap1-Nrf2 pathway
To evaluate the effect of cucurbitacin I on
oxidative stress, the level of ROS in SKVO3 cells was measured. As
shown in Fig. 3A, when cells were
treated with 0.3 µM cucurbitacin I, the level of intracellular ROS
markedly increased, whereas that of the control group was very low.
Cells have protective mechanisms against oxidative stress,
including a number of antioxidant proteins and signaling pathways,
of which the Keap1-Nrf2 pathway is one of them. Therefore in the
present study, the expression of Keap1, Nrf2 and HO-1, which is
downstream of Nrf2, was measured. As shown in Fig. 3B-E, the protein expression of Nrf2
decreased by 63.9% (P<0.05), HO-1 decreased by 58.7% (P<0.05)
and Keap1 increased by 72.5% (P<0.05) compared with the control
group. These results indicated that that cucurbitacin I increased
the level of intracellular oxidative stress and therefore regulated
the Keap1-Nrf2 pathway.
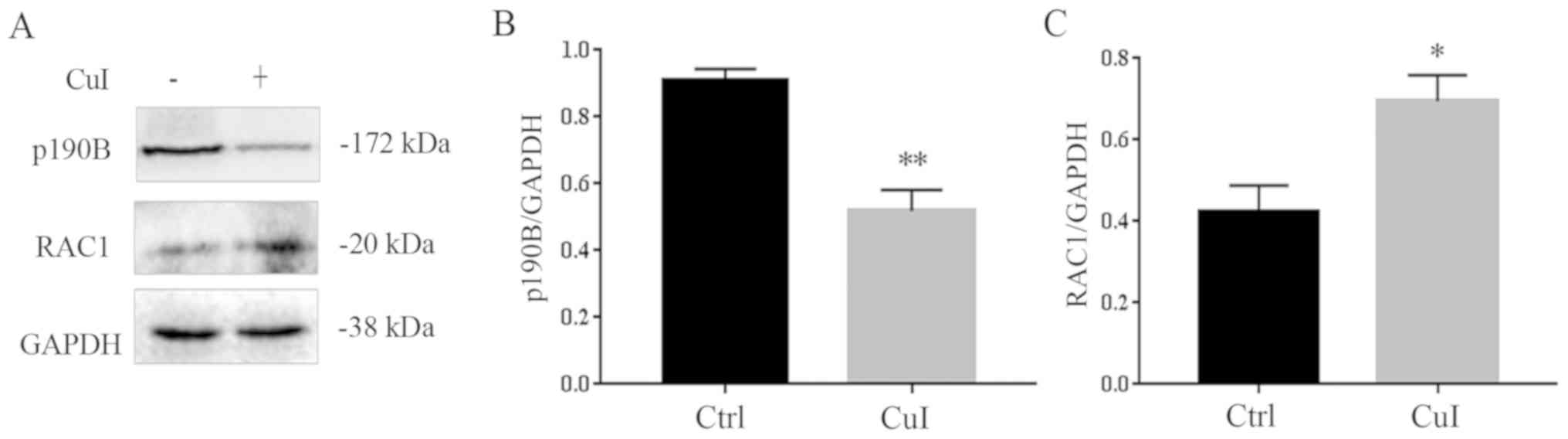
Cucurbitacin I alters cell morphology
by regulating the p190B-Rac1 pathway
It has been reported that the expression of p190B is
increased in ovarian cancer, which may affect the cell
cytoskeleton, morphology and migration (23). As shown in Fig. 1B, the number of cells decreased and
the cell surface had shrunk with the increase in cucurbitacin I
concentration. Therefore, the expression levels of
cytoskeleton-related protein p190B and its downstream protein Rac1
were detected. The results showed that, as compared with the
control group, the expression of p190B decreased by 42.8%
(P<0.01) and Rac1 expression increased by 66.3% (P<0.05) in
cells treated with 0.3 µM cucurbitacin I for 24 h (Fig. 4). Thus, suggesting that
cucurbitacin I regulated the p190B and Rac1 pathway to regulate
cell morphology.
Discussion
Ovarian cancer is one of the most common malignant
tumors in female reproductive organs. The incidence of ovarian
cancer is second only to that of cervical cancer and uterine body
cancer, and is a serious threat to women's lives (2–4). The
etiology of ovarian cancer is still unclear, but may be related to
genetic and endocrine factors. At present, in addition to surgery,
the development of antitumor drugs is also very important.
Cucurbitacin I has a strong antitumor activity (6,7).
Cucurbitacin I has been reported to induce death in various types
of tumor cells; for example it can induce apoptosis in non-small
cell lung cancer cells and osteosarcoma cells by inhibiting the
PI3K/AKT/p70S6K and STAT3 signaling, respectively. Zhang et
al (24) observed that
cucurbitacin I modulated the balance between autophagic and
apoptotic modes of cells to cause cancer cell death. In the present
study, the SKVO3 cell line was selected to investigate ovarian
cancer via various experiments. The present findings confirmed that
cucurbitacin I could kill SKVO2 cells in a concentration- and
time-dependent manner, indicating that its antitumor effect was
enhanced as the concentration increased. As shown in Fig. 1, a noteworthy phenomenon was found,
after cucurbitacin I administration, some of the shrunk cells that
had attached to the culture dish still had cell viability and did
not die completely. The morphology of SKVO3 cells changed in a
concentration-dependent manner.
Apoptosis is a form of programmed cell death that
brings about the orderly and efficient removal of damaged cells,
such as those resulting from DNA damage or during development
(25,26). The mechanism of apoptosis is very
complex and numerous signaling pathways are involved in this
process. However, caspases, a family of
cysteinyl-aspartate-specific proteases, are central to the
mechanism of apoptosis (27,28).
The activity of caspases is responsible for the hallmarks of
apoptosis, so the expression level of cleaved-caspase-3 was
detected. As shown in Fig. 2A,
cucurbitacin I caused a significant increase in the expression
level of cleaved-caspase-3 and pro-apoptotic protein BAX in SKVO3
cells, whereas the expression of anti-apoptotic protein Bcl-2
decreased and the ratio of Bcl-2/BAX decreased. In general, these
results indicated that cucurbitacin I induced SKVO3 cell
apoptosis.
Cancer development is characterized by the
uncontrolled growth and proliferation of transformed cells. A tumor
environment is characterized by low levels of oxygen and glucose,
which can induce ROS. At moderate concentrations, ROS activates the
cancer cell survival signaling cascade. At high concentrations, ROS
can cause cancer cell apoptosis (29). In the present study, the mechanism
through which cucurbitacin I induced cell death in ovarian cancer
was further explored. It was found that cucurbitacin I increased
the level of ROS in cells and decreased the expression of
antioxidant genes. Briefly, cucurbitacin I decreased the expression
of Nrf2, but increased that of the Keap1 protein, which can combine
with Nrf2 to further prevent Nrf2 from translocating into the
nucleus. Next, the expression of HO-1, an antioxidant gene
downstream of Nrf2, was detected, and it was found to have
decreased by 58.7%. Generally speaking, cucurbitacin I increased
the level of ROS and decreased the expression of antioxidant genes
via the Keap1-Nrf2 signaling axis, resulting in cell death.
When ovarian cancer occurs, the expression of
cytoskeleton-related proteins increases. Earp et al
(23) suggested that the
expression of the p190B protein increased significantly during the
development of ovarian cancer. Therefore, reducing the expression
of p190B could be a potential target for treatment. In the present
study, following the treatment of SKVO3 cells by cucurbitacin I,
the expression of p190B, a regulatory protein associated with the
cytoskeleton, decreased significantly, and that of the negative
regulatory protein Rac1 increased. This was associated with the
morphological changes of cells in Fig.
1B.
The anti-tumor signaling pathway of cucurbitacin I
is very complex. Although, it has been reported by Li et al
(30) that cucurbitacin I causes
ovarian cancer cell death via endoplasmic reticulum stress, the
present study demonstrated for the first time, to the best of the
our knowledge, that cucurbitacin I can also activate oxidative
stress by regulating the Keap1-Nrf2-HO1 signaling pathway, and
regulate the p190b-Rac1 signal axis to alter cell cytoskeleton
shape, so as to achieve the anti-tumor effect. A limitation of the
present study is that only one cell line was used and so there was
a lack of dose responses in this experiment, in future experiments
other cell lines, as well as primary cells, will be used to confirm
our findings. Additionally, further experiments to determine
concentration gradient are needed, and a variety of research
methods, such as flow cytometry and TUNEL fluorescence detection,
will be performed to further explore the findings of the present
study.
In conclusion, cucurbitacin I, a newly identified
potential chemotherapeutic drug, induced ovarian cancer cell death
in a concentration- and time-dependent manner. Cucurbitacin I
induced cell apoptosis by increasing BAX and cleaved-caspase-3, and
decreasing Bcl-2. In addition, cucurbitacin I increased the
intracellular ROS level and decreased antioxidant gene expression
via the Keap1-Nrf2 signaling pathway. In addition, the p190B-Rac1
signaling axis was inhibited by cucurbitacin I. These insights may
be used to develop novel therapeutic approaches to treat ovarian
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81900276), Nanchong
City & School Cooperation Project (grant no. 18SXHZ0546) and
Sichuan Provincial Health Commission's popularization and
application project(grant no. 20PJ146)
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RL and FL performed the experiments and drafted the
manuscript. JX, XL, HX and ST participated in the experiments. MY
and BH contributed to the final data analysis and edited the
manuscript. All the authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hunn J and Rodriguez GC: Ovarian cancer:
Etiology, risk factors, and epidemiology. Clin Obstet Gynecol.
55:3–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Farsinejad S, Cattabiani T, Muranen T and
Iwanicki M: Ovarian cancer dissemination-A cell Biologist's
perspective. Cancers (Basel). 11:19572019. View Article : Google Scholar
|
|
3
|
Yeung TL, Leung CS, Yip KP, Au Yeung CL,
Wong ST and Mok SC: Cellular and molecular processes in ovarian
cancer metastasis. A review in the theme: Cell and molecular
processes in cancer metastasis. Am J Physiol Cell Physiol.
309:C444–C456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moufarrij S, Dandapani M, Arthofer E,
Gomez S, Srivastava A, Lopez-Acevedo M, Villagra A and Chiappinelli
KB: Epigenetic therapy for ovarian cancer: Promise and progress.
Clin Epigenetics. 11:72019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu K, Xiao J, Zheng K, Feng X, Zhang J,
Song D, Wang C, Shen X, Zhao X, Wei C, et al: miR-21/STAT3 signal
is involved in odontoblast differentiation of human dental pulp
stem cells mediated by TNF-α. Cell Reprogram. 20:107–116. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ni Y, Wu S, Wang X, Zhu G, Chen X, Ding Y
and Jiang W: Cucurbitacin I induces pro-death autophagy in A549
cells via the ERK-mTOR-STAT3 signaling pathway. J Cell Biochem.
119:6104–6112. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu X, Huang H, Zhang J, Liu H, Ao R, Xiao
M and Wu Y: The anticancer effects of Cucurbitacin I inhibited cell
growth of human non-small cell lung cancer through PI3K/AKT/p70S6K
pathway. Mol Med Rep. 17:2750–2756. 2018.PubMed/NCBI
|
|
8
|
Yang Q, Qiu H, Xie H, Qi Y, Cha H, Qu J,
Wang M, Feng Y, Ye X, Mu J and Huang J: A Schistosoma
japonicum infection promotes the expansion of Myeloid-derived
suppressor cells by activating the JAK/STAT3 pathway. J Immunol.
198:4716–4727. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nguyen BC, Taira N, Maruta H and Tawata S:
Artepillin C and other Herbal PAK1-blockers: Effects on hair cell
proliferation and related PAK1-dependent biological function in
cell culture. Phytother Res. 30:120–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Yan T, Sun D, Xie C, Wang T, Liu
X, Wang J, Wang Q, Luo Y, Wang P, et al: Rutaecarpine inhibits
KEAP1-NRF2 interaction to activate NRF2 and ameliorate dextran
sulfate sodium-induced colitis. Free Radic Biol Med. 148:33–41.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Zhang W, Lv C, Wang Y, Ma B, Zhang
H, Fan Z, Li M and Li X: A novel biscoumarin compound ameliorates
cerebral ischemia reperfusion-induced mitochondrial oxidative
injury via Nrf2/Keap-1/ARE signaling. Neuropharmacology.
167:1079182020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tian T, Chen ZH, Zheng Z, Liu Y, Zhao Q,
Liu Y, Qiu H, Long Q, Chen M, Li L, et al: Investigation of the
role and mechanism of ARHGAP5-mediated colorectal cancer
metastasis. Theranostics. 10:5998–6010. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Héraud C, Pinault M, Lagrée V and Moreau
V: p190RhoGAPs, the ARHGAP35- and ARHGAP5-encoded proteins, in
health and disease. Cells. 8:3512019. View Article : Google Scholar
|
|
15
|
Frank SR, Köllmann CP, Luong P, Galli GG,
Zou L, Bernards A, Getz G, Calogero RA, Frödin M and Hansen SH:
p190 RhoGAP promotes contact inhibition in epithelial cells by
repressing YAP activity. J Cell Biol. 217:3183–3201. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bustos RI, Forget MA, Settleman JE and
Hansen SH: Coordination of Rho and Rac GTPase function via p190B
RhoGAP. Curr Biol. 18:1606–1611. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hwang M, Peddibhotla S, McHenry P, Chang
P, Yochum Z, Park KU, Sears JC and Vargo-Gogola T: P190B RhoGAP
regulates chromosome segregation in cancer cells. Cancers (Basel).
4:475–489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heckman-Stoddard BM, Vargo-Gogola T,
McHenry PR, Jiang V, Herrick MP, Hilsenbeck SG, Settleman J and
Rosen JM: Haploinsufficiency for p190B RhoGAP inhibits MMTV-Neu
tumor progression. Breast Cancer Res. 11:R612009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhai KF, Duan H, Cui CY, Cao YY, Si JL,
Yang HJ, Wang YC, Cao WG, Gao GZ and Wei ZJ: Liquiritin from
Glycyrrhiza uralensis attenuating rheumatoid arthritis via reducing
inflammation, suppressing angiogenesis, and inhibiting MAPK
signaling pathway. J Agric Food Chem. 67:2856–2864. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H and Joseph JA: Quantifying cellular
oxidative stress by dichlorofluorescein assay using microplate
reader. Free Radic Biol Med. 27:612–616. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhai KF, Zheng JR, Tang YM, Li F, Lv YN,
Zhang YY, Gao Z, Qi J, Yu BY and Kou JP: The Saponin D39 blocks
dissociation of non-muscular myosin heavy chain IIA from TNF
receptor 2, suppressing tissue factor expression and venous
thrombosis. Br J Pharmacol. 174:2818–2831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li RL, Wu SS, Wu Y, Wang XX, Chen HY, Xin
JJ, Li H, Lan J, Xue KY, Li X, et al: Irisin alleviates pressure
overload-induced cardiac hypertrophy by inducing protective
autophagy via mTOR-independent activation of the AMPK-ULK1 pathway.
J Mol Cell Cardiol. 121:242–255. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Earp M, Tyrer JP, Winham SJ, Lin HY,
Chornokur G, Dennis J, Aben KKH, Anton-Culver H, Antonenkova N,
Bandera EV, et al: Variants in genes encoding small GTPases and
association with epithelial ovarian cancer susceptibility. PLoS
One. 13:e01975612018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang T, Li Y, Park KA, Byun HS, Won M,
Jeon J, Lee Y, Seok JH, Choi SW, Lee SH, et al: Cucurbitacin
induces autophagy through mitochondrial ROS production which
counteracts to limit caspase-dependent apoptosis. Autophagy.
8:559–576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dietz A, Dalda N, Zielke S, Dittmann J,
van Wijk SJL, Vogler M and Fulda S: Proteasome inhibitors and Smac
mimetics cooperate to induce cell death in diffuse large B-cell
Lymphoma by stabilizing NOXA and triggering mitochondrial
apoptosis. Int J Cancer. 4:9822020.
|
|
26
|
Malinina A, Dikeman D, Westbrook R, Moats
M, Gidner S, Poonyagariyagorn H, Walston J and Neptune ER: IL10
deficiency promotes alveolar enlargement and lymphoid
dysmorphogenesis in the aged murine lung. Aging Cell.
19:e131302020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhai KF, Duan H, Chen Y, Khan GJ, Cao WG,
Gao GZ, Shan LL and Wei ZJ: Apoptosis effects of imperatorin on
synoviocytes in rheumatoid arthritis through
mitochondrial/caspase-mediated pathways. Food Funct. 9:2070–2079.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma M, Wang X, Liu N, Shan F and Feng Y:
Low-dose naltrexone inhibits colorectal cancer progression and
promotes apoptosis by increasing M1-type macrophages and activating
the Bax/Bcl-2/caspase-3/PARP pathway. Int Immunopharmacol.
83:1063882020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aggarwal V, Tuli HS, Varol A, Thakral F,
Yerer MB, Sak K, Varol M, Jain A, Khan MA and Sethi G: Role of
reactive oxygen species in cancer progression: Molecular mechanisms
and recent advancements. Biomolecules. 9:7352019. View Article : Google Scholar
|
|
30
|
Li H, Chen H, Li R, Xin J, Wu S, Lan J,
Xue K, Li X, Zuo C, Jiang W and Zhu L: Cucurbitacin I induces
cancer cell death through the endoplasmic reticulum stress pathway.
J Cell Biochem. Sep 11–2018.(Epub ahead of print).
|