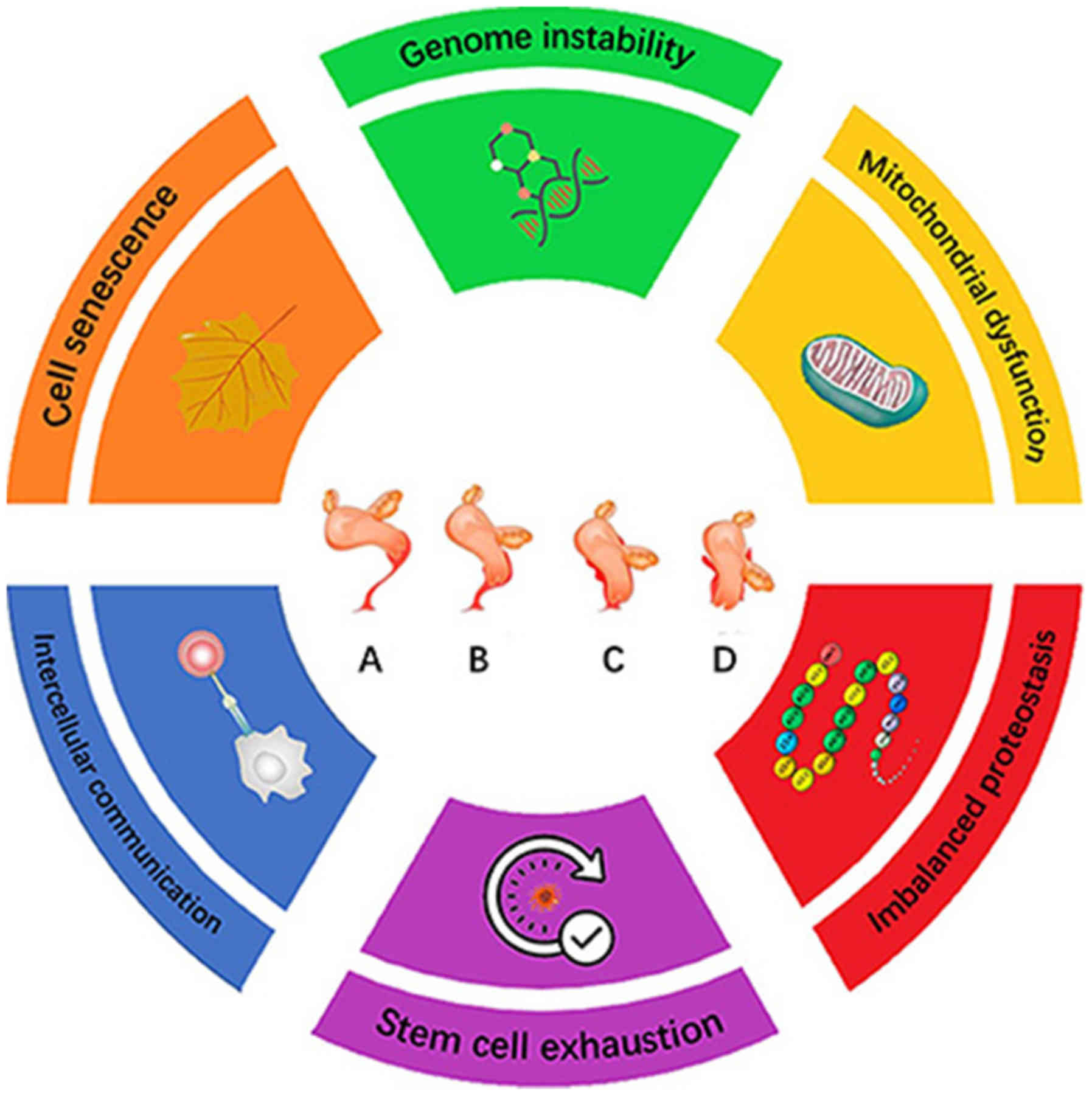
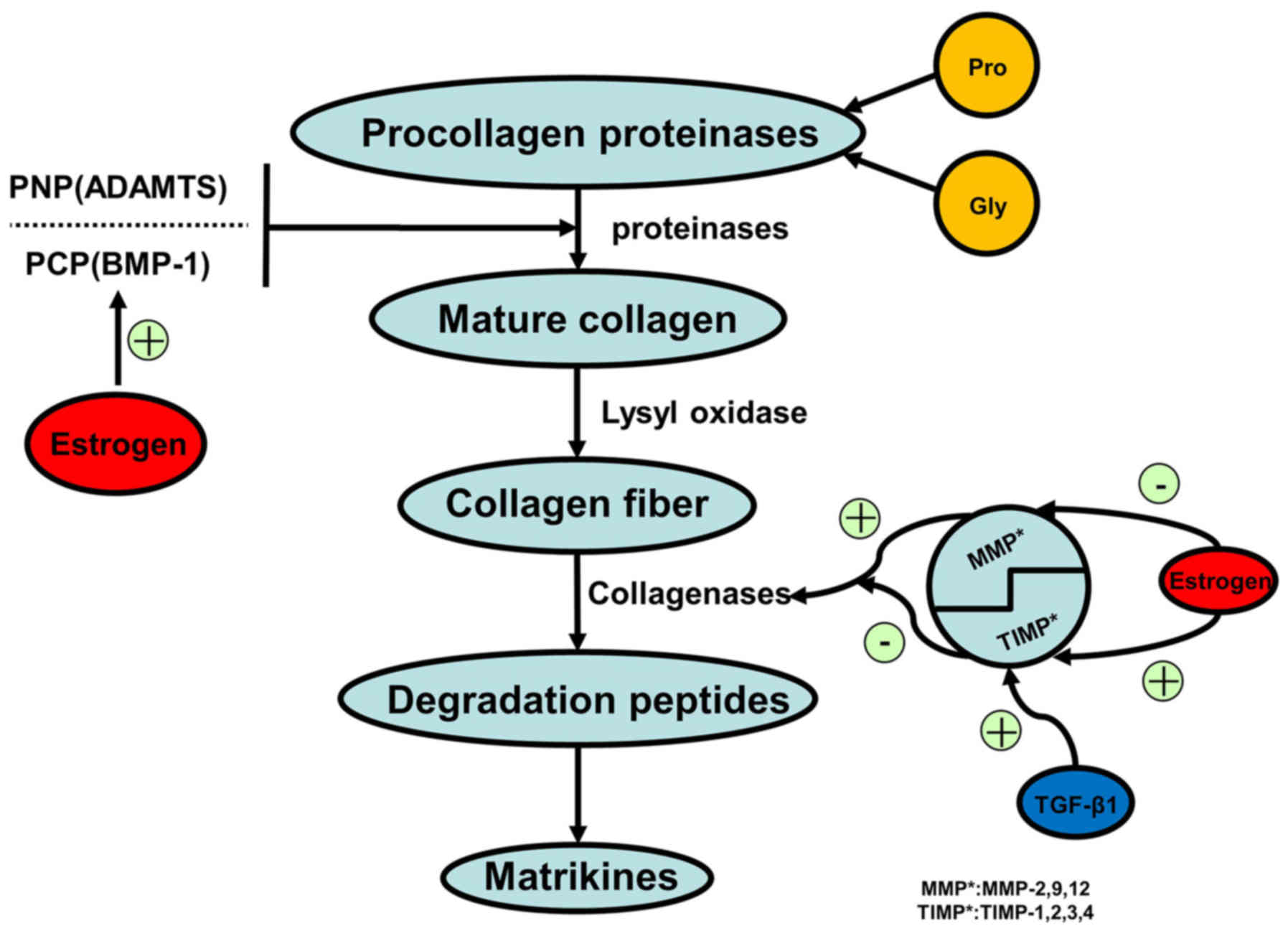
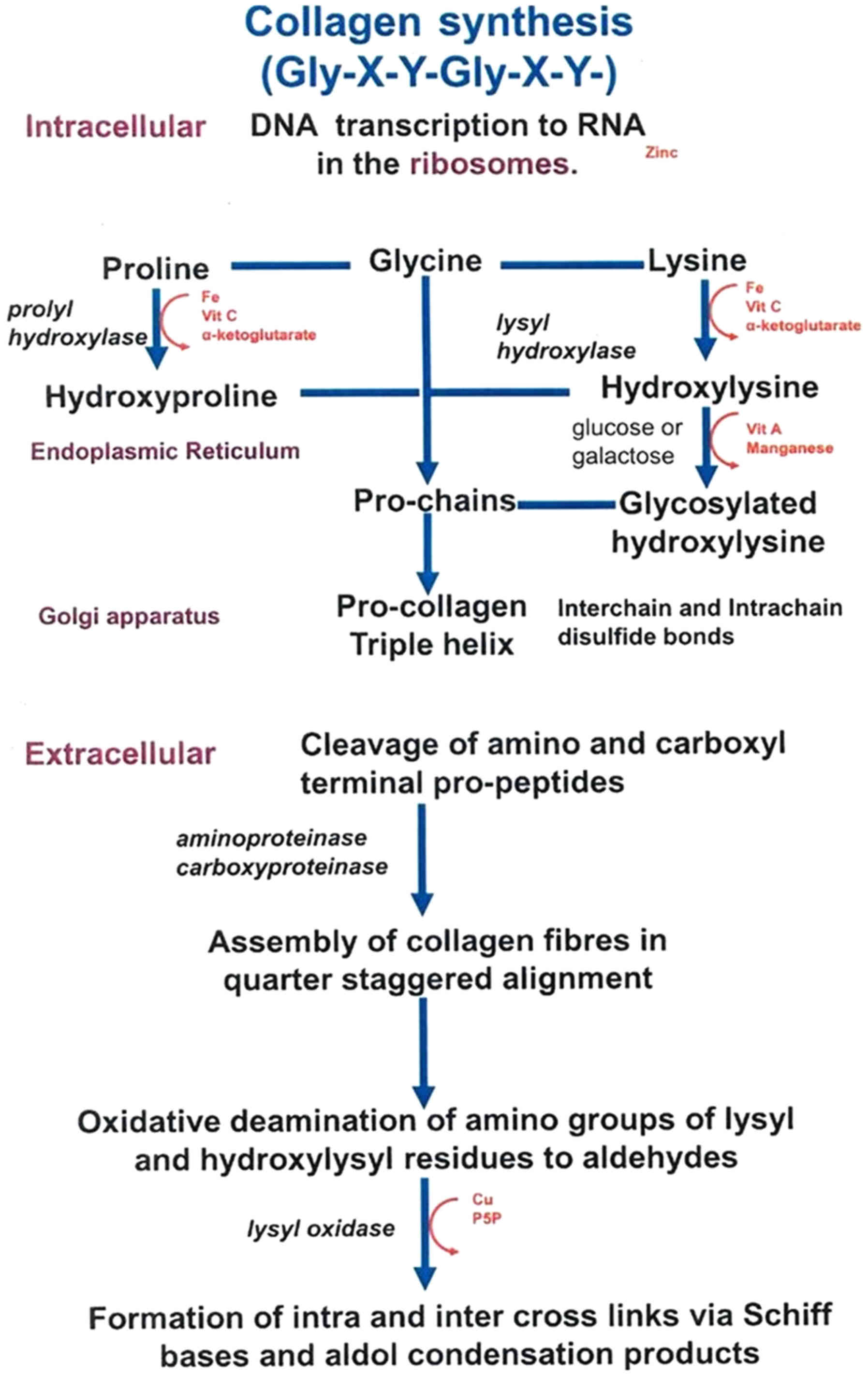
|
1
|
Iglesia CB and Smithling KR: Pelvic organ
prolapse. Am Fam Physician. 96:179–185. 2017.PubMed/NCBI
|
|
2
|
Nygaard I, Barber MD, Burgio KL, Kenton K,
Meikle S, Schaffer J, Spino C, Whitehead WE, Wu J and Brody DJ;
Pelvic floor disorders network, : Prevalence of symptomatic pelvic
floor disorders in US women. JAMA. 300:1311–1316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jelovsek JE, Maher C and Barber MD: Pelvic
organ prolapse. Lancet. 369:1027–1038. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kinman CL, Lemieux CA, Agrawal A, Gaskins
JT, Meriwether KV and Francis SL: The relationship between age and
pelvic organ prolapse bother. Int Urogynecol J Pelvic Floor
Dysfunct. 28:751–755. 2017. View Article : Google Scholar
|
|
5
|
Sun B, Zhou L, Wen Y, Wang C, Baer TM,
Pera RR and Chen B: Proliferative behavior of vaginal fibroblasts
from women with pelvic organ prolapse. Eur J Obstet Gynecol Reprod
Biol. 183:1–4. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Richardson AC, Lyon JB and Williams NL: A
new look at pelvic relaxation. Am J Obstet Gynecol. 126:568–573.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jackson SR, Avery NC, Tarlton JF, Eckford
SD, Abrams P and Bailey AJ: Changes in metabolism of collagen in
genitourinary prolapse. Lancet. 347:1658–1661. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
López-Otín C, Blasco MA, Partridge L,
Serrano M and Kroemer G: The hallmarks of aging. Cell.
153:1194–1217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tigges J, Krutmann J, Fritsche E,
Haendeler J, Schaal H, Fischer JW, Kalfalah F, Reinke H,
Reifenberger G, Stühler K, et al: The hallmarks of fibroblast
ageing. Mech Ageing Dev. 138:26–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim EJ, Chung N, Park SH, Lee KH, Kim SW,
Kim JY, Bai SW and Jeon MJ: Involvement of oxidative stress and
mitochondrial apoptosis in the pathogenesis of pelvic organ
prolapse. J Urol. 189:588–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li BS, Guo WJ, Hong L, Liu YD, Liu C, Hong
SS, Wu DB and Min J: Role of mechanical strain-activated PI3K/Akt
signaling pathway in pelvic organ prolapse. Mol Med Rep.
14:243–253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian X, Wang F, Luo Y, Ma S, Zhang N, Sun
Y, You C, Tang G, Li S, Gong Y, et al: Protective role of nuclear
factor-erythroid 2-related factor 2 against radiation-induced lung
injury and inflammation. Front Oncol. 8:5422018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang W, Sun Z, Yang B and Wang Q:
Nrf2-knockout protects from intestinal injuries in C57BL/6J mice
following abdominal irradiation with γ rays. Int J Mol Sci.
18:E16562017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Møller P, Løhr M, Folkmann JK, Mikkelsen L
and Loft S: Aging and oxidatively damaged nuclear DNA in animal
organs. Free Radic Biol Med. 48:1275–1285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gkogkolou P and Böhm M: Advanced glycation
end products: key players in skin aging? Dermatoendocrinol.
4:259–270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Willett TL, Pasquale J and Grynpas MD:
Collagen modifications in postmenopausal osteoporosis: Advanced
glycation end products may affect bone volume, structure and
quality. Curr Osteoporos Rep. 12:329–337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YS, Wang XJ, Feng W and Hua KQ:
Advanced glycation end products decrease collagen I levels in
fibroblasts from the vaginal wall of patients with POP via the
RAGE, MAPK and NF-κB pathways. Int J Mol Med. 40:987–998. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nunnari J and Suomalainen A: Mitochondria:
In sickness and in health. Cell. 148:1145–1159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim SJ, Cheresh P, Jablonski RP, Williams
DB and Kamp DW: The role of mitochondrial DNA in mediating alveolar
epithelial cell apoptosis and pulmonary fibrosis. Int J Mol Sci.
16:21486–21519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen H, Detmer SA, Ewald AJ, Griffin EE,
Fraser SE and Chan DC: Mitofusins Mfn1 and Mfn2 coordinately
regulate mitochondrial fusion and are essential for embryonic
development. J Cell Biol. 160:189–200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Brito OM and Scorrano L: Mitofusin 2
tethers endoplasmic reticulum to mitochondria. Nature. 456:605–610.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sebastián D, Hernández-Alvarez MI, Segalés
J, Sorianello E, Muñoz JP, Sala D, Waget A, Liesa M, Paz JC,
Gopalacharyulu P, et al: Mitofusin 2 (Mfn2) links mitochondrial and
endoplasmic reticulum function with insulin signaling and is
essential for normal glucose homeostasis. Proc Natl Acad Sci USA.
109:5523–5528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Wang X, Zhou Y, Peng C, Chen H and
Lu Y: Mitofusin2 regulates the proliferation and function of
fibroblasts: The possible mechanisms underlying pelvic organ
prolapse development. Mol Med Rep. 20:2859–2866. 2019.PubMed/NCBI
|
|
24
|
Lu Y, Chen HY, Wang XQ and Wang JX:
Correlations between Mitofusin 2 expression in fibroblasts and
pelvic organ prolapse: An in vitro study. Chin Med J (Engl).
130:2951–2959. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McHugh D and Gil J: Senescence and aging:
Causes, consequences, and therapeutic avenues. J Cell Biol.
217:65–77. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burton DG and Krizhanovsky V:
Physiological and pathological consequences of cellular senescence.
Cell Mol Life Sci. 71:4373–4386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kirkland JL and Tchkonia T: Cellular
senescence: A translational perspective. EBioMedicine. 21:21–28.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen B and Yeh J: Alterations in
connective tissue metabolism in stress incontinence and prolapse. J
Urol. 186:1768–1772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alcorta DA, Xiong Y, Phelps D, Hannon G,
Beach D and Barrett JC: Involvement of the cyclin-dependent kinase
inhibitor p16 (INK4a) in replicative senescence of normal human
fibroblasts. Proc Natl Acad Sci USA. 93:13742–13747. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takahashi A, Ohtani N, Yamakoshi K, Iida
S, Tahara H, Nakayama K, Nakayama KI, Ide T, Saya H and Hara E:
Mitogenic signalling and the p16INK4a-Rb pathway cooperate to
enforce irreversible cellular senescence. Nat Cell Biol.
8:1291–1297. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beauséjour CM, Krtolica A, Galimi F,
Narita M, Lowe SW, Yaswen P and Campisi J: Reversal of human
cellular senescence: Roles of the p53 and p16 pathways. EMBO J.
22:4212–4222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sampson N, Berger P and Zenzmaier C: Redox
signaling as a therapeutic target to inhibit myofibroblast
activation in degenerative fibrotic disease. BioMed Res Int.
2014:1317372014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cole EE, Leu PB, Gomelsky A, Revelo P,
Shappell H, Scarpero HM and Dmochowski RR: Histopathological
evaluation of the uterosacral ligament: Is this a dependable
structure for pelvic reconstruction? BJU Int. 97:345–348. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu C, Wang Y, Li BS, Yang Q, Tang JM, Min
J, Hong SS, Guo WJ and Hong L: Role of transforming growth factor β
1 in the pathogenesis of pelvic organ prolapse: A potential
therapeutic target. Int J Mol Med. 40:347–356. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamamoto K, Yamamoto M, Akazawa K, Tajima
S, Wakimoto H and Aoyagi M: Decrease in elastin gene expression and
protein synthesis in fibroblasts derived from cardinal ligaments of
patients with prolapsus uteri. Cell Biol Int. 21:605–611. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamamoto M, Aoyagi M, Akazawa K, Tajima S
and Yamamoto K: Decrease in p53 protein in cultured cardinal
ligament fibroblasts from patients with prolapsus uteri. Cell Biol
Int. 22:31–40. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Quan T, Qin Z, Robichaud P, Voorhees JJ
and Fisher GJ: CCN1 contributes to skin connective tissue aging by
inducing age-associated secretory phenotype in human skin dermal
fibroblasts. J Cell Commun Signal. 5:201–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jun JI and Lau LF: The matricellular
protein CCN1 induces fibroblast senescence and restricts fibrosis
in cutaneous wound healing. Nat Cell Biol. 12:676–685. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Powers ET, Morimoto RI, Dillin A, Kelly JW
and Balch WE: Biological and chemical approaches to diseases of
proteostasis deficiency. Annu Rev Biochem. 78:959–991. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hartl FU, Bracher A and Hayer-Hartl M:
Molecular chaperones in protein folding and proteostasis. Nature.
475:324–332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brennan M, Bhatti H, Nerusu KC,
Bhagavathula N, Kang S, Fisher GJ, Varani J and Voorhees JJ: Matrix
metalloproteinase-1 is the major collagenolytic enzyme responsible
for collagen damage in UV-irradiated human skin. Photochem
Photobiol. 78:43–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cuervo AM, Bergamini E, Brunk UT, Dröge W,
Ffrench M and Terman A: Autophagy and aging: The importance of
maintaining ‘clean’ cells. Autophagy. 1:131–140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bulteau AL, Moreau M, Nizard C and Friguet
B: Proteasome and photoaging: The effects of UV irradiation. Ann N
Y Acad Sci. 1100:280–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pereira L, D'Alessio M, Ramirez F, Lynch
JR, Sykes B, Pangilinan T and Bonadio J: Genomic organization of
the sequence coding for fibrillin, the defective gene product in
Marfan syndrome. Hum Mol Genet. 2:17621993. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang H, Hu W and Ramirez F: Developmental
expression of fibrillin genes suggests heterogeneity of
extracellular microfibrils. J Cell Biol. 129:1165–1176. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tyagi T, Alarab M, Leong Y, Lye S and
Shynlova O: Local oestrogen therapy modulates extracellular matrix
and immune response in the vaginal tissue of post-menopausal women
with severe pelvic organ prolapse. J Cell Mol Med. 23:2907–2919.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Calamini B and Morimoto RI: Protein
homeostasis as a therapeutic target for diseases of protein
conformation. Curr Top Med Chem. 12:2623–2640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tomaru U, Takahashi S, Ishizu A, Miyatake
Y, Gohda A, Suzuki S, Ono A, Ohara J, Baba T, Murata S, et al:
Decreased proteasomal activity causes age-related phenotypes and
promotes the development of metabolic abnormalities. Am J Pathol.
180:963–972. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Coolen AWM, Troost S, Mol BWJ, Roovers
JPWR and Bongers MY: Primary treatment of pelvic organ prolapse:
Pessary use versus prolapse surgery. Int Urogynecol J Pelvic Floor
Dysfunct. 29:99–107. 2018. View Article : Google Scholar
|
|
51
|
Cheng J, Zhao ZW, Wen JR, Wang L, Huang
LW, Yang YL, Zhao FN, Xiao JY, Fang F, Wu J, et al: Status,
challenges, and future prospects of stem cell therapy in pelvic
floor disorders. World J Clin Cases. 8:1400–1413. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shaw AC, Joshi S, Greenwood H, Panda A and
Lord JM: Aging of the innate immune system. Curr Opin Immunol.
22:507–513. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kirkwood TB: Understanding the odd science
of aging. Cell. 120:437–447. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jin M, Wu Y, Wang J, Ye W, Wang L, Yin P,
Liu W, Pan C and Hua X: MicroRNA-29 facilitates transplantation of
bone marrow-derived mesenchymal stem cells to alleviate pelvic
floor dysfunction by repressing elastin. Stem Cell Res Ther.
7:1672016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jin M, Chen Y, Zhou Y, Mei Y, Liu W, Pan C
and Hua X: Transplantation of bone marrow-derived mesenchymal stem
cells expressing elastin alleviates pelvic floor dysfunction. Stem
Cell Res Ther. 7:512016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ulrich D, Edwards SL, Su K, Tan KS, White
JF, Ramshaw JA, Lo C, Rosamilia A, Werkmeister JA and Gargett CE:
Human endometrial mesenchymal stem cells modulate the tissue
response and mechanical behavior of polyamide mesh implants for
pelvic organ prolapse repair. Tissue Eng Part A. 20:785–798.
2014.PubMed/NCBI
|
|
57
|
El Agha E, Kramann R, Schneider RK, Li X,
Seeger W, Humphreys BD and Bellusci S: Mesenchymal stem cells in
fibrotic disease. Cell Stem Cell. 21:166–177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ohshima S: Centrosome aberrations
associated with cellular senescence and p53 localization at
supernumerary centrosomes. Oxid Med Cell Longev. 2012:2175942012.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fumagalli M, Rossiello F, Clerici M,
Barozzi S, Cittaro D, Kaplunov JM, Bucci G, Dobreva M, Matti V,
Beausejour CM, et al: Telomeric DNA damage is irreparable and
causes persistent DNA-damage-response activation. Nat Cell Biol.
14:355–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Garm C, Moreno-Villanueva M, Bürkle A,
Petersen I, Bohr VA, Christensen K and Stevnsner T: Age and gender
effects on DNA strand break repair in peripheral blood mononuclear
cells. Aging Cell. 12:58–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
De Cecco M, Criscione SW, Peckham EJ,
Hillenmeyer S, Hamm EA, Manivannan J, Peterson AL, Kreiling JA,
Neretti N and Sedivy JM: Genomes of replicatively senescent cells
undergo global epigenetic changes leading to gene silencing and
activation of transposable elements. Aging Cell. 12:247–256. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Harley CB, Futcher AB and Greider CW:
Telomeres shorten during ageing of human fibroblasts. Nature.
345:458–460. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sedelnikova OA, Horikawa I, Zimonjic DB,
Popescu NC, Bonner WM and Barrett JC: Senescing human cells and
ageing mice accumulate DNA lesions with unrepairable double-strand
breaks. Nat Cell Biol. 6:168–170. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hoeijmakers JH: DNA damage, aging, and
cancer. N Engl J Med. 361:1475–1485. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Herbig U, Ferreira M, Condel L, Carey D
and Sedivy JM: Cellular senescence in aging primates. Science.
311:12572006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Krutmann J and Schroeder P: Role of
mitochondria in photoaging of human skin: The defective powerhouse
model. J Investig Dermatol Symp Proc. 14:44–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ressler S, Bartkova J, Niederegger H,
Bartek J, Scharffetter-Kochanek K, Jansen-Dürr P and Wlaschek M:
p16INK4A is a robust in vivo biomarker of cellular aging in human
skin. Aging Cell. 5:379–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Jeyapalan JC, Ferreira M, Sedivy JM and
Herbig U: Accumulation of senescent cells in mitotic tissue of
aging primates. Mech Ageing Dev. 128:36–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kreiling JA, Tamamori-Adachi M, Sexton AN,
Jeyapalan JC, Munoz-Najar U, Peterson AL, Manivannan J, Rogers ES,
Pchelintsev NA, Adams PD, et al: Age-associated increase in
heterochromatic marks in murine and primate tissues. Aging Cell.
10:292–304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hayflick L: The biology of human aging.
Adv Pathobiol. 7:80–99. 1980.PubMed/NCBI
|
|
71
|
Lee HC, Yin PH, Chi CW and Wei YH:
Increase in mitochondrial mass in human fibroblasts under oxidative
stress and during replicative cell senescence. J Biomed Sci.
9:517–526. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
d'Adda di Fagagna F, Reaper PM,
Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G,
Carter NP and Jackson SP: A DNA damage checkpoint response in
telomere-initiated senescence. Nature. 426:194–198. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Passos JF, Nelson G, Wang C, Richter T,
Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat
A, et al: Feedback between p21 and reactive oxygen production is
necessary for cell senescence. Mol Syst Biol. 6:3472010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Herbig U, Jobling WA, Chen BP, Chen DJ and
Sedivy JM: Telomere shortening triggers senescence of human cells
through a pathway involving ATM, p53, and p21(CIP1), but not
p16(INK4a). Mol Cell. 14:501–513. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Coppé JP, Desprez PY, Krtolica A and
Campisi J: The senescence-associated secretory phenotype: The dark
side of tumor suppression. Annu Rev Pathol. 5:99–118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Narita M, Nũnez S, Heard E, Narita M, Lin
AW, Hearn SA, Spector DL, Hannon GJ and Lowe SW: Rb-mediated
heterochromatin formation and silencing of E2F target genes during
cellular senescence. Cell. 113:703–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gabriel B, Denschlag D, Göbel H, Fittkow
C, Werner M, Gitsch G and Watermann D: Uterosacral ligament in
postmenopausal women with or without pelvic organ prolapse. Int
Urogynecol J Pelvic Floor Dysfunct. 16:475–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Bump RC, Mattiasson A, Bø K, Brubaker LP,
DeLancey JO, Klarskov P, Shull BL and Smith AR: The standardization
of terminology of female pelvic organ prolapse and pelvic floor
dysfunction. Am J Obstet Gynecol. 175:10–17. 1996. View Article : Google Scholar : PubMed/NCBI
|