Introduction
Lung cancer is a common malignancy and its
initiation and progression are complex processes associated with
the loss of normal regulatory pathways, including cell
proliferation, differentiation and apoptosis (1–4).
Current treatment strategies include chemotherapy; however, the
efficacy is limited and the side effects of these drugs pose major
challenges (5). Therefore, the
development of novel lung cancer drugs is imperative. Owing to its
long history in tumor treatment, the search for anti-tumor drugs
among Chinese herbal medicines has become a hot topic in cancer
research.
Artesunate (Art) is a water-soluble hemisuccinate
derivative of dihydroartemisinin and the most widely used member of
the family of artemisinin drugs. Artemisinin compounds are widely
used for the treatment of severe and complicated malaria in humans.
Art is a classic anti-malarial drug for the treatment of severe and
drug-resistant malaria (6–10). It also exerts anti-tumor activity
(11–17). Several in vitro and in
vivo studies have indicated that the anti-tumor effect of Art
is associated with the induction of apoptosis and cell cycle arrest
(11–13). It was also demonstrated to inhibit
tumor infiltration and metastasis (18,19).
However, studies investigating the mechanisms of the anti-tumor
effects of Art in lung cancer are limited, and hence, its role in
lung cancer therapy remains to be clarified. In the present study,
the anti-cancer efficacy of Art in the A549 lung adenocarcinoma
cell line was assessed and the underlying molecular mechanisms were
investigated.
The balance between apoptosis and cell proliferation
is essential for maintaining the function of normal cells and
growth of normal tissues (20,21).
Disruption of this balance leads to diseases, including tumors, in
the body. Cell apoptosis is closely linked to the occurrence of a
tumor. At present, apoptosis is the most studied mechanism in
anti-cancer therapy. Cellular apoptosis is an automated
gene-controlled death program mediated via complex regulatory
mechanisms. Bcl-2, Bax and the mitochondrial membrane potential are
essential effectors of the intrinsic pathway of apoptosis.
In the present study, it was investigated whether
Art is able to induce lung cancer cell apoptosis by regulating the
intrinsic apoptosis pathway, thereby providing a novel Art-mediated
mechanism for lung cancer treatment.
Materials and methods
Cell line and culture
The human lung cancer cell line A549 was purchased
form Procell Life Science & Technology Co., Ltd., and cultured
in RPMI 1640 medium supplemented with 10% fetal bovine serum (both
purchased from Gibco; Thermo Fisher Scientific, Inc.), 100 units/ml
penicillin and 100 µg/ml streptomycin at 37°C in a humidified
atmosphere with 5% CO2.
Chemicals and reagents
Art was purchased from Guilin Pharmaceutical
(Shanghai) Co., Ltd. The Annexin V-FITC/PI kit was purchased from
Beckman Coulter, Inc., while propidium iodide (PI) was purchased
from BD Biosciences.
Cytotoxicity assay
The sensitivity of A549 lung cancer cells to Art was
determined using an MTT assay, which is based on the capacity of
viable cells to metabolize a yellow tetrazolium salt, MTT, to form
purple formazan crystals. These are solubilized in acidified
2-propanol and the absorbance is measured spectrophotometrically at
490 nm. The cells were seeded in 96-well plates at a density of
5×104 cells/ml/well. Once cells were attached, serially
diluted art solution was added to reach a final concentration of 0,
0.1, 0.5, 1, 5, 10, 50, 100, 200, 400 or 800 µg/ml in a final
volume of 200 µl/well. Normal saline (NS) was used for the control
group (22). After drug treatment
for 24 h, the medium was replaced with an equivalent volume of
fresh RPMI 1640 medium containing 0.5 mg/ml MTT, followed by
incubation for an additional 4 h. Subsequently, the medium was
replaced with 180 µl DMSO, followed by incubation for 10 min at
room temperature. The cytotoxic effects of the drug concentrations
were determined by measuring the optical density values at 490 nm
with a microplate reader. Cell viability was expressed as the
relative synthesis of formazan in treated samples compared with the
control cells [(treated cells/control cells) ×100%].
Experimental groups and drug
intervention experiments
After the cultured A549 tumor cells were attached,
cells were grouped evenly and treated with serial concentrations of
artesunate (0, 25, 50 and 100 µg/ml) for 24 h. Then, cells were
harvested routinely; NS was used as the control. The cell
concentration of the suspension was adjusted to
1×106/ml. Each experiment was performed three times.
Assessment of the cell cycle
distribution using flow cytometry (FCM)
Single-cell suspension (1 ml containing
1×106 cells) was prepared, washed with cold PBS and
fixed with 70% ethanol at 4°C for 24 h prior to the addition of 1
ml PI (50 µg/ml). After incubation at 4°C for 30 min, FCM (FC-500;
Beckman Coulter, Inc.) was performed and MultiCycle AV software
(Beckman Coulter, Inc.) was used to analyze the cell cycle. The
proliferation status was expressed by the following proliferation
index =
(S+G2/M)/(G0/G1+S+G2/M)
×100%.
Assessment of cell apoptosis of A549
cells using FCM
To detect apoptosis, 1 ml of single-cell suspension
was stained with PI (12.5 µg/ml) and Annexin V-FITC (0.25 µg/ml)
and analyzed with an FC500 flow cytometer (Beckman Coulter). Cells
with positive staining for Annexin V and negative staining for PI
were considered to be in early apoptosis, whereas those that were
positive for both Annexin V and PI were considered to be in late
apoptosis.
Analysis of mitochondrial membrane
potential of A549 cells using FCM
To determine the mitochondrial membrane potential
following Art treatment, harvested A549 cells were washed with
ice-cold PBS and stained with 1 ml fluorescence reagent containing
10 µg/ml Rhodamine 123. After incubation for 30 min in the dark at
37°C, the stained cells were re-suspended in 1 ml PBS and analyzed
using the FC500 flow cytometer.
Assessment of the expression of Bcl-2
and Bax proteins in A549 cells using FCM
To determine the expression of Bcl-2 and Bax
proteins after Art treatment, the harvested cells were fixed
overnight in 70% ice-cold ethanol. After washing with ice-cold PBS,
the cells were incubated with anti-Bcl-2 (1:100; cat. no. sc-7382;
Santa Cruz Biotechnology, Inc.) and anti-Bax antibodies (1:100;
cat. no. sc-20067; Santa Cruz Biotechnology, Inc.) for 30 min in
the dark at room temperature. Subsequently, the cells were
incubated with IgG-FITC antibody (1:100; cat. no. 115-095-003;
Jackson ImmunoResearch Laboratories, Inc.) for 30 min in the dark
at room temperature. The stained cells were analyzed using the
FC500 flow cytometer, with the mean fluorescence intensity
representing the expression of Bcl-2 and Bax proteins.
Statistical analysis
Statistical analysis was performed using the SPSS
v21 software (IBM Corp.). Values are expressed as the mean ±
standard deviation. Multiple groups were compared using one-way
analysis of variance followed by Tukeys test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Art inhibits A549 cell survival and
proliferation
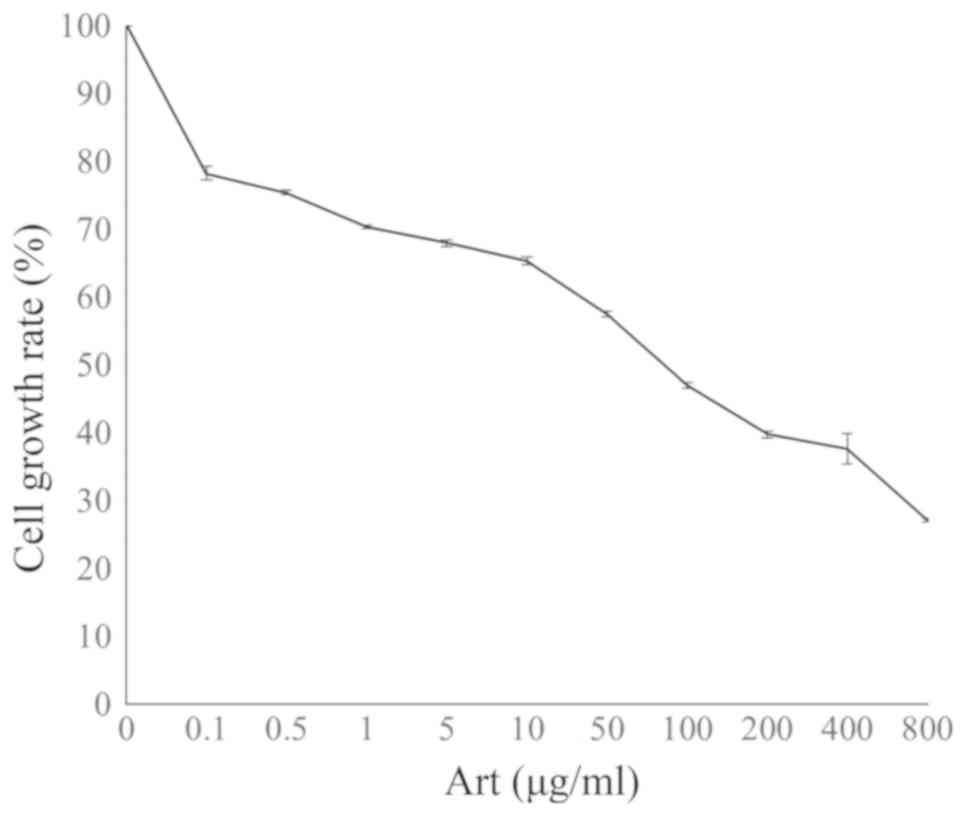
An MTT assay was used to determine the viability of
A549 lung adenocarcinoma cells treated with different
concentrations of Art at a dose range of 0.1–800 µg/ml for 24 h.
The survival of A549 cells was decreased by Art in a dose-dependent
manner, with a 50% inhibitory concentration (IC50) value
of 52.87±2.36 µg/ml (Fig. 1).
Treatment with Art exerted a growth inhibitory effect on A549 cells
in a concentration-dependent manner.
In addition, FCM revealed that the proliferation
index of A549 cells was significantly lower in the Art-treated
groups than that in the control group (P<0.01). The cell
proliferation index in the 100 µg/ml group was significantly lower
than that in the 25 and 50 µg/ml Art groups (P<0.01).
Furthermore, the cell population in G0/1 phase was significantly
higher in the Art-treated groups than in the control and the G0/1
phase population of the cell cycle increased with the concentration
of Art (P<0.01; Table I).
 | Table I.Cell cycle phase distribution of A549
cells after treatment with various concentrations of Art. |
Table I.
Cell cycle phase distribution of A549
cells after treatment with various concentrations of Art.
| Group | G0/1 (%) | S (%) | G2/M (%) | Proliferation index
(%) |
|---|
| Control | 59.56±0.11 | 36.68±1.79 | 3.76±1.72 | 40.44±0.11 |
| 25 µg/ml Art |
61.78±0.19a | 32.10±3.31 | 6.12±3.13 |
38.22±0.19a |
| 50 µg/ml Art |
63.48±0.18a |
28.41±1.18a | 8.11±1.10 |
36.52±0.18a |
| 100 µg/ml Art |
65.32±0.21a |
23.95±1.26a |
10.73±1.08a |
34.68±0.21a |
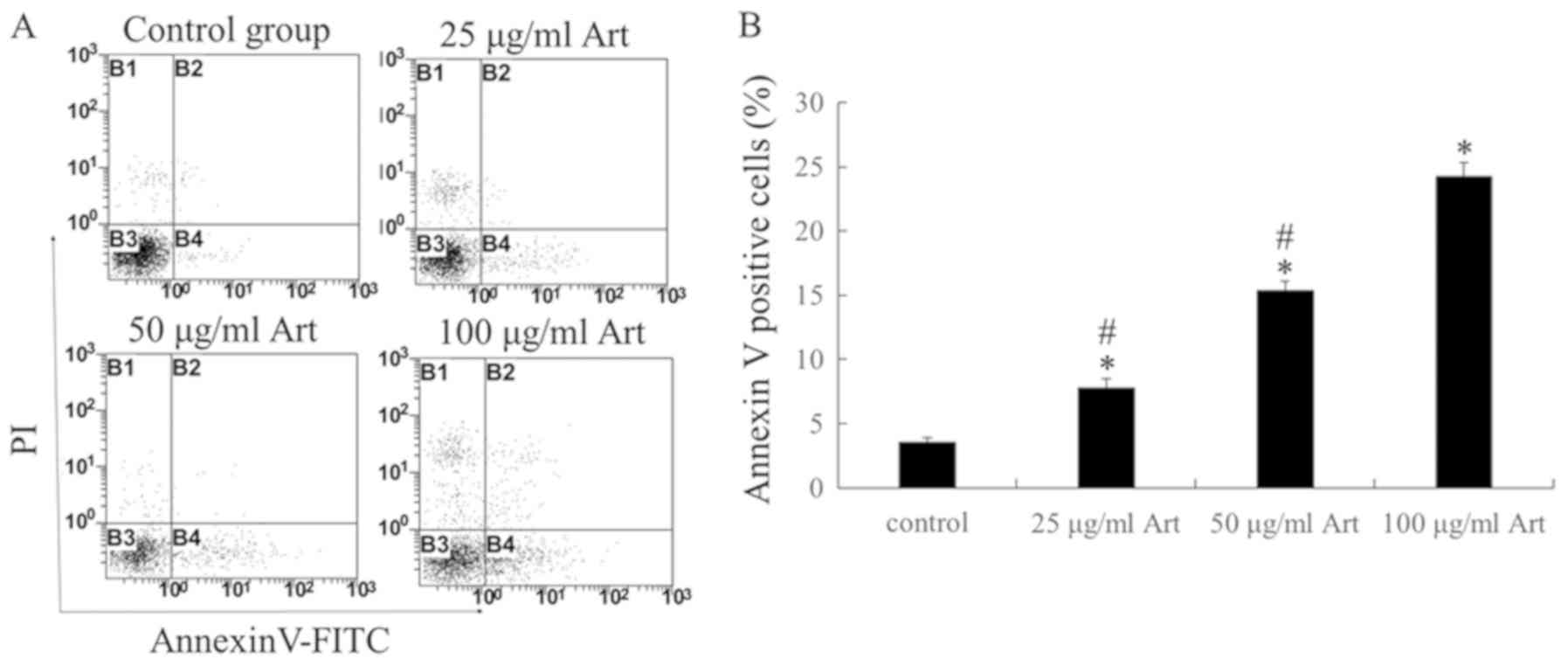
Art induces cell apoptosis and
modulates the mitochondrial membrane potential
In order to further investigate the mechanism of the
decrease in the number of viable A549 cells upon exposure to Art in
the MTT assay, Annexin V/PI staining was performed to determine
whether apoptosis was induced (Fig.
2). The results indicated that Art triggered apoptosis in a
dose-dependent manner within a range of 25–100 µg/ml; furthermore,
the cell apoptosis rate in the 100 µg/ml Art group was
significantly higher than that in the 25 and 50 µg/ml groups
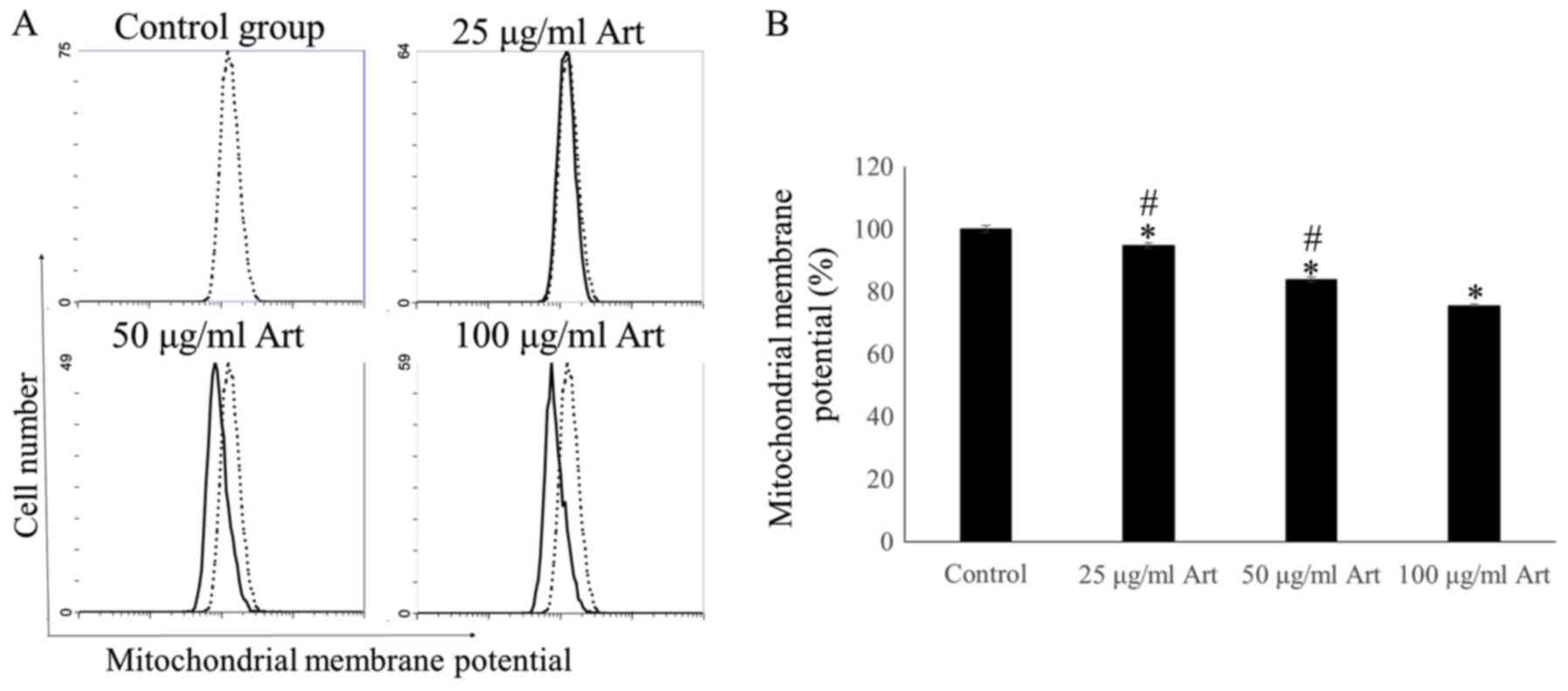
(P<0.01). In addition, the mitochondrial membrane potential was
determined in A549 cells using FCM. The mitochondrial membrane
potential in the 25, 50 and 100 µg/ml Art groups was significantly
lower than that in the control group (P<0.01). Treatment with
Art significantly decreased the mitochondrial membrane potential,
suggesting that Art triggered apoptosis in A549 cells in a
dose-dependent manner (P<0.01; Fig.
3).
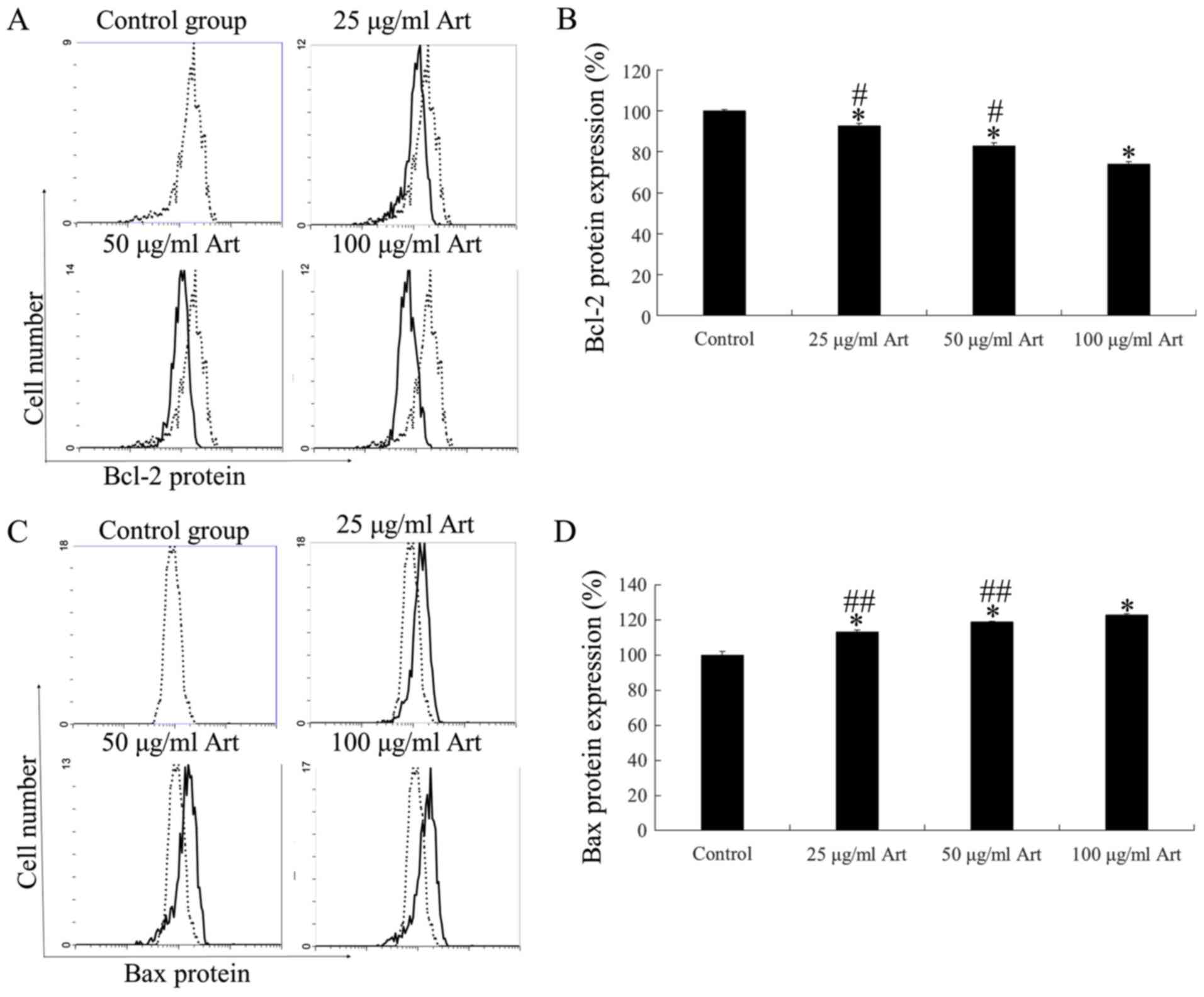
Art modulates Bcl-2 and Bax protein
expression in A549 cells
Bcl-2 and Bax regulate cell apoptosis via pore
formation in mitochondrial complexes, thereby serving as markers
for further analysis of the mechanism of action of Art in A549
cells. FCM revealed significant downregulation of Bcl-2 protein
expression compared with the control (P<0.01; Fig. 4A and B). Furthermore, Bax protein
expression was significantly upregulated compared with the control
following treatment with Art for 24 h (P<0.01; Fig. 4C and D). These expression profiles
were in line with the increased apoptotic activity via
mitochondrial membrane modulation in A549 lung cancer cells after
Art treatment.
Discussion
Lung cancer is one of the most common malignant
cancer types worldwide, exhibiting high morbidity and mortality
rates (1). Typical treatment
regimens include surgery, chemotherapy and radiotherapy (23,24).
Chemotherapy is the primary treatment for late-stage, inoperable
cases. However, toxic side effects of chemotherapeutic drugs have
severely affected the efficacy of such regimens, frequently
accounting for treatment failure. Thus, identifying anti-cancer
drugs for lung cancer with low toxicity and high efficacy is
imperative.
Chinese herbal medicine for tumor treatment has a
long history with minimal side effects. Art is a derivative of
artemisinin, derived from Artemisia annua, and is a classic
anti-malarial drug that was recently demonstrated to have
anti-tumor activity and is involved in the regulation of immune
function (25–29). Several in vitro and in
vivo studies have indicated that the anti-tumor effect of Art
is associated with the induction of apoptosis and cell cycle
arrest. Yang et al (30),
reported that Art induces mitochondrial apoptosis of retinoblastoma
cells via upregulating Kruppel-like factor 6 in in vivo and
in vitro experiments. Wang et al (31), indicated that Art inhibited the
proliferation and induced ferroptosis of CA-46cells in vivo.
Li et al (32), reported
that Art may be valuable as a therapeutic agent for osteoarthritis.
Chen et al (33) suggested
that Art promoted type I T-helper cell differentiation from
CD4+ T cells by downregulating Sirtuin 1 through
microRNA-142, thereby enhancing cell apoptosis in ovarian cancer.
Chen et al (34),
demonstrated that Art inhibited β-catenin expression and cell
proliferation as well as promoted apoptosis in MG-63 cells,
rendering it a promising drug for the clinical treatment of
osteosarcoma.
In addition to the anti-tumor effect, Art is also
able to reverse the drug resistance of tumors. Nunes et al
(35), reported that Art disrupts
the androgen receptor antagonist-mediated resistance observed in
metastatic castration-resistant prostate cancer. Jing et al
(36), suggested that the
combination of Art and sorafenib further improved the apoptosis of
hepatocellular carcinoma (HCC) and revealed that Art induces HCC
apoptosis via PI3K/AKT/mTOR pathway inhibition, thereby suggesting
that the combination of Art and sorafenib is a potential
therapeutic regimen for advanced HCC.
Previous studies by our group suggested that Art
inhibited the growth of esophageal cancer and gastric cancer cells
by inducing apoptosis (22,37,38)
and reversed the multidrug resistance of esophageal cancer by
modulating the expression of ATP binding cassette G2 (37,39).
The present study focused on the inhibitory effects of Art in lung
cancer. Compared with previous studies (22,40),
the present study investigated the inhibitory effects of Art on
lung cancer, including apoptosis. In addition, the present study
focused on the molecular mechanisms of apoptosis, which was
indicated to be mediated by the endogenous apoptosis pathway
centered on the mitochondrial membrane potential; furthermore, Art
inhibited the growth of lung cancer cells via induction of cell
cycle arrest.
In the present study, the lung cancer cell line A549
was selected to test the potential anti-cancer effects and the
tumor-suppressive effects of Art against lung cancer cells in
vitro. MTT assay suggested that Art inhibited the growth of
A549 cells in a dose-dependent manner. The IC50 of Art
on the A549 lung cancer cells over 24 h was 52.87 µg/ml and three
concentrations (25, 50 and 100 µg/ml) were selected for subsequent
experiments. However, the molecular mechanisms underlying
Art-induced cell death in lung cancer cells remained to be
clarified. In the present study, it was determined that Art exerts
potent cytotoxic effects on the human lung cancer cell line A549
in vitro. The cytotoxicity of Art was mediated by induction
of apoptosis and cell cycle arrest, which was further supported by
the detection of apoptosis-associated factors.
Malignant tumor cells have strong proliferative
abilities, typically with increasing S and G2/M phase
populations during the cell cycle. In the present study, it was
determined that the proliferation index of A549 lung cancer cells
decreased in a dose-dependent manner following Art treatment, which
blocked the cell cycle progression of these cells at the G0/1
stage. Thus, these results suggested that Art inhibits the growth
of lung cancer cells by inducing cell cycle arrest.
Dysregulation of apoptosis has a critical role in
the occurrence and development of tumors and induction of cell
apoptosis is vital for drug inhibition in aberrant cell growth.
Cellular apoptosis is an automated gene-controlled death mediated
via complex regulatory mechanisms. In the present study, cellular
apoptosis was determined by the detection of Annexin V/PI staining
by FCM. The results suggested that Art induced apoptosis of lung
cancer cells in a dose-dependent manner. The mitochondrial membrane
potential, Bcl-2 and Bax are essential effectors of the intrinsic
pathway of apoptosis, which were modulated by Art treatment. Since
the mitochondrial membrane potential is closely associated with
apoptosis, the decrease in the level may cause irreversible cell
death. Hence, the content of markers of the intrinsic pathway of
apoptosis, namely the Bcl-2 and Bax proteins, which regulate the
mitochondrial membrane potential, was determined. The expression of
Bcl-2 protein was indicated to be significantly decreased along
with the upregulation of Bax following Art administration, which
was consistent with decreased mitochondrial membrane potential that
results in cell apoptosis.
In conclusion, the present study explored the
specific mechanisms of lung cancer cell inhibition by Art. Art
induced lung cancer cell apoptosis and cell cycle arrest, reduced
the level of Bcl-2 protein and mitochondrial membrane potential and
increased the expression of Bax protein. A previous study found
that Art was associated with minimal toxic side effects (22), taken together with the present
findings, it could be speculated that Art may have potential as a
highly effective, non-toxic anti-cancer agent in chemotherapy. The
molecular mechanisms of growth inhibition of lung cancer cells by
Art are complex, necessitating further studies in the future. In
future investigations, a variety of lung cancer cell lines should
be used to further verify the growth inhibitory effect of Art on
lung cancer cells. In addition, non-cancerous cells should be used
to determine the side effects of Art.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors contributions
YZ performed the experiments and wrote the
manuscript. JL performed the experiments and statistical analysis.
LL designed the study, performed the experiments and revised the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de Groot P and Munden RF: Lung cancer
epidemiology, risk factors, and prevention. Radiol Clin North Am.
50:3017–876. 2012. View Article : Google Scholar
|
|
2
|
Kosacka M and Jankowska R: The
epidemiology of lung cancer. Pneumonol Alergol Pol. 75:76–80.
2007.(In Polish). PubMed/NCBI
|
|
3
|
Radziszewska A, Karczmarek-Borowska B,
Gradalska-Lampart M and Filip AA: Epidemiology, prevention and risk
morbidity factors for lung cancer. Pol Merkur Lekarski. 38:113–118.
2015.(In Polish). PubMed/NCBI
|
|
4
|
Steliga MA and Dresler CM: Epidemiology of
lung cancer: Smoking, secondhand smoke, and genetics. Surg Oncol
Clin N Am. 20:605–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tabchi S, Kassouf E, Rassy EE, Kourie HR,
Martin J, Campeau MP, Tehfe M and Blais N: Management of stage III
non-small cell lung cancer. Semin Oncol. 44:163–177. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abuaku BK, Mensah BA, Ofori MF,
Myers-Hansen J, Derkyi-Kwarteng AN, Essilfie F, Dokurugu M, Amoakoh
E, Koram KA and Ghansah A: Efficacy of artesunate/amodiaquine in
the treatment of uncomplicated malaria among children in Ghana. Am
J Trop Med Hyg. 97:690–695. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roussel C, Caumes E, Thellier M, Ndour PA,
Buffet PA and Jaureguiberry S: Artesunate to treat severe malaria
in travellers: review of efficacy and safety and practical
implications. J Travel Med. doi:10.1093/jtm/taw093. PubMed/NCBI
|
|
8
|
Jauréguiberry S, Thellier M, Ndour PA,
Ader F, Roussel C, Sonneville R, Mayaux J, Matheron S, Angoulvant
A, Wyplosz B, et al French Artesunate Working Group, :
Delayed-onset hemolytic anemia in patients with travel-associated
severe malaria treated with artesunate, France, 2011–2013. Emerg
Infect Dis. 21:804–812. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Raffray L, Receveur MC, Beguet M, Lauroua
P, Pistone T and Malvy D: Severe delayed autoimmune haemolytic
anaemia following artesunate administration in severe malaria: A
case report. Malar J. 13:3982014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rolling T, Agbenyega T, Krishna S,
Kremsner PG and Cramer JP: Delayed haemolysis after artesunate
treatment of severe malaria - review of the literature and
perspective. Travel Med Infect Dis. 13:143–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greenshields AL, Fernando W and Hoskin DW:
The anti-malarial drug artesunate causes cell cycle arrest and
apoptosis of triple-negative MDA-MB-468 and HER2-enriched SK-BR-3
breast cancer cells. Exp Mol Pathol. 107:10–22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang N, Chen H, Teng Y, Ding X, Wu H and
Jin X: Artesunate inhibits proliferation and invasion of mouse
hemangioendothelioma cells in vitro and of tumor growth
in vivo. Oncol Lett. 14:6170–6176. 2017.PubMed/NCBI
|
|
13
|
Zheng L and Pan J: The anti-malarial drug
artesunate blocks Wnt/β-catenin pathway and inhibits growth,
migration and invasion of uveal melanoma cells. Curr Cancer Drug
Targets. 18:988–998. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Verma S, Das P and Kumar VL:
Chemoprevention by artesunate in a preclinical model of colorectal
cancer involves down regulation of β-catenin, suppression of
angiogenesis, cellular proliferation and induction of apoptosis.
Chem Biol Interact. 278:84–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vandewynckel YP, Laukens D, Geerts A,
Vanhove C, Descamps B, Colle I, Devisscher L, Bogaerts E, Paridaens
A, Verhelst X, et al: Therapeutic effects of artesunate in
hepatocellular carcinoma: Repurposing an ancient antimalarial
agent. Eur J Gastroenterol Hepatol. 26:861–870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeong DE, Song HJ, Lim S, Lee SJ, Lim JE,
Nam DH, Joo KM, Jeong BC, Jeon SS, Choi HY, et al: Repurposing the
anti-malarial drug artesunate as a novel therapeutic agent for
metastatic renal cell carcinoma due to its attenuation of tumor
growth, metastasis, and angiogenesis. Oncotarget. 6:33046–33064.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim C, Lee JH, Kim SH, Sethi G and Ahn KS:
Artesunate suppresses tumor growth and induces apoptosis through
the modulation of multiple oncogenic cascades in a chronic myeloid
leukemia xenograft mouse model. Oncotarget. 6:4020–4035. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rasheed SA, Efferth T, Asangani IA and
Allgayer H: First evidence that the antimalarial drug artesunate
inhibits invasion and in vivo metastasis in lung cancer by
targeting essential extracellular proteases. Int J Cancer.
127:1475–1485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Qian H, Sha M, Luan Z, Lin M,
Yuan D, Li X, Huang J and Ye L: Downregulation of HOTAIR expression
mediated anti-metastatic effect of artesunate on cervical cancer by
inhibiting COX-2 expression. PLoS One. 11:e01648382016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alenzi FQ: Links between apoptosis,
proliferation and the cell cycle. Br J Biomed Sci. 61:99–102. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cvejic D, Selemetjev S, Savin S, Paunovic
I and Tatic S: Changes in the balance between proliferation and
apoptosis during the progression of malignancy in thyroid tumours.
Eur J Histochem. 53:65–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu L, Zuo LF, Zuo J and Wang J:
Artesunate induces apoptosis and inhibits growth of Eca109 and
Ec9706 human esophageal cancer cell lines in vitro and in
vivo. Mol Med Rep. 12:1465–1472. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duma N, Santana-Davila R and Molina JR:
Non-small cell lung cancer: Epidemiology, screening, diagnosis, and
treatment. Mayo Clin Proc. 94:1623–1640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
von Hagens C, Walter-Sack I, Goeckenjan M,
Osburg J, Storch-Hagenlocher B, Sertel S, Elsässer M, Remppis BA,
Edler L, Munzinger J, et al: Prospective open uncontrolled phase I
study to define a well-tolerated dose of oral artesunate as add-on
therapy in patients with metastatic breast cancer (ARTIC M33/2).
Breast Cancer Res Treat. 164:359–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Wang C, Wu Z, Xue J, Shen B, Zuo
W, Wang Z and Wang SL: Artesunate suppresses the growth of
prostatic cancer cells through inhibiting androgen receptor. Biol
Pharm Bull. 40:479–485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Q, Ni W, Deng Z, Liu M, She L and Xie
Q: Targeting nasopharyngeal carcinoma by artesunate through
inhibiting Akt/mTOR and inducing oxidative stress. Fundam Clin
Pharmacol. 31:301–310. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui C, Feng H, Shi X, Wang Y, Feng Z, Liu
J, Han Z, Fu J, Fu Z and Tong H: Artesunate down-regulates
immunosuppression from colorectal cancer Colon26 and RKO cells in
vitro by decreasing transforming growth factor β1 and
interleukin-10. Int Immunopharmacol. 27:110–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rassias DJ and Weathers PJ: Dried leaf
Artemisia annua efficacy against non-small cell lung cancer.
Phytomedicine. 52:247–253. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Y, Wu N, Wu Y, Chen H, Qiu J, Qian X,
Zeng J, Chiu K, Gao Q and Zhuang J: Artesunate induces
mitochondria-mediated apoptosis of human retinoblastoma cells by
upregulating Kruppel-like factor 6. Cell Death Dis. 10:8622019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang N, Zeng GZ, Yin JL and Bian ZX:
Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects
ferroptosis in Burkitts lymphoma. Biochem Biophys Res Commun.
519:533–539. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Mu W, Ren J, Wuermanbieke S, Wahafu
T, Ji B, Ma H, Amat A, Zhang K and Cao L: Artesunate alleviates
interleukin-1β-induced inflammatory response and apoptosis by
inhibiting the NF-κB signaling pathway in chondrocyte-like ATDC5
cells, and delays the progression of osteoarthritis in a mouse
model. Int J Mol Med. 44:1541–1551. 2019.PubMed/NCBI
|
|
33
|
Chen X, Zhang XL, Zhang GH and Gao YF:
Artesunate promotes Th1 differentiation from CD4+ T cells to
enhance cell apoptosis in ovarian cancer via miR-142. Braz J Med
Biol Res. 52:e79922019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen P, Gu WL, Gong MZ, Wang J and Li DQ:
Artesunate decreases beta-catenin expression, cell proliferation
and apoptosis resistance in the MG-63 human osteosarcoma cell line.
Cell Physiol Biochem. 43:1939–1949. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nunes JJ, Pandey SK, Yadav A, Goel S and
Ateeq B: Targeting NF-kappa B signaling by artesunate restores
sensitivity of castrate-resistant prostate cancer cells to
antiandrogens. Neoplasia. 19:333–345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jing W, Shuo L, Yingru X, Min M, Runpeng
Z, Jun X and Dong H: Artesunate promotes sensitivity to sorafenib
in hepatocellular carcinoma. Biochem Biophys Res Commun. 519:41–45.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu L, Zuo LF and Guo JW: Reversal of
multidrug resistance by the anti-malaria drug artesunate in the
esophageal cancer Eca109/ABCG2 cell line. Oncol Lett. 6:1475–1481.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang L, Liu L, Wang J and Chen Y:
Inhibitory effect of artesunate on growth and apoptosis of gastric
cancer cells. Arch Med Res. 48:623–630. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang L, Liu L, Chen Y, Du Y, Wang J and
Liu J: Correlation between adenosine triphosphate (ATP)-binding
cassette transporter G2 (ABCG2) and drug resistance of esophageal
cancer and reversal of drug resistance by artesunate. Pathol Res
Pract. 214:1467–1473. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang P, Luo HS, Li M and Tan SY:
Artesunate inhibits the growth and induces apoptosis of human
gastric cancer cells by downregulating COX-2. OncoTargets Ther.
8:845–854. 2015. View Article : Google Scholar
|