Introduction
Psoriasis is a common chronic inflammatory skin
disease in the clinic, its typical pathological changes include
hyperproliferation of keratinocytes and infiltration of
inflammatory cells (1). Although
it is commonly recognized as the result of interactions between
multiple factors, such as genetic and environmental factors
(2), the exact etiology of
psoriasis remains unclear. It has been reported that a large number
of inflammatory cells infiltrate the psoriatic lesions (3). T helper 17 (Th17) cells, a T cell
subpopulation that secretes pro-inflammatory cytokines, such as
interleukin (IL)-17 and IL-22, have been associated with the
pathogenesis of psoriasis (4).
Furthermore, in vitro studies have demonstrated that IL-22
induces excessive proliferation and abnormal differentiation of
human keratinocytes (5–7).
Keratinocytes are an important type of immune cell.
The excessive secretion of pro-inflammatory cytokines and
chemokines, the aggregation of neutrophils and the formation of new
blood vessels often occur in psoriasis (8). Therefore, a vicious circle is formed,
resulting in excessive proliferation and abnormal differentiation
of keratinocytes (2). In addition,
the abnormal activation of signal transduction pathways, associated
with immune responses, is considered to play a critical role in the
pathogenesis of psoriasis (9).
Previous studies have revealed that IL-22 activates the three main
mitogen-ctivated protein kinase (MAPK) pathways, namely p38 kinase,
mitogen-activated protein kinase (MEK)/ERK and JNK/stress-activated
protein kinase (SAPK) (10,11).
Furthermore, it has been reported that IL-22 upregulates the
expression of STAT3 and inhibits the differentiation and promotes
the proliferation of keratinocytes (12). However, the regulatory effects of
IL-22 on the MAPK pathway in keratinocytes have not yet been fully
investigated.
CCAAT enhancer binding protein α (C/EBPα), which is
a member of the C/EBP transcription factor family, serves a key
role in regulating the development, proliferation and
differentiation of keratinocytes (13). Additionally, C/EBPα regulates the
synthesis and metabolism of phospholipids in several types of
cells, including keratinocytes, fat cells and bone marrow cells
(14). Previous studies have
confirmed that C/EBPα is expressed in the basal lamina of epidermis
and its overexpression leads to keratinocyte cycle arrest (15).
In the present study, primary cultured keratinocytes
were used to evaluate the regulatory effects of IL-22 on the MAPK
signaling pathway and to further investigate the role of IL-22 in
the pathogenesis of psoriasis.
Materials and methods
Tissues
A total of 12 foreskin specimens were collected from
young children (6–18 years old) from September to October 2011 who
underwent circumcision in the Qilu Hospital of Shandong University
(China). The size of the skin biopsies was 0.6×1.5 cm. The study
was approved by the Ethics Committee of the Shandong University,
China and all subjects provided written informed consent by their
parents or guardians.
Cell culture
Foreskin samples were immediately cultured in
Epilife-HKGS medium (M-EPI-500-CA; Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with penicillin-streptomycin and
stored on ice. Foreskin tissue was removed under aseptic
conditions, following which it was rinsed, soaked and shred, then
digested with 0.25% pancreatin and incubated at 37°C for 5 min.
Digestion was terminated using 10% FBS in 1640 medium, then the
sample was centrifuged at 300 × g for 5 min at room temperature. A
100-mesh filter was used to collect the cell pellet from the
suspension and then the cells were incubated at 37°C in a MCO-5AC
incubator (Sanyo Electric Co., Ltd.) with 5% CO2.
Following incubation for 5 days and when cells reached 70–80%
confluency, cells were passaged and immunocytochemistry was
performed to detect cytokeratin (CK) expression on the cell
slides.
Immunocytochemistry
Cells were rinsed three times with PBS and fixed at
room temperature with 95% ethanol for ~1 h. Endogenous peroxidase
was blocked with 3% H2O2 in PBS at room
temperature for ~10 min and unspecific Ab binding was prevented
with 5% goat serum (cat. no. ZLI-9021; OriGene Technologies, Inc.).
Specific proteins were detected by immunohistochemistry using
primary antibody anti-CK10 (1:200; cat. no. ab76318). Primary
antibodies were added and samples were incubated in a humid chamber
at 4°C overnight. Secondary antibodies (supplied with the DAB
Immunochemistry kit; cat. no. PV-9000; OriGene Technologies, Inc)
conjugated with peroxidase were used and the Ag-Ab reaction was
visualized, according to the manufacturer's instructions. Images
were captured using a light microscope (magnification, ×200; cat.
no. CX31; Olympus Corporation).
IL-22 stimulation
Normal human epidermal keratinocytes (NHEKs) at
passage two were passaged and harvested using trypsin/EDTA. The
culture medium (M-EPI-500-CA; Invitrogen; Thermo Fisher Scientific,
Inc.) was added to prepare a single-cell suspension. When cells
adhered to the wall and cell fusion reached 60%, IL-22 (cat. no.
RC-212-33; Bio Basic, Inc.) was added to 6-well plates at
concentrations of 30, 60 and 90 ng/ml, and subsequently total RNA
and proteins were extracted from the cells for further
analysis.
Cell transfection
Before transfection, 2×105 cells were
inoculated and cultivated in the incubator at 37°C with 5%
CO2 until the cell confluence reached 50%. NHEKs were
transfected with 33 nM C/EBPα or control scramble siRNAs (cat. no.
siNoooooo1-1-10) (both Guangzhou RiboBio Co., Ltd.) with
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
sequence of C/EBPα siRNA was 5′-GGCAAAGAGAUUGACCUGG-3′. Subsequent
experiments were performed 24 h after cell transfection.
Reverse transcription (RT)PCR
analysis
Following stimulation of keratinocytes with IL-22
for 24 h at 37°C, total RNA was isolated using TRIzol (cat. no.
15596-026; Invitrogen; Thermo Fisher Scientific, Inc.) and its
purity and concentration were measured for further analysis.
Subsequently, total RNA was reverse transcribed into cDNA using a
BioTeke Super RT kit (cat. no. PR6601; BioTeke Corporation),
according to the manufacturer's instructions. The temperature
protocol for reverse transcription was as follows: 30°C for 5 min,
42°C for 60 min, and 95°C for 5 min. The C/EBPα mRNA expression
levels were detected using 2% agarose gel electrophoresis. A total
of 10 µl/lane PCR product was loaded with 2 µl 6X loading buffer
and separated at a constant voltage of 80 v. Bands were stained
with 0.5 µg/ml EB solution (cat. no. E1020; Beijing Solarbio
Science & Technology Co., Ltd.) at room temperature for 10 min.
Bands were visualized using a Tanon-2500R gel image analysis system
(Tanon Science and Technology Co., Ltd.). The primers used in the
present study were synthesized by Sangon Biotech, Co., Ltd., and
their sequences are presented in Table
I.
 | Table I.Genes and primer sequences. |
Table I.
Genes and primer sequences.
| Gene | Primer sequence
(5′-3′) |
|---|
| C/EBPα | F:
ACGTGGAGACGCAGCAGAA |
|
| R:
GTAGGCATTGGAGCGGTGA |
| β-actin | F:
CACCAACTGGGACGACAT |
|
| R:
CAGAGGCGTAGAGGGACA |
Western blot assay
Following stimulation of NHEKs with IL-22 (cat. no.
RC-212-33; Bio Basic, Inc.) at 37°C for 30, 60, 90 min and 48 h,
cells were harvested, and total protein extracts were isolated
using RIPA buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology). Protein concentration was determined using a BCA
protein assay kit (cat. no. P0010S; Beyotime Institute of
Biotechnology) and protein expression levels were determined using
western blotting. A total of 40 µg/lane total protein from each
sample was loaded and separated via 10% SDS-PAGE, then transferred
to a PVDF membrane. The membrane was blocked with blocking-buffer
(cat. no. P0023B; Beyotime Institute of Biotechnology) for 1 h at
room temperature, and then incubated with the primary antibody
overnight at 4°C. The next day, the membrane was washed with TBST
(0.1% Tween-20) and subsequently incubated with HRP-conjugated
secondary antibodies for 1 h at room temperature. The following
primary antibodies were used: Anti-C/EBPα (1:1,000; cat. no.
ab74404), anti-CK10 (1:1,000; cat. no. ab76318), anti-involucrin
(1:2,000; cat. no. ab53112; all from Abcam), anti-phosphorylated
(p)-JNK1/2/3 (1:2,000; cat. no. bs-1640R), anti-JNK1/2/3 (1:2,000;
cat. no. bs-2592R), anti-p38 (1:2,000; cat. no. bs-0637R),
anti-p-p38 (1:2,000; cat. no. bs-5476R), anti-ERK (1:2,000; cat.
no. bs-0022R) and anti-p-ERK (1:2,000; cat. no. bs-1522R; all
BIOSS) and anti-β-actin (1:1,000; cat. no. 4970s; Cell Signaling
Technology, Inc.). The following secondary antibody was used: Goat
anti-rabbit IgG H&L (HRP) (1:5,000; cat. no. ab6721; Abcam).
Visualization was performed using a BeyoECL Moon kit (cat. no.
PB0018FS; Beyotime Institute of Biotechnology). The protein bands
were analyzed with ImageJ software (version Java1.8.0_112;
imagej.nih.gov/ij/docs/index.html).
Cell viability assay
NHEKs were seeded into 96-well plates at a density
of 1×104/well and were then incubated at 37°C for 24 h.
Subsequently, IL-22 (RC-142; Bio Basic, Inc.) was added to the
wells at a final concentration of 30, 60 and 90 ng/ml. Finally,
following stimulation for 12, 24, 48 and 72 h, 10 µl Cell Counting
Kit-8 (CCK-8) reagent (Beyotime Institute of Biotechnology) was
added to each well and the cells were cultured at 37°C for an
additional 2 h, according to the manufacturer's instructions.
Optical density (OD) values were measured at 450 nm.
Statistical analysis
All statistical analyses were performed using SPSS
18.0 software (SPSS, Inc.). All data are presented as the mean ± SD
from independent experiments performed in triplicate. The
differences between 2 groups were analyzed using Student's t-test.
In addition, when ≥3 groups were compared, the differences were
analyzed using one-way ANOVA, followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression levels of CK10 in cells
assessed via immunocytochemistry
Brown staining in the cytoplasm demonstrated that
keratinocytes were successfully isolated from foreskin samples
(Fig. 1).
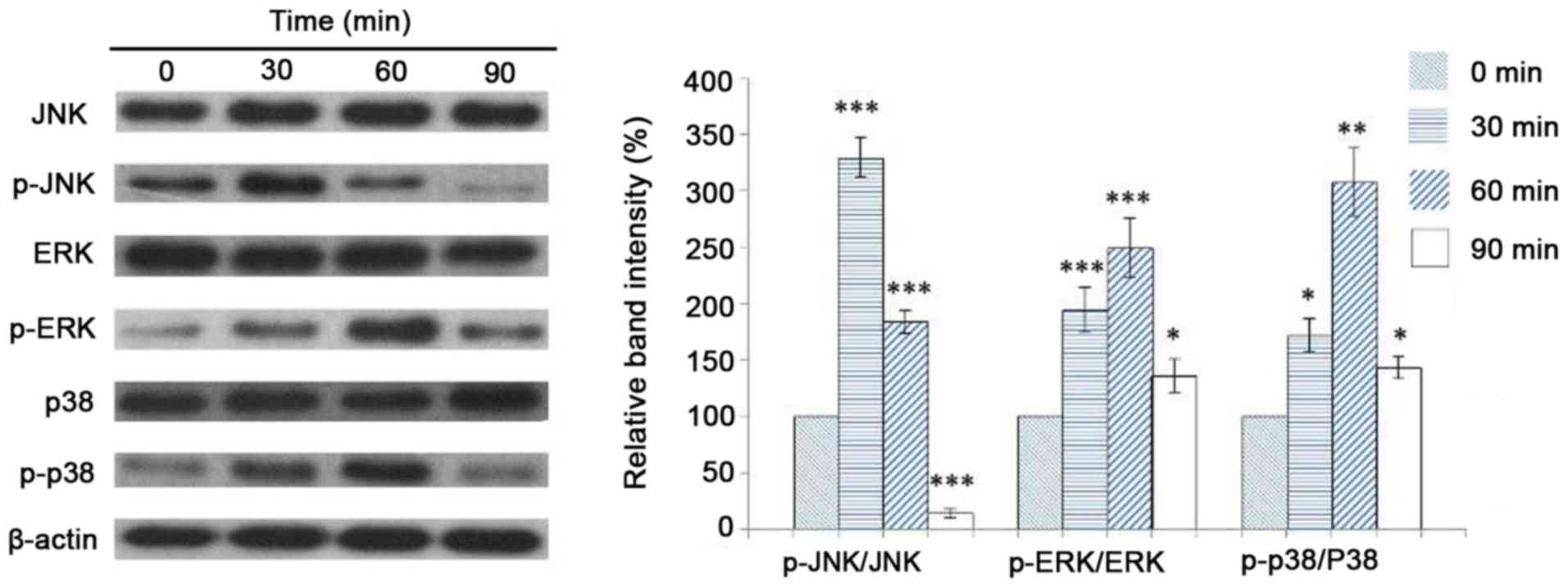
Expression levels of JNK, ERK and p38
in NHEKs following stimulation with IL-22
NHEKs were stimulated for 0, 30, 60 and 90 min with
60 ng/ml IL-22. Subsequently, total protein extracts were isolated
and western blot analysis was performed to detect the expression
levels of JNK, p-JNK, p38, p-p38, ERK and p-ERK. The results
revealed that JNK phosphorylation was significantly increased after
cell stimulation with IL-22 for 30 min and decreased at 60 min
post-stimulation (Fig. 2).
Accordingly, the expression levels of p-p38 and p-ERK were
significantly increased at 30 min and reached the highest levels at
60 min post-stimulation. However, expression levels decreased
following incubation for 90 min in the presence of IL-22.
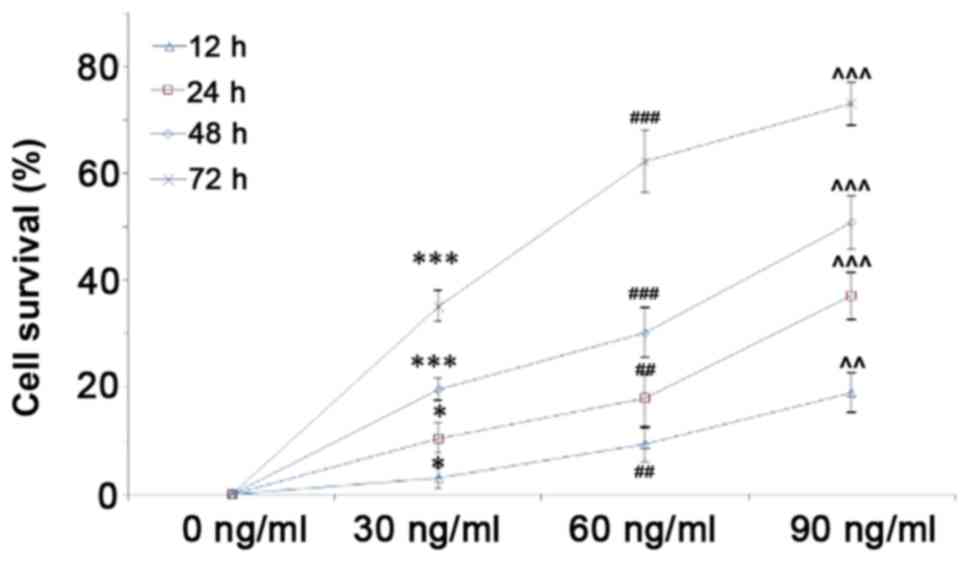
IL-22 promotes the proliferation of
keratinocytes
The effect of IL-22 on the proliferation of
keratinocytes was assessed using a CCK-8 assay. Therefore,
keratinocytes were stimulated for 12, 24, 48 and 72 h with 30, 60
and 90 ng/ml IL-22. The results demonstrated that IL-22 promoted
the proliferation of keratinocytes in a dose- and time-dependent
manner (Fig. 3).
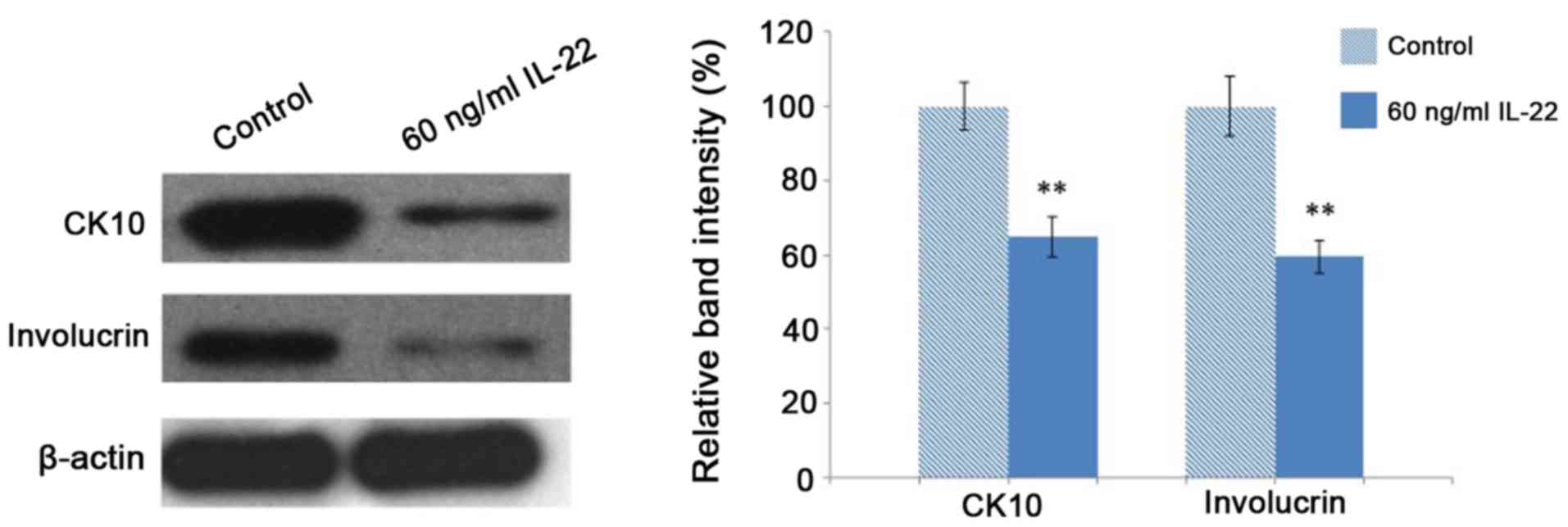
IL-22 inhibits the differentiation of
keratinocytes
Total protein extracts were isolated from
keratinocytes treated with 60 ng/ml IL-22 for 48 h to detect the
expression levels of keratinocyte differentiation markers, namely
CK10 and involucrin. IL-22 treatment for 48 h significantly reduced
the protein expression levels of CK10 and involucrin in
keratinocytes compared with the control (P<0.05; Fig. 4).
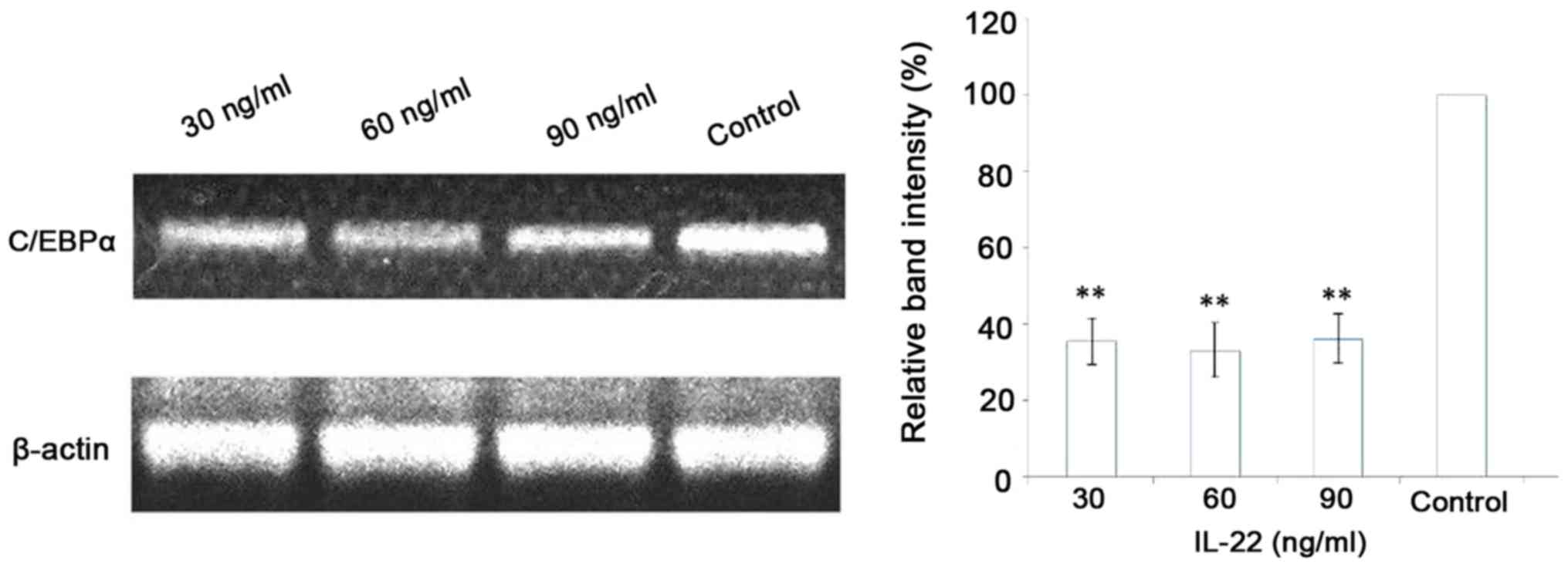
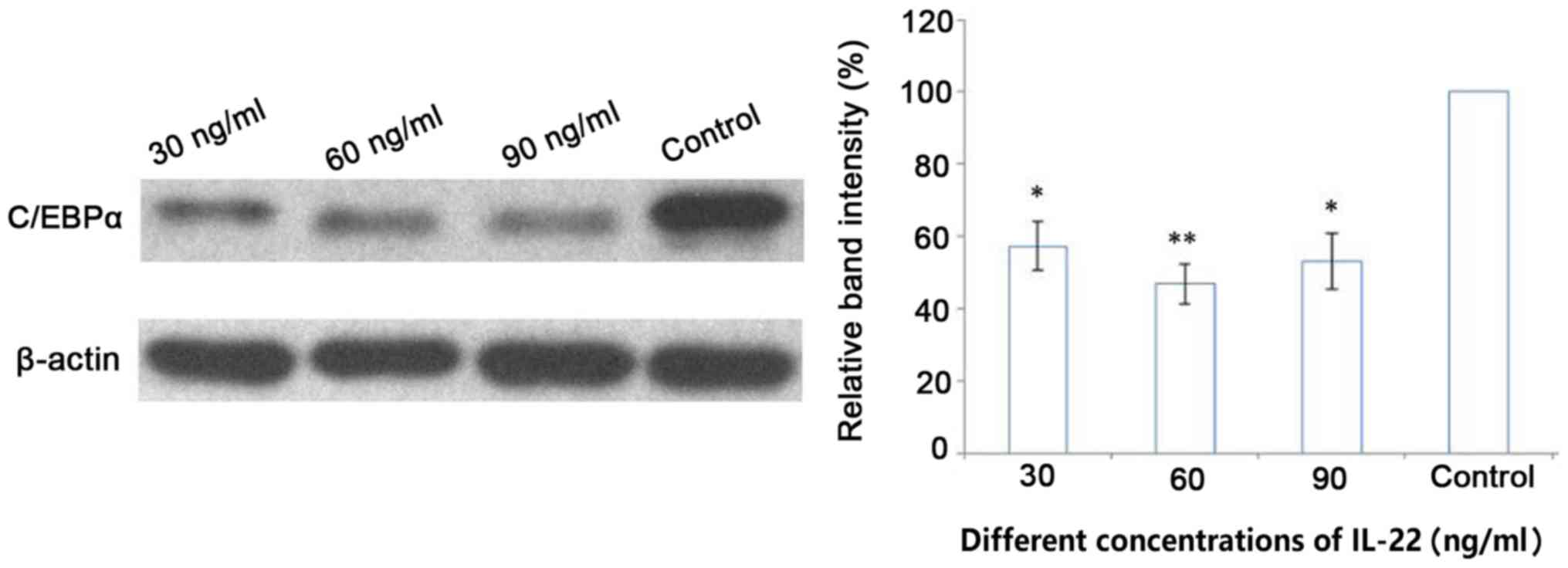
IL-22 decreases the expression of
C/EBPα at the mRNA and protein level
Following stimulation of keratinocytes with 30, 60
and 90 ng/ml IL-22 for 48 h, C/EBPα mRNA and protein expression
levels were assessed using RT-qPCR and western blot analysis,
respectively. The results showed that mRNA and protein expression
levels of C/EBPα were significantly downregulated in the IL-22
group compared with that in the control group (P<0.05) (Figs. 5 and 6). However, no statistically significant
differences were observed among the different concentration groups
(P>0.05).
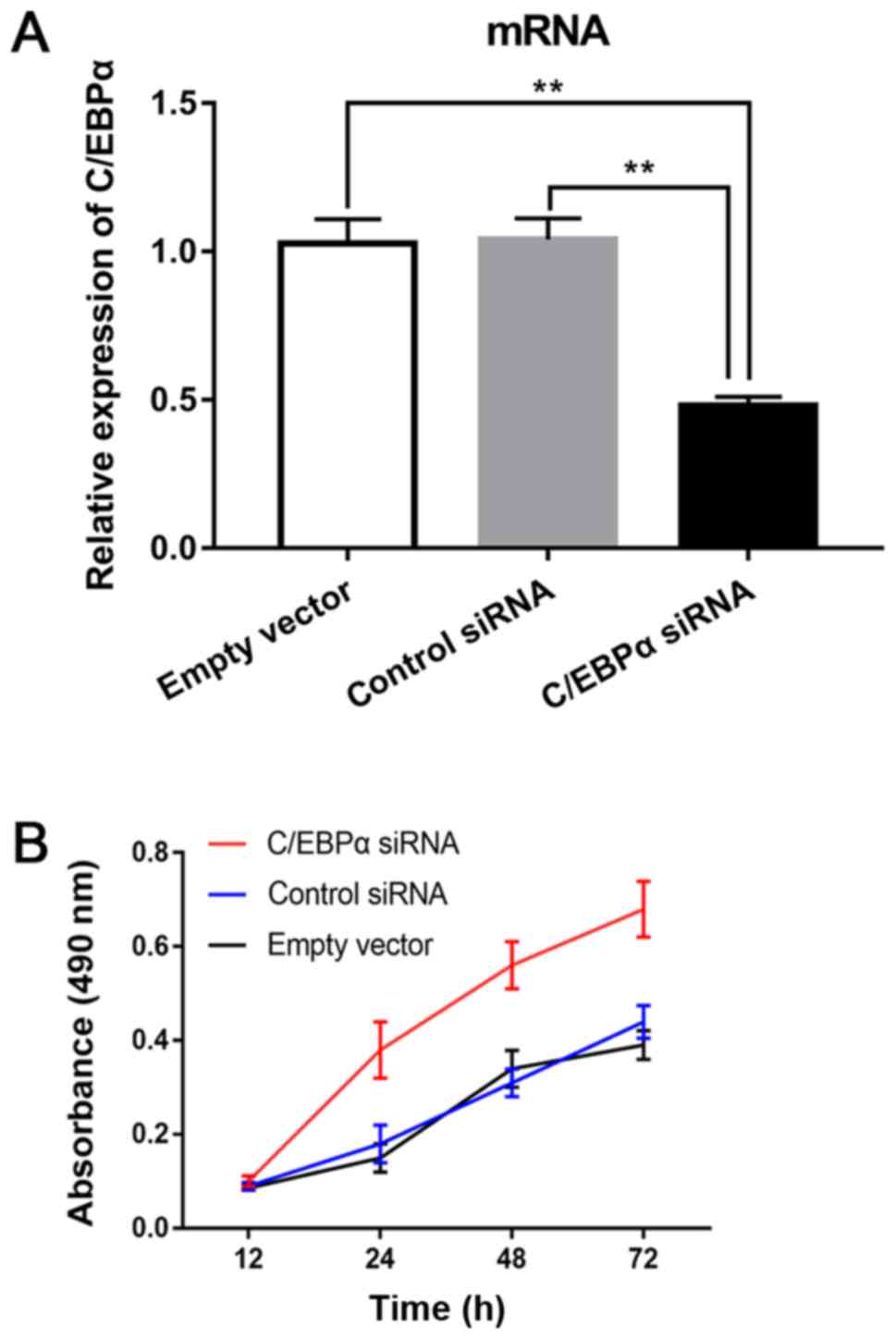
Downregulation of C/EBPα promotes
proliferation of keratinocytes
NHEKs, were transfected with C/EBPα siRNAs or
control using Lipofectamine 2000 to evaluate the effect of C/EBPα
on cell proliferation. The CCK-8 proliferation assay demonstrated
that C/EBPα siRNA promoted the proliferation of NHEKs at 24, 48 and
72 h (all P<0.05) compared with that of the control siRNA and
empty vector groups (Fig. 7). The
results suggested that downregulation of C/EBPα promoted
proliferation of keratinocytes.
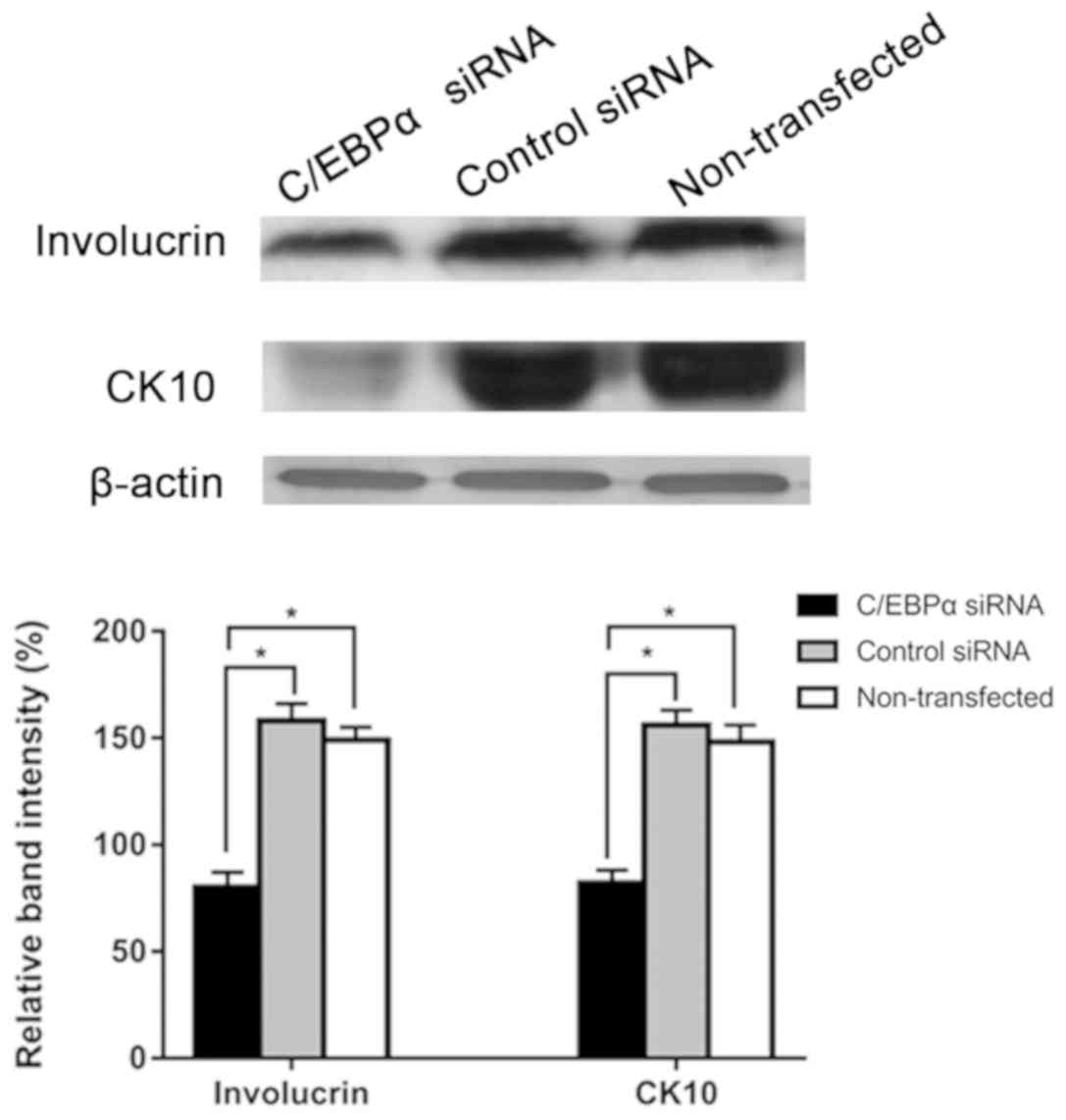
Downregulation of C/EBPα inhibits the
differentiation of keratinocytes
To investigate the effect of C/EBPα on the
differentiation of keratinocytes, cells were transfected with
C/EBPα siRNAs or control using Lipofectamine 2000. Western blot
analysis revealed that involucrin and CK10 expression levels were
significantly decreased in the C/EBPα siRNA group compared with
those in the control siRNA and non-transfected groups (Fig. 8). The aforementioned results
indicated that C/EBPα downregulation inhibited the differentiation
of keratinocytes.
Discussion
Psoriasis vulgaris, one of the most common chronic
inflammatory diseases in the clinic, is considered to occur as a
consequence of genetic and environmental factors, leading to the
induction of keratinocyte proliferation via immune-mediated
signaling pathways (1,2).
A previous study has demonstrated that in psoriasis
vulgaris the proliferation rate and the number of generated
keratinocytes is increased by 2 and 28 times, respectively,
compared with normal epidermis (16). However, the exact underlying
mechanism of the increased keratinocyte proliferation rate in
psoriasis remains unknown. The abnormal differentiation of
keratinocytes is one of the most important features of psoriatic
lesions, resulting from the abnormal expression of several keratins
(17). In addition, it has been
reported that transglutaminase 1 kinase, involucrin, peptidase
inhibitor 3, ATP binding cassette subfamily C member 8 and CK6/CK16
proteins are upregulated in psoriatic lesions (18). Furthermore, keratin K1/K10, a
characteristic protein of the terminal differentiation stage of
keratinocytes, is significantly reduced in the psoriatic lesions
compared with the normal skin (19). Therefore, in the present study,
CK10 and involucrin were selected as differentiation markers to
investigate the effect of IL-22 on keratinocyte differentiation.
The results revealed that IL-22 significantly decreased the
expression of CK10 and involucrin in keratinocytes. In addition,
IL-22 significantly promoted keratinocyte proliferation and
differentiation in a time- and dose-dependent manner.
Th17 cells, a subpopulation of CD4+ T
lymphocytes that differs from Th1, Th2 and regulatory T cells,
mainly secrete the cytokines IL-17 and IL-22 (20). Previous studies have confirmed that
IL-22 is one of the key pro-inflammatory cytokines in inflammatory
diseases (21,22). IL-22 has been found in
IL-9-stimulated BW5147 cells (mouse T lymphoma cells) and exerted
its biological effects by interacting with its receptor (23). An in vitro study
demonstrated that IL-22 promoted the excessive proliferation and
abnormal differentiation of human keratinocytes (5). Furthermore, Ma et al (24) established a psoriasis mouse model
by transferring CD4+CD45RBhi T cells from
BALB/cBy to C.B-17/Prkdcscid/scid mice and revealed that
subcutaneous injection of IL-22 antibodies prevented the
progression of psoriasis. They also reported that the levels of
Th17-related cytokines, namely IL-17A, IL-17F, IL-22 and IL-23p19,
were significantly reduced (24).
These findings suggest that IL-22 serves an important role in the
pathogenesis of psoriasis by promoting the secretion ability of
Th17 cells, which in turn acts on keratinocytes. Therefore, IL-22
may be considered the link between the immune system and
keratinocytes.
A previous study has confirmed that abnormal signal
transduction pathways play a critical role in the pathogenesis of
psoriasis (9). It has also been
demonstrated that three main pathways, namely MEK-ERK, JNK/SAP and
p38 kinase, are activated by IL-22 (25). Furthermore, IL-22 upregulates the
expression of STAT3, inhibits keratinocyte differentiation,
promotes keratinocyte proliferation and induces the formation of
psoriatic lesions (12).
Additionally, the activity of the MAPK signaling pathway is
significantly increased in psoriatic lesions (26). Notably, in psoriasis, p-ERK is
upregulated in the base layer and stratum spinosum cell nucleus,
while in the normal skin p-ERK is only expressed in the basal layer
(27). JNK and p38 kinase are
expressed in the granular layer of the normal skin and in both the
granular layer and stratum spinosum of the psoriatic lesions
(28). The results of the present
study revealed that primary cultured keratinocytes treated with 60
ng/ml IL-22 for 30 min upregulated the expression of p-ERK, p-JNK
and p-p38, indicating that IL-22 was involved in the activation of
the MAPK pathway in keratinocytes.
C/EBPα, a member of the C/EBP family, was first
isolated from rat liver cell nuclei by McKnight in 1987. C/EBPα is
a heat-stable transcription factor that interacts with the CCAAT
motif (29). Previous studies have
shown that C/EBPα is involved in the regulation of keratinocyte
differentiation and its expression is gradually elevated in stratum
spinosum, granular layer and stratum corneum keratinocytes in a
space- and time-dependent manner (13,15),
which indicated that C/EBPα was expressed in basal layer
keratinocytes, and is upregulated as keratinocytes exit the basal
layer, enter the spinous and granular layers and undergo terminal
differentiation. Maytin and Habener (30) found that CK10 was primarily
expressed in BALB/MK keratinocytes following stimulation with 0.12
mM Ca2+ for 24 h and its expression was decreased after
48 h. However, the expression levels of C/EBPα were increased by 5
times (31). In the terminal
differentiation stage, the markers of spinous and granular layer,
K1/K10, are induced by C/EBPα binding to DNA (13). The expression levels of C/EBPα and
CK10 over time (C/EBPα at first, CK10 later) and at different sites
(C/EBPα in the basal layer and CK10 in the spinous layer) suggests
that these molecules may be involved in the differentiation of
keratinocytes. C/EBPα is considered an important negative regulator
of cell proliferation and its anti-tumor effects have been reported
in numerous types of skin tumors (31). For example, Loomis et al
(32) demonstrated that
epidermal-specific C/EBPα knockout mice were highly susceptible to
skin squamous carcinoma. However, the underlying mechanisms of
C/EBPα in regulating cell proliferation remains unclear. It has
been considered that C/EBPα interacts with several cell cycle
proteins in its dimer form, it can interacts directly with CDK2 and
CDK4 enzymes to prevent cyclin binding thus regulating the cell
cycle (33). In addition, C/EBPα
regulates, stabilizes and activates the cell cycle inhibitor p21 or
directly inhibits CDK2-, CDK4- and E2F-induced transcription. These
findings indicate that C/EBPα may block the cell cycle process and
inhibit cell proliferation (34).
In the present study the expression levels of C/EBPα were
significantly decreased in keratinocytes following treatment with
IL-22, suggesting a potential role in the pathogenesis of psoriasis
vulgaris. Additionally, the MAPK signaling pathway may act as a key
molecule in the interaction between IL-22 and C/EBPα. Therefore,
elevated IL-22 expression levels in psoriasis vulgaris may activate
the MAPK signaling pathway, which in turn may mediate the
inactivation of C/EBPα. However, the mechanism of cell signal
transduction is complex. A signal transducer may not only
participate in the cell signal transduction of one pathway, and
functional molecules in one signal transduction pathway may affect
and regulate other pathways. The present study primarily
investigated the relationship between IL-22 and the MAPK signaling
pathway. Moreover, it was found that the effect of IL-22 on the
proliferation and differentiation of keratinocytes may be mediated
via the regulation of the MAPK signaling pathway. The result of
this study cannot exclude whether there are other signaling
pathways that play a role in the regulatory mechanism of IL-22.
This will be further studied in the future.
In conclusion, the present study demonstrated that
IL-22 promoted proliferation and inhibited differentiation of
keratinocytes. The C/EBPα expression levels were significantly
decreased in IL-22-treated keratinocytes. Furthermore,
downregulation of C/EBPα promoted keratinocyte proliferation and
decreased the expression of CK10 and involucrin. Therefore, IL-22
may contribute to the abnormal proliferation and differentiation of
keratinocytes via downregulating the expression of C/EBPα. Finally,
the results suggest that the IL-22/MAPK/C/EBPα axis may be involved
in the pathogenesis of psoriasis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81402607).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and LZ designed the present study and performed
the experiments, and should be regarded as corresponding and first
author, respectively. WM analyzed and interpreted patients' data.
JY contributed to the clinical diagnosis and histological
examination during the experiments. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Qilu Hospital of Shandong University, China and
was in accordance with The Declaration of Helsinki (1964) and its
later amendments. All patients provided written informed consent
for publication of the results.
Patient consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schön MP and Boehncke WH: Psoriasis. N
Engl J Med. 352:2715–1912. 2005. View Article : Google Scholar
|
|
2
|
Lowes MA, Bowcock AM and Krueger JG:
Pathogenesis and therapy of psoriasis. Nature. 445:866–873. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bos JD, de Rie MA, Teunissen MB and Piskin
G: Psoriasis: Dysregulation of innate immunity. Br J Dermatol.
152:1098–1107. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Di Cesare A, Di Meglio P and Nestle FO:
The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J
Invest Dermatol. 129:1339–1350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boniface K, Bernard FX, Garcia M, Gurney
AL, Lecron JC and Morel F: IL-22 inhibits epidermal differentiation
and induces proinflammatory gene expression and migration of human
keratinocytes. J Immunol. 174:3695–3702. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ekman AK, Bivik Eding C, Rundquist I and
Enerbäck C: IL-17 and IL-22 promote keratinocyte stemness in the
germinative compartment in psoriasis. J Invest Dermatol.
139:1564–1573.e8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang M, Ma W, Gao Y, Jia K, Zhang Y, Liu
H and Sun Q: IL-22-induced miR-122-5p promotes keratinocyte
proliferation by targeting Sprouty2. Exp Dermatol. 26:368–374.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boehncke WH: Etiology and pathogenesis of
psoriasis. Rheum Dis Clin North Am. 41:665–675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nestle FO, Kaplan DH and Barker J:
Psoriasis. N Engl J Med. 361:496–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lejeune D, Dumoutier L, Constantinescu S,
Kruijer W, Schuringa JJ and Renauld JC: Interleukin-22 (IL-22)
activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a
rat hepatoma cell line. Pathways that are shared with and distinct
from IL-10. J Biol Chem. 277:33676–33682. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu J, Xiao Z, Zhao R, Lu C and Zhang Y:
Paeoniflorin suppressed IL-22 via p38 MAPK pathway and exerts
anti-psoriatic effect. Life Sci. 180:17–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wolk K, Haugen HS, Xu W, Witte E, Waggie
K, Anderson M, Vom Baur E, Witte K, Warszawska K, Philipp S, et al:
IL-22 and IL-20 are key mediators of the epidermal alterations in
psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl).
87:523–536. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lopez RG, Garcia-Silva S, Moore SJ,
Bereshchenko O, Martinez-Cruz AB, Ermakova O, Kurz E, Paramio JM
and Nerlov C: C/EBPalpha and beta couple interfollicular
keratinocyte proliferation arrest to commitment and terminal
differentiation. Nat Cell Biol. 11:1181–1190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang ZC, Liu Y, Li SF, Guo L, Zhao Y,
Qian SW, Wen B, Tang QQ and Li X: Suv39h1 mediates AP-2α-dependent
inhibition of C/EBPα expression during adipogenesis. Mol Cell Biol.
34:2330–2338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thompson EA, Zhu S, Hall JR, House JS,
Ranjan R, Burr JA, He YY, Owens DM and Smart RC: C/EBPα expression
is downregulated in human nonmelanoma skin cancers and inactivation
of C/EBPα confers susceptibility to UVB-induced skin squamous cell
carcinomas. J Invest Dermatol. 131:1339–1346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weinstein GD, McCullough JL and Ross PA:
Cell kinetic basis for pathophysiology of psoriasis. J Invest
Dermatol. 85:579–583. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bernard BA, Asselineau D,
Schaffar-Deshayes L and Darmon MY: Abnormal sequence of expression
of differentiation markers in psoriatic epidermis: Inversion of two
steps in the differentiation program? J Invest Dermatol.
90:801–805. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duvic M, Asano AT, Hager C and Mays S: The
pathogenesis of psoriasis and the mechanism of action of
tazarotene. J Am Acad Dermatol. 39:S129–S133. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bernerd F, Magnaldo T and Darmon M:
Delayed onset of epidermal differentiation in psoriasis. J Invest
Dermatol. 98:902–910. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sallusto F and Lanzavecchia A:
Heterogeneity of CD4+ memory T cells: Functional modules for
tailored immunity. Eur J Immunol. 39:2076–2082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eyerich S, Eyerich K, Pennino D, Carbone
T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann
C, Behrendt H, et al: Th22 cells represent a distinct human T cell
subset involved in epidermal immunity and remodeling. J Clin
Invest. 119:3573–3585. 2009.PubMed/NCBI
|
|
22
|
Zenewicz LA and Flavell RA: IL-22 and
inflammation: Leukin′ through a glass onion. Eur J Immunol.
38:3265–3268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie MH, Aggarwal S, Ho WH, Foster J, Zhang
Z, Stinson J, Wood WI, Goddard AD and Gurney AL: Interleukin
(IL)-22, a novel human cytokine that signals through the interferon
receptor-related proteins CRF2-4 and IL-22R. J Biol Chem.
275:31335–31339. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma HL, Liang S, Li J, Napierata L, Brown
T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M,
et al: IL-22 is required for Th17 cell-mediated pathology in a
mouse model of psoriasis-like skin inflammation. J Clin Invest.
118:597–607. 2008.PubMed/NCBI
|
|
25
|
Weber GF, Gaertner FC, Erl W, Janssen KP,
Blechert B, Holzmann B, Weighardt H and Essler M: IL-22-mediated
tumor growth reduction correlates with inhibition of ERK1/2 and AKT
phosphorylation and induction of cell cycle arrest in the G2-M
phase. J Immunol. 177:8266–8272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Johansen C, Kragballe K, Westergaard M,
Henningsen J, Kristiansen K and Iversen L: The mitogen-activated
protein kinases p38 and ERK1/2 are increased in lesional psoriatic
skin. Br J Dermatol. 152:37–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang X, Yang D, Ma S and Liu H:
Up-regulation of activities of mitogen-activated protein kinase in
psoriatic lesions. J Dermatol Sci. 37:118–119. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Funding AT, Johansen C, Kragballe K and
Iversen L: Mitogen- and stress-activated protein kinase 2 and
cyclic AMP response element binding protein are activated in
lesional psoriatic epidermis. J Invest Dermatol. 127:2012–2019.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Johnson PF and McKnight SL: Eukaryotic
transcriptional regulatory proteins. Annu Rev Biochem. 58:799–839.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maytin EV and Habener JF: Transcription
factors C/EBP alpha, C/EBP beta, and CHOP (Gadd153) expressed
during the differentiation program of keratinocytes in vitro and in
vivo. J Invest Dermatol. 110:238–246. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oh HS and Smart RC: Expression of
CCAAT/enhancer binding proteins (C/EBP) is associated with squamous
differentiation in epidermis and isolated primary keratinocytes and
is altered in skin neoplasms. J Invest Dermatol. 110:939–945. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Loomis KD, Zhu S, Yoon K, Johnson PF and
Smart RC: Genetic ablation of CCAAT/enhancer binding protein alpha
in epidermis reveals its role in suppression of epithelial
tumorigenesis. Cancer Res. 67:6768–6776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McKnight SL: McBindall-a better name for
CCAAT/enhancer binding proteins? Cell. 107:259–261. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang H, Iakova P, Wilde M, Welm A, Goode
T, Roesler WJ and Timchenko NA: C/EBPalpha arrests cell
proliferation through direct inhibition of Cdk2 and Cdk4. Mol Cell.
8:817–828. 2001. View Article : Google Scholar : PubMed/NCBI
|