Introduction
Lung cancer has the highest morbidity and mortality
of all malignant tumors and is a serious threat to human health.
Non-small cell lung cancer (NSCLC) accounts for 80% of all lung
malignancies and is the main pathological type of lung cancer
(1). NSCLC has a young age of
onset and is characterized by increasing morbidity and mortality
rates. The current treatment strategies for patients with NSCLC
include surgery, radiotherapy, immunotherapy and chemotherapy, as
well as a combination of these treatment modalities (2). With the development of imaging
techniques and molecular biology techniques, great progress has
been made in the early diagnosis and treatment of patients with
NSCLC. Of note, epidermal growth factor receptor (EGFR)-tyrosine
kinase inhibitors may be used to treat patients with NSCLC with
EGFR mutations, and have been demonstrated to improve prognosis and
prolong the survival time of the aforementioned patients (3). However, the 5-year survival rate of
patients with NSCLC remains low and the 5-year recurrence rate
following complete surgical resection of stage I NSCLC is as high
as 50%. The majority of patients are diagnosed in the advanced
stages of the disease, and present with even higher recurrence and
metastasis rates (4).
Certain biological indicators, including
histological and tumor-node-metastasis staging, number of lymph
node metastases and molecular immune markers, such as EGFR mutation
status, currently serve as prognostic predictors and guide
treatment strategies in patients with NSCLC (5). However, chemotherapy resistance is
one of the key factors leading to poor prognosis in patients with
NSCLC and remains a challenging clinical problem (6,7).
Despite surgical intervention, radiotherapy and targeted therapies,
the majority of patients require standardized treatment with
paclitaxel. Paclitaxel is a type of taxane, and exerts its
cytotoxic effect predominantly by disrupting microtubule assembly.
Paclitaxel inhibits microtubule depolymerization and leads to cell
cycle arrest in the G2/M phase, resulting in apoptosis or necrosis
of tumor cells (8,9). However, in clinical treatment, the
majority of patients develop resistance to paclitaxel chemotherapy,
which eventually leads to treatment failure and affects the
survival time of patients (10,11).
The mechanisms of paclitaxel resistance are multifaceted and
complex, and include changes in cell membrane dynamics, increased
drug metabolism, alterations in DNA repair mechanisms, cell cycle
dysregulation and decreased apoptosis and autophagy (12,13).
Therefore, investigating the mechanisms of paclitaxel resistance
may improve the outcome of patients with NSCLC.
Improvements in molecular biology techniques have
allowed the identification of oncogenes and tumor suppressor genes,
which are an area of current interest in lung cancer research and
may provide a valuable basis for individualized treatment and
prognosis evaluation of patients with NSCLC (14). Mucin 1 (MUC1) is a member of the
membrane-bound mucin family of glycoproteins. MUC1 is located in
the apical surface of epithelial cells in the mammary glands,
pancreas and gastrointestinal, respiratory and urogenital tracts,
and under physiological conditions is not detected by the immune
system. MUC1 is characterized by polar expression, apical
distribution and rich glycosylation (15). MUC1 is ubiquitously expressed on
the cell surface in several types of cancer, including esophageal
and gastric cancer. Furthermore, MUC1 loses polarity and become
hypoglycosylated in cancer cells and may serve as a diagnostic
marker or a therapeutic target (16,17).
MUC1 is spontaneously hydrolyzed to produce two subunits, the α and
β subunits. The α subunit, also known as MUC1-N, is characterized
by 20–200 variable number of tandem repeats (VNTRs), which are
serine-, threonine- and proline-rich and may be modified by
O-glycosylation. The β subunit, also known as MUC1-C, is composed
of MUC1-extracullar domain, MUC1-transmembrane and MUC1-cytoplasmic
tail (CT). MUC1-CT is highly conserved and can interact with
phosphoinositide 3-kinase (PI3K), C-terminal Src kinase (CSK) and
nuclear factor κB (NF-κB) signaling pathways to regulate cell
activity (18–20). Previous studies have reported that
MUC1 is upregulated in several types of cancer, such as esophageal
squamous-cell carcinoma (16,21).
The high expression level of MUC1 in lung cancer has been closely
correlated with early recurrence, poor prognosis and a high
metastatic potential (22).
Furthermore, MUC1 knockdown suppressed lung cancer growth and
invasion by inhibiting cell proliferation and inducing apoptosis
(23). However, despite these
findings, the association between MUC1 and paclitaxel resistance in
patients with NSCLC remains unclear. Therefore, the aim of the
present study was to explore the role of MUC1 in
paclitaxel-resistant lung cancer cell line A549/PR, and to
investigate the associated mechanism.
Materials and methods
NSCLC tissue collection
A total of 30 patients with NSCLC patients were
recruited at The General Hospital of Western Theater Command from
February 23, 2016 to January 1, 2018. Tumor and adjacent
non-cancerous tissues were collected, snap frozen in liquid
nitrogen and stored at −80°C. The present study was approved by the
Ethics Committee of The General Hospital of Western Theater Command
and all patients provided written informed consent.
Cell culture and transfection
A549 cells were purchased from the Shanghai
Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences, and A549/PR cells were purchased from Nanjing KeyGen
Biotech, Co., Ltd. The two cell types were cultured in Dulbecco's
modified Eagle medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS; Invitrogen; Thermo
Fisher Scientific, Inc), 100 U/ml penicillin and 100 µg/ml
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.). All
cells were maintained in a humidified atmosphere containing 5%
CO2 at 37°C.
Paclitaxel-resistant NSCLC cell lines A549/PR were
treated with 0.5 µM of paclitaxel (Glpbio) in the culture for 3
months (24). Then cells were
maintained in 1 µM of PTX to maintain this drug-resistant
phenotype. Cellular assays were carried out when the cells were in
the logarithmic growth phase.
Two small interference (si) RNAs targeting MUC1 and
a scrambled siRNA were designed and synthesized by Ribobio. The 50
nM siRNAs were transfected into A549/PR cells (5×104
cells) using Lipofectamine® 2000 reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions when
cells reached 50% confluence. The sequences of MUC1 siRNA and its
NC were as follows: MUC1 siRNA, 5′-AAGACTGATGCCAGTAGCACT-3′; NC,
5′-AATTCTCCGAACGTGTCACGT-3′. After 72 h transfection,
reverse-transcription quantitative PCR (RT-qPCR) and western
blotting were used to assess the silence effect.
Cell proliferation assay
Cell proliferation was evaluated using the Cell
Counting Kit-8 assay (CCK-8; Dojindo Molecular Technology).
Briefly, A549/PR cells transfected with MUC1 siRNA or NC siRNA were
cultured in a 96-well plate (3×103 cells/well). The
cells were incubated for 0, 12, 24, 48 or 72 h at 37°C and a total
of 10 µl CCK-8 solution was added per well. The cells were
subsequently incubated for 4 h and the optical density at a
wavelength of 450 nm was measured using a microplate reader
(Peiou).
Cell apoptosis analysis
Cell apoptosis was analyzed with an Annexin V
Apoptosis Detection kit I (BD Biosciences). A total of 48 h
following transfection, A549/PR cells were digested and washed
twice in pre-chilled PBS. The cells were subsequently incubated
with fluorescein isothiocyanate annexin V and propidium iodide for
15 min in the dark. Apoptosis was analyzed using a flow cytometer
(FACScan; BD Biosciences) using FlowJo v7.6.1 (FlowJo LLC).
RT-qPCR
At 48 h post-transfection, the total RNA was
extracted from tissue specimens and transfected A549/PR cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. RNA was reversed
transcribed into cDNA using the TaqMan Reverse Transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.). qPCR was
subsequently performed using the Fast Start Universal SYBR Green
Master mix (Roche Applied Science) and a 7500 Real-time system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The following
primers were used: MUC1 forward, 5′-TCCTTTCTCTGCCCAGTCTG-3′ and
reverse, 5′-GTGTGGTAGGTGGGGTACTC-3′, GAPDH forward,
5′-GGACCTGACCTGCCGTCTAG-3′ and reverse, 5′-GTAGCCCAGGATGCCCTTGA-3′.
GAPDH was used as the reference gene.
Western blotting
At 48 h post-transfection, the total protein was
extracted from tissue specimens and transfected A549/PR cells
according to the manufacturer's protocol. Total protein was
quantified using a bicinchoninic acid assay kit (Vazyme) and 50 µg
protein/lane was separated via SDS-PAGE on a 10% gel. The separated
proteins were subsequently transferred onto polyvinylidene
difluoride membranes and blocked in 5% skim milk in TBST for 1.5 h
at 25°C. The membranes were incubated with primary antibodies
against MUC1 (cat. no. 14161), BCL2 associated X apoptosis
regulator (Bax; cat. no. 2774), BCL2 apoptosis regulator (Bcl-2;
cat. no. 2872), Caspase-3 (cat. no. 9662) and GAPDH (cat. no. 8884)
at 4°C overnight. All antibodies were used at a 1:1,000 dilution
and were purchased from Cell Signaling Technology, Inc. Membranes
were washed three times with TBST. Following the primary
incubation, membranes were incubated with secondary antibodies
(1:1,000) for 2 h at 25°C. Protein bands were visualized using an
Enhanced Chemiluminescence Detection system. GAPDH was used as the
loading control.
Statistical analysis
GraphPad Prism software (v5; GraphPad Software,
Inc.) was used to perform all the statistical analysis. The
Student's t-test was used to compare two groups and the one-way
analysis of variance (ANOVA) followed by Tukey's test was used for
the comparison of multiple groups. P<0.05 was considered to
indicate a statistically significant difference. All quantitative
data are expressed as the mean ± SD.
Results
MUC1 is upregulated in NSCLC
tissues
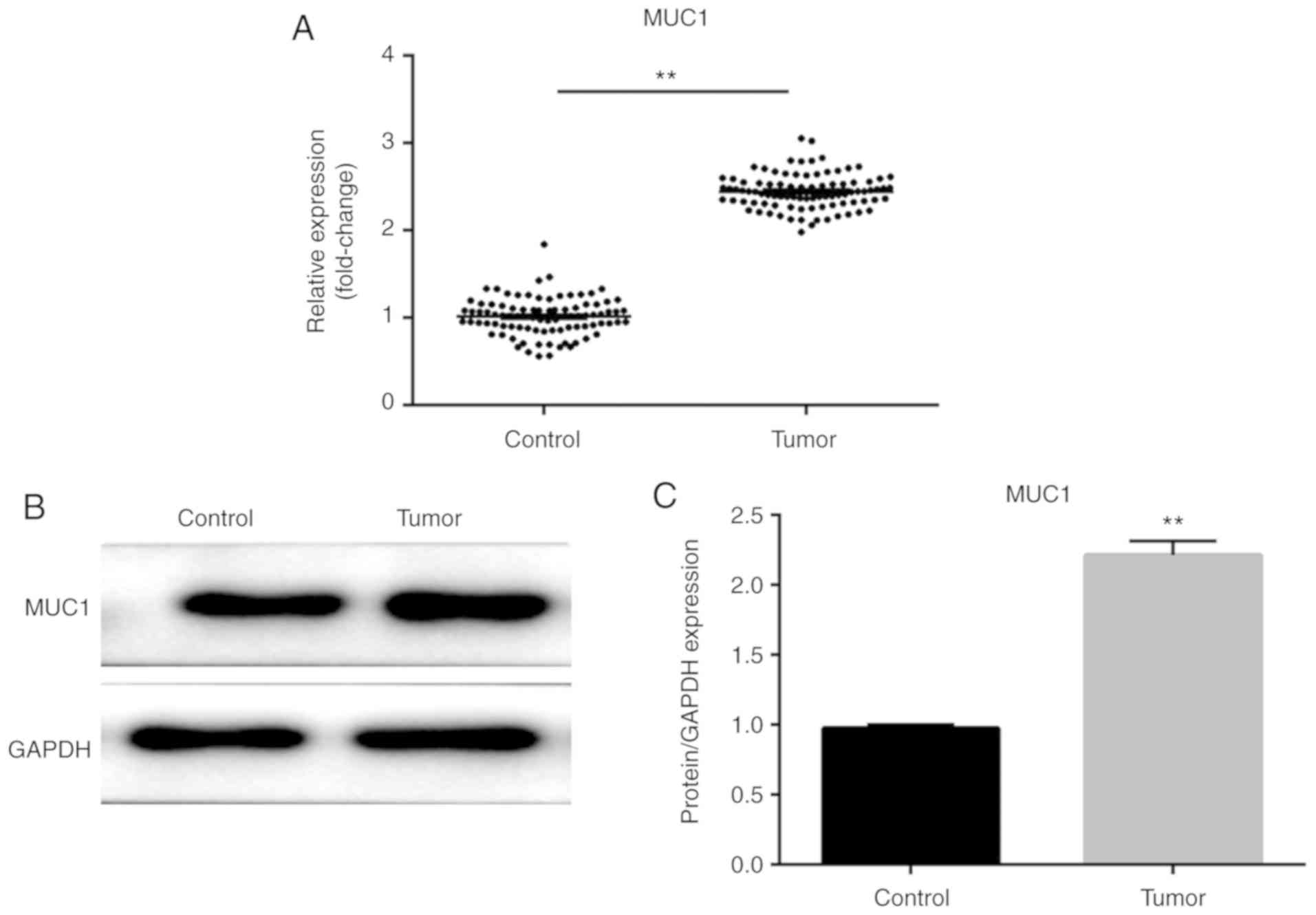
RT-qPCR and western blotting were performed to
detect the expression levels of MUC1 in NSCLC and adjacent
noncancerous tissues. As shown in Fig.
1A-C, the mRNA and protein expression levels of MUC1 were
upregulated in NSCLC tissues compared with adjacent noncancerous
tissues (P<0.01), suggesting that MUC1 may promote the
progression and development of NSCLC.
MUC1 is upregulated in A549/PR
cells
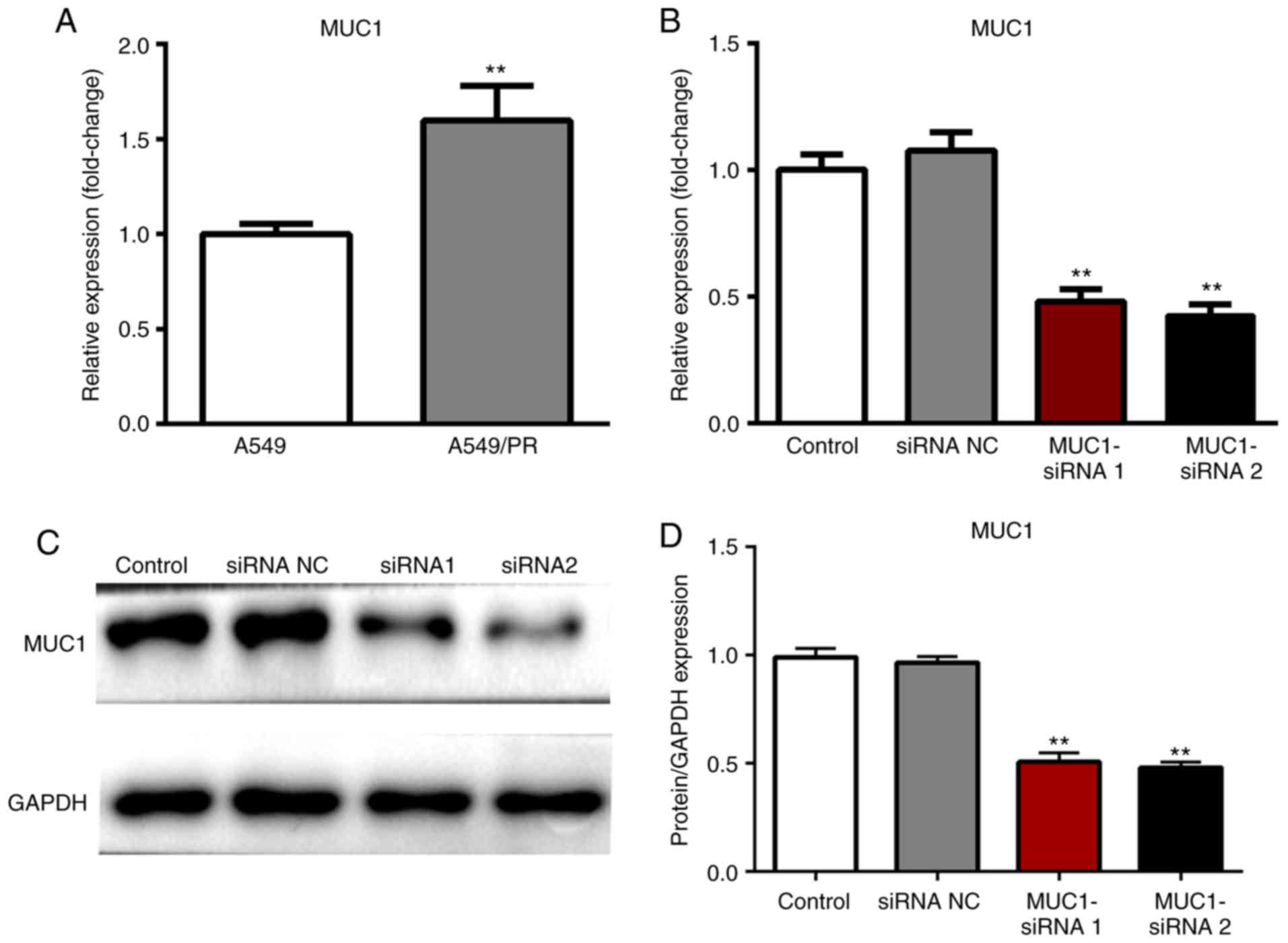
In order to further explore the role and underlying
mechanisms of MUC1 in paclitaxel resistance in NSCLC, at 72 h
post-transfection, the expression level of MUC1 was measured by
RT-qPCR and western blot respectively. The mRNA expression level of
MUC1 was significantly increased in A549/PR cells compared with
A549 cells (P<0.01; Fig. 2A).
The effect of MUC1 upregulation in NSCLC was further investigated
by transfecting A549/PR cells with NC siRNA or MUC1 siRNA. The
results revealed that the MUC1 siRNA significantly inhibited the
mRNA and protein expression levels of MUC1 in A549/PR cells
compared with the control (P<0.01; Fig. 2B-D).
Silencing of MUC1 suppresses the
proliferation of A549/PR cells following treatment with
paclitaxel
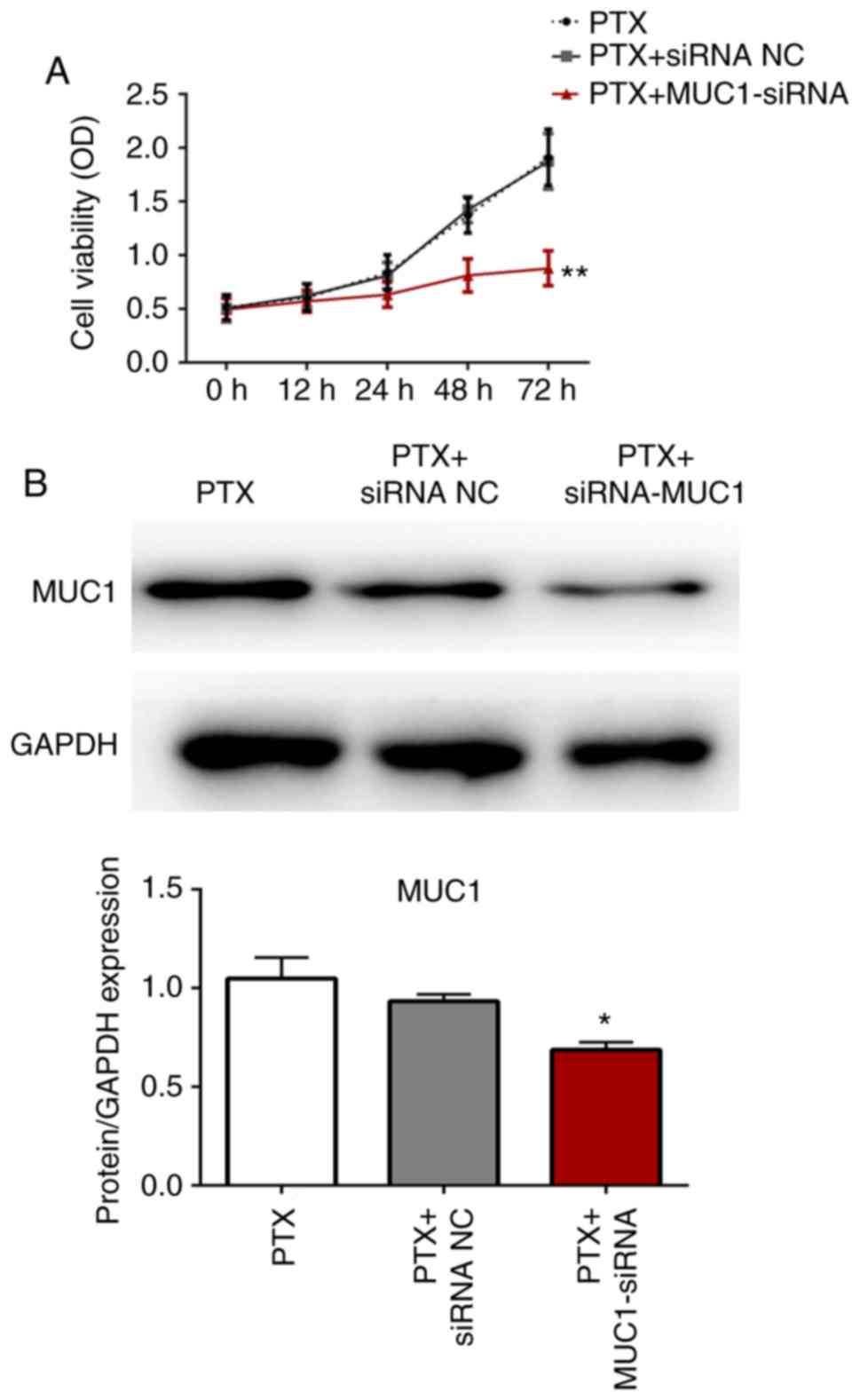
Paclitaxel resistance in control and transfected
cells A549/PR cells was investigated using the CCK-8 assay. As
shown in Fig. 3A and B, the
expression level of MUC1 was significantly decreased in MUC1-siRNA
group and there was no significant difference between the
proliferation rate of A549/PR cells and A549/PR cells transfected
with NC siRNA following paclitaxel treatment. However, the
proliferation rate of A549/PR cells transfected with MUC1 siRNA was
significantly decreased compared with the untransfected cells and
cells transfected with NC siRNA (P<0.01). These data suggested
that the inhibition of MUC1 significantly enhanced paclitaxel
sensitivity in A549/PR cells.
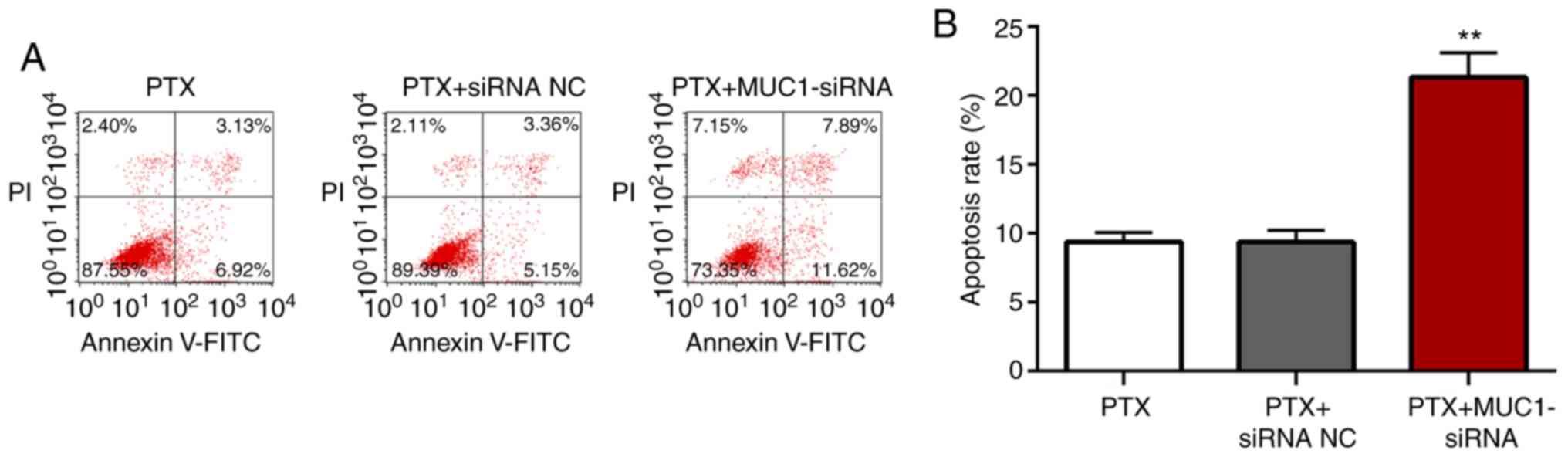
Inhibition of MUC1 promotes apoptosis
of A549/PR cells following treatment with paclitaxel
The apoptotic rates of A549/PR cells transfected
with NC siRNA or MUC1 siRNA following treatment with paclitaxel
were determined using flow cytometry. There were no significant
differences between the apoptotic rates of the untransfected cells
and cells transfected with NC siRNA following treatment with
paclitaxel (P<0.05; Fig. 4A and
B). However, cells transfected with MUC1 siRNA exhibited
significantly increased apoptosis compared with the untransfected
cells or cells transfected with NC siRNA (P<0.01). The data
suggested that inhibition of MUC1 promoted apoptosis of
paclitaxel-treated A549/PR cells.
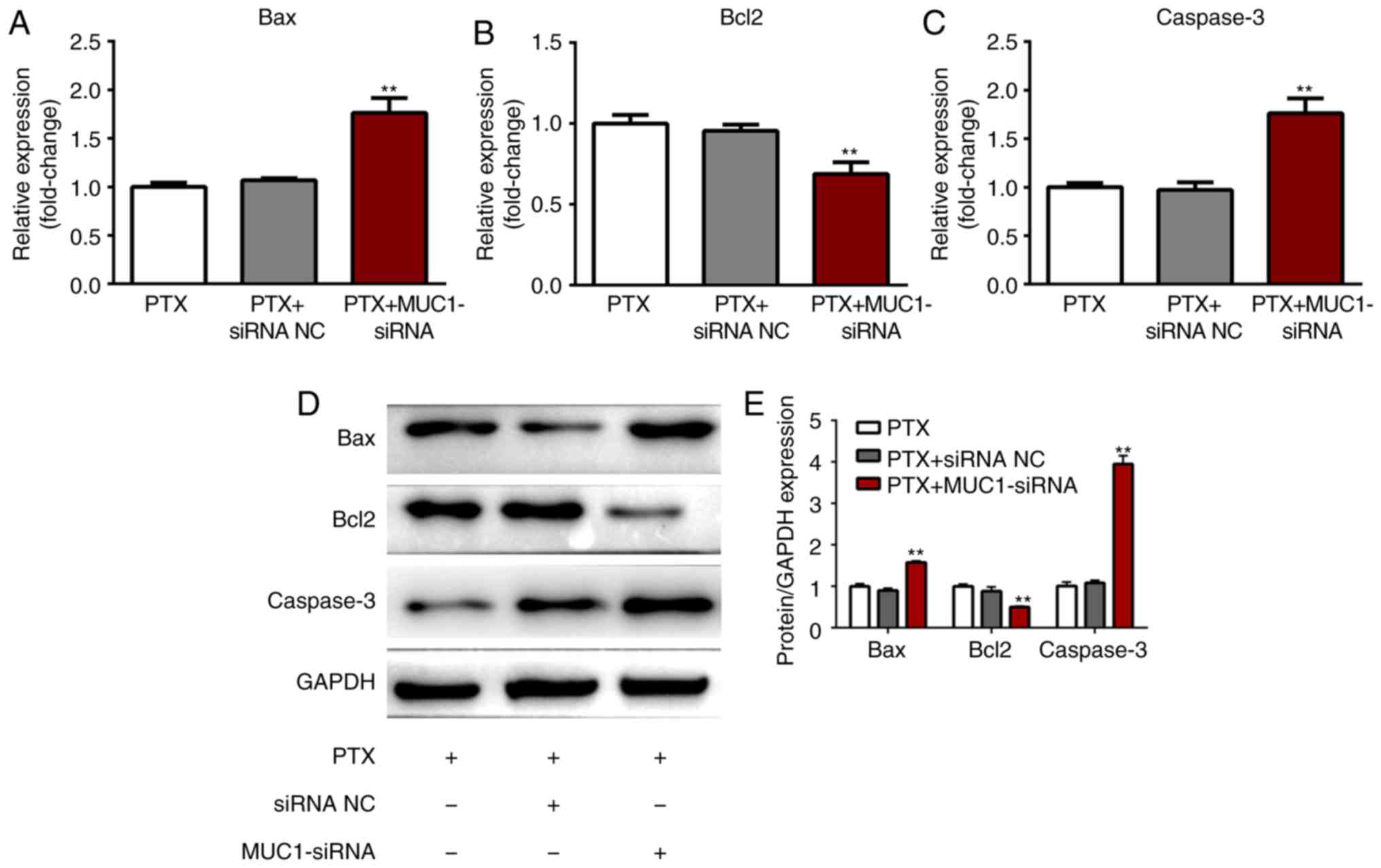
MUC1 regulates the expression of
Bcl2/BAX and caspase-3
Western blotting was performed to evaluate the
protein expression levels of apoptosis-associated proteins in
A549/PR cells transfected with NC siRNA or MUC1 siRNA following
treatment with paclitaxel. The protein levels of Bax, Bcl-2 and
caspase-3 were not significantly different between untransfected
cells and cells transfected with NC siRNA following treatment with
paclitaxel (P>0.05; Fig. 5).
The mRNA and protein levels of Bax and caspase-3 were significantly
upregulated while Bcl-2 was significantly downregulated in cells
transfected with MUC1 siRNA compared with untransfected cells or
cells transfected with NC siRNA (P<0.01). The results further
suggested that the inhibition of MUC1 promoted apoptosis of
paclitaxel-treated A549/PR cells.
Discussion
The increase in environmental pollution and smoking
in recent years has resulted in >500,000 new cases of lung
cancer and >400,000 lung cancer-associated mortalities annually.
Lung cancer has the highest incidence and mortality rates among all
tumors. Approximately ~80% of lung cancer cases are NSCLC. The
majority of patients with NSCLC are diagnosed in the advanced
stages of the disease and have inoperable tumors. Chemotherapy is
therefore the main treatment strategy for patients with advanced
NSCLC (25,26). However, the efficacy rate of
combined chemotherapy for NSCLC is 14–40%, and may result in
relapse. This phenomenon is closely associated with multidrug
resistance in NSCLC (27). Drug
resistance in tumors involves several mechanisms, and may lead to
multidrug resistance (28,29). Tumors with multidrug resistance are
a common clinical problem, and often result in chemotherapy failure
(27). Therefore, there has been
increased interest in overcoming or preventing drug resistance. The
main mechanisms of drug resistance in lung cancer include increased
expression of multidrug resistance genes such as P-glycoprotein,
glutathione transferases, decreased expression of topoisomerase and
promoting DNA damage repair and anti-apoptotic ability of cancer
cells (30).
Paclitaxel was originally isolated from the bark of
Taxus pacificus and has cytotoxic effects on many types of cancer
cells (31). Paclitaxel is a
tubulin-binding drug and has exhibited better prospects; however,
it is less cost-effective than other drugs (32). Additionally, paclitaxel is
associated with drug resistance and cross-resistance in cancer
cells. The mechanisms underlying paclitaxel resistance are complex
and are not fully understood. Paclitaxel resistance may occur due
to upregulation of P-glycoprotein, abnormal expression of
microtubule regulatory proteins or post- translational changes
expression of drug-resistant genes, abnormal signal transduction
and cell death pathway regulation, and alterations in tubulin
subtypes, proteins that regulate tubulin dynamics and paclitaxel
binding sites (33). The
elucidation of the pathways involved in paclitaxel resistance may
allow the identification of suitable patients and guide the
selection of treatment strategies. Furthermore, the emergence of
drug resistance may be avoided or reversed, thereby improving the
efficacy of chemotherapy.
The mucin family consists of high molecular weight
glycoproteins that protect and lubricate epithelial cells under
physiological conditions. MUC1, a member of the mucin family, is a
transmembrane heterodimer glycoprotein that is expressed at low
levels in the proximal side of secretory epithelial cells in the
mammary glands and respiratory, gastrointestinal and urogenital
tracts. In epithelial tumors, MUC1 expression is upregulated and
loses its polar distribution. Furthermore, MUC1 has been shown to
serve an important role in tumor proliferation, invasion,
metastasis, and chemotherapy resistance. MUC1 is highly expressed
in >90% of breast cancer cases and is associated with the
occurrence and development of breast cancer by interacting with
PI3K/AKT, MAP, NF-κB, Wnt, signal transducer and activator of
transcription, tumor protein P53 and estrogen receptor (ER) α
signaling pathways (34).
Following the stimulation of breast cancer cells with EGF, the MUC1
intracellular segment was phosphorylated, CSK and MAPK signaling
pathways were activated and cell proliferation was enhanced. In 17
β-estradiol-stimulated breast cancer cells, MUC1 enhanced
ERα-mediated transcription and promoted the survival and growth of
breast cancer cells. Ren et al (35), revealed that silencing MUC1
enhanced the sensitivity of A549 and ZR-75-1 cells to cytotoxic
drugs. Horn et al (36),
found that murine breast cancer DA3 cells overexpressing MUC1
increased the expression of interstitial markers, decreased the
expression of epithelial markers and enhanced the ability of cells
to produce extracellular matrix. In addition, previous studies have
revealed that MUC1 was upregulated in NSCLC tumors, and that
downregulation of MUC1 inhibited the progression of the disease
(37,38). In the future, we will conduct
further experiments concerning the effect of MUC1 on paclitaxel
resistance in H1299 or H1975 cell lines.
In the present study, MUC1 was upregulated in
clinical NSCLC tissues and A549/PR cells. MUC1 silencing
significantly suppressed the proliferation and promoted apoptosis
of paclitaxel-treated A549/PR cells by regulating Bax, Bcl-2 and
caspase-3 expression. The results obtained in the present study
suggested that the modulation of MUC1 may serve as a promising
therapeutic approach to overcome paclitaxel resistance in
NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by Youth Innovation
Fund of The General Hospital of Western Theater Command (grant no.
14732C119).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HYX, HG and HL designed the experiments and
performed the statistical analysis. DL, WWY and LZ were involved in
the study design, data acquisition and manuscript revision. PC,
XMS, ZHL and GJW were in charge of writing the manuscript and data
analysis. TZ collected all the samples and patients' clinical data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The General Hospital of Western Theater Command and
all patients provided prior written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Islami F, Miller KD, Siegel RL, Fedewa SA,
Ward EM and Jemal A: Disparities in liver cancer occurrence in the
United States by race/ethnicity and state. CA Cancer J Clin.
67:2966–289. 2017. View Article : Google Scholar
|
|
2
|
Breathnach OS, Freidlin B, Conley B, Green
MR, Johnson DH, Gandara DR, O'Connell M, Shepherd FA and Johnson
BE: Twenty-two years of phase III trials for patients with advanced
non-small-cell lung cancer: Sobering results. J Clin Oncol.
19:1734–1742. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: Recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cufer T, Ovcaricek T and O'Brien ME:
Systemic therapy of advanced non-small cell lung cancer:
Major-developments of the last 5-years. Eur J Cancer. 49:1216–1225.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yasukawa M, Sawabata N, Kawaguchi T, Kawai
N, Nakai T, Ohbayashi C and Taniguchi S: Histological grade:
Analysis of prognosis of non-small cell lung cancer after complete
resection. In Vivo. 32:1505–1512. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang L, Shang X and Feng Q: LncRNA TATDN1
contributes to the cisplatin resistance of non-small cell lung
cancer through TATDN1/miR-451/TRIM66 axis. Cancer Biol Ther.
20:261–271. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roach MC, Bradley JD and Robinson CG:
Optimizing radiation dose and fractionation for the definitive
treatment of locally advanced non-small cell lung cancer. J Thorac
Dis. 10 (Suppl 21):S2465–S2473. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chu T, Jiang L, Ying W and Han B: M30/M65
ratio predicts the outcome of paclitaxel chemotherapy for NSCLC.
Clin Transl Oncol. 19:326–331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu YL, Saijo N, Thongprasert S, Yang JC,
Han B, Margono B, Chewaskulyong B, Sunpaweravong P, Ohe Y, Ichinose
Y, et al: Efficacy according to blind independent central review:
Post-hoc analyses from the phase III, randomized, multicenter,
IPASS study of first-line gefitinib versus carboplatin/paclitaxel
in Asian patients with EGFR mutation-positive advanced NSCLC. Lung
Cancer. 104:119–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu Y, Wang J, Liu L, Yu L, Zhao N, Zhou X
and Lu X: Curcumin increases the sensitivity of
Paclitaxel-resistant NSCLC cells to Paclitaxel through
microRNA-30c-mediated MTA1 reduction. Tumour Biol.
doi:10.1177/1010428317698353.
|
|
11
|
Kubo N, Noda SE, Takahashi A, Yoshida Y,
Oike T, Murata K, Musha A, Suzuki Y, Ohno T, Takahashi T, et al:
Radiosensitizing effect of carboplatin and paclitaxel to carbon-ion
beam irradiation in the non-small-cell lung cancer cell line H460.
J Radiat Res (Tokyo). 56:229–238. 2015. View Article : Google Scholar
|
|
12
|
Ding J, Li M, Deng L and Li T: Study on
biological characteristics and mechanism of paclitaxel induced drug
resistance in endometrial carcinoma cells. BioMed Res Int.
2018:83720852018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sobue S, Mizutani N, Aoyama Y, Kawamoto Y,
Suzuki M, Nozawa Y, Ichihara M and Murate T: Mechanism of
paclitaxel resistance in a human prostate cancer cell line, PC3-PR,
and its sensitization by cabazitaxel. Biochem Biophys Res Commun.
479:808–813. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsakonas G, De Petris L and Ekman S:
Management of brain metastasized non-small cell lung cancer (NSCLC)
- From local treatment to new systemic therapies. Cancer Treat Rev.
54:122–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Liu Q, Kong L and Xu S: Mucin 1
downregulation impairs the anti-necroptotic effects of
glucocorticoids in human bronchial epithelial cells. Life Sci.
221:168–177. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song ZB, Gao SS, Yi XN, Li YJ, Wang QM,
Zhuang ZH and Wang LD: Expression of MUC1 in esophageal
squamous-cell carcinoma and its relationship with prognosis of
patients from Linzhou city, a high incidence area of northern
China. World J Gastroenterol. 9:404–407. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakamoto H, Yonezawa S, Utsunomiya T,
Tanaka S, Kim YS and Sato E: Mucin antigen expression in gastric
carcinomas of young and old adults. Hum Pathol. 28:1056–1065. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wreesmann VB, Sieczka EM, Socci ND, Hezel
M, Belbin TJ, Childs G, Patel SG, Patel KN, Tallini G, Prystowsky
M, et al: Genome-wide profiling of papillary thyroid cancer
identifies MUC1 as an independent prognostic marker. Cancer Res.
64:3780–3789. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morari EC, Silva JR, Guilhen AC, Cunha LL,
Marcello MA, Soares FA, Vassallo J and Ward LS: Muc-1 expression
may help characterize thyroid nodules but does not predict
patients' outcome. Endocr Pathol. 21:242–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abrosimov A, Saenko V, Meirmanov S,
Nakashima M, Rogounovitch T, Shkurko O, Lushnikov E, Mitsutake N,
Namba H and Yamashita S: The cytoplasmic expression of MUC1 in
papillary thyroid carcinoma of different histological variants and
its correlation with cyclin D1 overexpression. Endocr Pathol.
18:68–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nabavinia MS, Gholoobi A, Charbgoo F,
Nabavinia M, Ramezani M and Abnous K: Anti-MUC1 aptamer: A
potential opportunity for cancer treatment. Med Res Rev.
37:1518–1539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun ZG, Zhang M, Yang F, Gao W, Wang Z and
Zhu LM: Clinical and prognostic significance of signal transducer
and activator of transcription 3 and mucin 1 in patients with
non-small cell lung cancer following surgery. Oncol Lett.
15:4278–4288. 2018.PubMed/NCBI
|
|
23
|
Xu T, Li D, Wang H, Zheng T, Wang G and
Xin Y: MUC1 downregulation inhibits non-small cell lung cancer
progression in human cell lines. Exp Ther Med. 14:4443–4447.
2017.PubMed/NCBI
|
|
24
|
Sun H, Zhou X, Bao Y, Xiong G, Cui Y and
Zhou H: Involvement of miR-4262 in paclitaxel resistance through
the regulation of PTEN in non-small cell lung cancer. Open Biol.
9:1802272019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bocci G, Di Paolo A and Danesi R: The
pharmacological bases of the antiangiogenic activity of paclitaxel.
Angiogenesis. 16:481–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Milane L, Duan Z and Amiji M: Development
of EGFR-targeted polymer blend nanocarriers for combination
paclitaxel/lonidamine delivery to treat multi-drug resistance in
human breast and ovarian tumor cells. Mol Pharm. 8:185–203. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L, Liu X, Ren Y, Zhang J, Chen J,
Zhou W, Guo W, Wang X, Chen H, Li M, et al: Cisplatin-enriching
cancer stem cells confer multidrug resistance in non-small cell
lung cancer via enhancing TRIB1/HDAC activity. Cell Death Dis.
8:e27462017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lehnert M: Clinical multidrug resistance
in cancer: A multifactorial problem. Eur J Cancer. 32A:912–920.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gottesman MM and Pastan I: Biochemistry of
multidrug resistance mediated by the multidrug transporter. Annu
Rev Biochem. 62:385–427. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Volm M, Koomägi R, Efferth T and Mattern
J: Protein expression profiles of non-small cell lung carcinomas:
Correlation with histological subtype. Anticancer Res.
22:2321–2324. 2002.PubMed/NCBI
|
|
31
|
Wani MC, Taylor HL, Wall ME, Coggon P and
McPhail AT: Plant antitumor agents. VI. The isolation and structure
of taxol, a novel antileukemic and antitumor agent from Taxus
brevifolia. J Am Chem Soc. 93:2325–2327. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Waud WR, Gilbert KS, Harrison SD Jr and
Griswold DP Jr: Cross-resistance of drug-resistant murine P388
leukemias to taxol in vivo. Cancer Chemother Pharmacol. 31:255–257.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morin PJ: Drug resistance and the
microenvironment: Nature and nurture. Drug Resist Updat. 6:169–172.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kufe DW: MUC1-C oncoprotein as a target in
breast cancer: Activation of signaling pathways and therapeutic
approaches. Oncogene. 32:1073–1081. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ren J, Agata N, Chen D, Li Y, Yu WH, Huang
L, Raina D, Chen W, Kharbanda S and Kufe D: Human MUC1
carcinoma-associated protein confers resistance to genotoxic
anticancer agents. Cancer Cell. 5:163–175. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Horn G, Gaziel A, Wreschner DH,
Smorodinsky NI and Ehrlich M: ERK and PI3K regulate different
aspects of the epithelial to mesenchymal transition of mammary
tumor cells induced by truncated MUC1. Exp Cell Res. 315:1490–1504.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wei X, Lai Y, Li J, Qin L, Xu Y, Zhao R,
Li B, Lin S, Wang S, Wu Q, et al: PSCA and MUC1 in non-small-cell
lung cancer as targets of chimeric antigen receptor T cells.
OncoImmunology. 6:e12847222017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bouillez A, Adeegbe D, Jin C, Hu X, Tagde
A, Alam M, Rajabi H, Wong KK and Kufe D: MUC1-C promotes the
suppressive immune microenvironment in non-small cell lung cancer.
OncoImmunology. 6:e13389982017. View Article : Google Scholar : PubMed/NCBI
|