Introduction
Hepatocellular carcinoma (HCC) is the third most
common type of cancer in China (1). Early detection of HCC has always been
an important factor for its prevention and therapeutic
intervention. Imaging modalities such as ultrasonography can be
used for early screening of potentially malignant lesions and
provide guidance for further examination and treatment (2). However, it is often difficult to
distinguish malignant lesions from benign changes using only an
imaging method. Other in vitro diagnostic methods may be
required for differential diagnosis. Hepatic cirrhosis (HC) is the
most common precancerous condition that may require early
intervention (3). Hepatitis B
virus (HBV) infection is considered as a common cause of HC in
China. Other factors for HC include hepatitis C virus (HCV)
infection and alcoholic cirrhosis (2). The majority of patients with HC and
HCC generally exhibit >10 years of history of either hepatitis
virus infection or alcohol overuse (4,5).
The plasma-based septin 9 (SEPT9) gene methylation
assay, known as the SensiColon test, was approved by the Chinese
Food and Drug Administration (FDA) as the first blood-based early
detection test for colorectal cancer (CRC) (4,5).
Multiple case-control and prospective screening studies have
demonstrated its effectiveness in the early detection and screening
of CRC (4,5). The assay was designed to identify the
low copy number of aberrantly methylated SEPT9 (mSEPT9) DNA against
the strong background of normal genomic DNA. The assay includes a
high-perfromance, cell-free DNA extraction and bisulfite conversion
with a single PCR reaction to measure SEPT9 methylation in plasma
samples (6,7). Although the SensiColon assay was
modified from the US FDA-approved Epi proColon test, which includes
a triplicate PCR assay to detect mSEPT9, studies have demonstrated
that it has an equal detection sensitivity and a satisfactory
specificity (8,9).
It has been suggested that the sensitivity of the
mSEPT9 assay is enhanced with an increase in the severity of the
cancer lesions (6,8). Numerous studies have suggested that
the sensitivity of the mSEPT9 assay is associated with cancer stage
(6,7). Additionally, it has been demonstrated
that the detection sensitivity is influenced by age, but not by sex
(10). These factors are crucial
for evaluating the test performance and require further
investigation. Furthermore, previous studies have indicated that
the aberrant blood mSEPT9 levels can be detected in other types of
cancer, including lung cancer and HCC (11–13).
This further suggests that mSEPT9 detection may be used in the
diagnosis of multiple types of cancer, especially in combination
with other markers.
In the present study, a case-control study was
performed to evaluate the performance of the mSEPT9 assay in
patients with HCC and HC compared with the performance of
α-fetoprotein (AFP). The performance of the combination of the two
approaches was also examined. The association between mSEPT9
detection and HCC prognosis was investigated to determine whether
mSEPT9 could predict the long-term survival of patients with
HCC.
Materials and methods
Ethics
A detailed plan for the present study was submitted
to the Ethics Committees of the First Affiliated Hospital of Xian
Medical University (Xian, China) and Wuqi People's Hospital (Yanan,
China) for review and was approved prior to the initiation of the
clinical study. All subjects involved in the present study provided
written informed consent before blood collection, and were informed
of the usage of plasma and of the test results. Confirmation of
approval for clinical studies was received from the institutional
review board or ethics committee of the aforementioned hospitals.
The present clinical study was retrospectively registered to the
Chinese Clinical Trial Registry on April 4, 2020 (http://www.chictr.org.cn/enIndex.aspx;
registration no. ChiCTR2000031547).
Study design, patients and blood
collection
The present case-control study was designed and
performed at the First Affiliated Hospital of Xian Medical
University and Wuqi People's Hospital using the mSEPT9 assay
(SensiColon; BioChain Institute, Inc.). The clinical status of all
subjects was determined before blood collection for the mSEPT9
assay, and blood samples were obtained from all subjects who met
the selection criteria between June 2016 and April 2017 in the two
aforementioned hospitals. A total of 406 subjects were enrolled in
the present study, including 64 patients with HCC (mean age, 58
years; age range, 33–77 years), 44 patients with HC (mean age, 56
years; age range, 33–84 years) and 298 healthy individuals with no
evidence of disease (NED; Table I)
(mean age, 53 years; age range, 17–88 years). The classification of
all conditions was based on diagnosis from ultrasonic and computed
tomography (CT) scans, and subsequent pathological examinations.
Patients with HCC were divided into three subgroups according to
stage (A, B or C) based on the Barcelona Clinic Liver Cancer (BCLC)
staging system (14), and patients
with HC were divided into two categories (compensated and
decompensated) based on the assessment of patient condition, as
previously described (15). The
BCLC staging system was used since it takes into consideration the
liver function and was capable of predicating the prognosis, and
the status of mSEPT9 is closely associated with the functional
change and is predictive for survival (13), while the TNM staging does not
consider these factors.
 | Table I.Numbers of enrolled individuals
according to diagnosis group. |
Table I.
Numbers of enrolled individuals
according to diagnosis group.
|
|
| Sex |
| Age, years |
|---|
|
|
|
|
|
|
|---|
| Diagnosis
group | Total, n | Male | Female | <50 | 50-59 | 60-69 | ≥70 |
|---|
| Total, n | 406 | 303 | 103 | 165 | 126 | 70 | 45 |
| HCC | 64 | 50 | 14 | 16 | 19 | 19 | 10 |
| Stage
A | 23 | 17 | 6 | 6 | 6 | 8 | 3 |
| Stage
B | 23 | 19 | 4 | 5 | 7 | 7 | 4 |
| Stage
C | 18 | 14 | 4 | 5 | 6 | 4 | 3 |
| HC | 44 | 33 | 11 | 17 | 16 | 7 | 4 |
|
Decompensated | 11 | 7 | 4 | 5 | 3 | 2 | 1 |
|
Compensated | 33 | 26 | 7 | 12 | 13 | 5 | 3 |
| NED | 298 | 220 | 78 | 132 | 91 | 44 | 31 |
The inclusion criteria were that subjects had to be
adults >18 years old with complete clinicopathological
information and confirmed diagnosis of HCC, HC or NED (healthy
subjects) via imaging examination (including endoscopy, ultrasound,
magnetic resonance imaging and CT scans) and/or subsequent
pathological examination. The exclusion criteria included: Pregnant
women, history of any type of cancer or history of therapy for any
type of cancer. Subjects with incomplete clinicopathological or
follow-up information were also excluded. The NED group was
recruited from asymptomatic individuals who came to the hospitals
for physical examinations, and the HC group was recruited from
symptomatic individuals with known liver diseases (not malignant
liver diseases) who came to the hospitals for diagnosis and
therapy. All subjects underwent blood collection before examination
and before subsequent biopsies or surgery were performed. None of
the subjects received chemotherapy, radiotherapy or surgical
intervention before blood collection. All patients were followed up
to 630 days.
Sample size estimation
Sample size estimation was based on the following
equation for known detection sensitivity:
N=Z2x[p(1-p)]/E2. The parameters were defined
as follows: Z is a statistical parameter (Z=1.96 for 95% CI), E
represents the error (10% was selected for the present study) and p
represents the putative positive detection rate (PDR). A P-value of
0.80 was obtained from our previous pilot study investigating the
sensitivity of the mSEPT9 assay in HCC (data not published). It was
estimated that 61 patients with HCC were required. Using the same
method, the number of patients with HC was also estimated. When
P=0.12, the number of patients with HC required was 41. Healthy
subjects were recruited based on the ratio of HCC:NED (1:2), and
therefore the study goal was to recruit 122 healthy subjects, with
a total of 224 individuals.
Sample collection and storage
Samples were collected from outpatients and
inpatients, and sample information was recorded in sample
collection forms. A 10-ml peripheral blood sample was collected in
10 ml K2-EDTA anticoagulant tubes (BD Biosciences) to
ensure the accuracy of the assay. Sample storage and transportation
were performed following the manufacturers protocol of the
SensiColon assay.
DNA extraction, quantitative PCR
analysis of SEPT9 and the AFP test
DNA extraction from 10 ml plasma samples
(circulating tumor DNA) and bisulfite conversion were performed
according to the manufacturers protocol of the SensiColon assay
[BioChain (Beijing) Science and Technology, Co., Ltd.]. The
bisulfite-DNA was assayed with SensiColon kits on an ABI 7500 Fast
Dx Real Time PCR device (Thermo Fischer Scientific, Inc.). Primers
and conditions for qualitative PCR analysis of mSEPT9 were used
according to previously described methods (6,8–10).
Briefly, PCR was performed in triplicate with 15 µl template DNA
per well and run for 45 cycles. PCR results for β-actin (ACTB) and
mSEPT9 for each of the triplicate reactions were recorded using the
instrument software. PCR was performed using a Taqman-based assay
(Roche Diagnostics) with fluorophore detection. The sequence of
primers, blockers and probes for SEPT9 detection used in
methylation-specific PCR amplification are as follows: Forward
primer, 5-CCCACCAACCATCATAT-3 and reverse primer,
5-GTAGTAGTTAGTTTAGTATTTATTTT-3; probe1,
5-GTTCGAAATGATTTTATTTAGTTGC-3; and probe2, 5-CGTTGATCGCGGGGTTC-3
(9). β-actin was used as the
internal control to evaluate the plasma DNA quality and the
validity of PCR amplification. The sequence of primers and probes
for β-actin detection used in PCR amplification were as follows:
Forward, 5-GTGATGGAGGAGGTTTAGTAAGTT-3 and reverse,
5-CCAATAAAACCTACTCCTCCCTTAA-3; and probe,
5-ACCACCACCCAACACACAATAACAAACACA-3 (9). The thermocycling conditions were as
follows: Initial denaturation at 94°C for 20 min, followed by 45
cycles at 62°C for 5 sec, 55.5°C for 35 sec and 93°C for 30 sec;
and cooling at 40°C for 5 sec. The validity of each sample batch
was determined on the basis of mSEPT9 and ACTB threshold count (Cq)
values for the positive and negative controls (16). DNA from treated Jurkat cells was
used as the positive control and from treated HeLa cells was used
as the negative control. ACTB was used as an internal reference to
assess the integrity of each sample. The AFP assay used was a
commercial test adopted by the participating hospital, and its
cut-off was set to 20 µg/l according to the manufacturers protocol
[Roche Diagnostics (Shanghai) Co., Ltd.].
Data analysis and interpretation
The data from the PCR reactions of the SensiColon
assay were analyzed using the 1/1 algorithm, which means that a
sample was considered to be positive if the only PCR was positive
and was considered to be negative if it was negative (8,9). The
detailed methods for data analysis and interpretation have been
previously described (6,8–10).
For each sample, a relative methylation value was determined using
the 2−ΔΔCq method adapted for DNA methylation analyses
as previously described (16). In
brief, ΔΔCq values were calculated as follows:
ΔΔCqSample=ΔCqSample-ΔCqCalibrator,
where ΔCqSample=CqACTB of sample-CqSEPT9
of sample and ΔCqCalibrator=CqACTB of
calibrator-CqSEPT9 of calibrator. The cut-off of
the SensiColon assay in HCC detection was re-defined using receiver
operating characteristic (ROC) curves and the ΔΔCq values of the
control and the HCC group, and was determined by the best balance
between sensitivity and specificity. The cut-off was set to a Cq
value of 41.1 (ΔΔCq=−4.0) based on the aforementioned
considerations.
Statistical analysis
Analyses including unpaired Students t-test for
comparison of two groups with normal distribution (experiments were
repeated four times to ensure the repeatability and stability of
the test), one-way ANOVA followed by a post hoc test (Bonferronis
correction) for comparison of >3 groups with normal distribution
and χ2 test for comparison of rate or percentage between
groups. ROC curves and Kaplan-Meier survival curves were performed
using GraphPad Prism 5.0 (GraphPad Software, Inc.). Log-rank
(Mantel-Cox) test was used to compare two survival curves.
Bonferroni correction was performed for the P-values from
χ2 tests when >2 groups were compared using multiple
2×2 χ2 tests. P<0.05, P<0.0167, P<0.0125 or
P<0.01 were considered to indicate statistically significant
differences when comparing 2, 3, 4 or 5 groups, respectively. The
positive predictive value (PPV)=number of true positives/total
number of positives, and the negative predictive value (NPV)=number
of true negatives/total number of negatives. The positive
likelihood ratio (+LR)=sensitivity/(1-specificity), and the
negative likelihood ratio (−LR)=(1-sensitivity)/specificity. The
net reclassification index (NRI)=(sensitivitySEPT9 +
specificitySEPT9)-(sensitivityAFP +
specificityAFP). Data are presented in scatter plots or
histograms with mean values and 95% CI where appropriate, and in
ROC curves or Kaplan-Meier survival curves where appropriate. The
number of subjects for each statistical group was shown in Table I.
Results
mSEPT9 exhibits an improved overall
performance in HCC detection compared with AFP
In order to investigate the diagnostic sensitivity
of mSEPT9 in HCC and HC, the threshold value of mSEPT9 detection
was defined by examining plasma samples from individuals with HCC,
HC and NED. Combined analysis of ΔΔCq value distribution and ROC
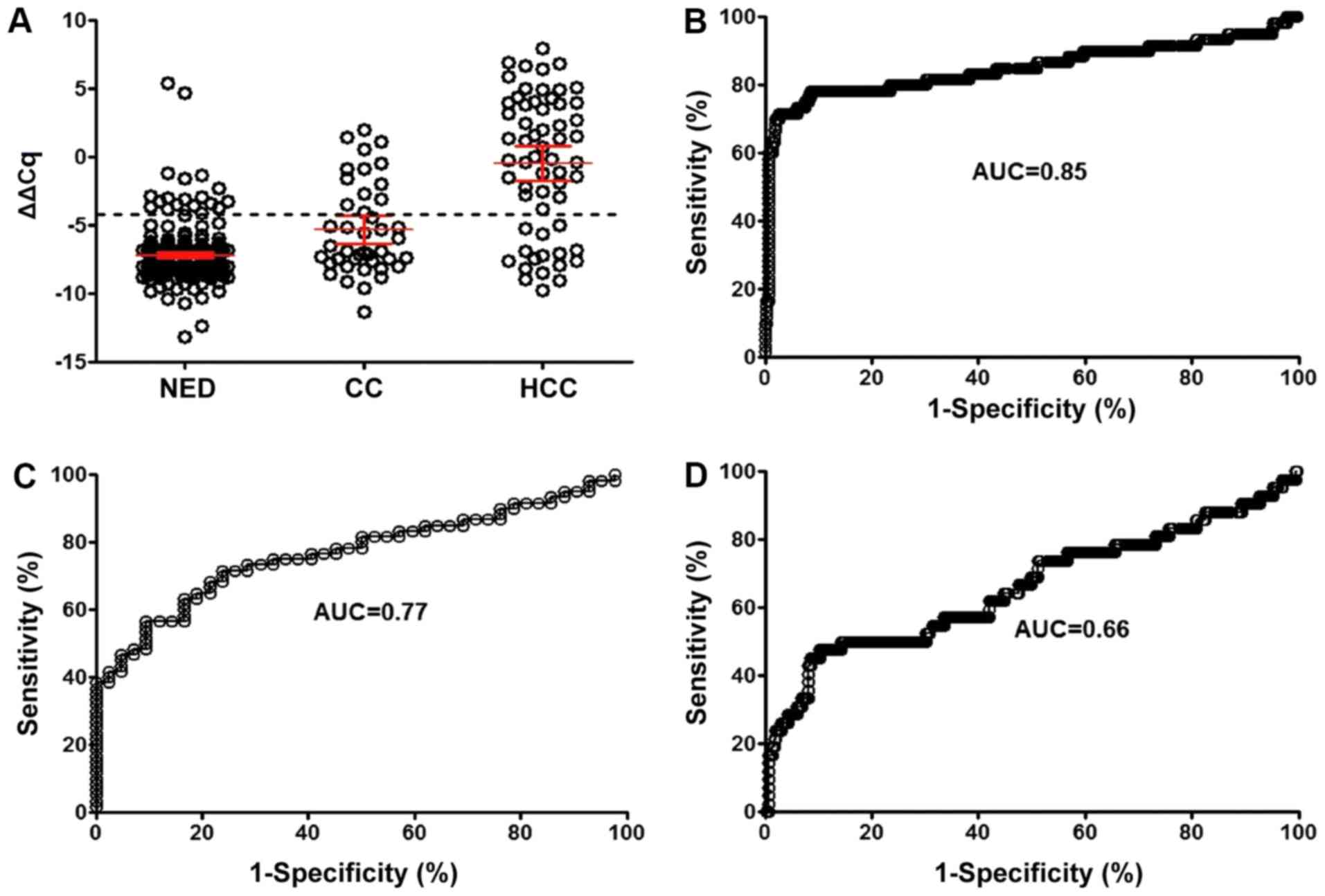
curve (Fig. 1A and B) suggested
that the optimal sensitivity (76.7%) and specificity (95.6%) of the
mSEPT9 assay for HCC were obtained at a Cq threshold of 41.1
(ΔΔCq=−4.0), with an area under the curve (AUC) of 0.85 (95% CI,
0.78-0.92). Under the same threshold, the detection sensitivity for
HC was 34.1% with an AUC of 0.77 (95% CI, 0.68-0.86; Fig. 1C), while the AUC for discrimination
between HCC and HC was 0.66 (95% CI, 0.56-0.76; Fig. 1D). The PPV for the mSEPT9 assay was
63.0%, and the NPV was 86.8%. The +LR was 18.7 and the -LR was
0.243, suggesting a high true positive probability when a test was
positive, and a high true negative probability when a test was
negative (data not shown). The NRI of mSPET9 for HCC was 0.212
compared with AFP, suggesting an improved diagnostic performance of
mSEPT9 compared with AFP.
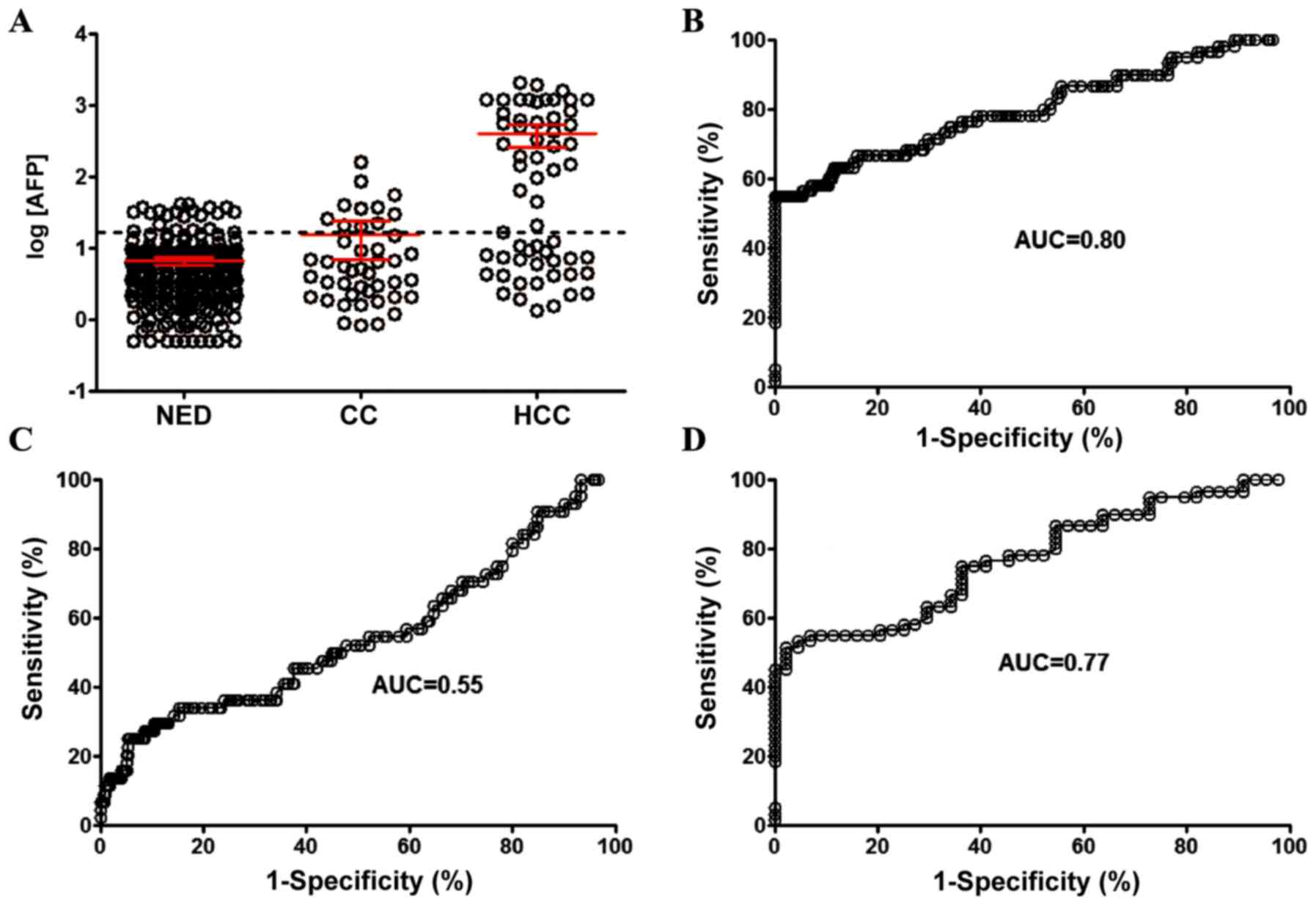
The detection sensitivity of AFP in the same groups
of individuals with HCC, HC and NED was also examined. When the
threshold for discriminating positive or negative detection was set
at 20 ng/ml (Fig. 2A), the
detection sensitivity for HCC was 56.7% with an AUC of 0.80 (95%
CI, 0.73-0.87; Fig. 2B), and the
sensitivity of HC detection was 25.0% with an AUC of 0.55 (95% CI,
0.45-0.65; Fig. 2C). The AUC for
discrimination between HCC and HC was 0.77 (95% CI, 0.68-0.86;
Fig. 2D).
Combination of mSEPT9 and AFP enhances
HCC detection performance
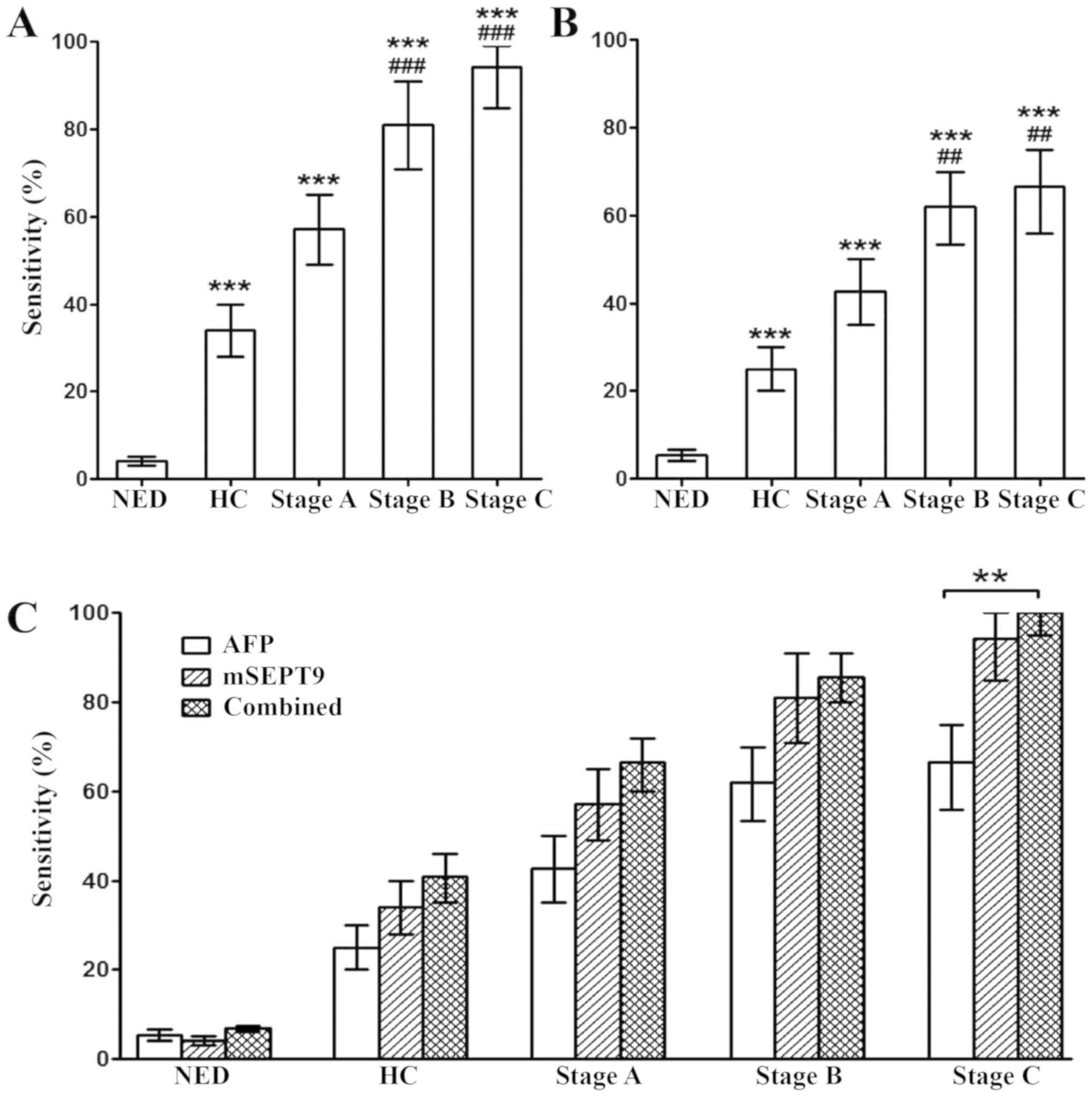
Furthermore, the stage-dependent sensitivity for HCC
in mSEPT9 and AFP detection was examined. The sensitivity of mSEPT9
for NED, HC and Stage A, B and C HCC was 4.1, 34.1, 57.1, 81.0 and
94.4%, respectively, at 95.9% specificity (Fig. 3A), while the sensitivity of AFP for
the aforementioned groups was 5.4, 25.0, 42.8, 61.9 and 66.7%,
respectively, at 94.6% specificity (Fig. 3B). Combined detection of mSPET9 and
AFP significantly enhanced the detection sensitivity compared with
AFP alone in stage C HCC (P<0.01), while a trend of increased
sensitivity was also observed in HC and stage A and B HCC, although
the differences were not statistically significant (Fig. 3C). In addition, the combined
sensitivity was not statistically different compared with the
sensitivity of mSEPT9 alone in the diagnosis of the aforementioned
groups (Fig. 3C).
mSEPT9 detection predicst long-term
survival in patients with HCC
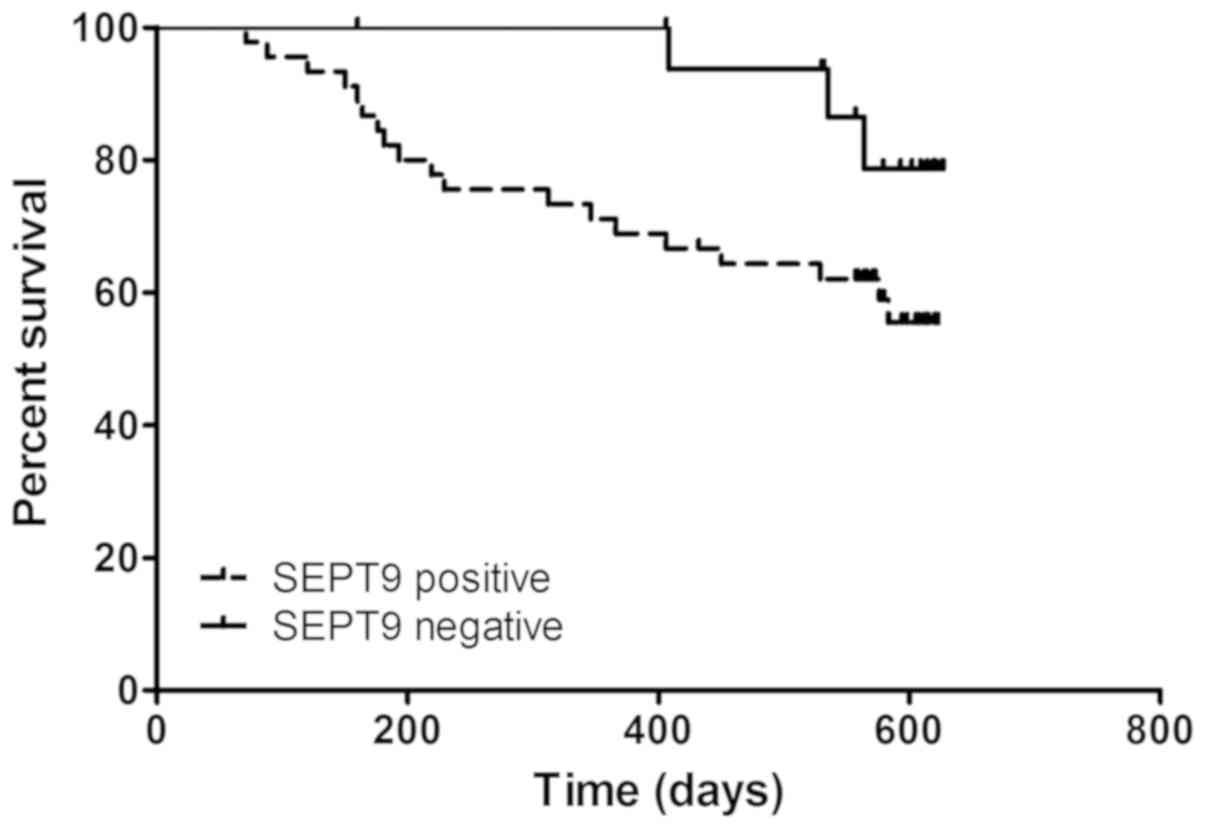
To investigate the potential role of mSEPT9 in the
prognosis of patients with HCC, these patients were followed for
~630 days (21 months) and a Kaplan-Meier survival analysis was
performed. As indicated in Fig. 4,
the Kaplan-Meier curves were plotted according to mSEPT9 positive
or negative detection. Patients with positive mSEPT9 detection
exhibited a significantly poorer survival rate than patients with
negative mSEPT9 (P=0.039; hazard ratio, 2.343; 95% CI,
0.957-5.736). The present findings suggested that mSEPT9 may
predict the long-term survival of patients with HCC, regardless of
the disease stage or therapy.
Diagnostic accuracy of mSEPT9 is
influenced by age, but not by sex or type of hepatitis virus
infection
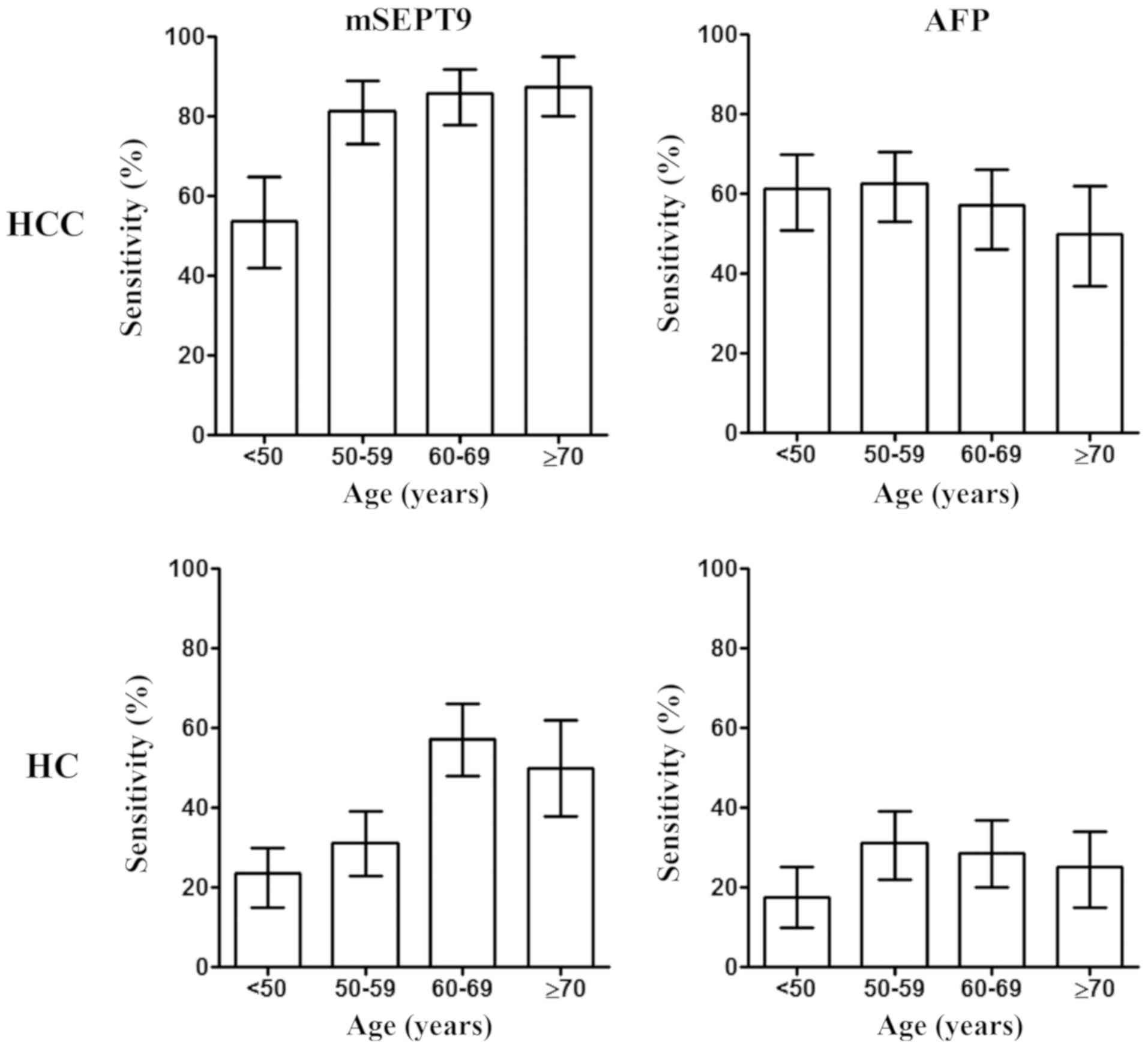
In order to investigate other factors influencing
the diagnostic accuracy of mSEPT9 in HCC, information about patient
age, sex and hepatitis infection status was collected and the
association between these factors and mSEPT9 detection performance
was analyzed (Fig. 5), which
suggested a higher sensitivity in patients >50 years old
(including groups: 50–59, 60–69 and ≥70 years; 84.2%) compared with
those <50 years (53.8%) (P=0.026; data not shown). Similarly, a
higher sensitivity in HC detection was observed in all patients
>60 years old (including groups: 60–69 and ≥70 years; 27.3%)
(P=0.098; data not shown) compared with those <60 years of age
(including groups: <50 and 50–59 years; 54.5%). By contrast, no
significant differences in sensitivity were detected across the
various age groups with AFP detection.
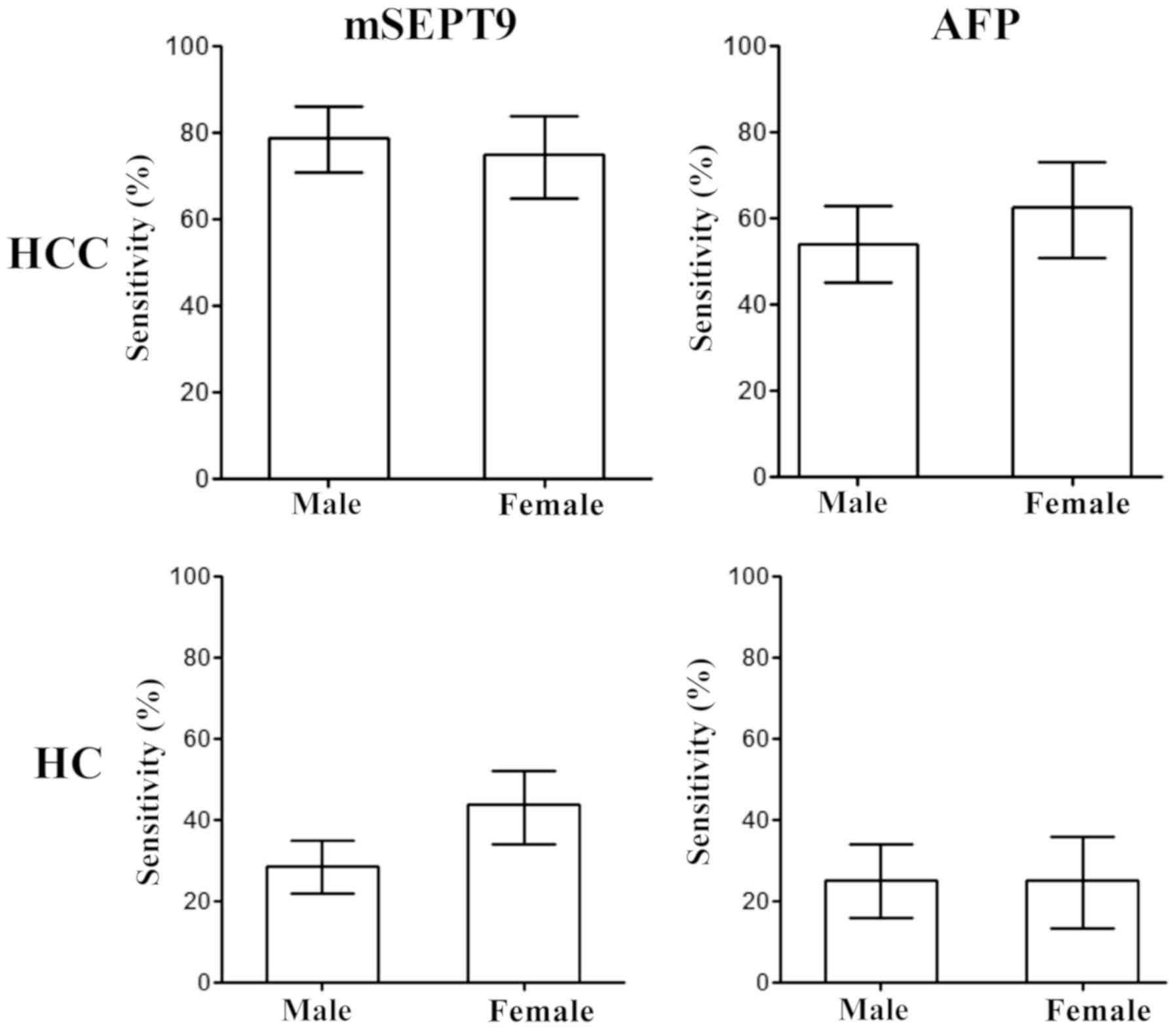
Furthermore, the influence of the sex of the
patients on HCC and HC diagnosis using mSEPT9 and AFP detection was
analyzed. As revealed in Fig. 6,
no significant differences were observed between males and females
in HCC or HC detection with either mSEPT9 or AFP, indicating a
non-discriminative detection for both sexes.
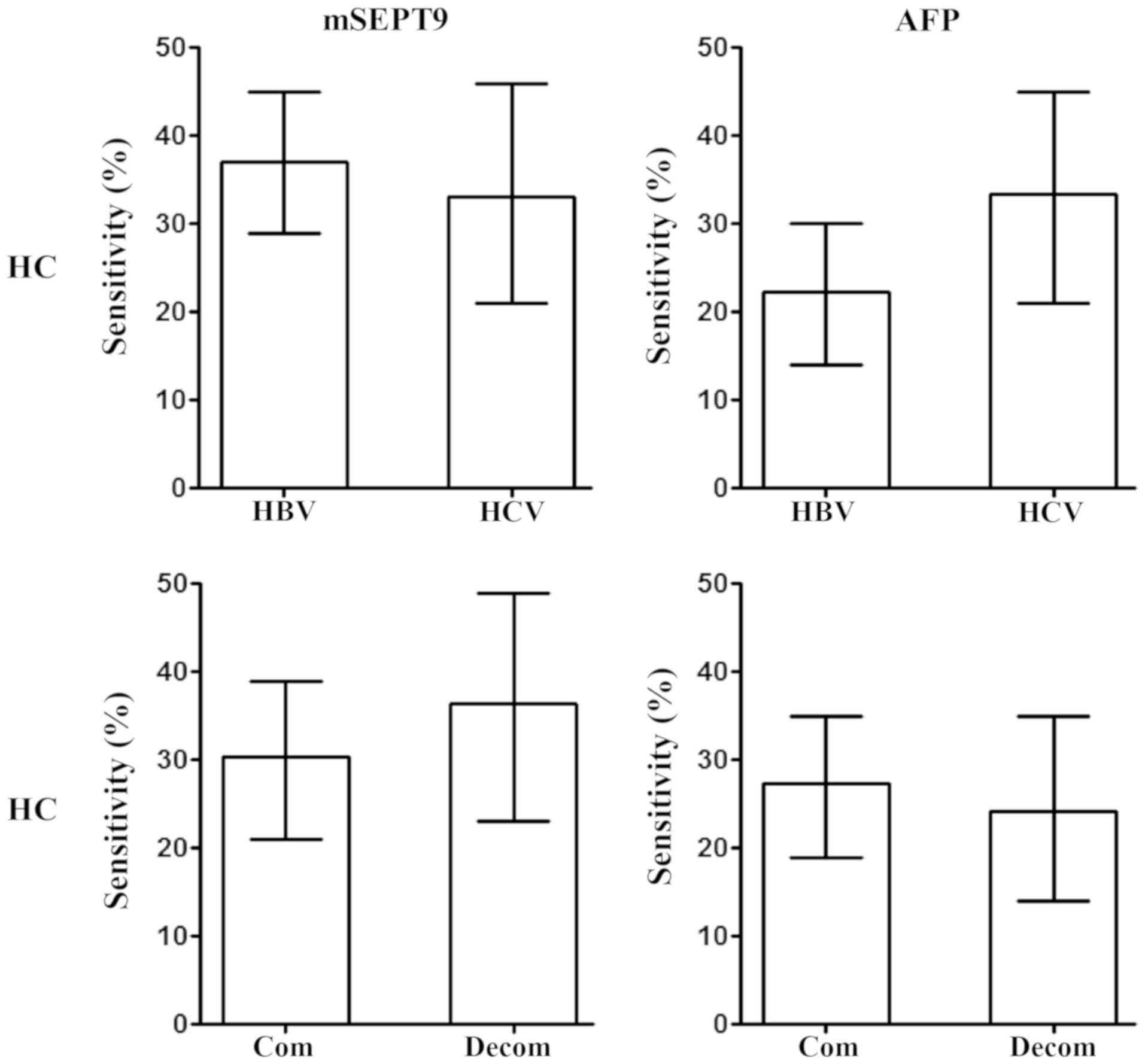
The majority of patients with HC have a history of
hepatitis virus infection, commonly HBV infection followed by HCV
infection (4,5). Therefore, the potential difference
between HBV- and HCV-infected patients with HC was analyzed. As
demonstrated in Fig. 7, no
difference in the sensitivity of HC diagnosis between HBV- and
HCV-infected patients was observed using mSEPT9 or AFP detection.
Additionally, since both compensated and decompensated patients
with HC are often observed clinically, the potential difference in
the detection sensitivity between these two groups was analyzed.
The present results suggested no significant differences between
the sensitivity of either mSEPT9 or AFP detection in patients with
compensated and decompensated HC (Fig.
7).
Discussion
The mSEPT9 assay has been used in the screening and
early detection of CRC for >5 years. The assay has been approved
by the US FDA as a screening test for the average-risk population
>50 years of age and by the Chinese FDA as an early detection
test for high-risk populations (9,17) as
a proven specific test for CRC. Additionally, it can effectively
detect colorectal precancerous conditions, such as adenoma
(18,19). Furthermore, aberrant mSEPT9 levels
have been detected in other types of cancer, such as lung cancer
and HCC (9–11), where they may serve as markers.
The performance of mSEPT9 and AFP assays in HC and
HCC detection has been investigated in a previous study conducted
in a European population using the Epi proColon 2.0 CE assay
(13). The Epi proColon 2.0 CE
test performs three parallel PCR reactions to detect the aberrant
methylation levels of the SEPT9 gene, which requires an appropriate
algorithm for result interpretation. The findings of the present
study suggest that the mSEPT9 assay may be used to detect HCC and
HC with high sensitivity in a Chinese population. In contrast to a
previous study, a modified version of the mSEPT9 assay approved by
the Chinese FDA for CRC early detection (9) was used to validate its applicability
for the diagnosis of HCC and HC. The modified mSEPT9 assay allowed
the detection of aberrant mSEPT9 with a single PCR reaction,
therefore facilitating its use in routine laboratory testing. The
overall sensitivity for HCC diagnosis (76.7%) was similar to that
for CRC diagnosis (9), and it was
the same as that reported for HCC in a European population
(13), indicating that mSEPT9 may
also detect HCC with a high sensitivity in a Chinese population.
Since the assay was initially designed and validated in CRC, the
present findings suggested that CRC and HCC may have similar mSEPT9
patterns, although this requires further study. Furthermore, the
present results indicated that mSEPT9 was not only specific to CRC
but can also be detected in other cancer types, such as HCC.
Additionally, a cancer stage dependent sensitivity of mSEPT9 was
observed, starting from benign HC to malignant HCC stages A-C. This
further indicated that mSEPT9 levels may reflect the severity of
the progression of hepatic lesions, in agreement with previous
studies on CRC where a stage-dependent sensitivity was observed
(6,10). Therefore, mSEPT9 levels may also be
used in HC or HCC therapeutic response assessment or monitoring, as
this application has been validated in surgery and late-stage CRC
therapies (20).
Notably, the present study revealed that the mSEPT9
assay had a higher sensitivity in patients >50 years of age than
in younger patients. This may reflect an increase in mSEPT9 levels
with increasing age in patients with HCC, which was also observed
in patients with CRC (4,5,10),
suggesting a common trend associated with increasing age. In
patients with CRC, a significant difference in positive detection
rate was observed between individuals >60 and <60 years of
age, which may be attributed to the late-onset age of CRC (4,5,10)
compared with that of HCC. Changes in gene methylation status with
age can occur in cancer as well as in normal tissues. It has been
demonstrated that low-level de novo methylation of CpG
islands occur in normal tissues and that their frequency increases
with age (21–23). Abnormal methylation levels observed
in tumor cells can also occur in normal cells (24). Overall, aberrant methylation
patterns detected in cancer may be present in normal aging cells,
which may result in malignant transformation due to the
accumulation of methylation aberrances with increasing age.
The present study suggested that the sex of the
patients did not influence the sensitivity of the mSEPT9 assay,
indicating that both sexes may share a similar mSEPT9 gene profile
and that the mechanism of HCC carcinogenesis may be similar between
them. This would further increase the applicability of the mSEPT9
assay for HCC detection.
The present findings suggested that the diagnostic
performance of mSEPT9 was improved compared with that of AFP in the
detection of HCC (sensitivity, 76.7 vs. 56.7%; AUC, 0.85 vs. 0.80)
and HC (sensitivity, 34.1 vs. 25.0%; AUC, 0.77 vs. 0.55), with no
marked difference in their specificity (95.9 vs. 94.6%).
Additionally, a higher sensitivity of mSEPT9 compared with AFP was
observed in diagnosing all HCC stages. By contrast, the performance
of mSEPT9 in discriminating HCC from HC was not higher compared
with that of AFP (AUC, 0.66 vs. 0.77). The present observations
suggested that mSEPT9 may be more suitable than AFP as a marker for
HCC detection. However, it was less effective in the differential
diagnosis between HCC and HC, possibly due to the higher detection
rate of HC by mSEPT9 increasing the difficulty in discriminating it
from HCC. A previous study has reported a similar behavior of
mSEPT9 when one marker is sensitive to a certain type of cancer and
its precancerous conditions (18).
Furthermore, the present findings demonstrated that abnormal mSEPT9
levels may also occur in HC, suggesting that aberrant methylation
may exist when lesions are at the benign stage. The transition from
benign HC to malignant HCC may be due to quantitative accumulation
of aberrancies that ultimately lead to malignant
transformation.
The combination of mSEPT9 and carcinoembryonic
antigen detection enhances the diagnostic performance of these
tests in CRC (9). However, in the
present study, the combination of mSEPT9 and AFP detection did not
significantly alter the performance of these tests in HCC and HC
compared with mSEPT9 detection alone. This may be due to the fact
that the proportion of overlapping detection between mSEPT9 and AFP
was substantial, which may result in insignificant complementation.
The present study suggested that detection of mSEPT9 alone may be
enough for HCC diagnosis. By contrast, a trend of increased
sensitivity was observed when the combination was compared with the
detection of AFP alone, suggesting that the combination may
potentially improve the detection performance of AFP compared with
using this protein marker alone. Furthermore, mSEPT9 detection may
be used in combination with AFP detection in the differential
diagnosis between HCC and HC, as the latter appeared more potent in
discriminating HCC from HC. In clinical practice, mSEPT9 and/or AFP
detection may be used in combination with ultrasonic examination to
support the differential diagnosis. Ultrasonic examination can
discriminate the majority of HC from HCC cases (25), and mSEPT9 and/or AFP may be used
for the remaining uncertain cases to maximize the definite
diagnosis and reduce misdiagnoses.
An important finding of the present study was that
the survival of patients with HCC may be predicted using mSEPT9
detection. Similar observations were reported for mSEPT9 in
patients with CRC and for methylated short stature homeobox 2 in
patients with lung cancer, independently of disease stage and
therapeutic interventions (20,26).
Additionally, previous studies have suggested that mSEPT9 may serve
as an independent risk factor for CRC (208) and HCC (13). Therefore, mSEPT9 may be used to
predict the long-term risk of patients with CRC and HCC
independently. AFP has been proposed as a predictive marker for HCC
in multiple studies (27,28). However, due to the lower
sensitivity of AFP detection in patients with HCC, only patients
with a positive AFP detection before therapy can be assessed, while
patients exhibiting negative AFP detection are often hard to assess
(29). By contrast, the positive
detection rate of mSEPT9 was higher than that of AFP (Fig. 3C), and may therefore be more
applicable in the assessment of prognosis or in predicting
survival. This would be another advantage of mSEPT9 over AFP
detection, especially for in vitro HCC diagnosis, in
addition to its higher sensitivity. Since the promoter region of
SEPT9 was reported to be highly methylated in HCC in a previous
study (13) and in the present
study, it is expected that the expression levels of SEPT9 would be
decreased, as it was demonstrated that hypermethylation of the
promoter region of SEPT9 decreased SEPT9 expression in CRC
(12,30,31).
There are a few of limitations to the present study.
Firstly, this was a case-control study, and thus whether mSEPT9
will exhibit a similar performance in a screening setting requires
further investigation. Moreover, it was difficult to discriminate
HCC from HC using mSEPT9, and it appeared that mSEPT9 alone may not
be sufficient for the differential diagnosis. High sensitivity of
mSEPT9 in both CRC and HCC means it is difficult to distinguish the
two cancer types in screening, and other methods should be combined
to overcome this issue. Future studies should focus on studying the
applicability of mSEPT9 as a predictive marker for long-term
prognosis in patients with HC, as ~34.1% of patients with HC in the
present study were mSEPT9 positive. Potential differences in the
prognosis or therapeutic response based on mSEPT9 dichotomization
may help in distinguishing different patients with HC and in
developing personalized therapeutic strategies. This would greatly
benefit patients with HC and may prevent its progression to
HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the project ‘The
key molecular events in the carcinogenesis and development of
gastrointestinal cancer and the clinical significance’ funded by
the Natural Science Foundation of China for innovative research
group (grant no. 81421003).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
YY and NH designed the study. NH, GF, CZ, FW and TZ
recruited the subjects and collected the samples. FW and TZ
arranged the test of samples and liaised with the laboratory. NH,
GF and CZ, FW and TZ analyzed the data. NH, GF, CZ, FW and TZ made
the figures and tables, and wrote the manuscript. YY proofread the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
A detailed plan of the present study was submitted
to the Ethics Committee of the First Affiliated Hospital of Xian
Medical University (Xian, China) and Wuqi People's Hospital (Yanan,
China) for review, and was approved before the initiation of the
clinical study. All subjects involved in the study provided written
informed consent before blood collection, and were informed of the
usage of plasma and the test results. Confirmation of approval for
clinical studies was received from the institutional review board
or ethics committee of the aforementioned hospitals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:2705–132. 2016. View Article : Google Scholar
|
|
2
|
Hung CH, Lu SN, Wang JH, Lee CM, Chen TM,
Tung HD, Chen CH, Huang WS and Changchien CS: Correlation between
ultrasonographic and pathologic diagnoses of hepatitis B and C
virus-related cirrhosis. J Gastroenterol. 38:153–157. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karhunen PJ and Penttilä A: Preneoplastic
lesions of human liver. Hepatogastroenterology. 34:10–15.
1987.PubMed/NCBI
|
|
4
|
Alberti A, Chemello L and Benvegnù L:
Natural history of hepatitis C. J Hepatol. 1 (Suppl 31):S17–S24.
1999. View Article : Google Scholar
|
|
5
|
Lyu X, Liu K, Chen Y, Wang Z, Yao J, Cai
G, Jiang Z, Wang Z, Jiang J and Gu H: Analysis of risk factors
associated with the development of hepatocellular carcinoma in
chronic HBV-infected Chinese: A meta-analysis. Int J Environ Res
Public Health. 13:6042016. View Article : Google Scholar
|
|
6
|
Song L, Jia J, Peng X, Xiao W and Li Y:
The performance of the SEPT9 gene methylation assay and a
comparison with other CRC screening tests: A meta-analysis. Sci
Rep. 7:30322017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song L and Li Y: Progress on the clinical
application of the SEPT9 gene methylation assay in the past 5
years. Biomark Med. 11:415–418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song L, Li Y, Jia J, Zhou G, Wang J, Kang
Q, Jin P, Sheng J, Cai G, Cai S and Han X: Algorithm optimization
in methylation detection with multiple RT-qPCR. PLoS One.
11:e01633332016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu D, Zhou G, Jin P, Zhu J, Li S, Wu Q,
Wang G, Sheng J, Wang J, Song L, et al: Detection of colorectal
cancer using a simplified SEPT9 gene methylation assay is a
reliable method for opportunistic screening. J Mol Diagn.
18:535–545. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song L, Jia J, Yu H, Peng X, Xiao W, Gong
Y, Zhou G, Han X and Li Y: The performance of the mSEPT9 assay is
influenced by algorithm, cancer stage and age, but not sex and
cancer location. J Cancer Res Clin Oncol. 143:1093–1101. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dietrich D, Jung M, Puetzer S, Leisse A,
Holmes EE, Meller S, Uhl B, Schatz P, Ivascu C and Kristiansen G:
Diagnostic and prognostic value of SHOX2 and SEPT9 DNA methylation
and cytology in benign, paramalignant and malignant pleural
effusions. PLoS One. 8:e842252013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Powrózek T, Krawczyk P, Kucharczyk T and
Milanowski J: Septin 9 promoter region methylation in free
circulating DNA-potential role in noninvasive diagnosis of lung
cancer: Preliminary report. Med Oncol. 31:9172014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oussalah A, Rischer S, Bensenane M, Conroy
G, Filhine-Tresarrieu P, Debard R, Forest-Tramoy D, Josse T,
Reinicke D, Garcia M, et al: Plasma mSEPT9: A novel circulating
cell-free DNA-based epigenetic biomarker to diagnose hepatocellular
carcinoma. EBioMedicine. 30:138–147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bruix J and Sherman M; Practice Guidelines
Committee, : American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
DAmico G, Morabito A, DAmico M, Pasta L,
Malizia G, Rebora P and Valsecchi MG: New concepts on the clinical
course and stratification of compensated and decompensated
cirrhosis. Hepatol Int. 12 (Suppl 1):S34–S43. 2018. View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Church TR, Wandell M, Lofton-Day C, Mongin
SJ, Burger M, Payne SR, Castaños-Vélez E, Blumenstein BA, Rösch T,
Osborn N, et al: Prospective evaluation of methylated SEPT9 in
plasma for detection of asymptomatic colorectal cancer. Gut.
63:317–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song L, Peng X, Li Y, Xiao W, Jia J, Dong
C, Gong Y, Zhou G and Han X: The SEPT9 gene methylation assay is
capable of detecting colorectal adenoma in opportunistic screening.
Epigenomics. 9:599–610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song L, Wang J, Wang H, Chen Y, Jia J, Guo
S, Liu H, Peng X, Xiao W, Gong Y, et al: The quantitative profiling
of blood mSEPT9 determines the detection performance on colorectal
tumors. Epigenomics. 10:1569–1583. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song L, Guo S, Wang J, Peng X, Jia J, Gong
Y, Yang B, Xiao W, Dong C, Liu H and Li Y: The blood mSEPT9 is
capable of assessing the surgical therapeutic effect and the
prognosis of colorectal cancer. Biomark Med. 12:961–973. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kulis M, Merkel A, Heath S, Queirós AC,
Schuyler RP, Castellano G, Beekman R, Raineri E, Esteve A, Clot G,
et al: Whole-genome fingerprint of the DNA methylome during human B
cell differentiation. Nat Genet. 47:746–756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Issa JP: Aging and epigenetic drift: A
vicious cycle. J Clin Invest. 124:24–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Teschendorff AE, Menon U, Gentry-Maharaj
A, Ramus SJ, Weisenberger DJ, Shen H, Campan M, Noushmehr H, Bell
CG, Maxwell AP, et al: Age-dependent DNA methylation of genes that
are suppressed in stem cells is a hallmark of cancer. Genome Res.
20:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maegawa S, Hinkal G, Kim HS, Shen L, Zhang
L, Zhang J, Zhang N, Liang S, Donehower LA and Issa JP: Widespread
and tissue specific age-related DNA methylation changes in mice.
Genome Res. 20:332–340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nagato Y, Kondo F, Kondo Y, Ebara M and
Ohto M: Histological and morphometrical indicators for a biopsy
diagnosis of well-differentiated hepatocellular carcinoma.
Hepatology. 14:473–478. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng X, Liu X, Xu L, Li Y, Wang H, Song L
and Xiao W: The mSHOX2 is capable of assessing the therapeutic
effect and predicting the prognosis of stage IV lung cancer. J
Thorac Dis. 11:2458–2469. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bai DS, Zhang C, Chen P, Jin SJ and Jiang
GQ: The prognostic correlation of AFP level at diagnosis with
pathological grade, progression, and survival of patients with
hepatocellular carcinoma. Sci Rep. 7:128702017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chan MY, She WH, Dai WC, Tsang SHY, Chok
KSH, Chan ACY, Fung J, Lo CM and Cheung TT: Prognostic value of
preoperative alpha-fetoprotein (AFP) level in patients receiving
curative hepatectomy-an analysis of 1,182 patients in Hong Kong.
Transl Gastroenterol Hepatol. 4:522019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aoyagi Y, Oguro M, Yanagi M, Mita Y, Suda
T, Suzuki Y, Hata K, Ichii K and Asakura H: Clinical significance
of simultaneous determinations of alpha-fetoprotein and
des-gamma-carboxy prothrombin in monitoring recurrence in patients
with hepatocellular carcinoma. Cancer. 77:1781–1786. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Potter NT, Hurban P, White MN, Whitlock
KD, Lofton-Day CE, Tetzner R, Koenig T, Quigley NB and Weiss G:
Validation of a real-time PCR-based qualitative assay for the
detection of methylated SEPT9 DNA in human plasma. Clin Chem.
60:1183–1191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ravegnini G, Zolezzi Moraga JM, Maffei F,
Musti M, Zenesini C, Simeon V, Sammarini G, Festi D, Hrelia P and
Angelini S: Analysis of SEPT9 promoter methylation status,
micronuclei frequency, and folate-related gene polymorphisms: The
potential for a novel blood-based colorectal cancer biomarker. Int
J Mol Sci. 16:28486–28497. 2015. View Article : Google Scholar : PubMed/NCBI
|