Introduction
Gastric cancer, one of the most common types of
human gastrointestinal cancer, is the second most common cause of
cancer-associated death worldwide, with ~720,000 gastric
cancer-related deaths annually (1,2). A
national survey assessment reported that the mortality rate of
gastric cancer in India is ≤12.5% (3). In East Asia, the burden of gastric
cancer is especially high, accounting for >50% of gastric cancer
incidence (4,5). In China, the 5-year overall survival
rate of patients with gastric cancer is ~30% (6). Furthermore, the overall survival of
patients with gastric cancer globally has been continuing to
decline since the beginning of the 2000s, with the proportion of
gastric cancer patients with metastasis at ~40% (3,7–9).
Currently, the most common treatment methods for gastric cancer are
surgical resection, radiotherapy and chemotherapy (10). Most patients with gastric cancer
are diagnosed at an advanced stage accompanied by cancer
metastasis, and the current therapeutic strategies are limited and
have a range of adverse effects, including kidney damage and hair
and hearing loss (11,12). Human epidermal growth factor
receptor 2 (HER-2), a member of the HER-2 family, is associated
with an increased risk of recurrence and poor outcomes of certain
malignancies, including breast and gastric cancer (13). HER-2 has been identified to be
involved in cancer initiation and progression, and the
dysregulation of HER-2 serves as an independent prognostic factor
in gastric cancer (14). Thus,
HER-2 is a common therapeutic target for gastric cancer.
MicroRNAs (miRNAs/miRs) are a class of non-coding
small RNA that consist of 17–25 nucleotides. miRNAs negatively
regulate the expression levels of a range of genes by binding to
the 3′ untranslated regions (3′UTR) of the mRNA of target genes.
miRNAs have been demonstrated to be involved in various
physiological and pathological processes, including cancer
(15). miRNAs play an important
role in tumorigenesis, cell proliferation, migration, apoptosis and
metastasis (16,17). miR-204-5p has been identified to be
downregulated in several types of cancers, and is a potential
regulator in human tumorigenesis (1,18).
Based on expression profiling data of gastrointestinal tumor
tissues and adjacent noncancerous tissues, miR-204-5p is one of the
most significantly downregulated miRNAs, and further study suggests
that miR-204-5p inhibits gastric cancer cell proliferation, while
inhibition of miR-204-5p promotes cell proliferation in gastric
cancer (1,19). It has been reported that miRNA-495
interacts with HER-2 to exert its function in gastric cancer;
however, whether miR-204-5p also interacts with HER-2 to exert its
function remains unclear.
The present study aimed to investigate the function
of miR-204-5p and to explore the interaction between miR-204-5p and
HER-2, in order to clarify the underlying mechanisms of miR-204-5p
and HER-2 in gastric cancer and to provide a valuable therapeutic
strategy for gastric cancer treatment.
Materials and methods
Cell culture
The human normal gastric epithelial HEGC cell and
two gastric cancer cell lines, MKN-45 (metastatic gastric cancer
cell line derived from a poorly differentiated gastric
adenocarcinoma) and AGS (a non-metastatic gastric cancer cell line
derived from poorly differentiated gastric adenocarcinoma) were
obtained from American Type Culture Collection and cultured in
RPMI-1640 medium supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) in a humidified incubator containing 5%
CO2.
Cell transfection
For transfection, MKN-45 cells (5×104
cells/well) were plated into 6-well plates and cultured to 90%
confluence. Subsequently, miR-204-5p mimics
(5′-UUCCCUUUGUCAUCCUAUGCCU-3′; 50 nM), miR-204-5p inhibitor
(5′-AGGCAUAGGAUGACAAAGGGAA-3′; 50 nM), the negative control
(5′-UUCUCCGAACGUGUCACGUTT-3′; 50 nM) and siRNA targeting HER-2
(si-HER-2; 5′-GGUGAAGGUGCUUGGAUCUUU-3′; 500 ng/µl) were transfected
into cells using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
After 48 h transfection, transfection efficiency was detected by
reverse transcription-quantitative (RT-q) PCR. Subsequent
experiments were conducted 48 h post-transfection.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was measured using a CCK-8 reagent
(Dojindo Molecular Technologies, Inc.) according to the
manufacturer's instructions. Briefly, MKN-45 cells
(5×103 cells/well) were cultured in 96-well plates and
incubated for 24 h at 37°C. Subsequently, CCK-8 reagent (10
µl/well) was added, and the cells were incubated for another 4 h.
Finally, the absorbance of each group at 450 nm was measured using
a microplate reader (Molecular Devices, LLC).
Colony formation assay
After transfection for 24 h, MKN-45 cells
(1,000-1,500 cells/well) were seeded into 6-well plates and
incubated for 7 days at 37°C. During this period, the medium was
refreshed every 3 days. Subsequently, cells were fixed with 4%
paraformaldehyde for 15 min and stained with Giemsa reagent for
10–30 min at room temperature. Finally, colonies were imaged under
light microscopy (magnification, ×100) and the number of colonies
(≥50 cells/colony) was counted. Three independent experiments were
performed for each assay.
Flow cytometry analysis
The rate of cell apoptosis (at early and late phase)
was determined using the Apoptosis Detection kit (BD Biosciences)
according to the manufacturer's protocol. MKN-45 cells were
collected 48 h post-transfection and resuspended in 500 µl binding
buffer. Subsequently, MKN-45 cells were incubated with Annexin
V-FITC and propidium iodide at room temperature for 15 min in the
dark, and then analyzed using a BD FACSCalibur™ flow cytometer (BD
Biosciences) and BD FACSDiva 6.1.3 software (BD Biosciences).
RT-qPCR
Total RNA was extracted from MKN-45 cells using
TRIzol® reagent (Thermo Fisher, Scientific, Inc.)
according to the manufacturer's instructions. Total RNA was reverse
transcribed into cDNA using the PrimeScript RT Master Mix kit
(Takara, Bio, Inc.) at 37°C for 15 min, followed by an incubation
at 85°C for 5 sec. Determination of gene expression was performed
using FastStart Universal SYBR Master Mix (Roche Diagnostics GmbH)
and analyzed using the 2−ΔΔCq method (20). The primer sequences were as
follows: miR-204-5p forward, 5′-ACACTCCAGCTGGGTTCCCTTTGTCATCCTAT-3′
and reverse, 5′-CTCAACTGGTGTCGTGGA-3′; HER-2 forward,
5′-CTGAACTGGTGTATGCAGATTGC-3′ and reverse, 5′-TTCCGAGCGGCCAAGTC-3′;
GAPDH forward, 5′-CCATCTTCCAGGAGCGAGAT-3′ and reverse,
5′-TGCTGATGATCTTGAGGCTG-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The thermocycling conditions
consisted of an initial denaturation at 95°C for 5 min followed by
40 cycles at 95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec.
GAPDH and U6 served as internal controls for the detection of HER-2
and miRNA, respectively.
Western blotting
Proteins were extracted from MKN-45 cells using
radio immunoprecipitation assay protein extraction reagent
(Beyotime Institute of Biotechnology) containing 0.5 mM
phenylmethanesulfonyl fluoride, and quantified by the Bradford
assay. Equal masses of protein sample (30 µg) were resolved by
SDS-PAGE on a 10% gel, and subsequently transferred to PVDF
membranes. Following blocking in 10% skimmed milk for 1 h at room
temperature, membranes were incubated at 4°C overnight with primary
antibodies against Bax (1:1,000; cat. no. ab182733), Bcl-2
(1:1,000; cat. no. ab196495), HER-2 (1:1,000; cat. no. ab16901) and
GAPDH (1:1,000; cat. no. ab181603; all purchased from Abcam). Then,
membranes were washed and incubated with the corresponding
horseradish peroxidase-conjugated anti-rabbit (1:2,000; cat. no.
sc-2004) or anti-mouse IgG secondary antibodies (1:2,000; cat. no.
sc-2005) (both Santa Cruz Biotechnology, Inc.) for 60 min at room
temperature. Protein bands were visualized with ECL Super Signal
reagent (Pierce; Thermo Fisher Scientific, Inc.). The relative
intensity of the bands was determined using ImageJ software version
1.46 (National Institutes of Health).
Wound healing assay
To determine the migratory ability of cells, cells
were grown to 100% confluence and a scratch was created using a
pipette tip. Subsequently, the culture medium was changed to
serum-free RPMI-1640 medium and the detached cells were removed.
After 24 h, cells were visualized under a light microscope
(magnification, ×100). The cell migration rate was calculated using
the following formula: (0 h scratch width-24 h scratch width)/0 h
scratch width.
Transwell assay
To determine the invasive ability of cells,
5×104 MKN-45 cells were resuspended in 100 µl serum-free
medium and seeded into the upper Transwell chamber (EMD Millipore),
which was pre-coated with Matrigel™ at room temperature for 25 min.
Complete medium supplemented with 10% FBS was added to the lower
chamber. After 24 h, the upper surface of the membrane was wiped
with a cotton swab, and the cells attached to the lower surface
were fixed with 4% formaldehyde for 10 min at room temperature and
stained with 0.1% crystal violet for 10 min at room temperature.
The invasive cells were observed and counted under a light
microscope (magnification, ×100) from at least five fields. The
relative cell invasion rate was calculated using the following
formula: Invasive cell count/invasive cell count of control
group.
Bioinformatics analysis
The Cancer Genome Atlas (TCGA) database (cancer.gov/tcga) was used to identify the association
of miR-204-5p with gastric cancer by collecting the profiles of
miR-204-5p in gastric cancer tissues and normal tissues (project
no.; TCGA-STAD). The expression of miR-204-5p between normal and
tumor tissues or between primary tumor and metastasis tumor tissues
was analyzed using the Wilcoxon-Mann-Whitney test. A putative
binding site of miR-204-5p in the 3′UTR of HER-2 was predicted
using StarBase (http://starbase.sysu.edu.cn/).
Luciferase reporter assay
HER-2, which contains a putative miR-204-5p binding
site, was cloned and inserted into the pmirGLO vector (Promega
Corporation). For the luciferase assay, MKN-45 cells were
co-transfected with pmirGLO-HER-2-WT or pmirGLO-HER-2-MUT, and
miR-204-5p or negative control using Lipofectamine®
(Invitrogen; Thermo Fisher Scientific, Inc.). After 24 h,
luciferase activity was measured using the dual luciferase reporter
assay system (Promega Corporation). The data were standardized to
Renilla luciferase activity.
Statistical analysis
Data are presented as the mean ± SD of three
independent experiments. Data analysis was performed using SPSS
software version 17.0 (SPSS, Inc.). Differences between 2 groups
were analyzed using the unpaired Student's t-test. Differences
among >2 groups were analyzed by one-way ANOVA followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
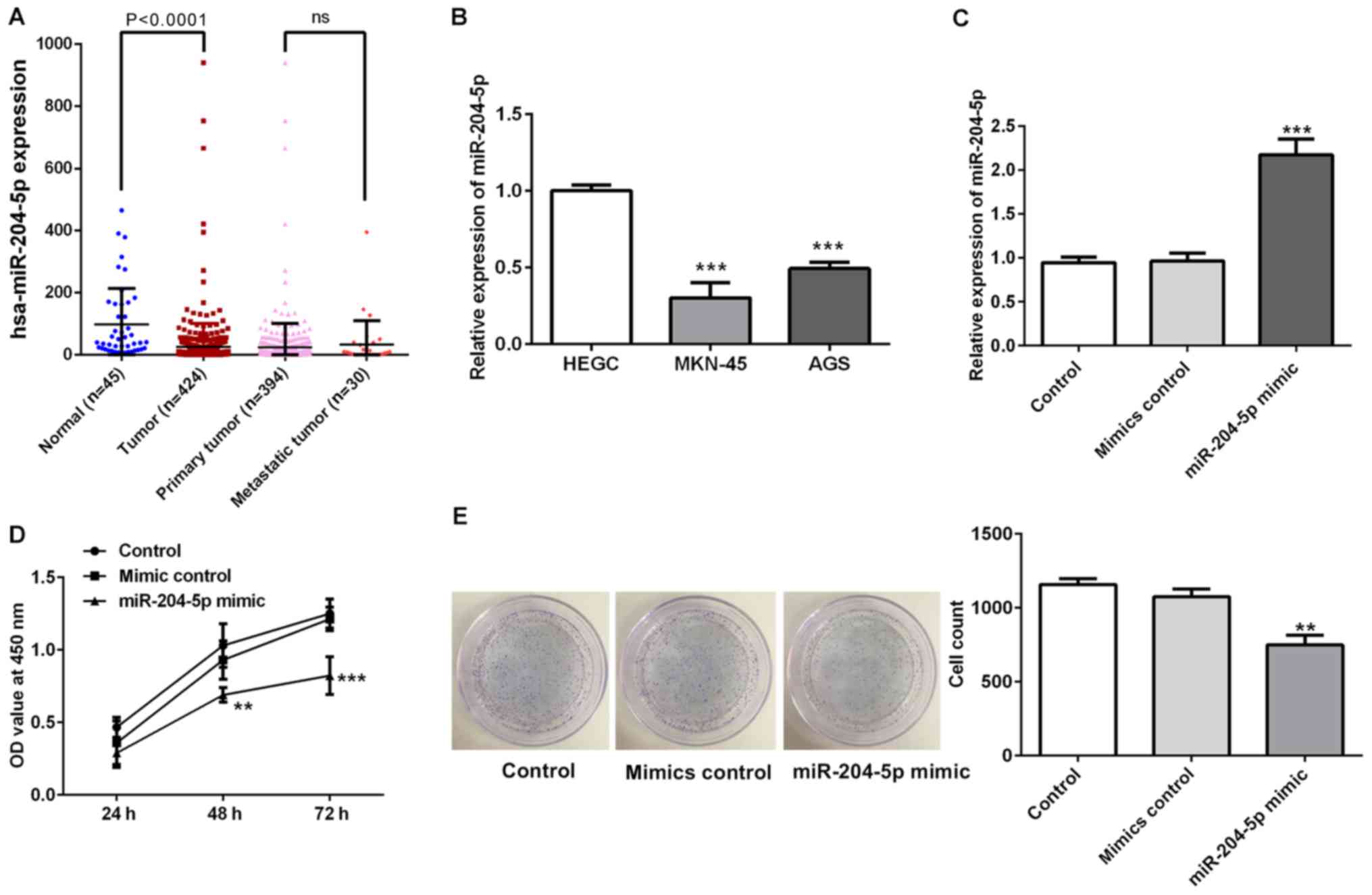
miR-204-5p is downregulated in gastric
cancer and inhibits cell proliferation
The result from the TCGA analysis revealed a
significant lower miR-204-5p expression in gastric cancer tissues
than that in normal tissues. Besides, there was no significant
difference between the expression level of miR-204-5p in primary
and metastatic tumors of patients with gastric cancer (Fig. 1A). To understand the biological
function of miR-204-5p in gastric cancer progression, the present
study detected miR-204-5p mRNA expression in a normal gastric
epithelial cell line (HEGC) and two gastric cancer cell lines
(Fig. 1B). The RT-qPCR assay
revealed that the expression levels of miR-204-5p were lower in the
gastric cancer cell lines, particularly in MKN-45 cells. Therefore,
MKN-45 cells were used in subsequent experiments.
First, MKN-45 cells were transfected with miR-204-5p
mimics to overexpress miR-204-5p (Fig.
1C). The effect of miR-204-5p on cell proliferation was
determined using CCK-8 and colony formation assays. As shown in
Fig. 1D and E, the optical density
value in the miR-204-5p group was significantly decreased compared
with that in the other groups, and the colony numbers in the
miR-204-5p mimic group were also decreased, suggesting that
overexpression of miR-204-5p could inhibit cell proliferation.
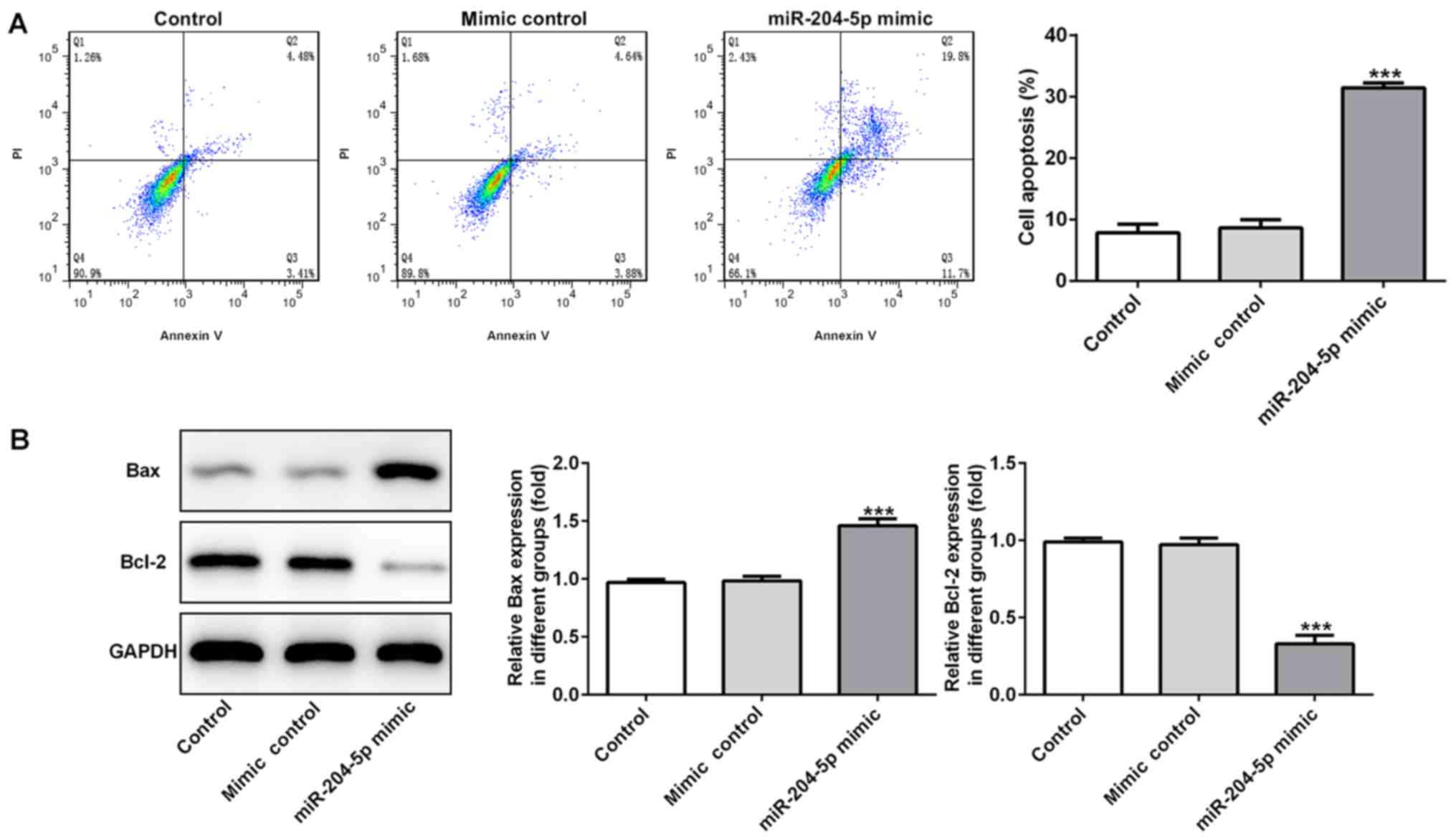
miR-204-5p induces cell apoptosis in
gastric cancer
Subsequently, to determine the effect of miR-204-5p
on cell apoptosis, flow cytometry analysis was performed and
expression levels of apoptosis-related proteins were detected. The
flow cytometry results revealed that the apoptotic cell rate was
significantly increased when miR-204-5p was overexpressed (Fig. 2A). Bax is a pro-apoptotic protein,
whereas Bcl-2 is an anti-apoptotic protein. Increased Bax protein
expression and decreased Bcl-2 protein expression were observed in
the miR-204-5p mimic group (Fig.
2B), suggesting that overexpression of miR-204-5p could promote
MKN-45 cell apoptosis.
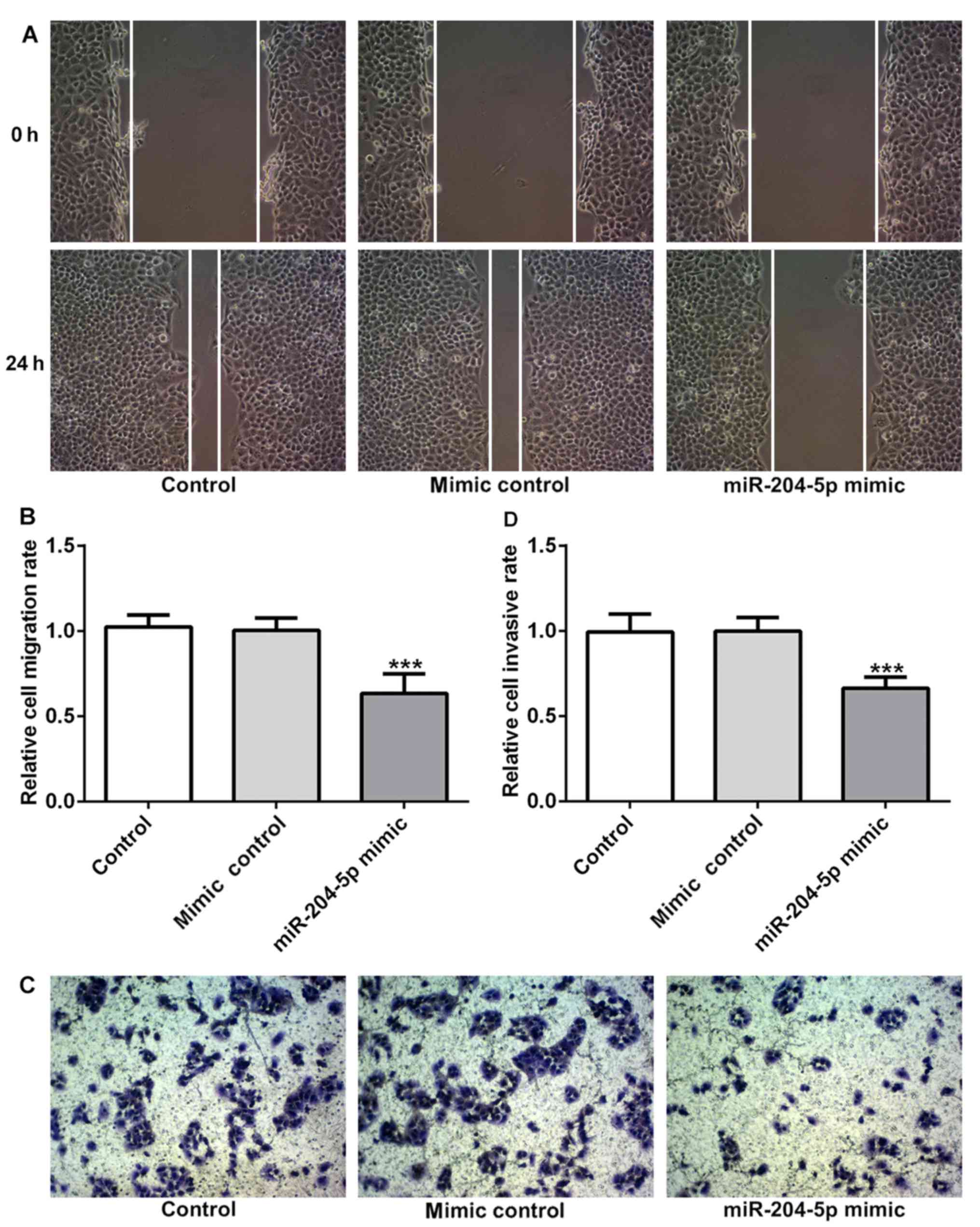
miR-204-5p inhibits cell migration and
invasion in gastric cancer
To determine the effect of miR-204-5p on cell
migration and invasion, wound healing and Transwell assay were
performed. As shown in Fig. 3A and
B, the size of the wound was decreased after incubation for 24
h, but the wound size in the miR-204-5p mimic group was markedly
larger than that in the other groups, suggesting that cells
overexpressing miR-204-5p have a decreased migratory ability.
Meanwhile, the staining results in Fig. 3C and D demonstrated that the number
of cells that were attached to the lower surface of the chamber was
decreased in the miR-204-5p mimic group, suggesting that the
invasive ability was decreased in the miR-204-5p mimic group.
Therefore, overexpression of miR-204-5p could suppress cell
migration and invasion in gastric cancer.
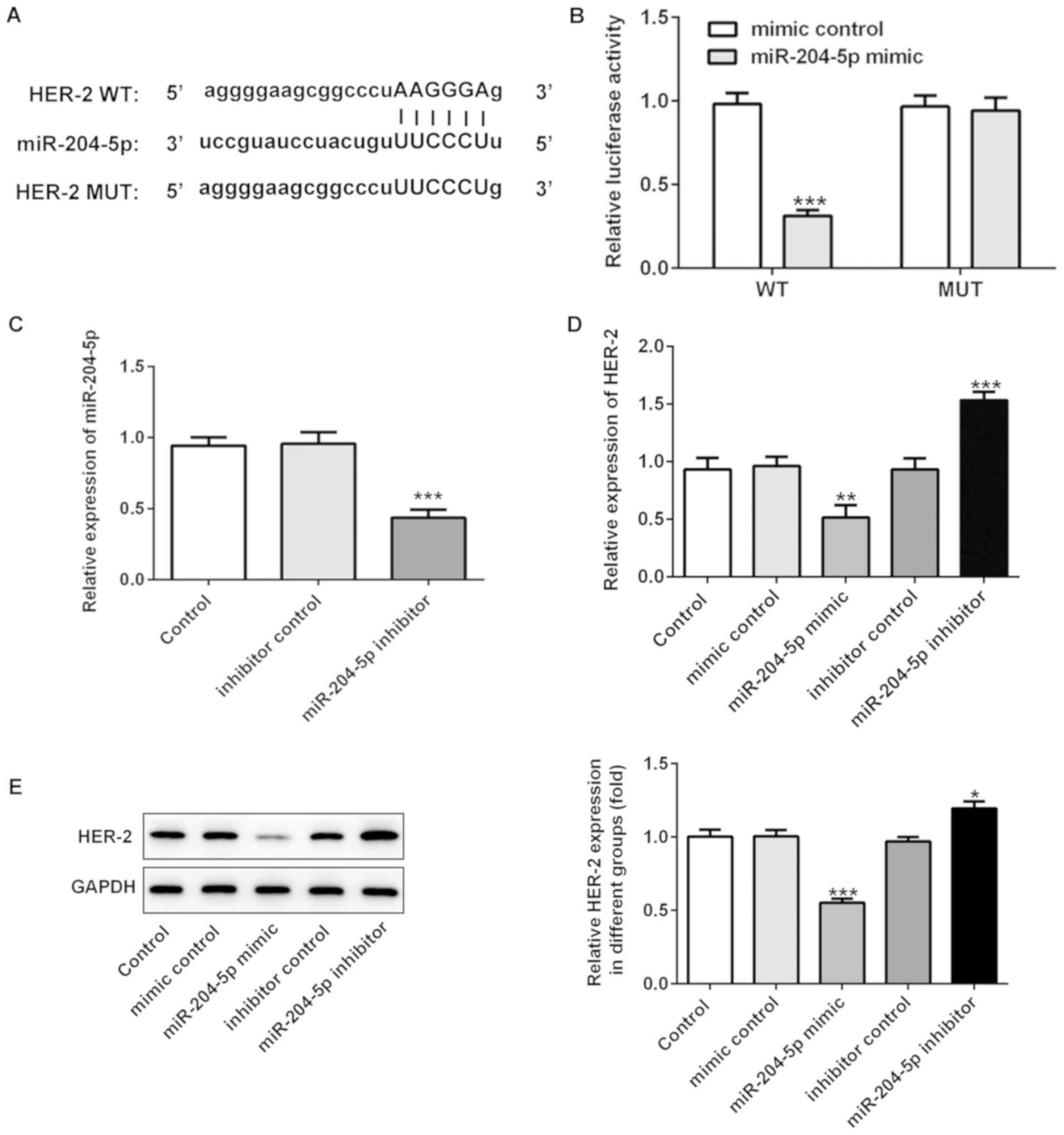
miR-204-5p directly targets HER-2
Furthermore, the present study examined whether
miR-204-5p could directly bind to the 3′ UTR of HER-2. miR-204-5p
was predicted to target HER-2 using StarBase (Fig. 4A), and the dual-luciferase activity
indicated that miR-204-5p could bind to the 3′UTR of HER-2 in
MKN-45 cells, thus inhibiting the translation of HER-2 mRNA
(Fig. 4B). To further determine
the association between miR-204-5p and HER-2, HER-2 expression was
detected when miR-204-5p was overexpressed or inhibited. RT-qPCR
and western blotting revealed that overexpression of miR-204-5p
inhibited the expression of HER-2, whereas downregulation of
miR-204-5p increased HER-2 expression in MKN-45 cells (Fig. 4C-E).
miR-204-5p regulates tumorigenesis of
gastric cancer by targeting HER-2
To further investigate the interaction between
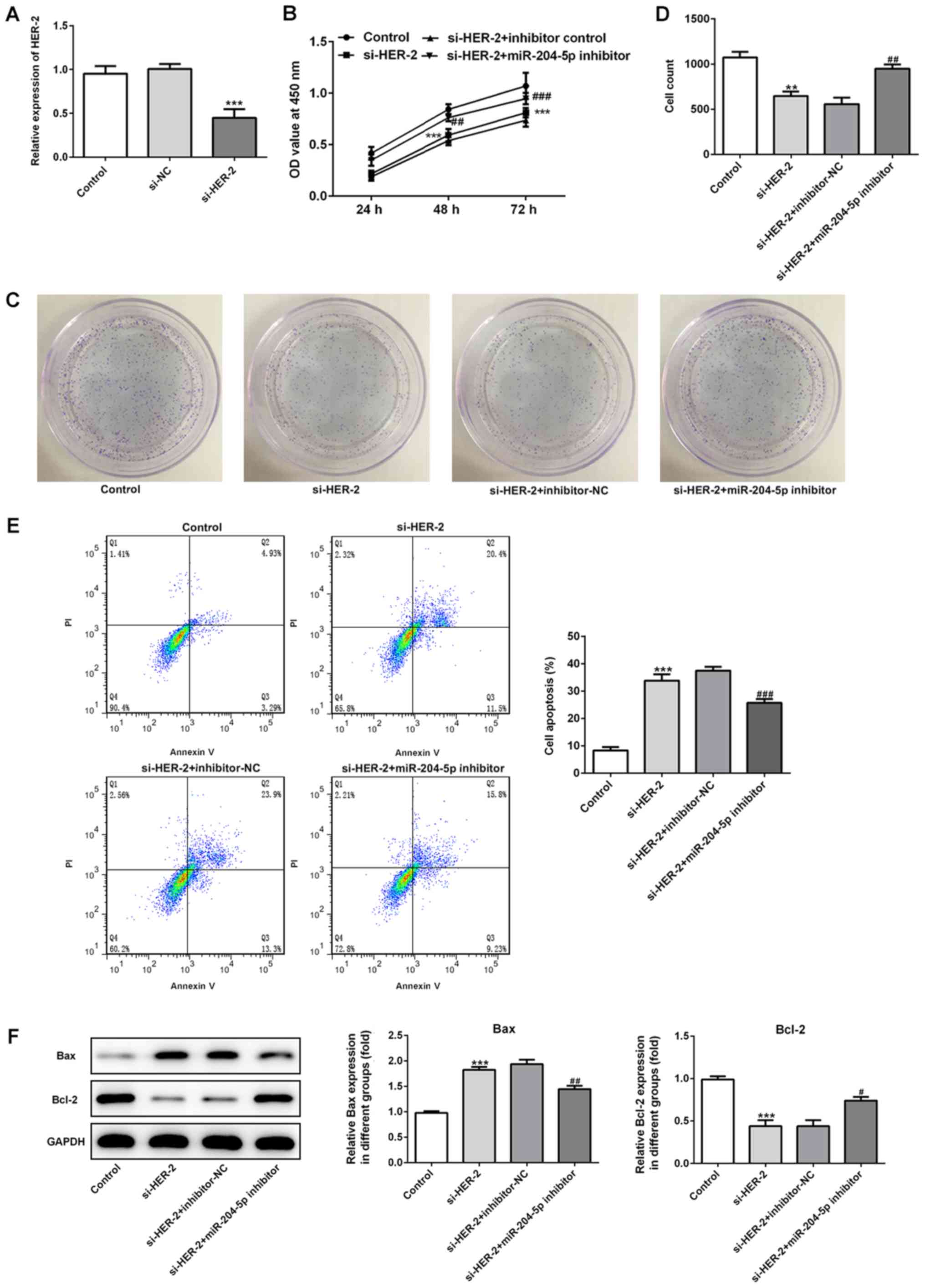
miR-204-5p and HER-2, cells were transfected with si-HER-2 with or
without miR-204-5p inhibitor. As shown in Fig. 5A and B, the expression level of
HER-2 was significantly decreased when cells were transfected with
si-HER-2. In the si-HER-2 group, cell proliferation was
significantly inhibited, which was reversed by inhibition of
miR-204-5p. The results of the colony formation assay in Fig. 5C and D were consistent with those
of the CCK-8 assay. Furthermore, si-HER-2 transfection
significantly induced cell apoptosis, which was also reversed by
inhibition of miR-204-5p (Fig.
5E). Increased Bax expression and decreased Bcl-2 expression
were observed in the si-HER-2 group, which was also reversed by
inhibition of miR-204-5p (Fig.
5F), indicating that inhibition of HER-2 induces cell apoptosis
via the regulation of Bax and Bcl-2, and inhibition of miR-204-5p
reverses this change by regulating Bax and Bcl-2.
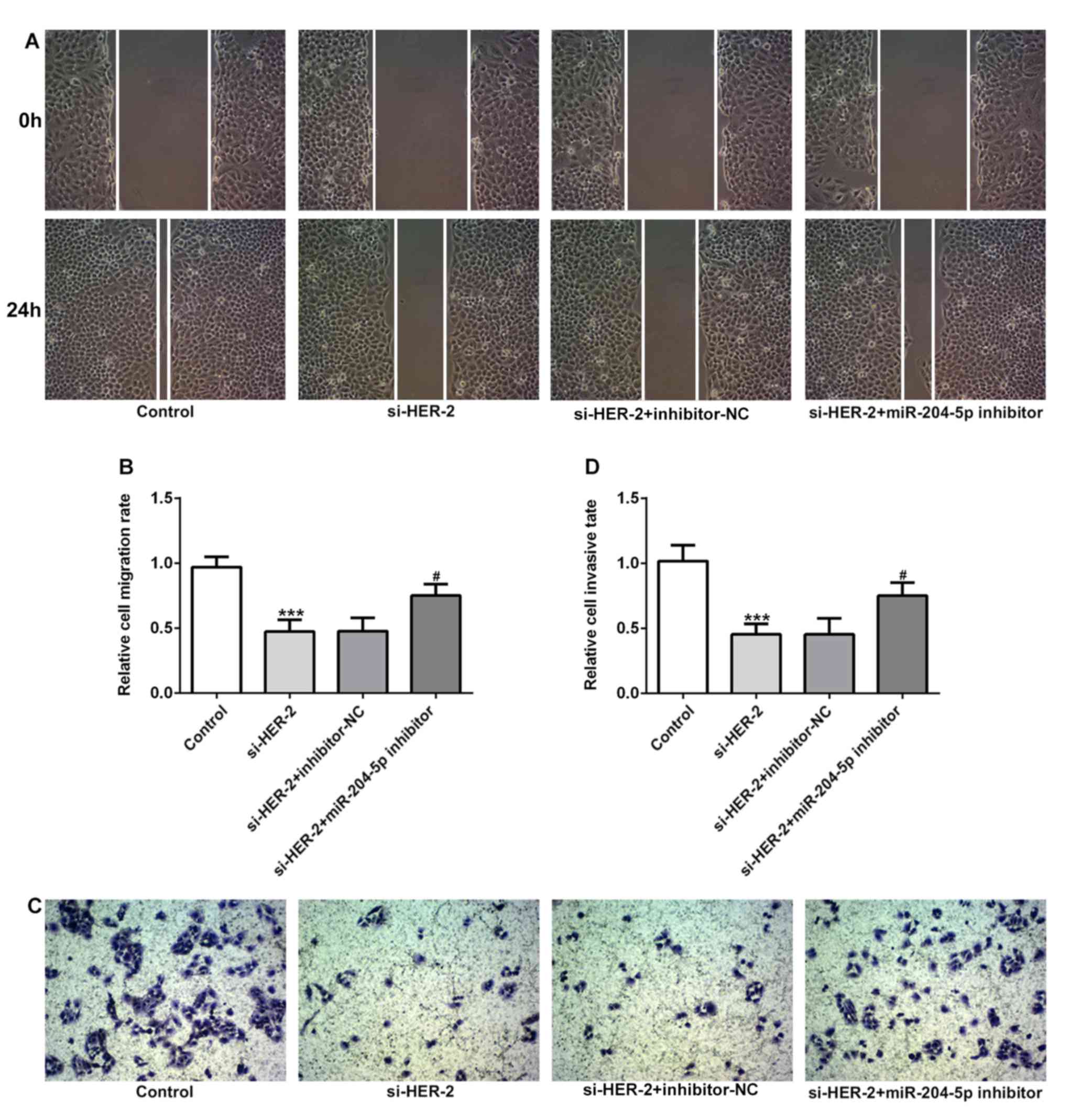
Furthermore, the present study explored the
migratory and invasive abilities of gastric cancer cells following
the regulation of miR-204-5p and HER-2 expression. The results in
Fig. 6A and B showed that
inhibition of HER-2 significantly decreased cell migration rate,
which was reversed following treatment with miR-204-5p inhibitor.
Similarly, results in Fig. 6C and
D demonstrated that inhibition of HER-2 significantly
suppressed the cell invasion rate, which was reversed following
treatment with miR-204-5p inhibitor. The results suggested that
inhibition of miR-204-5p could regulate cell migration and invasion
by targeting HER-2.
Discussion
Gastric cancer is one of the most common types of
cancer worldwide, with a high incidence rate of ≤70% in developing
contrries, particularly in Asia, including China and Korea, as well
as parts of South America. At diagnosis, approximately two-thirds
of patients with gastric cancer present with local invasion or
tumor metastasis (21,22). Therefore, early diagnosis of
gastric cancer is critical for effective therapy and prolonging the
survival of patients. It has been reported that miRNAs serve an
important role during the tumorigenesis and development of gastric
cancer (22). Furthermore, an
increased number of miRNAs have been demonstrated to have
diagnostic and prognostic values in gastric cancer (23). For example, the expression of
miR-1246 can be used to differentiate patients with gastric cancer
at TNM stage I from healthy controls, indicating that is a
potential biomarker for the early diagnosis of gastric cancer
(24). miR-381 has a higher
sensitivity and specificity in the diagnosis of gastric cancer
compared with other miRNAs. Furthermore, downregulated miR-381 has
been identified to be positively associated with lymph node
metastasis and development of gastric cancer, suggesting the use of
miR-381 as a biomarker for early diagnosis in gastric cancer
(25). miR-204-5p has been
reported to be downregulated and to act as a tumor suppressor in a
range of cancer types, such as colorectal cancer, papillary thyroid
cancer, malignant melanoma and hepatocellular cancer (18,26–28).
It has been demonstrated that miR-204-5p expression is
downregulated in gastric cancer tissues, and inhibition of
miR-204-5p could suppress cell proliferation by regulating
ubiquitin-specific protease 47 and Ras-related protein Rab-22A in
gastric cancer cells (1). Based on
the current study that investigated miR-204-5p in gastric cancer,
the various functions of miR-204-5p and its underlying mechanism of
action remain to be explored in further detail to help us
understand the role of miR-204-5p in the tumorigenesis and
development of gastric cancer, and to provide a more precise
diagnostic value for clinical application.
To evaluate the effect of miR-204-5p on the
biological characteristics of gastric cancer, the present study
overexpressed miR-204-5p in gastric cancer cells. The present study
explored the potential mechanism underlying the suppressive effect
of miR-204-5p in gastric cancer. Following overexpression of
miR-204-5p, the proliferation, migration, invasion and apoptosis
rates of cells were detected, and the molecular mechanism was
analyzed. In addition to the suppressive effect on cell
proliferation in gastric cancer, which was reported in a previous
study (19), miR-204-5p also
significantly suppressed cell migration and invasion, and promoted
cell apoptosis in gastric cancer. The rapid proliferation,
migration and invasion of gastric cancer cells is an important
reason for tumor metastasis, thus contributing to the development
of cancer. The inhibitory effect of miR-204-5p on proliferation,
migration and invasion of gastric cancer cells could be an
effective strategy for cancer treatment. Apoptosis is an important
target for therapeutic intervention in cancer. The Bcl-2 family
proteins, such as pro-apoptotic protein Bax and anti-apoptotic
protein Bcl-2, play critical roles in the regulation of apoptosis
of gastric cancer cells (29,30).
In the present study, miR-204-5p could promote cell apoptosis of
gastric cancer cells by regulating the expression levels of Bax and
Bcl-2, indicating that miR-204-5p is a promising target for
inhibiting gastric cancer metastasis.
Recently, studies have focused on the molecular
mechanism of miR-204-5p in the regulation of gastric cancer
progression, it was demonstrated that miR-204-5p could bind to the
3′UTR of its target genes, such as epidermal growth factor
receptor, CXC-C-X-C motif chemokine receptor 4 and its ligand C-X-C
motif chemokine ligand 12 to influence the expression of these
genes, thus regulating the progression of gastric cancer (31,32).
In the present study, HER-2 was demonstrated to be a direct target
of miR-204-5p, and miR-204-5p exhibited suppressive effects on the
progression of gastric cancer by inhibiting cell proliferation,
migration and invasion, and promoted cell apoptosis by regulating
HER-2. HER-2 (also known as ERBB-2), is a proto-oncogene that
encodes a 185 kDa plasma membrane-bound tyrosine kinase receptor,
located on chromosome 17 at q21 (33,34).
mRNA and protein expression levels of HER-2 are upregulated in
several types of cancers, therefore it is considered to be a factor
associated with poor prognosis (35,36).
Currently, anti-HER-2 therapy is usually administered after surgery
or in the neoadjuvant setting in breast cancer and gastric cancer
(37,38). Inhibition of HER-2 is an effective
and promising target in cancer research. Increasing evidence has
demonstrated that HER-2 is a direct target of several miRNAs, and
miRNAs can regulate cancer progression by inhibiting HER-2
expression. For example, miR-9 targets HER-2 to increase
responsiveness of breast cancer cells to cyclophosphamide or
docetaxel treatment (39).
miR-4319 suppresses growth and increases apoptosis of prostate
cancer cells via inhibition of HER-2 (40). In the present study, it was
demonstrated that HER-2 was a direct target of miR-204-5p, and
miR-204-5p inhibited the protein expression of HER-2. Further
experiments revealed that miR-204-5p or inhibition of HER-2
inhibited proliferation, migration and invasion, and promoted
apoptosis in gastric cancer cells. Furthermore, the suppressive
effect of miR-204-5p on gastric cancer cells was associated with
the inhibition of HER-2 expression.
However, the present study still had some
limitations. First, the experiments in this study were conducted at
the cellular level, therefore it would be useful to study mouse
models and patients with gastric cancer to corroborate these in
vitro findings. For example, whether miR-204-5p is an effective
biomarker to indicate prognosis in patients with gastric cancer
needs to be investigated in clinical settings. Additionally, the
therapeutic effect of miR-204-5p in gastric cancer needs to be
verified in a mouse model. Secondly, the gastric cancer cell lines
used in the present study were limited, the role of miR-204-5p
expression was only investigated in MKN-45 cells, and further data
concerning the role of miR-204-5p in gastric cancer should be
obtained from multiple gastric cancer cell lines. Thirdly, the
bioinformatics analysis in the present study is also limited. It
would be useful to perform analysis using multiple online databases
to learn more about the role and effect of miR-204-5p in gastric
cancer, and other types of cancer. Our future work will focus on
resolving these limitations to gain an increased understanding of
the role of miR-204-5p in gastric cancer.
In conclusion, the present study revealed a direct
interaction between miR-204-5p and HER-2 in gastric cancer.
miR-204-5p exhibited suppressive effects on the progression of
gastric cancer via the inhibition of cell proliferation, migration
and invasion, and promoted cell apoptosis by regulating HER-2. The
data presented in the current study suggest that miR-204-5p and
HER-2 could be potential targets for the development of treatments
for gastric cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Traditional
Chinese Medicine Science and Technology of Fujian (grant no.
2017FJZYLC502).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or available from The Cancer
genome Atlas (cancer.gov/tcga) under project no.
TCGA-STAD.
Authors' contributions
ZH and SY contributed to the study design. BC, BZ,
CL, YQ and HY conducted the experiments and performed data
analysis. SY and BC wrote the manuscript. ZH revised the manuscript
and gave the final approval of the version to be submitted. All
authors contributed to data analysis, drafting and revising the
manuscript and agreed to be accountable for all aspects of the
work. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang B, Yin Y, Hu Y, Zhang J, Bian Z,
Song M, Hua D and Huang Z: MicroRNA-204-5p inhibits gastric cancer
cell proliferation by downregulating USP47 and RAB22A. Med Oncol.
32:26452015.
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sheikh IA, Mirza Z, Ali A, Aliev G and
Ashraf GM: A proteomics based approach for the identification of
gastric cancer related markers. Curr Pharm Des. 22:804–811. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Allemani C, Weir HK, Carreira H, Harewood
R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A,
et al: Global surveillance of cancer survival 1995–2009: Analysis
of individual data for 25,676,887 patients from 279
population-based registries in 67 countries (CONCORD-2). Lancet.
385:977–1010. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hundahl SA: Staging, stage migration, and
patterns of spread in gastric cancer. Semin Radiat Oncol.
12:141–149. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thomassen I, van Gestel YR, van Ramshorst
B, Luyer MD, Bosscha K, Nienhuijs SW, Lemmens VE and de Hingh IH:
Peritoneal carcinomatosis of gastric origin: A population-based
study on incidence, survival and risk factors. Int J Cancer.
134:622–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tan MC, Balakrishnan M and Graham DY:
Gastric cancer worldwide except Japan. Gastric Cancer. Shiotani A:
Springer; Singapore: pp. 17–28. 2019, View Article : Google Scholar
|
|
10
|
Song H, Zhu J and Lu D: Molecular-targeted
first-line therapy for advanced gastric cancer. Cochrane Database
Syst Rev. 7:CD0114612016.PubMed/NCBI
|
|
11
|
Ohno T, Yokoyama Y, Aihara R, Mochiki E,
Asao T and Kuwano H: Sudden bilateral sensorineural hearing loss as
the presenting symptom of meningeal carcinomatosis of gastric
cancer: Report of a case. Surg Today. 40:561–565. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jansen EP, Saunders MP, Boot H, Oppedijk
V, Dubbelman R, Porritt B, Cats A, Stroom J, Olmos RV, Bartelink H
and Verheij M: Prospective study on late renal toxicity following
postoperative chemoradiotherapy in gastric cancer. Int J Radiat
Oncol Biol Phys. 67:781–785. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boku N: HER2-positive gastric cancer.
Gastric Cancer. 17:1–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kepil N, Batur S, Sonmez Wetherilt C and
Erdamar Cetin S: Human epidermal growth factor receptor 2 (HER-2)
status evaluation in advanced gastric cancer using
immunohistochemistry versus silver in situ hybridization. Bosn J
Basic Med Sci. 17:109–113. 2017.PubMed/NCBI
|
|
15
|
Mishra S, Yadav T and Rani V: Exploring
miRNA based approaches in cancer diagnostics and therapeutics. Crit
Rev Oncol Hematol. 98:12–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu J, Feng J, Zhi X, Tang J, Li Z, Xu Y,
Yang L, Hu Z and Xu Z: Let-7b inhibits cell proliferation,
migration, and invasion through targeting Cthrc1 in gastric cancer.
Tumour Biol. 36:3221–3229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu ED, Li N, Li BS, Li W, Zhang WJ, Mao
XH, Guo G, Zou QM and Xiao B: miR-30b, down-regulated in gastric
cancer, promotes apoptosis and suppresses tumor growth by targeting
plasminogen activator inhibitor-1. PLoS One. 9:e1060492014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin Y, Zhang B, Wang W, Fei B, Quan C,
Zhang J, Song M, Bian Z, Wang Q, Ni S, et al: miR-204-5p inhibits
proliferation and invasion and enhances chemotherapeutic
sensitivity of colorectal cancer cells by downregulating RAB22A.
Clin Cancer Res. 20:6187–6199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang Y, Zhang CD, Zhang C and Dai DQ:
DLX6-AS1/miR-204-5p/OCT1 positive feedback loop promotes tumor
progression and epithelial-mesenchymal transition in gastric
cancer. Gastric Cancer. 23:212–227. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang QX, Zhu YQ, Zhang H and Xiao J:
Altered miRNA expression in gastric cancer: A systematic review and
meta-analysis. Cell Physiol Biochem. 35:933–944. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shin VY and Chu KM: MiRNA as potential
biomarkers and therapeutic targets for gastric cancer. World J
Gastroenterol. 20:10432–10439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi Y, Wang Z, Zhu X, Chen L, Ma Y, Wang
J, Yang X and Liu Z: Exosomal miR-1246 in serum as a potential
biomarker for early diagnosis of gastric cancer. Int J Clin Oncol.
25:89–99. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Sun H, Guan J, Ji T and Wang X:
Serum microRNA-381: A potential marker for early diagnosis of
gastric cancer. Yonsei Med J. 60:720–726. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu L, Wang J, Li X, Ma J, Shi C, Zhu H,
Xi Q, Zhang J, Zhao X and Gu M: miR-204-5p suppresses cell
proliferation by inhibiting IGFBP5 in papillary thyroid carcinoma.
Biochem Biophys Res Commun. 457:621–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luan W, Qian Y, Ni X, Bu X, Xia Y, Wang J,
Ruan H, Ma S and Xu B: miR-204-5p acts as a tumor suppressor by
targeting matrix metalloproteinases-9 and B-cell lymphoma-2 in
malignant melanoma. Onco Targets Ther. 10:1237–1246. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chu Y, Jiang M, Du F, Chen D, Ye T, Xu B,
Li X, Wang W, Qiu Z, Liu H, et al: miR-204-5p suppresses
hepatocellular cancer proliferation by regulating homeoprotein SIX1
expression. FEBS Open Bio. 8:189–200. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jia X, Wen Z, Sun Q, Zhao X, Yang H, Shi X
and Xin T: Apatinib suppresses the proliferation and apoptosis of
gastric cancer cells via the PI3K/Akt signaling pathway. J BUON.
24:1985–1991. 2019.PubMed/NCBI
|
|
30
|
Shang HS, Lu HF, Lee CH, Chiang HS, Chu
YL, Chen A, Lin YF and Chung JG: Quercetin induced cell apoptosis
and altered gene expression in AGS human gastric cancer cells.
Environ Toxicol. 33:1168–1181. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Xing L, Xu H, Wang K, She J, Shi
F, Wu H, Sun Y, Gao J and He S: miR-204-5p suppress lymph node
metastasis via regulating CXCL12 and CXCR4 in gastric cancer. J
Cancer. 11:3199–3206. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Zhang H, Ge S, Fan Q, Zhou L, Li
H, Bai M, Ning T, Liu R, Wang X, et al: Effects of miR1385p and
miR2045p on the migration and proliferation of gastric cancer cells
by targeting EGFR. Oncol Rep. 39:2624–2634. 2018.PubMed/NCBI
|
|
33
|
Slamon DJ, Godolphin W, Jones LA, Holt JA,
Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J and Ullrich A:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fanotto V, Ongaro E, Rihawi K, Avallone A,
Silvestris N, Fornaro L, Vasile E, Antonuzzo L, Leone F, Rosati G,
et al: HER-2 inhibition in gastric and colorectal cancers: Tangible
achievements, novel acquisitions and future perspectives.
Oncotarget. 7:69060–69074. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Begnami MD, Fukuda E, Fregnani JH,
Nonogaki S, Montagnini AL, da Costa WL Jr and Soares FA: Prognostic
implications of altered human epidermal growth factor receptors
(HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor
outcome. J Clin Oncol. 29:3030–3036. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Di Oto E, Brandes AA, Cucchi MC and
Foschini MP: Prognostic impact of HER-2 Subclonal Amplification in
breast cancer. Virchows Arch. 471:313–319. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oke OW, Carla L; Gregor and Mariana
Chavez-Mac: Outcomes related to delayed initiation of anti-HER 2
therapy in pregnant HER2 positive breast cancer patients. 53rd
Annual Meeting of the American-Society-of-Clinical-Oncology (ASCO).
2017.
|
|
38
|
Shabbir A, Qureshi MA, Mirza T and Khalid
AB: Human epidermal growth factor (Her-2) in gastric and colorectal
adenocarcinoma. J Pak Med Assoc. 67:1085–1090. 2017.PubMed/NCBI
|
|
39
|
Sun G, Sun L, Liu Y, Xing H and Wang K:
Her-2 expression regulated by downregulation of miR-9 and which
affects chemotherapeutic effect in breast cancer. Cancer Gene Ther.
24:194–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao D, Sui Y and Zheng X: miR-331-3p
inhibits proliferation and promotes apoptosis by targeting HER2
through the PI3K/Akt and ERK1/2 pathways in colorectal cancer.
Oncol Rep. 35:1075–1082. 2016. View Article : Google Scholar : PubMed/NCBI
|