Introduction
Renal ischemia reperfusion injury (RIRI) refers to
the injury caused by the restoration of blood supply and
reperfusion of the kidney after a period of ischemia due to various
causes, such as reduced blood flow to the kidney from renal artery
disease, systemic hypotension or maldistribution of blood flow
(1,2). The pathological mechanism of RIRI is
complex, which includes generation of reactive oxygen species,
apoptosis, hypoxia and inflammation (3), has not been fully elucidated. At
present, the innate immune pattern recognition receptor NACHT LRR
and PYD domains-containing protein (NLRP3) inflammasome is a
research hotspot (4,5). NLRP3 is an important member of the
NOD-like receptor (NLR) family. When cells are stimulated by
external stimuli such as infection, NLRP3 inflammasomes are formed,
which are composed of NLRP3, apoptosis-associated speck-like
protein containing a caspase recruitment domain (ASC) and
pro-caspase 1. Meanwhile, NLRP3 inflammasomes activate pro-caspase
1 to form caspase 1, which further cleaves pro-interleukin (IL)-18
and pro-IL-1β into the important bioactive inflammatory factors
IL-18 and IL-1β; these then participate in inflammatory process
in vivo (6,7). A study by Mezzaroma et al
(8) suggested that NLRP3 may be
the initial receptor in myocardial I/R injury. Sandanger et
al (9) further verified that
NLRP3 is the initial receptor activated by the inflammasome, which
may be an important factor in the occurrence of the
ischemia-reperfusion (I/R) stage after myocardial infarction.
NLRP3 was also reported to be involved in kidney
injury, including renal I/R injury. It was demonstrated that
NLRP3/E3 ubiquitin-protein ligase XIAP signaling aggravated renal
fibrotic injury, and also NLRP3 inflammasome activation negatively
regulated podocyte autophagy in diabetic nephropathy (10,11).
Additionally, a humanized IL-6 receptor (IL-6R) antibody could
reduce the activation of NLRP3 inflammasome in diabetic
nephropathy, partly via suppressing IL-17A, which could be
suppressed by neutralization of IL-17C in renal I/R (12,13).
NLRP3 knockout could protect against ischemic acute kidney injury
(14). Therefore, the present
study focused on NLRP3 inflammasome.
Hypoxia serves a crucial role in the development of
RIRI (15). Hypoxia inducible
factor (HIF), which belongs to a transcription factor family, is
involved in the response to hypoxia and is sensitive to alterations
in oxygen availability. HIF-1 is associated with the acute response
and is involved in the transactivation of a variety of genes in
cellular life activity (16). In
addition, kidney injury molecule-1 (KIM-1) is a specific marker of
renal acute injury (17);
therefore, KIM-1 was examined to assess the renal injury in the
present study.
Tetramethylpyrazine (TMP) is a bioactive alkaloid
extracted from the traditional Chinese medicine chuanxiong. TMP was
originally identified to treat cardiovascular and cerebrovascular
diseases (18). TMP is a calcium
antagonist with various pharmacological effects. For example, TMP
improves microcirculation and displays antioxidation and
antifibrosis effects (19,20). At present, it has been widely used
in the treatment of acute and chronic renal failure, diabetic
nephropathy (21) and kidney
cancer (22). However, the
mechanism by which TMP alleviates RIPI is still unclear.
Since the pathogenic mechanism of RIPI is related to
inflammation, we hypothesized that the effect of TMP in reducing
RIPI is related to its anti-inflammatory effect. In the present
study, the effect of TMP on the NLRP3 inflammatory corpuscle in rat
kidneys was studied using a renal I/R model.
Materials and methods
Animal groups
A total of 27 male Sprague-Dawley rats (age, 8
weeks; weight, 200–220 g) were purchased from the Animal Experiment
Center of Shandong University (China). The rats were randomly
divided into the following groups (n=9 per group): i) Sham group;
ii) kidney I/R injury group (I/R group); and iii) kidney I/R injury
with TMP treatment group (I/R+TMP group). Rats were kept in a clean
and well-ventilated environment at a constant room temperature of
20–25°C, a humidity of 40–70%, with free access to food and water
and a 12-h circadian rhythm. TMP hydrochloride for injection was
purchased from Shijiazhuang Yiling Pharmaceutical Co., Ltd. This
study was approved by the Animal Ethics Committee of Binzhou
Medical University (China).
Establishment of RIPI model in
rats
Rats were fasted for 12 h before surgery, but had
free access to drinking water. According to the body weights of the
rats, 3% pentobarbital sodium (50 mg/kg body weight) was
intraperitoneally injected for anesthesia. The hair was removed
from the backs of the animals, and the skin was disinfected using
tincture of iodine. The skin was cut 0.5 cm along the spine and 0.5
cm at the lower edge of the ribs, and the muscles were separated
layer by layer. Carefully, the renal arteries of both kidneys were
separated, and the bilateral renal arteries were quickly closed
with artery clips. After 45 min of ischemia, the artery clip was
released, blood flow was restored, the wound was sutured, and
antibiotics and a certain amount of saline (30 µl/g body weight)
were given. After 24 h of reperfusion, the anesthetized rats were
given a PBS cardiac perfusion, and kidney tissues were taken for
the follow-up experiments. In the Sham group, the renal artery was
isolated and no ischemic treatment was performed. All other
procedures were the same. In the I/R+TMP group, TMP hydrochloride
was given immediately after reperfusion (40 mg/kg, 6-h interval) by
intraperitoneal injection (23).
At 24 h after the PBS cardiac perfusion, kidney tissues were taken;
some tissues were stored at −80°C for protein and reverse
transcription-quantitative (RT-q) PCR detection, while some tissues
were fixed with 4% paraformaldehyde at 4°C for 24 h, embedded in
paraffin, and sliced for morphological detection.
Cell culture
Rat proximal tubule epithelial cells (NRK cells; BD
Biosciences) were adjusted to 1×105 and cultured in
RPMI-1640 medium (HyClone; Cytiva) containing 10% FBS (HyClone;
Cytiva), with the addition of 100 U/ml penicillin-streptomycin, and
placed at 37°C in a 5% CO2 incubator.
Renal function test
Before cardiac perfusion, 0.5–1 ml of blood was
extracted from the heart, and after standing for 30 min, the serum
was separated by centrifugation at 3,000 × g for 10 min at 4°C. The
blood urea nitrogen and serum creatinine were detected using urea
assay kits (cat. no. C013-1-1) and creatinine assay kits (cat. no.
C011-1-1), in accordance with the manufacturer's instructions
(Nanjing Jiancheng Bioengineering Institute Co., Ltd.).
Periodic acid-Schiff (PAS)
staining
Paraffin-embedded sections (4-µm thick) were dewaxed
at 60°C for 2 h, washed with xylene at room temperature and
dehydrated in a descending alcohol series (100, 90, 80 and 75%).
The sections were washed three times with PBS at room temperature.
Periodate (1%) was added for oxidation for 10 min at room
temperature. The sections were counterstained with Harris
hematoxylin for 5 min after immersion in Schiff's solution for 15
min. The slides were rinsed and ammonia was added until a blue
color was achieved. All staining steps were performed at room
temperature, and cells were rinsed with tap water after each step
for 5 min. The slides were further washed in distilled water,
dehydrated, cleared, mounted in resin and observed using a Leica DM
6000 B light microscope (Leica Microsystems GmbH).
Renal tubular injury score
criteria
The renal tubules were observed under magnification
×200. The following scoring criteria were used: i) 0, no damage;
ii) 1, 0–10%; iii) 2, 11–25%; iv) 3, 26–45%; v) 4, 46–75%; vi) and
5, >75%.
Detection of cytokines and KIM-1
Sample diluent (100 µl) and rat serum (100 µl) was
added to the wells and incubated at 37°C for 2 h. Subsequently, the
liquid was discarded and the plate dried. Biotin-labeled antibody
working fluid (100 µl; 1X antibody) was added to each well and
incubated at 37°C for 1 h. This was discarded and the plate was
washed with 0.01 M PBS three times. Avidin-Biotin-Peroxidase
Complex working solution (100 µl; 1X solution) was added to each
well and the plate was incubated at room temperature for 1 h. This
was discarded and the plate was shaken dry. TMB color developing
agent (90 µl) was added to each well, incubated at 37°C in the dark
for 30 min and then 50 µl termination solution was added. The
optical density value was measured using a SpectraMax®
microplate reader (Molecular Devices, LLC) at 450 nm. All the
reagents were from the following ELISA kits: Tumor necrosis factor
(TNF)-α (cat. no. RTA00: R&D Systems, Inc.), IL-6 (cat. no.
R6000B; R&D Systems, Inc.) and KIM-1 (cat. no. ab119597;
Abcam).
TUNEL-DAPI double staining
According to the instructions of the TUNEL kit
(Roche Diagnostics). The 4 µm thick paraffin sections were dewaxed
in the oven at 60°C for 2 h, washed with xylene at room
temperature, dehydrated in a descending alcohol series (100, 90, 80
and 75%) and washed three times with PBS at room temperature. Cell
permeability solution was added for 8 min, and 500 µl TUNEL
reaction mixture was added (50 µl TdT and 450 µl
fluorescein-labeled dUTP) at 37°C for 1 h in the dark box. After
DAPI staining (10 µg/ml; at room temperature for 5 min), the cells
were sealed. Under fluorescence microscopy, 10 high-power
microscope fields were randomly selected from each glass slide to
count the number of apoptotic cells and calculate the apoptosis
rate using the following formula: Apoptosis rate/%=(number of
positive cells/total number of cells) ×100%.
Western blot analysis of NLRP3
expression
Kidney tissues were extracted, and RIPA (1% PMSF)
tissue lysate (Beyotime Institute of Biotechnology) was added and
the samples were homogenized with a tissue grinder to extract the
total protein. A BCA protein concentration kit was used to measure
the total protein concentration. Equal amounts of protein extract
(40 µg) were loaded per lane and separated via 8–12% SDS-PAGE
followed by membrane transfer to polyvinylidene fluoride membranes
(EMD Millipore) and blocking in 5% skimmed milk powder for 15 min
at room temperature. The primary antibody was added and incubated
overnight in a 4°C refrigerator with agitation. After washing with
1X TBST (0.1% Tween-20; cat. no. ST671; Beyotime Institute of
Biotechnology), the membrane was incubated with the secondary
antibody at room temperature for 2 h. Protein bands were visualized
using an ECL kit (Sparkjade Science Co., Ltd.). β-actin was used as
an internal reference. ImageJ software (version 1.8.0; National
Institutes of Health) was used to quantify the western blot bands.
Primary antibodies against NLRP3 (1:1,000; cat. no. ab214185;
Abcam), HIF1-α (1:1,000; cat. no. 14179; Cell Signaling Technology,
Inc.), cleaved caspase-3 (1:1,000; cat. no. ab49822; Abcam) and
β-actin (1:5,000; cat. no. 60008-1-Ig; ProteinTech Group, Inc.)
were used in the present study. The secondary antibodies included
horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG
(1:3,000; cat. no. SA00001-1; ProteinTech Group, Inc.) and
HRP-conjugated goat anti-rabbit IgG (1:3,000; cat. no. SA00001-2;
ProteinTech Group, Inc.).
RT-qPCR
Renal tissue (50 mg) was taken, RNA was extracted
using TRIzol® (Tiangen Biotech Co., Ltd.). cDNA was
obtained through reverse transcription of 500 ng RNA using a
reverse transcription kit (Tiangen Biotech Co., Ltd.) at 70°C for 5
min, 37°C for 5 min, 42°C for 60 min, 70°C for 10 min. The RT-qPCR
reaction was performed by taking 5 µl cDNA (1:5 diluted cDNA
solution) and reacting it in MicroAmp™ Fast Optical 96-well
reaction plates (Applied Biosystems; Thermo Fisher Scientific,
Inc.), with SYBR® Premix Ex Taq™ (Takara Bio Inc.). The
thermocycling conditions were: 94°C for 5 min, 95°C for 30 sec;
then, 40 cycles at 59°C for 30 sec, 72°C for 30 sec and 72°C for 10
min; and a final extension at 65–95°C for 15 min. The results were
analyzed using RQ Manager for SDS 2.3 with GenEx 7.0 (MultiD
Analyses AB). The following primers were used: NLRP3, forward:
5′-GGAGTGGATAGGTTTGCTGG-3′ and reverse: 5′-GGTGTAGGGTCTGTTGAGGT-3′;
housekeeping gene GAPDH, forward: 5′-TGCATCCTGCACCACCAACTGC-3′ and
reverse: 5′-ACAGCCTTGGCAGCACCAGTGG-3′. The 2−∆∆Cq method
was used to calculate the relative mRNA expression (24).
Immunohistochemical staining
Paraffin-embedded sections (4-µm thick) were dewaxed
and rehydrated using the GTVision™ III anti-rat/rabbit universal
immunohistochemical detection kit (cat. no. GK500710; Gene Tech
Co., Ltd.) according to the manufacturer's protocol. Antigen
retrieval was performed in 0.01 M citrate buffer at 100°C for 6
min. Following cooling, the sections were washed three times in
0.01 M PBS for 5 min each. Subsequently, the sections were
incubated with 3% H2O2 at room temperature
for 10 min. The sections were incubated with a primary polyclonal
antibody targeted against NLRP3 (cat. no. ab214185, 1:200; Abcam)
at 4°C overnight, and then with a secondary horseradish
peroxidase-conjugated goat anti-rabbit IgG antibody for 30 min at
room temperature, according to the rabbit polymer detection system
(cat. no. PV-6001; OriGene Technologies, Inc.). The sections were
stained with DAB reagent (1X) for 3 min at room temperature,
counterstained with hematoxylin for 3 min at room temperature and
washed by tap water. Stained sections were observed using a Leica
DM 6000 B light microscope (Leica Microsystems GmbH). Brown
staining indicated a positive reaction. ImageJ software (version
1.8.0; National Institutes of Health) was used for analysis.
Establishment of the oxygen and
glucose deprivation (OGD) model
The OGD model was constructed by replacing RPMI 1640
medium with Earle's balanced salt solution (EBSS; HyClone; Cytiva)
with 95% N2 and 5% CO2. NRK cells
(5×105) were cultured in the hypoxic incubator at 37°C
for 6 h, after which hypoxia was terminated and RPMI 1640 medium
with 10% FBS was added for 18 h for follow-up experiments.
CCK-8 assay
The cell density of NRK cells in the logarithmic
growth phase was adjusted to 1×105. Cell suspension (100
µl per well) was inoculated in 96-well plates, and the CCK-8
reagents (Beyotime Institute of Biotechnology) were added according
to the manufacturer's protocols. Each group was set up with three
duplicate wells and cultured at 37°C for 4 h. Then, the absorbance
was measured at 450 nm using the Infinite M200 PRO (Tecan Group,
Ltd.).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. The significance of the differences in mean values among
groups was examined by one-way ANOVA followed by Duncan's multiple
range tests. SPSS 20.0 (IBM, Corp.) and GraphPad Prism 6 software
(GraphPad Software, Inc.) were used for statistical analyses.
P<0.05 was considered to indicate a statistically significant
difference. All the experiments were performed in triplicate.
Results
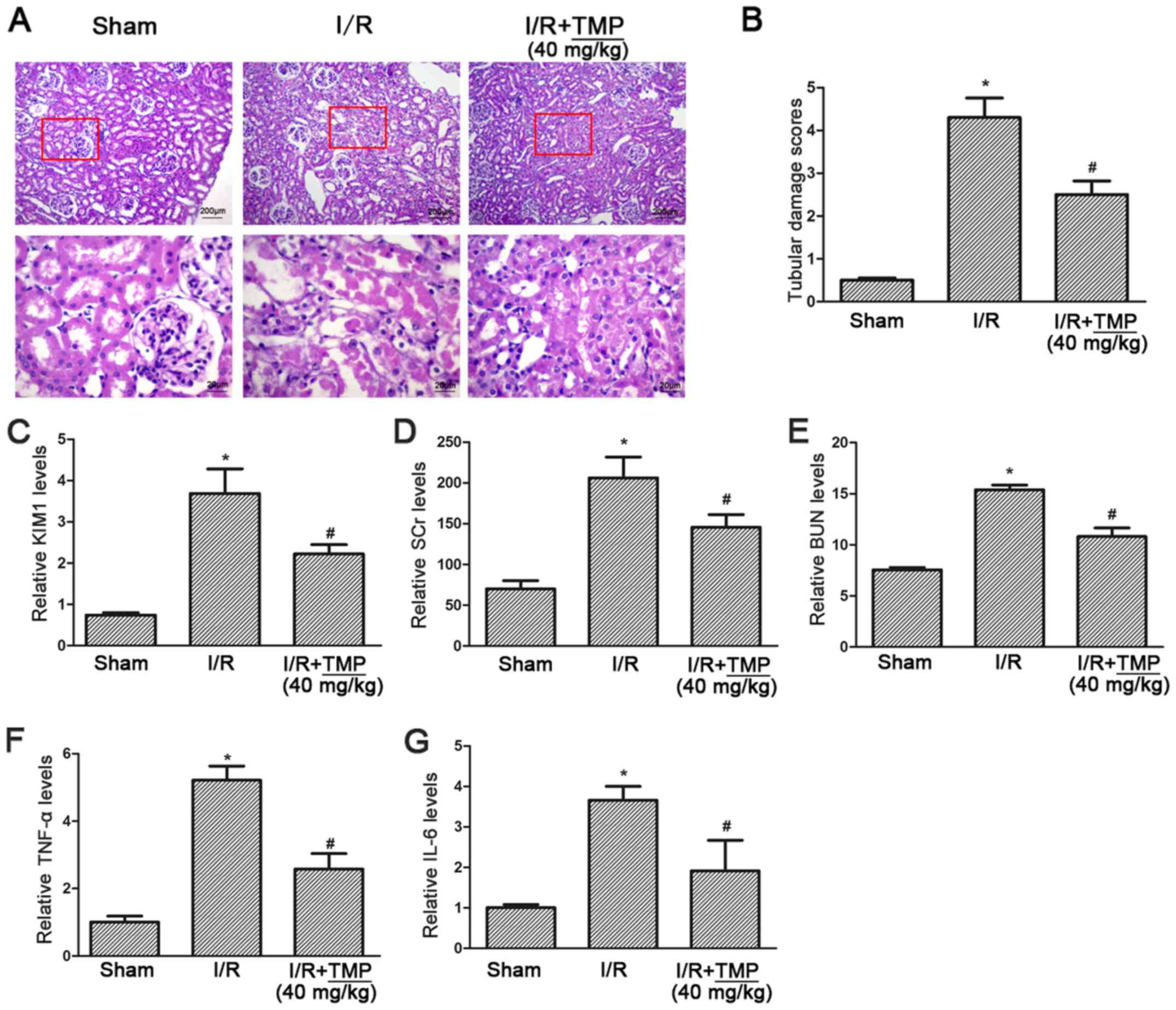
TMP alleviates renal tubular
pathological injury and improves renal function in rats with renal
I/R
PAS staining showed complete glomerular and renal
tubules in the sham operation group, with no obstruction in the
lumen and no obvious morphological abnormalities, while in the I/R
group the renal tubules showed extensive necrosis of epithelial
cells, obvious brush border membrane fraction and vacuolar tubules.
In the I/R+TMP group, the necrosis of renal tubular epithelial
cells was reduced, and the course of disease had slowed down. A
small number of exfoliated cells could be seen at the brush borders
of renal tubules, and some hollow vacuoles were observed in the
renal tubules (Fig. 1A).
Double-blind analysis of tubular injury by pathologists revealed a
significant decrease in tubular injury scores in TMP-treated rats
after renal I/R (P<0.05; Fig.
1B).
The level of KIM-1 was detected using an ELISA
assay, compared with in the Sham group the level of KIM-1 was
significantly increased in the I/R group and elevated KIM-1 was
significantly reduced in the I/R+TMP group (P<0.05; Fig. 1C). The serum creatinine and blood
urea nitrogen test results showed that, compared with the Sham
group, serum creatinine and urea nitrogen in the I/R group and
I/R+TMP group were both increased (P<0.05), indicating that
renal function was impaired by renal I/R. Compared with the I/R
group, serum creatinine and blood urea nitrogen in the I/R+TMP
group were significantly decreased (P<0.05; Fig. 1D and E), suggesting that TMP may
reduce renal functional injury.
The ELISA assay results for the inflammatory
cytokines TNF-α and IL-6 showed that, compared with the Sham group,
the levels of TNF-α and IL-6 in the I/R and I/R+TMP groups were
increased (P<0.05), and significantly decreased in the I/R+TMP
group compared with the I/R group (P<0.05; Fig. 1F and G), indicating that TMP can
reduce the production of inflammatory cytokines.
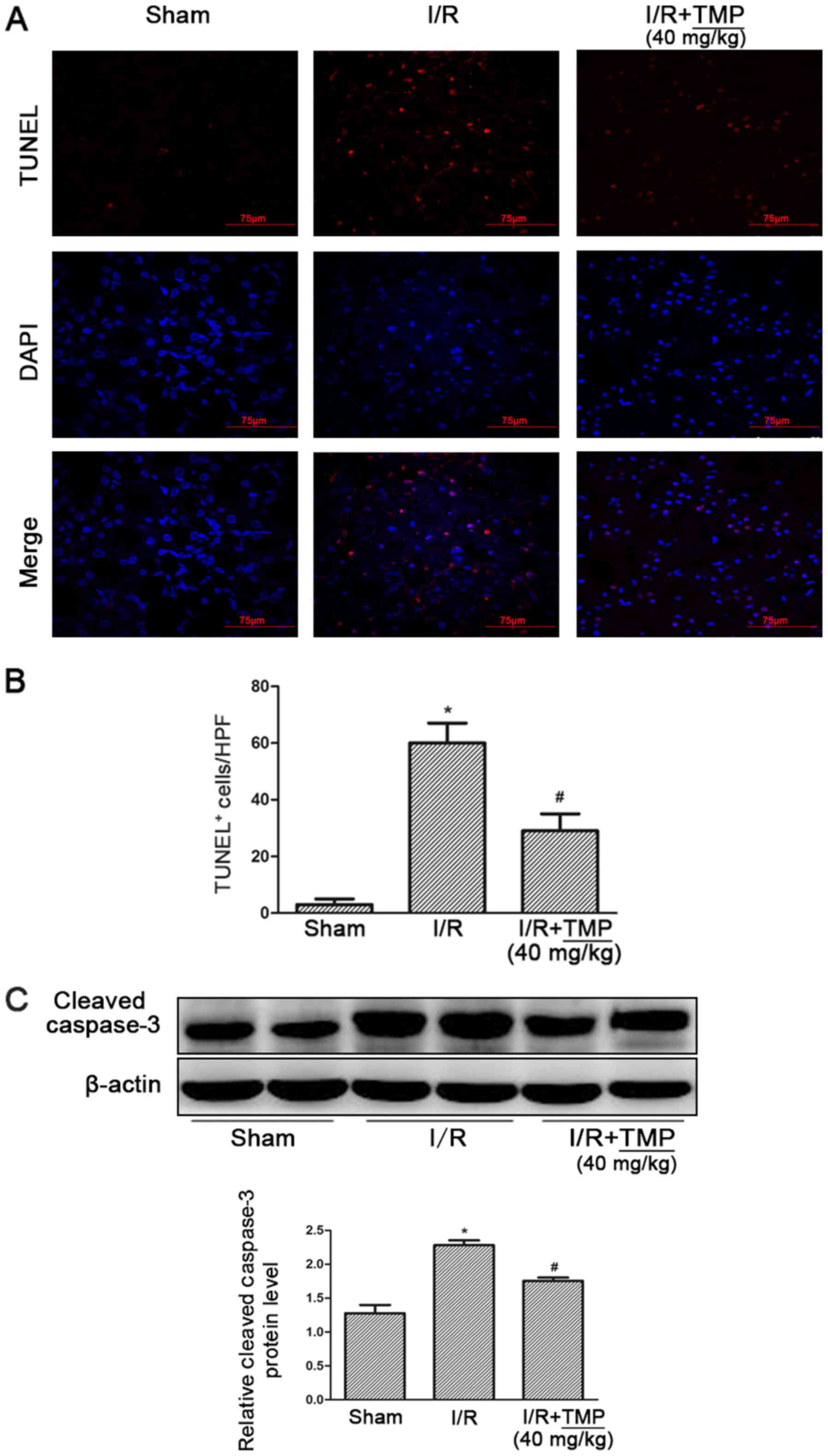
TMP reduces renal tubular apoptosis in
rats with renal I/R
After TUNEL-DAPI staining, it was found that
compared with the Sham group, the amount of renal tubular cell
apoptosis in the I/R and I/R+TMP groups was significantly
increased. Compared with the I/R group, the amount of renal tubular
cell apoptosis in the I/R+TMP group was significantly reduced after
TMP treatment (P<0.05; Fig. 2A and
B).
Meanwhile, the expression level of the apoptotic
protein caspase 3 in rat kidney tissues was detected. The
expression of caspase 3 was significantly increased in the I/R and
I/R+TMP groups. However, compared with the I/R group, the protein
expression of caspase 3 in the I/R+TMP group was significantly
reduced after TMP treatment (P<0.05; Fig. 2C).
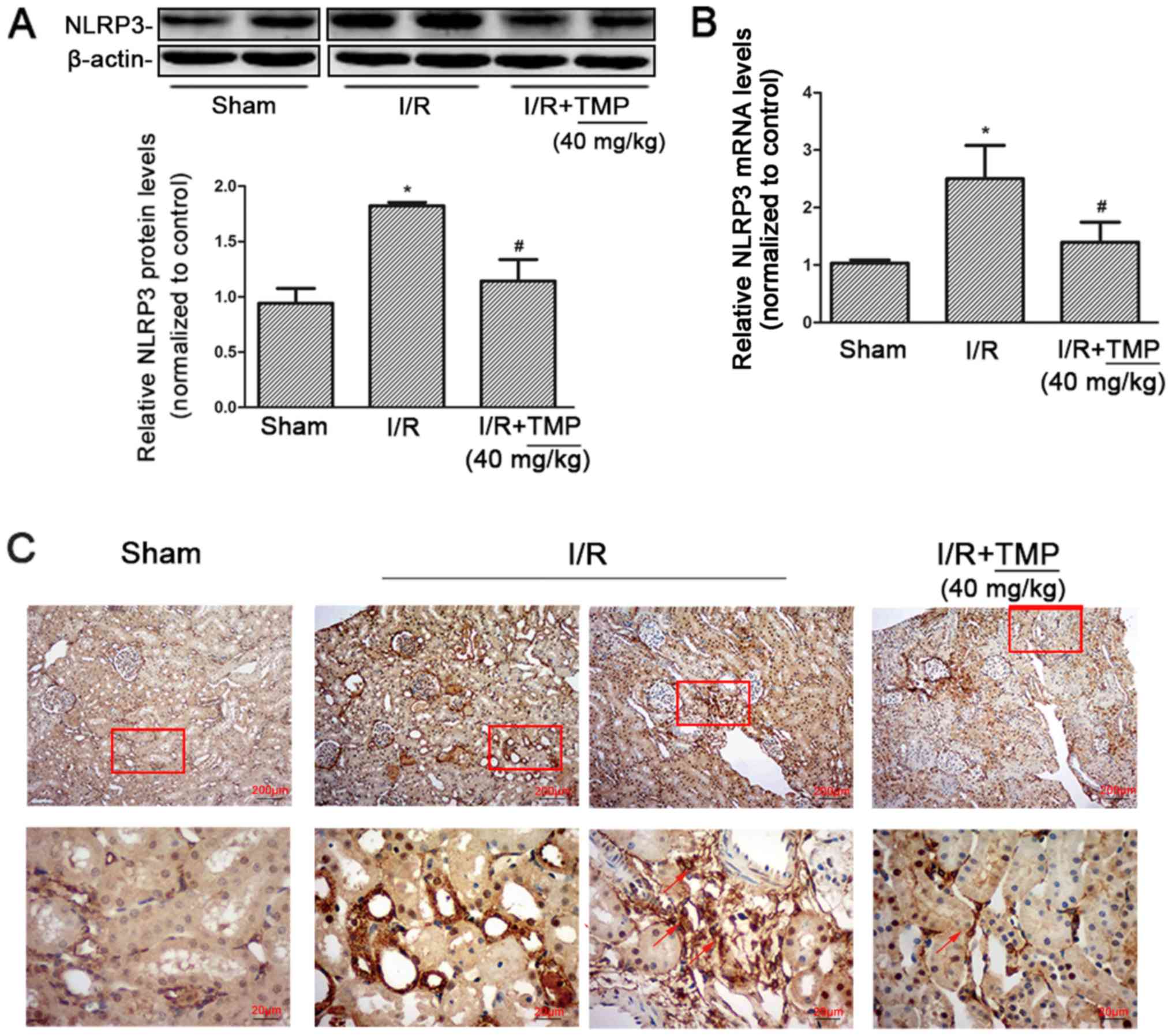
TMP reduces the expression of NLRP3 in
renal tissues of renal I/R rats
Western blot analysis of NLRP3 expression in the
kidney tissues of rats in each group showed that compared with the
Sham group, NLRP3 protein expression in the I/R and I/R+TMP groups
significantly increased. NLRP3 protein expression significantly
decreased in the I/R+TMP group compared with in the I/R group
(P<0.05; Fig. 3A).
According to RT-qPCR, the expression of NLRP3 mRNA
in rat kidney tissues increased. Compared with the Sham group, the
expression of NLRP3 mRNA in the I/R and I/R+TMP groups increased.
NLRP3 mRNA expression was reduced in the I/R+TMP group compared
with that in the I/R group (P<0.05; Fig. 3B). The PCR results were consistent
with the western blot analysis measuring protein expression.
After immunohistochemical staining, it was found
that compared with the Sham group, the positive expression of NLRP3
in renal tissues from the I/R and I/R+TMP groups significantly
increased. Besides renal tubules, NLRP3 was positively expressed in
infiltrating inflammatory cells in the renal interstitium in the
I/R group and the number of infiltrated inflammatory cells was
increased, which was markedly decreased in the I/R+TMP group.
Compared with the I/R group, the positive rate of NLRP3 expression
in renal tissues of the I/R+TMP group was notably reduced (Fig. 3C). The immunohistochemical results
showed that TMP could not only reduce the expression of NLRP3 in
renal tubules, but also mitigate renal tissue damage by reducing
the infiltration of inflammatory cells.
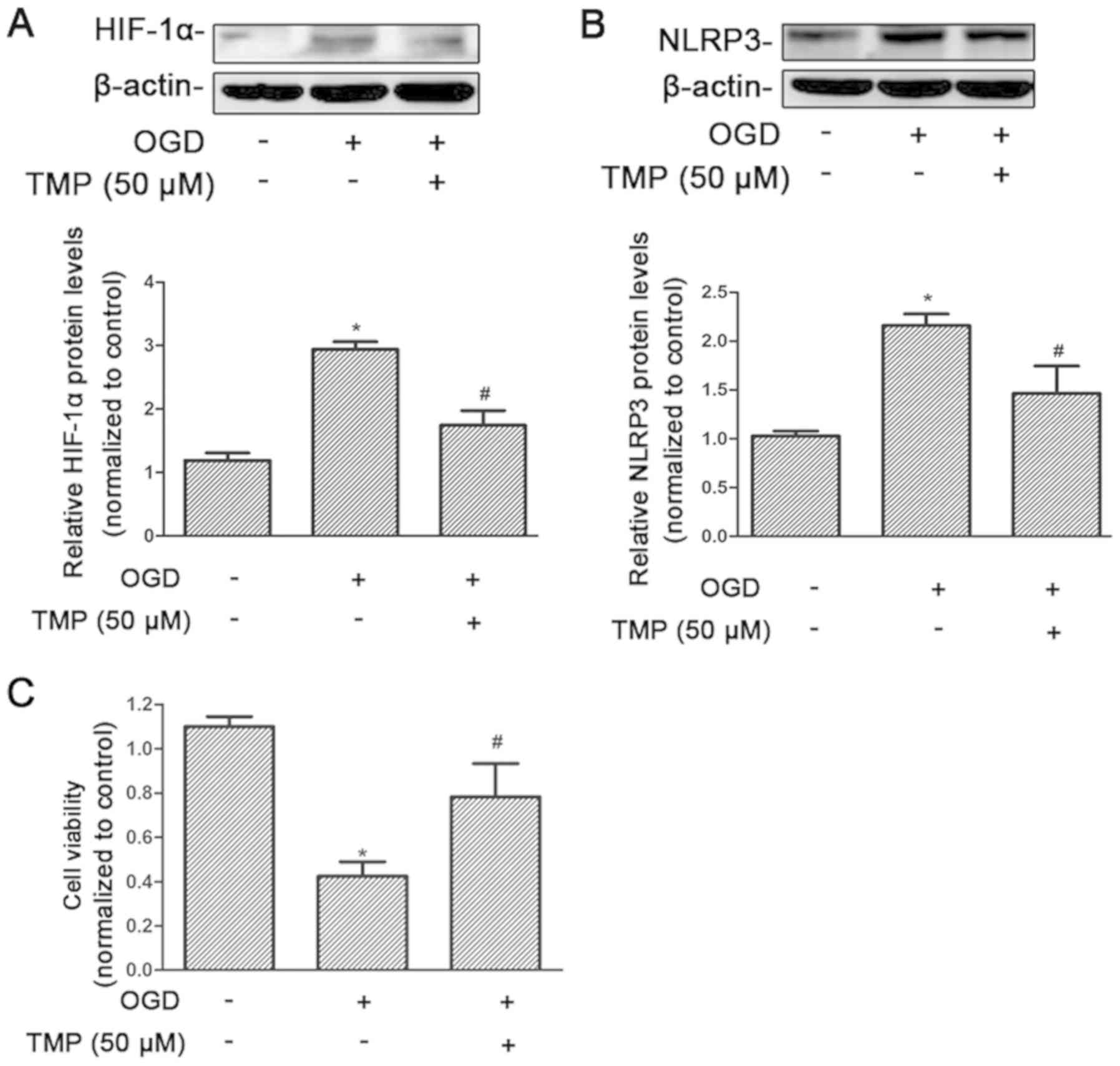
TMP may suppress NLRP3 expression
induced by hypoxia and improve cell viability in rat NRK-52E
cells
The response to hypoxia at the cellular level relies
on the activity of the transcription factor family, HIF. Therefore,
the effect of TMP on HIF-1α was assessed under hypoxia. HIF-1α was
significantly increased after treatment with OGD, and significantly
reduced after treatment with TMP (P<0.05; Fig. 4A). The level of NLRP3 expression
was significantly increased after OGD, and reduced after treatment
with TMP, which was consistent with the changes in HIF-1α
expression (P<0.05; Fig. 4B).
At the same time, cell viability was significantly decreased after
OGD treatment, and significantly increased after TMP treatment
(P<0.05; Fig. 4C).
Discussion
TMP can improve microcirculation, prevent platelet
aggregation and activate blood circulation (25). Studies have reported that TMP has a
cardioprotective effect in myocardial I/R injury, mainly through
antioxidant, anti-inflammatory and antiapoptotic activities, and
improves coronary blood flow and myocardial metabolism by enhancing
the expression of NO via upregulating the expression of NOS
(26). However, the mechanism by
which TMP alleviates RIPI is still unclear. The main pathological
mechanisms of kidney I/R involves free radicals, intracellular
calcium overload (27),
inflammatory reactions and apoptosis (28). Therefore, the present study focused
on the effect of TMP on the NLRP3 inflammasome and apoptosis.
Previous studies have demonstrated that inflammation
is involved in the process of I/R-induced kidney damage. Chen et
al (29) found that TMP can
inhibit liver inflammation by reducing the production of TNF-α and
IL-6, reactive oxygen species (ROS) generation and the activation
of NF-κB. Bai et al (30)
reported that TMP inhibits inflammatory cell activation and the
production of proinflammatory cytokines, and has been shown to
alleviate cerebral ischemia injury. Consistent with previous
findings, the present study found that after treatment with TMP,
the renal tubular injury scores of renal I/R rats were
significantly reduced (Fig. 1A and
B), and KIM-1 was also significantly decreased (Fig. 1C). The expression levels of
inflammatory factors were decreased (Fig. 1F and G), suggesting that TMP can
significantly reduce the inflammatory injury caused by renal
I/R.
Apoptosis plays an important role in RIPI. A number
of studies have demonstrated that TMP can reduce cell apoptosis.
Feng et al (31) found that
TMP could alleviate RIPI by inhibiting apoptosis. Qing et al
(32) reported that TMP can
participate in the Akt/transcription factor Nrf2 signaling pathway
to promote angiogenesis and reduce apoptosis to improve
multiterritory perforator flap survival in reconstructive surgery.
The present results showed that after treatment with TMP, renal
tissue apoptosis significantly decreased; and the expression levels
of apoptosis-related proteins were also reduced. These results
indicated that TMP could inhibit apoptosis (Fig. 2A and C).
Numerous studies have reported that TMP can inhibit
the inflammatory response. The inflammatory response is a complex
process, and there are a number of factors that cause inflammation.
Therefore, the current study focused on the relationship between
TMP and NLRP3 inflammasome. Previous studies have demonstrated that
NLRP3 is associated with cardiovascular and cerebrovascular
diseases, acute lung injury, hepatitis cirrhosis and kidney injury.
Zhang et al (33) reported
that lipopolysaccharide (LPS) effectively activates the NLRP3
inflammasome/caspase 1 pathway, causing liver cells to produce the
inflammatory cytokine IL-1β. However, TMP blocks the LPS-activated
NLRP3 inflammasome signaling pathway, which is achieved by
inhibiting toll-like receptor (TLR)4 and thereby reducing the
production of hepatocyte IL-1β. Wu et al (34) suggested that TMP may target liver
stem cells via the platelet derived growth factor-β
receptor/NLRP3/caspase-1 pathway to reduce the inflammatory
response in liver fibrosis. The present study also demonstrated
that TMP could inhibit the NLRP3 inflammasome during renal I/R, at
both the gene and protein levels, as verified in vivo and
in vitro.
The response to hypoxia at the cellular level relies
on the activity of the HIF transcription factor family (16). Huang et al (35) reported that when myocardial I/R
occurs, the expression of HIF-1α is the initiating factor of the
body's endogenous protective mechanism, which can promote the
course of myocardial I/R. HIF-1α plays a primary role in the body's
inflammatory response, which includes inflammatory cell activation
in various pathological conditions (16). In an HIF-1α-dependent manner, the
TLR7/8-mediated inflammatory reaction results in activation of the
NLRP3 inflammasome via activation of xanthine oxidase, which
produces uric acid and ROS (36).
NLRP3 inflammasome activity could be sustained via the cyclic AMP
(cAMP)/protein kinase A/cAMP response element/HIF-1α pathway as a
result of adenosine activity (37). Under pathological conditions of
venous thrombosis, the expression of NLRP3 is regulated by HIF-1α
(38). A novel HIF-1α-induced
eotaxin pathway identifies an unknown connection between hypoxia
and the regulation of the severity of inflammation regulated by
TLR4 in asthma (39). Hypoxia and
elevated HIF-1α promotes inflammatory signals, including
NLRP3/inflammasome/caspase-1 activation in fat-laden hepatocytes
(40). In the present study, the
effect of TMP on HIF-1α was examined under hypoxia. It was also
demonstrated that HIF-1α expression increased in NRK cells treated
with OGD, while the levels of HIF-1α were significantly reduced
after treatment with TMP. This is consistent with the changes in
NLRP3 expression. Therefore, it is suggested that TMP can reduce
NLPR3 by reducing HIF-1α expression.
At present, there are no effective drug treatments
and renal replacement therapy is used to prolong the survival of
patients with renal I/R nephropathy. Some drugs have been reported
to have action in only animal models of renal I/R.
Hydroxychloroquine attenuated renal injury via downregulation of
cathepsin (CTS) B and CTSL-mediated NLRP3 inflammasome activation
(41). Isoquercitrin has been
demonstrated to attenuate RIRI through its antioxidative,
anti-inflammatory and antiapoptotic effects to preserve renal
function (42). In addition,
recent studies indicated that cordycepin was effective for renal
I/R (43). These drugs, including
TMP, have been shown to regulate inflammation, apoptosis and
oxidative stress. However, no study has compared their efficacy in
the treatment of I/R in an animal model. At present, an ongoing
study in our research group is investigating the efficacy of TMP
compared with erythropoietin. ROS are involved in the activation of
NLRP3 inflammation (44). In the
present study, the effect of TMP on ROS was not investigated, and
only the alterations to NLRP3 expression levels were shown rather
than the activation of the NLRP3 inflammasome in in vivo
experiments. We will further explore the specific mechanism of TMP
in the reduction of hypoxia-induced elevation of NLRP3 in
vitro and investigate the relationship between NLRP3 and HIF-1α
in order to understand the interaction between hypoxic injury and
innate immune response.
In conclusion, the present study demonstrated that
TMP can reduce protein expression of NLRP3 in renal tissue and
renal tubular cell apoptosis, improve renal tubule pathological
injury, improve kidney serum creatinine and blood urea nitrogen
levels, and improve renal function to relieve RIPI. This study
represents a novel idea, broadening the uses of traditional Chinese
medicines. With increased understanding of the mechanism of action
of TMP, it is hoped that it may be applied to the treatment of a
wide range of kidney diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Nature Science Foundation of China (grant no. 81500557); the
Foundation for Excellent Young and Middle-Aged Scientists of
Shandong Province (grant no. BS2014YY018); Shandong Natural Science
Fund of Shandong Province (grant no. ZR2017BH055); Undergraduate
Innovation and Entrepreneurship Training Program of Shandong
Province (grant no. 201910440028).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WS, AL and ZW were responsible for designing the
study, collecting the data, performing the statistical analysis and
drafting the manuscript. XS, MD, FQ, LW and YZ collected the data,
performing the statistical analysis and conducted the literature
search. PD supervised the project, helped to design the study,
analyzed the data and wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Animal Ethics
Committee of Binzhou Medical University (approval no.
2015-008).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Malek M and Nematbakhsh M: Renal
ischemia/reperfusion injury; From pathophysiology to treatment. J
Renal Inj Prev. 4:2655–27. 2015.
|
|
2
|
Kellum JA, Unruh ML and Murugan R: Acute
kidney injury. BMJ Clin Evid. 2011:20012011.PubMed/NCBI
|
|
3
|
Sethi K, Rao K, Bolton D, Patel O and
Ischia J: Targeting HIF-1α to prevent renal ischemia-reperfusion
injury: Does it work? Int J Cell Biol. 2018:98527912018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo Z, Yu S, Chen X, Ye R, Zhu W and Liu
X: NLRP3 is involved in ischemia/reperfusion injury. CNS Neurol
Disord Drug Targets. 15:699–712. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Minutoli L, Puzzolo D, Rinaldi M, Irrera
N, Marini H, Arcoraci V, Bitto A, Crea G, Pisani A, Squadrito F, et
al: ROS-mediated NLRP3 inflammasome activation in brain, heart,
kidney, and testis ischemia/reperfusion injury. Oxid Med Cell
Longev. 2016:21830262016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shao BZ, Xu ZQ, Han BZ, Su DF and Liu C:
NLRP3 inflammasome and its inhibitors: A review. Front Pharmacol.
6:2622015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Latz E: The inflammasomes: Mechanisms of
activation and function. Curr Opin Immunol. 22:28–33. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mezzaroma E, Toldo S, Farkas D, Seropian
IM, Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF and
Abbate A: The inflammasome promotes adverse cardiac remodeling
following acute myocardial infarction in the mouse. Proc Natl Acad
Sci USA. 108:19725–19730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sandanger Ø, Ranheim T, Vinge LE, Bliksøen
M, Alfsnes K, Finsen AV, Dahl CP, Askevold ET, Florholmen G,
Christensen G, et al: The NLRP3 inflammasome is up-regulated in
cardiac fibroblasts and mediates myocardial ischemia-reperfusion
injury. Cardiovasc Res. 99:164–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xin R, Sun X, Wang Z, Yuan W, Jiang W,
Wang L, Xiang Y, Zhang H, Li X, Hou Y, et al: Apocynin inhibited
NLRP3/XIAP signalling to alleviate renal fibrotic injury in rat
diabetic nephropathy. Biomed Pharmacother. 106:1325–1331. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hou Y, Lin S, Qiu J, Sun W, Dong M, Xiang
Y, Wang L and Du P: NLRP3 inflammasome negatively regulates
podocyte autophagy in diabetic nephropathy. Biochem Biophys Res
Commun. 521:791–798. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu R, Liu X, Yin J, Wu H, Cai X, Wang N,
Qian Y and Wang F: IL-6 receptor blockade ameliorates diabetic
nephropathy via inhibiting inflammasome in mice. Metabolism.
83:18–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang F, Yin J, Lin Y, Zhang F, Liu X,
Zhang G, Kong Y, Lu Z, Wu R, Wang N, et al: IL-17C has a pathogenic
role in kidney ischemia/reperfusion injury. Kidney Int.
97:1219–1229. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wen Y, Liu YR, Tang TT, Pan MM, Xu SC, Ma
KL, Lv LL, Liu H and Liu BC: mROS-TXNIP axis activates NLRP3
inflammasome to mediate renal injury during ischemic AKI. Int J
Biochem Cell Biol. 98:43–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nangaku M and Eckardt KU: Hypoxia and the
HIF system in kidney disease. J Mol Med (Berl). 85:1325–1330. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Batie M, Druker J, D'Ignazio L and Rocha
S: KDM2 family members are regulated by HIF-1 in hypoxia. Cells.
6:82017. View Article : Google Scholar
|
|
17
|
Ichimura T, Bonventre JV, Bailly V, Wei H,
Hession CA, Cate RL and Sanicola M: Kidney injury molecule-1
(KIM-1), a putative epithelial cell adhesion molecule containing a
novel immunoglobulin domain, is up-regulated in renal cells after
injury. J Biol Chem. 273:4135–4142. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lv L, Jiang SS, Xu J, Gong JB and Cheng Y:
Protective effect of ligustrazine against myocardial ischaemia
reperfusion in rats: The role of endothelial nitric oxide synthase.
Clin Exp Pharmacol Physiol. 39:20–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen HY, Xu DP, Tan GL, Cai W, Zhang GX,
Cui W, Wang JZ, Long C, Sun YW, Yu P, et al: A potent
multi-functional neuroprotective derivative of tetramethylpyrazine.
J Mol Neurosci. 56:977–987. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tuttle RS, Marmelstein L, Trad T, Reddy S
and Radley T: In vitro uterine response to tetramethylpyrazine, the
active constituent of Chung Chong (a traditional Chinese medicine).
Am J Obstet Gynecol. 161:1319–1323. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang WJ, Li YR, Gao H, Wu XY, Wang XL,
Wang XN, Xiang L, Ren DM, Lou HX and Shen T: Protective effect of
the ethanol extract from Ligusticum chuanxiong rhizome against
streptozotocin-induced diabetic nephropathy in mice. J
Ethnopharmacol. 227:166–175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luan Y, Liu J, Liu X, Xue X, Kong F, Sun
C, Wang J, Liu L and Jia H: Tetramethypyrazine inhibits renal cell
carcinoma cells through inhibition of NKG2D signaling pathways. Int
J Oncol. 49:1704–1712. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang G, Xin R, Yuan W, Zhang L, Meng X,
Sun W, Han H, Hou Y, Wang L and Du P: Ligustrazine ameliorates
acute kidney injury through downregulation of NOD2-mediated
inflammation. Int J Mol Med. 45:731–742. 2020.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo M, Liu Y and Shi D: Cardiovascular
actions and therapeutic potential of tetramethylpyrazine (active
component isolated from rhizoma chuanxiong): Roles and mechanisms.
Biomed Res Int. 2016:24303292016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng Q, Huang YY, Zhu PC, Tong Q, Bao XY,
Zhang QH, Zheng GQ and Wang Y: Ligustrazine exerts cardioprotection
in animal models of myocardial ischemia/reperfusion injury:
Preclinical evidence and possible mechanisms. Front Pharmacol.
9:7292018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mehrotra P, Sturek M, Neyra JA and Basile
DP: Calcium channel Orai1 promotes lymphocyte IL-17 expression and
progressive kidney injury. J Clin Invest. 129:4951–4961. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kar F, Hacioglu C, Senturk H, Donmez DB
and Kanbak G: The role of oxidative stress, renal inflammation, and
apoptosis in post ischemic reperfusion injury of kidney tissue: The
protective effect of dose-dependent boric acid administration. Biol
Trace Elem Res. 195:150–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen B, Ma Y, Xue X, Wei J, Hu G and Lin
Y: Tetramethylpyrazine reduces inflammation in the livers of mice
fed a high fat diet. Mol Med Rep. 19:2561–2568. 2019.PubMed/NCBI
|
|
30
|
Bai XY, Wang XF, Zhang LS, Du PC, Cao Z
and Hou Y: Tetramethylpyrazine ameliorates experimental autoimmune
encephalomyelitis by modulating the inflammatory response. Biochem
Biophys Res Commun. 503:1968–1972. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng L, Ke N, Cheng F, Guo Y, Li S, Li Q
and Li Y: The protective mechanism of ligustrazine against renal
ischemia/reperfusion injury. J Surg Res. 166:298–305. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qing L, Wu P, Zhou Z, Yu F and Tang J:
Tetramethylpyrazine improved the survival of multiterritory
perforator flaps by inducing angiogenesis and suppressing apoptosis
via the Akt/Nrf2 pathway. Drug Des Devel Ther. 13:1437–1447. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang F, Jin H, Wu L, Shao J, Wu X, Lu Y
and Zheng S: Ligustrazine disrupts lipopolysaccharide-activated
NLRP3 inflammasome pathway associated with inhibition of Toll-like
receptor 4 in hepatocytes. Biomed Pharmacother. 78:204–209. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu X, Zhang F, Xiong X, Lu C, Lian N, Lu Y
and Zheng S: Tetramethylpyrazine reduces inflammation in liver
fibrosis and inhibits inflammatory cytokine expression in hepatic
stellate cells by modulating NLRP3 inflammasome pathway. IUBMB
Life. 67:312–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang X, Zuo L, Lv Y, Chen C, Yang Y, Xin
H, Li Y and Qian Y: Asiatic acid attenuates myocardial
ischemia/reperfusion injury via Akt/GSK-3β/HIF-1α signaling in rat
H9c2 cardiomyocytes. Molecules. 21:12482016. View Article : Google Scholar
|
|
36
|
Nicholas SA, Bubnov VV, Yasinska IM and
Sumbayev VV: Involvement of xanthine oxidase and hypoxia-inducible
factor 1 in Toll-like receptor 7/8-mediated activation of caspase 1
and interleukin-1β. Cell Mol Life Sci. 68:151–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ouyang X, Ghani A, Malik A, Wilder T,
Colegio OR, Flavell RA, Cronstein BN and Mehal WZ: Adenosine is
required for sustained inflammasome activation via the
A2A receptor and the HIF-1α pathway. Nat Commun. 4:2909.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cosin-Roger J, Simmen S, Melhem H, Atrott
K, Frey-Wagner I, Hausmann M, de Vallière C, Spalinger MR,
Spielmann P, Wenger RH, et al: Hypoxia ameliorates intestinal
inflammation through NLRP3/mTOR downregulation and autophagy
activation. Nat Commun. 8:982017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sokulsky LA, Goggins B, Sherwin S, Eyers
F, Kaiko GE, Board PG, Keely S, Yang M and Foster PS: GSTO1-1 is an
upstream suppressor of M2 macrophage skewing and HIF-1α-induced
eosinophilic airway inflammation. Clin Exp Allergy. 50:609–624.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hernández A, Geng Y, Sepúlveda R, Solís N,
Torres J, Arab JP, Barrera F, Cabrera D, Moshage H and Arrese M:
Chemical hypoxia induces pro-inflammatory signals in fat-laden
hepatocytes and contributes to cellular crosstalk with Kupffer
cells through extracellular vesicles. Biochim Biophys Acta Mol
Basis Dis. 1866:1657532020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tang TT, Lv LL, Pan MM, Wen Y, Wang B, Li
ZL, Wu M, Wang FM, Crowley SD and Liu BC: Hydroxychloroquine
attenuates renal ischemia/reperfusion injury by inhibiting
cathepsin mediated NLRP3 inflammasome activation. Cell Death Dis.
9:3512018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liang S, Xu Z, Ruan Y, Niu T, Guo W, Jiang
W and Hou J: Isoquercitrin attenuates renal ischemia/reperfusion
injury through antioxidation, anti-inflammation, and antiapoptosis
in mice. Transplant Proc. 52:1014–1019. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Han F, Dou M, Wang Y, Xu C, Li Y, Ding X,
Xue W, Zheng J, Tian P and Ding C: Cordycepin protects renal
ischemia/reperfusion injury through regulating inflammation,
apoptosis, and oxidative stress. Acta Biochim Biophys Sin
(Shanghai). 52:125–132. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang F, Wang Z, Wei X, Han H, Meng X,
Zhang Y, Shi W, Li F, Xin T, Pang Q and Yi F: NLRP3 deficiency
ameliorates neurovascular damage in experimental ischemic stroke. J
Cereb Blood Flow Metab. 34:660–667. 2014. View Article : Google Scholar : PubMed/NCBI
|