Introduction
An increasingly aging population and deterioration
in the quality of the environment are contributing to the increased
incidence of central nervous system (CNS) diseases such as stroke,
traumatic brain injury, brain tumors and neurodegenerative
disorders (1). According to
previous statistics, in 2015 the number of mortalities and
disability-adjusted life-years from neurological disorders were
9.40 and 250.7 million individuals, respectively, which suggests
that CNS diseases are amongst the leading causes of disability and
mortality worldwide (2). It is
reported that the median survival of patients with glioblastoma is
only 4–15 months, and the 5-year survival rate is <5% (3). One of the reasons for the poor
therapeutic effect of drugs used in the treatment of CNS diseases
is that they cannot effectively be delivered to the lesions in the
brain due to the blood-brain barrier (BBB) (4). The BBB consists of non-fenestrated
brain microvascular endothelial cells (BMECs), which form the
lining of brain capillaries, and adjacent cells including
astrocytes and pericytes (5).
BMECs are highly specialized structures due to their extensive
tight junctions (TJs) between them (6). They play an important role in BBB
function by restricting and precisely regulating the exchange of
molecules between the peripheral circulatory system and CNS
(7). Consequently, as a result of
the presence of the BBB, numerous drugs with a molecular weight of
>400 Da cannot reach the lesions in the brain (8), severely reducing the therapeutic
effect of drugs used in the treatment of CNS diseases. Therefore,
safe regulation of the permeability of the BBB has become a key
issue in the treatment of CNS diseases.
During previous decades, various methods have been
evaluated to overcome the problems associated with BBB
permeability, such as local delivery strategies to bypass the BBB
and physical mechanisms to breach the BBB (8). The former, which is extremely
invasive, primarily refers to convection-enhanced delivery (CED)
that uses positive pressure to inject anti-cancer agents directly
into brain tumors and thus bypass the BBB (9). The latter includes focused
ultrasound, photodynamic therapy and electromagnetic microwave
pulsed (EMP) radiation (10). All
these processes have been reported to open the BBB reversibly in a
less invasive manner than CED (11–13).
However, focused ultrasound and photodynamic therapy must be used
with microbubbles and photosensitizers, respectively, which makes
these two methods more complicated and higher-risk (14,15).
Our previous studies demonstrated that in
vivo (non-invasive) exposure of rats to EMP with a field
strength of 200 kV/m and a pulse width of 14 nsec caused a
temporary and reversible increase in BBB permeability; the
permeability peaked at 3 h after EMP exposure (13). Moreover, the transendothelial
electrical resistance of an in vitro BBB model, established
by co-culturing primary rat BMECs and astrocytes, is lower after
EMP exposure, which indicates increasing BBB permeability (16,17).
Li et al (18) also
reported that exposure of rats to EMP facilitated lomustine to
penetrate the BBB and be delivered the site of a brain tumor.
Nevertheless, for clinical application of EMP, it is important to
evaluate the relationship between the biological effect of EMP and
parameters such as electric field strength and exposure duration.
Compared with traditional EMP, ultra-wide band EMP (UWB-EMP)
exhibits a shorter rise time (within the psec range), higher
instantaneous peak power and wider spectrum (19). Thus far, there have been very few
studies focusing on the effects of UWB-EMP on BBB permeability. In
the present study, a range of EMP field strengths and post-exposure
durations were used to examine the potential of UWB-EMP to alter
BBB permeability in rats with the aim of facilitating the
development of techniques to be applied in clinical settings.
Materials and methods
Animals
A total of 138 male Sprague-Dawley (SD) rats,
weighing 220±20 g (age, 7 weeks), were obtained from the Laboratory
Animal Center of Air Force Medical University. Rats were
acclimatized for 1 week, and housed in groups of 6 in polycarbonate
cages under a 12:12-h light-dark cycle, 60% humidity and a
temperature of 23±2°C with ad libitum access to food and tap
water. They were fasted for 12 h prior to use in the experiments,
which were conducted in accordance with the National Institutes of
Health Guide for the Care and Use of Laboratory Animals (20) and approved by the Animal
Experimentation Ethics Committee of Airforce Medical University
(approval no. IACUC-20180503).
UWB-EMP exposure and experiment
protocols
The UWB-EMP exposures were conducted in a microwave
chamber which housed the UWB-EMP generator (Northwest Institute of
Nuclear Technology) and a platform to place animals. During
exposure, rats were kept individually in a special transparent
polymethyl methacrylate box (6×6×20 cm) and placed on the platform.
UWB-EMP pulses were generated by a spark gap pulse generator and
transmitted as previously described (21). The pulse repetition rate was 10 Hz
and the pulse duration was 0.9 nsec. Appropriate electric field
strength was achieved by modifying the distance between the UWB-EMP
generator and the platform.
The first experiment was conducted to investigate
BBB permeability at different time periods following a single
UWB-EMP exposure at 400 kV/m (total 20,000 pulses). A total of 90
SD rats were randomly divided into 5 groups (n=18/group, evenly
split for Evans blue (EB) staining, immunohistochemistry and
western blotting): Group 1, sham exposure (without UWB-EMP
transmission in the same microwave chamber); and groups 2–5,
examination of BBB at 0.5, 3, 6 and 24 h, respectively, after a
single UWB-EMP exposure at a field strength of 400 kV/m for 20,000
pulses.
A second experiment was conducted to investigate the
effect of different field strengths of UWB-EMP on BBB. A total of
48 SD rats were randomly divided into 4 groups (n=12/group, evenly
split for EB staining and albumin immunohistochemistry): Group 1,
sham exposure (without UWB-EMP transmission in the same microwave
chamber); and groups 2–4, UWB-EMP exposure at a field strength of
50, 200 and 400 kV/m for a total of 20,000 pulses, respectively.
Thus, the average specific absorption rates of groups 2–4 were
1.10×10−4, 1.75×10−3 and 7.01×10−3
W/kg, respectively. The BBB permeability was examined at 3 h after
UWB-EMP exposure.
All animals were anesthetized by intraperitoneal
administration of 1% sodium pentobarbital (45 mg/kg). In the first
experiment, animals were sacrificed at different time periods after
UWB-EMP exposure according to the grouping. In the second
experiment, animals were sacrificed at 3 h after exposure, prior to
subsequent procedures, including cardiac puncture and
perfusion.
EB staining
EB staining is frequently used as a marker to
evaluate BBB opening due to its ability to bind to plasma albumin
(22). At 30 min prior to
sacrifice, each animal was injected intravenously (4 ml/kg) with 4%
EB (MP Biomedicals, LLC). After perfusion with 0.9% normal saline
and subsequently 4% paraformaldehyde, brains were removed and
frozen at −80°C, and then embedded in Tissue-Tek®
O.C.T.™ cryostat-embedding compound (Sakura Finetek USA, Inc.).
Brains were sectioned at a thickness of 30 µm. Randomly selecting
six visual fields, EB extravasation was visualized using a
fluorescence microscope (Leica Microsystems GmbH; total
magnification, ×400) with green light excitation. Quantification
was performed using a scoring system by three experienced, blinded,
independent lab technicians as previously described (13). Briefly, four frozen sections
containing the frontal cortex from each rat were selected and a
total of 24 sections were examined in each group. The comprehensive
score (CS) of EB leakage was determined based on: i) Quantity of EB
fluorescence spots; ii) intensity of fluorescence; and iii) range
of fluorescence spots. Fluorescence range and intensity were
divided into four levels from weak to strong as follows: Negative,
0; small range/weak intensity; 1; moderate range/intensity, 2;
large range/strong intensity, 3. The CS of EB leakage for each
section was obtained by adding the quantity of EB spots, the value
for EB intensity and the value for EB range. The average score of 4
sections was the final CS of EB leakage for each rat.
ELISA
Blood was collected from each rat via cardiac
puncture prior to perfusion. Whole blood samples were stored at 4°C
overnight to separate serum via natural precipitation. A rat S100β
ELISA kit (sensitivity of 37.5 pg/ml; cat. no. E-EL-R0868; Wuhan
Elabscience Biotechnology Co., Ltd.) was used to measure serum
S100β levels according to the manufacturer's instructions. The
absorbance of the final yellow product was detected
spectrophotometrically at 450 nm wavelength (Bio-Rad Laboratories,
Inc.) and the optical density values were converted into
concentrations via a standard curve.
Immunohistochemistry
After anesthesia and perfusion, the brains were
dissected and fixed in 4% paraformaldehyde at room temperature
overnight. Paraffin-embedded sections with a thickness of 5 µm were
prepared, dewaxed and hydrated. Hydrogen peroxide-methanol solution
(3%) was used to eliminate the endogenous peroxidase activity at
room temperature for 15 min, and 0.01 M citrate buffer was used for
antigen retrieval in the microwave. Sections were washed three
times with PBS and blocked with 5% normal donkey serum (cat. no.
SL050; Beijing Solarbio Science & Technology Co., Ltd.) in PBS
at room temperature for 30 min. Then, the slides were incubated
with sheep anti-albumin antibody (1:500; cat. no. A110-134A; Bethyl
Laboratories, Inc.) and rabbit anti-zonula occludens 1 (ZO-1)
antibody (1:100; cat. no. 61-7300; Zymed; Thermo Fisher Scientific,
Inc.) at 4°C overnight. After washing with PBS, horseradish
peroxidase (HRP)-conjugated donkey anti-sheep antibody (1:500; cat.
no. ab6900; Abcam) or FITC-conjugated goat anti-rabbit antibody
(1:100; cat. no. A22120; Abbkine Scientific Co., Ltd.) was added to
the slides and incubated for 2 h at room temperature. For albumin
immunohistochemistry, the color was developed using
2,2′-diaminobenzidine (Boster Biological Technology) at room
temperature for ~5 min. Following hematoxylin counterstaining (room
temperature; 1 min), slides were sealed with neutral gum. A total
of 24 sections in each group were observed and photographed using a
light microscope (Nikon Corporation; total magnification, ×400).
Semi-quantitative evaluation of albumin immunohistochemistry was
conducted in a similar manner to quantification of EB
fluorescence.
Western blot analysis of ZO-1 and
heat-shock protein 70 (HSP70)
Brains were immediately dissected after euthanasia,
and the cortex was separated on ice and stored at −80°C. The total
protein in the cortex was obtained with a whole protein extraction
kit (cat. no. KGP250; Nanjing KeyGen Biotech Co., Ltd.) and then
quantified using a bicinchoninic acid assay kit (Boster Biological
Technology). A total of 40 µg of each protein sample was loaded per
lane and then separated via 8% SDS-PAGE and transferred onto PVDF
membranes (EMD Millipore). After blocking in TBS-0.1% Tween-20 and
5% nonfat dry milk at room temperature for 1 h, the membranes were
incubated with rabbit polyclonal anti-ZO-1 antibody (1:1,000; cat.
no. 61-3700; Zymed; Thermo Fisher Scientific, Inc.), rabbit
anti-HSP70 antibody (1:1,000; cat. no. ab181606; Abcam) and mouse
anti-GAPDH antibody (1:2,000; cat. no. ab8245; Abcam) at 4°C
overnight. After rinsing with TBS-0.1% Tween 20, membranes were
then incubated HRP-conjugated goat anti-rabbit IgG (1:5,000; cat.
no. ab6721; Abcam) and HRP-conjugated goat anti-mouse IgG (1:5,000;
cat. no. ab6789; Abcam) at room temperature for 2 h. Finally, the
membranes were washed four times and developed using an Immobilon
Western Chemiluminescent HRP Substrate kit (EMD Millipore). Gray
value analysis was performed using QuantityOne software version
4.6.8 (Bio-Rad Laboratories, Inc.).
H&E staining
Paraffin-embedded sections (5 µm) were prepared as
described above. After conventional gradient dewaxing, three
sections containing the frontal cortex from each rat (total of 18
sections of each group) were stained in hematoxylin staining
solution for 5 min. Following rinsing with tap water for 2 min,
sections were placed in an alcohol hydrochloride differentiation
fluid for 10 sec and rinsed with tap water for ~10 min until the
blue color returned. Then, the sections were stained with eosin for
1 min, then washed with distilled water and dehydrated with
gradient alcohol. Finally, the sections were rendered transparent
with xylene and sealed with neutral gum. All procedures were
performed at room temperature. The morphology of brain tissue was
observed in five random visual fields of each section using a light
microscope (Nikon Corporation; total magnification, ×200).
Statistical analysis
The normality of variables was evaluated using
Lilliefors test. Data were expressed as the mean ± SD if they were
normally distributed (S100β concentration, relative amount of HSP70
and ZO-1). If non-normally distributed (CS of EB fluorescence, CS
of albumin immunohistochemistry), the data were expressed as the
median (interquartile range). One-way ANOVA and Tukey's multiple
comparison test were applied for comparisons of normally
distributed data, whereas Kruskal-Wallis H test followed by
Dunn-Bonferroni test for post hoc comparisons was used to analyze
non-distributed data. All data were analyzed using SPSS 22.0
software (IBM Corp.), and P<0.05 was considered to indicate a
statistically significant difference.
Results
Influence of a single UWB-EMP exposure
on BBB permeability at different time periods after exposure
The permeability of the BBB was evaluated via EB and
albumin extravasation into the brain parenchymal cells, and the
levels of S100β in serum at different time periods after a single
exposure to UWB-EMP at the field strength of 400 kV/m. The
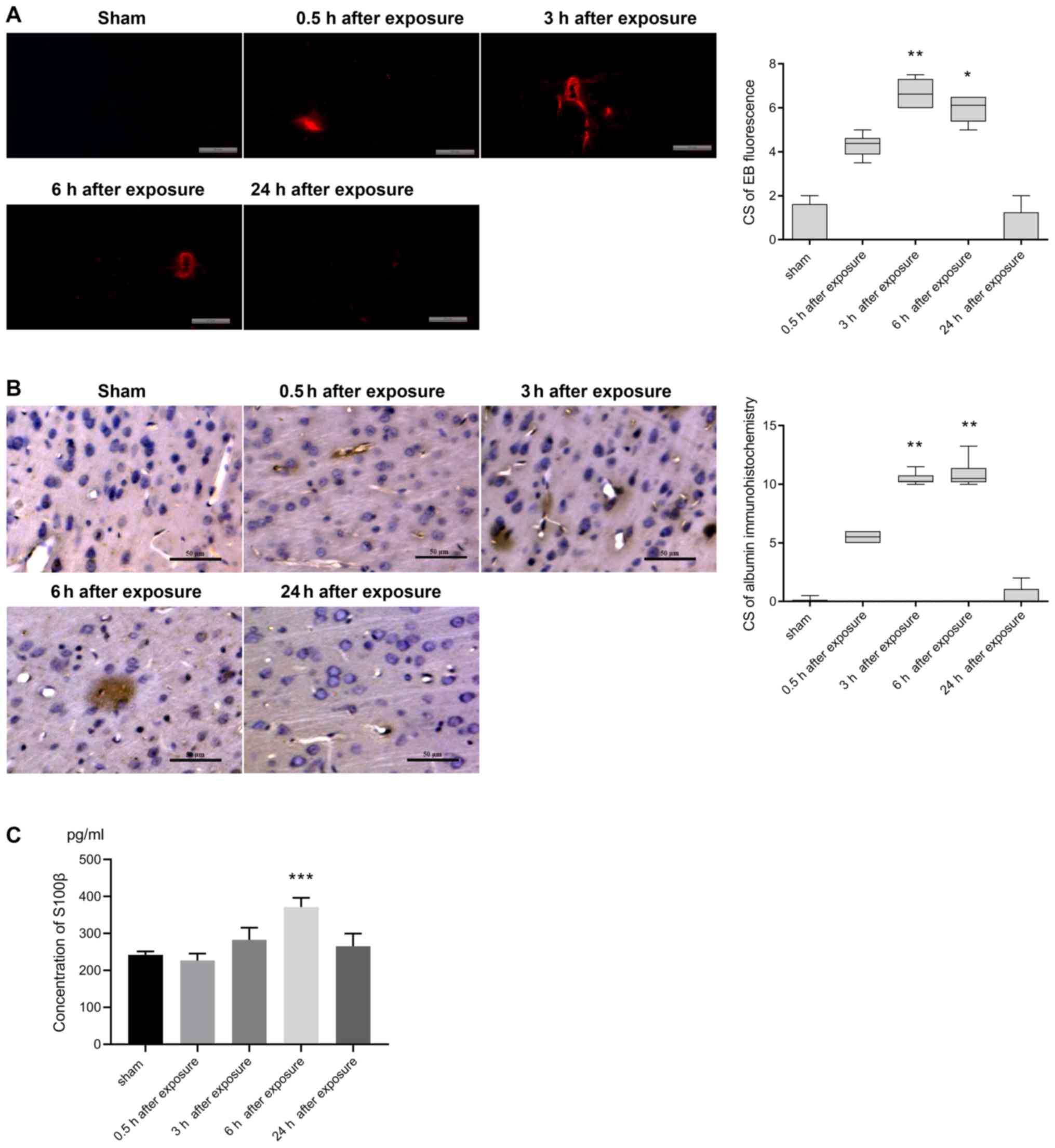
fluorescence images of EB in sham-exposed controls revealed very
few or no red fluorescence spots, which are indicative of
perivascular albumin leakage, in the cortex (Fig. 1A). In animals exposed to UWB-EMP,
there was a greater number of fluorescence spots at 0.5 h, which
was further increased at 3 and 6 h. However, at 24 h after UWB-EMP
exposure, the leakage was similar to that observed in sham-exposed
controls (Fig. 1A). The CS for EB
fluorescence in 3 h post-exposure group [6.625 (6.000, 7.313);
P=0.001] and 6 h post-exposure group [6.125 (5.375, 6.500);
P=0.013] was significantly higher compared with the sham group
[0.000 (0.000, 1.625); Fig. 1A].
The CSs obtained from immunohistochemical analysis of albumin
extravasation showed perivascular albumin leakage at 0.5 h after
UWB-EMP exposure [5.500 (5.000, 6.000); P=0.535] compared with the
sham group [0.000 (0.000, 0.125); Fig.
1B]. At 3 h after exposure, both the number of microvessels
with albumin exudation and the extent of exudation increased
[10.250 (10.188, 10.750); P=0.001], and the CS of albumin
extravasation peaked at 6 h [10.500 (10.188, 11.375); P=0.003].
However, at 24 h, albumin distribution in UWB-EMP-exposed animals
resembled that in sham-exposed controls (Fig. 1B). The levels of S100β in serum
were also significantly increased at 6 h after UWB-EMP exposure
(371.292±25.190 ng/ml; P<0.001), while at other exposure time
points, there was no significant difference between UWB-EMP-exposed
and sham-exposed animals (Fig.
1C).
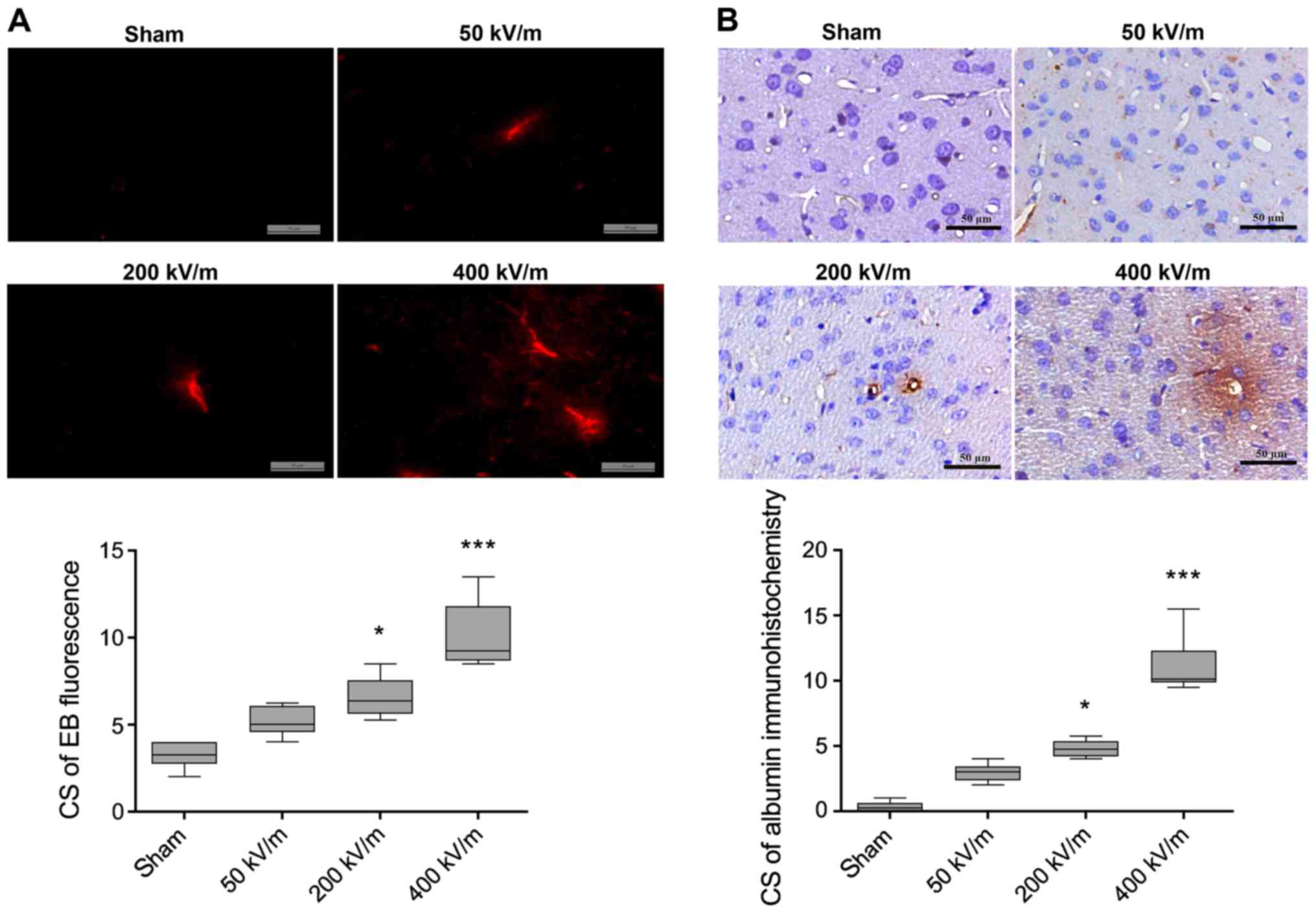
Influence of UWB-EMP field strength on
BBB
Following the observation that the highest increase
in BBB permeability occurred between 3 and 6 h after a single
UWB-EMP exposure, the influence of field strength on the BBB was
then examined. The CSs for albumin immunostaining and EB
fluorescence in sham-exposed animals were not significantly
difference compared with those exposed to UWB-EMP at 50 kV/m
(Fig. 2). However, when UWB-EMP
exposure was conducted with a field strength of 200 or 400 kV/m,
marked perivascular extravasation of albumin and EB fluorescence
was observed in the cerebral cortex of the rats (Fig. 2). The CSs of EB fluorescence and
exudation of albumin in animals exposed to UWB-EMP at 400 kV/m
[9.250 (8.688, 11.813) and 10.125 (9.875, 12.313), respectively]
were notably increased compared with in the 200 kV/m UWB-EMP group
[6.375 (5.625, 7.563) and 4.750 (4.188, 5.375), respectively]
although the differences were not statistically significant
(P=0.637 and P=0.813, respectively; Fig. 2).
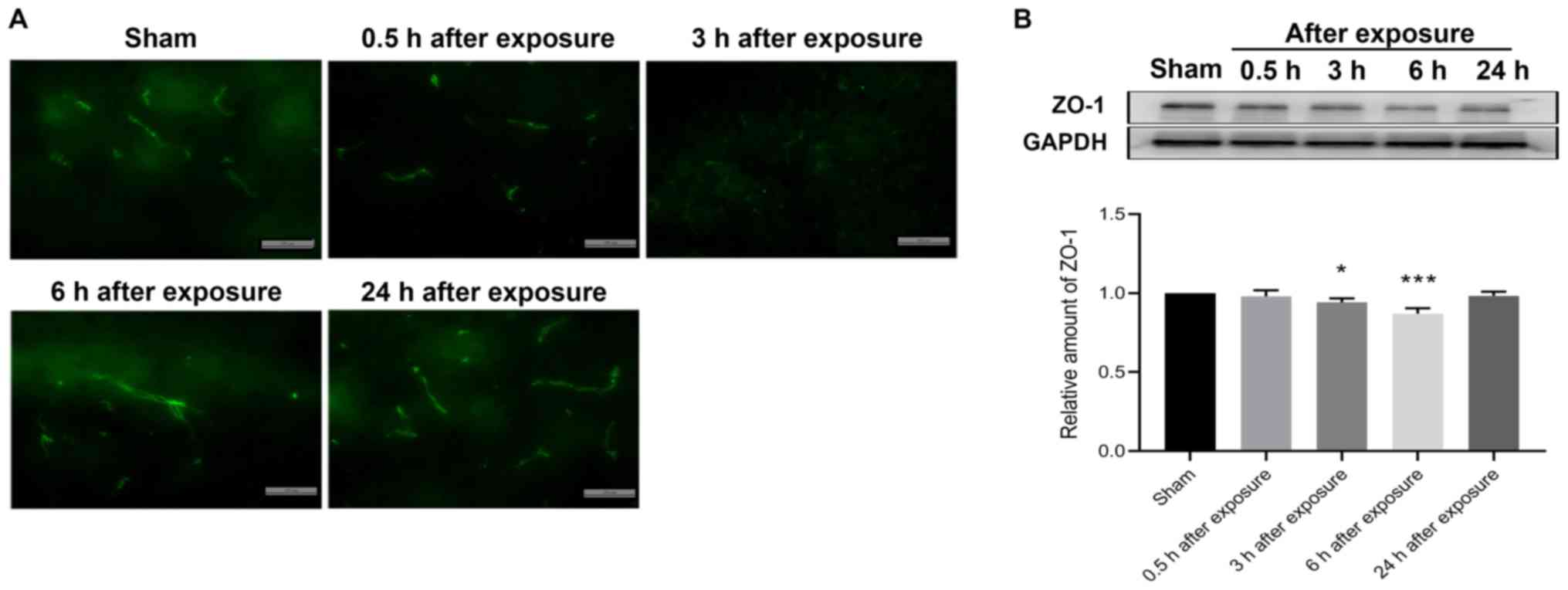
Effect of UWB-EMP exposure on ZO-1 in
the cortex
ZO-1 is a major TJ protein important for maintaining
BBB permeability in the brain (23). Western blot analysis was conducted
to evaluate the expression of ZO-1, while immunohistochemical
staining was performed to observe the distribution of ZO-1. The
results showed that the expression levels of ZO-1 at 3 and 6 h
after UWB-EMP exposure (0.942±0.025 and 0.871±0.033, respectively)
were significantly decreased (P=0.012 and P<0.001, respectively)
and, at 24 h after UWB-EMP exposure, the expression of ZO-1
(0.984±0.025) was slightly lower than that of sham-exposed controls
(the difference was not statistically significant; P=0.758;
Fig. 3B). However, the
distribution of ZO-1 did not notably vary at different exposure
time points following UWB-EMP exposure (Fig. 3A).
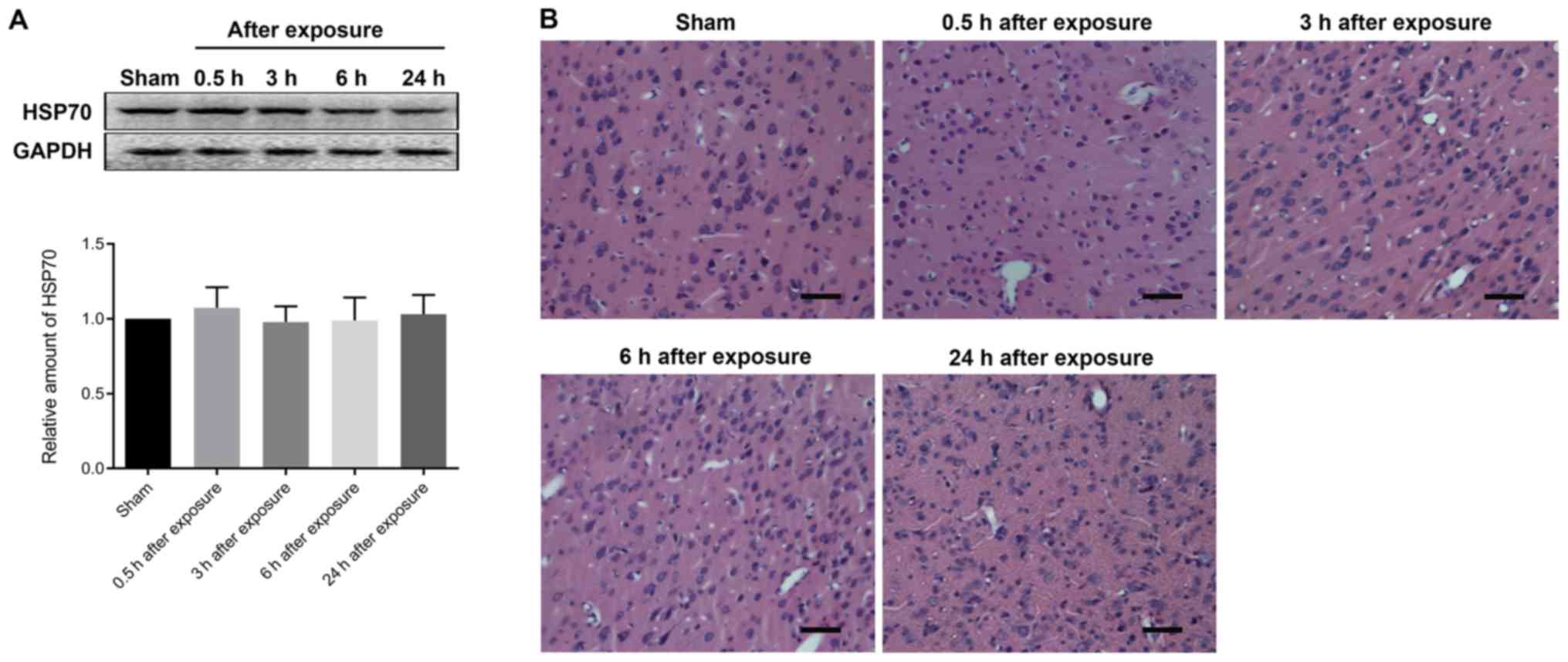
Effect of UWB-EMP on HSP70 levels in
cortex
Western blot analysis of HSP70 expression showed
that the expression levels of HSP70 at different time points after
UWB-EMP exposure were not significantly altered (Fig. 4A).
Effect of UWB-EMP on the morphology of
cerebral cortex
The safety of UWB-EMP exposure was evaluated by
analyzing morphological changes in H&E-stained cerebral cortex
sections. The data indicated no markedly histopathological
abnormalities in any animals exposed to UWB-EMP (Fig. 4B).
Discussion
The development of safe and effective technology to
permit access through the BBB is of paramount importance to
combating CNS diseases. Oscar and Hawkins (24) observed that a single exposure of
rats to 1.3 GHz of continuous or pulsed microwave radiation can
increase the permeability of the BBB in the medulla, cerebellum and
hypothalamus, but not in the hippocampus and cortex. Albert and
Kerns (25) also observed
reversible opening of the BBB in random areas of the brains of
hamsters exposed to 2.45 GHz microwave radiation. A number of other
in vitro and in vivo studies in rats reported similar
observations of enhanced permeability of the BBB (26–29).
Conversely, Soderqvist et al (30,31)
conducted studies in humans, using serum S100β, transthyretin and
β-trace protein as markers to examine the impact of 890 MHz
microwave radiation on BBB integrity; however, they failed to
observe an association or an effect of microwave exposure on the
human BBB. These contradictory results may be due to differences in
experimental subjects, varying methodologies and, most importantly,
diverse microwave exposure parameters. Furthermore, most of the
studies that reported impaired BBB integrity following exposure to
microwaves focused on adverse effects of long-term exposure and did
not consider the thermal effect of microwave radiation, which can
damage normal tissue seriously. The ideal microwave exposure that
can be applied to assist drugs in penetrating the BBB is preferably
a single non-thermal exposure.
UWB-EMP is widely used in radar technology,
electronic countermeasures and wireless communications (32). They have a sub-nsec rise time and a
pulse width within a small number of nsec. The ultra-short rise
time results in its ultra-wide spectrum, and the ultra-short pulse
width allows a high peak power density without producing
hyperthermia (33). Therefore, the
influence of a single UWB-EMP exposure of varying electric field
strength was examined on BBB permeability in the present study. The
results indicated that the BBB permeability of rats began to
increase immediately after UWB-EMP exposure, reaching a maximum
between 3–6 h after exposure and returning to normal within 24 h.
Subsequent experiments indicated that UWB-EMP exposure with
different field strength induced field strength-dependent effects
on BBB permeability, as BBB permeability increased with increasing
UWB-EMP field strength.
S100β is a protein secreted by glial cells (34). Generally, the concentration of
S100β in peripheral blood is very low; a clinical cutoff value
≤0.10 µg/l has been considered normal (35). When the BBB is damaged, S100β can
enter the circulatory system in large quantities; thus, the S100β
concentration in serum can indirectly reflect the degree of BBB
disruption (36). As the
biological half-life of plasma S100β is 1–2 h (24), its content also indicates the
cumulative degree of BBB opening. In the present study, the S100β
concentration in the serum was increased significantly at 6 h after
UWB-EMP exposure, which was similar to the results of the albumin
immunohistochemistry experiments.
HSP70 is a major stress-inducible protein, which can
be induced by stimuli such as injury, heat and ultraviolet exposure
(37). The upregulation of HSP70
often indicates a stress state or even disease state (37). In the present study, the expression
levels of HSP70 and brain histomorphology were analyzed to evaluate
the safety of a single UWB-EMP exposure. The results indicated that
UWB-EMP did not induce significant damage in the brain tissue of
rats. Kovacs et al (38)
reported that BBB opening induced by MRI-guided focused ultrasound
combined with microbubbles resulted in an immediate
damage-associated molecular pattern response, including elevated
HSP70 levels. Compared with focused ultrasound combined with
microbubbles, UWB-EMP exposure may cause insufficient damage in the
brain to induce expression of HSP70. However, a comprehensive
safety evaluation needs to be conducted.
The extensive TJs between BMECs are the structural
basis of the BBB, regulating the diffusion of water, ions and
macromolecules through the paracellular pathway (39). TJ consists of transmembrane
proteins, cytoplasmic accessory proteins and cytoskeletal proteins
(39). The ZO proteins are amongst
the most integral cytoplasmic accessory proteins, which play a role
in connecting transmembrane proteins to cytoskeletal proteins,
signal transduction and transcriptional modulation (40). In the present study, the expression
and distribution of ZO-1, a 220-kDa protein belonging to the ZO
protein family, were evaluated. Previous studies have shown that
the abnormal expression or distribution of ZO-1 can lead to TJ
integrity disruption, causing an increase in BBB permeability
(41–44). In the present study, western blot
and immunofluorescence analyses were conducted to determine whether
ZO-1 was involved in the disruption of BBB permeability following
UWB-EMP exposure. The results indicated that the expression of ZO-1
was decreased significantly at 3 and 6 h after exposure. However,
there was no notable effect on its distribution. The parallel
changes in ZO-1 expression and BBB integrity suggested an important
role for ZO-1 in UWB-EMP-induced BBB opening in the rat brain.
Nevertheless, the present study has certain
limitations. Although EB and albumin are common tracers to examine
BBB leakage, their large molecular weights (~67 kDa) make it
difficult to detect very small increases in BBB permeability. The
use of tracers across a varied size range should be considered in
further studies. Additionally, the present study only examined a
single UWB-EMP exposure. However, further investigations into the
impact of repeated and long-term exposure to UWB-EMP are required
to find an effective field strength and time period during which
BBB permeability is increased.
In conclusion, UWB-EMP exposure increased BBB
permeability transiently and safely in a field strength-dependent
manner. This was associated with changes in the expression of ZO-1,
which could be a mechanism involved in BBB permeability.
Acknowledgements
We wish to acknowledge Dr Hu Long (School of
Electronic Science and Engineering, Xi'an Jiaotong University) and
Mr Xu Shenglong (Faculty of Preventive Medicine, Airforce Medical
University) for their contribution to the maintenance and
administration of the UWB-EMP equipment.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 51437008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GG and GD conceived the study. PG and QC designed
the study, and performed the western blot analysis and ELISA. YL,
QG and HY performed H&E staining and immunohistochemical
staining. JinL provided UWB-EMP equipment, performed exposure
protocols and confirmed the exposure parameters in the study. JH
and JiaL performed analysis of data. YZ and LZ helped interpret the
staining data and create the figures. GG and GD curated data. PG
drafted the original version of the manuscript. LZ, GD and GG
revised the manuscript critically. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All experiments were conducted in accordance with
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals and approved by the Animal Experimentation
Ethics Committee of Airforce Medical University (approval no.
IACUC-20180503).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hahad O, Lelieveld J, Birklein F, Lieb K,
Daiber A and Munzel T: Ambient air pollution increases the risk of
cerebrovascular and neuropsychiatric disorders through induction of
inflammation and oxidative stress. Int J Mol Sci. 21:27752020.
View Article : Google Scholar
|
|
2
|
The Lancet Neuroiogy: Global analysis of
neurological disease: Burden and benefit. Lancet Neurol.
16:8572017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ostrom QT, Gittleman H, Liao P, Rouse C,
Chen Y, Dowling J, Wolinsky Y, Kruchko C and Barnholtz-Sloan J:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 16
(Suppl 4):iv1–iv63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arvanitis CD, Ferraro GB and Jain RK: The
blood-brain barrier and blood-tumour barrier in brain tumours and
metastases. Nat Rev Cancer. 20:26–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hawkins BT and Davis TP: The blood-brain
barrier/neurovascular unit in health and disease. Pharmacol Rev.
57:173–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang Z, Guo D, Xiong L, Wu B, Xu X, Fu J,
Kong L, Liu Z and Xie C: TLR4/PKCα/occludin signaling pathway may
be related to blood-brain barrier damage. Mol Med Rep.
18:1051–1057. 2018.PubMed/NCBI
|
|
7
|
Lee MR and Jayant RD: Penetration of the
blood-brain barrier by peripheral neuropeptides: New approaches to
enhancing transport and endogenous expression. Cell Tissue Res.
375:287–293. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Z, Nelson AR, Betsholtz C and
Zlokovic BV: Establishment and dysfunction of the blood-brain
barrier. Cell. 163:1064–1078. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhan W and Wang CH: Convection enhanced
delivery of chemotherapeutic drugs into brain tumour. J Control
Release. 271:74–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parodi A, Rudzinska M, Deviatkin AA, Soond
SM, Baldin AV and Zamyatnin AA Jr: Established and emerging
strategies for drug delivery across the blood-brain barrier in
brain cancer. Pharmaceutics. 11:2452019. View Article : Google Scholar
|
|
11
|
McDannold N, Zhang Y, Supko JG, Power C,
Sun T, Peng C, Vykhodtseva N, Golby AJ and Reardon DA: Acoustic
feedback enables safe and reliable carboplatin delivery across the
blood-brain barrier with a clinical focused ultrasound system and
improves survival in a rat glioma model. Theranostics. 9:6284–6299.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Semyachkina-Glushkovskaya O, Chehonin V,
Borisova E, Fedosov I, Namykin A, Abdurashitov A, Shirokov A,
Khlebtsov B, Lyubun Y, Navolokin N, et al: Photodynamic opening of
the blood-brain barrier and pathways of brain clearing. J
Biophotonics. 11:e2017002872018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding GR, Qiu LB, Wang XW, Li KC, Zhou YC,
Zhou Y, Zhang J, Zhou JX, Li YR and Guo GZ: EMP-induced alterations
of tight junction protein expression and disruption of the
blood-brain barrier. Toxicol Lett. 196:154–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McMahon D, Poon C and Hynynen K:
Evaluating the safety profile of focused ultrasound and
microbubble-mediated treatments to increase blood-brain barrier
permeability. Expert Opin Drug Deliv. 16:129–142. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kimura S, Kuroiwa T, Ikeda N, Nonoguchi N,
Kawabata S, Kajimoto Y and Ishikawa T: Assessment of safety of
5-aminolevulinic acid-mediated photodynamic therapy in rat brain.
Photodiagnosis Photodyn Ther. 21:367–374. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou JX, Ding GR, Zhang J, Zhou YC, Zhang
YJ and Guo GZ: Detrimental effect of electromagnetic pulse exposure
on permeability of in vitro blood-brain-barrier model. Biomed
Environ Sci. 26:128–137. 2013.PubMed/NCBI
|
|
17
|
Zhou Y, Qiu LB, An GZ, Zhou JX, Du L, Ma
YH, Guo GZ and Ding GR: Effects of electromagnetic pulse exposure
on gelatinase of blood-brain barrier in vitro. Electromagn Biol
Med. 36:1–7. 2017.PubMed/NCBI
|
|
18
|
Li K, Zhang K, Xu S, Wang X, Zhou Y, Zhou
Y, Gao P, Lin J, Ding G and Guo G: EMP-induced BBB-disruption
enhances drug delivery to glioma and increases treatment efficacy
in rats. Bioelectromagnetics. 39:60–67. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang X, Wang Y, Wu S and Gulliver TA:
Experimental study of wireless monitoring of human respiratory
movements using UWB impulse radar systems. Sensors (Basel).
18:30652018. View Article : Google Scholar
|
|
20
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the care and use of laboratory animals. 8th.
Washington (DC): National Academies Press (US); 2011
|
|
21
|
Jiang DP, Li J, Zhang J, Xu SL, Kuang F,
Lang HY, Wang YF, An GZ, Li JH and Guo GZ: Electromagnetic pulse
exposure induces overexpression of beta amyloid protein in rats.
Arch Med Res. 44:178–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang HL and Lai TW: Optimization of evans
blue quantitation in limited rat tissue samples. Sci Rep.
4:65882014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu WY, Wang ZB, Zhang LC, Wei X and Li L:
Tight junction in blood-brain barrier: An overview of structure,
regulation, and regulator substances. CNS Neurosci Ther.
18:609–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oscar KJ and Hawkins TD: Microwave
alteration of the blood-brain barrier system of rats. Brain Res.
126:281–293. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Albert EN and Kerns JM: Reversible
microwave effects on the blood-brain barrier. Brain Res.
230:153–164. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang YM, Zhou Y, Qiu LB, Ding GR and Pang
XF: Altered expression of matrix metalloproteinases and tight
junction proteins in rats following PEMF-Induced BBB permeability
change. Biomed Environ Sci. 25:197–202. 2012.PubMed/NCBI
|
|
27
|
Tang J, Zhang Y, Yang L, Chen Q, Tan L,
Zuo S, Feng H, Chen Z and Zhu G: Exposure to 900 MHz
electromagnetic fields activates the mkp-1/ERK pathway and causes
blood-brain barrier damage and cognitive impairment in rats. Brain
Res. 1601:92–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang LF, Li X, Gao YB, Wang SM, Zhao L,
Dong J, Yao BW, Xu XP, Chang GM, Zhou HM, et al: Activation of
VEGF/Flk-1-ERK pathway induced blood-brain barrier injury after
microwave exposure. Mol Neurobiol. 52:478–491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sirav B and Seyhan N: Effects of GSM
modulated radio-frequency electromagnetic radiation on permeability
of blood-brain barrier in male & female rats. J Chem Neuroanat.
75:123–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soderqvist F, Carlberg M and Hardell L:
Use of wireless telephones and serum S100B levels: A descriptive
cross-sectional study among healthy Swedish adults aged 18–65
years. Sci Total Environ. 407:798–805. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Soderqvist F, Carlberg M and Hardell L:
Biomarkers in volunteers exposed to mobile phone radiation. Toxicol
Lett. 235:140–146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dorsey WC, Ford BD, Roane L, Haynie DT and
Tchounwou PB: Induced mitogenic activity in AML-12 mouse
hepatocytes exposed to low-dose ultra-wideband electromagnetic
radiation. Int J Environ Res Public Health. 2:24–30. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ruan C, Zhao W, Chen GF and Zhu SL:
Characteristic of the ultra-wideband electromagnetic radiation
generated by PCSS. Microwave Opt Technol Lett. 49:1118–1122. 2007.
View Article : Google Scholar
|
|
34
|
Michetti F, D'Ambrosi N, Toesca A, Puglisi
MA, Serrano A, Marchese E, Corvino V and Geloso MC: The S100B
story: From biomarker to active factor in neural injury. J
Neurochem. 148:168–187. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Astrand R and Unden J: Clinical Use of the
Calcium-Binding S100B Protein, a Biomarker for Head Injury. Methods
Mol Biol. 1929:679–690. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kanner AA, Marchi N, Fazio V, Mayberg MR,
Koltz MT, Siomin V, Stevens GH, Masaryk T, Aumayr B, Vogelbaum MA,
et al: Serum S100beta: A noninvasive marker of blood-brain barrier
function and brain lesions. Cancer. 97:2806–2813. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yun CW, Kim HJ, Lim JH and Lee SH: Heat
shock proteins: Agents of cancer development and therapeutic
targets in anti-cancer therapy. Cells. 9:602019. View Article : Google Scholar
|
|
38
|
Kovacs ZI, Kim S, Jikaria N, Qureshi F,
Milo B, Lewis BK, Bresler M, Burks SR and Frank JA: Disrupting the
blood-brain barrier by focused ultrasound induces sterile
inflammation. Proc Natl Acad Sci USA. 114:E75–E84. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Haseloff RF, Dithmer S, Winkler L, Wolburg
H and Blasig IE: Transmembrane proteins of the tight junctions at
the blood-brain barrier: Structural and functional aspects. Semin
Cell Dev Biol. 38:16–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tietz S and Engelhardt B: Brain barriers:
Crosstalk between complex tight junctions and adherens junctions. J
Cell Biol. 209:493–506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fan X, Jiang Y, Yu Z, Liu Q, Guo S, Sun X,
van Leyen K, Ning M, Gao X, Lo EH and Wang X: Annexin A2 plus
low-dose tissue plasminogen activator combination attenuates
cerebrovascular dysfunction after focal embolic stroke of rats.
Transl Stroke Res. 8:549–559. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Goodall EF, Wang C, Simpson JE, Baker DJ,
Drew DR, Heath PR, Saffrey MJ, Romero IA and Wharton SB:
Age-associated changes in the blood-brain barrier: Comparative
studies in human and mouse. Neuropathol Appl Neurobiol. 44:328–340.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim BJ, Hancock BM, Bermudez A, Del Cid N,
Reyes E, van Sorge NM, Lauth X, Smurthwaite CA, Hilton BJ, Stotland
A, et al: Bacterial induction of Snail1 contributes to blood-brain
barrier disruption. J Clin Invest. 125:2473–2483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zihni C, Mills C, Matter K and Balda MS:
Tight junctions: From simple barriers to multifunctional molecular
gates. Nat Rev Mol Cell Biol. 17:564–580. 2016. View Article : Google Scholar : PubMed/NCBI
|