Introduction
Small interfering RNAs (siRNAs) are the widely used
for the suppression of targeted gene expression in cells (1). For the analysis of gene function with
siRNA, reproducible transfections with a large set of siRNAs in a
multi-well plate is required (2).
For effective transfection of siRNAs into cells, cationic liposomes
are currently the most widely used carrier (3). However, siRNA and cationic liposome
complexes (siRNA lipoplexes) are unstable when stored in solution
at room temperature, but they can be stabilized by drying.
Therefore, for transfection of a large set of siRNAs, reverse (Rev)
transfection with freeze-dried siRNA lipoplexes are the validated
means. In Rev-transfection with freeze-dried siRNA lipoplexes, the
siRNA lipoplex solution is added into the well of cell culture
plates, followed by freeze-drying, and at the time of transfection,
a cell suspension is added to the culture plate well.
Rev-transfection can reduce the time for transfection if a large
set of siRNA lipoplexes are freeze-dried in advance in multi-well
plates.
A typical freeze-drying process consists of three
main phases: Freezing and primary and secondary drying (4). The size of siRNA lipoplexes usually
increases after freeze-drying because of the influence of severe
stress during the freezing, lyophilizing, and rehydration
processes, which can damage the siRNA lipoplexes (5). Therefore, disaccharides such as
sucrose and trehalose have been used as cryoprotectants for the
stabilization of siRNA lipoplexes during the freeze-drying and
rehydration processes (6–9). During freeze-drying, water molecules
associated with the polar head groups of hydrated phospholipids of
the liposomal membrane are replaced by disaccharide molecules,
which protect liposomes from aggregation and fusion between siRNA
lipoplexes. Regarding the Rev-transfection of siRNA or micro
(mi)RNA, chitosan and TransIT-TKO® transfection
reagent (cationic polymer/lipid formulation) exhibited effective
gene-silencing in H1299 and RAW264.3 cell lines when their
complexes with siRNA were freeze-dried in the presence of 10% (292
mM) sucrose in a 24-well plate (7). Furthermore, Rev-transfection with
commercially available Lipofectamine 2000 lipoplexes introduced
siRNA at high efficiency into various types of cell lines when they
were vacuum-dried in the presence of dextran and polyvinyl alcohol
(PVA) in a 24-well plate (10). In
addition, Rev-transfection with Lipofectamine 2000 lipoplexes
freeze-dried in Opti-MEM on a tissue culture plate showed high
transfection efficiency of miRNA in mesenchymal stem cells
(11). Previously, we reported
that the presence of trehalose or sucrose in the freeze-drying of
siRNA lipoplexes increased the long-term stability of siRNA
lipoplexes without apparent loss of gene-silencing activity by
Rev-transfection (12). However,
to the best of our knowledge, there are still few reports on the
application of Rev-transfection with freeze-dried siRNA lipoplexes
in multi-well plates for the transfection of siRNAs into cells.
In this study, we examined the effect of freezing
and saccharide types in the freeze-drying of siRNA lipoplexes on
the efficiency of gene-silencing in cells using Rev-transfection.
Here, we found that vacuum-drying of siRNA lipoplexes in trehalose
or sucrose solution without a pre-freezing process also resulted in
high gene-silencing activity upon Rev-transfection. In addition,
the presence of disaccharides such as maltose, lactose, lactulose,
and cellobiose during the freeze-drying of siRNA lipoplexes
exhibited gene-silencing activity without cytotoxicity by
Rev-transfection, compared with those of monosaccharides or
trisaccharides. This study provides valuable information about the
Rev-transfection with freeze-dried or vacuum-dried siRNA lipoplexes
for efficient siRNA delivery into the cells with a large set of
siRNAs in a multi-well plate.
Materials and methods
Materials
Cholesteryl
(3-((2-hydroxyethyl)amino)propyl)carbamate hydroiodide (HAPC-Chol)
was synthesized as described previously (13).
N-(2-(2-Hydroxyethylamino)ethyl)cholesteryl- 3-carboxamide
(OH-Chol) and cholesteryl (2-((2-hydroxyethyl)amino)ethyl)carbamate
(OH-C-Chol) were synthesized as described previously (14). 1,2-Dioleoyl- 3-
trimethylammonium-propane methyl sulfate salt (DOTAP) was obtained
from Avanti Polar Lipids Inc. Dimethyldioctadecylammonium bromide
(DDAB, product name: DC-1-18) and 11- ((1,3- bis(dodecanoyloxy)- 2-
((dodecanoyloxy)methyl)propan- 2- yl) amino)- N,N,N-
trimethyl-11- oxoundecan-1- aminium bromide (product name: TC-1-12)
were obtained from Sogo Pharmaceutical Co., Ltd.. 1,2-Dioleoyl-
sn- glycero- 3-phosphoethanolamine (DOPE, COATSOME ME-8181)
was obtained from NOF Co., Ltd.. Glucose, fructose, galactose,
mannose, sucrose, trehalose dihydrate, maltose monohydrate, lactose
monohydrate, lactulose, cellobiose, and raffinose pentahydrate were
obtained from Wako Pure Chemical Industries, Ltd.. Melezitose was
purchased form Sigma-Aldrich Co. LLC. All other chemicals were of
the highest grade available.
Small interfering RNAs
siRNAs targeting nucleotides of firefly
luciferase (Luc siRNA), and non-silencing siRNA [control (Cont)
siRNA] as a negative control for Luc siRNA were synthesized by
Sigma Genosys. The siRNA sequences of the Luc siRNA were: Sense
strand: 5′-CCGUGGUGUUCGUGUCUAAGA-3′, and antisense strand:
5′-UUAGACACGAACACCACGGUA-3 (15).
The siRNA sequences of the Cont siRNA were: Sense strand:
5′-GUACCGCACGUCAUUCGUAUC-3′, and antisense strand:
5′-UACGAAUGACGUGCGGUACGU-3′ (14).
Preparation of cationic liposomes and
siRNA lipoplexes
Cationic cholesterol derivative-based liposomes were
prepared from OH-Chol/DOPE (composition designated as LP-OH),
OH-C-Chol/DOPE (composition designated as LP-OH-C), and
HAPC-Chol/DOPE (composition designated as LP-HAPC) at a molar ratio
of 3:2 (16). The cationic
liposomes including dialkyl or trialkyl cationic lipids were
prepared from DOTAP/DOPE (composition designated as LP-DOTAP),
DDAB/DOPE (composition designated as LP-DDAB), and TC-1-12/DOPE
(composition designated as LP-TC-1-12) at a molar ratio of 1:1
(16).
For the preparation of cationic liposomes using a
thin-film hydration method, cationic lipid and DOPE were dissolved
in chloroform, and the chloroform was evaporated under vacuum on a
rotary evaporator at 60°C to obtain a thin film. The thin film was
hydrated with water at 60°C by vortex mixing. The hydrated
liposomes were placed in an eggplant flask and sonicated using a
bath-type sonicator (Bransonic® 2510J-MTH, 42 kHz, 100
W, Branson UL Trasonics Co.) for 5–10 min at room temperature.
To prepare cationic liposome/siRNA complexes (siRNA
lipoplexes), each liposome preparation was added to 50 pmol siRNA
at a charge ratio (+:-) of 7:1 for cationic liposomes composed of
cationic cholesterol derivatives and DOPE (14,16)
or 4:1 for cationic liposomes composed of dialkyl or trialkyl
cationic lipids and DOPE (16,17)
with vortex-mixing for 10 sec and left at room temperature for 15
min. The charge ratio (+:-) of liposomes:siRNA is expressed as the
molar ratio of cationic lipid to siRNA phosphate.
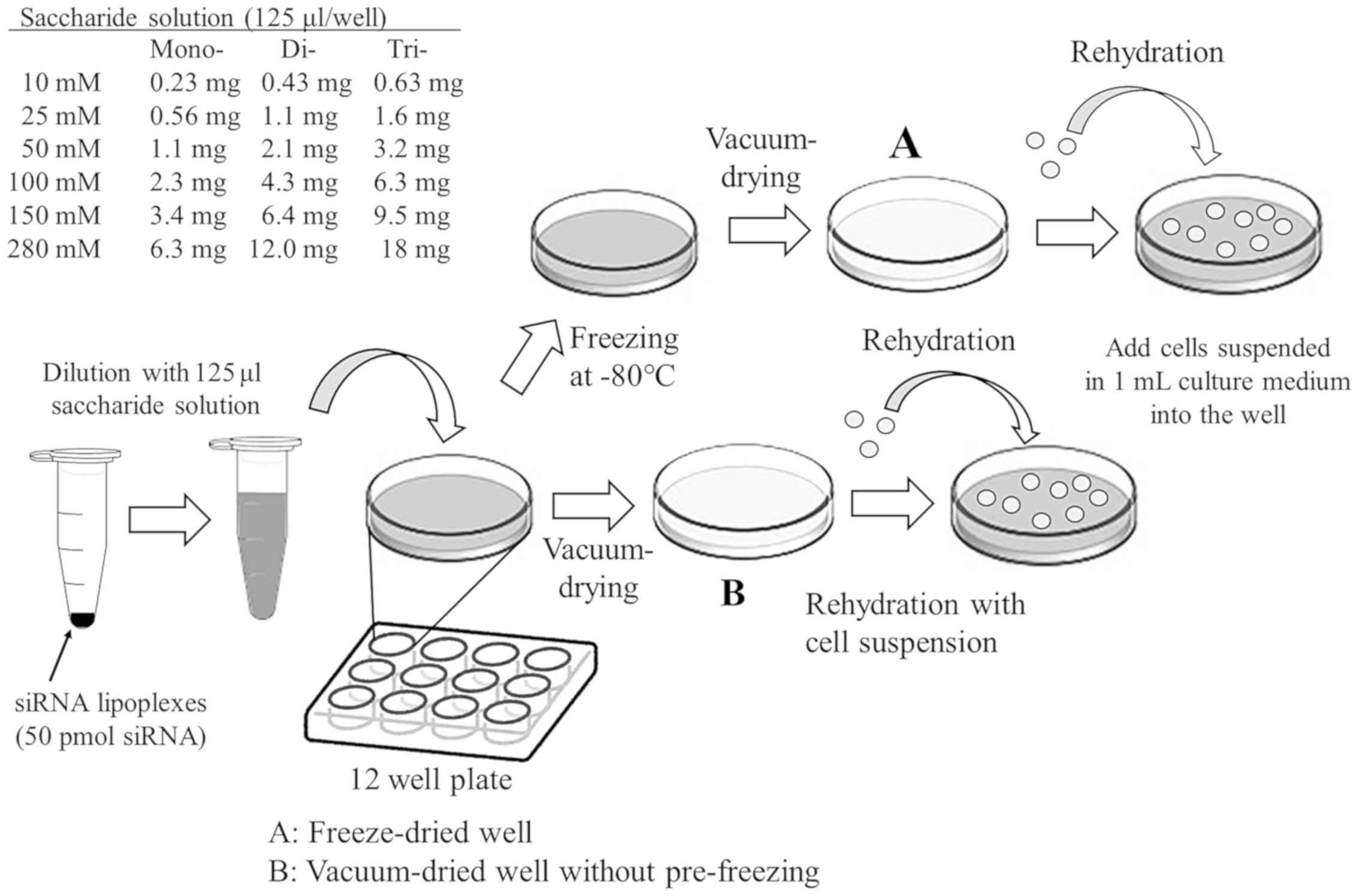
Appearance of cakes after
freeze-drying or vacuum-drying of saccharide solution
For comparison of appearance of cakes (dry powder)
after freeze-drying and vacuum-drying of saccharide solution in
12-well plates, 125 µl of 10, 25, 50, 100, or 150 mM (0.34, 0.86,
1.71, 3.42 or 5.13% (w/v), respectively) trehalose or sucrose
solutions were sterilized using a 0.45 µm filter, and then
transferred into a 12-well plate. In preparation for the
freeze-dried saccharides, the plate was frozen at −80°C, and then
dried in a high vacuum (10–20 Pa) using a freeze-dryer [(FDU-540,
Tokyo Rikakikai Co. (EYELA)], equipped with dry chamber (DRC-2L,
EYELA). In the preparation of vacuum-dried saccharides, the plate
was dried in a high vacuum (10–20 Pa) without pre-freezing.
For comparison of cake volume between freeze-dried
saccharides in 5 ml vial, 5 ml of 25 and 100 mM glucose, fructose,
galactose, mannose, sucrose, trehalose, maltose, lactose,
lactulose, cellobiose, raffinose, or melezitose solution were
transferred into 5 ml vial. The vials were frozen at −80°C and then
dried in a high vacuum (10–20 Pa).
Size and ζ-potential of reconstituted
siRNA lipoplexes
For measurement of the size and ζ-potential of siRNA
lipoplexes, siRNA lipoplexes were formed by the addition of
cationic liposomes to 5 μg Cont siRNA with vortex-mixing for 10 sec
and left at room temperature for 15 min. In the preparation of
vacuum-dried siRNA lipoplexes, each lipoplex was diluted in 933 µl
of 10, 50 or 100 mM trehalose or sucrose solution sterilized using
a 0.45 µm filter [125 µl saccharide solution per 50 pmol (0.67 µg)
siRNA], and then transferred to a 6-well plate. The plates were
dried in a high vacuum. In the preparation of freeze-dried siRNA
lipoplexes, each lipoplex with 5 µg Cont siRNA was diluted in 933
µl of 25 or 100 mM mono-, di-, or trisaccharide solution sterilized
using a 0.45 µm filter, and then transferred to a 6-well plate,
followed by freezing at −80°C. The frozen plates were dried in a
high vacuum.
Vacuum-dried and freeze-dried siRNA lipoplexes were
reconstituted with an appropriate volume (~1 ml) with water, and
the particle size distributions (cumulant average particle size) of
siRNA lipoplexes were measured by the cumulant method using a
light-scattering photometer (ELS-Z2, Otsuka Electronics Co., Ltd.)
at 25°C. The ζ-potentials were measured using an electrophoresis
light-scattering method with ELS-Z2 at 25°C.
Cell culture
Human breast cancer MCF-7 cells stably expressing
firefly luciferase (MCF-7-Luc) which constructed by
transfection of plasmid pcDNA3 containing the firefly
luciferase (hLuc) gene from plasmid psiCHECK2 (Promega Corp.) were
donated by Dr Kenji Yamato (University of Tsukuba, Tsukuba, Japan).
MCF-7-Luc cells were grown in RPMI-1640 medium, supplemented with
10% heat-inactivated fetal bovine serum (FBS) and 1.2 mg/ml G418 at
37°C in a 5% CO2 humidified atmosphere.
Effect of pre-freezing of siRNA
lipoplexes before vacuum-drying on gene-silencing by
Rev-transfection
For the preparation for Rev-transfection with
vacuum-dried siRNA lipoplexes, siRNA lipoplexes were formed by the
addition of cationic liposomes to 50 pmol (0.67 µg) Cont siRNA or
Luc siRNA by vortex-mixing for 10 sec and left at room temperature
for 15 min. Each lipoplex was diluted in 125 µl of 10, 25, 50, 100,
150, or 280 mM [0.34, 0.86, 1.71, 3.42, 5.13, or 9.52% (w/v),
respectively] trehalose or sucrose solution sterilized using a 0.45
µm filter, and then transferred into 12-well plates (50 pmol
siRNA/well). These plates were dried in a high vacuum and then
stored at room temperature in a desiccator until use.
For Rev-transfection with vacuum-dried siRNA
lipoplexes on 12-well plate, 1×105 MCF-7-Luc cells were
suspended in 1 ml of medium supplemented with 10% FBS, and then the
suspension was added to the well (final 50 nM siRNA concentration).
The molarity of the medium after rehydration (final 1.25, 3.125,
6.25, 12.5, 18.75, or 35 mM [0.04, 011, 0.21, 0.43, 0.64, and 1.12%
(w/v), respectively] trehalose or sucrose in medium) was maintained
at ~286–320 mOsm, which was approximately equivalent to the
physiological molarity. Forty-eight hours after transfection, the
cells were lysed by the addition of 125 µl of cell lysis buffer
(Pierce™ Luciferase cell lysis buffer, Thermo Fisher
Scientific Inc.) after washing with PBS, and subjected to one cycle
of freezing (−80°C) and thawing at 37°C, followed by centrifugation
at 15,000 g for 10 sec. Aliquots of 10 µl of the supernatants of
cell lysates were mixed with 50 µl of PicaGene MelioraStar-LT
Luminescence Reagent (Toyo Ink Mfg. Co., Ltd.), and the
luminescence was measured as counts per sec (cps) using a
chemoluminometer (ARVO X2, Perkin Elmer). The protein concentration
of the supernatants was determined using BCA reagent (Thermo Fisher
Scientific Inc.), with bovine serum albumin as a standard, and the
luciferase activity (cps/µg protein) was calculated. Luciferase
activity (%) was calculated as relative to the luciferase activity
(cps/µg protein) of untransfected cells.
Effect of saccharide types in
freeze-drying of siRNA lipoplexes on gene-silencing by
Rev-transfection
For the preparation for Rev-transfection with
freeze-dried siRNA lipoplexes, siRNA lipoplexes were formed by the
addition of cationic liposomes to 50 pmol Cont siRNA or Luc siRNA
by vortex-mixing for 10 sec and left at room temperature for 15
min. Each lipoplex was diluted in 125 µl of various concentrations
of mono-, di-, or trisaccharide solution sterilized using a 0.45-µm
filter, and then transferred to a 12-well plate (50 pmol
siRNA/well), followed by freezing at −80°C. The frozen plates were
dried in a high vacuum and then stored at room temperature in a
desiccator until use.
For Rev-transfection with freeze-dried siRNA
lipoplexes, 1×105 MCF-7-Luc cells were suspended in 1 ml
of medium supplemented with 10% FBS, and then the suspension was
added to the well (final 50 nM siRNA concentration). Forty-eight
hours after transfection, luciferase activity was measured as
described above.
Cytotoxicity of Rev-transfection with
freeze-dried siRNA lipoplexes
Each lipoplex with 5 pmol Cont siRNA was diluted in
12.5 µl of various concentrations of mono-, di-, or trisaccharide
solution, and then the mixture was transferred to the wells of
96-well plates (5 pmol siRNA/well). After freezing at −80°C, the
plates were dried under high vacuum. MCF-7-Luc cells
(1×104) were suspended in 100 µl of medium supplemented
with 10% FBS and then the suspension was added to the well (final
50 nM siRNA concentration). After a 24-h incubation period, cell
numbers were determined using a Cell Counting Kit-8. Cell viability
was expressed as relative to the absorbance at 450 nm of cells
added into wells without freeze-dried lipoplexes.
Statistical analysis
Data are presented as the mean ± standard deviation
of triple determinations. The statistical significance of
differences between mean values was determined by Student's t-test
using GraphPad Prism 4.0 (GraphPad Software, Inc.). Multiple
measurement comparisons were performed by analysis of variance
followed by one-way analysis of variance on ranks with post hoc
Tukey test using GraphPad Prism 4.0. Each experiment was repeated
≥3 times. P≤0.05 was considered to indicate a statistically
significant difference
Results and Discussion
Characterization of vacuum-dried siRNA
lipoplexes after reconstitution
Lyoprotectants such as sucrose and trehalose are
often used to improve the stability of liposomes or lipoplexes in
freeze-drying (18). Previously,
we demonstrated that regardless of the cationic lipid types used in
cationic liposomes, Rev-transfection with siRNA lipoplexes
freeze-dried in trehalose or sucrose solution could induce
efficient gene-silencing in the cells (12). The freeze-drying process of siRNA
lipoplexes consisted of freezing and primary and secondary drying
in a high vacuum, and the freezing process of siRNA lipoplexes
solution was performed before vacuum-drying. In this study, first,
to investigate whether the freezing process before vacuum-drying of
siRNA lipoplexes affected gene-silencing in the cells after
Rev-transfection, we prepared vacuum-dried siRNA lipoplexes without
pre-freezing process. Here, for the preparation of cationic
liposomes, we used OH-Chol, OH-C-Chol, and HAPC-Chol as cationic
cholesterol derivatives; DOTAP and DDAB as dialkyl cationic lipids;
and TC-1-12 as a trialkyl cationic lipid (Fig. 1). For cationic liposomes with
cationic cholesterol derivatives, LP-OH, LP-OH-C, and LP-HAPC were
prepared from OH-Chol/DOPE, OH-C-Chol/DOPE, and HAPC-Chol/DOPE,
respectively, at a molar ratio of 3:2. In contrast, for cationic
liposomes with dialkyl or trialkyl cationic lipids, LP-DOTAP,
LP-DDAB, and LP-TC-1-12 were prepared from DOTAP/DOPE, DDAB/DOPE,
and TC-1-12/DOPE, respectively, at a molar ratio of 1:1.
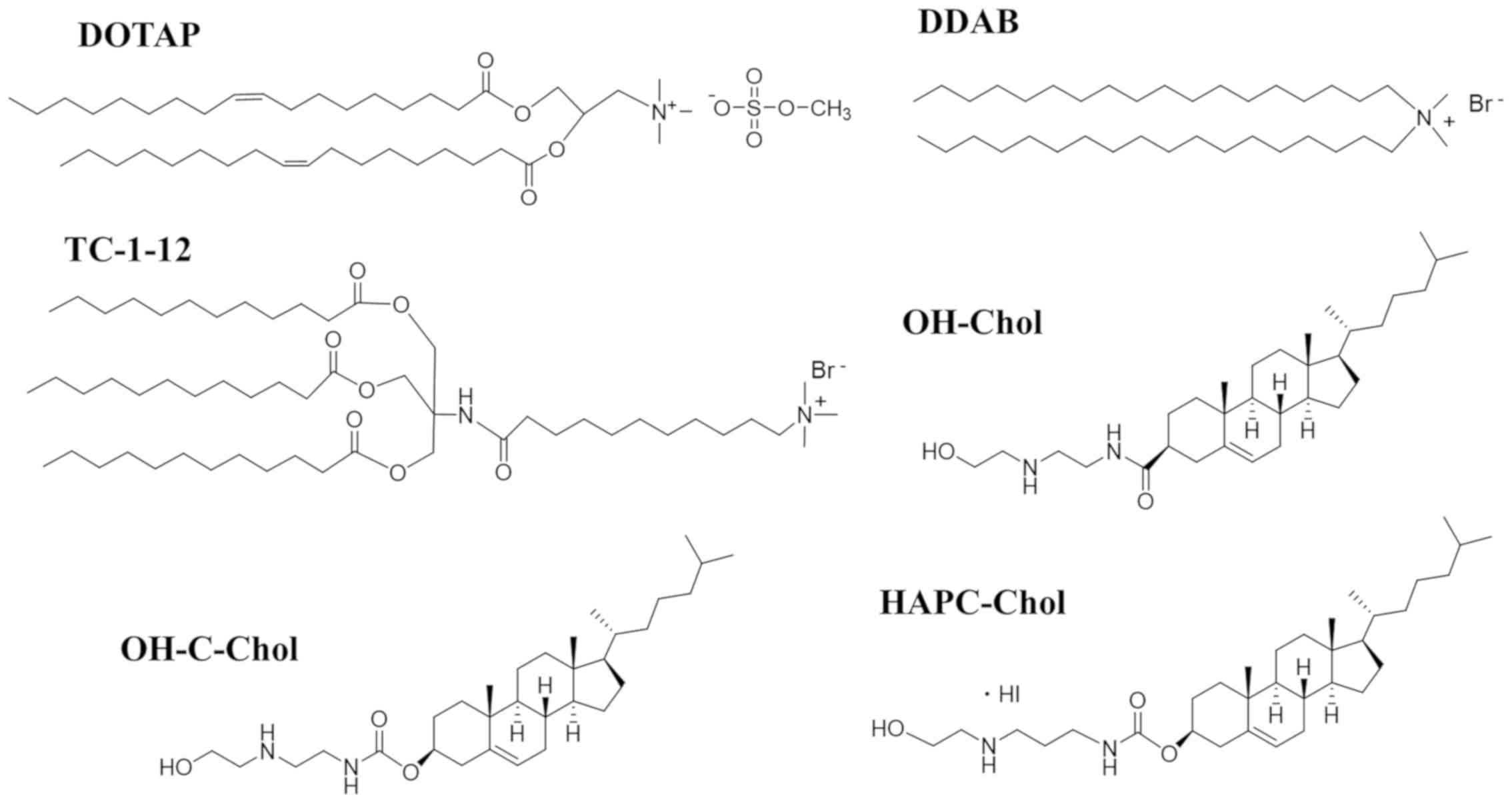 | Figure 1.Structure of cationic cholesterol
derivatives and cationic lipids with dialkyl or trialkyl chains.
OH-Chol,
N-(2-(2-hydroxyethylamino)ethyl)cholesteryl-3-carboxamide;
OH-C-Chol, cholesteryl (2-((2-hydroxyethyl)amino)ethyl)carbamate;
HAPC-Chol, cholesteryl (3-((2-hydroxyethyl)amino)propyl)carbamate
hydroiodide; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane methyl
sulfate salt; DDAB, dimethyldioctadecylammonium bromide; TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide. |
The sizes of the cationic liposomes prepared in this
study were approximately 80–100 nm with a monodisperse
distribution, and the ζ-potentials were approximately 41–56 mV
(12). In the preparation of siRNA
lipoplexes, we reported previously that the optimal charge ratios
(+:-) were 7:1 for cationic liposomes composed of cationic
cholesterol derivatives and 4:1 for cationic liposomes composed of
dialkyl or trialkyl cationic lipids (16,19);
therefore, in subsequent experiments, we used siRNA lipoplexes
formed at charge ratios (+:-) of 7:1 for LP-OH, LP-OH-C, and
LP-HAPC and 4:1 for LP-DOTAP, LP-DDAB, and LP-TC-1-12,
respectively. The sizes of siRNA lipoplexes were approximately
150–190 nm, and the ζ-potentials were approximately 34–47 mV
(12).
To examine whether vacuum-drying without
pre-freezing affected the size of siRNA lipoplexes after
rehydration, vacuum-dried siRNA lipoplexes were prepared in the
presence of 10, 50, or 100 mM trehalose or sucrose solution, and
measured the sizes of the siRNA lipoplexes after rehydration with
water (Table I). In vacuum-drying
in 10 mM disaccharide, siRNA lipoplexes exhibited a larger size
(approximately 260–570 nm), and in 50 mM disaccharide, they were
approximately 180–330 nm in size. However, siRNA lipoplexes were
approximately 170–240 nm in size (0.19-0.26 in PDI) when they were
vacuum-dried in 100 mM trehalose or sucrose solution. These results
indicated that the presence of 100 mM disaccharide during
vacuum-drying of siRNA lipoplexes did not greatly increase the size
of siRNA lipoplexes regardless of the cationic lipid types in
cationic liposomes.
 | Table I.Particle size and ζ-potential of small
interfering RNA lipoplexes after rehydration of vacuum-dried
lipoplexes. |
Table I.
Particle size and ζ-potential of small
interfering RNA lipoplexes after rehydration of vacuum-dried
lipoplexes.
| A, 10 mM
trehalose |
|---|
|
|---|
| Lipoplexc | Sizeb, nm | PDI |
ζ-potentiala, mV |
|---|
| LP-OH | 483.9±22.0 | 0.24±0.01 | 39.4±1.1 |
| LP-OH-C | 329.7±88.7 | 0.16±0.03 | 35.0±0.2 |
| LP-HAPC | 296.6±12.1 | 0.15±0.01 | 43.3±0.7 |
| LP-DOTAP | 477.1±29.8 | 0.21±0.01 | 50.3±1.0 |
| LP-DDAB | 573.2±25.8 | 0.25±0.01 | 56.2±1.5 |
| LP-TC-1-12 | 467.8±15.6 | 0.21±0.01 | 47.9±1.7 |
|
| B, 10 mM
sucrose |
|
|
Lipoplexc | Sizeb, nm | PDI |
ζ-potentiala, mV |
|
| LP-OH | 553.8±60.8 | 0.25±0.02 | 37.5±1.0 |
| LP-OH-C | 343.6±99.6 | 0.19±0.03 | 36.2±0.6 |
| LP-HAPC | 264.9± 9.9 | 0.20±0.04 | 37.8±1.0 |
| LP-DOTAP | 435.9± 6.1 | 0.20±0.01 | 38.9±0.9 |
| LP-DDAB | 545.6±94.1 | 0.26±0.02 | 47.2±0.5 |
| LP-TC-1-12 | 505.0±97.6 | 0.23±0.04 | 40.9±0.3 |
|
| C, 50 mM
trehalose |
|
|
Lipoplexc |
Sizeb,
nm | PDI |
ζ-potentiala, mV |
|
| LP-OH | 247.1±48.2 | 0.17±0.06 | 45.0±1.3 |
| LP-OH-C | 248.5±28.6 | 0.14±0.01 | 45.5±0.7 |
| L-HAPC | 184.3±6.2 | 0.26±0.00 | 40.6±0.7 |
| LP-DOTAP | 295.4±30.1 | 0.25±0.07 | 49.4±0.7 |
| LP-DDAB | 265.5±7.8 | 0.26±0.01 | 49.6±1.1 |
| LP-TC-1-12 | 327.8±14.8 | 0.16±0.00 | 55.4±2.5 |
|
| D, 50 mM
sucrose |
|
|
Lipoplexc |
Sizeb,
nm | PDI |
ζ-potentiala, mV |
|
| LP-OH | 297.9±37.7 | 0.15±0.02 | 37.4±1.1 |
| P-OH-C | 256.3±21.8 | 0.12±0.01 | 38.4±0.5 |
| LP-HAPC | 201.8±14.6 | 0.17±0.06 | 40.8±0.7 |
| LP-DOTAP | 207.0±3.6 | 0.26±0.01 | 46.2±1.2 |
| LP-DDAB | 308.8±18.0 | 0.15±0.01 | 45.6±1.0 |
| LP-TC-1-12 | 176.0±4.6 | 0.22±0.02 | 47.9±2.5 |
|
| E, 100 mM
trehalose |
|
|
Lipoplexc |
Sizeb,
nm | PDI |
ζ-potentiala, mV |
|
| LP-OH | 188.2±2.2 | 0.25±0.05 | 39.7±0.9 |
| LP-OH-C | 168.8±5.2 | 0.26±0.02 | 41.1±1.7 |
| LP-HAPC | 174.6±4.9 | 0.23±0.01 | 40.2±1.1 |
| LP-DOTAP | 231.7±6.9 | 0.24±0.01 | 38.6±1.2 |
| LP-DDAB | 240.5±9.9 | 0.26±0.02 | 44.5±1.4 |
| LP-TC-1-12 | 186.9±6.4 | 0.20±0.02 | 42.6±0.4 |
|
| F, 100 mM
sucrose |
|
|
Lipoplexc |
Sizeb,
nm | PDI |
ζ-potentiala, mV |
|
| LP-OH | 175.0±0.4 | 0.26±0.01 | 41.1±0.9 |
| LP-OH-C | 190.0±5.7 | 0.23±0.01 | 39.6±0.6 |
| LP-HAPC | 179.2±5.1 | 0.26±0.02 | 37.8±0.5 |
| LP-DOTAP | 240.2±8.2 | 0.25±0.01 | 38.7±1.7 |
| LP-DDAB | 230.6±2.7 | 0.26±0.02 | 49.5±0.2 |
| LP-TC-1-12 | 177.5±6.8 | 0.19±0.02 | 49.0±1.3 |
Effect of freezing before
vacuum-drying of siRNA lipoplexes on gene-silencing effect in the
cells by Rev-transfection
To examine the effect of pre-freezing before
vacuum-drying of siRNA lipoplexes on gene-silencing effect in the
cells after Rev-transfection, each of the lipoplexes was diluted
with solutions containing various concentrations of trehalose or
sucrose, and then the mixtures were added into the wells of a
12-well plate, followed by vacuum-drying without pre-freezing
(Fig. 2). In Rev-transfection with
vacuum-dried siRNA lipoplexes in 12-well plates, we added 125 µl of
disaccharide solution per well for vacuum-drying of the siRNA
lipoplexes. Because, in our previous study, siRNA lipoplexes
freeze-dried with 125 µl of 150 mM disaccharide solution did not
greatly induce the cytotoxic effect (12). The cakes after vacuum-drying of 125
µl of 10, 25, 50, 100, 150, and 280 mM disaccharide solution
contained 0.43, 1.1, 2.1, 4.3, 6.4, and 12.0 mg disaccharide per
well, respectively. With increasing concentrations of trehalose or
sucrose present during drying, larger cakes were observed on the
wells after freeze-drying or vacuum-drying, and the large
difference in appearance between the cakes after freeze-drying and
vacuum-drying was not observed (Fig.
S1).
After vacuum-drying of the siRNA lipoplexes, we
reconstituted siRNA lipoplexes with MCF-7-Luc cells suspended in
culture medium. As a result, LP-OH, LP-OH-C, LP-HAPC, LP-DOTAP,
LP-DDAB, and LP-TC-1-12 lipoplexes with Luc siRNA did not suppress
luciferase activity when their lipoplexes were vacuum-dried without
disaccharides (Figs. 3 and
4). However, increasing
concentrations of trehalose or sucrose present during vacuum-drying
were associated with increased gene-silencing activity. LP-OH and
LP-OH-C lipoplexes with Luc siRNA strongly suppressed luciferase
activity when their lipoplexes were vacuum-dried at above 25 mM
trehalose or sucrose; however, LP-HAPC, LP-DOTAP, LP-DDAB, and
LP-TC-1-12 lipoplexes with above 10 mM trehalose or sucrose showed
strong suppression of luciferase activity (Figs. 3 and 4). These results indicated that higher
concentration of disaccharide in vacuum-drying might be needed for
LP-OH and LP-OH-C lipoplexes to keep gene silencing activity
compared with other formulations. The largest difference in
gene-silencing activities of between vacuum-dried siRNA lipoplexes
(Figs. 3 and 4) and freeze-dried siRNA lipoplexes
(12) with trehalose or sucrose
was not observed in any of the cationic liposomes tested. From
these findings, in Rev-transfection, the freezing process before
vacuum-drying of siRNA lipoplexes did not affect the gene-silencing
activity of siRNA lipoplexes.
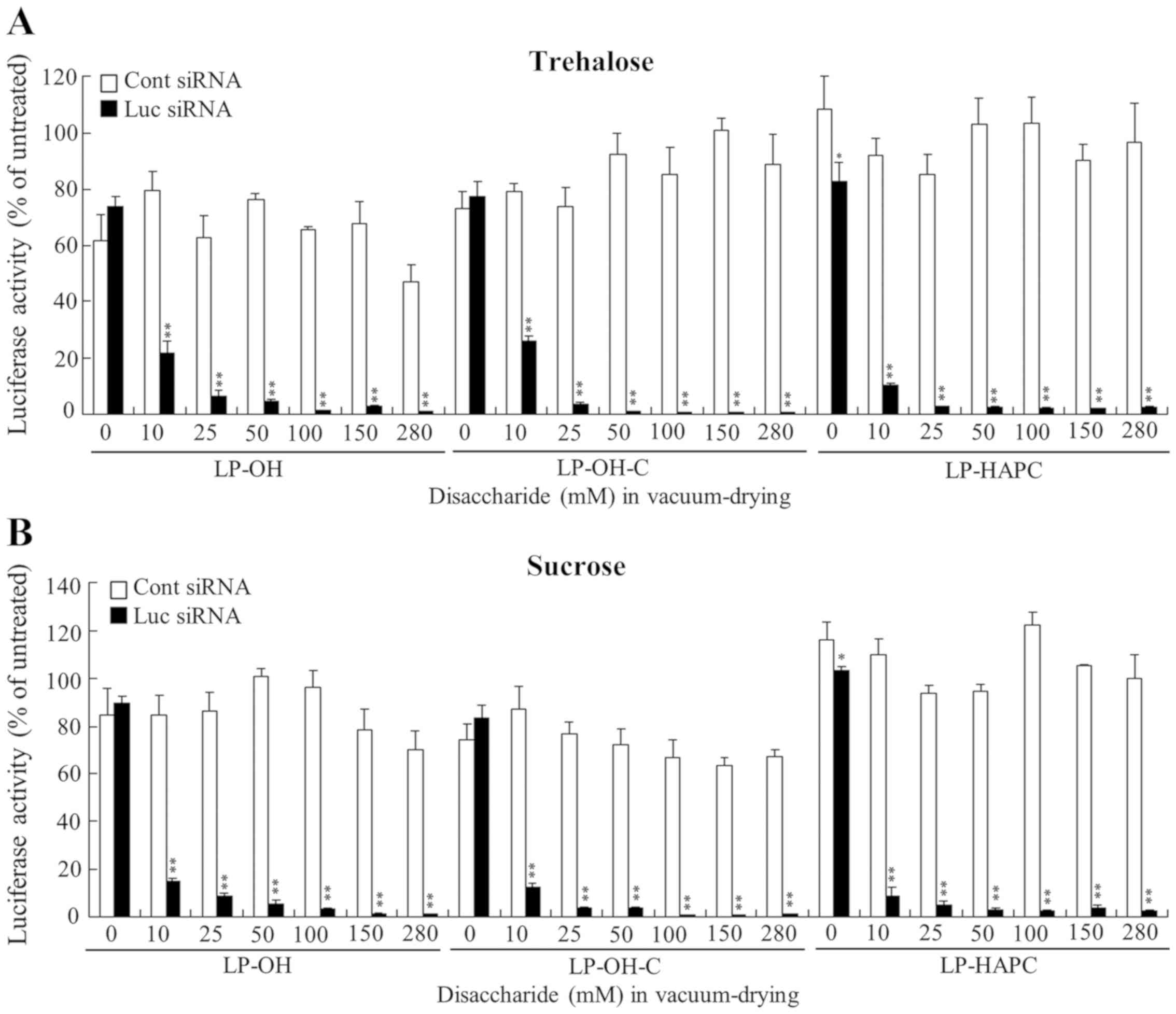 | Figure 3.Effect of trehalose and sucrose during
vacuum-drying of siRNA lipoplexes on the suppression of luciferase
expression in MCF-7-Luc cells after reverse transfection with siRNA
lipoplexes. LP-OH, LP-OH-C and LP-HAPC lipoplexes with 50 pmol Luc
siRNA or Cont siRNA were diluted in 125 µl of 10, 25, 50, 100, 150
or 280 mM (0.43, 1.1, 2.1, 4.3, 6.4 and 12.0 mg, respectively) (A)
trehalose or (B) sucrose and then transferred to 12-well plates,
followed by vacuum-drying. Data are presented as the mean ± SD
(n=3). *P<0.05 and **P<0.01 vs. Cont siRNA. siRNA, small
interfering RNA; Luc, luciferase; Cont, control; LP-OH, OH-Chol
liposome; LP-OH-C, OH-C-Chol liposome; LP-HAPC, HAPC-Chol
liposome. |
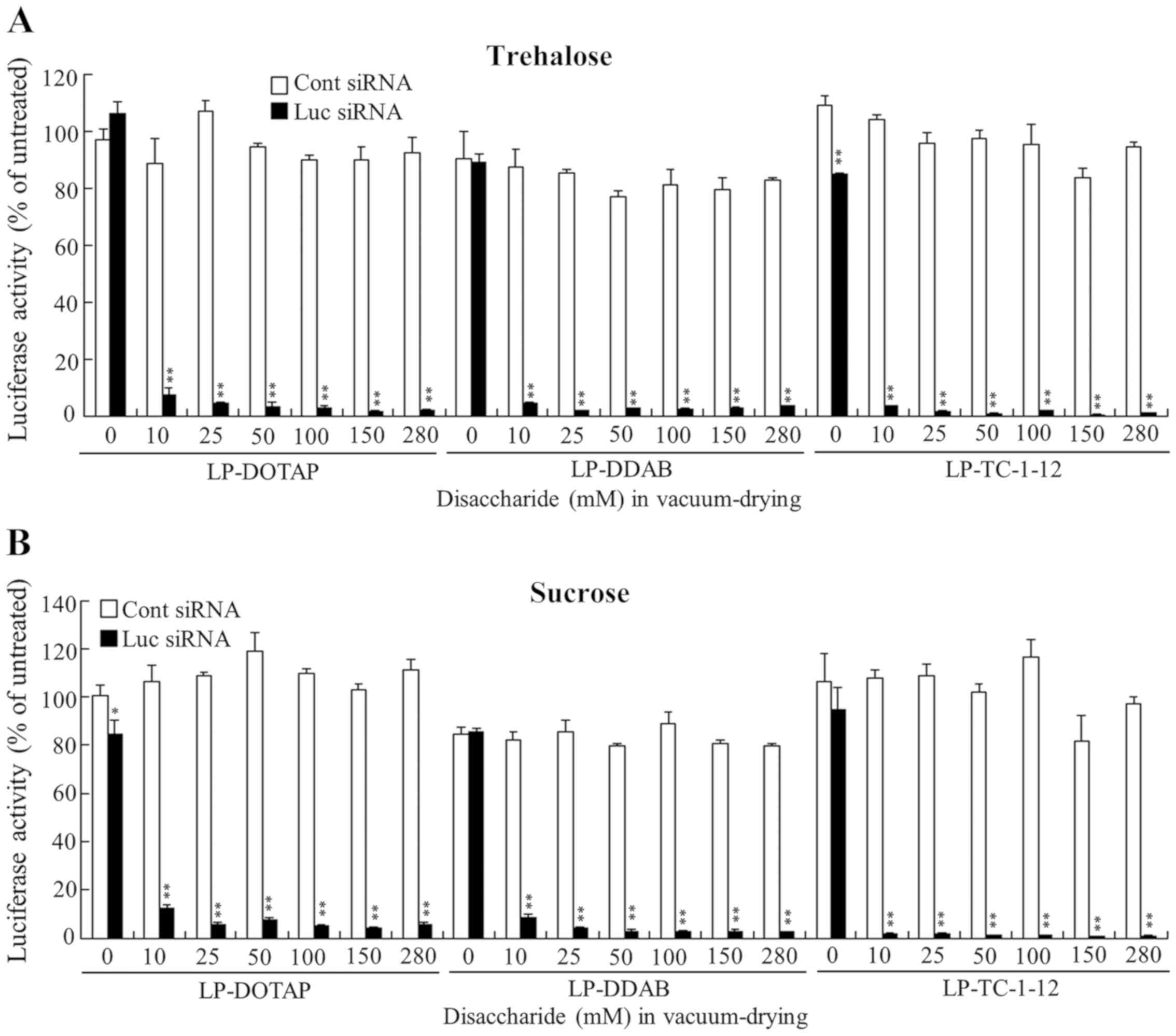 | Figure 4.Effect of trehalose and sucrose during
vacuum-drying of siRNA lipoplexes on the suppression of luciferase
expression in MCF-7-Luc cells after reverse transfection with siRNA
lipoplexes. LP-DOTAP, LP-DDAB and LP-TC-1-12 lipoplexes with 50
pmol Luc siRNA or Cont siRNA were diluted in 125 µl of 10, 25, 50,
100, 150, or 280 mM (0.43, 1.1, 2.1, 4.3, 6.4 and 12.0 mg,
respectively) (A) trehalose or (B) sucrose and then transferred to
12-well plates, followed by vacuum-drying. Data are presented as
the mean ± SD (n=3). *P<0.05 and **P<0.01 vs. Cont siRNA.
siRNA, small interfering RNA; Luc, luciferase; Cont, control;
LP-DOTAP, DOTAP liposome; LP-DDAB, DDAB liposome; LP-TC-1-12,
TC-1-12 liposome. |
Characterization of freeze-dried siRNA
lipoplexes after reconstitution
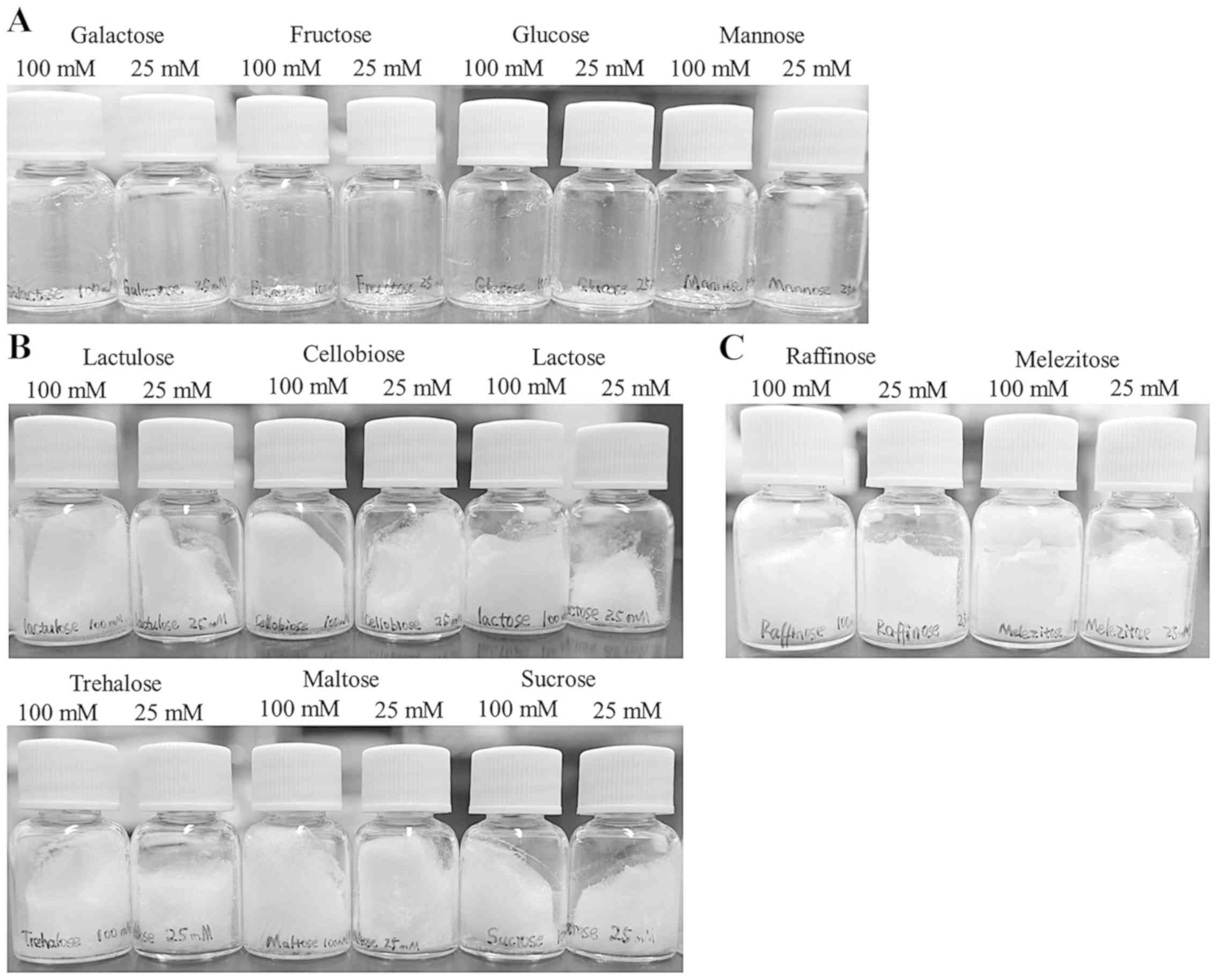
A variety of saccharides including glucose,
fructose, maltose, and lactose have also been shown to act as
cryoprotectants during dehydration/rehydration of liposomes and
nanoparticles (18,20). Yadava et al reported that
DOTAP/DOPE lipoplexes freeze-dried in the presence of 278 mM
glucose and lactose exhibited gene-silencing effects without loss
of transfection activity when they were rehydrated with water and
then transfected into cells (conventional transfection) (6). However, to the best of our knowledge,
in Rev-transfection with freeze-dried siRNA lipoplexes, there are
still few reports on the application of saccharides except
trehalose and sucrose as cryoprotectants. Therefore, we prepared
cakes of mono-, di-, and trisaccharides by freeze-drying, and
compared their appearance among saccharide types. Here, we used
four monosaccharides (glucose, fructose, galactose, or mannose),
six disaccharides (sucrose, trehalose, maltose, lactose, lactulose,
or cellobiose), and two trisaccharides (raffinose or melezitose)
for the preparation of freeze-dried saccharides. As a result, large
cakes were observed after freeze-drying of 25 or 100 mM di- and
trisaccharide solutions; however, insufficient cakes were observed
in freeze-drying of monosaccharide solutions (Fig. 5).
Next, to examine whether the saccharide types during
freeze-drying affected the size of siRNA lipoplexes after
rehydration, we prepared freeze-dried siRNA lipoplexes in the
presence of 25 or 100 mM mono-, di-, or trisaccharide solutions,
and measured the sizes of the siRNA lipoplexes after rehydration
(Table II). In subsequent
experiments, we decided to use LP-DDAB and LP-DOTAP for preparation
of freeze-dried siRNA lipoplexes. In addition, we used ten kind of
saccharides except the trehalose and sucrose for preparation of
freeze-dried siRNA lipoplexes, because we have already reported
that both trehalose and sucrose have been useful as a
cryoprotectant for Rev-transfection with freeze-dried siRNA
lipoplexes (12).
 | Table II.Particle size and ζ-potential of
siRNA lipoplexes after the rehydration of freeze-dried
lipoplexes. |
Table II.
Particle size and ζ-potential of
siRNA lipoplexes after the rehydration of freeze-dried
lipoplexes.
| A, LP-DOTAP
lipoplexesa |
|---|
|
|---|
| Saccharide, 25
mM | Sizeb, nm | PDI |
ζ-potentialb, mV |
|---|
| Glucose | 322.3±5.5 | 0.27±0.01 | 45.1±2.2 |
| Fructose | 321.3±13.9 | 0.28±0.02 | 47.6±1.0 |
| Galactose | 231.6±7.3 | 0.29±0.01 | 40.4±0.7 |
| Mannose | 312.0±44.3 | 0.22±0.05 | 43.0±0.7 |
| Maltose | 185.0±3.9 | 0.25±0.01 | 50.1±0.3 |
| Lactose | 182.4±6.5 | 0.26±0.01 | 37.2±0.7 |
| Lactulose | 195.4±2.5 | 0.26±0.02 | 41.0±0.8 |
| Cellobiose | 184.1±1.6 | 0.25±0.01 | 44.6±1.1 |
| Raffinose | 183.2±2.7 | 0.25±0.01 | 36.3±0.4 |
| Melezitose | 206.0±11.1 | 0.26±0.01 | 27.5±1.3 |
|
| B, LP-DDAB
lipoplexesa |
|
| Saccharide, 25
mM |
Sizeb,
nm | PDI |
ζ-potentialb, mV |
|
| Glucose | 459.3±56.2 | 0.21±0.02 | 46.6±0.9 |
| Fructose | 1,041.8±266.1 | 0.42±0.09 | 45.7±1.4 |
| Galactose | 648.0±21.9 | 0.29±0.01 | 47.6±1.6 |
| Mannose | 323.9±27.7 | 0.15±0.01 | 53.9±2.7 |
| Maltose | 215.9±23.4 | 0.19±0.06 | 45.2±1.3 |
| Lactose | 162.3±3.0 | 0.18±0.00 | 36.7±2.8 |
| Lactulose | 217.0±13.8 | 0.23±0.01 | 38.8±0.4 |
| Cellobiose | 173.3±3.8 | 0.22±0.02 | 44.6±1.2 |
| Raffinose | 174.7±1.3 | 0.22±0.03 | 46.9±2.2 |
| Melezitose | 247.8±34.4 | 0.20±0.06 | 36.4±1.6 |
|
| C, LP-DOTAP
lipoplexesa |
|
| Saccharide, 100
mM |
Sizeb,
nm | PDI |
ζ-potentialb, mV |
|
| Glucose | 173.5±3.6 | 0.24±0.01 | 47.8±3.5 |
| Fructose | 219.6±0.6 | 0.24±0.02 | 46.5±0.7 |
| Galactose | 187.6±4.9 | 0.24±0.01 | 50.5±1.1 |
| Mannose | 219.6±0.6 | 0.24±0.02 | 46.5±0.7 |
| Maltose | 182.2±7.7 | 0.19±0.01 | 30.6±0.6 |
| Lactose | 187.3±1.3 | 0.14±0.01 | 30.9±0.6 |
| Lactulose | 188.8±6.4 | 0.14±0.01 | 46.3±0.7 |
| Cellobiose | 169.9±1.9 | 0.21±0.02 | 42.8±0.7 |
| Raffinose | 207.7±8.5 | 0.24±0.00 | 40.8±0.8 |
| Melezitose | 185.0±4.7 | 0.18±0.01 | 37.2±0.9 |
|
| D, LP-DDAB
lipoplexesa |
|
| Saccharide, 100
mM |
Sizeb,
nm | PDI |
ζ-potentialb, mV |
|
| Glucose | 171.9±4.1 | 0.23±0.01 | 43.8±1.9 |
| Fructose | 232.2±30.4 | 0.22±0.09 | 40.9±1.6 |
| Galactose | 235.6±38.3 | 0.20±0.05 | 43.3±2.3 |
| Mannose | 193.0±8.6 | 0.25±0.04 | 36.1±1.6 |
| Maltose | 193.1±0.8 | 0.22±0.00 | 43.5±2.2 |
| Lactose | 188.9±2.7 | 0.20±0.01 | 35.8±0.3 |
| Lactulose | 196.7±8.5 | 0.23±0.04 | 44.8±1.3 |
| Cellobiose | 179.8±3.2 | 0.24±0.00 | 40.1±0.4 |
| Raffinose | 162.5±0.8 | 0.18±0.01 | 44.1±1.5 |
| Melezitose | 197.7±1.7 | 0.21±0.01 | 15.3±1.0 |
In freeze-drying in 25 mM monosaccharide solution,
LP-DOTAP, and LP-DDAB lipoplexes exhibited larger in size
(~230–1000 nm) than those (160–250 nm) in 25 mM di- and
trisaccharide solutions. However, siRNA lipoplexes were
approximately 160–240 nm in size (0.14-0.25 in PDI) when they were
freeze-dried in 100 mM saccharide solution regardless of the
saccharide type. These results indicated that the presence of 100
mM saccharide during freeze-drying did not greatly increase the
size of siRNA lipoplexes.
Effect of saccharide types in
freeze-drying of siRNA lipoplexes on gene-silencing effects in the
cells by Rev-transfection
To examine whether the saccharide types during
freeze-drying affected the gene-silencing effects by
Rev-transfection with siRNA lipoplexes, freeze-dried siRNA
lipoplexes were reconstituted with MCF-7-Luc cells suspended in
culture medium. LP-DDAB, and LP-DOTAP lipoplexes with Luc siRNA did
not suppress luciferase activity when their lipoplexes were
freeze-dried without mono-, di-, or trisaccharides (Figs. 6–8). However, increasing concentrations of
saccharides present during freeze-drying were associated with
increased gene-silencing activity regardless of the saccharide
types, and those with above 25 mM saccharide showed strong
suppression of luciferase activity (>80% knockdown, compared
with untreated cells) (Figs.
6–8). However, in
Rev-transfection with LP-DDAB lipoplexes of Cont siRNA,
freeze-drying in glucose, galactose, or mannose induced
non-specific gene-silencing (Fig.
6B), indicating that the off-targeted effect induced by
Rev-transfection with siRNA lipoplexes may be dependent on the
combination of cationic liposomes and saccharide.
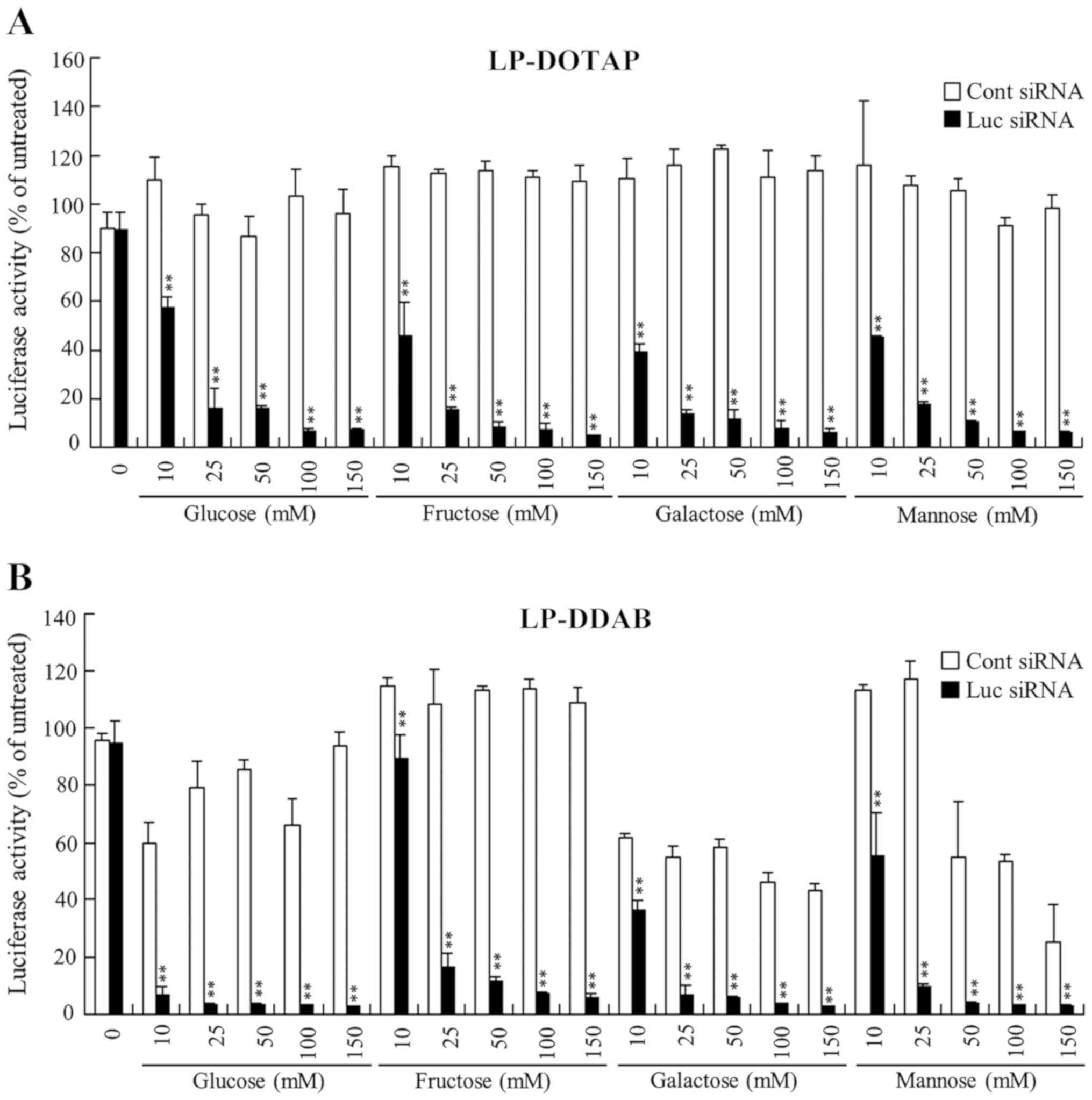 | Figure 6.Effect of monosaccharides during the
freeze-drying of siRNA lipoplexes on the suppression of luciferase
expression in MCF-7-Luc cells after reverse transfection. (A)
LP-DOTAP and (B) LP-DDAB lipoplexes with 50 pmol Luc siRNA or Cont
siRNA were diluted in 125 µl of 10, 25, 50, 100, or 150 mM glucose,
fructose, galactose or mannose solution and then transferred to
12-well plates, followed by freeze-drying. MCF-7-Luc cells
suspended in culture medium (1 ml) were added to the well and
luciferase assays were performed after incubation for 48 h at 37°C.
Data are presented as the mean ± SD (n=3). **P<0.01 vs. Cont
siRNA. siRNA, small interfering RNA; Luc, luciferase; Cont,
control; LP-DOTAP, DOTAP liposome; LP-DDAB, DDAB liposome. |
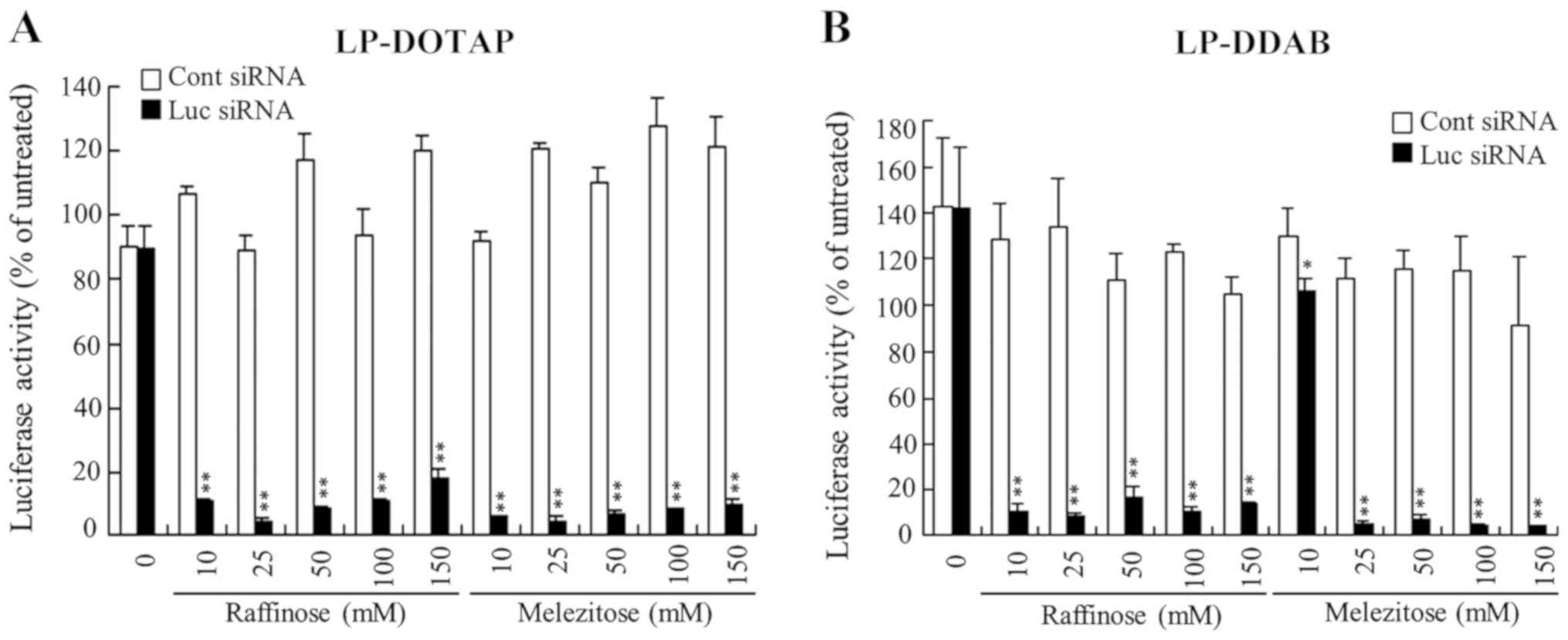 | Figure 8.Effect of trisaccharide during the
freeze-drying of siRNA lipoplexes on the suppression of luciferase
expression in MCF-7-Luc cells after reverse transfection. (A)
LP-DOTAP and (B) LP-DDAB lipoplexes with 50 pmol Luc siRNA or Cont
siRNA were diluted in 125 µl of 10, 25, 50, 100, or 150 mM
raffinose or melezitose solution and then transferred to 12-well
plates, followed by freeze-drying. MCF-7-Luc cells suspended in
culture medium (1 ml) were added to the well, and luciferase assays
were performed after incubation for 48 h at 37°C. Data are
presented as the mean ± SD (n=3). *P<0.05 and **P<0.01 vs.
Cont siRNA. siRNA, small interfering RNA; Luc, luciferase; Cont,
control; LP-DOTAP, DOTAP liposome; LP-DDAB, DDAB liposome. |
Cytotoxicity by Rev-transfections with
freeze-dried siRNA lipoplexes
To examine cytotoxicity by Rev-transfection with
freeze-dried siRNA lipoplexes, we measured cell viabilities at 24 h
after Rev-transfections with siRNA lipoplexes into MCF-7 cells. For
all the siRNA lipoplexes tested, Rev-transfection did not show high
cytotoxicity when the siRNA lipoplexes were freeze-dried with
disaccharides (Fig. 9A and B);
however, with an increase in the concentration of monosaccharide in
freeze-drying, the cytotoxicity after Rev-transfection was
increased (Fig. 9A and B). In
addition, the presence of trisaccharide in freeze-drying of LP-DDAB
lipoplexes also exhibited moderate cytotoxicity after
Rev-transfection (Fig. 9). We
speculated that insufficient cakes after freeze-drying of
monosaccharide solutions (Fig. 5)
might affect cell viability by Rev-transfection. However, the
mechanism why the presence of monosaccharide or trisaccharide
during freeze-drying induced cytotoxicity for the cells was not
clear. We reported previously that LP-DOTAP and LP-DDAB lipoplexes
freeze-dried in 100 mM trehalose or sucrose exhibited minimal
toxicity (~70–80% cell viability) (12). These findings suggested that
disaccharides may be suitable as a cyroprotectant during the
freeze-drying of siRNA lipoplexes for Rev-transfection.
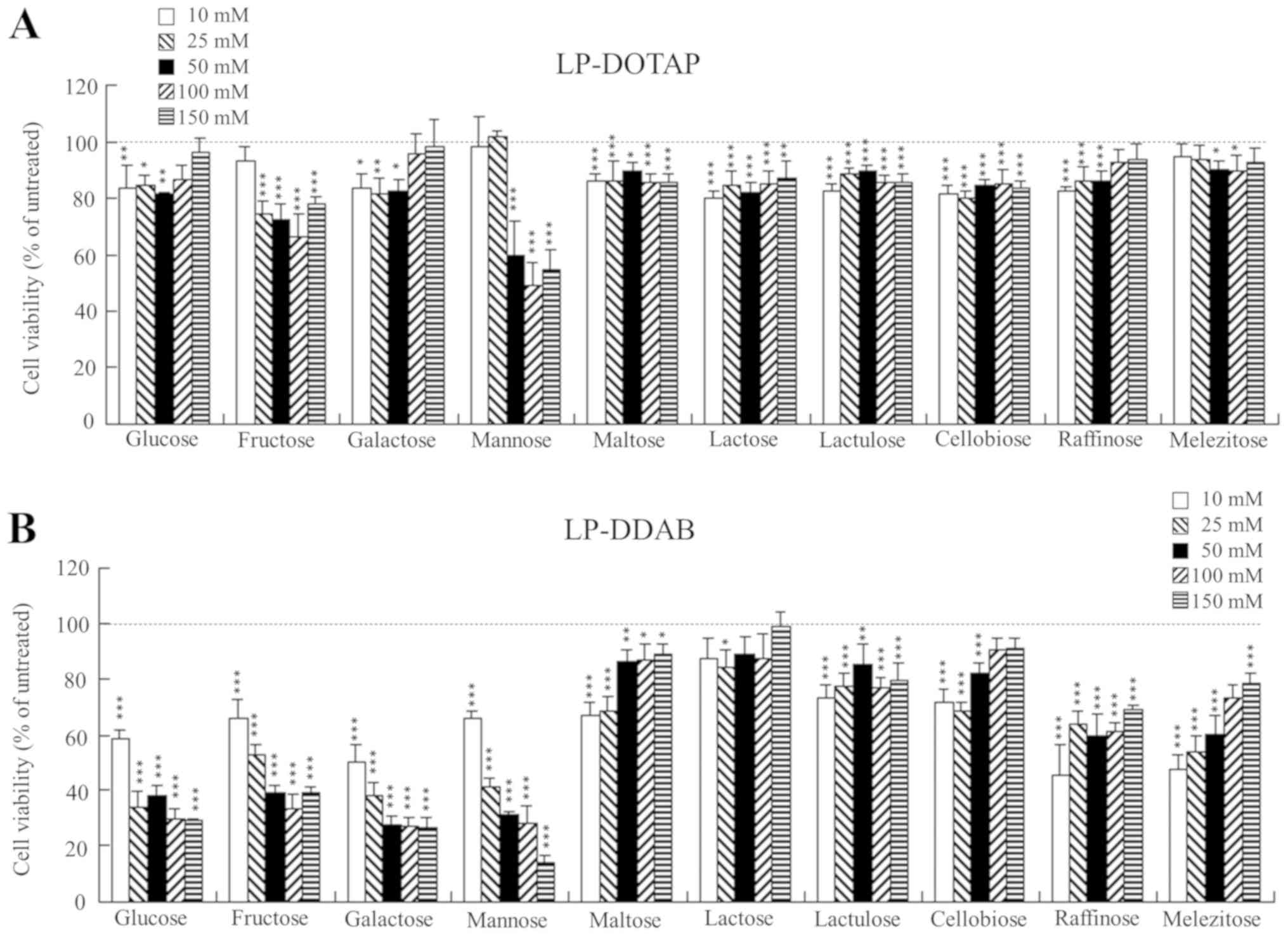 | Figure 9.MCF-7-Luc cell viability 24 h after
reverse transfection with freeze-dried siRNA lipoplexes. (A)
LP-DOTAP and (B) LP-DDAB lipoplexes of 5 pmol Cont siRNA were
diluted in 12.5 µl of 10, 25, 50, 100, or 150 mM mono-, di- or
trisaccharide solution and then transferred to 96-well plates,
followed by freeze-drying. MCF-7-Luc cells suspended in culture
medium (100 µl) were added to the plate (final, 50 nM siRNA). After
a 24-h incubation period at 37°C, cell viabilities were measured
using Cell Counting Kit-8, and were expressed as relative to that
of untransfected cells. Data are presented as the mean ± SD (n=3).
Luc, luciferase; siRNA, small interfering RNA; Cont, control.
*P<0.05, **P<0.01 and ***P<0.001 vs. untransfected cells.
LP-DOTAP, DOTAP liposome; LP-DDAB, DDAB liposome. |
In Rev-transfection with polyethylenimine
(PEI)/plasmid DNA (pDNA) complexes (polyplexes), the presence of
low concentrations of sucrose [<1% (29.2 mM)] during
freeze-drying did not have a notable influence to pDNA transfection
efficacy whereas higher concentration [>5% (146 mM)] reduced the
transfection efficiency (21). In
contrast, in our study, regardless of the saccharide types used in
the preparation of freeze-dried siRNA lipoplexes, the presence of
25–150 mM saccharides during the freeze-drying of siRNA lipoplexes
exhibited efficient gene-silencing, indicating that
Rev-transfection using cationic liposomes might not be notably
affected by concentration of saccharide, compared with that using
cationic polymer. However, DDAB lipoplexes freeze-dried with mono-
and trisaccharides induced off-targeted effects (Fig. 6B) or moderate toxicity (Fig. 9B) although they exhibited efficient
gene-silencing effects. Therefore, for Rev-transfection with
freeze-dried siRNA lipoplexes, it will be necessary to find optimal
saccharides that can induce efficient gene-silencing without
off-target effects and cytotoxicity. Here, we found that the
presence of maltose, lactose, lactulose, and cellobiose during the
freeze-drying of siRNA lipoplexes could induce gene-silencing
activity without cytotoxicity by Rev-transfection as well as
sucrose and trehalose. This study provides valuable information
about the Rev-transfection with freeze-dried or vacuum-dried siRNA
lipoplexes for efficient siRNA delivery into the cells.
In this study, we evaluated the effects of freezing
before vacuum-drying of siRNA lipoplexes on gene-silencing by
Rev-transfection, and found that freezing process did not affect
the gene-silencing activity by siRNA lipoplexes. In addition,
regardless of the type of mono-, di-, and trisaccharides in
freeze-drying of siRNA lipoplexes, Rev-transfection can induce
efficient gene-silencing; however, disaccharides exhibited higher
cell viability after Rev-transfection compared with mono- and
trisaccharides. These findings suggested that use of disaccharides
will be suitable for the preparation of freeze-dried or
vacuum-dried siRNA lipoplexes in Rev-transfection.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Mr. Reo Yamagishi
and Mr. Shun Fujishita (Department of Molecular Pharmaceutics,
Hoshi University) for assistance with the experimental work
assessing the in vitro gene-silencing effect.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH conceived and designed the study. Experiments
were performed by MT and SH. YH wrote the manuscript. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wilson RC and Doudna JA: Molecular
mechanisms of RNA interference. Annu Rev Biophys. 42:3233–239.
2013. View Article : Google Scholar
|
|
2
|
Erfle H, Neumann B, Liebel U, Rogers P,
Held M, Walter T, Ellenberg J and Pepperkok R: Reverse transfection
on cell arrays for high content screening microscopy. Nat Protoc.
2:392–399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang S, Zhi D and Huang L: Lipid-based
vectors for siRNA delivery. J Drug Target. 20:724–735. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen C, Han D, Cai C and Tang X: An
overview of liposome lyophilization and its future potential. J
Control Release. 142:299–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Franzé S, Selmin F, Samaritani E,
Minghetti P and Cilurzo F: Lyophilization of liposomal
formulations: Still necessary, still challenging. Pharmaceutics.
10:1392018. View Article : Google Scholar
|
|
6
|
Yadava P, Gibbs M, Castro C and Hughes JA:
Effect of lyophilization and freeze-thawing on the stability of
siRNA-liposome complexes. AAPS PharmSciTech. 9:335–341. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andersen MØ, Howard KA, Paludan SR,
Besenbacher F and Kjems J: Delivery of siRNA from lyophilized
polymeric surfaces. Biomaterials. 29:506–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kundu AK, Chandra PK, Hazari S, Ledet G,
Pramar YV, Dash S and Mandal TK: Stability of lyophilized siRNA
nanosome formulations. Int J Pharm. 423:525–534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ball RL, Bajaj P and Whitehead KA:
Achieving long-term stability of lipid nanoparticles: Examining the
effect of pH, temperature, and lyophilization. Int J Nanomedicine.
12:305–315. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujita S, Ota E, Sasaki C, Takano K,
Miyake M and Miyake J: Highly efficient reverse transfection with
siRNA in multiple wells of microtiter plates. J Biosci Bioeng.
104:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu K, Xu J, Liu M, Song W, Yan J, Gao S,
Zhao L and Zhang Y: Induction of osteogenic differentiation of stem
cells via a lyophilized microRNA reverse transfection formulation
on a tissue culture plate. Int J Nanomedicine. 8:1595–1607.
2013.PubMed/NCBI
|
|
12
|
Hattori Y, Hu S and Onishi H: Effects of
cationic lipids in cationic liposomes and disaccharides in the
freeze-drying of siRNA lipoplexes on gene silencing in cells by
reverse transfection. J Liposome Res (In press).
|
|
13
|
Ding W, Hattori Y, Higashiyama K and
Maitani Y: Hydroxyethylated cationic cholesterol derivatives in
liposome vectors promote gene expression in the lung. Int J Pharm.
354:196–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hattori Y, Hara E, Shingu Y, Minamiguchi
D, Nakamura A, Arai S, Ohno H, Kawano K, Fujii N and Yonemochi E:
siRNA delivery into tumor cells by cationic cholesterol
derivative-based nanoparticles and liposomes. Biol Pharm Bull.
38:30–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hattori Y, Nakamura T, Ohno H, Fujii N and
Maitani Y: siRNA delivery into tumor cells by lipid-based
nanoparticles composed of hydroxyethylated cholesteryl triamine.
Int J Pharm. 443:221–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hattori Y, Nakamura M, Takeuchi N, Tamaki
K, Shimizu S, Yoshiike Y, Taguchi M, Ohno H, Ozaki KI and Onishi H:
Effect of cationic lipid in cationic liposomes on siRNA delivery
into the lung by intravenous injection of cationic lipoplex. J Drug
Target. 27:217–227. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hattori Y, Nakamura A, Arai S, Kawano K,
Maitani Y and Yonemochi E: siRNA delivery to lung-metastasized
tumor by systemic injection with cationic liposomes. J Liposome
Res. 25:279–286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abdelwahed W, Degobert G, Stainmesse S and
Fessi H: Freeze-drying of nanoparticles: Formulation, process and
storage considerations. Adv Drug Deliv Rev. 58:1688–1713. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hattori Y, Nakamura M, Takeuchi N, Tamaki
K, Ozaki KI and Onishi H: Effect of cationic lipid type in
PEGylated liposomes on siRNA delivery following the intravenous
injection of siRNA lipoplexes. World Acad Sci J. 1:74–85. 2019.
|
|
20
|
Crowe LM, Crowe JH, Rudolph A, Womersley C
and Appel L: Preservation of freeze-dried liposomes by trehalose.
Arch Biochem Biophys. 242:240–247. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reinisalo M, Urtti A and Honkakoski P:
Freeze-drying of cationic polymer DNA complexes enables their
long-term storage and reverse transfection of post-mitotic cells. J
Control Release. 110:437–443. 2006. View Article : Google Scholar : PubMed/NCBI
|