Introduction
Ischemic reperfusion (I/R) commonly occurs after
shock therapy, artery bypass and cardiopulmonary cerebral
resuscitation (1–3). The functions of tissues and organs
normally recover after I/R; however, I/R injury may aggravate and
cause severe complications, including stroke (4). The outcome of myocardial surgery I/R
is closely associated with the occurrence of myocardial ischemic
reperfusion injury (MIRI). Previous studies have reported that
oxygen free radical injury (5),
energy metabolism disorder in cardiomyocytes (6), calcium overload (7), apoptosis of cardiomyocytes and other
inflammatory diseases (8–10) serve important roles in MIRI. During
reperfusion, a large amount of inflammatory factors is produced to
promote tissue infiltration of inflammatory cells, thus affecting
cell structures and functions (11). Hypoxia/reoxygenation (H/R) in cells
is a major characteristic of I/R, and is frequently used to
simulate the activity of I/R (12,13).
Low cell viability, high apoptosis and inflammatory responses occur
after cell injury induced by H/R (14).
Dexmedetomidine (Dex) is a new α2-adrenergic
receptor agonist with high selectivity that and exerts analgesic
(15), anti-stress (16) and anti-inflammatory (17) effects. Chen et al (18) have demonstrated that Dex exerts
protective effects on postoperative cognitive dysfunction by
downregulating the expression levels of inflammatory factors
interleukin (IL)-1β, tumor necrosis factor (TNF)-α and NF-κB in
rats. Wang et al (19) have
investigated the mechanism of lidocaine-induced cytotoxicity and
reported that the anti-inflammatory effect of Dex may be achieved
by suppressing the mitogen-activated protein kinase (MAPK)
signaling pathway to affect the expression of caspase-3 and Bcl-2
and reduce apoptosis. Additionally, Dex has been reported to reduce
the expression of macrophage inflammatory protein-2 and other
cytokines (TNF-α, IL-6 and IL-1β) in rat lung cells (20). Through regulating the peroxisome
proliferator-activated receptor-γ coactivator (PGC)-1α signaling
pathway, Dex alleviates encephala edema and apoptosis in the
presence of PGC-1α expression, thus protecting neurons from
oxidative stress (21). In
addition, Liu et al (22)
explored the effects of Dex on injury caused by ischemia in
neuronal cells, and the results demonstrated that Dex promoted
neuronal cell viability and reduced apoptosis, as well as the ratio
of Bax/Bcl-2 expression levels. In the present study, an
H/R-induced H9C2 cell model was constructed and pretreated with Dex
to investigate the protective effects of Dex on H/R injury in
cardiomyocytes and its mechanism in the C/EBP-homologous protein
(CHOP) signaling pathway.
Materials and methods
Cell culture and grouping
The H9C2 (2–1) cell
line was obtained from The Cell Bank of Type Culture Collection of
the Chinese Academy of Sciences (cat. no. GNR 5). The cells were
incubated in 90% DMEM (GIBCO; Thermo Fisher Scientific, Inc.)
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 100
U/ml penicillin and 0.1 mg/ml streptomycin (Sigma-Aldrich; Merck
KGaA) with 5% CO2 at 37°C. The cells were divided into
control, Dex, H/R, and Dex+H/R groups. The control group was
normally cultured; Dex group was pretreated with 1 µM Dex (Beijing
Huamaike Biotechnology Co., Ltd.) for 1 h and cultured normally;
the H/R group was deoxidized using 4 mM sodium dithionite for 1 h
and reoxidized for 12 h; the Dex+H/R group was pretreated with 1 µM
Dex followed by H/R treatment.
Cell transfection
Cell transfection was performed as previously
described (23). Small interfering
(si)RNA targeting CHOP (siCHOP; cat. no. siB078266608-1-5;
Guangzhou RiboBio Co.), si-negative control (NC; siNC; cat. no.
siN0000002-1-5), pCMV6-XL5-CHOP (cat. no. SC117581; Origene
Technologies, Inc.) and its NC overexpression vector (pCMV6-XL5;
cat. no. PCMV6XL5; Origene Technologies, Inc.) were dissolved in 50
μl DMEM (HyClone; GE Healthcare Life Sciences) and used to
overexpress or knock down the expression of CHOP. Prior to cell
transfection, the cells were digested, thoroughly mixed, seeded
into a 6-well plate (1×106 cells/ml) and evenly
distributed in an orifice plate. After culturing overnight until
the cells reached 60–70% confluency, the cells were transfected
with 20 pmol siCHOP, CHOP siNC and NC in DMEM using
Lipofectamine® 2000 transfection reagent (Thermo Fisher
Scientific, Inc.) for 48 h at 37°C. At 24 h post-transfection,
reverse transcription-quantitative PCR (RT-qPCR) and western
blotting were used to detect the transfection efficiency. After the
transfection, the H9C2 cells were divided into control, H/R,
Dex+H/R, siNC+Dex+H/R and siCHOP+Dex+H/R groups to detect the
effects of silencing CHOP on H/R injury to the cells, whereas those
in the H/R, Dex+H/R, NC+Dex+H/R, CHOP+Dex+H/R groups were used to
explore the effects of CHOP overexpression in H9C2 cells.
MTT assay
H9C2 cells were seeded (2×104 cells/well)
into 96-well plates. MTT (10 µl; Amresco) was added to each well
and cultured for 4 h at room temperature. Subsequently, 100 µl DMSO
(Sigma-Aldrich; Merck KGaA) was added into each well and gently
agitated for 10 min at room temperature. The optical density (OD)
was detected using a microplate reader at 490 nm.
2,4-dinitrophenylhydrazine
colorimetric assay
The cells were collected and centrifuged at 255 × g
for 2 min at room temperature to obtain the supernatant. The
lactate dehydrogenase (LDH) levels were determined by
2,4-dinitrophenylhydrazine colorimetric assay (cat. no. A020-1-2;
Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer's instructions. The OD value was determined using a
microplate reader at 440 nm.
Flow cytometry
Early and late apoptosis were detected by flow
cytometry to explore the effects of Dex, overexpression or
knockdown of CHOP on H9C2 cells using the FITC/propidium iodide
(PI) Apoptosis Detection Kit [cat. no. 70-AP101-100; Multisciences
(Lianke) Biotech Co., Ltd.]. The cells were centrifuged at 1,000 ×
g at 4°C for 5 min to collect the sediments, blocked with 70%
ethanol for 2 h at 4°C and dyed with Annexin V-fluorescein
isothiocyanate and PI. Finally, the cells were analyzed by flow
cytometry (BD FACScanto II; BD Biosciences) and FlowJo software
(version 7.6.1; FlowJo LLC).
RT-qPCR
The relative mRNA expression levels of TNF-α, IL-1β,
IL-6, CHOP and 78 kDa glucose-regulated protein (Grp78) in H9C2
cells were determined by RT-qPCR. Total RNAs were extracted from
H9C2 cells using QIAshredder and an RNeasy Kit (Qiagen GmbH), and
the RNA concentration was detected. A total of 1.0 µg RNA was
reverse transcribed into cDNA using SuperScirpt™ II Reverse
Transcriptase (Thermo Fisher Scientific, Inc.) with 5 µl
First-Strand Buffer, 1 µl dNTP mix and 100 ng primers at 37°C for
60 min and 4°C for 5 min. Subsequently, qPCR was performed using
SYBR Fast qPCR Mix (Invitrogen; Thermo Fisher Scientific, Inc.).
The following thermocycling conditions were used for qPCR: Initial
denaturation at 95°C for 15 min; denaturation 95°C for 10 sec; 40
cycles at 60°C for 30 sec (annealing) and at 72°C for 30 sec
(extension); and final extension at 72°C for 7 min. The expression
levels of RT-qPCR products were determined by the 2−ΔΔCq
method (24). GADPH served as the
internal reference, and the primer sequences are presented in
Table I.
 | Table I.Primer sequences used for
RT-qPCR. |
Table I.
Primer sequences used for
RT-qPCR.
| Primer | Sequences
(5′→3′) |
|---|
| TNF-α | Forward:
ATGGGCTCCCTCTCATCAGT |
|
| Reverse:
GCTTGGTGGTTTGCTACGAC |
| IL-1β | Forward:
TCCTCGTGACTCGTGGGAT |
|
| Reverse:
GGTGTGCAGATGCCGGTTCAG |
| IL-6 | Forward:
CCAGTTGCCTTCTTGGGACT |
|
| Reverse:
GGTCTGTTGTGGGTGGTATCC |
| Grp78 | Forward:
GGTGCAGCAGGACATCAAGTT |
|
| Reverse:
CCCACCTCCAATATCAACTTGA |
| CHOP | Forward:
CTGGAAGCCTGGTATGAGGAT |
|
| Reverse:
CAGGGTCAAGAGTAGTGAAGGT |
| GAPDH | Forward: AGAAGGCTGG
GGCTCATTTG |
|
| Reverse: AGGGGCCATC
CACAGTCTTC |
Western blotting
The protein expression levels of CHOP and Grp78 were
measured by western blotting. The total proteins of H9C2 cells were
harvested by radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology), and the protein concentration was
measured by a bicinchoninic acid protein assay (Bio-Rad
Laboratories, Inc.). The proteins (20 µg/lane) were separated by
SDS-PAGE and electrotransferred to PVDF membranes, which were
blocked with 5% skimmed milk at room temperature for 1 h. The
membranes were then incubated with CHOP (1:200; cat. no. ab11419;
Abcam), Grp78 (1:100; cat. no. ab21685; Abcam) and GAPDH (1:10,000;
cat. no. ab181602; Abcam) primary antibodies at 4°C overnight. The
membranes were rinsed with TBST (0.05% Tween-20) and incubated with
the specific horseradish peroxide-conjugated secondary antibodies
for CHOP (goat anti-mouse; 1:2,000; cat. no. ab205719; Abcam),
Grp78 (goat anti-rabbit; 1:5,000; cat. no. ab205718; Abcam) and
GAPDH (goat anti-rabbit; 1:1,000; cat. no. ab6721; Abcam) for 2 h
at room temperature. The protein bands were visualized by enhanced
chemiluminescence reagent (Thermo Fisher Scientific, Inc.). Protein
expression was quantified using Quantity One software (version 4.4;
Bio-Rad Laboratories, Inc.).
ELISA
The cells were treated and suspended, the culture
supernatants were collected into a 1-ml centrifuge tube and
centrifuged at 3,000 × g for 10 min at room temperature, and the
supernatant was collected. The levels of superoxide dismutase
(SOD), nitric oxide (NO) and malondialdelyde (MDA) were measured
using ELISA kits (SOD, cat. no. 19160; Sigma-Aldrich; Merck KGaA;
NO, cat. no. S0021; Beyotime Institute of Biotechnology; MDA, cat.
no. MAK085c; Sigma-Aldrich; Merck KGaA) according to the
manufacturers' instructions. The absorbance was measured at 450 nm
using an Infinite M200 PRO microplate reader (Tecan Group,
Ltd.).
Statistical analysis
SPSS 17.0 (SPSS, Inc.) software was used for data
analysis. The data are presented as the mean ± standard deviation
from three independent experiments. One-way ANOVA with Tukey's post
hoc test was used for multiple group analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of Dex on cell viability, LDH
level, apoptosis and expression of inflammatory factors in H9C2
cells
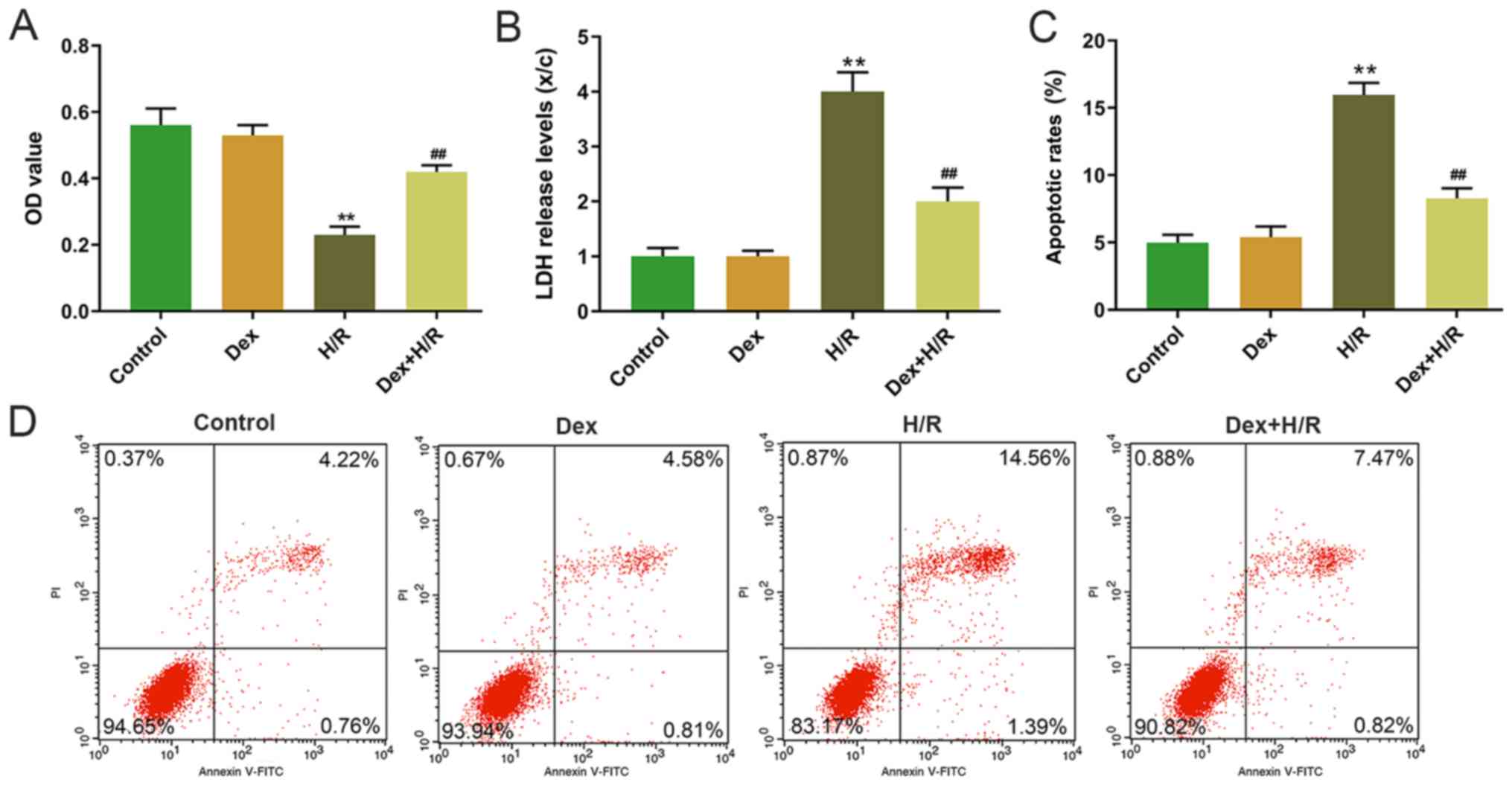
The H9C2 cells were induced by H/R, and cell
viability was detected by MTT assay. The results demonstrated that
H9C2 cell viability was significantly lower in the H/R group
compared with that in the control group, and that the Dex+H/R group
exhibited significantly higher cell viability compared with that in
the H/R group (Fig. 1A).
Additionally, LDH levels were significantly higher in the H/R group
compared with the control and Dex+H/R groups (Fig. 1B). The results also demonstrated a
higher apoptotic rate in the H/R group compared with that in the
control group, and the apoptotic rate was significantly lower in
the Dex+H/R group compared with the H/R group (Fig. 1C and D). In the H/R group, the
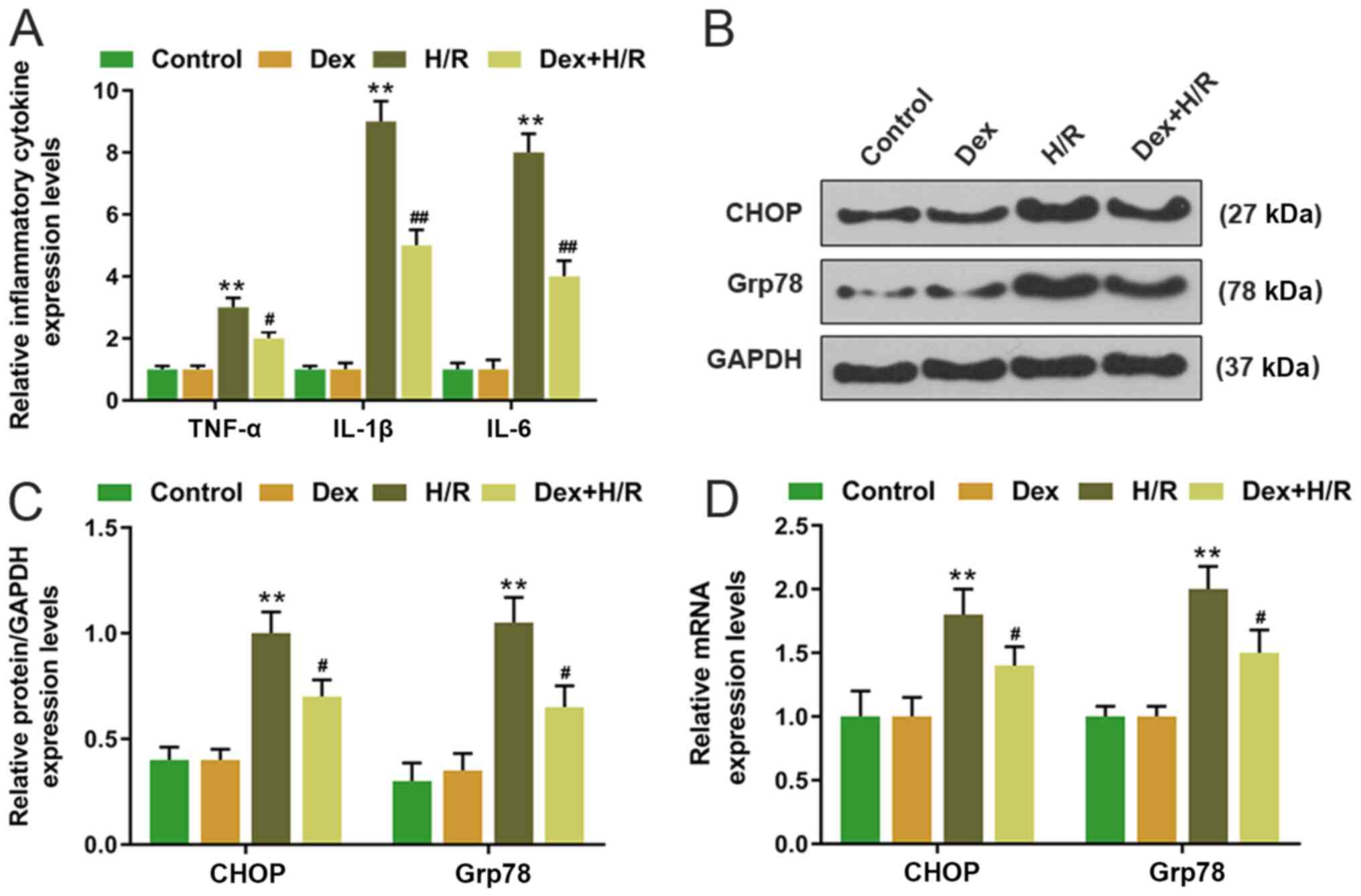
expression levels of TNF-α, IL-1β and IL-6 were significantly
higher compared with those in the control group, whereas the levels
of TNF-α, IL-1β and IL-6 were significantly lower in the Dex+H/R
group compared with those in the H/R group (Fig. 2A).
Dex affects the CHOP signaling
pathway
CHOP and Grp78 expression levels were determined by
RT-qPCR and western blotting. Both mRNA and protein expression
levels of CHOP and Grp78 were significantly upregulated in the H/R
group compared with the control group, whereas in the Dex-+H/R
group, CHOP and Grp78 expression levels were significantly lower
compared with those in the H/R group (Fig. 2B-D).
Effects of silencing CHOP on cell
viability, LDH level, apoptosis and expression of inflammatory
factors and Grp78 in H9C2 cells
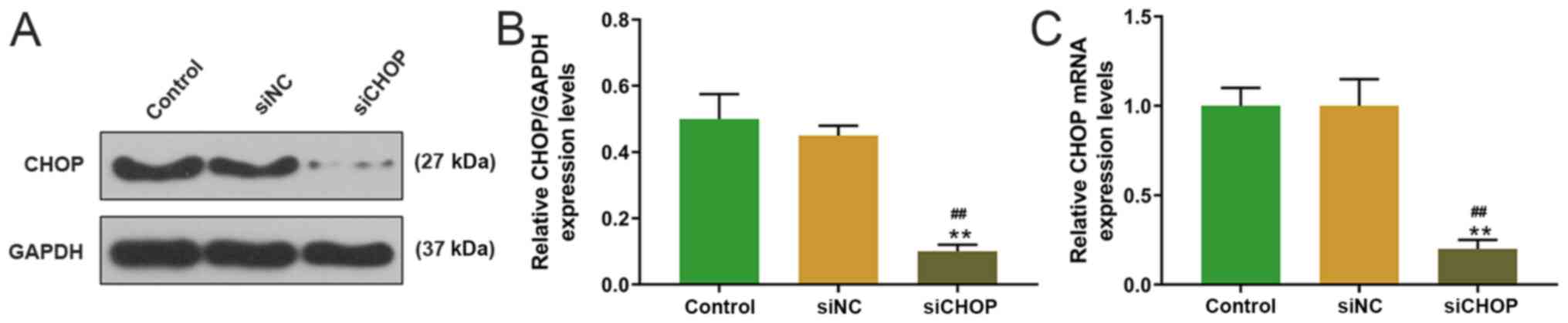
siCHOP was transfected into H9C2 cells, and the
transfection efficiency was measured by western blotting. The
expression of CHOP in the siCHOP group was significantly lower
compared with the control and siNC groups (Fig. 3). In addition, MTT assay revealed
that the cell viability was significantly higher in the
siCHOP+Dex+H/R group compared with the siNC+Dex+H/R group (Fig. 4A). In addition, the LDH levels and
apoptotic rates were significantly lower in the siCHOP+ Dex+H/R
group compared with the H/R and siNC+ Dex+H/R groups (Fig. 4B-D). In the H/R group, the
expression levels of TNF-α, IL-1β and IL-6 were significantly
higher compared with the control group; by contrast, the expression
levels of TNF-α, IL-1β and IL-6 were significantly lower in the
Dex+H/R and siCHOP+ Dex+H/R groups compared with the H/R and siNC+
Dex+H/R groups (Fig. 5A).
Furthermore, the protein and mRNA expression levels of Grp78 were
significantly elevated in the H/R group compared with the control,
whereas the expression levels of Grp78 were lower in the Dex+H/R
and siCHOP+ Dex+H/R groups compared with the H/R and siNC+H/R
groups (Fig. 5B-D).
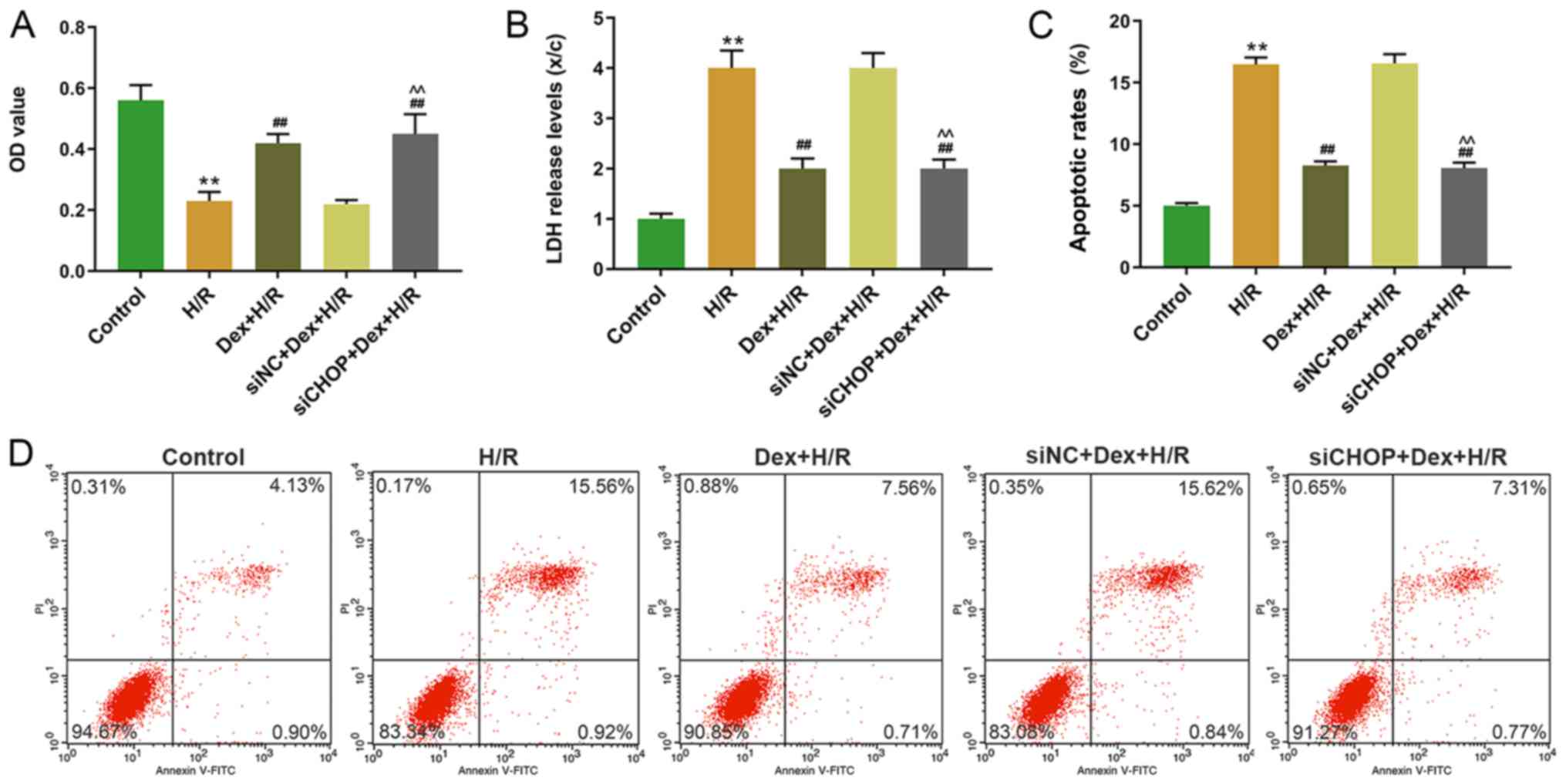 | Figure 4.Effects of CHOP silencing on cell
viability, LDH level and apoptosis in H9C2 cells. (A) Cell
viability of H9C2 cells was determined by MTT assay at 24 h. (B)
LDH levels of H9C2 cells were determined by
2,4-dinitrophenylhydrazine colorimetric assay. (C and D) Apoptotic
rates of H9C2 cells were determined by flow cytometry. **P<0.001
vs. control; ##P<0.001 vs. H/R;
^^P<0.001 vs. siNC+Dex+HR. CHOP, C/EBP-homologous
protein; LDH, lactate dehydrogenase, H/R, hypoxia/reoxygenation;
Dex, dexmedetomidine; si, small interfering; NC, negative control;
OD, optical density; PI, propidium iodide. |
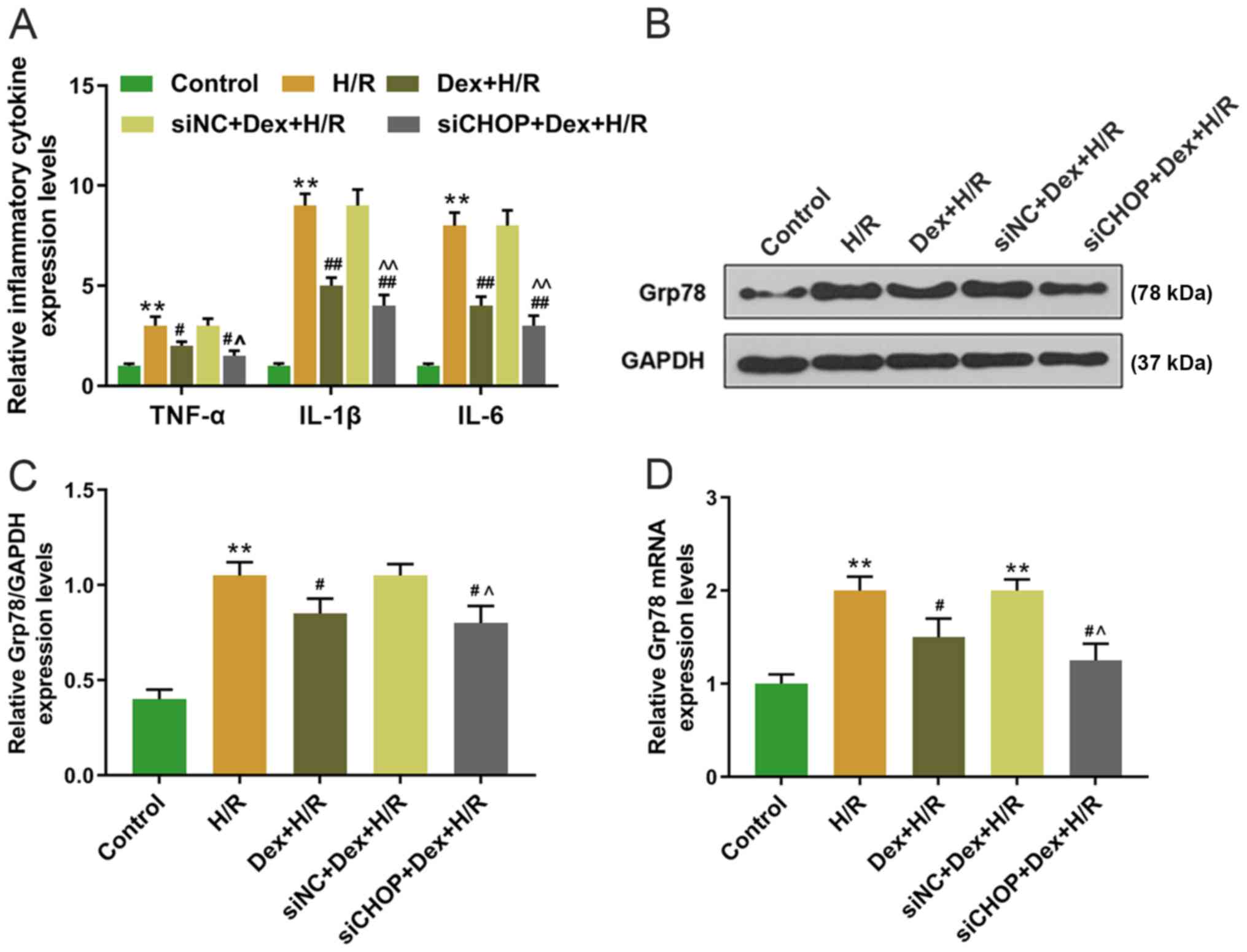 | Figure 5.Effects of CHOP silencing on the
expression of inflammatory factors and Grp78 in H9C2 cells. (A)
Expression levels of TNF-α, IL-1β and IL-6 in H9C2 cells were
detected by RT-qPCR. (B-D) Expression levels of Grp78 in H9C2 cells
were determined by (B and C) western blotting and (D) RT-qPCR.
**P<0.001 vs. control; #P<0.05,
##P<0.001 vs. H/R; ^P<0.05,
^^P<0.001 vs. siNC+Dex+HR. TNF, tumor necrosis
factor; IL, interleukin; RT-qPCR, reverse
transcription-quantitative PCR; Dex, dexmedetomidine; H/R,
hypoxia/reoxygenation; si, small interfering; NC, negative control;
CHOP, C/EBP-homologous protein. |
Effects of CHOP overexpression on cell
viability, LDH level, apoptosis and expression of inflammatory
factors and Grp78 in H9C2 cells
The CHOP overexpression plasmid was transfected into
H9C2 cells, and significantly higher levels of CHOP were observed
in the CHOP group compared with the control and NC groups (Fig. 6A-C). The effect of CHOP
overexpression on cell viability was then measured; compared with
the H/R and Dex+H/R groups, cell viability was significantly lower
in the CHOP+Dex+H/R group (Fig.
6D). The CHOP+Dex+H/R group exhibited significantly higher LDH
levels and apoptotic rates compared with the H/R and Dex+H/R groups
(Fig. 6E-G). Furthermore, the
expression levels of TNF-α, IL-1β and IL-6 were significantly lower
in the Dex+H/R and NC+Dex+H/R groups, but significantly higher in
the CHOP+Dex+H/R group compared with the H/R group (Fig. 7A). The Grp78 protein and mRNA
expression levels were significantly higher in the CHOP+Dex+H/R
group compared with the Dex+H/R group (Fig. 7B-D).
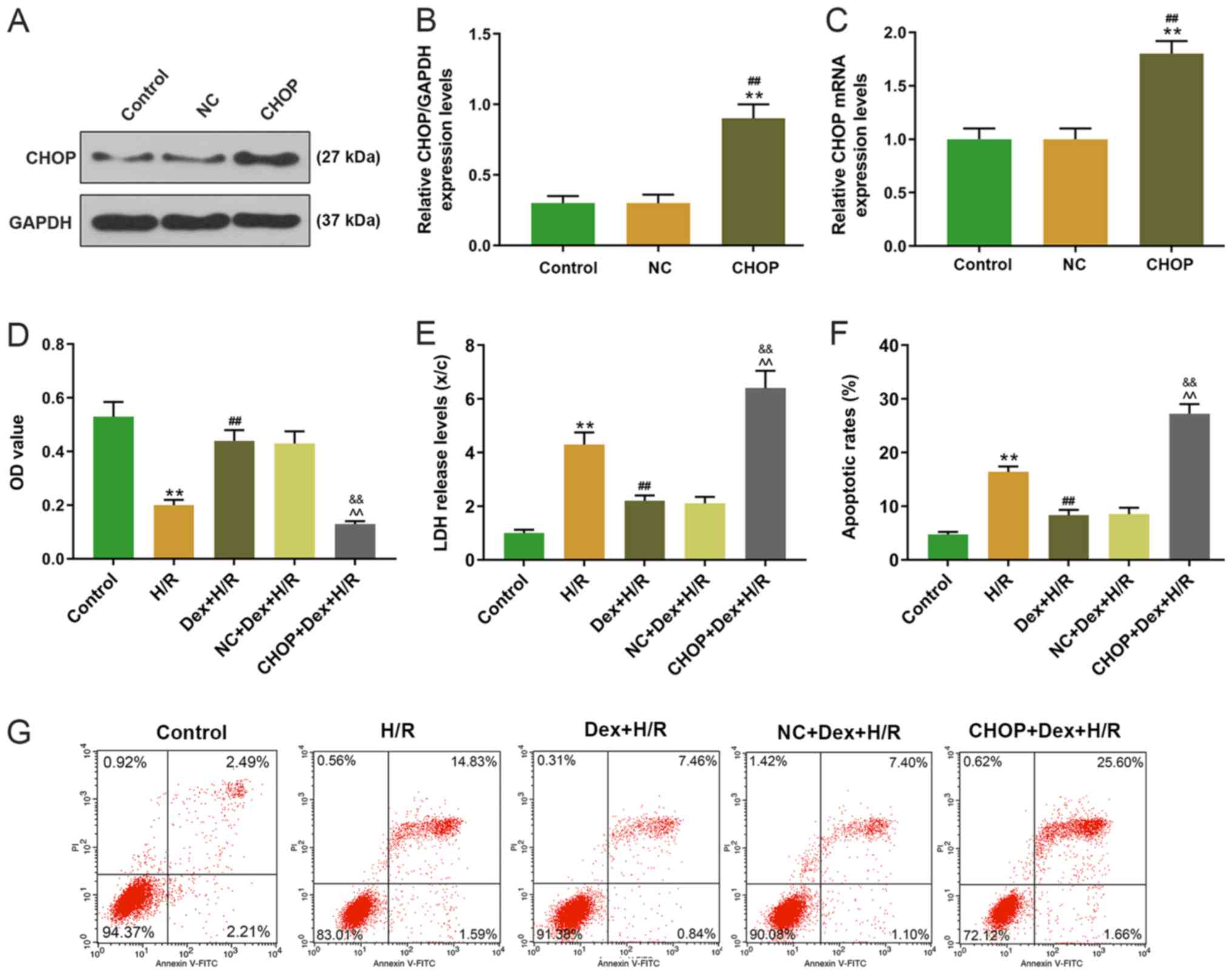 | Figure 6.Transfection efficiency of CHOP and
effects of CHOP overexpression on cell viability, LDH level and
apoptosis in H9C2 cells. (A-C) Expression levels of CHOP were
determined by (A and B) western blotting and (C) RT-qPCR. (D) Cell
viability of H9C2 cells was determined by MTT assay at 24 h. (E)
LDH levels of H9C2 cells determined by 2,4-dinitrophenylhydrazine
colorimetric assay. (F and G) Apoptotic rates of H9C2 cells were
determined by flow cytometry. **P<0.001 vs. Control;
##P<0.001 vs. NC or H/R;
&&P<0.001 vs. Dex+H/R;
^^P<0.001 vs. NC+Dex+HR. CHOP, C/EBP-homologous
protein; RT-qPCR, reverse transcription-quantitative PC; LDH,
lactate dehydrogenase; H/R, hypoxia/reoxygenation; Dex,
dexmedetomidine; NC, negative control; OD, optical density; PI,
propidium iodide. |
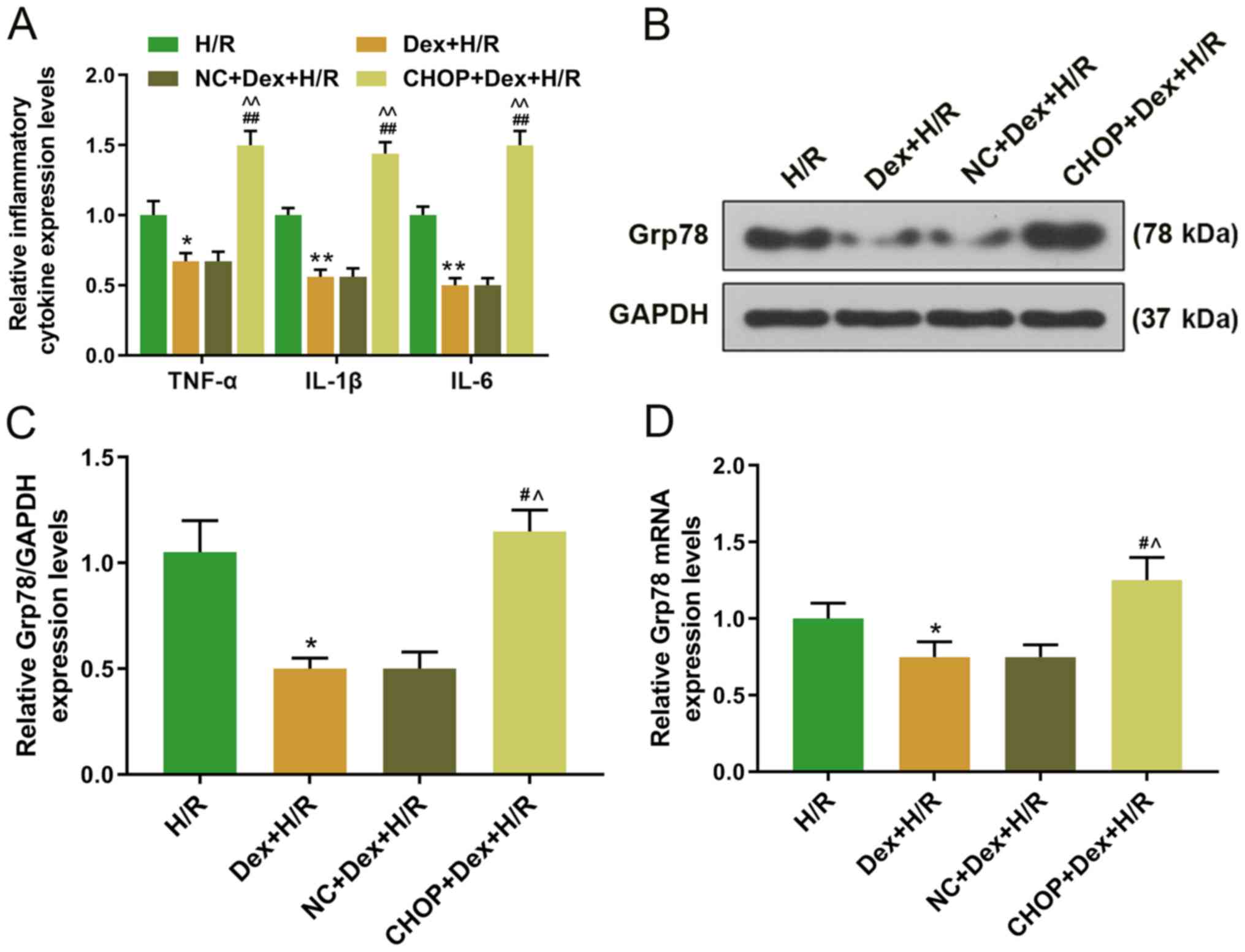 | Figure 7.Effects of CHOP overexpression on the
expression levels of inflammatory factors and Grp78 in H9C2 cells.
(A) Expressions of TNF-α, IL-1β and IL-6 in H9C2 were detected by
RT-qPCR. (B and C) Protein and (D) mRNA expression levels of Grp78
in H9C2 cells were determined by (B and C) western blotting (D) and
RT-qPCR. *P<0.05, **P<0.001 vs. H/R; #P<0.05,
##P<0.001 vs. Dex+H/R; ^P<0.05,
^^P<0.001 vs. NC+Dex+H/R. TNF, tumor necrosis factor;
IL, interleukin; RT-qPCR, reverse transcription-quantitative PCR;
Dex, dexmedetomidine; H/R, hypoxia/reoxygenation; NC, negative
control; CHOP, C/EBP-homologous protein. |
Effects of silencing CHOP on the SOD
activity and NO and MDA levels in H/R-induced H9C2 cells
The effects of silencing CHOP on H/R-induced H9C2
cells were further observed by measuring SOD activity, as well as
MDA and NO levels. The results demonstrated that the SOD activity
was significantly decreased in the H/R-group compared with the
control group, and that the SOD activity was significantly higher
in the Dex+H/R and siCHOP+Dex+H/R groups compared with that in the
H/R and siNC+Dex+H/R groups, respectively (Fig. 8A). In addition, the NO and MDA
levels were significantly higher in the H/R group compared with the
control group, but significantly lower in the Dex+H/R and siCHOP+
Dex+H/R groups compared with the HR and siNC+ Dex+H/R groups
(Fig. 8B and C).
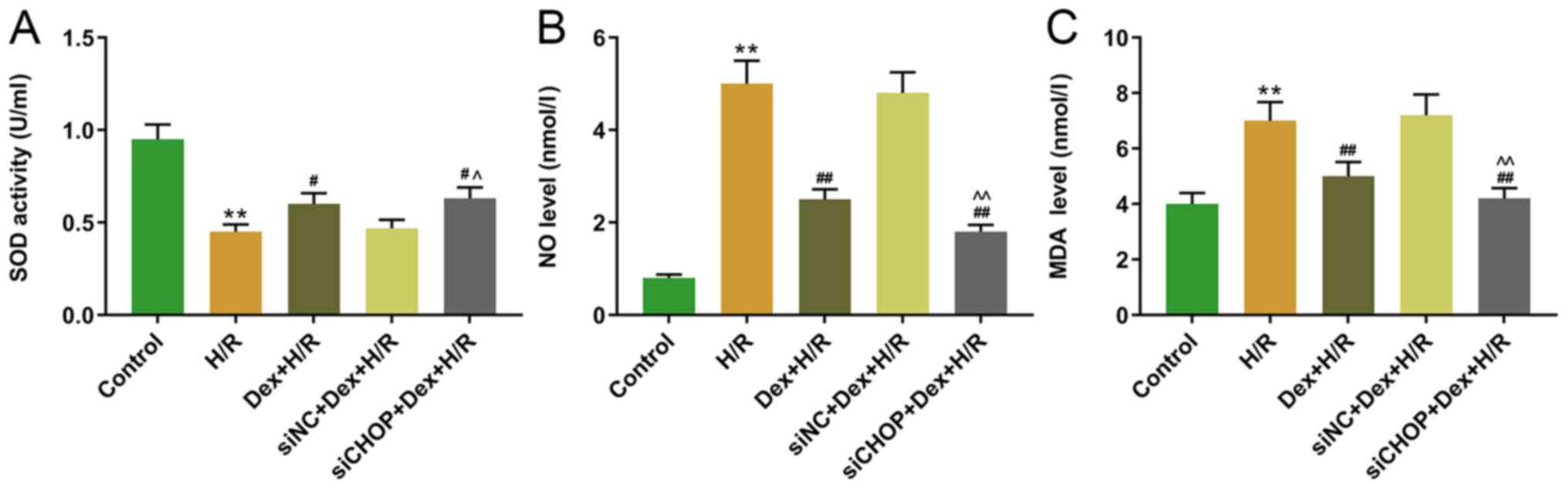 | Figure 8.Effects of CHOP knockdown on the SOD
activity, and NO and MDA levels in H/R-induced H9C2 cells. The
levels of (A) SOD, (B) NO and (C) MDA were measured using ELISA
kits. **P<0.001 vs. H/R; #P<0.05 and
##P<0.001 vs. Dex+H/R; ^P<0.05 and
^^P<0.001 vs. siNC+Dex+H/R. CHOP, C/EBP-homologous
protein; SOD, superoxide dismutase; NO, nitric oxide; MDA,
malondialdelyde; H/R, hypoxia/reoxygenation; Dex, dexmedetomidine;
si, small interfering; NC, negative control. |
Discussion
Dex has been demonstrated to reduce H/R-induced
damage in primary neonatal rat cardiomyocytes (25). In addition, Dex reduces
H2O2-induced cardiomyocyte apoptosis in
neonatal rats through mitochondrial and endoplasmic
reticulum-mediated oxidative stress pathways (26). To explore the mechanism of Dex
pretreatment in the present study, an H/R model was constructed in
H9C2 cells using sodium dithionite. Sodium dithionite depletes
oxygen in the culture medium and simulates an anoxic environment,
which causes hypoxic injury in cells without damaging the cell
membrane; thus, it is frequently used to induce H/R injury in cells
(27,28). In the present study, the H/R model
was established with or without the pretreatment of Dex; the
results demonstrated that H/R significantly reduced the viability
of H9C2 cells, promoted apoptosis and increased the expression
levels of LDH and inflammatory factors, whereas Dex pretreatment
prior to H/R increased the cell viability and suppressed the
release of LDH and inflammatory factors. LDH occurs ubiquitously in
cardiomyocytes and is released into the blood upon damage to the
cell membrane (29). Accordingly,
the level of LDH in the supernatant of the culture medium may
reflect the degree of cardiomyocyte injury (30–32).
In corroboration with the results of the present study, previous
studies have demonstrated that the level of LDH is significantly
higher in the H/R-induced I/R injury compared with the control in
cardiomyocytes (33–35). The inflammatory factors TNF-α,
IL-1β and IL-6 are commonly recognized as inflammatory indicators
(36). TNF-α and IL-1β are
responsible for the stimulation of chemokines and the secretion of
adhesion molecules in ischemic tissues, and IL-6 is responsible for
the regulation of inflammation (37,38).
Previous studies have reported that Dex induces multiple protective
effects on MIRI (39), and such
findings were further supported by the present study. Yu et
al (40) have reported that
microRNA-665 and Dex protect cardiomyocytes against I/R injury
caused by oxide stress and suppress apoptosis. Additionally, by
inhibiting the expression of inflammatory cytokines, Dex attenuates
the I/R injury induced by bilateral renal pedicle clamping
(41).
The present study further explored the effects of
CHOP on H9C2 cells induced by H/R. Yang et al (42) have observed that the expression
levels of CHOP and Grp78 are significantly upregulated in
cardiomyocytes following I/R. During the H/R process, high CHOP and
Grp78 expression levels in H9C2 cells are present (43). CHOP serves a role in the apoptosis
signaling pathways (44,45). CHOP and Grp78 are endoplasmic
reticulum (ER) stress proteins and are widely recognized as ER
stress markers (46). Evidence
suggests important roles for ER stress proteins in the
cardiomyocyte apoptosis caused by H/R process and its regulation on
CHOP in ER stress-induced autophagy (47,48).
In H/R-induced H9C2 cell autophagy, the high expression level of
CHOP is reduced by the inhibition of ER stress (49). Liu et al (26) have reported that Dex alleviates
cardiomyocyte apoptosis induced by H2O2 via
ER stress pathways. Furthermore, Dex treatment has been observed to
attenuate cerebral I/R injury by moderating the protein kinase
RNA-like endoplasmic reticulum kinase/CHOP/Caspase-11 pathway
through inhibition of the expression of ER stress-related apoptotic
pathway proteins (50). In human
umbilical vein endothelial cells, pretreatment with Dex increases
cell the survival rate, decreases expression of CHOP and caspase-3
protein, and the apoptotic rate of H/R-induced cells, suggesting a
possible association between Dex and the CHOP signaling pathway in
H/R induced apoptosis (51). In
the present study, western blotting and RT-qPCR demonstrated that
pretreatment with Dex significantly suppressed the H/R-induced high
expression levels of CHOP and Grp78, suggesting a potential
association between Dex and the CHOP signaling pathway.
Additionally, in H/R-injured cells, knockdown of CHOP expression
promoted cell viability, inhibited apoptosis and suppressed the
release of LDH and expression of inflammatory factors and Grp78,
whereas the overexpression of CHOP aggravated the cell injury
induced by H/R. These results suggested that the block of CHOP may
attenuate the negative effects of H/R on cell viability and
apoptosis, release of LDH and the expression of inflammatory
factors and Grp78 of H9C2 cells, and that the overexpression of
CHOP inhibited the protective effects of Dex on cardiomyocytes.
However, the present study is still not rigorous
enough; the effects of Dex on the cardiomyocytes of H/R-injured
neonatal mice need further research. In future studies, an H/R
injury mouse model will be established or neonatal mouse
cardiomyocytes will be selected for research.
In conclusion, the present study explored the
effects of Dex on H/R-induced H9C2 cells and observed that the
pretreatment with Dex may alleviate H/R-induced cell injury through
regulating the CHOP pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS, ZL and JL made substantial contributions to the
conception and design of the study, data acquisition, analysis and
interpretation, drafted the manuscript and critically revised it
for important intellectual content. All authors are accountable for
all aspects of the work in ensuring that questions related to the
accuracy or integrity of the work are appropriately investigated
and resolved. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kloner RA, Shi J, Dai W, Carreno J and
Zhao L: Remote ischemic conditioning in acute myocardial infarction
and shock states. J Cardiovasc Pharmacol Ther. 25:3307–109. 2020.
View Article : Google Scholar
|
|
2
|
Cooper WA, Corvera JS, Thourani VH, Puskas
JD, Craver JM, Lattouf OM and Guyton RA: Perfusion-assisted direct
coronary artery bypass provides early reperfusion of ischemic
myocardium and facilitates complete revascularization. Ann Thorac
Surg. 75:1132–1139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walker AC and Johnson NJ: Critical care of
the post-cardiac arrest patient. Cardiol Clin. 36:419–428. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu D, Wang J, Li H, Xue M, Ji A and Li Y:
Role of hydrogen sulfide in ischemia-reperfusion injury. Oxid Med
Cell Longev. 2015:1869082015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Faust KB, Chiantella V, Vinten-Johansen J
and Meredith JH: Oxygen-derived free radical scavengers and
skeletal muscle ischemic/reperfusion injury. Am Surg. 54:709–719.
1988.PubMed/NCBI
|
|
6
|
Dong HJ, Li J, Zhan H, Li Y and Su RB: Tea
polyphenols promote cardiac function and energy metabolism in ex
vivo rat heart with ischemic/reperfusion injury and inhibit calcium
inward current in cultured rat cardiac myocytes. Nan Fang Yi Ke Da
Xue Xue Bao. 36:604–608. 2016.PubMed/NCBI
|
|
7
|
Li JP, Guo LL, Chen Z, Wang R and Wang J:
Relationship between calcium overload and myocardial ischemia
reperfusion injury and intervention strategy of Chinese herbal
medicine. Zhongguo Zhong Yao Za Zhi. 41:2168–2173. 2016.(In
Chinese). PubMed/NCBI
|
|
8
|
Francis A and Baynosa R:
Ischaemia-reperfusion injury and hyperbaric oxygen pathways: A
review of cellular mechanisms. Diving Hyperb Med. 47:110–117.
2017.PubMed/NCBI
|
|
9
|
Zendedel A, Gharibi Z, Anbari K,
Abbaszadeh A, Khayat ZK, Khorramabadi RM, Soleymaninejad M and
Gholami M: Selenium ameliorate peripheral nerve
ischemic-reperfusion injury via decreased TNF-α. Biol Trace Elem
Res. 176:328–337. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Halladin NL: Oxidative and inflammatory
biomarkers of ischemia and reperfusion injuries. Dan Med J.
62:B50542015.PubMed/NCBI
|
|
11
|
Laubach VE and Sharma AK: Mechanisms of
lung ischemia-reperfusion injury. Curr Opin Organ Transplant.
21:246–252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Zhu X, Liu X, Du A and Yu B:
miR-200a mediates protection of thymosin β-4 in cardiac
microvascular endothelial cells as a novel mechanism under
hypoxia-reoxygenation injury. J Cell Biochem. 120:19098–19106.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao C, Wang R, Li B, Guo Y, Yin T, Xia Y,
Zhang F, Lian K, Liu Y, Wang H, et al: TXNIP/Redd1 signalling and
excessive autophagy: A novel mechanism of myocardial
ischaemia/reperfusion injury in mice. Cardiovasc Res. 116:645–657.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Manning JR, Thapa D, Zhang M, Stoner MW,
Traba J, Corey C, Shiva S, Sack MN and Scott I: Loss of GCN5L1 in
cardiac cells disrupts glucose metabolism and promotes cell death
via reduced Akt/mTORC2 signaling. Biochem J. 476:1713–1724. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El Baz MM and Farahat TEM: Efficacy of
adding dexmedetomidine to intra-articular levobupivacaine on
postoperative pain after knee arthroscopy. Anesth Essays Res.
13:254–258. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sha J, Zhang H, Zhao Y, Feng X, Hu X, Wang
C, Song M and Fan H: Dexmedetomidine attenuates
lipopolysaccharide-induced liver oxidative stress and cell
apoptosis in rats by increasing GSK-3β/MKP-1/Nrf2 pathway activity
via the α2 adrenergic receptor. Toxicol Appl Pharmacol.
364:144–152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen L, Cao J, Cao D, Wang M, Xiang H,
Yang Y, Ying T and Cong H: Protective effect of dexmedetomidine
against diabetic hyperglycemia-exacerbated cerebral
ischemia/reperfusion injury: An in vivo and in vitro study. Life
Sci. 235:1165532019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen N, Chen X, Xie J, Wu C and Qian J:
Dexmedetomidine protects aged rats from postoperative cognitive
dysfunction by alleviating hippocampal inflammation. Mol Med Rep.
20:2119–2126. 2019.PubMed/NCBI
|
|
19
|
Wang Q, Tan Y, Zhang N, Xu Y, Wei W, She
Y, Bi X, Zhao B and Ruan X: Dexmedetomidine inhibits activation of
the MAPK pathway and protects PC12 and NG108-15 cells from
lidocaine-induced cytotoxicity at its maximum safe dose. Biomed
Pharmacother. 91:162–166. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang CL, Tsai PS and Huang CJ: Effects of
dexmedetomidine on regulating pulmonary inflammation in a rat model
of ventilator-induced lung injury. Acta Anaesthesiol Taiwan.
46:151–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jacob H, Stanisavljevic L, Storli KE,
Hestetun KE, Dahl O and Myklebust MP: A four-microRNA classifier as
a novel prognostic marker for tumor recurrence in stage II colon
cancer. Sci Rep. 8:61572018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu YJ, Wang DY, Yang YJ and Lei WF:
Effects and mechanism of dexmedetomidine on neuronal cell injury
induced by hypoxia-ischemia. BMC Anesthesiol. 17:1172017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gonzalez Villarreal C, Said Fernandez S,
Soto Dominguez A, Padilla Rivas G, Garza Treviño E, Rodriguez Rocha
H and Martinez Rodriguez H: Bone marrow mesenchymal stem cells:
Improving transgene expression level, transfection efficiency and
cell viability. J BUON. 23:1893–1903. 2018.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peng K, Qiu Y, Li J, Zhang ZC and Ji FH:
Dexmedetomidine attenuates hypoxia/reoxygenation injury in primary
neonatal rat cardiomyocytes. Exp Ther Med. 14:689–695. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu XR, Li T, Cao L, Yu YY, Chen LL, Fan
XH, Yang BB and Tan XQ: Dexmedetomidine attenuates H2O2-induced
neonatal rat cardiomyocytes apoptosis through mitochondria- and
ER-medicated oxidative stress pathways. Mol Med Rep. 17:7258–7264.
2018.PubMed/NCBI
|
|
27
|
Jiang J, Chen DY, Liu ZT, Chen F, Zhang
JJ, Cui J and Pang J: Effect of N-perfluorooctane on
hypoxia/reoxygenation injury in human umbilical vein endothelial
cells. Acta Cardiol Sin. 32:716–722. 2016.PubMed/NCBI
|
|
28
|
Chai YL, Xu JZ, Zhang YL and Sheng GT:
Effects of probucol on cultured human umbilical vein endothelial
cells injured by hypoxia/reoxygenation. Genet Mol Res.
15:150167522016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin CX, Gu JL and Cao JM: The acute toxic
effects of platinum nanoparticles on ion channels, transmembrane
potentials of cardiomyocytes in vitro and heart rhythm in vivo in
mice. Int J Nanomedicine. 14:5595–5609. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fan L, Zhou W, Zhang L, Jiang D, Zhao Q
and Liu L: Sitagliptin protects against hypoxia/reoxygenation
(H/R)-induced cardiac microvascular endothelial cell injury. Am J
Transl Res. 11:2099–2107. 2019.PubMed/NCBI
|
|
31
|
Kong QR, Ji DM, Li FR, Sun HY and Wang QX:
MicroRNA-221 promotes myocardial apoptosis caused by myocardial
ischemia-reperfusion by down-regulating PTEN. Eur Rev Med Pharmacol
Sci. 23:3967–3975. 2019.PubMed/NCBI
|
|
32
|
Zhang Y, Zhang H, Zhang Z, Li S, Jiang W,
Li X and Lv J: lncRNA MALAT1 cessation antagonizes
hypoxia/reoxygenation injury in hepatocytes by inhibiting apoptosis
and inflammation via the HMGB1-TLR4 axis. Mol Immunol. 112:22–29.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ge L, Cai Y, Ying F, Liu H, Zhang D, He Y,
Pang L, Yan D, Xu A, Ma H and Xia Z: miR-181c-5p exacerbates
hypoxia/reoxygenation-induced cardiomyocyte apoptosis via targeting
PTPN4. Oxid Med Cell Longev. 2019:19579202019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen Y, Wang H, Zhang Y, Wang Z, Liu S and
Cui L: Pretreatment of ghrelin protects H9c2 cells against
hypoxia/reoxygenation-induced cell death via PI3K/AKT and AMPK
pathways. Artif Cells Nanomed Biotechnol. 47:2179–2187. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang H, Wang C, Zhang L, Lv J and Ni H:
Rutin alleviates hypoxia/reoxygenation-induced injury in myocardial
cells by up-regulating SIRT1 expression. Chem Biol Interact.
297:44–49. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soomro AH, Khan E, Noori S, Lone MA, Syal
Z and Sheikh S: Assessment of cytokine release against oral mucosal
cell line culture (TR146) stimulated by neutrophil elastase
associated with Behcet's disease. Int J Dent. 2019:60956282019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Q, Weng Y, Jiang Y, Zhao S, Zhou D
and Xu N: Overexpression of miR-140-5p inhibits
lipopolysaccharide-induced human intervertebral disc inflammation
and degeneration by downregulating toll-like receptor 4. Oncol Rep.
40:793–802. 2018.PubMed/NCBI
|
|
38
|
Peters MC, McGrath KW, Hawkins GA, Hastie
AT, Levy BD, Israel E, Phillips BR, Mauger DT, Comhair SA, Erzurum
SC, et al: Plasma interleukin-6 concentrations, metabolic
dysfunction, and asthma severity: A cross-sectional analysis of two
cohorts. Lancet Respir Med. 4:574–584. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ren J, Li C, Liu Y, Liu H and Dong Z:
Protective effect of dexmedetomidine against myocardial
ischemia-reperfusion injury in rabbits. Acta Cir Bras. 33:22–30.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu J, Yang W, Wang W, Wang Z, Pu Y, Chen
H, Wang F and Qian J: Involvement of miR-665 in protection effect
of dexmedetomidine against oxidative stress injury in myocardial
cells via CB2 and CK1. Biomed Pharmacother. 115:1088942019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu Z, Wang D, Zhou Z, Chen Q, Zhang D,
Chen S, Jiang H, Jia C and Liu X: Dexmedetomidine attenuates renal
and myocardial ischemia/reperfusion injury in a dose-dependent
manner by inhibiting inflammatory response. Ann Clin Lab Sci.
49:31–35. 2019.PubMed/NCBI
|
|
42
|
Yang TR, Zhang T, Mu NH, Ruan LB, Duan JL,
Zhang RP and Miao YB: Resina draconis inhibits the
endoplasmic-reticulum-induced apoptosis of myocardial cells via
regulating miR-423-3p/ERK signaling pathway in a tree shrew
myocardial ischemia-reperfusion model. J Biosci. 44:532019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guan G, Zhang J, Liu S, Huang W, Gong Y
and Gu X: Glucagon-like peptide-1 attenuates endoplasmic reticulum
stress-induced apoptosis in H9c2 cardiomyocytes during
hypoxia/reoxygenation through the GLP-1R/PI3K/Akt pathways. Naunyn
Schmiedebergs Arch Pharmacol. 392:715–722. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu H, Tian M, Ding C and Yu S: The C/EBP
homologous protein (CHOP) transcription factor functions in
endoplasmic reticulum stress-induced apoptosis and microbial
infection. Front Immunol. 9:30832019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao J, Xiang X, Zhang H, Jiang D, Liang
Y, Qing W, Liu L, Zhao Q and He Z: CHOP induces apoptosis by
affecting brain iron metabolism in rats with subarachnoid
hemorrhage. Exp Neurol. 302:22–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lei Y, Wang S, Ren B, Wang J, Chen J, Lu
J, Zhan S, Fu Y, Huang L and Tan J: CHOP favors endoplasmic
reticulum stress-induced apoptosis in hepatocellular carcinoma
cells via inhibition of autophagy. PLoS One. 12:e01836802017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu JQ, Chen B, Hu HX, Yue RC, Zhang S, Xu
L, Wang H, Li Q, Tan CY, Chen HY and Zhang RY: Lycopene protects
against hypoxia/reoxygenation injury in mouse cardiomyocytes by
inhibiting endoplasmic reticulum stress induced apoptosis. Zhonghua
Xin Xue Guan Bing Za Zhi. 44:518–523. 2016.(In Chinese). PubMed/NCBI
|
|
48
|
Cao L, Chen Y, Zhang Z, Li Y and Zhao P:
Endoplasmic reticulum stress-induced NLRP1 inflammasome activation
contributes to myocardial ischemia/reperfusion injury. Shock.
51:511–518. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Guan G, Yang L, Huang W, Zhang J, Zhang P,
Yu H, Liu S and Gu X: Mechanism of interactions between endoplasmic
reticulum stress and autophagy in hypoxia/reoxygenation-nduced
injury of H9c2 cardiomyocytes. Mol Med Rep. 20:350–358.
2019.PubMed/NCBI
|
|
50
|
Liu C, Fu Q, Mu R, Wang F, Zhou C, Zhang
L, Yu B, Zhang Y, Fang T and Tian F: Dexmedetomidine alleviates
cerebral ischemia-reperfusion injury by inhibiting endoplasmic
reticulum stress dependent apoptosis through the
PERK-CHOP-Caspase-11 pathway. Brain Res. 1701:246–254. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang C, Gao C, Yu M, Li B, Lu Y and Lü G:
Effect of dexmedetomidine pretreatment on
hypoxia/reoxygenation-induced injury in human umbilical vein
endothelial cells and the possible mechanism. Zhonghua Yi Xue Za
Zhi. 94:3527–3530. 2014.(In Chinese). PubMed/NCBI
|