Introduction
Cardiac disease is currently the largest risk factor
for of adult mortality and is associated with ≤17 million cases of
mortality per year worldwide (1).
Myocarditis is an inflammatory condition of the heart, which can be
induced by viral infection, hypersensitivity to certain substances
and autoimmune reactions (2). If
it is not treated properly, myocarditis may lead to dilated
cardiomyopathy, which may result in the development of heart
failure. Although research in this area has advanced rapidly in
recent years, the therapeutic strategies used to cure myocarditis
remain limited. At present, physicians generally believe that
myocarditis is the result of various pathogenic factors. The
development of myocarditis is accompanied by lipid metabolism
disorders, and inflammatory and immune responses (3,4).
Apolipoprotein E (ApoE) is an arginine-rich alkaline
protein, which is present in plasma chylomicron, low-density
lipoprotein and very low-density lipoprotein (5). ApoE can be synthesized in various
tissues, mainly in the liver, brain and kidney. ApoE is a ligand
for low-density lipoprotein receptors and for hepatocyte remnant
receptors; therefore it is closely associated with lipoprotein
metabolism. ApoE is polymorphic, and is dependent on individual
lipid levels and is closely associated with the development of
atherosclerosis. In addition, ApoE is involved in the activation of
hydrolyzed fats, immunoregulation and regeneration of nerve tissue
(6–8). However, to the best of our knowledge,
the relationship between ApoE and myocarditis has not yet been
investigated. Given the important role of ApoE in cardiac
development, the present study aimed to determine whether ApoE is
also involved in myocarditis.
Phosphoinositide 3-kinase (PI3K) is an intracellular
phosphatidylinositol kinase, which is known to regulate
inflammatory responses in numerous diseases (9,10).
The inhibition of PI3K is also able to promote infarct resorption
and prevent adverse cardiac remodeling following myocardial
infarction in mice (11). A study
using PI3Kγ-deficient mice demonstrated a complex contribution of
PI3Kγ to reparative angiogenesis in myocardial infarction (12). PI3K catalytic subunit δ isoform
(P110δ) is an enzyme that in humans is encoded by the PIK3CD gene
(13), which is widely involved in
cell growth, differentiation, and immune regulation and other
effects. P110δ is mainly expressed in white blood cells, and it is
involved in T and B lymphocyte differentiation, maturation and the
neutrophil chemotaxis process; therefore, P110δ is considered an
important molecule that regulates the leukocyte immune response
(14). In addition, it has been
suggested that P110δ is an attractive pharmacological target that
modulates unwanted immune responses and certain blood cancers
(15). Notably, P110δ-selective
inhibitors are currently being tested in clinical trials to treat
autoimmunity, allergies and lymphoid malignancies (16).
In recent years, there have been many reports about
the relationship between P110δ and inflammation. Previous studies
have reported that B-cell lymphoma 2 (Bcl-2)-associated X protein
(Bax) and BH3 interacting-domain death agonist (Bid) have an
important role in transforming growth factor (TGF)-β1-induced
myocarditis and apoptosis. TGF-β1 was able to promote the
expression and activation of P110δ, and phosphorylate p21 through
Bax and Bid (15). The lack of
P110δ can effectively reverse TGF-β1-induced myocarditis (17); therefore, it may be suggested that
P110δ and myocarditis are closely related. A previous study
suggested that P110δ is able to inhibit monocyte infiltration in
ApoE knockout (KO) mice (18). In
present study, ApoE−/−/P110δ−/− mice were
constructed on the basis of ApoE−/− mice and
investigated the effects of P110δ deletion on myocarditis in ApoE
KO mice.
Materials and methods
Animal model
ApoE−/− mice were purchased from Jackson
Laboratory (Ben Harbor, ME, USA). P110δ−/− mice were
constructed by ourselves as described previously (19). Wild-type (WT) mice were purchased
from the Experimental Animal Center of Guangdong Province
(Guangzhou, China). The 12 mice (6 female and 6 male, weighted
20–25 g) were weaned on the 22nd day after birth and fed a normal
diet and food and water was free including 4% fat and 0.07%
cholesterol). The mice were maintained under a specific
pathogen-free conditions with 12-h light/dark cycles at 26–28°C and
50–65% humidity. DNA was extracted from the tail tissues according
to the MagBeads Tissues Gen DNA Extraction kit (D1700, Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China). The
expression levels of ApoE and P110δ were determined by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). The
primers used are as follows: ApoE, sense
5′-GCCTAGCCGAGGGAGAGCCG-3′, antisense 5′-TGTGACTTGGGAGCTCTGCAGC-3′;
and P110δ, sense 5′-CTGTCATCTCACCTTGCTCC-3′ and antisense
5′-AGCGAACCGCCCTATGAC-3′. The reaction conditions were as follows:
94°C for 3 min, followed by 35 cycles at 94°C for 20 sec, 65°C for
30 sec and 72°C for 15 sec. Mice with ApoE/P110δ deletion were
screened from the hybrid F2 generation and were used as
experimental mice, whereas ApoE−/− mice were used as
control mice. Mice in the experimental group (n=6) and control
group (n=6) were 50% female and 50% male. The present study was
approved by the Institutional Animal Care and Use Committee of The
Second Hospital of Shandong University (Jinan, China).
Characterizing myocarditis by
hematoxylin and eosin (H&E) staining
After obtaining mouse myocardial tissue samples
(n=6), the myocardium tissues were fixed for 24 h using 10%
formalin at room temperature and embedded in paraffin and sectioned
to 3–5 µm; tissue sections were floated in a water bath and placed
onto glass slides. The glass slides were then placed in staining
racks. Paraffin was cleared from the samples in three changes of
xylene (2 min per change). After hydrating the samples, the
sections were stained in hematoxylin solution for 1 min and were
washed under running tap water at room temperature for ≥5 min.
Samples were then stained in working eosin Y solution for 10 sec,
after which the samples were dehydrated and cleared in three
changes of xylene (2 min per change). The slides were viewed under
a light microscope. Myocarditis was characterized by inflammatory
cell infiltration, myocardial cell degeneration, necrosis and a
disordered myocardial arrangement (20).
Characterizing myocarditis by
echocardiogram examination
Cardiac function and morphology were monitored by M-
and B-mode transthoracic echocardiography, respectively (21). Left ventricular fractional
shortening (LVFS) and left ventricular ejection fraction (LVEF)
were assessed by cardiac magnetic resonance imaging in all mice
(n=6/group).
Counting of white blood cells and
monocytes
Ascites were collected from the mice following
sacrifice, and measurement of white blood cells was performed using
an automated blood cell analyzer (Beckman Coulter, Inc., Brea, CA,
USA). The whole blood was used to measure monocytes and
neutrophils. The number of cells was expressed as means ± standard
deviation of three independent measurements.
Western blotting
Proteins were extracted from mouse myocardial
tissues using general protein kits from Beyotime Institute of
Biotechnology (Haimen, China). All protein samples were adjusted to
equal concentrations via a Bicinchoninic Acid protein assay,
followed by the addition of bromophenol blue. Equal amounts of
proteins were loaded on 10% SDS-PAGE. A total of 6 µl protein
marker (EMD Millipore, Billerica, MA, USA) was added at the same
time. The protein samples were separated according to a
predetermined voltage. Subsequently, the protein was transferred to
nitrocellulose membranes. Then the membrane was blocked in room
temperature for 2 h with TBS containing 5% skim milk and 0.1%
Tween-20, and incubated with primary antibodies against caspase-3
(ab13585), Bax (ab32503), Bcl-2 (ab32124) and GAPDH (ab8245)
(Abcam, Cambridge, MA, USA) at a dilution of 1:1,000 at 4°C
overnight, followed by incubation with
horseradish-peroxidase-conjugated goat anti-rabbit (A11008,
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
anti-mouse IgG secondary antibodies (A-11077, Invitrogen; Thermo
Fisher Scientific, Inc.) for 60 min at room temperature. Detection
was performed using the LI-COR Odyssey Scanning Infrared
Fluorescence Imaging system (LI-COR Biosciences, Lincoln, NE,
USA).
Flow cytometry
The red blood cell lysis buffer, ACK Lysis Buffer
(Beyotme Institute of Biotechnology) was added to the blood
samples, and centrifuged at 350 × g for 5 min at 4°C. The
precipitate was then washed three times with 0.5% bovine serum
albumin (BSA, Beyotme Institute of Biotechnology) for 5 min.
Anti-CD4-fluorescein isothiocyanate antibody (F1773, 1:100;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added and
incubated for 30 min at 4°C in the dark, followed by centrifugation
at 350 × g for 5 min at 4°C. The precipitate was washed with 0.5%
BSA three times and the supernatant was discarded. The final
precipitate was resuspended with 200 µl 0.5% BSA for detection. The
protein was detected using FACSCanto™ II (Becton Dickinson,
Franklin Lakes, NJ, USA). The results are expressed as the means ±
standard deviation of three independent measurements.
Statistical analysis
All experimental data are expressed as the means ±
standard deviation and were analyzed by Image-Pro-Plus 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA) and Graph Pad Prism 5
(GraphPad Software, Inc., La Jolla, CA, USA). One-way analysis of
variance was used for multi-group comparisons followed by
Bonferroni method as a post hoc test for multiple comparisons.
Paired t-test was used to compare two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Construction of a mouse model
Male ApoE−/− mice and female
P110δ−/− mice (age, 6 weeks) were placed in one cage,
and F1 mice were identified by DNA detection. Male and female
ApoE−/− mice from the F1 generation were hybridized with
F1 generation P110δ−/− mice to obtain the F2 generation.
F2 generation mice were also identified with DNA detection.
ApoE−/− mice were selected as the control group, whereas
ApoE−/−/P110δ−/− mice from the F2 generation
were selected as the experimental group. The results of the
identification are presented in Fig.
1A; lanes 1, 6, 7, 8, 9 and 10 are ApoE+/− with two
bands at 165 and 330 bp; lanes 2, 3, 4, 5, 11 and 12 are ApoE
−/− with one band at 330 bp. As shown in Fig. 1B, lanes 1, 2 and 3 are
P110δ+/− heterozygous with two bands at 145 and 485 bp;
lanes 4, 5, 6, 7 and 8 are P110δ−/− homozygous with one
band at 485 bp.
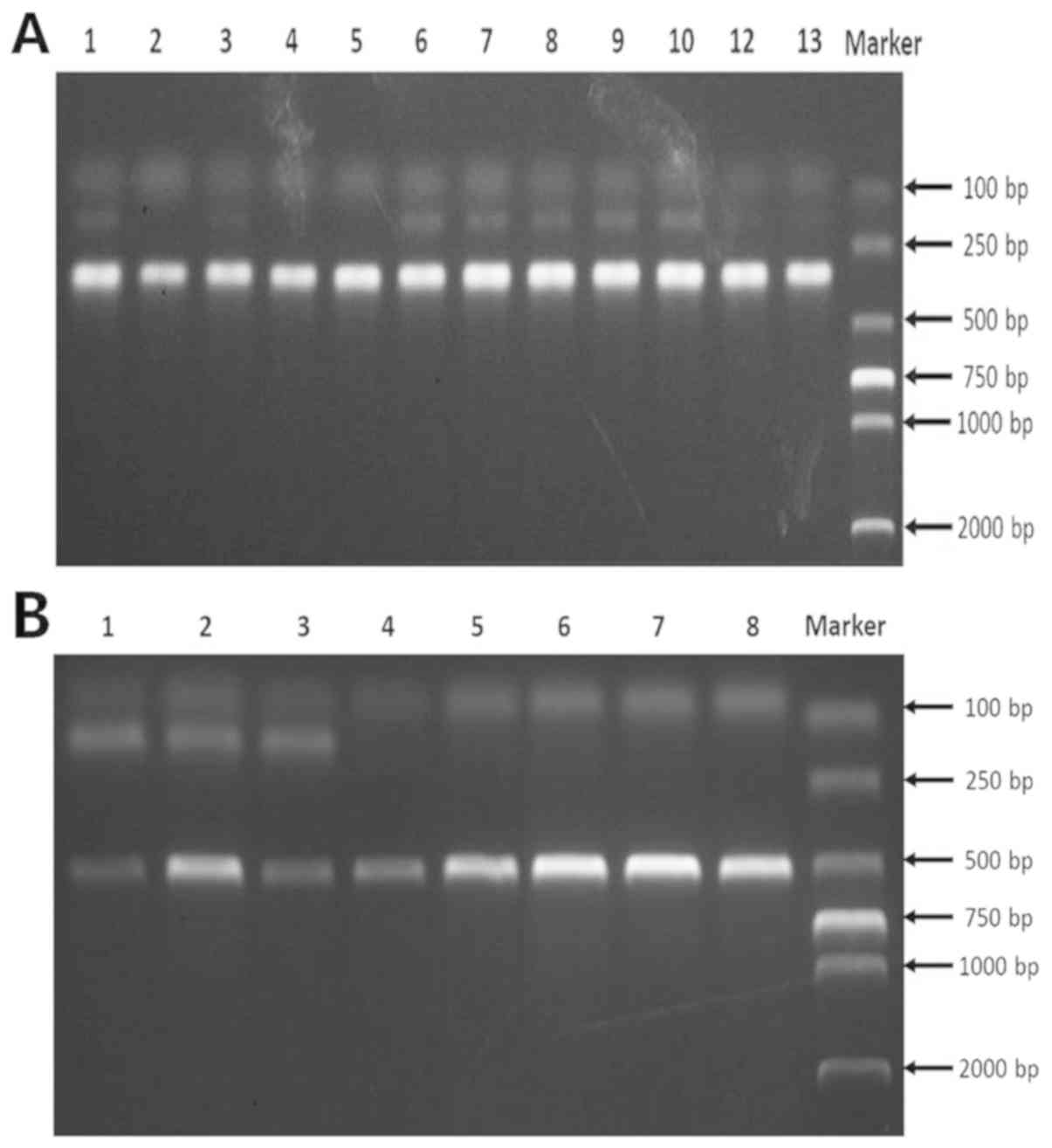 | Figure 1.Construction of a mouse model. (A)
Identification of ApoE−/− mice. ApoE+/− with
two bands at 165 and 330 bp. Lanes 1, 6, 7, 8, 9 and 10 are
ApoE+/− with two bands at 165 and 330 bp; Lanes 2, 3, 4,
5, 11 and 12 are ApoE−/−with one band at 330 bp. (B)
Identification of P110δ−/− mice. P110δ−/−
homozygous with one band at 485 bp. Lanes 1, 2 and 3 are
P110δ+/− heterozygous with two bands at 145 and 485 bp;
Lanes 4, 5, 6, 7 and 8 are P110δ−/− homozygous with one
band at 485 bp. ApoE, apolipoprotein E; P110δ, phosphoinositide
3-kinase catalytic subunit δ isoform. |
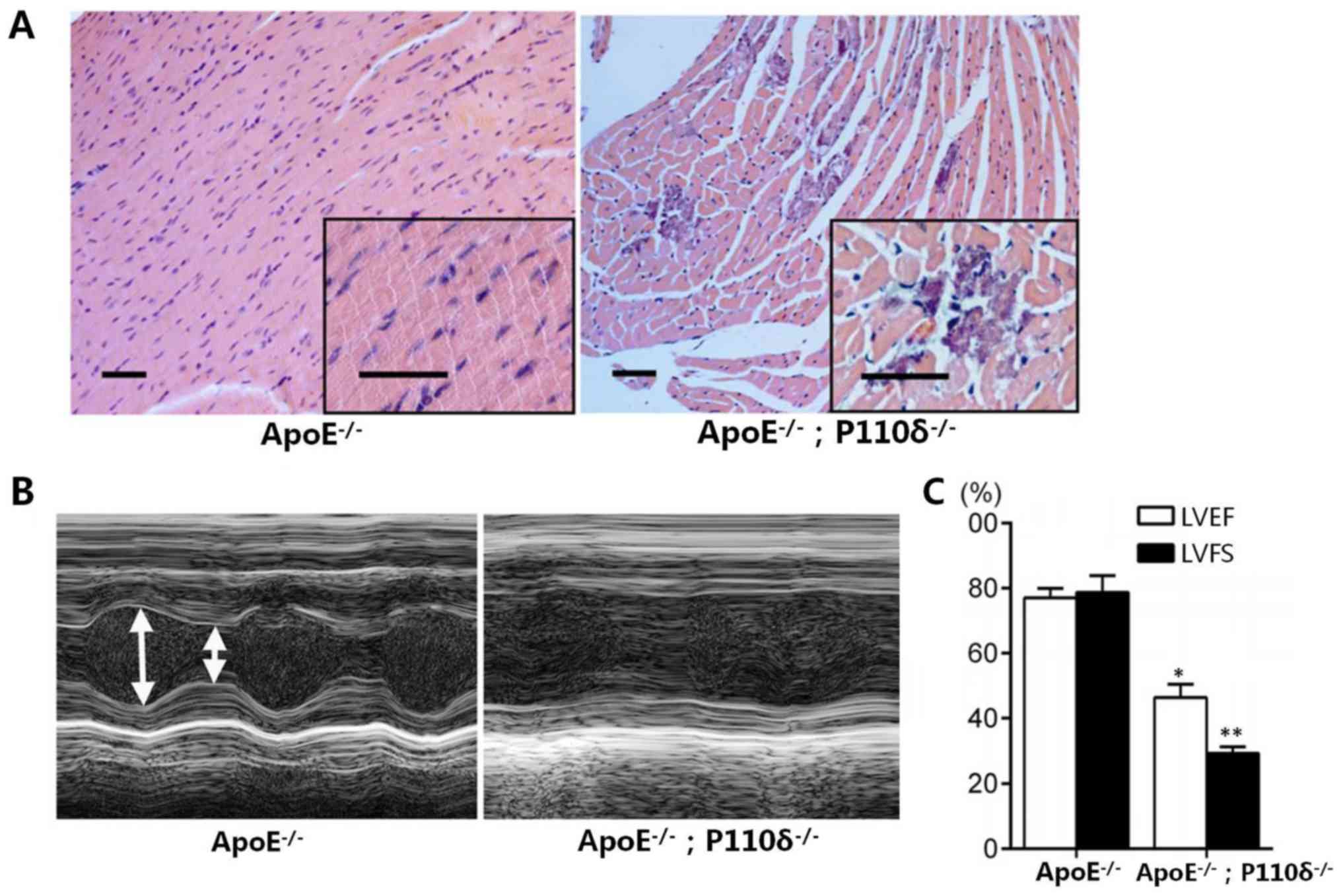
Deletion of P110δ promotes myocarditis
in mice
P110δ is involved in T and B cell differentiation,
lymphocyte maturation and the neutrophil chemotaxis process.
Therefore, the present study aimed to investigate whether the
absence of P110δ would induce myocarditis in ApoE−/−
mice. As shown in Fig. 2A, cells
were spindle-shaped and regular in ApoE−/− mice;
however, in the ApoE−/−/P110δ −/− mice the
nuclei of cells were smaller and the number of inflammatory cells
was markedly increased. The electrocardiogram results presented in
Fig. 2B demonstrated that
contractions in the control group were normal, whereas in the
ApoE−/−/P110δ−/− mice, the systolic and
diastolic contractions were notably smaller; and further
examination indicated that LVFS and LVEF were decreased by 38.5 and
61.3% compared with in the control group (Fig. 2C).
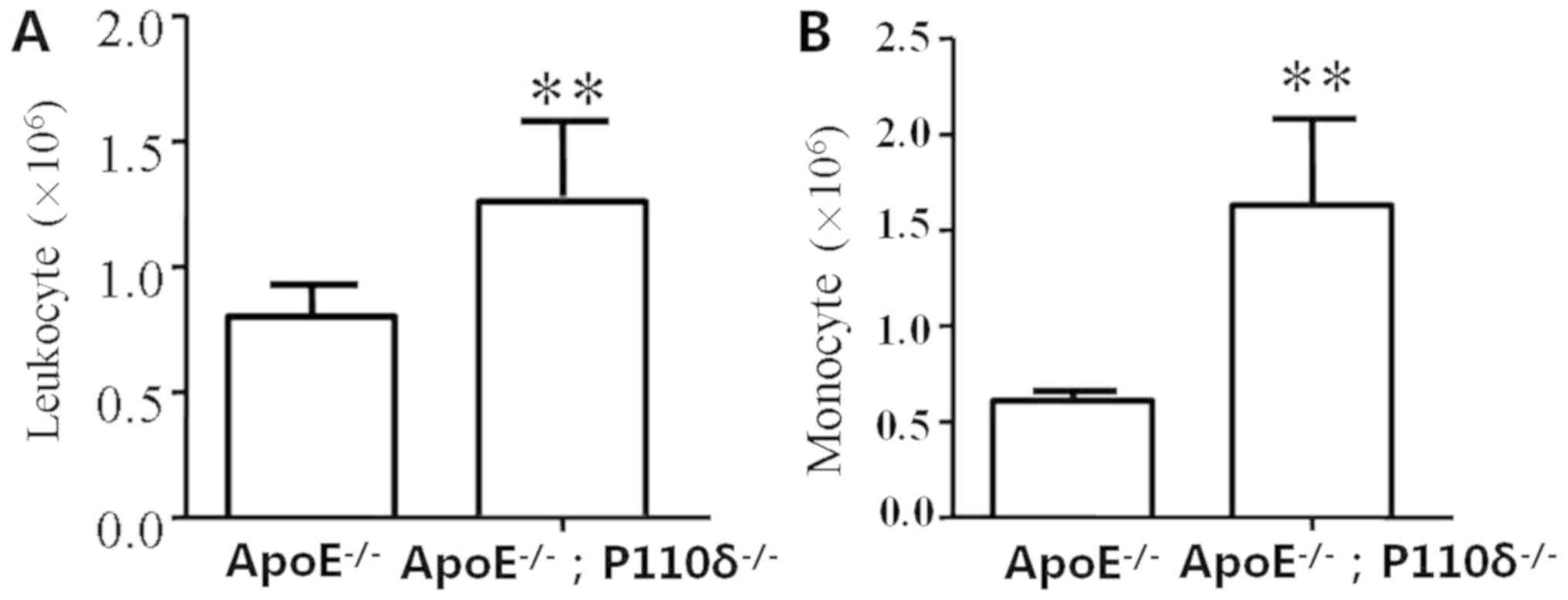
Deletion of P110δ promotes
infiltration of inflammatory cells
H&E staining revealed that infiltration of
inflammatory cells occurs in P110δ-deficient mice. Leukocyte
adhesion and infiltration is achieved through endothelial cells;
therefore, these functional alterations may lead to a series of
heart diseases. In particular, monocytes serve an important role in
initiating cell adhesion. Therefore, peritoneal cells were
extracted and analyzed in the present study. Blood cell analysis
demonstrated that the amount of white blood cells in the
experimental group was significantly higher than in the control
group, and there was a statistically significant difference
(Fig. 3A). In addition, the number
of monocytes in ApoE−/−/P110δ−/− mice was
significantly increased compared with in the control group
(Fig. 3B).
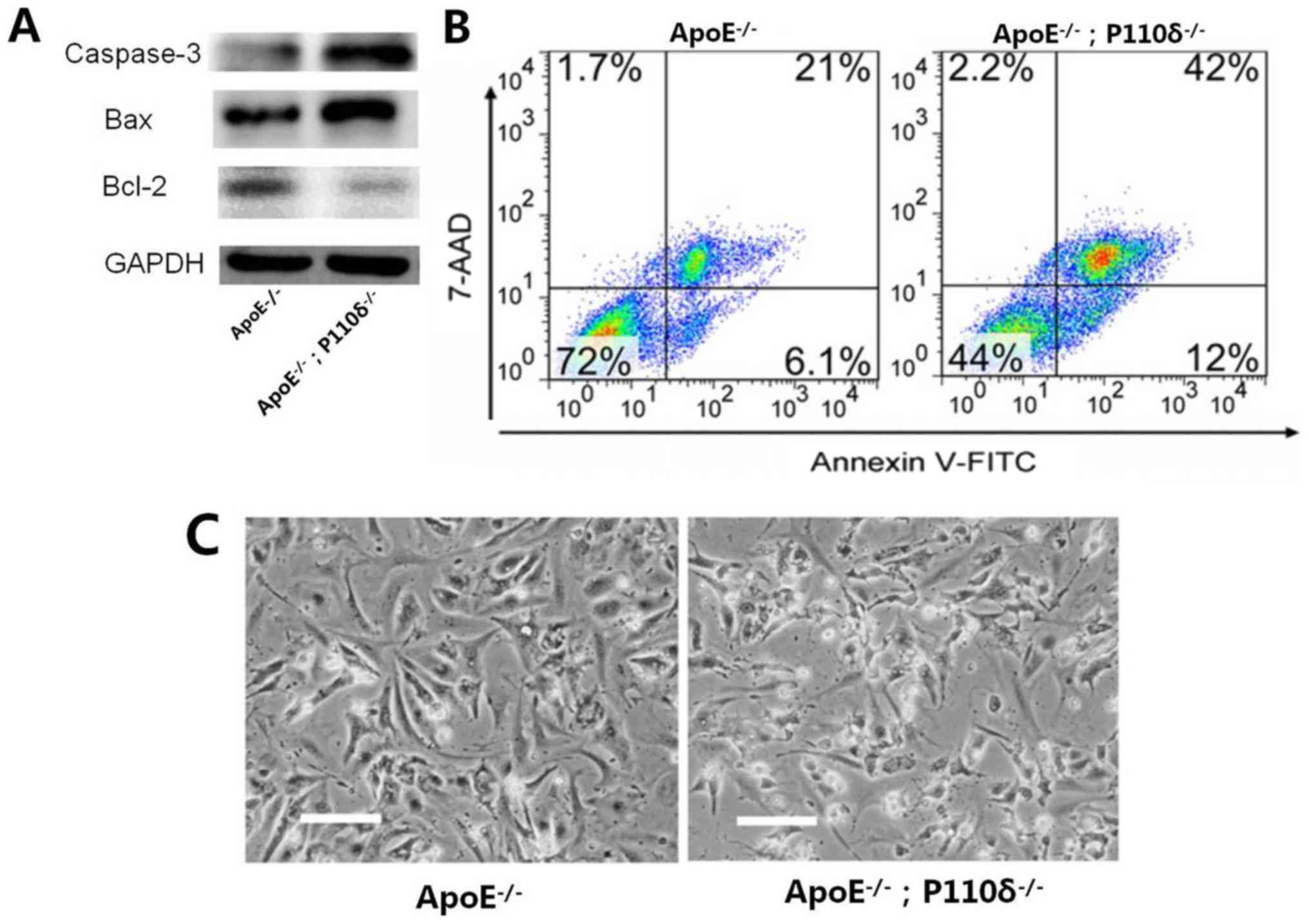
Deletion of P110δ promotes apoptosis
of mouse cardiomyocytes
Apoptosis is a process of programmed cell death,
which induced by naturally occurring cellular processes that is
different from pathological cell necrosis. Apoptosis of myocardial
cells in the two mouse groups was analyzed. Western blotting
demonstrated that the expression levels of Bcl-2 in
ApoE−/−/P110δ−/− mice were markedly decreased
compared with in the control group, while the expression levels of
Bax and caspase-3 were notably increased within
ApoE−/−/P110δ−/− mice (Fig. 4A). As shown in Fig. 4B, apoptosis of myocardial cells in
ApoE−/−/P110δ−/− mice was increased by 2-fold
compared with in the control group as measured by flow cytometry.
In addition, the morphology of myocardial cells in the control
group were spindle-shaped, whereas cells in the experimental group
appeared shrunken and apoptotic by florescence microscopy (Fig. 4C). Therefore, it may be
hypothesized that deletion of P110δ promotes apoptosis of mouse
cardiomyocytes in ApoE−/−/P110δ−/− mice.
Discussion
Numerous clinical and experimental animal studies
have confirmed that inflammation serves an important role in the
pathophysiology of myocarditis (22,23).
In an experimental model of myocarditis, it was revealed that
inflammatory cells, such as monocytes, macrophages and dendritic
cells, may infiltrate the vascular adventitia. These inflammatory
cells could improve myocarditis by increasing the infiltration of
inflammatory cells into the adventitia (24). The present study indicated that
cardiomyocytes were significantly infiltrated by inflammatory cells
and the nuclei were clearly disorganized in
ApoE−/−/P110δ−/− mice (data not shown). An
electrocardiogram revealed that the control group exhibited normal
systolic contractions; however, in
ApoE−/−/P110δ−/− mice systole and diastole
were markedly reduced, and LVFS and LVEF were significantly
decreased. Furthermore, the results revealed that
ApoE−/−/P110δ−/− mice had a significant
increase in leukocyte and monocyte levels. Apoptosis is a process
of programmed cell death. A previous study reported that
anti-apoptotic drugs could pass the blood-brain barrier to reach
the drug-target site, thereby reducing intracranial excitotoxicity
and release of cytochrome c. Blocking the release of
cytochrome c would prevent the transcriptional translation
of apoptotic genes, thereby increasing the expression of the
anti-apoptotic protein, Bcl-2, through a series of cascade
reactions, which have a role in the regulation of apoptosis
(25). Western blotting
demonstrated that the levels of apoptotic proteins, such as Bax and
caspase-3, were significantly increased in
ApoE−/−/P110δ−/− mice compared with in the
control group. Apoptosis of myocardial cells in
ApoE−/−/P110δ−/− mice was also higher than
that in the control group (42 vs. 21%). Furthermore, the morphology
of myocardial cells was spindle-shaped in the control group,
whereas, the cells were shrunken and apoptotic in the experimental
group. Therefore, it may be hypothesized that deletion of P110δ
promotes apoptosis of mouse cardiomyocytes in
ApoE−/−/P110δ−/− mice.
In conclusion, it may be hypothesized that deletion
of P110δ is an inducing factor in the development of myocarditis in
mice. In addition, P110δ may have an important role in the absence
of ApoE. The mechanism of action underlying the development of
myocarditis may be associated with monocyte infiltration and
apoptosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZZ, AYX, WW and FL conducted the experiments. QZZ,
AYX, AMP, LNC and WW drafted the manuscript and acquired and
analyzed the data, and made substantial contributions to the
concept and design of the present study. QZZ, AYX, WW, AMP, LNC and
FL interpreted the data and revised the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of The Second Hospital of Shandong
University (Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mensah K and Anderson JR: Diagnostic
investigations of adult cardiac disease. Surg (Oxford). 36:52–56.
2018. View Article : Google Scholar
|
|
2
|
Chen QQ, Chen M, Zhang LH, Zeng Y, Qi-Cai
L, Yang LQ and Lin XC: Costimulation blockade by combining CTLA4Ig
with anti-CD40L mAb markedly inhibits the inflammatory response of
experimental autoimmune myocarditis. Eur J Inflamm. 15:28–34. 2017.
View Article : Google Scholar
|
|
3
|
Ramos C, Becerril C, Montaño M,
García-De-Alba C, Ramírez R, Checa M, Pardo A and Selman M: FGF-1
reverts epithelial-mesenchymal transition induced by TGF-{beta}1
through MAPK/ERK kinase pathway. Am J Physiol Lung Cell Mol
Physiol. 299:L222–L231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adamson IY, Bowden DH, Cote MG and Witschi
H: Lung injury induced by butylated hydroxytoluene: cytodynamic and
biochemical studies in mice. Lab Invest. 36:26–32. 1977.PubMed/NCBI
|
|
5
|
Bachmeier C, Paris D, Beaulieu-Abdelahad
D, Mouzon B, Mullan M and Crawford F: A multifaceted role for apoE
in the clearance of beta-amyloid across the blood-brain barrier.
Neurodegener Dis. 11:13–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bangen KJ, Beiser A, Delano-Wood L, Nation
DA, Lamar M, Libon DJ, Bondi MW, Seshadri S, Wolf PA and Au R: APOE
genotype modifies the relationship between midlife vascular risk
factors and later cognitive decline. J Stroke Cerebrovasc Dis.
22:1361–1369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bell RD, Winkler EA, Singh I, Sagare AP,
Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, et
al: Apolipoprotein E controls cerebrovascular integrity via
cyclophilin A. Nature. 485:512–516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bender AR and Raz N: Age-related
differences in memory and executive functions in healthy APOE ε4
carriers: The contribution of individual differences in prefrontal
volumes and systolic blood pressure. Neuropsychologia. 50:704–714.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vanhaesebroeck B, Leevers SJ, Ahmadi K,
Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ and
Waterfield MD: Synthesis and function of 3-phosphorylated inositol
lipids. Annu Rev Biochem. 70:535–602. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marone R, Cmiljanovic V, Giese B and
Wymann MP: Targeting phosphoinositide 3-kinase: Moving towards
therapy. Biochim Biophys Acta. 1784:159–185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schu PV, Takegawa K, Fry MJ, Stack JH,
Waterfield MD and Emr SD: Phosphatidylinositol 3-kinase encoded by
yeast VPS34 gene essential for protein sorting. Science. 260:88–91.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kok K, Nock GE, Verrall EA, Mitchell MP,
Hommes DW, Peppelenbosch MP and Vanhaesebroeck B: Regulation of
p110delta PI 3-kinase gene expression. PLoS One. 4:e51452009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vanhaesebroeck B, Welham MJ, Kotani K,
Stein R, Warne PH, Zvelebil MJ, Higashi K, Volinia S, Downward J
and Waterfield MD: P110delta, a novel phosphoinositide 3-kinase in
leukocytes. Proc Natl Acad Sci USA. 94:4330–4335. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harris SJ, Foster JG and Ward SG: PI3K
isoforms as drug targets in inflammatory diseases: Lessons from
pharmacological and genetic strategies. Curr Opin Investig Drugs.
10:1151–1162. 2009.PubMed/NCBI
|
|
15
|
Al-Rasheed NM, Fadda LM, Attia HA, Ali HM
and Al-Rasheed NM: Quercetin inhibits sodium nitrite-induced
inflammation and apoptosis in different rats organs by suppressing
Bax, HIF1-α, TGF-β, Smad-2, and AKT pathways. J Biochem Mol
Toxicol. 31:2017. View Article : Google Scholar
|
|
16
|
Berndt A, Miller S, Williams O, Le DD,
Houseman BT, Pacold JI, Gorrec F, Hon WC, Liu Y, Rommel C, et al:
The p110 delta structure: Mechanisms for selectivity and potency of
new PI(3)K inhibitors. Nat Chem Biol. 6:117–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang HR, Cho SJ, Lee CG, Homer RJ and
Elias JA: Transforming growth factor (TGF)-beta1 stimulates
pulmonary fibrosis and inflammation via a Bax-dependent,
bid-activated pathway that involves matrix metalloproteinase-12. J
Biol Chem. 282:7723–7732. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang F, Li X, Gui Y, Qi C, Lu M, Dai C,
Wang H and Wang L: p110Delta inhibits monocyte infiltration by
thioglycollate-induced periotoneal inflammation but not HCD-induced
inflammation and atherosclerosis in APOE KO mice. Lipids.
50:839–846. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bucher K, Schmitt F, Mothes B,
Blumendeller C, Schäll D, Piekorz R, Hirsch E, Nürnberg B and
Beer-Hammer S: Deficiency of PI3-Kinase catalytic isoforms p110γ
and p110δ in mice enhances the IL-17/G-CSF axis and induces
neutrophilia. Cell Commun Signal. 15:282017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schindelin J, Arganda-Carreras I, Frise E,
Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S
and Schmid B: Fiji: An open-source platform for biological-image
analysis. Nat Methods. 9:676–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sehgal A, Allison BJ, Gwini SM, Miller SL
and Polglase GR: Cardiac morphology and function in preterm growth
restricted infants: Relevance for clinical sequelae. J Pediatr.
188:128–134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hueso M, De Ramon L, Navarro E, Ripoll E,
Cruzado JM, Grinyo JM and Torras J: Datasets for the validation of
the ‘in vivo’ siRNA-silencing of CD40 and for the detection of new
markers of atherosclerosis progression in ApoE-deficient mice. Data
Brief. 9:1105–1112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li M, Su Y, Yu Y, Yu Y, Wang X, Zou Y, Ge
J and Chen R: Dual roles of calpain in facilitating Coxsackievirus
B3 replication and prompting inflammation in acute myocarditis. Int
J Cardiol. 221:1123–1131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heymans S, Eriksson U, Lehtonen J and
Cooper LT Jr: The quest for new approaches in myocarditis and
inflammatory cardiomyopathy. J Am Coll Cardiol. 68:2348–2364. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Liu L, Yang L and Wang H:
Dracorhodin perchlorate induced human breast cancer MCF-7 cell
apoptosis through mitochondrial pathway. Mod Chin Med.
17:1263–1266. 2015.
|