Introduction
Although paraquat is one of the most widely used
herbicides, it is highly toxic and lethal (1,2).
Paraquat poisoning has been identified to cause severe damage to
the lungs, digestive tract, liver, kidney, heart and brain
(3). Nonetheless, lung damage is
the most apparent, which often culminates in death due to
respiratory failure (3). In fact,
the survival of the patients with paraquat poisoning is notably
affected by the development of pulmonary interstitial fibrosis
(4,5). Current treatments for paraquat
poisoning include hormones, antioxidants, immunosuppressants and
hemoperfusion (6,7). Cyclophosphamide is also commonly used
as an immunosuppressive therapy for paraquat poisoning (8,9);
however, it can cause severe adverse effects (10).
Tacrolimus (also known as FK506) is a macrolide
immunosuppressive drug, which inhibits the transcription of several
cytokine genes (NF-AT, IL-2) by binding with calcineurin (11). Tacrolimus was discovered to inhibit
T cell activation and T helper cell-dependent B cell proliferation
and it has been widely used in organ transplantation and for the
treatment of numerous types of autoimmune disease, such as
myasthenia gravis (11–14). However, to the best of our
knowledge, only a few studies have documented the use of tacrolimus
for the treatment of paraquat-induced lung injury and its
underlying mechanism of action remains unclear. A previous study by
Nagano et al (15)
indicated that tacrolimus inhibited collagen synthesis in murine
pulmonary fibroblasts treated with bleomycin. In addition,
Staab-Weijnitz et al (16)
demonstrated that the intracellular chaperone FK506 binding protein
10 (FKBP10) was upregulated in idiopathic pulmonary fibrosis
stromal fibroblasts. Furthermore, FKBP10 inhibition in primary
idiopathic pulmonary fibrosis fibroblasts reduced the expression of
fibrinogen and prevented collagen synthesis.
Fibrosis of various organs is a highly complex
process. Previous studies have suggested that the transforming
growth factor-β1/SMAD signaling pathway served an important role in
the process of organ fibrosis (17,18).
It has been shown that the upregulation of TGF-β1 is associated
with the pathological changes of pulmonary fibrosis and the
severity of the disease. Notably, TGF-β1 increased the formation of
connective tissue and prevented its degradation through the SMAD
signaling, thereby initiating pulmonary fibrosis (17,18).
After paraquat enters the human body, alveolar type
I and II epithelial cells will actively ingest paraquat, causing
serious lung injury (1). The
present study aimed to determine the role of tacrolimus as a
putative therapeutic agent for pulmonary injury in rat alveolar
epithelial type II cells (RLE-6TN cells) exposed to paraquat. The
potential effects of tacrolimus were evaluated in vitro by
analyzing the expression levels of inflammatory factors involved in
the TGF-β1/SMAD signaling pathway and known to serve a role in
pulmonary fibrosis. The present findings may provide supporting
evidence for the use of tacrolimus in the treatment of pulmonary
interstitial fibrosis induced by paraquat.
Materials and methods
Main reagents
Paraquat and tacrolimus were purchased from Shanghai
Aladdin Bio-Chem Technology Co., Ltd. High glucose DMEM and FBS
were obtained from HyClone; Cytiva. MTT reagent was purchased from
Amresco, LLC. Rat TGF-β1 (cat. no. CSB-E04727r), SMAD3 (cat. no.
CSB-EL021788RA), SMAD7 (cat. no. CSB-E09225r) and connective tissue
growth factor (CTGF; cat. no. CSB-E07876r) levels were analyzed
using ELISA kits purchased from Cusabio Technology LLC. BSA (cat.
no. A8020), the TGF-β1 receptor type I/II dual inhibitor
(LY2109761; cat. no. ab29286) and anti-SMAD3 antibody (cat. no.
ab40854) were obtained from Abcam, and the anti-SMAD7 antibody
(cat. no. 42-0400) was purchased from Thermo Fisher Scientific,
Inc. The Cy3 conjugated Goat Anti-Rabbit IgG secondary antibody
(cat. no. GB21303) was obtained from Wuhan ServiceBio Technology
Co., Ltd. RNA extracting fluid (cat. no. G3013) was obtained from
Wuhan ServiceBio Technology Co., Ltd. HyPure™ Molecular Biology
Grade Water (cat. no. SH30538.02) was obtained from HyClone.
RevertAid First Strand cDNA Synthesis kit was obtained from Thermo
Fisher Scientific, Inc. FastStart Universal SYBR Green Master (Rox)
was obtained from Roche Diagnostics. Primers were obtained from
Wuhan ServiceBio Technology Co., Ltd.
Cell culture
The rat alveolar epithelial type II RLE-6TN cell
line was obtained from the Beijing Beina Chuanglian Biotechnology
Research Institute (http://www.bnbio.com/default.html). RLE-6TN cells were
cultured in high glucose DMEM, supplemented with 10% FBS, and
maintained at 37°C with 5% CO2. Upon reaching 70–80%
confluence, the cells were incubated with 0.25% trypsin solution
and passaged at a ratio of 1:3. The culture medium was changed
every 48 h. Cells in the exponential growth phase were used for
further experiments.
MTT assay
RLE-6TN cells in logarithmic growth phase were
digested with trypsin to prepare cell suspension. A total of
1×104 cells were inoculated into 96-well plate with 200
µl cells in each well. The cells were cultured in CO2
(5%) incubator overnight at 37°C and adhered to the wall. The
marginal pores were filled with sterile PBS. Following 24 h of
incubation, the culture medium was discarded and new culture medium
containing different concentrations of paraquat (10, 50, 100, 200,
400, 600, 800, 1,000 or 2,000 nmol/l) was added for an additional
24 h at 37°C. Following the treatment, 20 µl MTT (5 g/l) was added
to each well and incubated at 37°C for 4 h. Culture medium
containing MTT reagent was removed, 150 µl DMSO was added into each
well and spun at low speed 120 × g for 10 min to fully dissolve the
crystal. The absorbance was measured at 490 nm (A490nm;
RT-2100c; Rayto Life and Analytical Sciences Co., Ltd.) in order to
assess the paraquat concentration required for 50% inhibition of
cellular viability (IC50). GraphPad Prism 8 was used to
calculate the IC50 (GraphPad Software, Inc.). In
addition, an inhibitory rate (%) was calculated using the following
formula: [(A490nm of control group-A490nm of
experimental group)/A490nm of control group] ×100.
In further experiments, RLE-6TN cells
(1×104 cells/well) were treated with a range of
concentrations of tacrolimus (0, 0.1, 1, 10 or 20 ng/ml) for 4 h at
37°C and subsequently incubated in the presence of tacrolimus and
200 nmol/l paraquat for 24 h at 37°C to determine the effects of
different concentrations of tacrolimus on the survival rate of
RLE-6TN cells exposed to paraquat. LY2109761 was also incubated
with RLE-6TN cells (1×104 cells/well) at a range of
concentrations (0, 1, 5, 10, 20 or 30 µmol/l) for 16 h at 37°C and
subsequently incubated in the presence of LY2109761 and 200 nmol/l
paraquat for 16 h at 37°C. Following these treatments, the
inhibitory rate (%) was performed as described above.
Experimental groupings
RLE-6TN cells were divided into the following seven
groups: i) control group, which contained untreated cells incubated
in culture medium for 24 h at 37°C; ii) paraquat group, which
contained cells cultured in DMEM containing 200 nmol/l paraquat for
24 h at 37°C; iii) tacrolimus group, which was comprised of cells
cultured in DMEM containing 10 ng/ml tacrolimus for 4 h and
subsequently in DMEM containing 10 ng/ml tacrolimus and 200 nmol/l
paraquat for 24 h at 37°C; and iv-vii) LY2109761 groups, which was
comprised of cells cultured in DMEM containing 5 µmol/l LY2109761
for 16 h and subsequently in DMEM containing 5 µmol/l LY2109761 and
200 nmol/l paraquat for 1, 4, 8 or 16 h at 37°C, which was used to
assess the action of LY2109761 at different time points. Each group
had three experimental wells.
ELISAs
A total of 106 RLE-6TN cells/ml were
diluted in PBS (pH 7.2–7.4) and lysed using repeated freeze-thaw
cycles. The cells were frozen below −20°C and thawed at room
temperature ten times; due to the formation of ice particles in the
cells, and the increase in salt concentration in the cell solution,
the cells swelled and the cell structure was broken. The samples
were then centrifuged at 3,000 × g for 20 min at room temperature.
Following centrifugation, the supernatant was collected and the
concentrations of TGF-β1, SMAD3, SMAD7 and CTGF were detected using
their respective ELISA kits, according to the manufacturers'
protocols.
Immunofluorescence staining
RLE-6TN cells (1×104 cells/well) were
fixed with 4% paraformaldehyde for 30 min at room temperature and
washed with PBS three times for 5 min each time. A total of 50–100
µl 0.5% Triton X-100 was added, and the samples were incubated at
room temperature for 10 min. The samples were subsequently washed
with PBS three times for 5 min and incubated with 5% BSA for 30 min
at room temperature. The primary antibodies (1:200, LY2109761
inhibitor; 1:500, anti-SMAD3 antibody; 1:100, anti-SMAD7 antibody)
were diluted in PBS and added dropwise to the samples, then
incubated overnight at 4°C. Following the primary antibody
incubation, the samples were washed three times with PBS for 5 min
each time and incubated with a Cy3 conjugated Goat Anti-Rabbit IgG
secondary antibody (1:300) at room temperature for 50 min. The
samples were then washed three times with PBS for 5 min each time
and subsequently stained with 0.5 µg/ml DAPI dye at room
temperature for 10 min. The samples were washed three times with
PBS for 5 min each time, dried, then the surface containing the
cells was sealed with anti-fluorescence quenching, which involved
adding a sealing tablet on a clean slide, holding the cells on the
slide onto the sealing agent. The stained slides were visualized
under fluorescence microscope (magnification, ×200 and ×400;
Eclipse Ci-L; Nikon Corporation) and the images were analyzed using
ImageJ 1.51K (National Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
The mRNA expression levels of TGF-β1, SMAD3, SMAD7
and CTGF were analyzed using RT-qPCR. Briefly, total RNA was
extracted from RLE-6TN cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was then
reverse transcribed into cDNA using RevertAid First Strand cDNA
Synthesis kit, according to the manufacturer's instructions. Target
genes were amplified by qPCR using FastStart Universal SYBR Green
Master on a StepOne Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and the expression levels were
normalized to the internal reference gene, β-actin. The following
thermocycling conditions were used for the qPCR: Initial
denaturation at 95°C for 10 min; followed by 40 cycles of
denaturation at 95°C for 15 sec and annealing/elongation at 60°C
for 60 sec. The melting curve was analyzed via heating at 0.3°C
every 15 sec from 60–90°C. The primer sequences used for the qPCR
are presented in Table I. qPCR was
performed and the data were analyzed using ABI 7500 Real-Time PCR
(Thermo Fisher Scientific, Inc.). The relative gene expression
levels were calculated using the 2−ΔΔCq method (19).
 | Table I.Primer sequences used for the reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for the reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′→3′) | Product length,
bp |
|---|
| β-actin | F:
TGCTATGTTGCCCTAGACTTCG | 240 |
|
| R:
GTTGGCATAGAGGTCTTTACGG |
|
| Transforming growth
factor-β1 | F:
CTTTAGGAAGGACCTGGGTTG | 140 |
|
| R:
GGTTGTGTTGGTTGTAGAGGG |
|
| SMAD3 | F:
CGAGAACACTAACTTCCCCGCT | 112 |
|
| R:
GTGGTTCATCTGGTGGTCGCTA |
|
| SMAD7 | F:
TCGGAAGTCAAGAGGCTGTGTT | 148 |
|
| R:
GTTTGAGAAAATCCATCGGGTA |
|
| Connective tissue
growth factor | F:
CCAACTATGATGCGAGCCAACT | 272 |
|
| R:
TTAGCCCGGTAGGTCTTCACACT |
|
Statistical analysis
SPSS 18.0 (SPSS, Inc.) statistical software was used
to analyze the data. Each experiment was repeated three times. All
data are presented as the mean ± SD. A Shapiro-Wilk test was used
to analyze the distribution of the data and multi-group comparisons
were performed using a Tukey's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of paraquat, tacrolimus and
LY2109761 on the viability of RLE-6TN cells
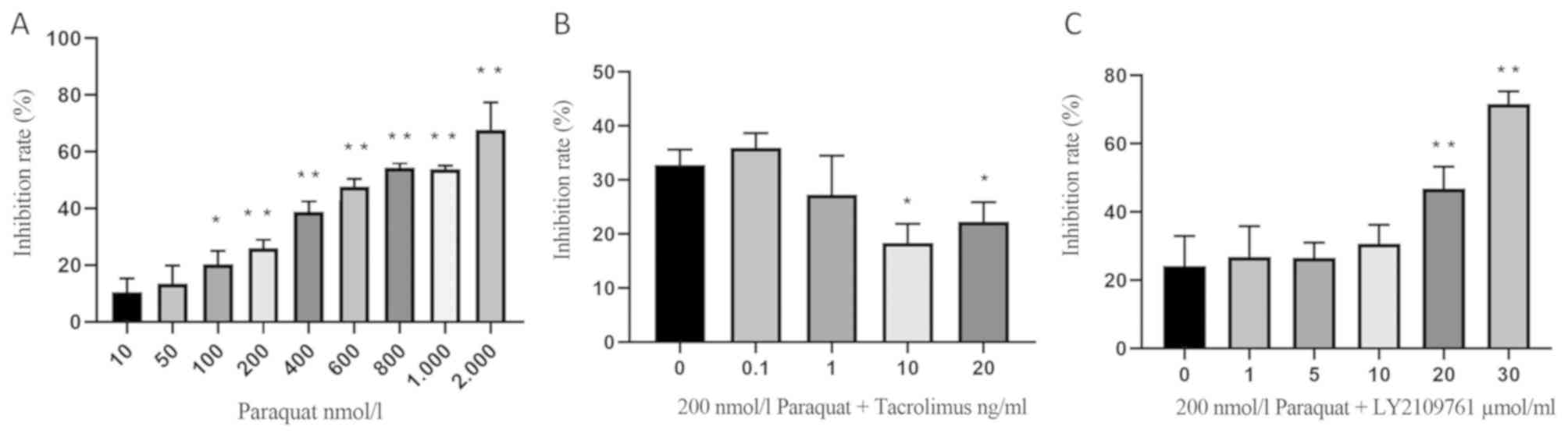
RLE-6TN cells were treated with 10, 50, 100, 200,
400, 600, 800, 1,000 or 2,000 nmol/l paraquat for 24 h and the cell
inhibitory rate was determined using an MTT assay; the results
revealed that the cell inhibitory rate increased with increasing
concentrations of paraquat (Fig.
1A). Notably, the IC50 of paraquat was estimated to
be 700 nmol/l; however, this induced high levels of cell death.
Therefore, in subsequent experiments, 200 nmol/l paraquat was used,
corresponding to an inhibitory rate of 26.05±2.99% (Fig. 1A).
In addition, RLE-6TN cells were pretreated with 0.1,
1, 10 or 20 ng/ml tacrolimus for 4 h, then incubated with 200
nmol/l paraquat for an additional 24 h. The inhibition rate of 10
ng/ml tacrolimus on paraquat-exposed alveolar type II epithelial
cells was 18.40±3.49%. Therefore, this concentration was used for
subsequent experiments (Fig.
1B).
Lastly, RLE-6TN cells were cultured for 16 h in
medium containing 1, 5, 10, 20 or 30 µmol/l LY2109761 and 200
nmol/l paraquat (Fig. 1C). The
inhibitory rate was significantly increased following the treatment
with 20 and 30 µmol/l LY2109761. Furthermore, the IC50
of LY2109761 was discovered to be 20 µmol/l; however, this
concentration resulted in increased cell death. The inhibitory rate
caused by 5 µmol/l was 26.56±4.49%. (Fig. 1C), and thus this concentration was
used in further experiments.
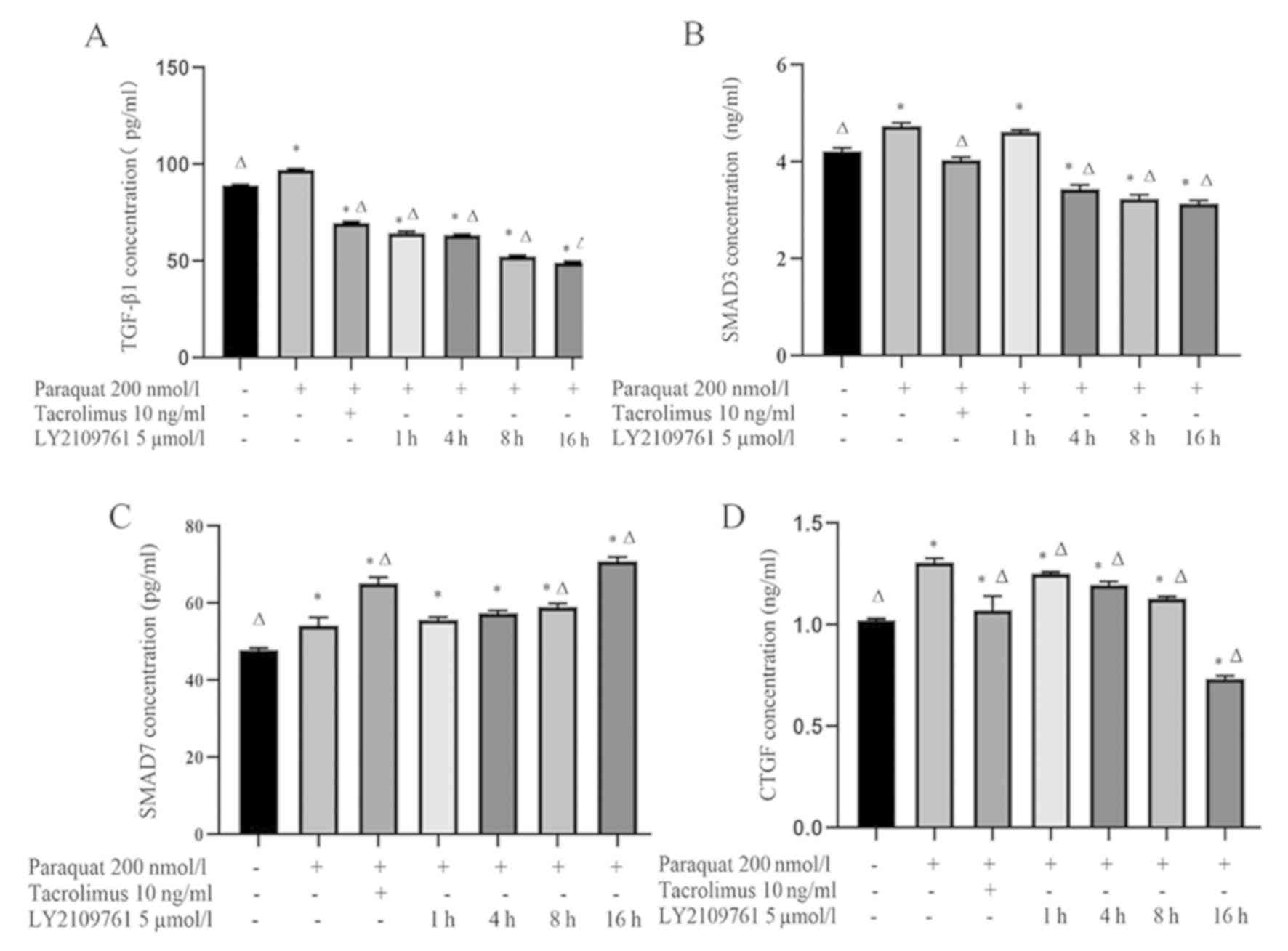
Tacrolimus decreases the concentration
of TGF-β1 and SMAD3 in the supernatant of paraquat treated cells,
and increases the concentration of SMAD7
The concentration of TGF-β1 in the supernatant of
RLE-6TN cells treated with 200 nmol/l paraquat was significantly
increased compared with the control group (Fig. 2A). However, the concentration of
TGF-β1 in the tacrolimus group was significantly decreased compared
with the control and paraquat groups. Notably, the levels of TGF-β1
gradually decreased following a 1, 4, 8 and 16 h incubation with 5
µmol/l LY2109761 compared with the paraquat group (Fig. 2A).
In addition, the concentration of SMAD3 in the
paraquat group was significantly increased compared with the
control cells (Fig. 2B). Following
the treatment with tacrolimus, SMAD3 levels were significantly
decreased compared with the paraquat groups. Moreover, the
concentration of SMAD3 in RLE-6TN cells was decreased following the
treatment with LY2109761 for 4, 8 and 16 h compared with the
paraquat and control groups (Fig.
2B).
Compared with the control group and paraquat group,
the concentration of SMAD7 increased significantly after tacrolimus
treatment. Compared with paraquat group, the levels of SMAD7 in
LY2109761 8 and 16 h groups were significantly increased (Fig. 2C).
Finally, the concentration of CTGF in the paraquat
group was significantly increased compared with the control group.
However, CTGF levels in cells pretreated with tacrolimus were
significantly decreased compared with the paraquat group.
Consistent with the treatment with tacrolimus, CTGF levels in the
LY2109761 groups were gradually decreased with time compared with
the paraquat group (Fig. 2D).
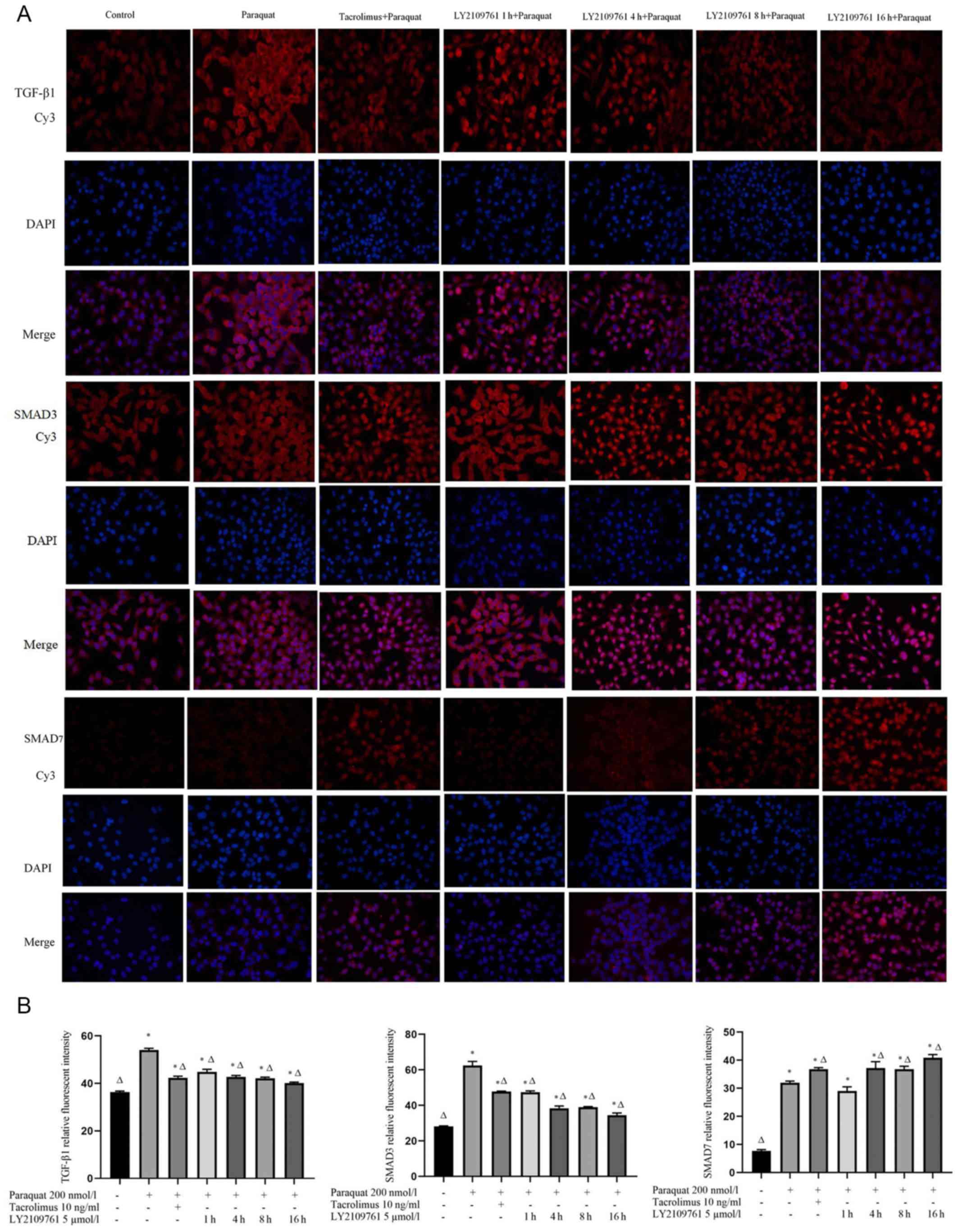
Tacrolimus downregulates the
expression levels of TGF-β1 and SMAD3 in paraquat treated cells and
upregulates the expression of SMAD7
The TGF-β1, SMAD3 and SMAD7 proteins were discovered
to be localized in both the nucleus and cytoplasm (Fig. 3A). The expression levels of TGF-β1
and SMAD 3 in the paraquat group were upregulated compared with the
control group. The pretreatment with tacrolimus downregulated the
expression levels of TGF-β1 and SMAD3 compared with the cells
exposed to paraquat alone, while the expression levels of SMAD7
were upregulated in the presence of tacrolimus compared with the
paraquat group. The treatment with LY2109761 downregulated the
expression levels of TGF-β1 and SMAD3. The expression levels of
SMAD7 were upregulated following a 4 h incubation with LY2109761
compared with paraquat treatment alone (Fig. 3A and B).
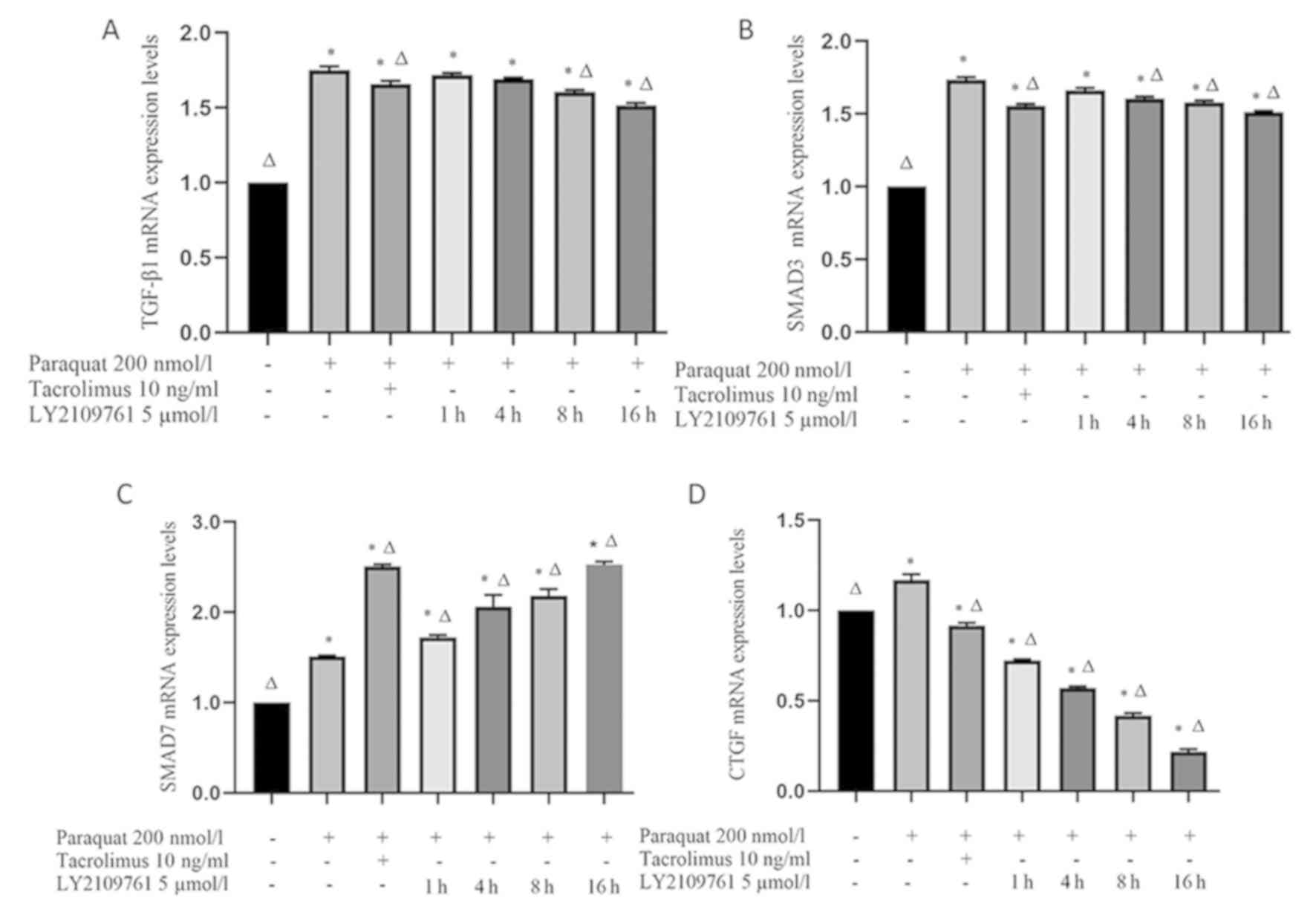
Tacrolimus downregulates the mRNA
expression levels of TGF-β1, SMAD3 and CTGF in paraquat treated
cells, and upregulates the expression of SMAD7 mRNA
The expression levels of TGF-β1 and SMAD3 in RLE-6TN
cells exposed to paraquat were significantly upregulated compared
with the control group (Fig. 4A and
B). However, both TGF-β1 and SMAD3 mRNA expression levels were
significantly downregulated following tacrolimus treatment compared
with the paraquat group. Following the treatment with LY2109761,
the expression levels of TGF-β1 gradually decreased over time; at
the 8 and 16 h time points, TGF-β1 mRNA expression levels in the
LY2109761 groups were significantly downregulated compared with the
paraquat group. Similarly, SMAD3 mRNA expression levels were also
downregulated following 4, 8 and 16 h of LY2109761 treatment
compared with the paraquat group (Fig.
4A and B).
SMAD7 mRNA expression levels in RLE-6TN cells were
significantly upregulated following paraquat exposure compared with
the control group (Fig. 4C).
Furthermore, following tacrolimus pretreatment, SMAD7 expression
levels were significantly upregulated compared with paraquat
treatment alone. Notably, SMAD7 mRNA expression levels were also
significantly upregulated following the treatment with LY2109761
for 1, 4, 8 and 16 h compared with the paraquat and control groups
(Fig. 4C).
Finally, CTGF expression levels were discovered to
be significantly upregulated in paraquat-exposed cells compared
with the control group (Fig. 4D);
however, tacrolimus pretreatment significantly downregulated the
mRNA expression levels of CTGF compared with the control and
paraquat groups. Similarly, the expression levels of CTGF in the
LY2109761 treatment for 1, 4, 8 and 16 h groups were significantly
downregulated compared with those in the paraquat and control
groups (Fig. 4D).
Discussion
The common poisoning method of paraquat poisoning is
via oral administration into the digestive system. The toxicant is
absorbed into the blood via the digestive tract, and the clinical
manifestations associated with poisoning appear (20). Type I and II epithelial cells of
the lung and Clara cells of the trachea include a polyamine
transport system, which has been discovered to facilitate the
transport of paraquat to the lungs (21). This process was reported to be
characterized by inflammatory cell infiltration and pulmonary
fibrosis in the lung tissue, and the transition of epithelial cells
into interstitial cells was identified as an important pathological
process of pulmonary fibrosis (22,23).
As a result, alveolar epithelial cells were revealed to lose
polarity, leading to cytoskeletal reconstruction and a
spindle-shape type cellular morphology (22,23).
The TGF-β1/SMAD signaling pathway was identified to be involved in
pulmonary fibrosis. Briefly, TGF-β1 binding to TGF-β receptor leads
to the phosphorylation of SMAD2 and SMAD3, and phosphorylated SMAD2
and SMAD3 subsequently form a complex with the phosphorylated form
of SMAD4 (24). This complex then
translocates to the nucleus and interacts with nuclear
transcription factors, such as CTGF, where it regulates gene
transcription (25). CTGF has been
discovered to promote the transcription of genes involved in cell
proliferation, cell adhesion and migration, as well as angiogenesis
and other biological functions (25,26).
Meanwhile, SMAD6 and SMAD 7 were reported to be strong inhibitors
of SMAD2 and SMAD3 activation, resulting in the negative regulation
of the TGF-β1/SMAD signaling pathway, and thus could prevent
fibrosis (27,28). SMAD 6 and SMAD 7 were also
identified to serve as intracellular antagonists of the TGF-β
receptor (27,28).
In the present study, the levels of TGF-β1, SMAD 3
and CTGF were increased in RLE-6TN cells following paraquat
exposure. However, tacrolimus treatment reversed this effect and
led to TGF-β1, SMAD3 and CTGF downregulation, and SMAD7
upregulation. Moreover, the TGF-β receptor inhibitor LY2109761
produced similar effects to tacrolimus treatment. These findings
suggested that tacrolimus may inhibit fibrosis through the
TGF-β1/SMAD signaling pathway. Tacrolimus was found to inhibit the
fibrosis of alveolar type II epithelial cells by inhibiting the
expression of TGF-β1, SMAD 3 and CTGF, which are all known to
promote fibrosis. Conversely, tacrolimus also prevented fibrosis
through upregulating SMAD7, a protein known to inhibit fibrosis in
alveolar type II epithelial cells. Therefore, due to its strong
immunosuppressive effects, and its ability to inhibit the
transformation of epithelial cells into mesenchymal cells,
tacrolimus may represent a promising drug candidate for the
treatment of acute paraquat poisoning.
Nonetheless, the present study had several
limitations. Firstly, the current findings were solely based on
in vitro experimentation and thus, the effects of tacrolimus
require further validation in vivo. Other aspects also
require further validation, including the effect of tacrolimus on
cell viability, apoptosis and the necrosis of RLE-6TN cells, for
instance. Moreover, the relationship between tacrolimus and
fibrosis markers, such as N-cadherin and E-cadherin, remains to be
examined. Lastly, whether tacrolimus also affects the dynamics of
SMAD3 and SMAD7 phosphorylation, the hallmark of SMAD signaling
pathway activation, also requires further study.
In conclusion, the present study demonstrated that
paraquat can increase the expression levels of TGF-β1, SMAD3 and
CTGF in RLE-6TN cells, and tacrolimus can inhibit the increase of
these pro-fibroinflammatory factors. Moreover, tacrolimus can serve
an antifibrotic role by increasing the expression of the fibrosis
inhibitor SMAD7, which reduces the lung injury caused by
paraquat.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Shandong Key
Research and Development Plan Project (grant nos. 2015GSF118038 and
2016GSF201041).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XJ conceived the study; ZZ and QN contributed
significantly to the data analysis and manuscript preparation; YR
performed the data analysis and wrote the manuscript; and BK and LK
interpreted the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
ORCID nos.: YR, 0000-0002-2136-0123; XJ,
0000-0002-2277- 6817; ZZ, 0000-0002-2469-1577; QN,
0000-0003-0039-9202; BK, 0000-0002-0312-686X; LK,
0000-0002-0988-1022.
References
|
1
|
Dinis-Oliveira RJ, Duarte JA,
Sánchez-Navarro A, Remião F, Bastos ML and Carvalho F: Paraquat
poisonings: Mechanisms of lung toxicity, clinical features, and
treatment. Crit Rev Toxicol. 38:13–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jeyaratnam J: Acute pesticide poisoning: A
major global health problem. World Health Stat Q. 43:139–144.
1990.PubMed/NCBI
|
|
3
|
Gil HW, Hong JR, Jang SH and Hong SY:
Diagnostic and therapeutic approach for acute paraquat
intoxication. J Korean Med Sci. 29:1441–1449. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamashita M, Yamashita M and Ando Y: A
long-term follow-up of lung function in survivors of paraquat
poisoning. Hum Exp Toxicol. 19:99–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He X, Wang L, Szklarz G, Bi Y and Ma Q:
Resveratrol inhibits paraquat-induced oxidative stress and
fibrogenic response by activating the nuclear factor erythroid
2-related factor 2 pathway. J Pharmacol Exp Ther. 342:81–90. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eddleston M, Wilks MF and Buckley NA:
Prospects for treatment of paraquat-induced lung fibrosis with
immunosuppressive drugs and the need for better prediction of
outcome: A systematic review. QJM. 96:809–824. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qian J, Ye Y, Lv L, Zhu C and Ye S: FTY720
attenuates paraquat-induced lung injury in mice. Int
Immunopharmacol. 21:426–431. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Botella DMJ and Belenguer TJ: Paraquat
poisoning. A study of 29 cases and evaluation of the effectiveness
of the ‘Caribbean scheme’. Med Clin (Barc). 115:530–533.
2000.PubMed/NCBI
|
|
9
|
Gao J, Feng S and Li Y: Prolonged low-dose
cyclophosphamide treatment after pulse therapy attenuates lung
injury in rats with paraquat intoxication. Korean J Intern Med.
33:1137–1142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barnes H, Holland AE, Westall GP, Goh NSL
and Glaspole IN: Cyclophosphamide for connective tissue
disease-associated interstitial lung disease. Cochrane Database Sys
Rev. 1:CD0109082018.
|
|
11
|
Thomson AW and Starzl TE: New
immunosuppressive drugs: Mechanistic insights and potential
therapeutic advances. Immunol Rev. 136:71–98. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Artz MA, Boots JMM, Ligtenberg G, Roodnat
JI, Christiaans MHL, Vos PF, Blom HJ, Sweep FCG, Demacker PNM and
Hilbrands LB: Improved cardiovascular risk profile and renal
function in renal transplant patients after randomized conversion
from cyclosporine to tacrolimus. J Am Soc Nephrol. 14:1880–1888.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Grady JG, Hardy P, Burroughs AK and
Elbourne D; UK; Ireland Liver Transplant Study Group, : Randomized
controlled trial of tacrolimus versus microemulsified cyclosporin
(TMC) in liver transplantation: Poststudy surveillance to 3 years.
Am J Transplant. 7:137–141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim YH, Shin HY and Kim SM: Long-term
safety and efficacy of tacrolimus in myasthenia gravis. Yonsei Med
J. 60:633–639. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagano J, Iyonaga K, Kawamura K, Yamashita
A, Ichiyasu H, Okamoto T, Suga M, Sasaki Y and Kohrogi H: Use of
tacrolimus, a potent antifibrotic agent, in bleomycin-induced lung
fibrosis. Eur Respir J. 27:460–469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Staab-Weijnitz CA, Fernandez IE, Knüppel
L, Maul J, Heinzelmann K, Juan-Guardela BM, Hennen E, Preissler G,
Winter H, Neurohr C, et al: FK506-binding protein 10, a potential
novel drug target for idiopathic pulmonary fibrosis. Am J Resp Crit
Care. 192:455–467. 2015. View Article : Google Scholar
|
|
17
|
Dhainaut JF, Charpentier J and Chiche JD:
Transforming growth factor-beta: A mediator of cell regulation in
acute respiratory distress syndrome. Crit Care Med. 31 (Suppl
4):S258–S264. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bartram U and Speer CP: The role of
transforming growth factor beta in lung development and disease.
Chest. 125:754–765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sukumar CA, Shanbhag V and Shastry AB:
Paraquat: The poison potion. Indian J Crit Care Med. 23 (Suppl
4):S263–S266. 2019.PubMed/NCBI
|
|
21
|
Hoet PH, Lewis CP, Demedts M and Nemery B:
Putrescine and paraquat uptake in human lung slices and isolated
type II pneumocytes. Biochem Pharmacol. 48:517–524. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Willis BC and Borok Z: TGF-beta-induced
EMT: Mechanisms and implications for fibrotic lung disease. Am J
Physiol Lung Cell Mol Physiol. 293:L525–L534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim KK, Kugler MC, Wolters PJ, Robillard
L, Galvez MG, Brumwell AN, Sheppard D and Chapman HA: Alveolar
epithelial cell mesenchymal transition develops in vivo during
pulmonary fibrosis and is regulated by the extracellular matrix.
Proc Natl Acad Sci USA. 103:13180–13185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blank U and Karlsson S: The role of Smad
signaling in hematopoiesis and translational hematology. Leukemia.
25:1379–1388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chung AC, Zhang H, Kong YZ, Tan JJ, Huang
XR, Kopp JB and Lan HY: Advanced glycation end-products induce
tubular CTGF via TGF-beta-independent Smad3 signaling. J Am Soc
Nephrol. 21:249–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen QK, Lee K, Radisky DC and Nelson CM:
Extracellular matrix proteins regulate epithelial-mesenchymal
transition in mammary epithelial cells. Differentiation.
86:126–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krafft E, Lybaert P, Roels E, Laurila HP,
Rajamaki MM, Farnir F, Myllarniemi M, Day MJ, Mc EK and Clercx C:
Transforming growth factor beta 1 activation, storage, and
signaling pathways in idiopathic pulmonary fibrosis in dogs. J Vet
Intern Med. 28:1666–1675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nie Y, Li S, Yi Y, Su W, Chai X, Jia D and
Wang Q: Effects of astragalus injection on the TGFβ/Smad pathway in
the kidney in type 2 diabetic mice. BMC Complement Altern Med.
14:1482014. View Article : Google Scholar : PubMed/NCBI
|