Introduction
Epilepsy affects ~50 million people worldwide
(1). Currently, epilepsy is the
second most common neurological disorder after headache; in China
alone, there are >9 million epileptic patients and the
prevalence is increasing every year (2). Almost one third of patients with
epilepsy require lifelong treatment with one or more anti-epileptic
drugs. At present, the majority of anti-epileptic drugs aim to
prevent the abnormal discharge of neurons by affecting the function
or structure of ion channels and neurotransmitters; however, ~30%
of patients have seizures that are refractory to available
medications (3,4). Therefore, it is important to develop
new effective alternative and complementary methods to treat
epilepsy. The pathological characteristics of epilepsy include
hippocampal sclerosis, the decrease or even complete disappearance
of significant numbers of neurons and significantly activated
proliferation of astrocytes and microglia in brain tissue (5,6).
Zhvaniia et al (6)
demonstrated that the predominant characteristic of epilepsy is
proliferation and hypertrophy of astrocytes and activation of
microglia. Therefore, inhibiting the activation and proliferation
of microglial cells may be an effective means to prevent the
occurrence and development of epilepsy.
Osthole, 7-methoxy-8-(3-methyl-2-butenyl) coumarin,
is a coumarin derivative clinically ingested as an important
ingredient of medicinal plants and herbs in Traditional Chinese
Medicine and exhibits a number of pharmacological and biological
activities (7,8). Osthole can serve an anticancer role
by inducing apoptosis of cancer cells, exhibits antioxidant effects
by increasing adenylate cyclase activity and cyclic adenosine
monophosphate content and can inhibit NF-κB signal translocation by
regulating phosphorylation of nuclear factor of κ light polypeptide
gene enhancer in B-cells inhibitor α and IκB kinase, thus serving
an anti-inflammatory role by inhibiting chemokine and
proinflammatory cytokine production (9). In addition, osthole also possesses
the effects of improving learning disabilities and
anti-osteoporosis (10,11), but its mechanism is seldom studied
and remains to be elucidated. Luszczki et al (12) demonstrated that osthole exerts
anti-electroshock and anticonvulsant actions in mice. In our
previous study (13), osthole
could improve behavioral manifestations, including convulsions and
spasms, during seizures and prolong the seizure incubation period
in a rat model of epilepsy induced by kainic acid (KA) and could
reduce spike and spike-slow waves in an electroencephalogram during
epileptic seizures; however, the underlying mechanism remains to be
elucidated. Thus, osthole warrants further evaluation as a
potential therapeutic agent for epilepsy.
The Notch signaling pathway is a short-range signal
transduction pathway that mediates numerous cell outcomes during
development through interactions between adjacent cells (14). Notch signaling is involved in the
regulation of multiple biological processes, including cell
proliferation, differentiation and apoptosis (15). The Notch signaling pathway consists
of Notch receptors (Notch 1–4), Notch ligands (DLL-1, DLL-3, DLL-4,
Jagged-1 and Jagged-2), intracellular effector molecules,
regulatory molecules and other effector substances in mammals.
Notch signaling is activated following Notch receptor-ligand
binding at the cell surface, which induces cleavage of tumor
necrosis factor-α (TNF-α)-converting enzyme, a metalloprotease
family member, to release the Notch intracellular domain (NICD) of
the Notch receptor into the nucleus. The NICD binds to
recombinant-recognition-sequence-binding protein (RBP) at the Jκ
site (RBP-Jκ), which subsequently recruits coactivator proteins
including mastermind-like 1. The transcriptional targets of Notch
signaling are mostly genes of basic helix-loop-helix family
transcription factors, primarily HES and HEY
(14,16). Evidence indicates that Notch
signaling serves an important role in regulating the activation of
immune cells. In fact, Grandbarbe et al (17) demonstrated that inhibition of Notch
signaling reduced the numbers of activated microglia. Therefore,
Notch signaling may be a novel and important target to inhibit
microglial proliferation and improve function in epilepsy. In the
present study, KA-activated BV-2 cells were used to evaluate the
effect of osthole on microglial proliferation and to further
explore its mechanism.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM) and
Trypsin-EDTA (0.25%) were obtained from Gibco (Thermo Fisher
Scientific, Inc.). Fetal bovine serum (FBS) was obtained from
Macgene Biotech Co., Ltd. KA was obtained from Sigma-Aldrich (Merck
KGaA) and osthole was obtained from Dalian Meilun Biotech Co., Ltd.
Penicillin-streptomycin solution and Anti-Fade Mounting Medium were
obtained from Beyotime Institute of Biotechnology. Cell Counting
Kit-8 (CCK-8) was obtained from Dojindo Molecular Technologies,
Inc. TriQuick reagent, DAPI solution, phosphate-buffered solution
(PBS) and dimethyl sulfoxide were obtained from Beijing Solarbio
Science & Technology Co., Ltd. PrimeScript™ RT Master Mix and
TB Green® Premix Ex Taq™ II (Tli RNaseH Plus) were
obtained from Takara Bio, Inc. Rabbit monoclonal anti-mouse Notch-1
antibody (product code ab52627), rabbit monoclonal anti-mouse
Jagged-2 antibody (product code ab109627), rabbit monoclonal
anti-mouse RBP-Jκ antibody (product code ab180588), rabbit
anti-mouse Hes-1 (product code ab108937) and goat anti-Rabbit IgG
H&L (Cy3®) pre-adsorbed antibody (product code
ab6939) were obtained from Abcam. Rabbit monoclonal anti-mouse
Notch-2 antibody (cat. no. 5732) was obtained from Cell Signaling
Technology, Inc. Rabbit monoclonal anti-mouse Jagged-1 antibody
(cat. no. E-AB-30148), mouse interleukin (IL)-6 ELISA kit (cat. no.
E-EL-M0044c), mouse TNF-α ELISA kit (cat. no. E-EL-M0049c) and
mouse nitric oxide synthase 2 (NOS2)/inducible i) NOS ELISA Kit
(cat. no. E-EL-M0696c) were purchased from Elabscience.
Cell cultures and treatments
BV-2 cells, obtained from Professor Jinyan Wang
(Chinese Medical Sciences University, Liaoning, China), were
cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin and
100 µg/ml streptomycin in a humidified atmosphere with 5%
CO2/95% air at 37°C. To evaluate the effect of osthole
on the proliferation of KA-activated BV-2 cells, the cells were
divided into a control group (Con group), a KA activation group (KA
group) and a KA activation + Osthole (Ost group). For the Con
group, BV-2 cells were cultured in DMEM at 37°C without any
treatment. For the KA group, cells were incubated with KA (100 µM)
at 37°C for 2 h. For the Ost group, cells were pretreated with
osthole for 24 h prior to stimulation with KA at 37°C.
CCK-8 assay for optimal culture
concentration
Exponentially growing cells were plated into a
96-well plate (Corning, Inc.) at 1×105 cells/well and
stabilized for 24 h. Osthole was added to the wells with final
concentrations of 20, 40, 60, 80, 100, 120, 140, 160, 180, 200, 300
and 400 µM for 24 h at 37°C, while the wells with DMEM only were
set as the control. Following incubation, 10 µl of the CCK-8
solution was added to each well, followed by incubation for 3 h at
37°C. The optical density (OD) at 450 nm was measured by a
microplate reader (BioTek Instruments, Inc.). The cell viability
was calculated by the following formula: Cell viability
(%)=(ODosthole/ODcontrol) ×100%.
CCK-8 assay for optimal culture
time
Exponentially growing cells were plated into a 96
well plate (Corning, Inc.) at 1×105 cells/well and
stabilized for 24 h. Following treatment with 100 µM KA for 2 h at
37°C, the medium was replaced with either common medium (control)
or 131.2 µM osthole for 2, 4, 6, 8, 16, 24, 48, 72 or 96 h at 37°C
and 10 µl of the CCK-8 solution was added to each well, followed by
incubation for 3 h at 37°C. The inhibition rate was calculated by
the following formula: Inhibition rate
(%)=[1-(ODosthole/ODcontrol)] ×100%.
Cell morphology analysis
Cell morphological changes were observed using an
inverted microscope (Olympus Corporation; magnification, ×100, 200
and 400).
ELISA
The cell culture supernatant of each group was
collected and the samples were centrifuged for 20 min at 1,000 × g
at 2–8°C. The supernatant was collected to perform the assay. The
levels of TNF-α, IL-6 and NOS2/iNOS were evaluated by ELISA kits
according to the manufacturer's protocols. Absorbance was
determined at 450 nm using a microplate reader (BioTek Instruments,
Inc.).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from each group cells
(1×106 cells) using TriQuick reagent and
reverse-transcribed into cDNA using PrimeScript RT Master Mix.
Relative quantitation of NOTCH-1, NOTCH-2, JAGGED-1, JAGGED-2,
RBP-Jκ, HES-1 and β-ACTIN (Table I) genes were performed using SYBR
Premix Ex Taq II on a CFX 96 instrument (Bio-Rad Laboratories,
Inc.) according to the manufacturer's instructions. The PCR cycling
conditions were 95°C for 30 sec, 40 cycles at 95°C for 5 sec and
60°C for 30 sec. The experiment was repeated three times. Relative
changes in gene expression were determined using the
2−ΔΔCq method (18),
normalized to the reference gene β-actin.
 | Table I.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table I.
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Primer | Sequences |
|---|
| NOTCH-1 | Sense |
5′-GAGGCATAAGCAGAGGTAGGAG-3′ |
|
| Antisense |
5′-TGCGAAGTGGACATTGACG-3′ |
| NOTCH-2 | Sense |
5′-TACCAGTGCAACTGCCAACCA-3′ |
|
| Antisense |
5′-GATTGATGCCGTCCACACAGA-3′ |
|
JAGGED-1 | Sense |
5′-ACAGGGAAAACTCACAGG-3′ |
|
| Antisense |
5′-CAGGTCTTACCACCGAACA-3′ |
|
JAGGED-2 | Sense |
5′-AGTTCCTGGATGGAAGACTGCAA-3′ |
|
| Antisense |
5′-TGACCAGAGAGCAGGCAAGG-3′ |
| RBP-Jκ | Sense |
5′-TCCAACCACTGCCCATAA-3′ |
|
| Antisense |
5′-TCCACCCAAACGACTCAC-3′ |
| HES-1 | Sense |
5′-ATTCTTGCCCTTCGCCTC-3′ |
|
| Antisense |
5′-ACGACACCGGACAAACCA-3′ |
| β-actin | Sense |
5′-CATCCGTAAAGACCTCTATGCCAAC-3′ |
|
| Antisense |
5′-ATGGAGCCACCGATCCACA-3′ |
Western blot analysis
Proteins from each group were extracted using
ice-cold RIPA lysis buffer (Beyotime Institute of Biotechnology)
supplemented with 1% phenylmethanesulfonyl fluoride then
centrifuged at 9,636 × g for 20 min at 4°C. The protein
concentration for each sample was determined using a bicinchoninic
acid protein concentration determination kit (Beijing Solarbio
Science & Technology Co., Ltd.). An equal amount of sample
proteins (30 µg) was loaded and separated by 15% SDS-PAGE gel and
transferred onto 0.45 µm (Notch-1, Notch-2, Jagged-1, Jagged-2 and
RBP-Jκ) and 0.22 µm (Hes-1) polyvinylidene fluoride membranes.
Following transfer, the membranes were washed three times with PBS
and blocked with 5% (w/v) skimmed milk powder in PBS for 1 h at
room temperature. Subsequently, the membranes were incubated with
rabbit anti-Notch-1 (1:1,500), rabbit anti-Notch-2 antibody
(1:1,000), rabbit anti-Jagged-1 (1:1,500), rabbit anti-Jagged-2
(1:5,000), rabbit anti-RBP-Jκ (1:5,000) and rabbit anti-Hes-1
(1:500) overnight at 4°C with gentle agitation. Following washing,
appropriate secondary peroxidase-conjugated antibodies (1:1,000)
were added and the membranes were incubated for 1 h at room
temperature. Band intensity was analyzed using an ECL kit on a
ChemiDoc™ XRS+ imaging system (Bio-Rad Universal Hood II; Bio-Rad
Laboratories, Inc.). Densitometry was analyzed using ImageJ
software (version 1.51j8; National Institutes of Health). The
results were expressed as arbitrary units after being normalized to
mouse anti-β-actin antibodies (1:1,000).
Immunofluorescence
Cells (1×105) seeded on cover slips were
fixed with 4% paraformaldehyde for 20 min at 4°C, then
permeabilized in 0.5% Triton X-100 (Beijing Solarbio Science &
Technology Co., Ltd.) for 20 min, blocked in 10% BSA (Beyotime
Institute of Biotechnology) for 1 h at room temperature.
Subsequently, the slips were incubated with rabbit anti-Notch-1
(1:150), rabbit anti-Notch-2 antibody (1:1,000), rabbit
anti-Jagged-1 (1:150), rabbit anti-Jagged-2 (1:150), rabbit
anti-RBP-Jκ (1:100) and rabbit anti-Hes-1 (1:100) at 4°C overnight
followed by incubation with Alexa 488-conjugated goat anti-rabbit
IgG H&L (Cy3®) secondary antibody for 30 min at room
temperature. Cells were washed three times with PBS and incubated
with DAPI for 5–10 min at room temperature and then washed with PBS
5 times. Images were captured using a DP73 fluorescence microscope
(Olympus Corporation; magnification, ×200).
Statistical analysis
All experimental data were expressed as the mean ±
standard deviation. Statistical comparison of differences between
groups were analyzed using one-way analysis of variance (ANOVA)
followed by Student-Newman-Keuls and Fisher's least significant
difference post hoc tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
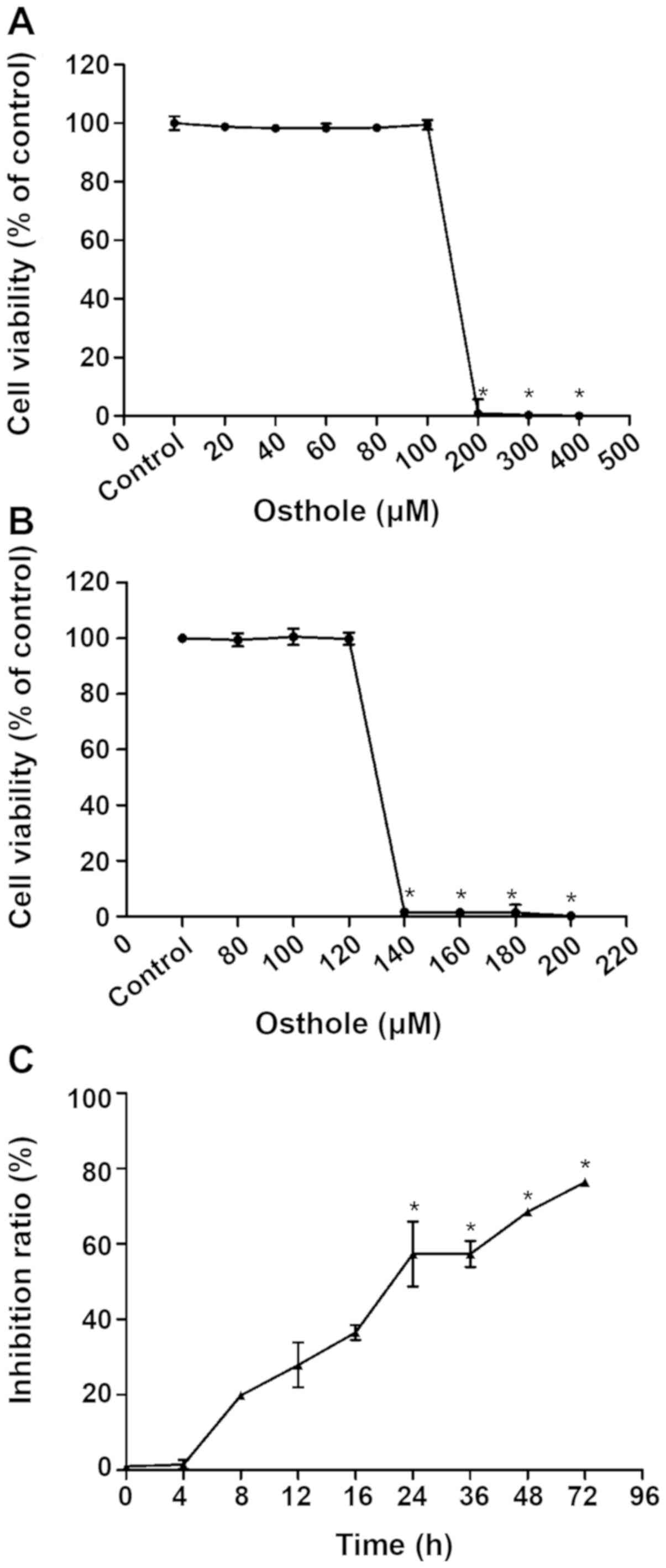
Effect of various concentrations of
osthole on BV-2 cell proliferation
To determine an optimal concentration of osthole for
subsequent experiments, the effects of various concentrations of
osthole on BV-2 cell proliferation were examined by CCK-8 assay.
CCK-8 results of BV-2 cells exposed to 20–400 µM osthole for 24 h
are demonstrated in Fig. 1A. Cell
viability was significantly decreased when the concentration of
osthole was 200–400 µM (P<0.05). However, further experimental
results demonstrated in Fig. 1B,
indicated that cell viability was significantly reduced with
concentrations of osthole between 120 and 140 µM (P<0.05),
yielding an IC50 value of 131.2 µM. Therefore, 131.2 µM
was used in subsequent experiments as a safe dose of osthole for
BV-2 cells.
Time-effect association of osthole on
inhibited proliferation of KA-activated BV-2 cells
The results presented in Fig. 1C indicated significantly inhibited
proliferation of BV-2 cells treated with 131.2 µM osthole for at
least 24 h compared with the KA group (P<0.05). Therefore, 24 h
was selected as the osthole exposure time for subsequent
experiments.
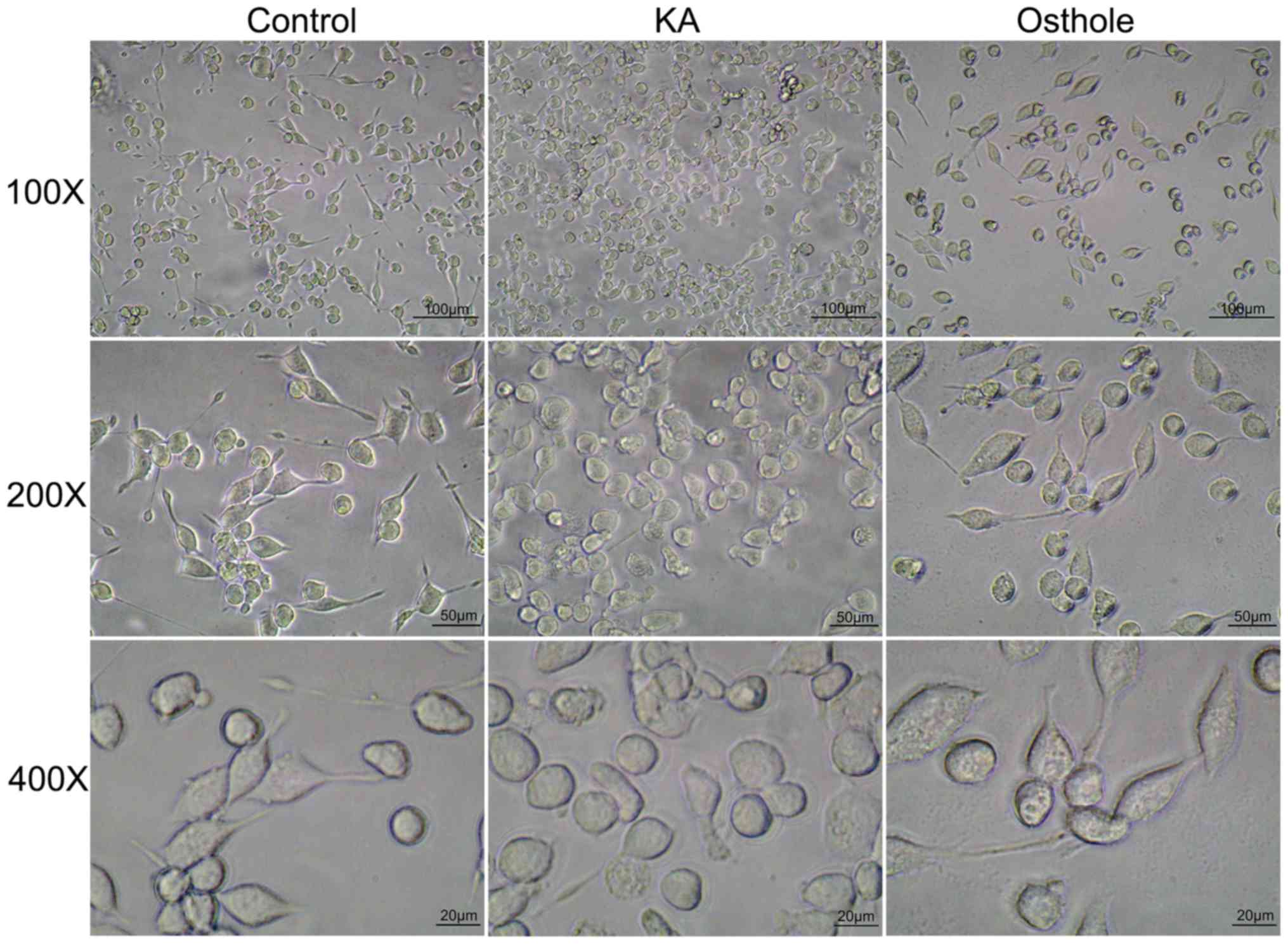
Effect of osthole on the morphology of
KA-activated BV-2 cells
Morphological changes associated with BV-2 cells of
each group were assessed as number of BV-2 cells and cell body
size. The results of inverted microscopy indicated adherent growth
of BV-2 cells in the Control group, which exhibited uniform
distribution, regular morphology and small nuclei; in addition, the
majority of cells had slender protrusions and the cell body was
bright and refractive (Fig. 2).
Compared with the Control group, cells in the KA group were denser,
had larger bodies and short or absent protrusions and formed
amoeba-like cells; characteristics of an activated state. In the
Osthole group, cells were reduced in number and had slightly
smaller cell bodies compared with the KA group and the protrusions
became longer, indicating a proliferation-inhibited state.
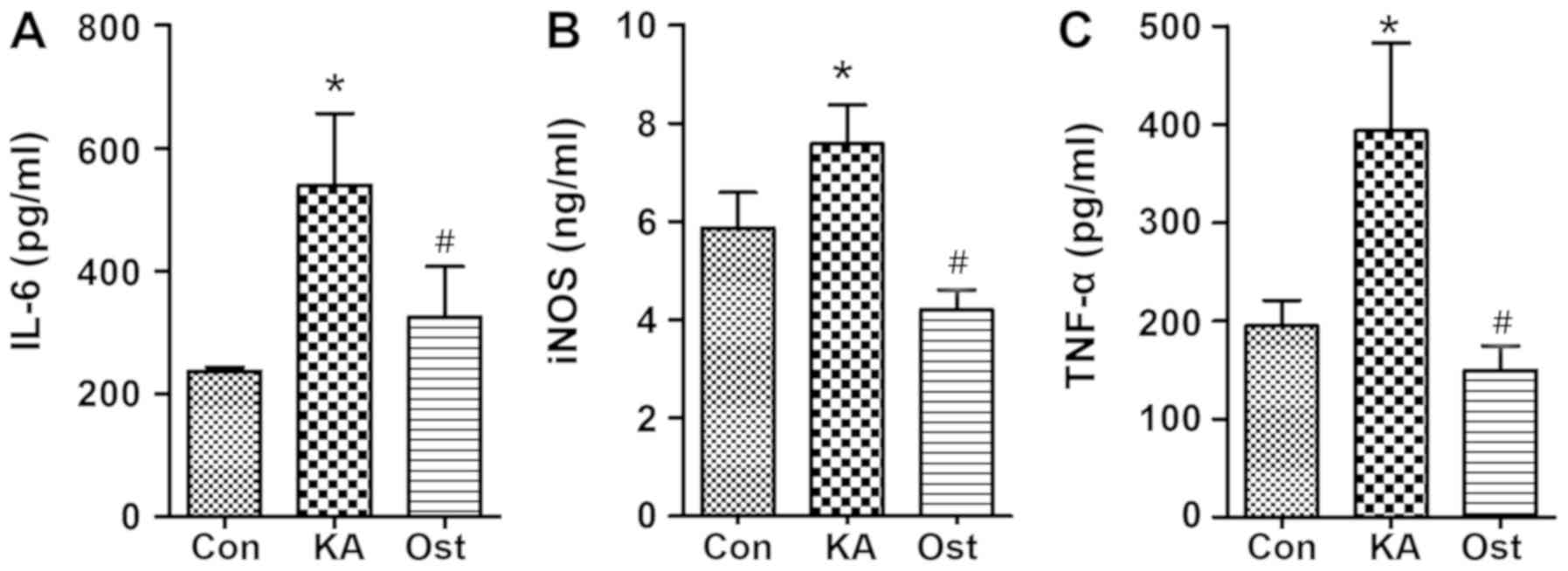
Effect of osthole on TNF-α, IL-6 and
NOS2/iNOS release by KA-activated BV-2 cells
Compared with the Con group, levels of IL-6,
NOS2/iNOS and TNF-α were significantly increased in the KA group
(Fig. 3). However, increases in
the levels of TNF-α, IL-6 and NOS2/iNOS were significantly
inhibited by osthole treatment (P<0.05).
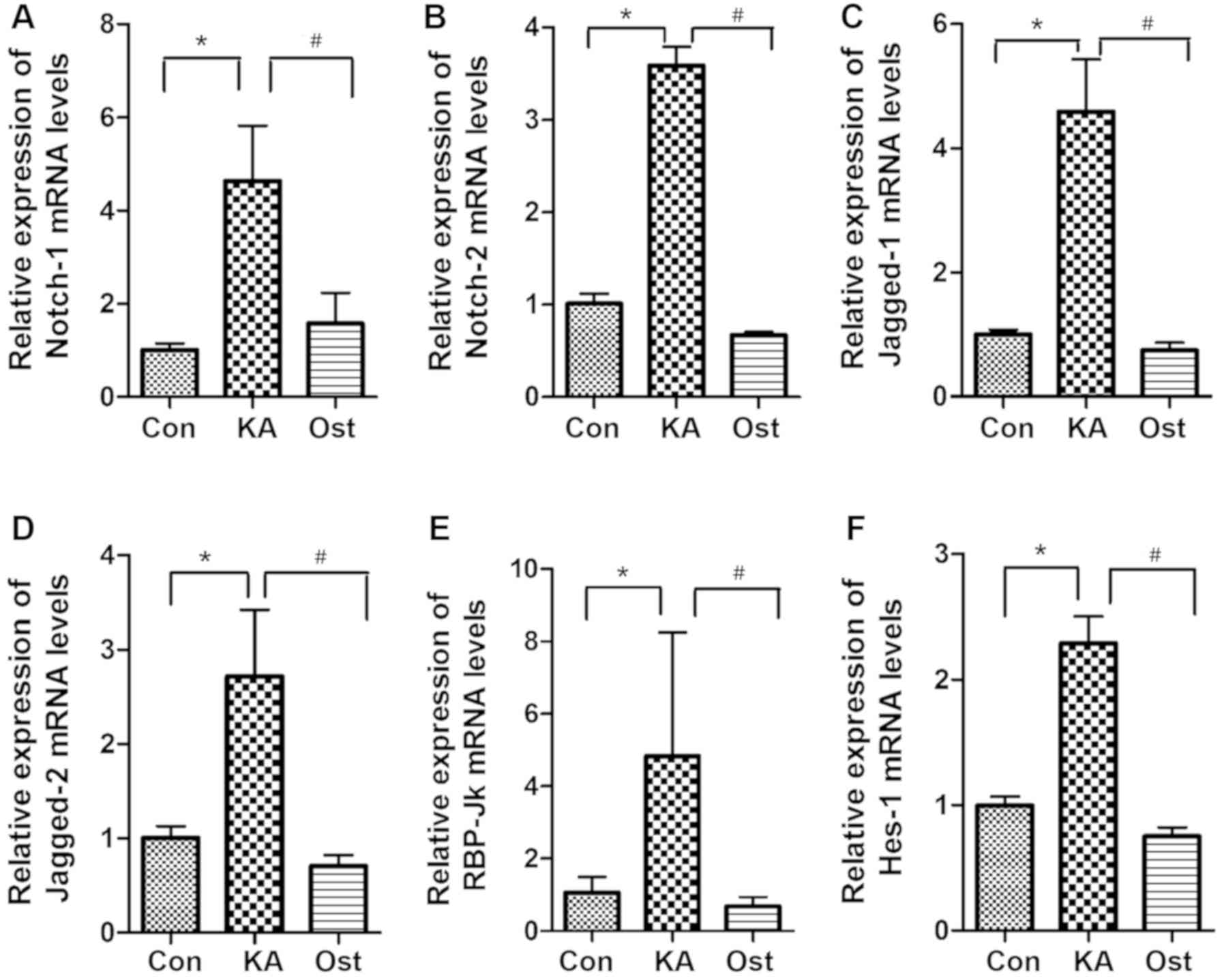
Effect of osthole on mRNA expression
of Notch signaling components in KA-activated BV-2 cells
mRNA levels of Notch-1, Notch-2, Jagged-1, Jagged-2,
RBP-Jκ and Hes-1 were assessed by RT-qPCR (Fig. 4). The results demonstrated that
mRNA levels of Notch-1, Notch-2, Jagged-1, Jagged-2, RBP-Jκ and
Hes-1 were upregulated in the KA group compared with the Con group
(P<0.05). In the Ost group, the mRNA levels were significantly
suppressed compared with the KA group (P<0.05).
Effect of osthole on expression of
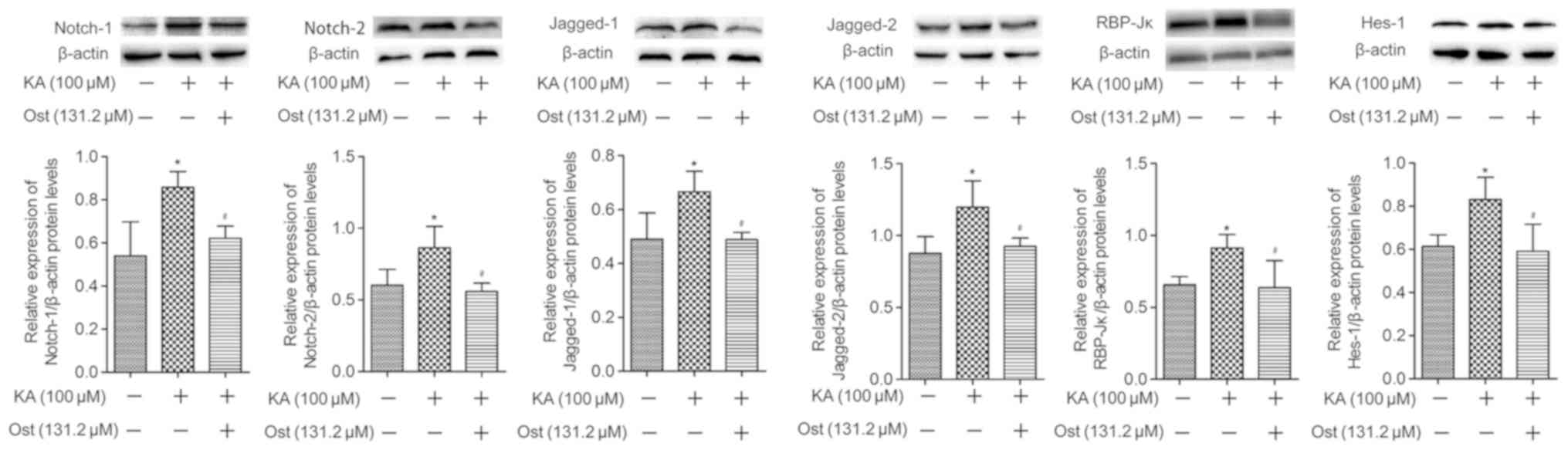
Notch signaling proteins in KA-activated BV-2 cells
Notch-1, Notch-2, Jagged-1, Jagged-2, RBP-Jκ and
Hes-1 protein expression levels were measured by western blotting
(Fig. 5) and immunofluorescence
assay (Fig. 6). Western blotting
results revealed significantly higher protein expression levels of
Notch-1, Notch-2, Jagged-1, Jagged-2, RBP-Jκ and Hes-1 in the KA
group compared with the Con group. However, pretreatment with
osthole significantly inhibited KA-induced increases of Notch-1,
Notch-2, Jagged-1, Jagged-2, RBP-Jκ and Hes-1 expression (Fig. 5). These results were confirmed by
immunofluorescence assay (Fig. 6),
which also indicated increased levels of Notch-1, Notch-2,
Jagged-1, Jagged-2, RBP-Jκ and Hes-1 in KA-activated BV-2 cells, in
addition to decreased levels of these proteins in the BV-2 cells of
the Ost group (P<0.05).
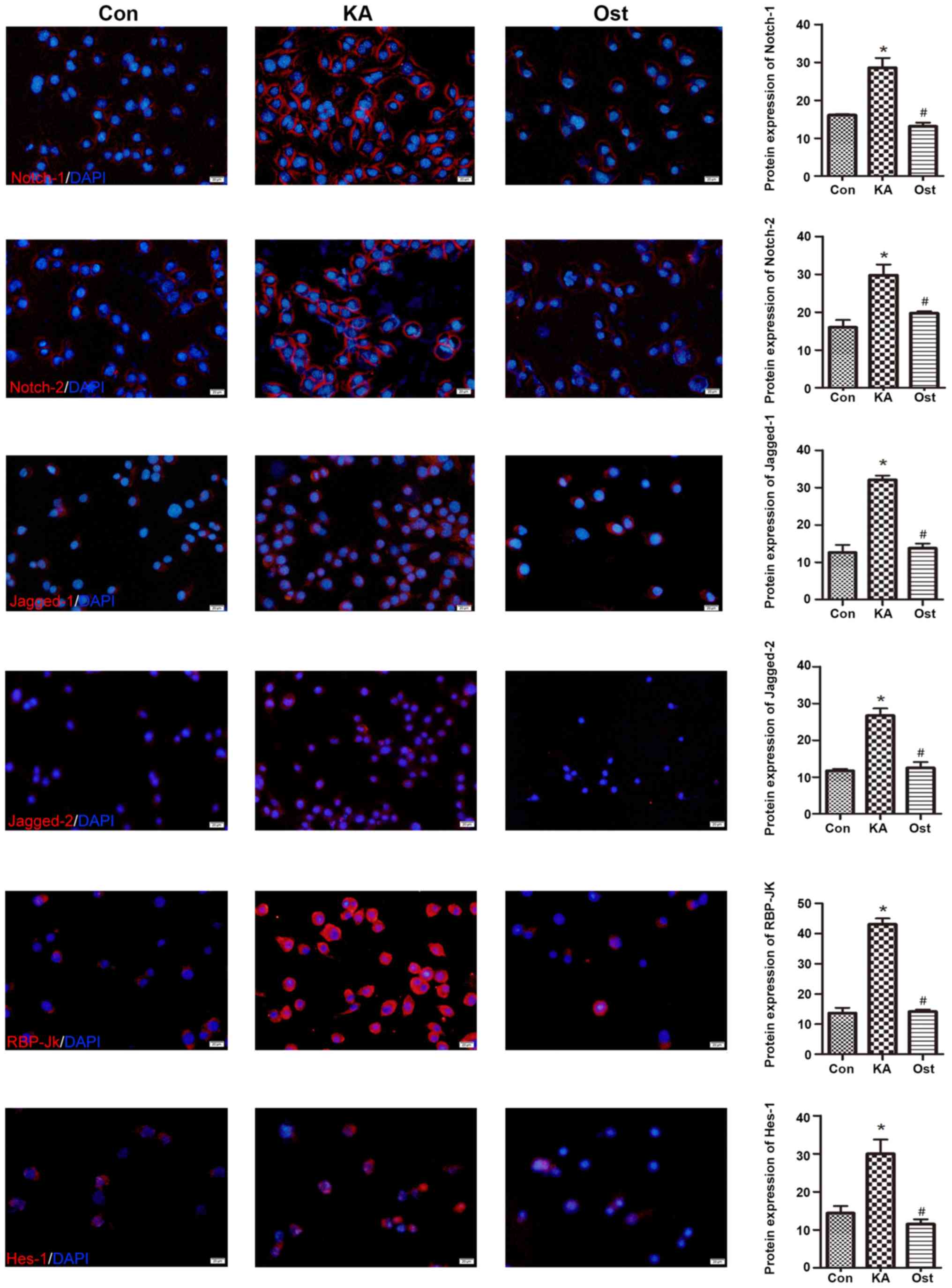 | Figure 6.Effect of osthole on the expression
of Notch-1, Notch-2, Jagged-1, Jagged-2, RBP-Jκ and Hes-1 proteins
in KA-activated BV-2 cells by immunofluorescence staining.
Magnification, ×200; scale bars, 20 µm. Values are expressed as the
mean ± standard deviation. *P<0.05 vs. the Con group,
#P<0.05 vs. the KA group. Con, control; KA, kainic
acid; Ost, osthole. |
Discussion
Discovered by Del Rio Hortega in 1932, microglia are
the fourth major cell type in the central nervous system (CNS)
after neurons, astrocytes and oligodendrocytes, and account for
~5–20% of the total number of glia (19). Microglia possess important
immune-related effects in the CNS and can secrete a large number of
inflammation-related factors following activation (20,21).
Avignone et al (22),
identified that microglial proliferation and morphological
modifications increase inflammatory mediators and cause significant
neurodegeneration in KA-induced epileptic mice. In the present
study, BV-2 cells were amoeba-like and increased in number
following activation by KA. In addition, activated microglia could
release a number of inflammatory mediators, including TNF-α, IL-6
and NOS2/iNOS. Dambach et al (23) observed that strongly activated
microglia can induce a CNS inflammatory reaction, which is directly
associated with epilepsy. Recently, Zhao et al (24) demonstrated that epilepsy can be
induced by microglial proliferation. Therefore, microglial
activation and proliferation is an important mechanism for the
progressive development of epilepsy and its inhibition could be an
opportune target for alleviating post-epileptic injury.
Osthole can cross the blood-brain barrier and exert
neuroprotective effects against Alzheimer's disease, traumatic
brain injury and transient cerebral ischemia (12,25).
Luszczki et al (26),
observed that osthole suppresses seizure activity in a mouse
maximal electroshock-induced seizure model. These findings provide
new indications for osthole as an anti-epileptic treatment. Our
previous study identified that osthole can inhibit epileptic
seizures in KA-induced epileptic rats and delay the progressive
development of epilepsy (27), but
its mechanism has yet to be clarified. The present study identified
that 131.2 µM osthole could inhibit the proliferation of microglia
without destroying cell activity, as 24 h exposure of BV-2 cells to
osthole significantly inhibited the cell activities induced by KA
activation. Bao et al (9),
demonstrated that osthole can reduce TNF-α, IL-6 and IL-1β released
by lipopolysaccharide (LPS)-stimulated BV-2 cells via NF-κB and
nuclear factor erythroid 2-related factor 2 pathways. In the
present study, osthole inhibited the proliferation of KA-activated
BV-2 cells and reduced the release of TNF-α, IL-6 and NOS2/iNOS via
the Notch signaling pathway, thus serving a neuroprotective role in
neuroinflammation.
Notch signaling serves critical roles in neural stem
cell maintenance and neurogenesis and regulation of Notch signaling
is involved in a number of neurodegenerative diseases and brain
disorders (28). Purow et
al (29), identified that
Notch signaling was closely associated with the proliferation of
glioma cells. Notch-1 and Jagged-1 are overexpressed in a number of
glioma cell lines and primary human gliomas and downregulation of
these factors by RNA interference induces apoptosis and inhibits
proliferation of multiple glioma cell lines. Grandbarbe et
al (30) reported that Notch
signaling was activated in LPS-stimulated microglial cells;
specifically, RT-qPCR revealed that the expression levels of
NOTCH-1, NOTCH-2 and JAGGED-1 genes were increased in
LPS-stimulated microglial cells, while JAGGED-2 expression
remained at a markedly low level. Previously published western blot
results revealed increased Notch-1 protein expression in
LPS-stimulated microglial cells. Zeng et al (31) reported that the expression of
Notch-1, RBP-Jκ and Hes-1 was increased in activated microglia
following cerebral ischemia in vivo and in vitro, and
that hypertonic saline could improve ischemic stroke by suppressing
Notch signaling. Additionally, Wu et al (32) identified that Notch signaling was
activated following status epilepticus in a pilocarpine-induced rat
model of epilepsy. Specifically, Hes-1 protein expression was
increased and inhibition of Notch signaling could suppress
microglial activation and attenuate neuronal apoptosis and loss.
Collectively, the results of these studies indicated the
involvement of the Notch signaling pathway in microglial activation
and proliferation.
In the present study, expression of Notch-1,
Notch-2, Jagged-1, Jagged-2, RBP-Jκ and Hes-1 were increased
following KA activation and Notch signaling was activated. However,
osthole could significantly inhibit KA-induced upregulation of
Notch signaling, microglial activation and subsequent release of
proinflammatory cytokines. Consequently, the inhibitory effect of
osthole on KA-activated BV-2 cells may partly occur through
downregulation of the Notch pathway. However, there were two major
limitations in the present study that should be addressed in future
research. First, it has not been confirmed in animal experiments
whether osthole can inhibit microglia proliferation via the Notch
signaling pathway to improve epilepsy. Secondly, the site of action
of osthole requires further investigation.
Acknowledgements
The authors would like to thank Professor Jinyan
Wang (Chinese Medical Sciences University, Liaoning, China) for
providing the BV-2 microglia cells.
Funding
No funding was received.
Availability of data and material
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
CQZ and QGZ designed the experiments and revised the
work for important intellectual content. YZL and ZS designed and
performed the experiments. YZL, ZS and HRX analyzed the data. YZL
wrote the paper. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nesbitt G, McKenna K, Mays V, Carpenter A,
Miller K and Williams M; EPGP Investigators, : The epilepsy
phenome/genome project (EPGP) informatics platform. Int J Med
Inform. 82:248–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Keezer MR, Sisodiya SM and Sander JW:
Comorbidities of epilepsy: Current concepts and future
perspectives. Lancet Neurol. 15:106–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen H, He H, Xiao Y, Luo M, Luo H and
Wang J: Losigamone add-on therapy for focal epilepsy. Cochrane
Database Syst Rev. 12:CD0093242019.PubMed/NCBI
|
|
4
|
Engel J: Etiology as a risk factor for
medically refractory epilepsy: A case for early surgical
intervention. Neurology. 51:1243–1244. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shaw JA, Perry VH and Mellanby J: MHC
Class II expression by microglia in tetanus toxin-induced
experimental epilepsy in the rat. Neuropathol Appl Neurobiol.
20:392–398. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhvaniia MG, Bolkvadze TN, Chkhikvishvili
TsG, Kotariia NT, Dzhaparidze HD, Lordkipanidze TG and Bikashvili
TZ: Quantitative analysis of gliocytes and macrogliocyte-neuronal
ratio in the rat hippocampus after kindling. Morfologiia.
136:18–21. 2009.(In Russian). PubMed/NCBI
|
|
7
|
Yu C, Li P, Qi D, Wang L, Qu HL, Zhang YJ,
Wang XK and Fan HY: Osthole protects sepsis-induced acute kidney
injury via down-regulating NF-κB signal pathway. Oncotarget.
8:4796–4813. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liao M, Diao X, Cheng X, Sun Y and Zhang
L: Nontargeted SWATH acquisition mode for metabolites
identification of osthole in rats using ultra-high-performance
liquid chromatography coupled to quadrupole time-of-flight mass
spectrometry. RSC Adv. 8:14925–14935. 2018. View Article : Google Scholar
|
|
9
|
Bao Y, Meng X, Liu F, Wang F, Yang J, Wang
H and Xie G: Protective effects of osthole against inflammation
induced by lipopolysaccharide in BV2 cells. Mol Med Rep.
17:4561–4566. 2018.PubMed/NCBI
|
|
10
|
Yao Y, Wang Y, Kong L, Chen Y and Yang J:
Osthole decreases tau protein phosphorylation via PI3K/AKT/GSK-3β
signaling pathway in Alzheimer's disease. Life Sci. 217:16–24.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du G, Song Y, Wei L, Li L, Wang X, Xu Q,
Zhan H, Cao Y, Zheng Y and Ding D: Osthole inhibits proliferation
and induces catabolism in rat chondrocytes and cartilage tissue.
Cell Physiol Biochem. 36:2480–2493. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luszczki JJ, Wojda E, Andres-Mach M,
Cisowski W, Glensk M, Glowniak K and Czucuwar SJ: Anticonvulsant
and acute neurotoxic effects of imperatorin, osthole and valproate
in the maximal electroshock seizure and chimney tests in mice: A
comparative study. Epilepsy Res. 85:293–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li ZQ, Zou SF, Zeng CQ, Cui JH, Li XY, Pan
X and Duan CM: Effect of osthole on expression of voltage-gated
potassium channel Kv1.2 in epileptic rats. J Apoplexy Nervous Dis.
29:40–43. 2012.
|
|
14
|
Miele L: Notch signaling. Clin Cancer Res.
12:1074–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tzou WS, Lo YT, Pai TW, Hu CH and Li CH:
Stochastic simulation of notch signaling reveals novel factors that
mediate the differentiation of neural stem cells. J Comput Biol.
21:548–567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shang Y, Smith S and Hu X: Role of Notch
signaling in regulating innate immunity and inflammation in health
and disease. Protein Cell. 7:159–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grandbarbe L, Bouissac J, Rand M, Hrabé de
Angelis M, Artavanis-Tsakonas S and Mohier E: Delta-Notch signaling
controls the generation of neurons/glia from neural stem cells in a
stepwise process. Development. 130:1391–1402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Varnholt H, Drebber U, Schulze F,
Wedemeyer I, Schirmacher P, Dienes HP and Odenthal M: MicroRNA gene
expression profile of hepatitis C virus-associated hepatocellular
carcinoma. Hepatology. 47:1223–1232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ling EA and Wong WC: The origin and nature
of ramified and amoeboid microglia: A historical review and current
concepts. Glia. 7:9–18. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan Y, Zha H, Rangarajan P, Ling EA and
Wu C: Anti-inflammatory effects of edaravone and scutellarin in
activated microglia in experimentally induced ischemia injury in
rats and in BV-2 microglia. BMC Neurosci. 15:1252014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao L, Kan EM, Lu J, Hao A, Dheen ST, Kaur
C and Ling EA: Toll-like receptor 4 mediates microglial activation
and production of inflammatory mediators in neonatal rat brain
following hypoxia: Role of TLR4 in hypoxic microglia. J
Neuroinflammation. 10:232013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Avignone E, Ulmann L, Levavasseur F,
Rassendren F and Audinat E: Status epilepticus induces a particular
microglial activation state characterized by enhanced purinergic
signaling. J Neurosci. 28:9133–9144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dambach H, Hinkerohe D, Prochnow N,
Stienen MN, Moinfar Z, Haase CG, Hufnagel A and Faustmann PM: Glia
and epilepsy: Experimental investigation of antiepileptic drugs in
an astroglia/microglia co-culture model of inflammation. Epilepsia.
55:184–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao X, Liao Y, Morgan S, Mathur R,
Feustel P, Mazurkiewicz J, Qian J, Chang J, Mathern GW, Adamo MA,
et al: Noninflammatory changes of microglia are sufficient to cause
epilepsy. Cell Rep. 22:2080–2093. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li SH, Gao P, Wang LT, Yan YH, Xia Y, Song
J, Li HY and Yang JX: Osthole stimulated neural stem cells
differentiation into neurons in an Alzheimer's disease cell model
via upregulation of MicroRNA-9 and rescued the functional
impairment of hippocampal neurons in APP/PS1 transgenic mice. Front
Neurosci. 11:3402017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luszczki JJ, Andres-Mach M, Cisowski W,
Mazol I, Glowniak K and Czuczwar SJ: Osthole suppresses seizures in
the mouse maximal electroshock seizure model. Eur J Pharmacol.
607:107–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeng CQ, Li DP, Tang W, Wang W, Cao PA and
Zou SF: Relevance of potassium channels Kv1.2 to pathogenesis of
epileptic rat. J Apoplexy Nervous Dis. 27:700–703. 2010.
|
|
28
|
Jeske R, Albo J, Marzano M, Bejoy J and Li
Y: Engineering brain-specific pericytes from human pluripotent stem
cells. Tissue Eng Part B Rev. Jun 22–2020.(Online ahead of print).
View Article : Google Scholar
|
|
29
|
Purow BW, Haque RM, Noel MW, Su Q, Burdick
MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I, et al:
Expression of Notch-1 and its ligands, Delta-Like-1 and Jagged-1,
is critical for glioma cell survival and proliferation. Cancer Res.
65:2353–2363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grandbarbe L, Michelucci A, Heurtaux T,
Hemmer K, Morga E and Heuschling P: Notch signaling modulates the
activation of microglial cells. Glia. 55:1519–1530. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeng WX, Han YL, Zhu GF, Huang LQ, Deng
YY, Wang QS, Jiang WQ, Wen MY, Han QP, Xie D and Zeng HK:
Hypertonic saline attenuates expression of notch signaling and
proinflammatory mediators in activated microglia in experimentally
induced cerebral ischemia and hypoxic bv-2 microglia. BMC Neurosci.
18:322017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu L, Li Y, Yu M, Yang F, Tu M and Xu H:
Notch signaling regulates microglial activation and inflammatory
reactions in a rat model of temporal lobe epilepsy. Neurochem Res.
6:1269–1282. 2018. View Article : Google Scholar
|