Introduction
Adenomyosis (AM) is a heterogenous gynecological
disease, which is characterized by uterine enlargement, and deep
location of endometrial glands and stroma within the myometrium
(1). Menorrhagia, dysmenorrhea and
metrorrhagia are the most frequently identified symptoms in two
thirds of patients with AM; notably, one third of patients with AM
are asymptomatic (2). A previous
report revealed that preterm birth and premature rupture of
membranes, uterine atony and ectopic pregnancy are also associated
with AM (3). However, the exact
pathogenesis and etiology of AM have not been fully elucidated, due
to its complex pathogenesis and variable presentation. Hysterectomy
is a standard treatment for AM, and suppressive hormonal treatments
cam be used to temporarily suppress AM and improve the symptoms;
however, there is no currently available medical therapy to cure AM
(4). Therefore, exploring the
pathogenesis of AM is critical for developing strategies for
treating the disease.
Aquaporins (AQPs) are a family of membrane water
channels, which are distributed widely in specific cell types in
different organs and tissues, and primarily function as regulators
of intracellular and intercellular water flow (5). A previous study identified the
significance of AQPs in mammalian pathophysiology, and suggested
that AQPs and AQP modulators (mostly inhibitors) could act as
underlying drug targets (6). In
addition to the research achievements in drug-like AQP inhibitors,
regulation of AQP function may be desirable in some
pathophysiological conditions, such as in tumors (7). AM is characterized by the malignant
biological behaviors of invasion and migration (8); these biological behaviors are similar
to those of tumors. Therefore, AQP regulation may be a potential
therapeutic option for treating AM. Additionally, AM and
endometriosis are closely linked diseases, although there are
several differences in their pathogenesis and pathogenic mediators
(9). Some similarities exist
between AM and endometriosis at least in some subgroups, more often
the two conditions coexist (10).
AQP2, AQP5 and AQP8 have all been reported to play roles in
endometriosis (11), but their
roles in the occurrence of AM have not been clearly reported.
Notably, AQP5 is known to play a role in estrogen-induced ectopic
implantation of endometrial stromal cells (ESCs) in endometriosis
(12). Furthermore, AQP5 gene
silencing has been shown to inhibit the proliferation and migration
of ectopic endometrial glandular epithelial cells in endometriosis
(13). Therefore, it is of great
value to explore the role of AQP2, AQP5 and AQP8 in the progression
of AM; such an exploration based on the regulation of AQP2, AQP5
and AQP8 might contribute to the discovery of a novel therapy for
AM. To the best of our knowledge, no currently used drug
specifically targets AQP5.
In the present study, the expression levels of AQP2,
AQP5 and AQP8 were detected in eutopic and ectopic endometrial
samples from mice with AM, and the effects of AQP5 silencing on
cell viability, migration, invasion, epithelial-mesenchymal
transition (EMT), and matrix metalloproteinase (MMP)-2 and MMP-9
expression were detected in eutopic and ectopic ESCs.
Materials and methods
Animals
Sexually mature virgin female Institute of Cancer
Research (ICR) mice (age, 8 weeks) and 8-week-old male ICR mice
(Chengdu Dossy Experimental Animals Co., Ltd.) were used in the
present study. The mice weighed ~29 g were freely fed a standard
rodent diet from Animal Laboratory of Chengdu University of
Traditional Chinese Medicine. The mice were maintained under
specific pathogen-free laboratory conditions at 20°C under a 12-h
light/dark cycle with 48% humidity. The animal health and behavior
were monitored every 3 days. All animal experiments were approved
by the Institutional Committee for Animal Research of The Second
Affiliated Hospital of Hainan Medical University (Haiku, China) and
in accordance with National Guidelines for the Care and Use of
Laboratory Animals (14).
Animal model of AM and collection of
specimens
The mice were divided into control (without any
surgery), sham (the needle was punctured into the right-side of the
uterus and saline solution was injected into the uterine cavity),
AM-I (estrus) and AM-II (non-estrus) groups (n=12 mice/group). Mice
in the AM-I and AM-II groups received surgery for modeling AM. All
groups of mice were fed for 3 months to the end point of the
experiment.
The AM animal model was constructed by the
transplantation of the pituitary gland of mice into the uterus of
female mice, as previously reported (15,16).
Briefly, 8-week old female mice were administered with an
appropriate amount of anesthetic (3% pentobarbital sodium, 30–35
mg/kg) through intraperitoneal injection. The skin layer and
subcutaneous muscle layer were stratified in the 8-week-old female
mice, and uterine bifurcation under the bladder was used to isolate
the uterus. Male mice (age, 8 weeks) were sacrificed by overdose
with 100–150 mg/kg pentobarbital sodium through intraperitoneal
injection, followed by cervical dislocation used as a secondary
physical method of sacrifice; euthanasia was verified by checking
the heartbeat of the mice. Subsequently, craniotomy was performed
to extract the pituitary glands from the mice, and the extractions
were mixed with saline solution. Part of the pituitary glands was
maintained in saline solution suspension (0.5–1 ml) in a needle.
The needle was injected into the right-side of the uterus of female
mice, and the pituitary-saline suspension was slowly pushed into
the uterine cavity. The isolated uterus was put back in the
abdominal cavity while the needle and the inner core were removed.
A total of 3 months later, all mice were sacrificed (via cervical
dislocation) at AM-I or AM-II according to vaginal secretion
smears, and the right uterine specimen was immediately collected by
laparotomy. Nodule numbers on the uterine surface of mice was
counted under a light microscope. The uterine diameter of mice, and
uterine, ovarian and body weight were measured by a Vernier caliper
(segmented measurement) and electronic balance. Half of uterine
tissues were added into Eppendorf tubes and stored in liquid
nitrogen, whereas the other uterine tissues of mice were stored in
4% paraformaldehyde at 4°C for protein detection using western
blotting. Uterine index (%) was calculated as follows: Uterine
weight (mg)/mouse body weight (mg) ×100. Ovary index (%) was
calculated as follows: Ovary weight (mg)/mouse body weight (mg)
×100.
Hematoxylin-eosin (HE) staining and
grading of AM severity
Uterine tissues of mice fixed with 4%
paraformaldehyde for 24 h at room temperature were embedded in
paraffin and cut into 4-µm sections, which were then stained with
HE for 5 min at 60°C. After staining, the sections were dehydrated
with gradually increasing concentrations of ethanol and xylene.
Longitudinal and cross sections of uterine tissues
of the mice were selected. Under an optical microscope, the areas
of eutopic and ectopic endometrium were observed under
magnification, ×10 and the grade of AM severity of endometrial
glands and interstitial permeability of the uterine muscle were
observed under magnification, ×20.
The specific grading indicators of AM severity were
as follows: Grade 0 (normal uterus), grade 1 (ESCs invaded the
inner layer of the muscles), grade 2 (endometrial stroma and gland
invaded the inner layer of muscles), grade 3 (endometrial stroma
and gland invaded the junction of the internal and external muscle
layer) and grade 4 (endometrial gland exhibited cystic hyperplasia
and nodules appeared under the serous layer). In each case, the
cross section area of the inner membrane was determined by
calipers, and AM severity was evaluated according to the degree of
permeability (grade 0–4), the average value was calculated to
assess the severity of the uterine gland. The experiment was
conducted at least in triplicate.
Cells isolation, culture and
identification
Six cases of AM (age, 33–51 years) were confirmed by
pathology after total hysterectomy at the Second Affiliated
Hospital of Hainan Medical University (Hainan, China) between
January 16, 2018 and March 16, 2019. The present study was approved
by the Ethics Committee of the Second Affiliated Hospital of Hainan
Medical University (SA20180111022). Written informed consent was
obtained from all patients.
Under aseptic conditions, eutopic and ectopic
endometrial samples were collected in a sterile cell culture dish,
and were then washed 2–3 times with D-Hanks (Beijing Solarbio
Science & Technology Co., Ltd.) to remove the mucus and blood
clots attached to the surface of the tissue specimens. The tissue
samples were rinsed in sterile PBS and then sliced into small
pieces (4–5 µm). Subsequently, the sliced tissues were maintained
in 50-ml centrifuge tubes, centrifuged at 4°C, 500 × g for 5 min
and the liquid supernatant was removed. Collagenase I (Invitrogen;
Thermo Fisher Scientific, Inc.) was added to eutopic or ectopic
samples until the volume was raised by 1 or 3 cm, respectively. The
eutopic or ectopic endometrial suspension was digested with
Collagenase I for 1 or 2 h respectively in a water bath at 37°C. In
order to promote the effect of Collagenase I, the mixed suspension
was shaken every 15 min. Subsequently, DMEM (Hyclone; GE Healthcare
Life Sciences) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) was added into the mixed
suspension at an equal volume to Collagenase I to terminate the
digestion. The suspension was filtered through a 40-µm cell
strainer into a fresh 50-ml centrifuge tube. The filtered
suspension was centrifuged at 4°C, 800 × g for 5 min, and the
liquid supernatant was discarded. Precipitates (cells) in the tube
were resuspended in DMEM supplemented with 10% FBS. The cells were
plated into a 25-cm2 culture flask at density of
1×105 cells/ml and cultured at 37°C with 5%
CO2.
After the ESCs were cultured to generation 2–3, the
expression levels of Vimentin were detected by immunofluorescence
with an anti-Vimentin antibody (cat. no. PA5-27231; Invitrogen;
Thermo Fisher Scientific, Inc.).
Immunofluorescence staining
ESCs were cultured on 1×1 cm cover slips, fixed with
4% cold polyoxymethylene (cat. no. P1110; Beijing Solarbio Science
& Technology Co., Ltd.) for 20 min at 4°C and then washed three
times with PBS. The fixed cells were subsequently incubated in PBS
containing 0.2% Triton X-100 for 2–5 min and then blocked with 5%
BSA (Sigma-Aldrich; Merck KGaA) for 1 h at room temperature. Next,
the cells were incubated with an anti-Vimentin antibody (1:100;
cat. no. PA5-27231; Invitrogen; Thermo Fisher Scientific, Inc.)
overnight at 4°C, washed and incubated with biotin-conjugated
anti-rabbit IgG (1:8,000; cat. no. 65–6140; Invitrogen; Thermo
Fisher Scientific, Inc.) at 37°C for 1 h. The nuclei were stained
with 4′,6-diamidino-2-phenylindole (DAPI; cat. no. D9542;
Sigma-Aldrich; Merck KGaA) for 10 min at room temperature in the
dark. Fluorescence images were captured using an immunofluorescence
laser confocal microscope (Nikon Corporation). The experiment was
performed at least in triplicate.
Transfection
Parental non-transfected cells were used as control
cells. The small interfering RNA (siRNA) targeting AQP5 (siAQP5,
5′-GCGCUCAACAACAACACAA-3′) and scrambled/nonsense siRNA negative
control (siNC, 5′-ACGUGACACGUUCGGAGAATT-3′) were purchased from
Guangzhou RiboBio Co., Ltd. The cells transfected with siNC served
as the negative control for siAQP5. The siRNAs (0.25 µg) were
transfected into the eutopic and ectopic ESCs (5×105) at
37°C for 48 h to knockdown AQP5 expression using
Lipofectamine® 2000 Transfection Reagent (cat. no.
11668; Invitrogen; Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from eutopic and ectopic ESCs was
extracted using TRIzol® reagent (cat. no. 15596018;
Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA (1 µg) was
reverse transcribed to cDNA using PrimeScript™ RT Master Mix (cat.
no. RR036B; Takara Biotechnology Co., Ltd.) at 42°C for 1 h.
RT-qPCR was performed in a 7300 real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using TB
Green® Premix Ex Taq™ II (cat. no. RR820Q; Takara
Biotechnology Co., Ltd.) under the following conditions: Incubation
at 95°C for 30 min, followed by amplification at 95°C for 35 sec,
60°C for 34 sec and 72°C for 35 sec, for a total of 40 cycles, and
a final extension step at 72°C for 5 min. The relative expression
levels were calculated using the 2−ΔΔCq method (17) and normalized to the expression
levels of GAPDH. The sequences of primers (provided by Sangon
Biotech Co., Ltd.) used are shown in Table I.
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Primer | Primer sequences
(5′-3′) |
|---|
| AQP5 F |
CAAAGACAGCGGCAGAGACTAT |
| AQP5 R |
CTGTCTCCTTTTCCAGGTTATCC |
| MMP-2 F |
TGGCAAGTACGGCTTCTGTC |
| MMP-2 R |
TTCTTGTCGCGGTCGTAGTC |
| MMP-9 F |
GCTGACTACGATAAGGACGGCA |
| MMP-9 R |
TAGTGGTGCAGGCAGAGTAGGA |
| E-cadherin F |
CGAGAGCTACACGTTCACGG |
| E-cadherin R |
GGGTGTCGAGGGAAAAATAGG |
| N-cadherin F |
TCAGGCGTCTGTAGAGGCTT |
| N-cadherin R |
ATGCACATCCTTCGATAAGACTG |
| GAPDH F |
CCAGGTGGTCTCCTCTGA |
| GAPDH R |
GCTGTAGCCAAATCGTTGT |
CCK-8 assay
The cells (5×104/well) were plated in
96-well plates. After siRNA transfection for 24 h, cell viability
was determined using the CCK-8 kit (cat. no. CA1210; Beijing
Solarbio Science & Technology Co., Ltd.). After siRNA
transfection, the cells were cultivated for 24, 48 and 72 h.
Subsequently, CCK-8 solution (10 µl) was added to the 96-well
plates, and the cells were cultured for another 4 h. The absorbance
was then determined at 450 nm using a microplate reader (SpectraMax
iD5; Molecular Devices, LLC).
Scratch wound-healing assay
The cells (5×105/well) were cultivated to
80–90% confluence in 6-well plates. After the medium had been
discarded, confluent cells were scratched using a 10-µl pipette
tip, washed with serum-free DMEM and incubated at 37°C for 48 h.
Migration was evaluated by counting migrating cells from five
random fields under a 100× inverted microscope (Ts2r-FL; Nikon
Corporation). The following formula [(1-the distance following
healing/the distance prior to healing) ×100] was used to calculated
relative migration rate of cells.
Transwell invasion assay
Transwell chambers (8-µm pores; Corning Inc.) coated
in Matrigel (BD Biosciences) were used to determine the
invasiveness of cells. The cells (1×105/well in a
24-well plate) were inserted into the upper chambers of Transwell
chambers, which contained 200 µl DMEM, whereas 500 µl DMEM
containing 10% FBS was added into the lower chamber as the
chemoattractant. The cells were incubated at 37°C for 48 h.
Subsequently, the cells adhered to the upper surface were removed
using a cotton swab, whereas those on the lower surface were fixed
with 4% pre-cold methanol for 15 min, and stained with 0.1% crystal
violet solution (Sigma-Aldrich; Merck KGaA) for 20 min at room
temperature. Cells were counted from a minimum of 10 fields per
filter under a 100× light microscope (Ts2r-FL; Nikon
Corporation).
Western blotting
Tissue and cell lysates of eutopic and ectopic
endometrium samples were lysed for protein isolation and analyzed
as previously described (18).
Total proteins were extracted using RIPA buffer (cat. no. R0010;
Beijing Solarbio Science & Technology Co., Ltd.). The
supernatant from total proteins was collected and the protein
concentration was determined using the BCA protein assay kit (cat.
no. PC0020; Beijing Solarbio Science & Technology Co., Ltd.).
The proteins (30 µg/lane) were separated by SDS-PAGE on 12% gels
and transferred onto PVDF membranes (cat. no. IPVH00010; EMD
Millipore) using electroblotting apparatus. The membranes were then
blocked with 5% (w/v) skimmed milk in Tris-buffered saline
containing 0.5% Tween-20 (w/v) buffer for 1 h at 37°C. The
membranes were probed with specific primary antibodies (Abcam), as
listed in Table II, at 4°C
overnight, and then washed with TBS containing 0.1% Tween-20. The
membranes were then incubated with secondary antibodies
[horseradish peroxidase (HRP)-conjugated secondary antibody (goat
anti-mouse; cat. no. ab205719; Abcam) and HRP-conjugated secondary
antibody (Goat Anti-Rabbit; cat. no. ab205718; Abcam)] at a
dilution of 1:2,000 for 1 h at 37°C. After washing the membranes
three times at an interval of 10 min, the signals were visualized
using an ECL detection kit (Promega Corporation) and normalized to
GAPDH. Bio-Rad ChemiDoc™ XRS+ system with Image Lab™ Software
version 4.1 (Bio-Rad Laboratories, Inc.) were used to semi-quantify
band intensities.
 | Table II.List of primary antibodies used for
western blotting. |
Table II.
List of primary antibodies used for
western blotting.
| Target | Antibody name | Cat. no. | Dilution |
|---|
| AQP2 | Rabbit
Anti-Aquaporin 2 antibody (EPR21080) | ab199975 | 1:500 |
| AQP5 | Rabbit
Anti-Aquaporin 5 antibody | ab78486 | 1:1,000 |
| AQP8 | Rabbit Anti-AQP8
antibody (EPR8397) | ab133667 | 1:1,000 |
| MMP-2 | Rabbit Anti-MMP2
antibody (EPR17003-25) | ab181286 | 1:1,000 |
| MMP-9 | Rabbit Anti-MMP9
antibody | ab73734 | 1:1,000 |
| E-cadherin | Rabbit Anti-E
Cadherin antibody (EP700Y) | ab40772 | 1:10,000 |
| N-cadherin | Rabbit Anti-N
Cadherin antibody | ab18203 | 1:10,000 |
| GAPDH | Mouse Anti-GAPDH
antibody (6C5)-Loading Control | ab8245 | 1:1,000 |
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical significance was analyzed by one-way ANOVA followed by
Dunnett's post hoc test to compare the differences. P<0.05 was
considered to indicate a statistically significant difference. The
analyses were performed using SPSS 17.0 software (SPSS, Inc.).
Results
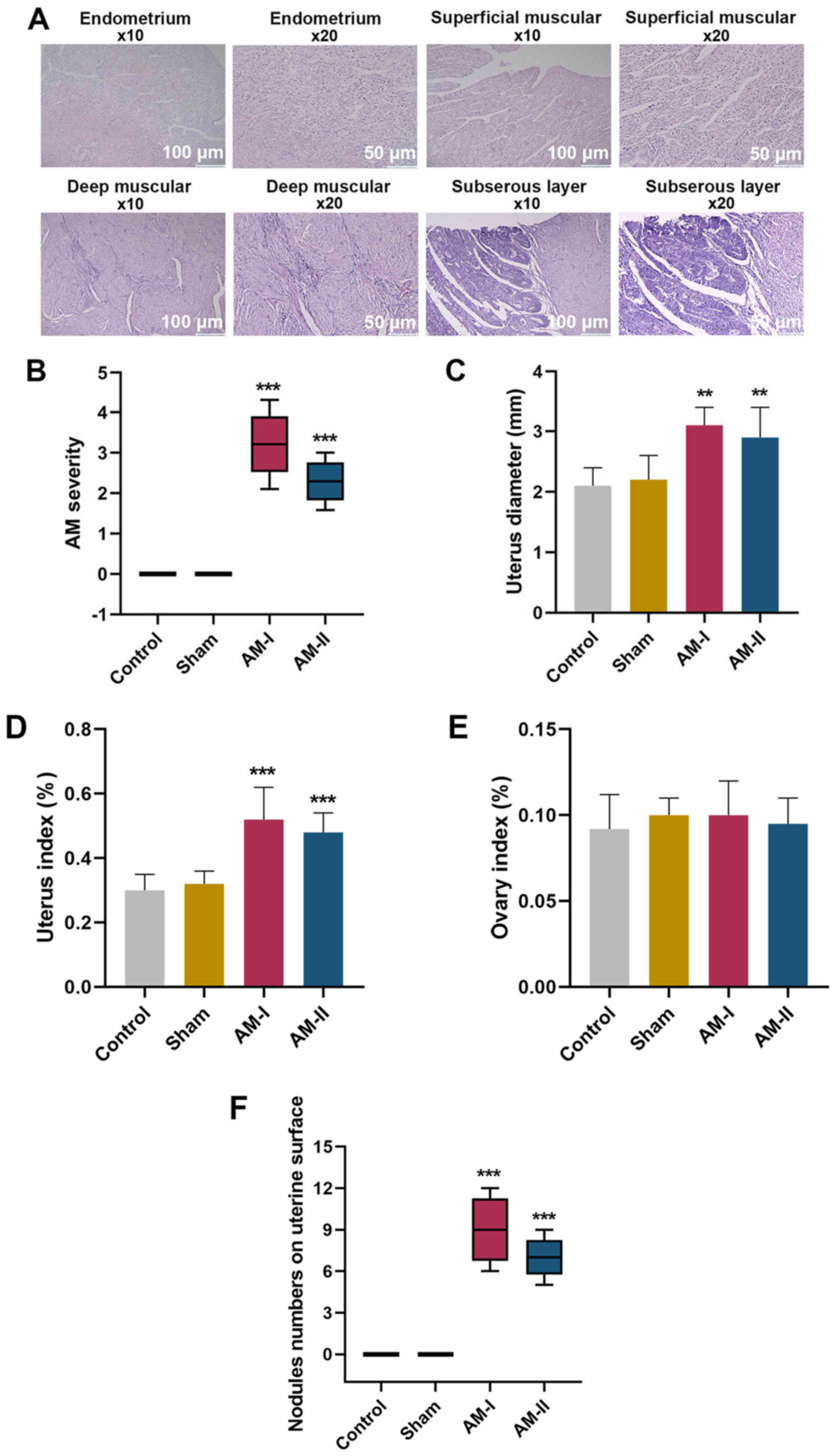
Observation of pathological features
in AM
The normal endometrium, deep muscular, superficial
muscular and subserous layers of the pathological sections of the
mice are shown in Fig. 1A.
Notably, ESCs and glands infiltrated the deep muscular, superficial
muscular and subserous layers of the pathological sections of the
mice in AM groups. It was revealed that AM severity was higher in
the AM-I and AM-II groups compared with the sham group (Fig. 1B; P<0.001), which indicated that
the model was successfully established. Similarly, uterus diameter
was longer (Fig. 1C; P<0.01),
uterus index was higher (Fig. 1D;
P<0.001) and there were a greater number of nodules on the
uterine surface (Fig. 1F;
P<0.001) in the AM-I and AM-II groups compared with the sham
treatment group. However, no statistically significant differences
were found in ovary index among the control, sham, AM-I or AM-II
groups, which may indicate that that there was no effect on the
ovaries (Fig. 1E; P>0.05).
These data suggested that the AM model was successfully constructed
and could be used in subsequent experiments.
AQP2, AQP5 and AQP8 are
highly-expressed in eutopic and ectopic endometrial tissues from AM
mice, particularly in ectopic endometrium
AQP2 (Fig. 2A and
B; P<0.001), AQP5 (Fig. 2A and
C; P<0.001) and AQP8 (Fig. 2A
and D; P<0.001) were highly expressed in the eutopic
endometrium of AM-I- and AM-II-treated mice compared with the sham
treatment group. In addition, the expression levels of AQP2
(Fig. 2E and F), AQP5 (Fig. 2E and G) and AQP8 (Fig. 2E and H) were highly expressed in
the ectopic endometrium of AM-I and AM-II mice compared with the
sham treatment group.
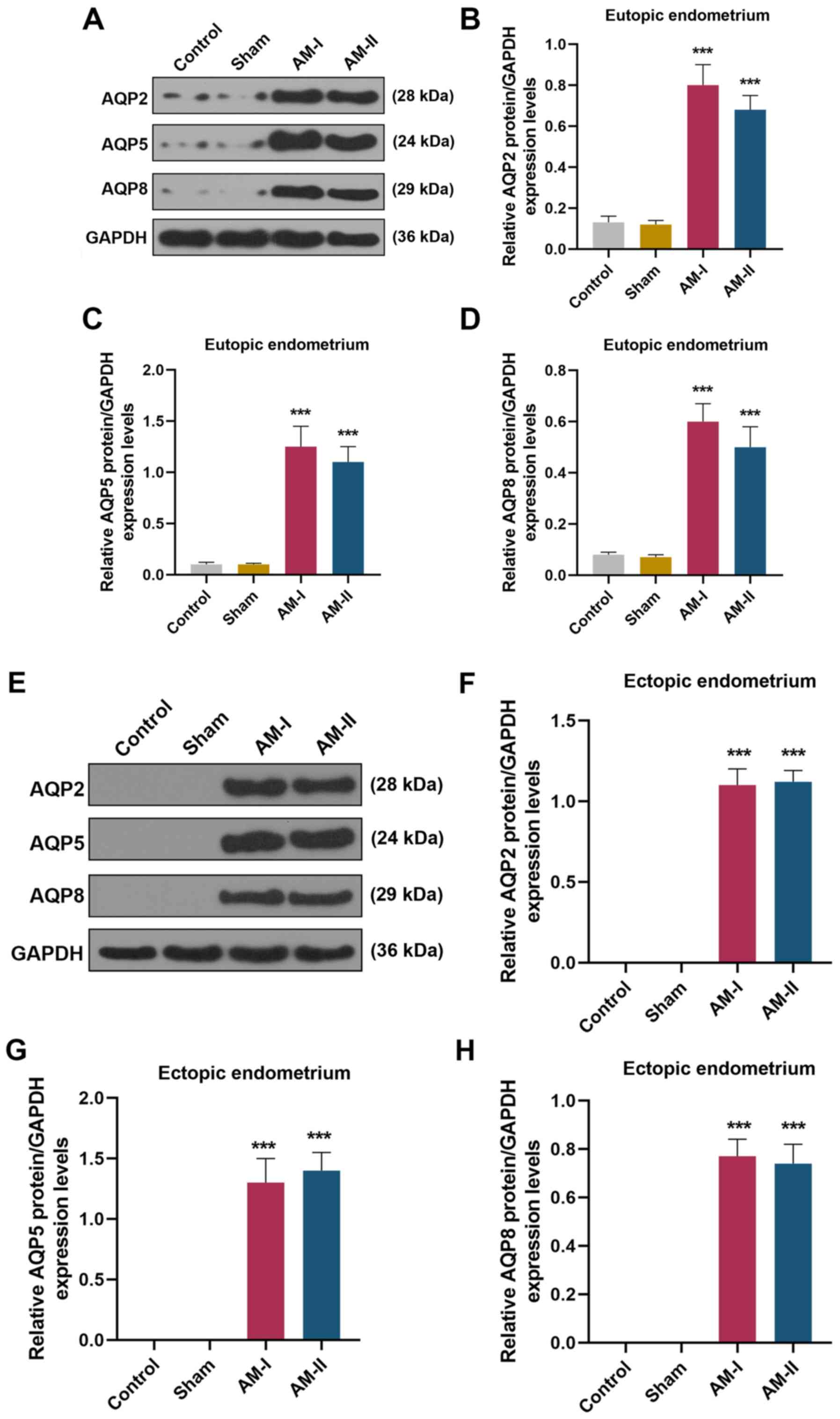 | Figure 2.Expression levels of AQP2, AQP5 and
AQP8 in eutopic and ectopic endometrial samples were detected by
western blot analysis. (A) Western blot analysis of AQP2, AQP5 and
AQP8 from the eutopic endometrium. Protein expression levels of (B)
AQP2, (C) AQP5 and (D) AQP8 in the eutopic endometrium of control,
sham, AM-I and AM-II groups were semi-quantified. (E) Western blot
analysis of AQP2, AQP5 and AQP8 from ectopic endometrium. Protein
expression levels of (F) AQP2, (G) AQP5 and (H) AQP8 in the ectopic
endometrium of control, sham, AM-I and AM-II groups were
semi-quantified. Data are presented as the mean ± SD. ***P<0.001
vs. sham. AM, adenomyosis; AQP, aquaporin. |
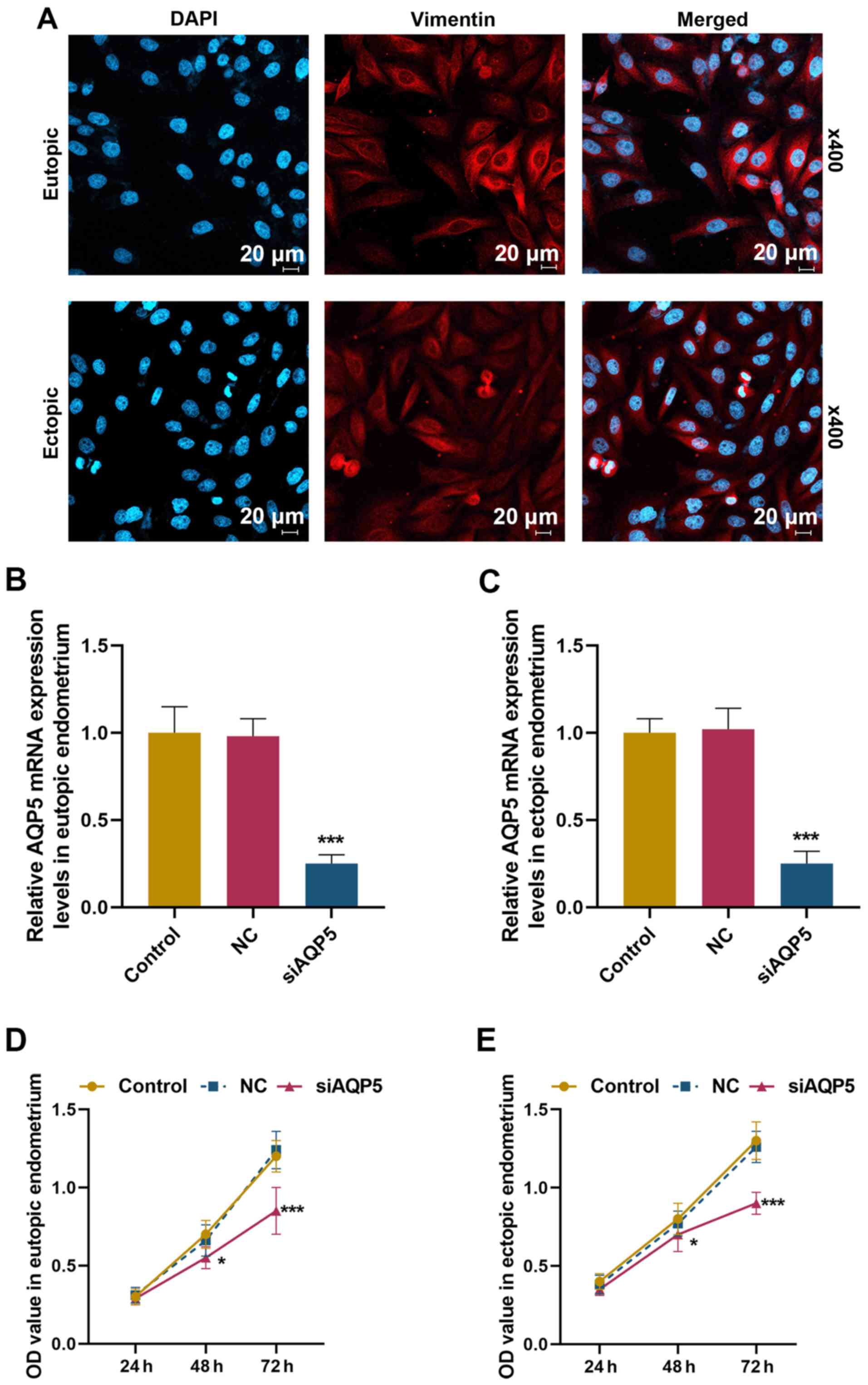
siAQP5 suppresses the viability,
migration, invasion and EMT of ESCs
As shown in Fig.
3A, vimentin was positively expressed in cells from eutopic and
ectopic endometrium, which according to previous research (19) indicated that these cells were ESCs
(purity >99%). To investigate if low expression levels of AQP5
can alleviate AM, the effects of knockdown of AQP5 expression were
examined on cell viability, migration, invasion and EMT in the
cells obtained from eutopic and ectopic endometrium. As shown in
Fig. 3B and C, the mRNA expression
levels of AQP5 were significantly reduced in eutopic and ectopic
ESCs following transfection with siAQP5 (P<0.001). In addition,
as shown in Fig. 3D and E,
compared with the control, reduced expression of AQP5 suppressed
viability of eutopic and ectopic ESCs at 48 h (P<0.05) and 72 h
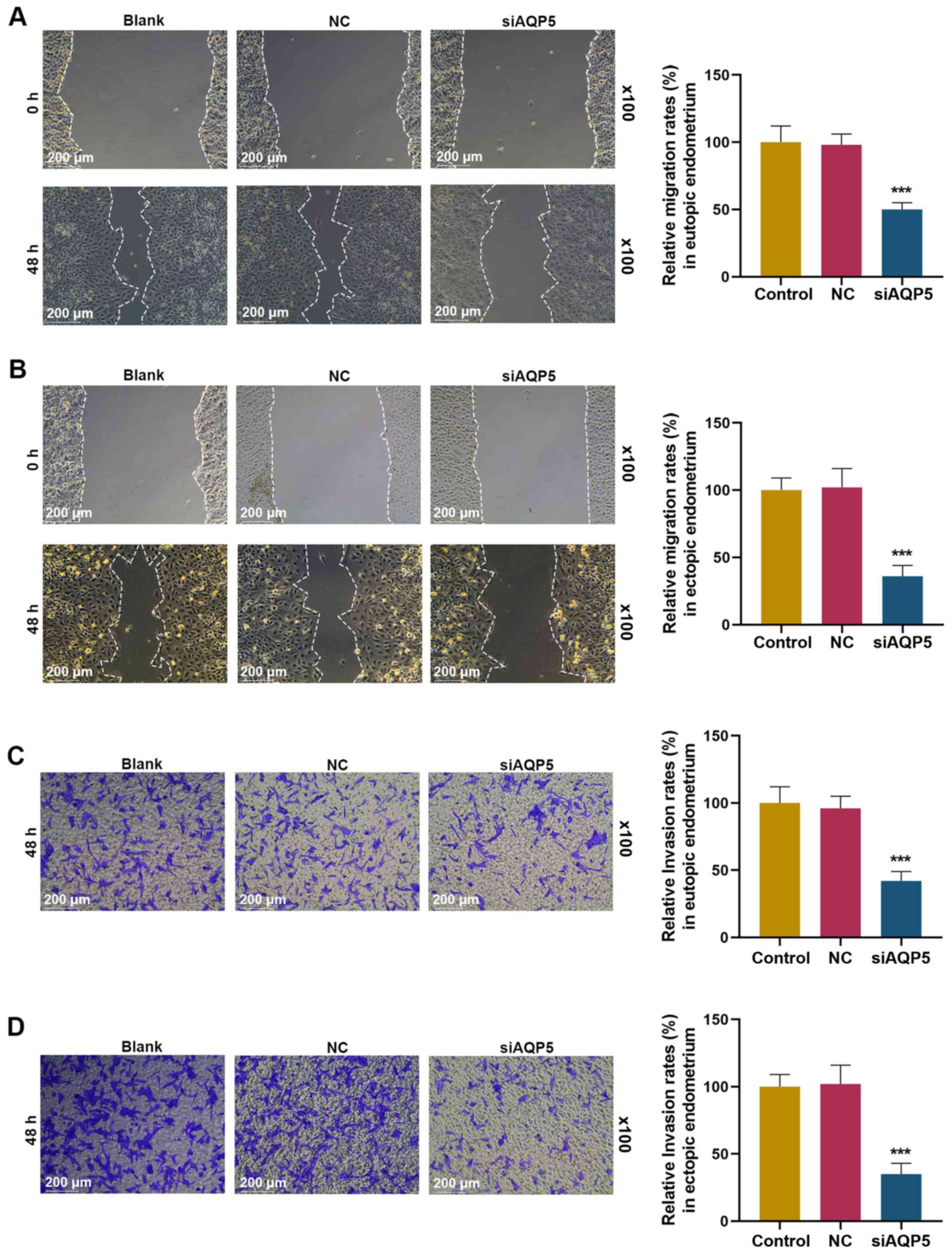
(P<0.001). The effects of siAQP5 on migration and invasion of
eutopic and ectopic ESCs were evaluated by performing scratch
wound-healing assays and Transwell assays, respectively. Compared
with the blank control group, siAQP5 significantly suppressed the
migration of eutopic and ectopic ESCs (Fig. 4A and B; P<0.001). Similarly,
compared with the blank control group, siAQP5 significantly
suppressed the invasion of eutopic and ectopic ESCs (Fig. 4C and D; P<0.001). As shown in
Fig. 5A-F, compared with the
control group, siAQP5 markedly reduced the mRNA and protein
expression levels of MMP-2, MMP-9 and N-cadherin, but increased the
mRNA and protein expression levels of E-cadherin in eutopic and
ectopic ESCs (P<0.001).
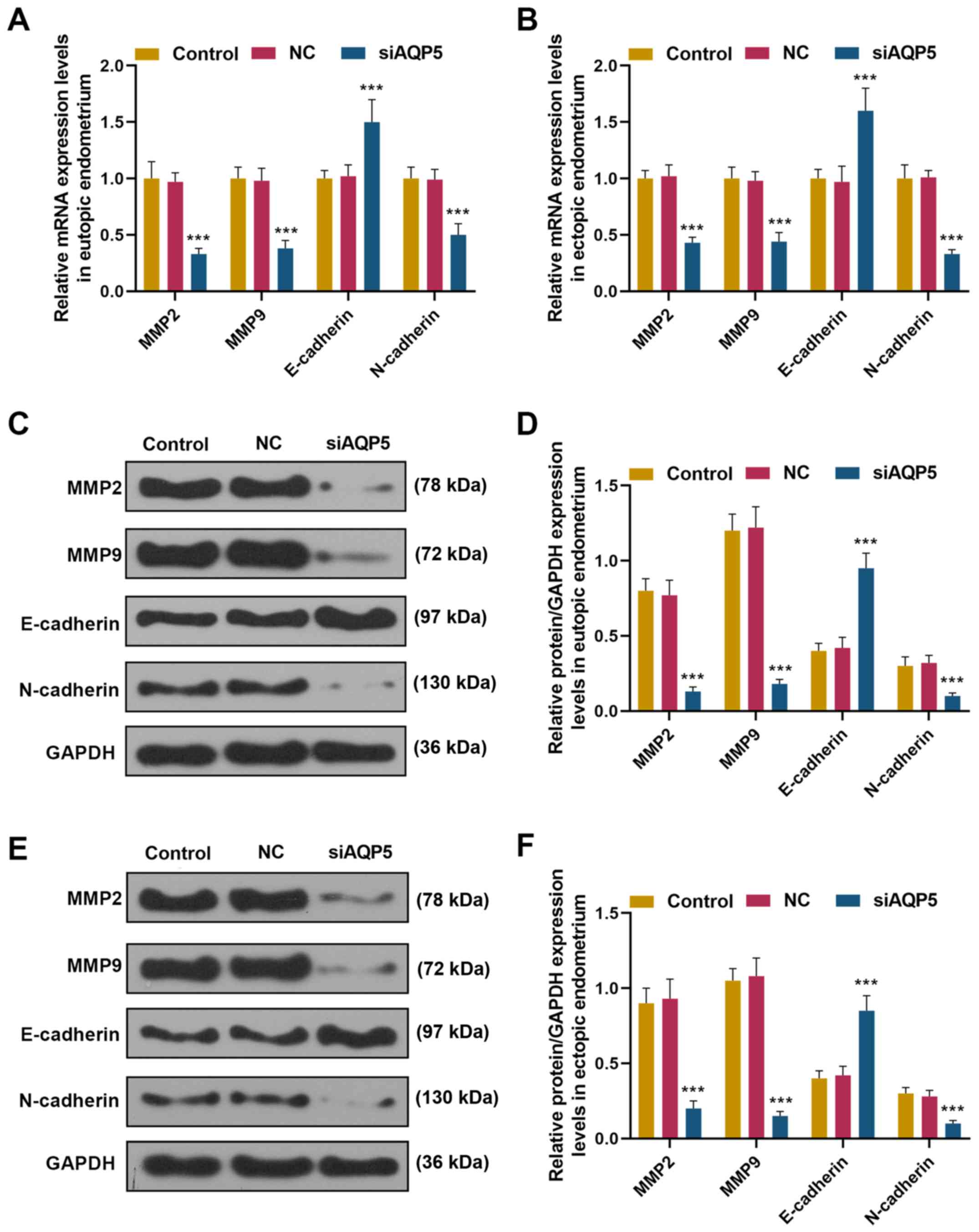 | Figure 5.Knockdown of AQP5 suppressed
epithelial-mesenchymal transition, and MMP-2 and MMP-9 expression
in eutopic and ectopic ESCs mRNA expression levels of E-cadherin,
N-cadherin, MMP-2 and MMP-9 from (A) eutopic and (B) ectopic ESCs
from the control, NC and siAQP5 groups were detected by reverse
transcription-quantitative PCR. Protein expression levels of
E-cadherin, N-cadherin, MMP-2 and MMP-9 in the (C and D) eutopic
and (E and F) ectopic ESCs of the control, NC and siAQP5 groups
were detected by western blotting. Data are presented as the mean ±
SD. ***P<0.001 vs. control. AQP, aquaporin; MMP, matrix
metalloproteinase; NC, negative control; siAQP5, small interfering
RNA targeting AQP5; ESCs, endometrial stromal cells. |
Discussion
Cell attachment, adhesion and invasion are important
in maintaining the normal functions of the endometrium, but it is
believed that these properties may participate in the development
of endometriosis (20). In
addition, adhesion, invasion and growth of the endometrium towards
the muscle layer to form ectopic endometrium is seen as a key event
in the pathogenesis of AM (21).
Thus, understanding the mechanisms underlying cell viability,
migration and invasion of the endometrium may help understand and
potentially help develop a future treatment for AM.
The present study revealed that AM severity and
uterus index were higher, uterus diameter was longer and there were
a greater number of nodules on the uterine surface in the AM model
of female mice compared with in the control mice. This suggested
that estrus could regulate the severity of AM in female mice. In
addition, AQP2, AQP5 and AQP8 were highly expressed in the eutopic
and ectopic endometrium of AM-I and AM-II model mice. Therefore,
the role of AQP5 in ESCs extracted from the eutopic and ectopic
endometrium of patients with AM was further investigated. The
results indicated that AQP5 silencing suppressed the viability of
ESCs from the eutopic and ectopic endometrium. In addition, it was
found that AQP5 silencing inhibited the migration of ESCs, similar
to a previous study which reported that AQP5 silencing inhibited
proliferation and migration of ectopic endometrial glandular
epithelial cells in endometriosis (13). It has also previously been
suggested that AQP5 may affect cell proliferation and migration
during tumor development. For example, downregulating AQP5
expression inhibited the proliferation and migration of human
gastric carcinoma cells (22),
human glioma cells (23) and
prostate cancer cells (24).
Downregulating AQP5 in Ishikawa cells has also been shown to reduce
cell migration (25).
The current study found that AQP5 silencing
inhibited cell viability, migration and invasion, which aligns with
a previous study in human non-small cell lung cancer, which
demonstrated that AQP5 expression promoted cell invasion (26). In addition, the mRNA and protein
expression levels of MMP-2 and MMP-9, and some EMT-related genes
(N-cadherin and E-cadherin) were determined; the results revealed
that AQP5 silencing inhibited the mRNA and protein expression
levels of MMP-2, MMP-9 and N-cadherin, but promoted E-cadherin of
ESCs. MMPs are a family of zinc-dependent proteolytic enzymes
capable of degradation and reconstruction of the extracellular
matrix, which can lead to cell migration and invasion (27). MMP-2 and MMP-9 degrade type IV
collagen and gelatin substrates (28). MMP-2 and MMP-9 expression levels
have been reported to be higher in the eutopic and ectopic
endometrial tissues of patients with AM compared with healthy
tissues (29). Thus, increased
MMP-2 and MMP-9 expression may play an important role in the
development of AM, potentially through increasing the invasiveness
of endometrial tissues into the myometrium (30). EMT transforms epithelial cells into
mesenchymal cells (31). During
EMT, loss of cell-cell adhesion is related to the migratory and
invasive characteristics of cells (32). EMT is characterized by decreased
E-cadherin and corresponding increased N-cadherin expression
(33). Therefore, the current data
suggested that AQP5 silencing inhibited the invasion and migration
of cells, which may be caused by the regulation of EMT, and MMP-2
and MMP-9 expression. A similar report revealed that downregulation
of AQP5 inhibited hepatocellular carcinoma cell invasion and EMT
process through modulating EMT-related molecules, such as
E-cadherin and N-cadherin (34).
Wang et al (35) reported
that AQP5 silencing inhibited proliferation, migration and the
enzyme activity of MMP-9 in human umbilical vein endothelial cells,
but did not affect the enzyme activity of MMP-2. The discrepancy
between this previous report and the current study with regards to
MMP-2 may have resulted from different detection indexes (i.e.
enzyme activity and protein expression). Collectively, silencing of
AQP5 expression and/or function might be a potential useful
treatment to prevent the development of AM; however, the method by
which AQP5 could be silenced requires further study.
This study indicated that the effects of AQP5 on AM
may be fundamental for the development of novel therapeutic
targets, or reliable diagnostic and prognostic biomarkers. However,
the pathological images were not high quality. In addition,
cytokeratin, von Willebrand factor and α-smooth muscle actin could
be applied to identify ESCs, which were not applied in this study.
Further research is required to identify the underlying mechanisms
and signaling pathways of AQP5 on AM, and such studies will reveal
whether AQP5 and downstream effectors could be potential targets
for developing new therapeutics for AM.
Taken together, these findings revealed that AQP5
was highly expressed in eutopic and ectopic endometrial samples in
a mouse model of AM. In addition, AQP5 silencing suppressed
viability, migration, invasion, EMT, and MMP-2 and MMP-9 protein
expression in ESCs derived from patients with AM. These results may
provide a new basis for the development of future therapeutics for
AM through the inhibition of the expression and function of AQP
channels. The effects of depleting AQP2 and AQP8, along with the
pathogenic role of these transmembrane channels in malignancy, AM
and endometriosis should also be further explored.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Medical
Scientific Research Project of Hainan Province (grant no.
18A200093).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJ and YL developed the concept and designed the
study. YH and XZ conducted data acquisition, analysis and
interpretation, LJ, YL and FW conducted the majority of
experiments, drafted the manuscript and critically revised it for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of the Second Affiliated Hospital of Hainan Medical
University (SA20180111022). Written informed consent was obtained
from all patients. All animal experiments were approved by the
Institutional Committee for Animal Research of The Second
Affiliated Hospital of Hainan Medical University and in accordance
with National Guidelines for the Care and Use of Laboratory
Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AM
|
adenomyosis
|
|
AQP2
|
aquaporin 2
|
|
ESCs
|
endometrial stromal cells
|
|
MMP
|
matrix metalloproteinase
|
|
EMT
|
epithelial-mesenchymal transition
|
|
AQPs
|
aquaporins
|
|
ICR
|
Institute of Cancer Research
|
|
HE
|
hematoxylin-eosin
|
References
|
1
|
Struble J, Reid S and Bedaiwy MA:
Adenomyosis: A clinical review of a challenging gynecologic
condition. J Minim Invasive Gynecol. 23:164–185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harada T, Khine YM, Kaponis A, Nikellis T,
Decavalas G and Taniguchi F: The lity. Obstet Gynecol Surv.
71:557–568. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vlahos NF, Theodoridis TD and
Partsinevelos GA: Myomas and adenomyosis: Impact on reproductive
outcome. Biomed Res Int. 2017:59264702017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pontis A, D'Alterio MN, Pirarba S, de
Angelis C, Tinelli R and Angioni S: Adenomyosis: A systematic
review of medical treatment. Gynecol Endocrinol. 32:696–700. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li C and Wang W: Molecular biology of
aquaporins. Adv Exp Med Biol. 969:1–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soveral G and Casini A: Aquaporin
modulators: A patent review (2010–2015). Expert Opin Ther Pat.
27:49–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beitz E, Golldack A, Rothert M and von
Bulow J: Challenges and achievements in the therapeutic modulation
of aquaporin functionality. Pharmacol Ther. 155:22–35. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He Y, Zhou L, Fan Z, Liu S and Fang W:
Palmitic acid, but not high-glucose, induced myocardial apoptosis
is alleviated by N-acetylcysteine due to attenuated
mitochondrial-derived ROS accumulation-induced endoplasmic
reticulum stress. Cell Death Dis. 9:5682018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
García-Solares J, Donnez J, Donnez O and
Dolmans MM: Pathogenesis of uterine adenomyosis: Invagination or
metaplasia? Fertil Steril. 109:371–379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Benagiano G, Brosens I and Habiba M:
Structural and molecular features of the endomyometrium in
endometriosis and adenomyosis. Hum Reprod Update. 20:386–402. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou YF, Mori T, Nagasawa H, Nakayama T,
Kubota T and Sakamoto S: Probucol, a hypocholesterolemic agent,
prevents the development of uterine adenomyosis induced by
pituitary grafting in mice. Anticancer Res. 24:2209–2212.
2004.PubMed/NCBI
|
|
12
|
Jiang XX, Fei XW, Zhao L, Ye XL, Xin LB,
Qu Y, Xu KH, Wu RJ and Lin J: Aquaporin 5 plays a role in
estrogen-induced ectopic implantation of endometrial stromal cells
in endometriosis. PLoS One. 10:e01452902015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xin LB, Jiang XX, Ye XL, Wu RJ, Xu KH, Ma
JY and Lin J: AQP5 gene silencing inhibits proliferation and
migration of ectopic endometrial glandular epithelial cells in
endometriosis. Zhejiang Da Xue Xue Bao Yi Xue. 44:285–292. 2015.(In
Chinese).
|
|
14
|
National Research Council Institute for
Laboratory Animal Research. Guide for the Care and Use of
Laboratory Animals. National Academies Press (US) Copyright 1996 by
the National Academy of Sciences. All rights reserved.; Washington
(DC): 1996
|
|
15
|
Koujyo T, Hatakeyama S, Yamada H, Iwabuchi
K, Kajino K, Ogasawara K, Onoe K and Fujimoto S: Induction of
endometriosis and adenomyosis by transvaginal pituitary
transplantation in mice with and without natural killer cell
activity. Am J Reprod Immunol. 40:441–446. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mori T, Kyokuwa M and Nagasawa H: Animal
model of uterine adenomyosis: Induction of the lesion in rats by
ectopic pituitary isografting. Lab Anim Sci. 48:64–68.
1998.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression using different real-time quantitative PCR
and 2(-Delta Delta C(T)) method. Methods. 402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Q, Yang T, Li D, Ding F, Bai G, Wang W
and Sun H: Knockdown of aquaporin-5 sensitizes colorectal cancer
cells to 5-fluorouracil via inhibition of the Wnt-β-catenin
signaling pathway. Biochem Cell Biol. 96:572–579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheong ML, Lai TH and Wu WB: Connective
tissue growth factor mediates transforming growth factor β-induced
collagen expression in human endometrial stromal cells. PLoS One.
14:e02107652019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sundqvist J, Andersson KL, Scarselli G,
Gemzell-Danielsson K and Lalitkumar PG: Expression of adhesion,
attachment and invasion markers in eutopic and ectopic endometrium:
A link to the aetiology of endometriosis. Hum Reprod. 27:2737–2746.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamin Hu and Bing ZH: Expression of VEGF-C
and ICAM-1 in adenomyosis and clinical significance. Acta Med Univ
Sci Technol Huazhong. 38:217–219. 2009.
|
|
22
|
Huang YH, Zhou XY, Wang HM, Xu H, Chen J
and Lv NH: Aquaporin 5 promotes the proliferation and migration of
human gastric carcinoma cells. Tumour Biol. 34:1743–1751. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang S, Zhao X, Yang S, Chen B and Shi J:
Salidroside alleviates high glucose-induced oxidative stress and
extracellular matrix accumulation in rat glomerular mesangial cells
by the TXNIP-NLRP3 inflammasome pathway. Chem Biol Interact.
278:48–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J, Wang Z, Chong T, Chen H, Li H, Li G,
Zhai X and Li Y: Over-expression of a poor prognostic marker in
prostate cancer: AQP5 promotes cells growth and local invasion.
World J Surg Oncol. 12:2842014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang XX, Xu KH, Ma JY, Tian YH, Guo XY,
Lin J and Wu RJ: Reduced migration of Ishikawa cells associated
with downregulation of aquaporin-5. Oncol Lett. 4:257–261. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chae YK, Woo J, Kim MJ, Kang SK, Kim MS,
Lee J, Lee SK, Gong G, Kim YH, Soria JC, et al: Expression of
aquaporin 5 (AQP5) promotes tumor invasion in human non small cell
lung cancer. PLoS One. 3:e21622008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Q, Jin M, Yang F, Zhu J, Xiao Q and
Zhang L: Matrix metalloproteinases: Inflammatory regulators of cell
behaviors in vascular formation and remodeling. Mediators Inflamm.
2013:9283152013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Webb AH, Gao BT, Goldsmith ZK, Irvine AS,
Saleh N, Lee RP, Lendermon JB, Bheemreddy R, Zhang Q, Brennan RC,
et al: Inhibition of MMP-2 and MMP-9 decreases cellular migration,
and angiogenesis in in vitro models of retinoblastoma. BMC Cancer.
17:4342017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yi KW, Kim SH, Ihm HJ, Oh YS, Chae HD, Kim
CH and Kang BM: Increased expression of p21-activated kinase 4 in
adenomyosis and its regulation of matrix metalloproteinase-2 and −9
in endometrial cells. Fertil Steril. 103:1089–1097.e2. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li T, Li YG and Pu DM: Matrix
metalloproteinase-2 and −9 expression correlated with angiogenesis
in human adenomyosis. Gynecol Obstet Invest. 62:229–235. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tulchinsky E, Demidov O, Kriajevska M,
Barlev NA and Imyanitov E: EMT: A mechanism for escape from
EGFR-targeted therapy in lung cancer. Biochim Biophys Acta Rev
Cancer. 1871:29–39. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jakobsen KR, Demuth C, Sorensen BS and
Nielsen AL: The role of epithelial to mesenchymal transition in
resistance to epidermal growth factor receptor tyrosine kinase
inhibitors in non-small cell lung cancer. Transl Lung Cancer Res.
5:172–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ramamurthy VP, Ramalingam S, Gediya LK and
Njar VCO: The retinamide VNLG-152 inhibits f-AR/AR-V7 and MNK-eIF4E
signaling pathways to suppress EMT and castration-resistant
prostate cancer xenograft growth. FEBS J. 285:1051–1063. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He Z, Dong W, Hu J and Ren X: AQP5
promotes hepatocellular carcinoma metastasis via NF-κB-regulated
epithelial-mesenchymal transition. Biochem Biophys Res Commun.
490:343–348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang W, Li Q, Yang T, Li D, Ding F, Sun H
and Bai G: RNA interference-mediated silencing of aquaporin (AQP)-5
hinders angiogenesis of colorectal tumor by suppressing the
production of vascular endothelial growth factor. Neoplasma.
65:55–65. 2018. View Article : Google Scholar : PubMed/NCBI
|