Introduction
The adult body contains ~24 g Mg, of which ~60% is
located in the skeleton, accounting for 0.26–0.55% of the mass
percentage of the natural bone. Park et al (1,2)
demonstrated that Mg2+ promoted osteoblast adhesion,
stimulated alkaline phosphatase (ALP) and osteocalcin (OC)
syntheses, and increased the expression levels of integrin,
transcription factor distal-less homeobox 5 (Dlx5), osteogenesis
marker gene ALP, bone sialoprotein (BSP), osteoblast-specific
transcription factor Osterix (Osx) and OC. Crespi et al
(3) reported that Mg2+
enhanced the expression of runt-related transcription factor 2
(Runx2), and initiated osteoblast synthesis of OC and bone matrix.
Magnesium phosphate increases the expression of Runx2, ALP and
osteopontin in osteoblasts (4). In
our previous study, mouse mesenchymal stem cells (MMSCs) were
cultured in Mg-containing hydroxyapatite (Mg-HA) solution for 72 h.
The reverse transcription-quantitative PCR (RT-qPCR) results
demonstrated that the mRNA expression levels of ALP, Osx and Runx2
in the Mg-HA group were significantly higher compared with MMSCs
cultured without Mg-HA, suggesting that Mg-HA promoted MMSC
osteogenic differentiation (5). To
meet clinical requirements, several bone-related ions were added to
synthetic HA to improve its effects, which indicated that Mg-HA was
suitable for clinical application (6). Magnesium and hydroxyapatite
composites can overcome the shortcomings of traditional
bioceramics, including lack of bone formation inducing ability
(7). In addition, extracts of
biodegradable Mg alloys exhibited superior effects on human
mesenchymal stem cell proliferation and differentiation compared
with culture media (8).
Mitogen-activated protein kinases (MAPKs) are a
group of serine-threonine protein kinases that can be activated by
different extracellular stimuli, such as cytokines,
neurotransmitters, hormones, cell stress and cell adhesion
(9,10). There are four independent MAPK
signaling pathways composed of four signaling families: The
MAPK/ERK family or classical pathway, the Big MAP kinase-1 family,
c-Jun N-terminal kinase family and p38 family (9). MAPK signaling pathways are crucial
for several physiological activities in the cell, including
proliferation, differentiation and apoptosis (10–12).
MAPK has been reported to be pivotal in regulating signaling
pathways involved in osteogenic differentiation, specifically the
p38/Osx signaling pathway, where its activation promotes osteogenic
differentiation (13). Yi et
al (14) demonstrated that
activation of the p38 signaling pathway facilitates osteogenic
differentiation over fat cell differentiation during mesenchymal
stem cell differentiation. In vitro, the inhibition of p38α
and p38β can block early osteogenic differentiation (15,16).
In addition, p38α favors the mineralization of long bones (17). The p38ignaling pathway can also
phosphorylate the key transcriptional factor Runx2, and thus
facilitate osteogenic differentiation (18). A combination of Runx2 and Osx can
activate osteogenic lineage transcriptional patterns, such as
increased mRNA expression levels of various bone-related genes,
including ALP, BSP, osteocalcin, osteopontin and type I collagen
(19). Independent of Runx2, the
p38 signaling pathway can regulate the transcription factor Dlx5,
which promotes osteogenic differentiation by activating BSP and Osx
(20).
The 27 kDa heat shock protein (Hsp27) is expressed
in a differentiation-related pattern and can regulate osteocalcin
synthesis (21). Activating
transcription factor 4 (Atf4) is an important transcription factor
that serves a pivotal role in osteoblastic differentiation and bone
formation (22,23). CCAAT/enhancer binding protein
homologous protein (Ddit3) also increases osteoblastic
differentiation potential (24,25).
Myocyte enhancer factor 2C (Mef2c) significantly regulates
osteogenic differentiation of various stem cells (26). Moreover, the functions of Hsp27,
Atf4, Ddit3 and Mef2c are dependent on the p38 signal transduction
pathway (27–29).
In the present study, the expression levels of
phosphorylated (p)-p38, Osx, Runx2 and p38 downstream genes,
including Hsp27, Atf4, Ddit3 and Mef2c, were assessed following
Mg2+ treatment. Furthermore, the osteogenic
differentiation potential of MMSCs in the Mg2+-treated
group was evaluated via ALP and Alizarin Red staining. The
relationship between Mg2+ and the p38/Osx/Runx2
signaling pathway was explored to determine the mechanisms
underlying the osteogenesis-boosting effect of
MgCl2.
Materials and methods
Cell line and cell culture
MMSCs (ScienCell Research Laboratories, Inc.) were
cultured in mesenchymal stem cell culture medium (ScienCell
Research Laboratories, Inc.; Mg concentration, 0.8112 mM)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
and 100 µg/ml streptomycin and penicillin (Gibco; Thermo Fisher
Scientific, Inc.) in a humidified incubator containing 5%
CO2 at 37°C.
ALP staining assay
MMSCs were seeded into 12-well plates
(1×105 cells/well). After culture with mesenchymal stem
cell culture medium for 24 h, different concentrations of
MgCl2 (0 and 20 mM) were added to the osteoblast
differentiation medium (MODM; Mg concentration, 0.8112 mM;
ScienCell Research Laboratories, Inc.) and the culture medium was
changed every 2 days. MMSCs continuously cultured with mesenchymal
stem cell culture medium, changed every 2 days, were considered as
the undifferentiated negative control. Throughout osteogenic
differentiation, the concentration of MgCl2 remained
constant. After 14 days of induction, cells were fixed with 4%
paraformaldehyde for 15 min at room temperature and washed twice
with PBS. NBT/BCIP color substrate solution was freshly prepared by
adding the Ready-to-Use tablet (Roche Diagnostics) into 10 ml
double distilled water according to the manufacturer's protocol.
Cells were stained with NBT/BCIP color substrate solution for 15
min at 25°C, washed twice with distilled water and observed under
an IX71-F22FL/DIC optical microscope (Olympus Corporation;
magnification, ×10).
ELISA
MMSCs were induced with MgCl2 as
aforementioned. After 14 days of induction, cells were harvested,
rinsed with PBS, homogenized in 500 µl PBS and stored overnight at
−20°C. Subsequently, two freeze-thaw cycles were performed to break
the cell membranes. The lysate was centrifuged for 5 min at 5,000 ×
g at 4°C. The supernatant was collected and immediately assayed
using a mouse osteocalcin ELISA kit (cat. no. CSB-E06917m; Cusabio
Technology LLC) according to the manufacturer's protocol. The
results were read using a microplate reader at a wavelength of 450
nm.
Alizarin Red staining assay
MMSCs were induced with MgCl2 as
aforementioned. After 21 days of induction, cells were fixed with
4% paraformaldehyde for 10 min at room temperature, and washed once
with distilled water. Subsequently, cells were stained with
Alizarin Red (Beijing Solarbio Science & Technology Co., Ltd.)
for 30 min at room temperature, washed with distilled water and
observed under an IX71-F22FL/DIC optical microscope (Olympus
Corporation; magnification, ×10). Alizarin Red staining analysis
was conducted using Image-Pro Plus software (version 6.0; Media
Cybernetics, Inc).
Western blotting
MMSCs were seeded into 6-well plates
(3×105 cells/well). After 24 h, MgCl2 (20 mM)
was added to the culture medium for 0, 5, 30 or 60 min to determine
the immediate effect of p38 activation. In the other group, 20 mM
MgCl2 was applied to the culture medium for a longer
period (1, 24 and 72 h) to detect the continuous activating effect.
Subsequently, cells were washed with cold PBS at least three times
and lysed with RIPA lysis buffer (Beyotime Institute of
Biotechnology) at 4°C. Then laemmli sample buffer (2% SDS) was
added. Total protein was quantified using a BCA protein
quantification kit (Thermo Fisher Scientific, Inc.). Proteins (40
µg) were denatured in 1X SDS loading buffer, separated via 10%
SDS-PAGE and transferred to PVDF membranes (EMD Millipore). The
membranes were blocked for 1 h with 5% non-fat milk in TBS-Tween-20
(0.1% Tween-20) at room temperature with gentle agitation.
Subsequently, the membranes were incubated overnight at 4°C with
primary antibodies targeted against: Runx2 (cat. no. ab76956;
Abcam), anti-Osx (cat. no. ab22552; Abcam), p38 (cat. no. 8690S;
Cell Signaling Technology, Inc.), p-p38 (cat. no. 9216S; Cell
Signaling Technology, Inc.) and β-actin (cat. no. 3700S; Cell
Signaling Technology, Inc.). All primary antibodies were used at a
dilution of 1:1,000. Following primary incubation, the membranes
were incubated with horseradish peroxidase-conjugated goat
anti-mouse (cat. no. 7074S; Cell Signaling Technology, Inc.) and
goat anti-rabbit (cat. no. 7076S; Cell Signaling Technology, Inc.)
secondary antibodies for 2 h at room temperature. All secondary
antibodies were used at a dilution of 1:2,000. Protein bands were
visualized using SuperSignal™ West Femto Maximum Sensitivity
substrate (cat. no. 34095; Thermo Fisher Scientific, Inc.). Protein
expression was semi-quantified using Quantity One software (version
4.6.2; Bio-Rad Laboratories, Inc.) with β-actin as the loading
control.
RT-qPCR
MMSCs were seeded into 6-well plates
(3×105 cells/well). After 24 h, MgCl2 (20 mM)
and 10 µM SB203580 (cat. no. S1076; Selleck Chemicals), a specific
inhibitor of the p38 signaling pathway, were added to the culture
medium. After 48 h, total RNA was extracted from cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) and
reverse transcribed into cDNA using the High Capacity cDNA Reverse
Transcription kit (cat. no. 4368814; Applied Biosystems; Thermo
Fisher Scientific, Inc.) according to manufacturer's instruction.
Briefly, 1 ng total RNA was reverse transcribed with 3 steps: 10
min incubation at 25°C, 120 min incubation at 37°C and 5 min
incubation at 85°C. Subsequently, qPCR was performed using SYBR
Select Master Mix (cat. no. 4368577; Applied Biosystems; Thermo
Fisher Scientific, Inc.) and a ViiA 7 real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for qPCR: 95°C for 30 sec;
followed by 40 cycles of 95°C for 5 sec, 58°C for 15 sec and 72°C
for 30 sec. The sequences of the primers used for qPCR were: Hsp27
forward, 5′-GTCCCTGGATGTCAACCACT-3′ and reverse,
5′-GACTGGGATGGTGATCTCGT-3′; Atf4 forward,
5′-CCTGAACAGCGAAGTGTTGG-3′ and reverse,
5′-TGGAGAACCCATGAGGTTTCAA-3′; Ddit3 forward,
5′-AAGCCTGGTATGAGGATCTGC-3′ and reverse,
5′-TTCCTGGGGATGAGATATAGGTG-3′; Mef2c forward,
5′-ATCCCGATGCAGACGATTCAG-3′ and reverse,
5′-AACAGCACACAATCTTTGCCT-3′; and GAPDH forward,
5′-AGCTTCGGCACATATTTCATCTG-3′ and reverse,
5′-CGTTCACTCCCATGACAAACA-3′. mRNA expression levels were quantified
using the 2−ΔΔCq method (30) and normalized to the internal
reference gene GAPDH.
Cell apoptosis
MMSCs were seeded into 6-well plates at
3×105 cells per well overnight with mesenchymal stem
cell culture medium. Subsequently, the medium was changed to MODM
with different concentration of MgCl2 (0, 2, 20, 50 and
100 mM). Cells were harvest to analyze with FITC Annexin V
Apoptosis Detection Kit I (BD Biosciences) according to
manufacturer's protocols. In brief, the MMSCs were washed twice
with cold PBS and then harvest with trypsin digestion. Resuspended
in 100 µl binding buffer, cells were then incubated with 5 µl FITC
Annexin V and 5 µl propidium iodide (PI) for 15 min at room
temperature avoiding light. Then, 400 µl extra binding buffer was
added to each sample before they were analyzed with a flow
cytometer (FACSCanto II; BD Biosciences). The results were analyzed
with FlowJo v10 software (FlowJo LLC).
Cell proliferation
MMSCs were seeded into 96-well plates
(5×103 cells/well) overnight with mesenchymal stem cell
culture medium in triplicate. MODM with different concentrations of
magnesium (0, 2, 20 and 100 mM) were changed every other day. Cell
proliferation assay was performed using CellTiter 96™ Aqueous One
Solution Cell Proliferation (MTS) assay (Promega Corporation) every
day for 5 days according to the manufacturer's instructions.
Statistical analysis
Statistical analyses were conducted using GraphPad
Prism software (version 7; GraphPad Software, Inc.) and SPSS
software (version 19.0; IBM, Corp.). Experiments were repeated at
least three times and quantified data are presented as the mean ±
standard deviation. Comparisons among multiple groups were analyzed
using one-way ANOVA followed by Bonferroni's post hoc test.
Comparisons between two groups were analyzed using unpaired
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
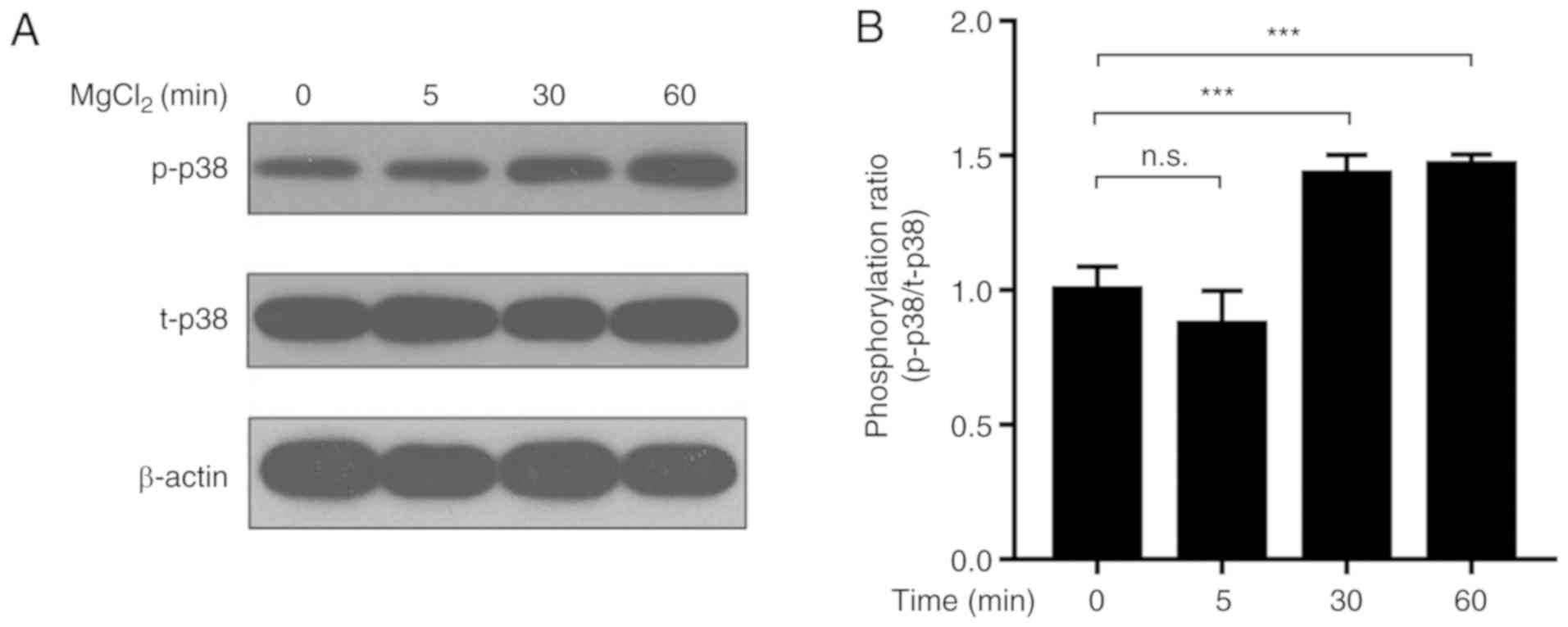
Effect of Mg2+ on the
levels of p-p38 and total (t)-p38 in MMSCs
Previously, it was reported that Mg2+
promoted mouse bone marrow-derived MMSC proliferation and
osteogenic differentiation (7).
Moreover, different concentrations of Mg2+ displayed
contrasting effects on osteoblast differentiation, suggesting there
would be a peak in its enhancing effect with the appropriate dose
(31). Based on the preliminary
findings of the present study, the optimal concentration of
Mg2+ for osteogenic differentiation was 20 mM (Fig. S1), as it notably facilitated
osteogenesis and partially decreased apoptosis induced by MODM (0
MgCl2 group). To verify our observations, cell
proliferation was analyzed with MTS at the early differentiating
stage (Fig. S1C). The 0, 2, and
20 mM MgCl2 groups exhibited better proliferation than
100 mM group although no significant difference was observed. Thus,
20 mM Mg2+ was selected for subsequent experiments. The
p38 signaling pathway has been hypothesized to be closely
associated with osteogenic differentiation (18); therefore, the effect of
Mg2+ on the p38 signaling pathway was further examined.
The results indicated that 20 mM Mg2+ increased the
ratio of p-p38/t-p38 in MMSCs in a time-dependent manner (Figs. 1 and S2).
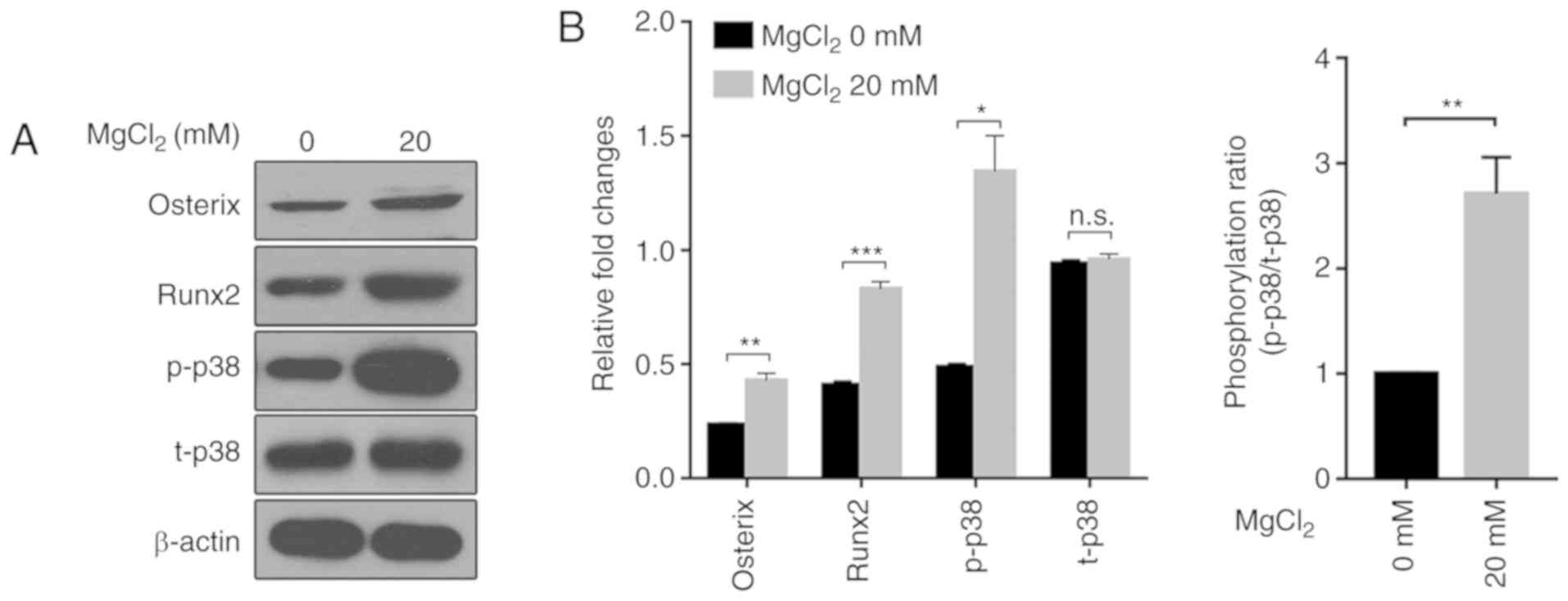
Effect of Mg2+ on the
expression levels of Osx and Runx2 in MMSCs
Downstream genes in the p38 signaling pathway, such
as Osx and Runx2, are key genes involved in osteogenic
differentiation (12). The western
blotting results indicated that Mg2+ treatment
significantly increased the expression levels of Osx and Runx2
compared with the control group, suggesting that Mg2+
enhanced the activity of the p38 signaling pathway (Fig. 2). Furthermore, compared with the
control group, Mg2+ also increased the ratio of
p-p38/t-p38, an indicator of activation of the signaling pathway,
highlighting a potential mechanism underlying
Mg2+-mediated promotion of MMSC osteogenic
differentiation.
Effect of Mg2+ on the
expression levels of p38 downstream genes
To further investigate the hypothesis that the p38
signaling pathway mediated the effects of Mg2+
treatment, the expression of other crucial p38 downstream genes,
specifically Hsp27, Atf4, Ddit3 and Mef2c, was evaluated. The mRNA
expression levels of Hsp27, Atf4, Ddit3 and Mef2c in the
MgCl2 group were significantly increased compared with
the control group (Fig. 3). The
results suggested that Mg2+ increased the expression
levels of p38 downstream target genes via activation of the p38
signaling pathway.
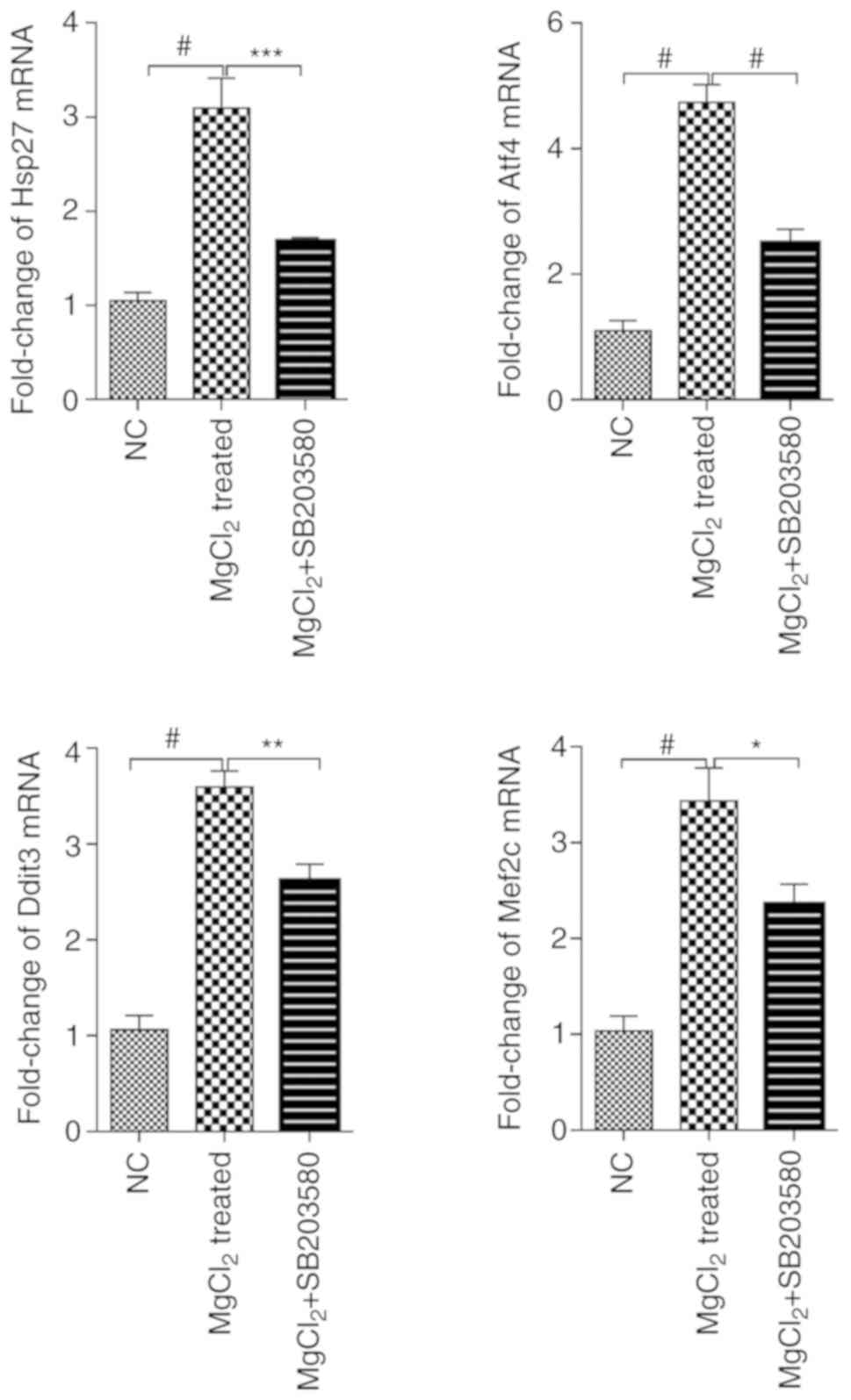 | Figure 3.Effect of Mg2+ on the
expression levels of Hsp27, Atf4, Ddit3 and Mef2c is abrogated by
SB203580-mediated p38 inhibition. MMSCs were cultured with media
containing 20 mM MgCl2 for 48 h, with or without 10 µM
SB203580. Data were analyzed using one-way ANOVA with Bonferroni's
post hoc testing. *P<0.05, **P<0.01, ***P<0.001;
#P<0.0001. Hsp27, 27 kDa heat shock protein; Atf4,
activating transcription factor 4; Ddit3, CCAAT/enhancer-binding
protein homologous protein; Mef2c, myocyte enhancer factor 2C;
MMSC, mouse mesenchymal stem cell; NC, negative control. |
Moreover, blocking of the p38 signaling pathway
using SB203580 resulted in downregulation of Hsp27, Atf4, Ddit3 and
Mef2c expression levels in MgCl2-treated cells (Fig. 3), supporting the hypothesis that
Mg2+ increased the expression of osteogenesis-associated
genes via activation of the p38 signaling pathway.
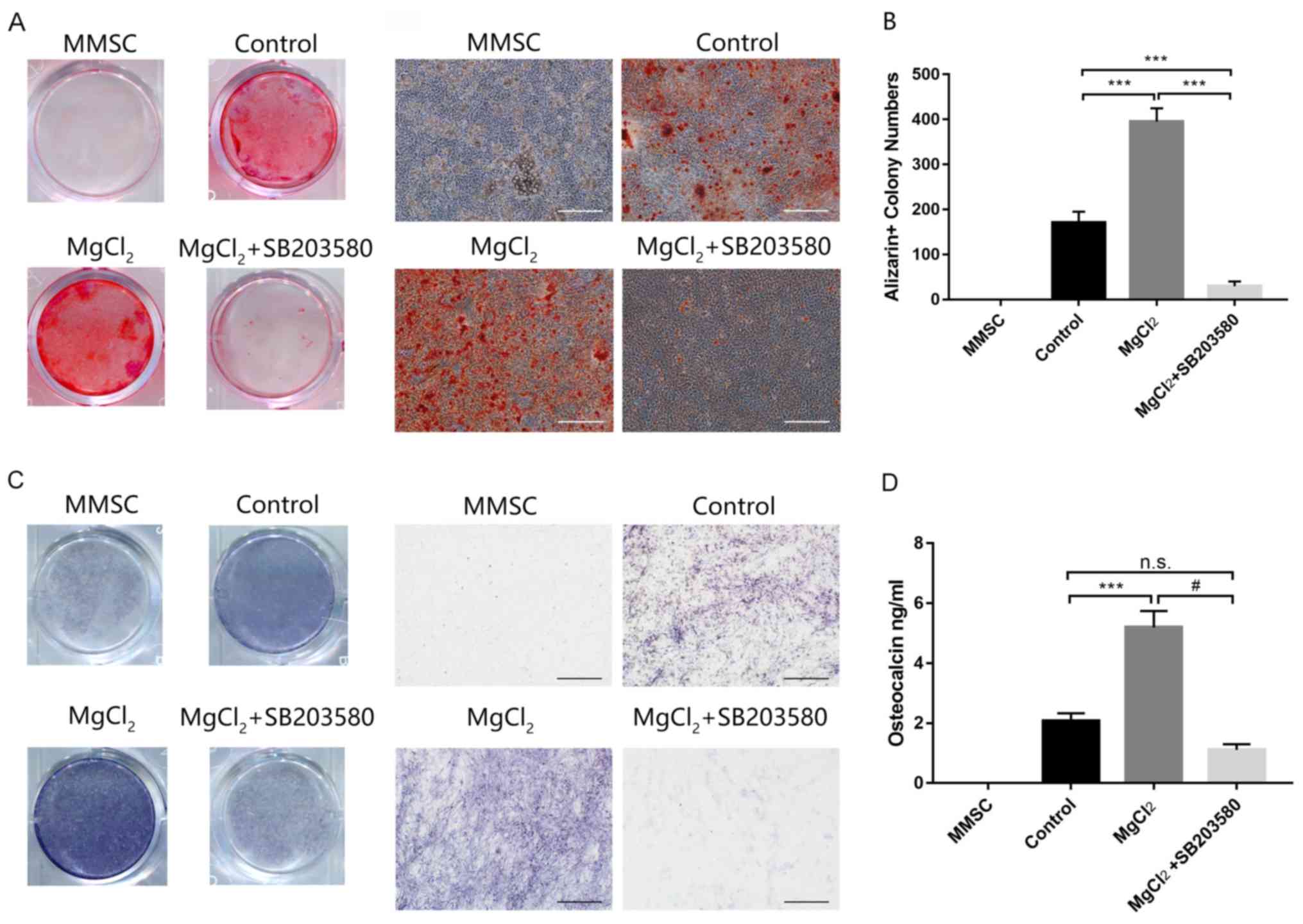
Effect of Mg2+ on MMSC
osteogenic differentiation
MMSCs were induced with MODM containing glutamine,
ascorbic acid, β-glycerol phosphate disodium and dexamethasone to
determine whether Mg2+ promoted osteogenic
differentiation. MMSCs were treated with MgCl2 20 mM
with or without SB203580. Control cells were incubated with MMSC
media. After 21 days, fully differentiated MMSCs were fixed, and
calcium node formation was analyzed by performing Alizarin Red
staining (Fig. 4A). Compared with
the control group, MgCl2 significantly increased the
number and size of calcium nodes. SB203580 significantly reduced
Mg2+-induced osteogenic differentiation (Fig. 4B). The ALP staining results and
quantification of osteocalcin protein levels via ELISA were
consistent with the Alizarin Red staining results (Fig. 4C and D). Collectively, the results
suggested that Mg2+ promoted MMSC osteogenic
differentiation, which was dependent on activation of the p38
signaling pathway.
Discussion
In the present study, the facilitatory effects of
MgCl2 on MMSC osteogenic differentiation, including the
effects on p38 signaling pathway activation, were assessed.
Magnesium, the second most abundant intracellular cation in the
human body, serves a key role in a number of essential cellular
processes, including DNA replication and repair, and cell
proliferation (32,33). As a result, magnesium sulfate is
the necessary component in most of the commercialized culture media
used for adhesive cells (34). In
the present study, the culture media used for MMSCs and
differentiation contained 0.8112 mM MgSO4 as background
magnesium. Due to the low background concentration, it was assumed
that the magnesium present in the media would not affect the
results of the present study. High concentrations of
MgCl2 (50 or 100 mM) resulted in a dose-dependent
increase in apoptosis compared with the 0 mM MgCl2
group. Conversely, 2 and 20 mM MgCl2 reduced apoptosis
rate compared with both the control group and the high
concentration groups. Furthermore, 20 mM MgCl2 displayed
an increased capacity to facilitate osteogenic differentiation
among the different concentrations of MgCl2 used in the
present study. The signaling pathways underlying
Mg2+-mediated increases in osteogenesis were evaluated,
and the results indicated that MgCl2 increased
activation of the p38 signaling pathway compared with the control
group. Additionally, the results suggested that the expression
levels of key p38 downstream osteogenic genes were significantly
increased by MgCl2 treatment compared with the control
group. Collectively, the results indicated a potential key role for
intracellular Mg2+ in MMSC osteogenesis.
It has been reported that four downstream genes are
closely associated with MMSC osteogenic differentiation, and the
characteristics and formation of osteoblasts (35–38).
Activation of the p38 signaling pathway phosphorylates Hsp27,
thereby stimulating osteoblast mineralization (35). Atf4 is an essential transcription
factor that participates in the synthesis of osteoblast proteins
(36). Pereira et al
(24) reported that Ddit3 induced
osteogenic differentiation, and it has also been demonstrated that
Mef2c serves an important role in regulating gene expression in
osteoblasts (37).
Apart from those four genes, Runx2, another
downstream gene of p38 pathway, is also considered as a key
effector and marker of osteoblast differentiation (3,4). Its
transcription also reported to be influenced by other pathways like
TRPM7/PI3K signaling pathway (38,39).
Transient receptor potential M-type 7 (TRPM7), a non-selective
cationic channel with constitutive activity, is an important
regulator of entry of several extracellular metal ions, and serves
an important regulatory role in bone sclerosis and remodeling
(38). It has been reported that
Mg2+ can upregulate the expression of Runx2 via a
TRPM7/PI3K signaling pathway, which results in increased expression
of the osteogenic marker ALP (39). Therefore, TRPM7 serves an important
role in Mg2+-mediated BMSC osteoblast
differentiation.
Moreover, magnesium accelerates osteogenesis by
affecting the secretion of the growth factor (40). Fibroblast growth factor (FGF)
regulates the proliferation of osteoblast precursor cells via the
p38 signaling pathway, and regulates Runx2 function, thereby
promoting osteoblast differentiation and maturation (40). Mg2+ can increase the
expression of FGF-2, and promote BMSC proliferation and osteogenic
differentiation (8). Therefore,
Mg2+ may promote BMSC proliferation and osteogenic
differentiation by activating the p38 signaling pathway via
FGF-2.
In the present study, Mg2+ was derived
from MgCl2. The means by which extracellular
Mg2+ ions from biomaterials are transported into cells
affects the expression levels of transcription factors and requires
further investigation.
In conclusion, Mg2+ promoted MMSC
osteogenic differentiation by activating the p38 signaling pathway,
and blocking the p38 signaling pathway abrogated the effects of
Mg2+ on differentiation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81871756), the
High-Level Medical Talents Training Project of Changzhou (grant no.
2016CZLJ004), the Municipal Social Development Project of the
Changzhou City (grant no. CJ20180073) and the Youth Project of
Changzhou Municipal Commission of Health and Family Planning (grant
no. QN201718).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XYN designed the study and contributed to writing
the manuscript. XBX and SN performed the experiments. SN
contributed to the critical revision of the manuscript and provided
important intellectual feedback. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Park JW: Osseointegration of two different
phosphate ion-containing titanium oxide surfaces in rabbit
cancellous bone. Clin Oral Implants Res. 24:145–151. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park JW, Kim YJ, Jang JH and Song H:
Osteoblast response to magnesium ion-incorporated nanoporous
titanium oxide surfaces. Clin Oral Implants Res. 21:1278–1287.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crespi R, Mariani E, Benasciutti E,
Capparè P, Cenci S and Gherlone E: Magnesium-enriched
hydroxyapatite versus autologous bone in maxillary sinus grafting:
Combining histomorphometry with osteoblast gene expression profiles
ex vivo. J Periodontol. 80:586–593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang TY, Su WT and Chen PH: Comparing the
effects of chitosan scaffolds containing various divalent metal
phosphates on osteogenic differentiation of stem cells from human
exfoliated deciduous teeth. Biol Trace Elem Res. 185:316–326. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xinbo X, Xinye N and Dong Z: Functions of
the Mg-HA coating on carbon/carbon composite surface to promote the
proliferation and osteogenic differentiation of mBMSCs. RSC
Advances. 6:105056–105062. 2016. View Article : Google Scholar
|
|
6
|
Sethmann I, Luft C and Kleebe HJ:
Development of phosphatized calcium carbonate biominerals as
bioactive bone graft substitute materials, part I: Incorporation of
magnesium and strontium ions. J Funct Biomater. 9:692018.
View Article : Google Scholar
|
|
7
|
Bertran O, del Valle LJ, Revilla-Lopez G,
Rivas M, Chaves G, Casas MT, Casanovas J, Turon P, Puiggalí J and
Alemán C: Synergistic approach to elucidate the incorporation of
magnesium ions into hydroxyapatite. Chemistry. 21:2537–2546. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li RW, Kirkland NT, Truong J, Wang J,
Smith PN, Birbilis N and Nisbet DR: The influence of biodegradable
magnesium alloys on the osteogenic differentiation of human
mesenchymal stem cells. J Biomed Mater Res A. 102:4346–4357.
2014.PubMed/NCBI
|
|
9
|
Fang JY and Richardson BC: The MAPK
signalling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rodríguez-Carballo E, Gámez B and Ventura
F: p38 MAPK signaling in osteoblast differentiation. Front Cell Dev
Biol. 4:402016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao WL, Zhang DZ, Fan CH and Yu BJ:
Intermittent stretching and osteogenic differentiation of bone
marrow derived mesenchymal stem cells via the p38MAPK-osterix
signaling pathway. Cell Physiol Biochem. 36:1015–1025. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yi C, Liu D, Fong CC, Zhang J and Yang M:
Gold nanoparticles promote osteogenic differentiation of
mesenchymal stem cells through p38 MAPK pathway. ACS Nano.
4:6439–6448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bain J, Plater L, Elliott M, Shpiro N,
Hastie CJ, McLauchlan H, Klevernic I, Arthur JSC, Alessi DR and
Cohen P: The selectivity of protein kinase inhibitors: A further
update. Biochem J. 408:297–315. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cuenda A, Rouse J, Doza YN, Meier R, Cohen
P, Gallagher TF, Young PR and Lee JC: SB 203580 is a specific
inhibitor of a MAP kinase homologue which is stimulated by cellular
stresses and interleukin-1. FEBS Lett. 364:229–233. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thouverey C and Caverzasio J: The p38α
MAPK positively regulates osteoblast function and postnatal bone
acquisition. Cell Mol Life Sci. 69:3115–3125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Greenblatt MB, Shim JH, Zou W, Sitara D,
Schweitzer M, Hu D, Lotinun S, Sano Y, Baron R, Park JM, et al: The
p38 MAPK pathway is essential for skeletogenesis and bone
homeostasis in mice. J Clin Invest. 120:2457–2473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun J, Zhou H, Deng Y, Zhang Y, Gu P, Ge S
and Fan X: Conditioned medium from bone marrow mesenchymal stem
cells transiently retards osteoblast differentiation by
downregulating runx2. Cells Tissues Organs. 196:510–522. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ulsamer A, Ortuño MJ, Ruiz S, Susperregui
ARG, Osses N, Rosa JL and Ventura F: BMP-2 induces osterix
expression through up-regulation of Dlx5 and its phosphorylation by
p38. J Biol Chem. 283:3816–3826. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kato K, Tokuda H, Mizutani J, Adachi S,
Matsushima-Nishiwaki R, Natsume H, Kozawa O and Otsuka T: Role of
HSP27 in tumor necrosis factor-α-stimulated interleukin-6 synthesis
in osteoblasts. Int J Mol Med. 28:887–893. 2011.PubMed/NCBI
|
|
22
|
Yang SY, Wei FL, Hu LH and Wang CL:
PERK-eIF2α-ATF4 pathway mediated by endoplasmic reticulum stress
response is involved in osteodifferentiation of human periodontal
ligament cells under cyclic mechanical force. Cell Signal.
28:880–886. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu S, Franceschi RT, Luo M, Zhang X, Jiang
D, Lai Y, Jiang Y, Zhang J and Xiao G: Parathyroid hormone
increases activating transcription factor 4 expression and activity
in osteoblasts: Requirement for osteocalcin gene expression.
Endocrinology. 149:1960–1968. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pereira RC, Delany AM and Canalis E:
CCAAT/enhancer binding protein homologous protein (DDIT3) induces
osteoblastic cell differentiation. Endocrinology. 145:1952–1960.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu Y, Sun H, Song F, Fu D and Wang J:
DDIT3 overexpression increases odontoblastic potential of human
dental pulp cells. Cell Prolif. 47:249–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen S, Huang D, Feng G, Zhu L, Zhang Y,
Cao P, Zheng K, Zhang D and Feng X: MEF2 transcription factor
regulates osteogenic differentiation of dental pulp stem cells.
Cell Reprogram. 18:237–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hell-Pourmojib M, Neuner P, Fischer H,
Rezaie S, Kindås-Mügge I, Knobler R and Trautinger F: Differential
expression of a novel gene in response to hsp27 and cell
differentiation in human keratinocytes. J Invest Dermatol.
119:154–159. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang Q, Li F, Shi K, Wu P, An J, Yang Y
and Xu C: ATF4 activation by the p38MAPK-eIF4E axis mediates
apoptosis and autophagy induced by selenite in Jurkat cells. FEBS
Lett. 587:2420–2429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han J, Jiang Y, Li Z, Kravchenko VV and
Ulevitch RJ: Activation of the transcription factor MEF2C by the
MAP kinase p38 in inflammation. Nature. 386:296–299. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wong ML and Medrano JF: Real-time PCR for
mRNA quantitation. Biotechniques. 39:75–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu WC, Pringa E and Chou L: Effect of
magnesium on the osteogenesis of normal human osteoblasts. Magnes
Res. 30:42–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hartwig A: Role of magnesium in genomic
stability. Mutat Res. 475:113–121. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wolf FI, Trapani V and Cittadini A:
Magnesium and the control of cell proliferation: Looking for a
needle in a haystack. Magnes Res. 21:83–91. 2008.PubMed/NCBI
|
|
34
|
Takeichi M and Okada TS: Roles of
magnesium and calcium ions in cell-to-substrate adhesion. Exp Cell
Res. 74:51–60. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kato K, Adachi S, Matsushima-Nishiwaki R,
Minamitani C, Natsume H, Katagiri Y, Hirose Y, Mizutani J, Tokuda
H, Kozawa O and Otsuka T: Regulation by heat shock protein 27 of
osteocalcin synthesis in osteoblasts. Endocrinology. 152:1872–1882.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang X, Guo B, Li Q, Peng J, Yang Z, Wang
A, Li D, Hou Z, Lv K, Kan G, et al: miR-214 targets ATF4 to inhibit
bone formation. Nat Med. 19:93–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stephens AS, Stephens SR, Hobbs C,
Hutmacher DW, Bacic-Welsh D, Woodruff MA and Morrison NA: Myocyte
enhancer factor 2C, an osteoblast transcription factor identified
by dimethyl sulfoxide (DMSO)-enhanced mineralization. J Biol Chem.
286:30071–30086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakano Y, Le MH, Abduweli D, Ho SP,
Ryazanova LV, Hu Z, Ryazanov AG, Den Besten PK and Zhang Y: A
critical role of TRPM7 as an ion channel protein in mediating the
mineralization of the craniofacial hard tissues. Front Physiol.
7:2582016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang X, Zu H, Zhao D, Yang K, Tian S, Yu
X, Lu F, Liu B, Yu X, Wang B, et al: Ion channel functional protein
kinase TRPM7 regulates Mg ions to promote the osteoinduction of
human osteoblast via PI3K pathway: In vitro simulation of the
bone-repairing effect of Mg-based alloy implant. Acta Biomater.
63:369–382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Takei Y, Minamizaki T and Yoshiko Y:
Functional diversity of fibroblast growth factors in bone
formation. Int J Endocrinol. 2015:7293522015. View Article : Google Scholar : PubMed/NCBI
|