Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide, among which non-small cell
lung cancer (NSCLC) accounts for >85% (1). Although numerous improvements have
been achieved in therapeutic methods including surgical strategy,
neo-adjuvant therapy, targeted drugs and molecular markers, the
prognosis of NSCLC remains far from satisfaction, particularly for
advanced cases who fail to benefit from tumor resection (2). Furthermore, chemotherapy-based
treatment remains the primary option for patients with advanced
NSCLC, while a proportion of patients experience chemoresistance,
post-response progression and metastasis after chemotherapy, which
represents a considerable bottleneck for their clinical treatment
(3). Therefore, it is an urgent
challenge to identify novel and effective treatment options to
improve the prognosis of these patients with advanced NSCLC.
Apatinib, as a novel small-molecule
anti-angiogenesis agent, exerts a favorable anti-tumor effect in
terms of inhibiting the proliferation and migration of endothelial
cells, reducing angiogenesis and micro-vascularization of tumors
via selectively binding or inhibiting vascular endothelial growth
factor receptor-2 (VEGFR-2) and suppressing its downstream signal
transduction pathways (4,5). Despite increased evidence of the
efficacy of apatinib in treating advanced cancer types, including
gastric cancer, colorectal cancer and ovarian cancer, its
application in NSCLC has rarely been investigated and only a few
studies with small sample sizes have been performed (6–9).
Apatinib has been reported to attenuate multi-chemotherapy drug
resistance in several cancer types using in vitro
experiments and may enhance the efficacy of chemotherapy in
treating patients with advanced cancer types in clinical settings
(10,11). Moreover, apatinib is able to
regulate autophagy in serval cancer types, including anaplastic
thyroid cancer, colon cancer and osteosarcoma, and autophagy is
considered a key factor in NSCLC chemoresistance and metastasis
(12). Based on the abovementioned
findings, it was hypothesized that apatinib may be able to
synergize the efficacy of chemotherapeutics in the treatment of
NSCLC via regulating autophagy.
Therefore, the present study aimed to investigate
the efficacy and safety of apatinib plus docetaxel vs. docetaxel
alone, and their effects on regulating autophagy markers in
patients with advanced NSCLC. In addition, it was evaluated whether
apatinib was able to sensitize the cells to docetaxel-induced
apoptosis in chemoresistant NSCLC cells via regulating
autophagy.
Materials and methods
Patients
A total of consecutive 39 patients (age, 47–75
years; 26 male patients and 13 female patients) with advanced
NSCLC, who underwent apatinib plus docetaxel treatment or docetaxel
alone treatment, were retrospectively enrolled in The First
Affiliated Hospital of Nanjing Medical University between January
2017 and December 2018. The inclusion criteria were as follows: i)
Diagnosed with primary NSCLC at stage IV or recurrent metastatic
NSCLC refractory/intolerant to standard therapy; ii) age ≥18 years;
iii) a baseline Eastern Cooperative Oncology Group performance
status score of 0–1 (13); iv)
adequate hematologic, hepatic and renal functions; v) cessation of
other anti-tumor therapies for ≥1 month prior to enrollment; vi)
presence of ≥1 measurable lesion defined by Response Evaluation
Criteria in Solid Tumors version 1.1 (RECIST 1.1) (14); and vii) a survival prognosis of
>3 months. Furthermore, the exclusion criteria were as follows:
i) Previously treated with immunotherapy; ii) untreated metastases
of the central nervous system; iii) uncontrolled blood pressure
with medication (>140/90 mmHg); iv) serious infection or
autoimmune disease; v) bleeding tendency; and vi) hepatopathy,
nephropathy, cardiopathy, respiratory disease or uncontrollable
diabetes.
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Nanjing Medical
University, and all patients provided written informed consent
prior to enrollment.
Treatment and assessment
Patients were divided into two groups: The Apatinib
plus docetaxel group (n=19) and the Docetaxel group (n=20). In the
Apatinib plus docetaxel group, patients received apatinib (500
mg/day, orally; Heng Rui Pharmaceutics; www.hrs.com.cn) and docetaxel (60 mg/m2,
intravenously; Heng Rui Pharmaceutics) on day 1 every 3 weeks for a
total of four treatment cycles. In the Docetaxel group, patients
received docetaxel (60 mg/m2, intravenously; Heng Rui
Pharmaceutics) on day 1 every 3 weeks for a total of four cycles.
The treatment response was evaluated after four cycles of
treatment, referring to the RECIST 1.1 criteria, as follows:
Complete remission (CR), partial remission (PR), stable disease
(SD) or progressive disease. Then, the overall remission rate (ORR)
was calculated as CR + PR, and the disease control rate (DCR) was
calculated as CR + PR + SD. In addition, adverse events (AE) were
recorded and evaluated according to the National Cancer Institute
Common Terminology Criteria for AE (version 4.0; www.meddramsso.com).
Immunohistochemistry (IHC)
analysis
It has been reported that apatinib regulates
autophagy in various cancer types and autophagy is considered as a
key factor in chemotherapy drug resistance (12,15–17).
Therefore, the expression levels of autophagy markers, light chain
3α (LC3A) and Beclin-1, were detected in tumor tissues
pre-treatment and post-treatment using IHC analysis. Lung tumor
tissues, obtained via biopsy pre-treatment (before treatment) and
post-treatment [after two cycles of treatment (~42 days)], were
available from only three patients in the Apatinib plus docetaxel
group and three patients in the Docetaxel group, and these tissues
were subjected to IHC analysis.
The tumor tissue section (thickness, 4 µm), which
was fixed with 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA) at
4°C for 24 h and embedded in paraffin (Sigma-Aldrich; Merck KGaA),
was deparaffinized, rehydrated and subjected to antigen retrieval,
which was followed by blocking with 10% goat serum (Sigma-Aldrich;
Merck KGaA) at 37°C for 1 h and 0.3% H2O2 to
block non-specific binding and peroxidase activity, respectively.
Subsequently, primary anti-Beclin-1 (1:100; cat. no. bsm-33315M)
and anti-LC3A antibodies (1:100; cat. no. bsm-33309M; both Beijing
Biosynthesis Biotechnology Co., Ltd.) were added and incubated at
4°C overnight. Subsequently, the samples were incubated with
secondary horseradish peroxidase (HRP)-conjugated goat anti-rabbit
IgG (H+L) antibody (1:10,000; cat. no. A27036; Invitrogen; Thermo
Fisher Scientific, Inc.) at 37°C for 60 min. The tissue sections
were stained with diaminobenzidine (Sigma-Aldrich; Merck KGaA) at
37°C for 5 min and counterstained with 0.5% hematoxylin
(Sigma-Aldrich; Merck KGaA) at room temperature for 5 min. The IHC
staining results were observed under a Nikon ECLIPSE E200 light
microscope (Nikon Corporation; magnification, ×400), and assessed
for staining intensity and density of positively stained cells
according to a previously described method (18), with an IHC staining score of 0–12
for each section.
Cell culture
Human NSCLC cells (wild-type A549) and
docetaxel-resistant A549 cells (A549/DTX) were purchased from the
Type Culture Collection of the Chinese Academy of Sciences. Cells
were cultured in 90% RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Zhejiang Tianhang
Biological Technology Co., Ltd.) and 100 U/ml penicillin
(Sigma-Aldrich; Merck KGaA) and streptomycin (Sigma-Aldrich; Merck
KGaA) at 37°C in a humidified atmosphere containing 5%
CO2.
Determination of A549/DTX cells
To determine the docetaxel resistance of A549/DTX
cells, 0, 2.5, 5, 10 or 20 µM docetaxel (Abmole Bioscience Inc.)
was added to treat A549/DTX cells and wild-type A549 cells for 24 h
at 37°C. Cell viability was detected using Cell Counting Kit-8
(Sigma-Aldrich; Merck KGaA) according to the manufacturer's
instructions at a wavelength of 450 nm. Then, the relative cell
viability was calculated by referring to the viability of cells
treated with 0 µM docetaxel in each cell type.
Docetaxel, rapamycin and
3-methyladenine (3-MA) treatments of A549/DTX cells and subsequent
analyses
In order to investigate whether autophagy promotes
docetaxel resistance in A549/DTX cells, 10 µM docetaxel (Abmole
Bioscience Inc.), 20 µM autophagy activator rapamycin
(Sigma-Aldrich; Merck KGaA) and 50 mM autophagy inhibitor 3-MA
(Sigma-Aldrich; Merck KGaA) were used alone or in combination to
treat the cells simultaneously at 37°C. The concentration of
docetaxel was selected based on the IC50 value of
docetaxel in A549/DTX cells reported in a previous study (19). The concentrations of rapamycin and
3-MA were in reference to our previous study (20).
After 48 h of treatment, the protein expression
levels of LC3A, Beclin-1, poly(ADP) ribose polymerase (PARP) and
phosphorylated (p)-AKT were determined via western blot analysis in
each group of cells. Moreover, the apoptotic rate in each group of
cells was determined with an Annexin V/PI double staining kit
(Calbiochem; Merck KGaA) using a FACSCanto II flow cytometer (BD
Biosciences), according to the manufacturer's protocol. The data
was analyzed using FlowJo software (version 7.6; BD Biosciences)
(21).
Apatinib and docetaxel treatment of
A549/DTX cells and subsequent experiments
In order to investigate whether apatinib synergizes
the anti-cancer effect of docetaxel on A549/DTX cells via
regulating autophagy, 10 µM apatinib (Selleck Chemicals) and 10 µM
docetaxel (Abmole Bioscience, Inc.) were used alone or in
combination to treat the cells at 37°C. After 48 h of incubation,
the protein expression levels LC3A, Beclin-1, PARP and p-AKT were
determined via western blot analysis, while the apoptosis rate was
determined using a Annexin V/PI double staining kit (Calbiochem;
Merck KGaA), according to the manufacturer's protocol, in each
group of cells.
Apatinib and docetaxel treatments of
wild-type A549 cells and subsequent assays
In order to investigate whether apatinib synergizes
the anti-cancer effect of docetaxel in wild-type A549 cells via
regulating autophagy, 10 µM apatinib (Selleck Chemicals) and 10 µM
docetaxel (Abmole Bioscience, Inc.) were used to treat wild-type
A549 cells alone or in combination at 37°C. After 48 h of
treatment, the expression levels of LC3A and Beclin-1 were
determined via western blot analysis, and the cell apoptosis rate
was determined with an Annexin V/PI double staining kit
(Calbiochem; Merck KGaA), according to the manufacturer's protocol,
in each group of cells.
Western blot analysis
After isolation of total protein with RIPA lysis and
extraction buffer (Thermo Fisher Scientific, Inc.), the protein
concentration was quantified with the Pierce™ BCA Protein assay kit
(Thermo Fisher Scientific, Inc.). Subsequently, 20 µg protein
samples were separated using 4–20% SDS-PAGE (Nanjing KeyGen Biotech
Co., Ltd.) and then transferred onto Immobilon®-P
Transfer membranes (Merck KGaA). After blocking with 5% non-fat
milk for 2 h at 37°C, membranes were incubated with primary
antibodies, including anti-Beclin-1 (1:1,000; cat. no. bs-1353R;
Beijing Biosynthesis Biotechnology Co., Ltd.), anti-LC3A (1:1,000;
cat. no. bsm-33309M; Beijing Biosynthesis Biotechnology Co., Ltd.),
anti-p-AKT antibody (1:1,000; cat. no. 4058; Cell Signaling
Technology, Inc.), anti-PARP antibody (1:1,000; cat. no. 9532; Cell
Signaling Technology, Inc.) and anti-β-actin antibody (1:10,000;
cat. no. sc-58679; Santa Cruz Biotechnology, Inc.), overnight at
4°C. Subsequently, the membranes were incubated with rabbit
anti-mouse IgG-HRP (1:5,000; cat. no. sc-358917; Santa Cruz
Biotechnology, Inc.) or goat anti-rabbit IgG-HRP (1:5,000; cat. no.
sc-2004; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. The bands were visualized using ECL kit (Nanjing
KeyGen Biotech Co., Ltd.) followed by exposure to X-ray film
(Kodak).
Reverse transcription-quantitative
(RT-q)PCR
After the extraction of total RNA using PureZOL RNA
isolation reagent (Bio-Rad Laboratories, Inc.), it was reverse
transcribed to cDNA using the ReverTra Ace qPCR RT kit (Toyobo Life
Science) at 37°C for 15 min. Subsequently, qPCR was performed using
the KOD SYBR qPCR mix (Toyobo Life Science) to quantify the mRNA
expression levels of LC3A and Beclin-1. The thermocycling
conditions used for qPCR were as follows: Initial denaturation at
98°C for 2 min; 40 cycles of denaturation at 98°C for 10 sec and
annealing and extension at 61°C for 30 sec. The quantified results
were determined using the 2−∆∆Cq method with β-actin as
the internal reference (22).
The sequences of the primers were as follows: LC3A
forward, 5′AGCGAGTTGGTCAAGATCATC3′ and reverse,
5′GGTTTCCTGGGAGGCGTAGA3′; Beclin-1 forward,
5′TCAGAGATACCGACTTGTTCCTTAC3′ and reverse,
5′ACTGCCTCCTGTGTCTTCAATC3′; and β-actin forward,
5′TCGTGCGTGACATTAAGGAGAA3′ and reverse,
5′AGGAAGGAAGGCTGGAAGAGT3′.
Statistical analysis
The experiments were performed in triplicate.
Statistical analyses were performed using SPSS software version
22.0 (IBM Corp.), and plots were generated using GraphPad Prism
Software version 7.00 (GraphPad Software, Inc.). Data are presented
as the mean ± standard deviation for continuous variables and n (%)
for categorical variables. Comparisons between two independent
groups of continuous data were analyzed using an unpaired Student's
t-test. Comparisons between two independent groups of categorical
data were analyzed usingthe χ2 test or Fisher's exact
test. Comparison between two paired groups was performed using the
paired t-test. Multiple comparisons among groups was performed
using one-way ANOVA followed by Tukey's multiple-comparisons test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Basic characteristics of patients
A total of 14 males and five females, aged 47–75
years, including six patients with adenocarcinomas and 13 with
squamous cell carcinomas, were included in the Apatinib plus
docetaxel group. Moreover, 12 males and eight females, aged 48–75
years, including 10 patients with adenocarcinomas and 10 with
squamous cell carcinomas, were included in Docetaxel group. There
was no difference in sex, age or histological type between the two
groups (all P>0.05).
Treatment efficacy
After the four cycles of treatment, the CR was the
same (0 vs. 0%; P=1.000), while the ORR (37 vs. 10%; P=0.047) and
DCR (84 vs. 45%; P=0.011) were increased in the Apatinib plus
docetaxel group compared with the Docetaxel group, respectively
(Table I). These results indicated
that apatinib plus docetaxel was more efficient compared with
docetaxel alone in treating patients with advanced NSCLC.
 | Table I.Treatment response between the
Apatinib plus docetaxel group and the Docetaxel group. |
Table I.
Treatment response between the
Apatinib plus docetaxel group and the Docetaxel group.
| Parameter | Docetaxel
group | Apatinib plus
docetaxel group | P-value |
|---|
| CR, n (%) | 0 (0) | 0 (0) | 1.000 |
| PR, n (%) | 2 (10) | 7 (37) | 0.047 |
| SD, n (%) | 7 (35) | 9 (47) | 0.433 |
| PD, n (%) | 11 (65) | 3 (16) | 0.011 |
| ORR, n (%) | 2 (10) | 7 (37) | 0.047 |
| DCR, n (%) | 9 (45) | 16 (84) | 0.011 |
AEs occurrence
The occurrences of total hypertension (58 vs. 0%,
P<0.001) and total hand-foot syndrome (26 vs. 0%, P=0.014) were
significantly higher in the Apatinib plus docetaxel group compared
with those in the Docetaxel group, respectively. However, no
differences in any other AEs, such as neutropenia, were present
between the Apatinib plus docetaxel group and the Docetaxel group
(Table II). Moreover, all the AEs
in the two groups were mild and tolerable.
 | Table II.Adverse events between the Apatinib
plus docetaxel group and the Docetaxel group. |
Table II.
Adverse events between the Apatinib
plus docetaxel group and the Docetaxel group.
|
| Docetaxel
group | Apatinib plus
docetaxel group |
|
|---|
|
|
|
|
|
|---|
| Parameter | Grade 1–2 | Grade 3–4 | Total | Grade 1–2 | Grade 3–4 | Total |
P-valuea |
|---|
| Neutropenia, n
(%) | 15 (75) | 0 (0) | 15 (75) | 16 (84) | 0 (0) | 16 (84) | 0.476 |
| Thrombocytopenia, n
(%) | 4 (20) | 0 (0) | 4 (20) | 5 (26) | 0 (0) | 5 (26) | 0.640 |
| Anemia, n (%) | 4 (20) | 0 (0) | 4 (20) | 2 (11) | 0 (0) | 2 (11) | 0.412 |
| Hypertension, n
(%) | 0 (0) | 0 (0) | 0 (0) | 10 (53) | 1 (5) | 11 (58) | <0.001 |
| Hand-foot syndrome,
n (%) | 0 (0) | 0 (0) | 0 (0) | 5 (26) | 0 (0) | 5 (26) | 0.014 |
| Fatigue, n (%) | 4 (20) | 0 (0) | 4 (20) | 7 (37) | 0 (0) | 7 (37) | 0.243 |
| Proteinuria, n
(%) | 0 (0) | 0 (0) | 0 (0) | 3 (16) | 0 (0) | 3 (16) | 0.064 |
| Hemorrhage, n
(%) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 1 (5) | 0.297 |
| Oral mucositis, n
(%) | 1 (5) | 0 (0) | 1 (5) | 0 (0) | 1 (5) | 1 (5) | 1.000 |
| Nausea, n (%) | 16 (80) | 0 (0) | 16 (80) | 15 (79) | 0 (0) | 15 (79) | 0.935 |
| Diarrhea, n
(%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Hyperbilirubinemia,
n (%) | 4 (20) | 0 (0) | 4 (20) | 3 (16) | 0 (0) | 3 (16) | 0.732 |
| Elevated
transaminase, n (%) | 2 (10) | 0 (0) | 2 (10) | 2 (11) | 0 (0) | 2 (11) | 0.957 |
Autophagy markers
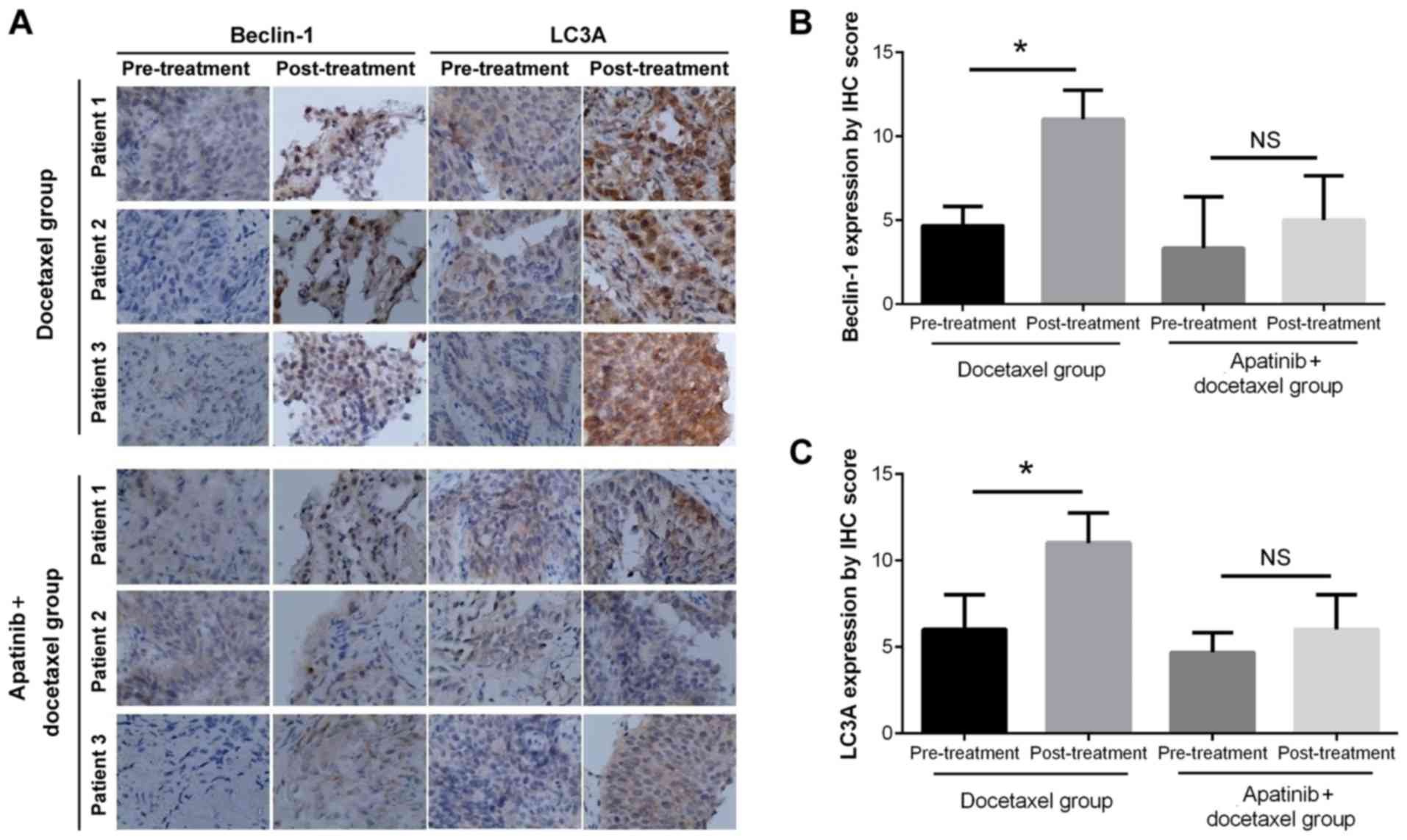
Representative IHC staining images are presented in
Fig. 1A. Semi-quantitative IHC
scoring demonstrated that in the Docetaxel group, Beclin-1
(P<0.01) and LC3A (P<0.05) expression levels were increased
after two cycles of treatment compared with the levels prior to
treatment (Fig. 1B and C), while
in the Apatinib plus docetaxel group, the expression levels
remained similar after two cycles of treatments compared with the
pre-treatment score (both P>0.05; Fig. 1B and C). These results suggested
that apatinib attenuated autophagy in advanced NSCLC induced by
docetaxel for ≥2 cycles (treatment duration, 42 days).
Validation of docetaxel resistance of
A549/DTX cells
A549/DTX cells demonstrated increased relative cell
viability compared with wild-type A549 cells under 2.5, 5, 10 and
20 µM docetaxel treatment (P<0.05), which suggested that
A549/DTX cells were docetaxel resistant (Fig. S1).
Combination treatments on A549/DTX
cells
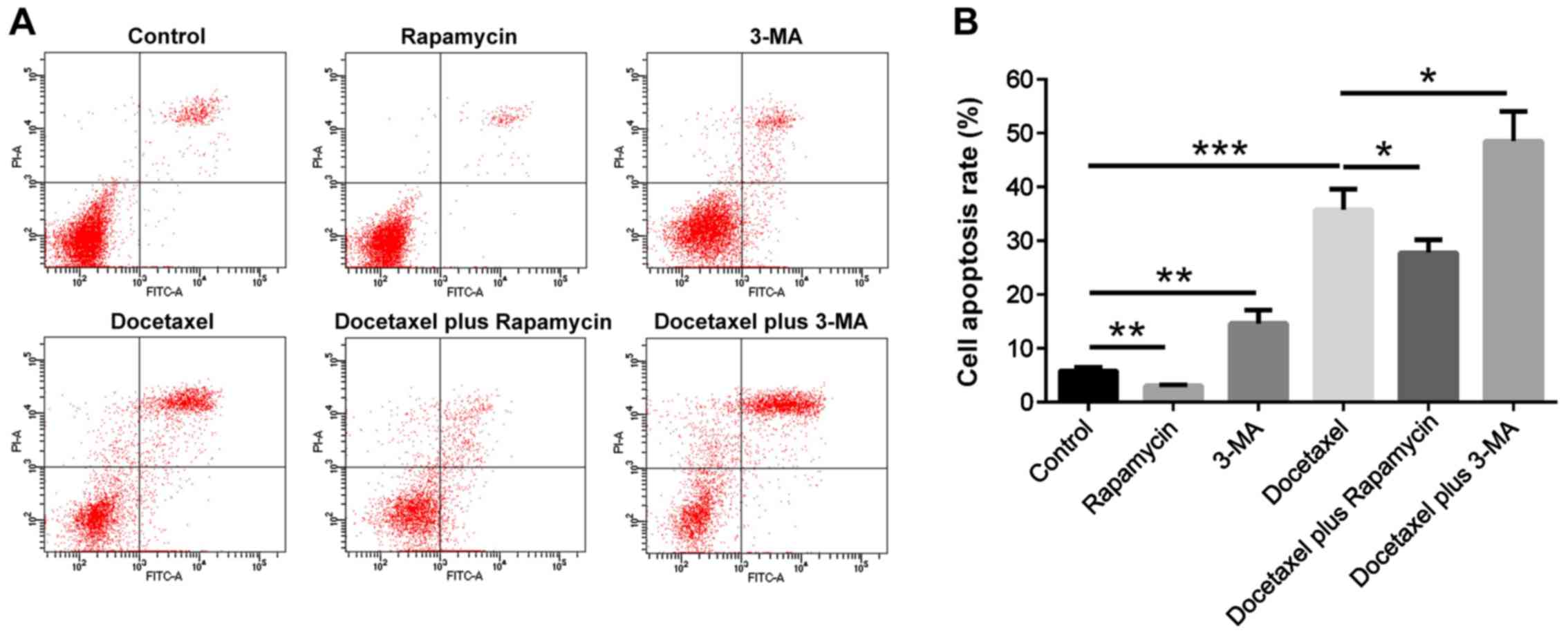
A549/DTX cells were treated with 10 µM docetaxel, 20
µM rapamycin and 50 mM 3-MA alone or in combination (Fig. 2). Docetaxel treatment increased the
protein expression levels of LC3A, Beclin-1, p-AKT and PARP in
A549/DTX cells compared with the control (P<0.05; Fig. 2A and D). Rapamycin plus docetaxel
treatments further increased the protein expression levels of LC3A,
Beclin-1, p-AKT and PARP compared with docetaxel treatment in
A549/DTX cells (P<0.05; Fig.
2A), while 3-MA plus docetaxel treatments reduced LC3A,
Beclin-1, p-AKT and PARP expression levels compared with docetaxel
treatment in A549/DTX cells in A549/DTX cells (P<0.05; Fig. 2D). Furthermore, the mRNA expression
levels of LC3A and Beclin-1 exhibited similar trends to the protein
expression levels in most groups of A549/DTX cells (P<0.05;
Fig. 2B, C, E and F).
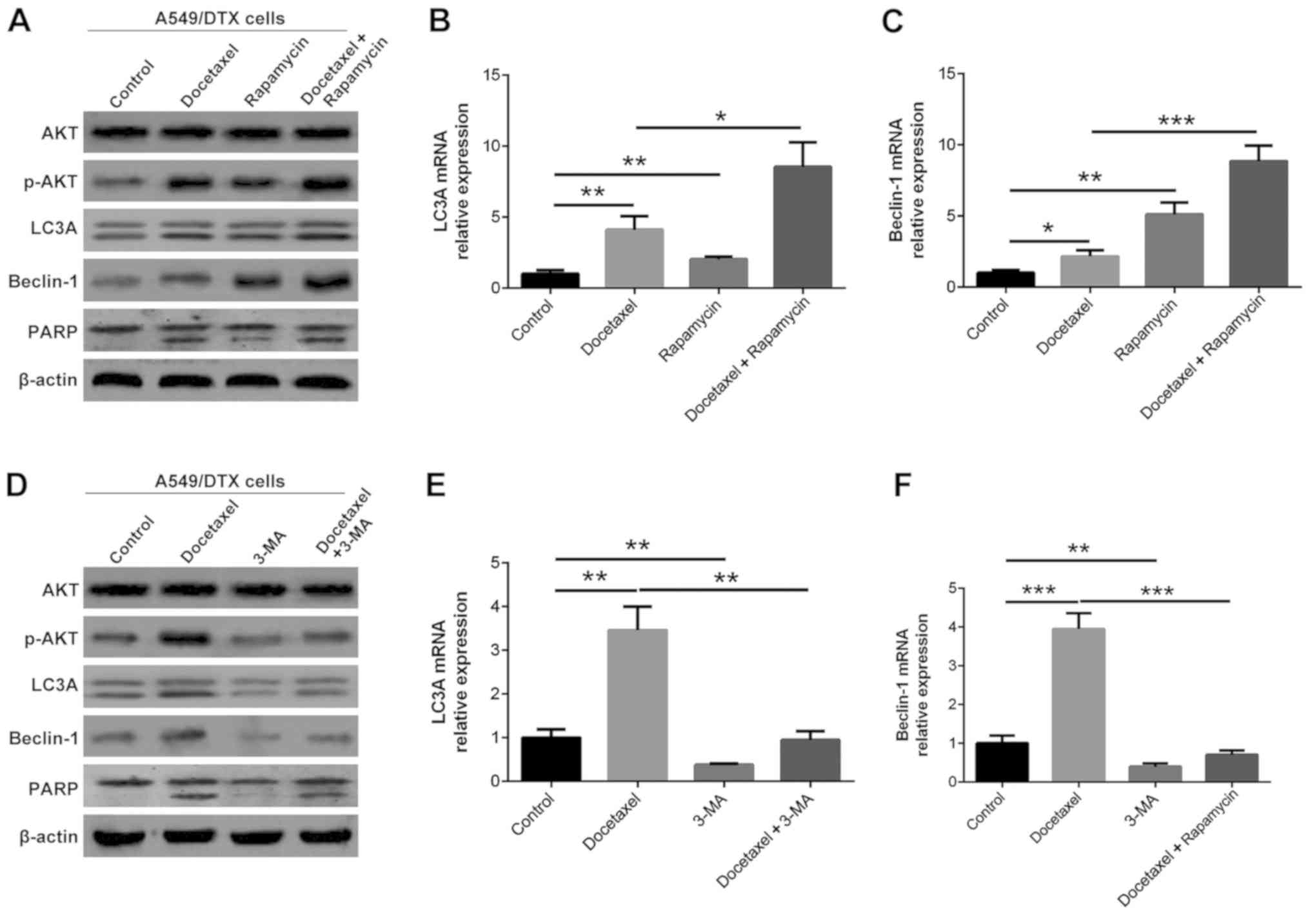 | Figure 2.LC3A, Beclin-1, AKT, p-AKT and PARP
expression levels following treatment with rapamycin, 3-MA and
docetaxel. (A) LC3A, Beclin-1, AKT, p-AKT and PARP protein
expression levels after rapamycin and docetaxel treatment. (B) LC3A
and (C) Beclin-1 mRNA expression levels after rapamycin and
docetaxel treatment. (D) LC3A, Beclin-1, AKT, p-AKT and PARP
protein expression levels after 3-MA and docetaxel treatment. (E)
LC3A and (F) Beclin-1 mRNA expression levels after 3-MA and
docetaxel treatment. *P<0.05, **P<0.01 and ***P<0.001.
LC3A, light chain 3α; p-AKT, phosphorylated AKT; 3-MA,
3-methyladenine; PARP, poly (ADP) ribose polymerase. |
The cell apoptotic rate was decreased in the
Rapamycin group (P<0.01), while it was increased in 3-MA group
(P<0.01) and Docetaxel group (P<0.001) compared with the
Control group (Fig. 3A and B). In
addition, the cell apoptotic rate was reduced in the Docetaxel plus
Rapamycin group (P<0.05), but it was enhanced in the Docetaxel
plus 3-MA group compared with Docetaxel group (P<0.05; Fig. 3A and B). These results suggested
that autophagy attenuated the effect of docetaxel to induce
apoptosis in A549/DTX cells.
Apatinib synergized the effect of
docetaxel in the treatment of A549/DTX cells via regulating
autophagy
Apatinib (10 µM) and docetaxel (10 µM) were used to
treat A549/DTX cells alone or in combination. Apatinib reduced the
protein expression levels of LC3A, Beclin-1 and p-AKT in
docetaxel-treated A549/DTX cells (P<0.05), while the expression
of PARP was not notably affected (P>0.05; Fig. 4A). Moreover, the mRNA expression
levels of LC3A and Beclin-1 exhibited similar trends to their
protein expression in each group of A549/DTX cells (P<0.05;
Fig. 4B and C).
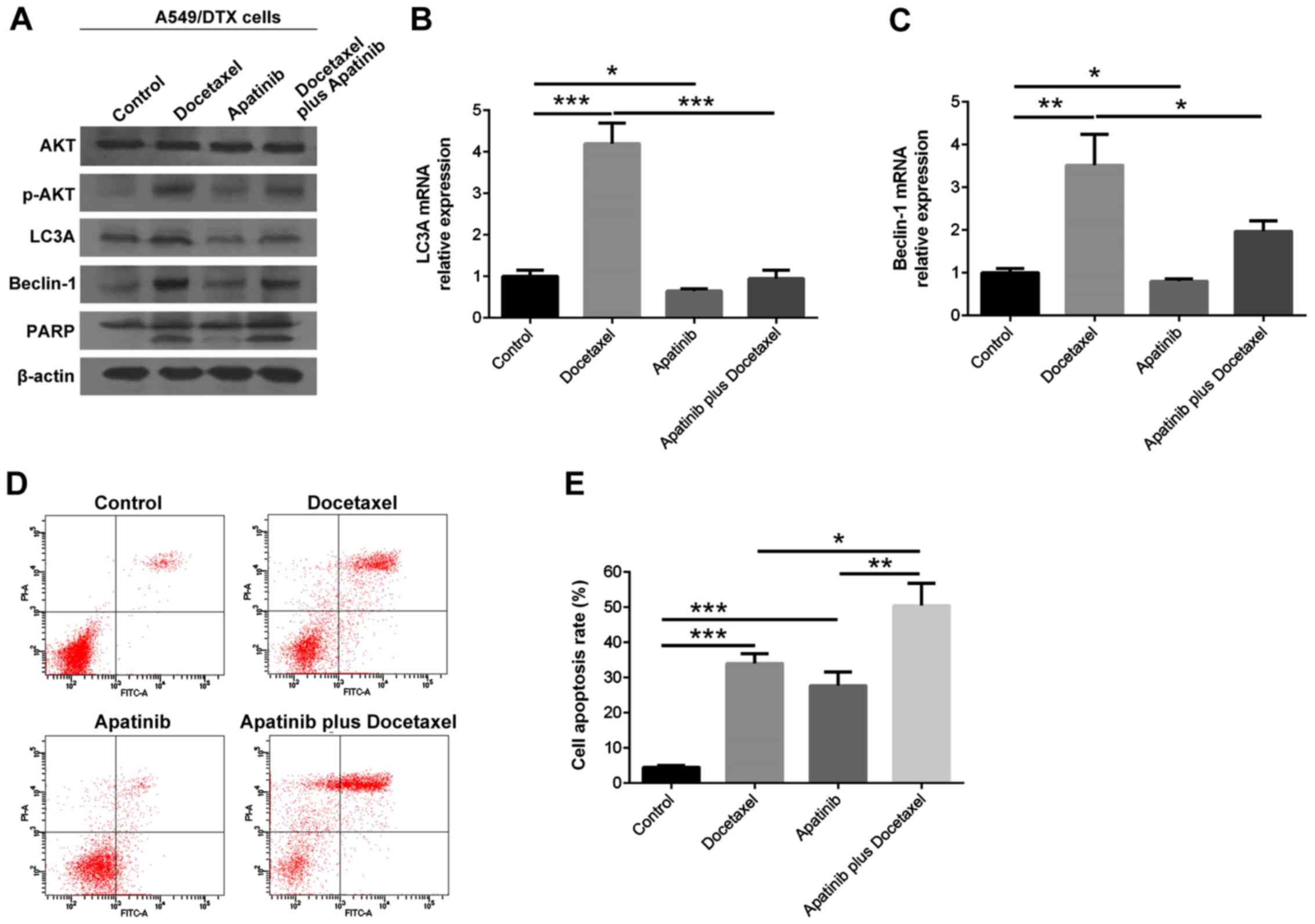 | Figure 4.LC3A, Beclin-1, AKT, p-AKT and PARP
expression levels and cell apoptosis rate of A549/DTX cells
following apatinib and docetaxel treatment. (A) LC3A, Beclin-1,
AKT, p-AKT and PARP protein expression levels after apatinib and
docetaxel treatment. (B) LC3A mRNA expression after apatinib and
docetaxel treatment. (C) Beclin-1 mRNA expression after apatinib
and docetaxel treatment. (D) Representative flow cytometry images
of cell apoptosis and (E) bar graph presenting the cell apoptosis
rate following treatment with apatinib and docetaxel. *P<0.05,
**P<0.01 and ***P<0.001. LC3A, light chain 3α; p-AKT,
phosphorylated AKT; PARP, poly (ADP) ribose polymerase. |
The cell apoptotic rate was increased in Docetaxel
alone and Apatinib alone groups compared with the control group
(both P<0.001), but also in the Apatinib plus docetaxel group
compared with the Docetaxel group (P<0.05) and Apatinib group
(P<0.01; Fig. 4D and E). Thus,
it was indicated that apatinib synergized the effect of docetaxel
in treating A549/DTX cells via suppressing autophagy.
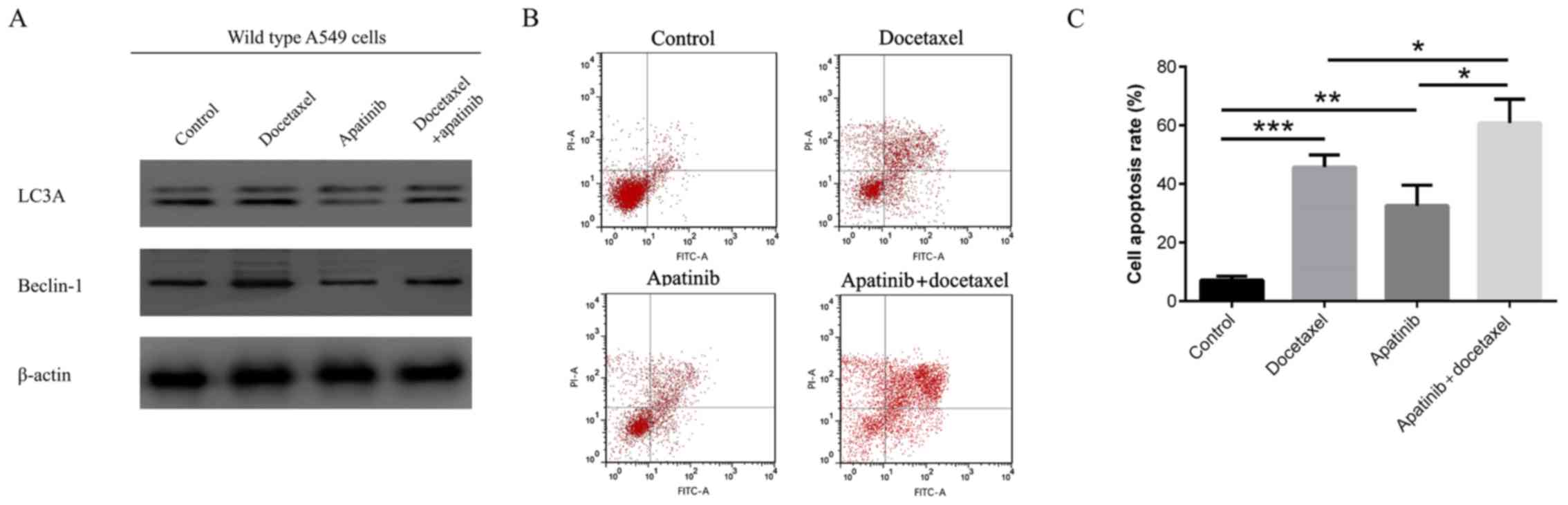
Synergism of apatinib with docetaxel
in treating wild-type A549 cells
Apatinib (10 µM) and docetaxel (10 µM) were used to
treat wild-type A549 cells alone or in combination. In wild-type
A549 cells, docetaxel had a less pronounced effect on enhancing the
autophagy markers LC3A and Beclin-1, while apatinib exhibited a
notable effect to repress autophagy markers (Fig. 5A). Furthermore, the cell apoptosis
rate was significantly increased in the Docetaxel alone and
Apatinib alone groups compared with the control group (both
P<0.001), and also in the Apatinib plus docetaxel group compared
with the Docetaxel group (P<0.05) and Apatinib group (P<0.05;
Fig. 5B and C). These results
suggested that apatinib exhibited a synergistic effect with
docetaxel in the treatment of wild-type A549 cells.
Discussion
The present study demonstrated that: i) Apatinib
plus docetaxel improved the treatment efficacy and attenuated
autophagy compared with docetaxel alone in patients with advanced
NSCLC; ii) autophagy reduced the cytotoxic effect of docetaxel on
A549/DTX cells; and iii) apatinib sensitized A549/DTX cells to the
cytotoxic effect of docetaxel by repressing autophagy.
Anti-angiogenesis drugs plus chemotherapy have been
introduced to treat several types of cancer at the advanced stage,
including advanced/metastatic urothelial carcinoma, advanced
gastric cancer and advanced NSCLC, as second-line treatments or
beyond (23–25). For instance, ramucirumab plus
docetaxel prolongs progression-free survival and overall survival
compared with docetaxel alone in patients with stage IV NSCLC, when
administered during or after a first-line platinum-based
chemotherapy regimen (25). As the
first independently developed small-molecule anti-angiogenesis
agent in China, apatinib has attracted increased attention in the
oncology field (4). Previous
studies examining apatinib plus chemotherapy in treating advanced
cancer have focused on cancer types including gastric, colorectal
and ovarian cancer, while studies reporting on its use in treating
NSCLC are currently limited (8,9). For
instance, in a single-center, open-label, dose-escalating phase I
trial it was observed that apatinib plus docetaxel was well
tolerated and exhibited promising efficacy in patients with
advanced lung adenocarcinoma; however, the study only included 12
patients and lacked a control group who only receive docetaxel
(8). Another multi-center,
prospective study revealed that apatinib plus docetaxel achieved an
ORR of 33% and a DCR of 67% in the treatment of patients with
advanced non-squamous NSCLC, but the study only included 14
patients and also lacked a group treated with docetaxel as a
control (9).
In the present study, in order to assess the
efficacy and safety of apatinib plus docetaxel in treating advanced
NSCLC, 39 patients with primary NSCLC at stage IV or recurrent
metastatic NSCLC refractory/intolerant to standard therapy were
enrolled and treated with apatinib plus docetaxel or with docetaxel
alone. The results indicated that apatinib plus docetaxel achieved
a higher ORR (37 vs. 10%) and DCR (84 vs. 45%) compared with
docetaxel alone in these patients. Furthermore, in the tumor tissue
of patients treated with docetaxel, the expression levels of
Beclin-1 and LC3A were increased post-treatment compared with to
those prior to treatment, while these remained unchanged in the
apatinib plus docetaxel-treated group. Thus, it was suggested that
apatinib may enhance the efficacy of docetaxel via attenuating
autophagy in patients with advanced NSCLC. A possible explanation
for the present findings is that autophagy significantly
contributes to the resistance of NSCLC to docetaxel, while apatinib
inhibits autophagy via regulating pathways, including AKT/mTOR and
VEGFR2/STAT3/Bcl-2, in cancer (15–17),
and therefore, its application enhances the efficacy of docetaxel
in patients with advanced NSCLC. This hypothesis was further
evaluated via the subsequent in vitro experiments of the
present study. Moreover, as previously indicated, apatinib
increases the uptake of docetaxel into NSCLC cells and
drug-resistant NSCLC cells (19),
which may explain how apatinib improved the treatment outcome of
docetaxel in patients with advanced NSCLC in the current study.
Docetaxel resistance is a critical issue in treating
advanced NSCLC, and its potential underlying mechanisms have been
elucidated, including the implication of intracellular multidrug
resistance-associated protein P-glycoprotein, lung resistance
protein, glutathione transferase and t-structural changes of
intracellular microtubules topoisomerase (26). In addition, it has been reported
that high-mobility group box 1-mediated autophagy contributes to
docetaxel resistance in lung adenocarcinoma (27), while another study revealed that
Klotho-mediated autophagy was closely involved in chemotherapy
resistance (including docetaxel resistance) in lung cancer
(28). In the present study, it
was also demonstrated that autophagy attenuated the cytotoxic
effects of docetaxel on A549/DTX cells, which was in line with
these previous findings.
With regards to the autophagy regulation of
apatinib, this drug was previously reported to regulate autophagy
via controlling the AKT/mTOR pathway in anaplastic thyroid cancer
(15). Moreover, apatinib can
modulate autophagy markers and the AKT/mTOR pathway in colon cancer
(16), as well as regulate
autophagy and apoptosis of osteosarcoma cells via the
VEGFR2/STAT3/Bcl-2 signaling pathway (17). However, to the best of our
knowledge, its regulation of autophagy in NSCLC has not been
previously reported. The present results suggested that in the
Docetaxel group, Beclin-1 and LC3A expression levels were increased
in NSCLC tumor tissues post-treatment compared with that prior to
treatment, while these remained unchanged in the Apatinib plus
docetaxel group. Therefore, it was speculated that apatinib may
also regulate autophagy in docetaxel-treated patients with advanced
NSCLC. Furthermore, the results of the in vitro experiments
indicated that autophagy attenuated the cytotoxic effect of
docetaxel on A549/DTX cells. Thus, the effect of apatinib to
regulate autophagy in the presence of docetaxel in A549/DTX cells
was further investigated, which demonstrated that apatinib
sensitized A549/DTX cells to the cytotoxic effects of docetaxel via
repressing autophagy. The possible mechanism may be that apatinib
inhibits autophagy via regulating the AKT/mTOR and
VEGFR2/STAT3/Bcl-2 pathways (15–17),
leading to an increase in the anti-cancer efficacy of docetaxel.
However, the detailed molecular mechanisms underlying
apatinib-mediated inhibition of autophagy were not investigated in
the present study, which was a limit of the present study and
requires further investigation.
In conclusion, apatinib synergizes the effect of
docetaxel in treating patients with advanced NSCLC or
recurrent/refractory NSCLC, as well as chemoresistant NSCLC cells
via regulating autophagy. The present findings may provide novel
evidence for the combined application of apatinib and docetaxel in
treating advanced NSCLC and recurrent/refractory NSCLC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by National Key Research
and Development Program of China (grant no. 2018YFC1313602), Major
International (Regional) Joint Research Project (grant no.
81820108001), National Natural Science Foundation of China
(81670029), Jiangsu Key Principal Investigator of Medicine (grant
no. ZDRCA2016018), Project 333 for Cultivation of Young and
Middle-aged Leading Talents (grant no. BRA2019078) and Jiangsu Key
Program of Social Development (grant no. BE2015651).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ conceived and designed the experiment, analyzed
the data and revised the manuscript. RH, TL and KH collected the
data and analyzed the data. ZC and NW performed data analysis and
provided interpretation. XW provided technical support, and
analyzed and interpreted the results. LG critically revised the
article and interpreted the data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The First Affiliated Hospital of Nanjing Medical University
(Nanjing, China) and all patients signed the informed consent
before the enrollment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
VEGFR-2
|
vascular endothelial growth factor
receptor-2
|
|
RECIST
|
Response Evaluation Criteria in Solid
Tumors
|
|
CR
|
complete remission
|
|
PR
|
partial remission
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
ORR
|
overall remission rate
|
|
DCR
|
disease control rate
|
|
AE
|
adverse event
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanino R, Tsubata Y, Harashima N, Harada M
and Isobe T: Novel drug-resistance mechanisms of pemetrexed-treated
non-small cell lung cancer. Oncotarget. 9:16807–16821. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y,
Li J and Lou L: YN968D1 is a novel and selective inhibitor of
vascular endothelial growth factor receptor-2 tyrosine kinase with
potent activity in vitro and in vivo. Cancer Sci. 102:1374–1380.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Zhao X, Chen L, Guo H, Lv F, Jia K,
Yv K, Wang F, Li C, Qian J, et al: Safety and pharmacokinetics of
novel selective vascular endothelial growth factor receptor-2
inhibitor YN968D1 in patients with advanced malignancies. BMC
Cancer. 10:5292010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maroufi NF, Rashidi MR, Vahedian V,
Akbarzadeh M, Fattahi A and Nouri M: Therapeutic potentials of
apatinib in cancer treatment: Possible mechanisms and clinical
relevance. Life Sci. 241:1171062020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu F, Zhang S, Gao G, Zhao J, Ren S and
Zhou C: Successful treatment using apatinib with or without
docetaxel in heavily pretreated advanced non-squamous non-small
cell lung cancer: A case report and literature review. Cancer Biol
Ther. 19:141–144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duan JC, Wang ZJ, Lin L, Li JL, Wang Y,
Bai H, Hu XS, Liu YT, Hao XZ, Wang HY, et al: Apatinib, a novel
VEGFR inhibitor plus docetaxel in advanced lung adenocarcinoma
patients with wild-type EGFR: A phase I trial. Invest New Drugs.
37:731–737. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang Q, Zhang NL, Ma DY, Tan BX, Hu X and
Fang XD: Efficacy and safety of apatinib plus docetaxel as the
second or above line treatment in advanced nonsquamous NSCLC: A
multi center prospective study. Medicine (Baltimore).
98:e160652019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP,
Wang F, Tao LY, Zhang CZ, Dai CL, Tiwari AK, et al: Apatinib
(YN968D1) reverses multidrug resistance by inhibiting the efflux
function of multiple ATP-binding cassette transporters. Cancer Res.
70:7981–7991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Jia Y, Gao Y, Chang Z, Han H, Yan J
and Qin Y: Clinical efficacy and survival analysis of apatinib
combined with docetaxel in advanced esophageal cancer. Onco Targets
Ther. 12:2577–2583. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye
WC, Zhang DM and Chen ZS: Autophagy and multidrug resistance in
cancer. Chin J Cancer. 36:522017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Azam F, Latif MF, Farooq A, Tirmazy SH,
AlShahrani S, Bashir S and Bukhari N: Performance status assessment
by using ECOG (Eastern Cooperative Oncology Group) score for cancer
patients by oncology healthcare professionals. Case Rep Oncol.
12:728–736. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng H, Cheng X, Kuang J, Chen L, Yuen S,
Shi M, Liang J, Shen B, Jin Z, Yan J and Qiu W: Apatinib-induced
protective autophagy and apoptosis through the AKT-mTOR pathway in
anaplastic thyroid cancer. Cell Death Dis. 9:10302018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu W, Ke H, Qianshan D, Zhen W, Guoan X
and Honggang Y: Apatinib has anti-tumor effects and induces
autophagy in colon cancer cells. Iran J Basic Med Sci. 20:990–995.
2017.PubMed/NCBI
|
|
17
|
Liu K, Ren T, Huang Y, Sun K, Bao X, Wang
S, Zheng B and Guo W: Apatinib promotes autophagy and apoptosis
through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death
Dis. 8:e30152017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu H, Jin C, Zhu Q, Liu T, Ke B, Li A and
Zhang T: Dysregulated expressions of PTEN, NF-kB, WWP2, p53 and
c-Myc in different subtypes of B cell lymphoma and reactive
follicular hyperplasia. Am J Transl Res. 11:1092–1101.
2019.PubMed/NCBI
|
|
19
|
Feng SQ, Wang GJ, Zhang JW, Xie Y, Sun RB,
Fei F, Huang JQ, Wang Y, Aa JY and Zhou F: Combined treatment with
apatinib and docetaxel in A549 ×enograft mice and its cellular
pharmacokinetic basis. Acta Pharmacol Sin. 39:1670–1680. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang L, Hui K, Hu C, Wen Y, Yang S, Zhu
P, Wang L, Xia Y, Qiao Y, Sun W, et al: Autophagy inhibition
potentiates the anti-angiogenic property of multikinase inhibitor
anlotinib through JAK2/STAT3/VEGFA signaling in non-small cell lung
cancer cells. J Exp Clin Cancer Res. 38:712019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bulatov E, Sayarova R, Mingaleeva R,
Miftakhova R, Gomzikova M, Ignatyev Y, Petukhov A, Davidovich P,
Rizvanov A and Barlev NA: Isatin-schiff base-copper (II) complex
induces cell death in p53-positive tumors. Cell Death Discov.
4:1032018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Petrylak DP, de Wit R, Chi KN, Drakaki A,
Sternberg CN, Nishiyama H, Castellano D, Hussain SA, Fléchon A,
Bamias A, et al: Ramucirumab plus docetaxel versus placebo plus
docetaxel in patients with locally advanced or metastatic
urothelial carcinoma after platinum-based therapy (RANGE): Overall
survival and updated results of a randomised, double-blind, phase 3
trial. Lancet Oncol. 21:105–120. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takahari D: Second-line chemotherapy for
patients with advanced gastric cancer. Gastric Cancer. 20:395–406.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garon EB, Ciuleanu TE, Arrieta O, Prabhash
K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel
J, et al: Ramucirumab plus docetaxel versus placebo plus docetaxel
for second-line treatment of stage IV non-small-cell lung cancer
after disease progression on platinum-based therapy (REVEL): A
multicentre, double-blind, randomised phase 3 trial. Lancet.
384:665–673. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen ZJ, Le HB, Zhang YK, Qian LY, Sekhar
KR and Li WD: Lung resistance protein and multidrug resistance
protein in non-small cell lung cancer and their clinical
significance. J Int Med Res. 39:1693–1700. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan B, Chen D, Huang J, Wang R, Feng B,
Song H and Chen L: HMGB1-mediated autophagy promotes docetaxel
resistance in human lung adenocarcinoma. Mol Cancer. 13:1652014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen T, Ren H, Thakur A, Yang T, Li Y,
Zhang S, Wang T and Chen MW: Decreased level of klotho contributes
to drug resistance in lung cancer cells: Involving in
klotho-mediated cell autophagy. DNA Cell Biol. 35:751–757. 2016.
View Article : Google Scholar : PubMed/NCBI
|