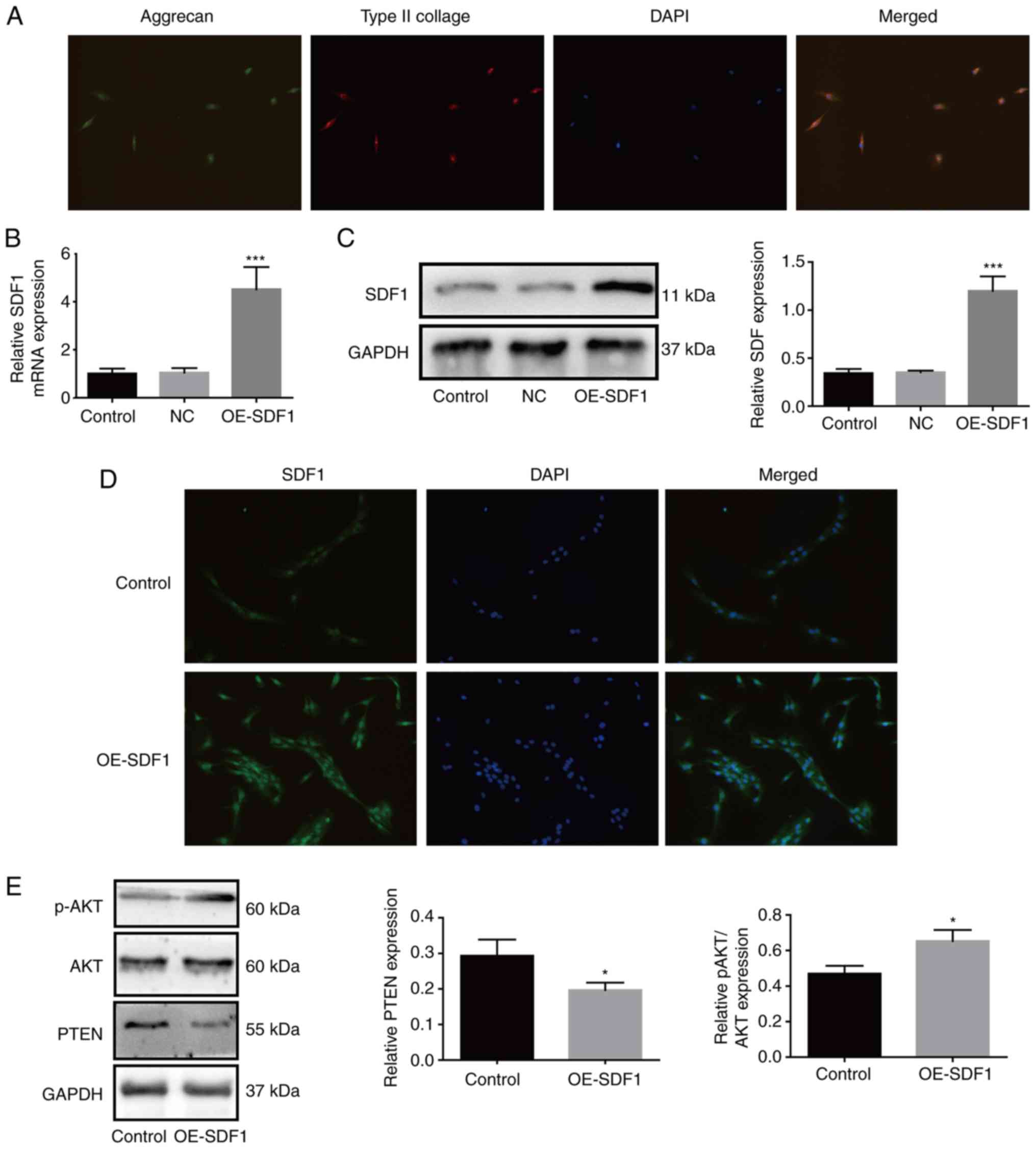
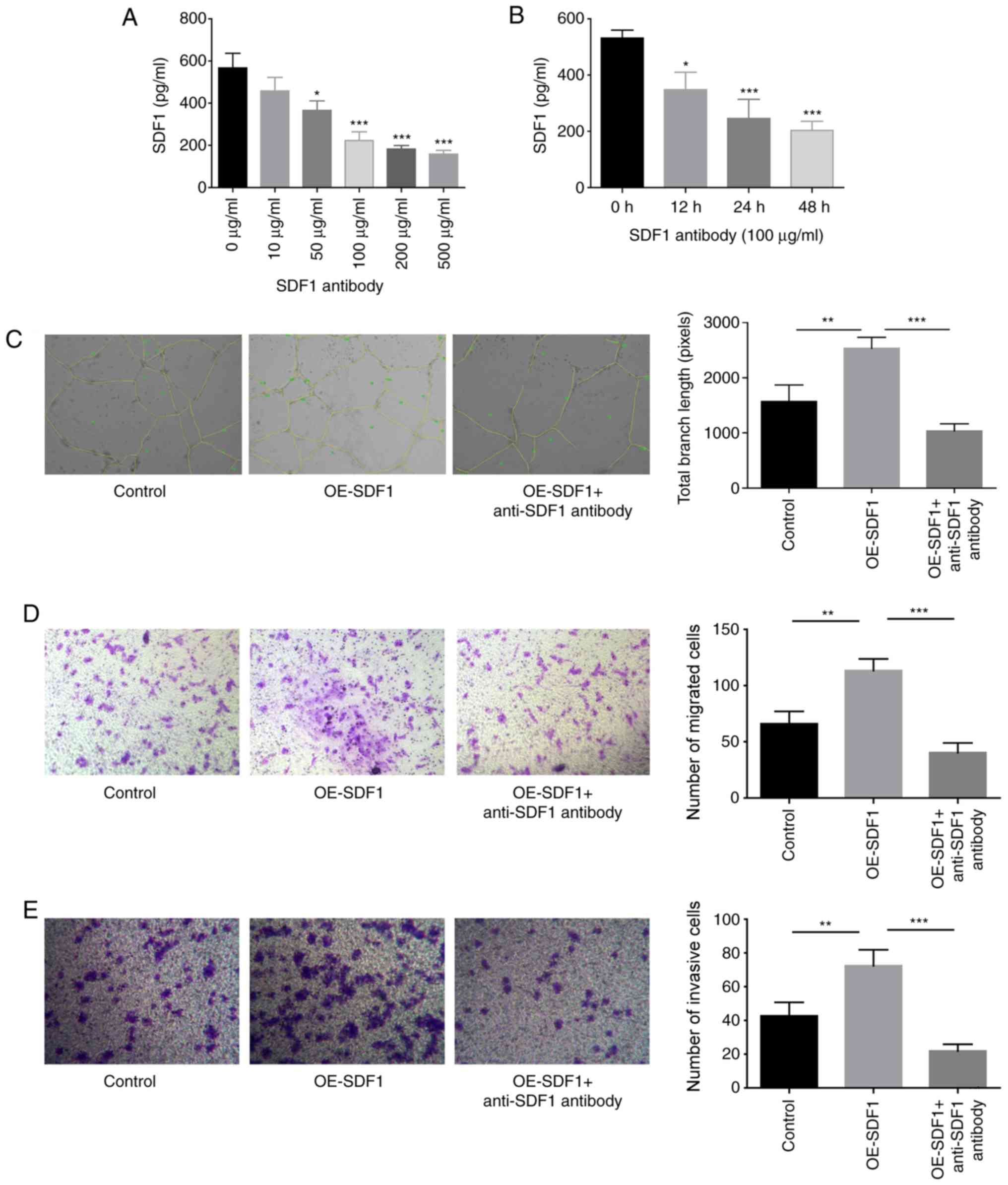
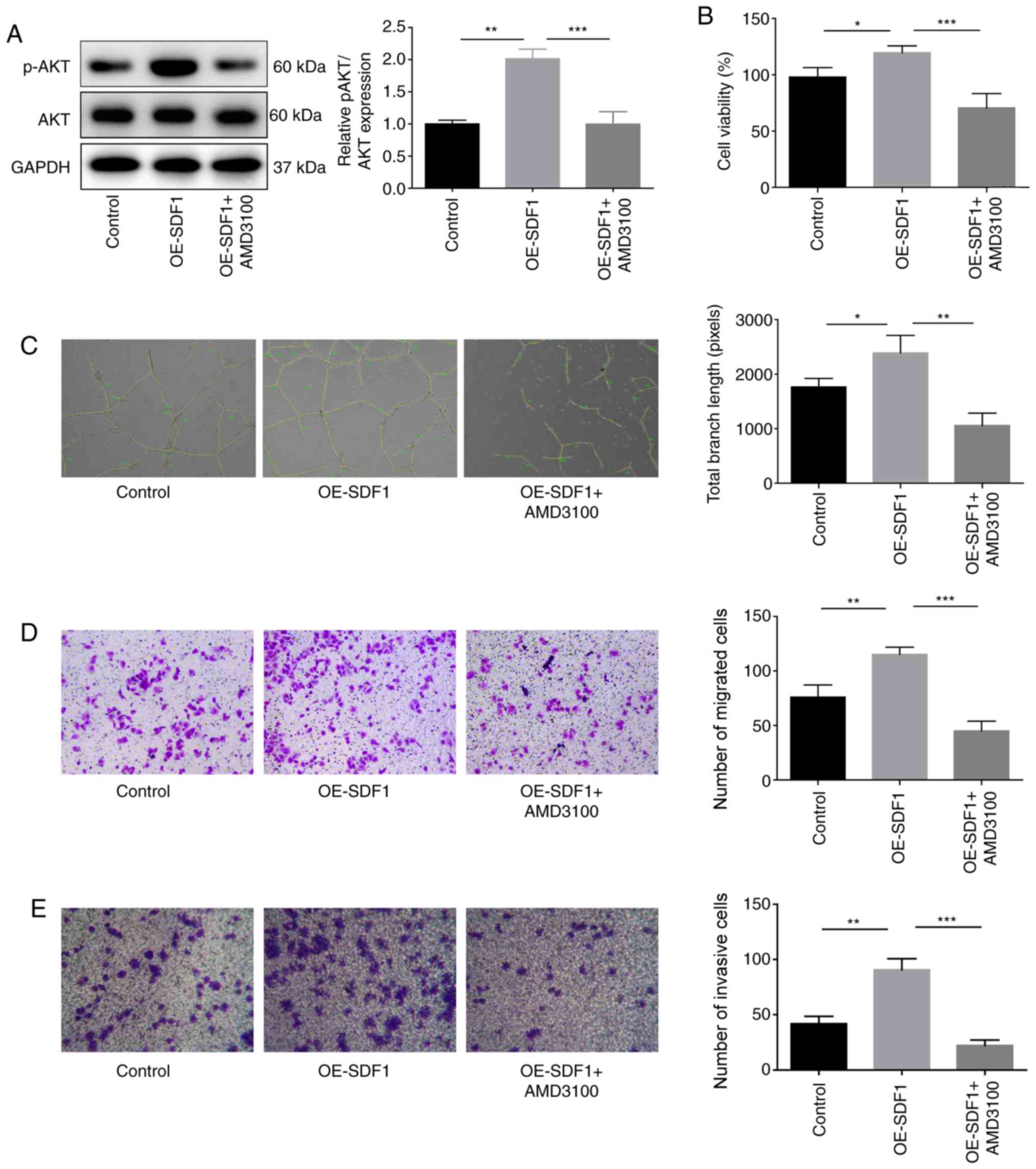
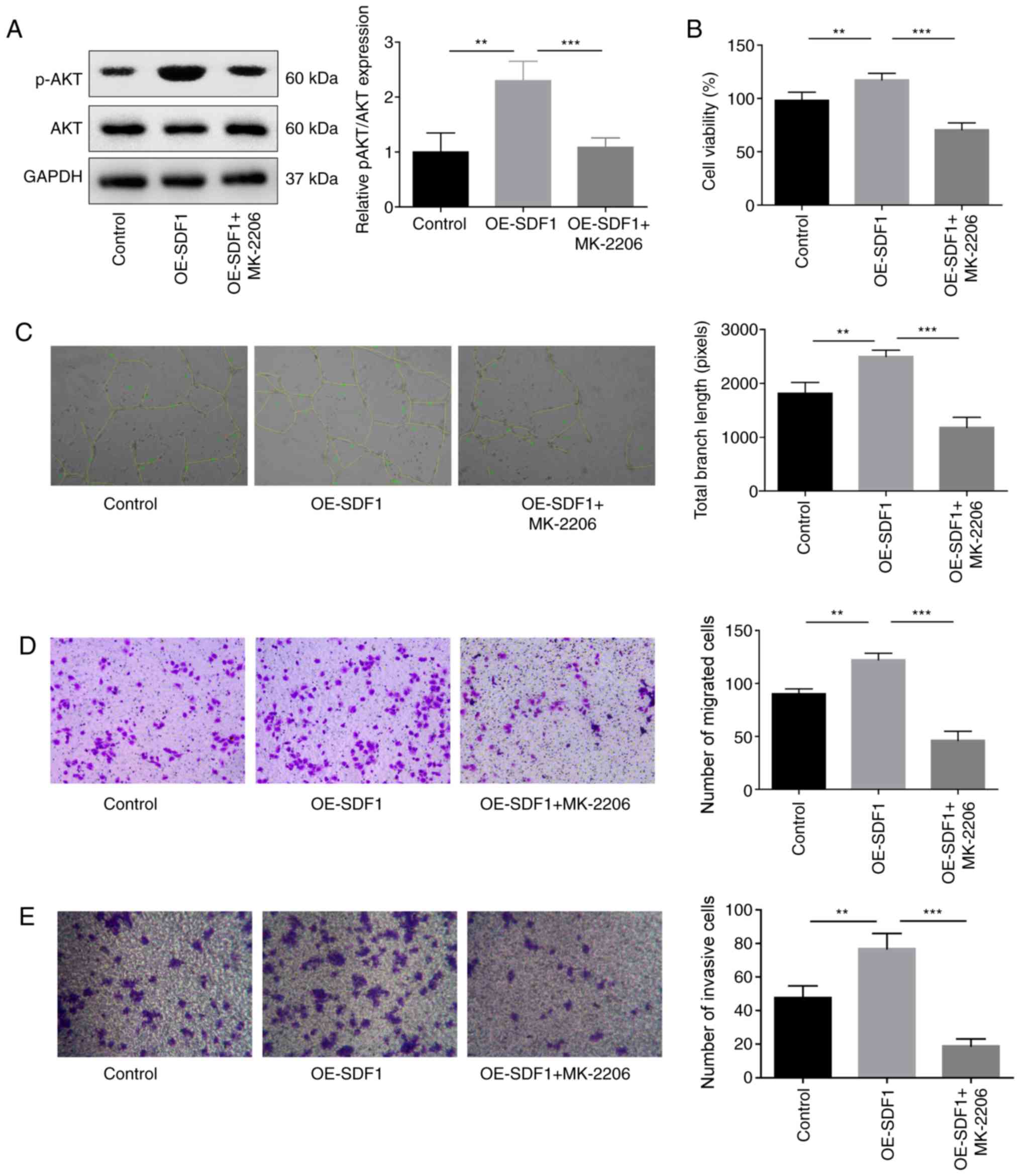
|
1
|
Savigny P, Watson P and Underwood M;
Guideline Development Group, : Early management of persistent
non-specific low back pain: Summary of NICE guidance. BMJ.
338:b18052009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dagenais S, Caro J and Haldeman S: A
systematic review of low back pain cost of illness studies in the
United States and internationally. Spine J. 8:8–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Freburger JK, Holmes GM, Agans RP, Jackman
AM, Darter JD, Wallace AS, Castel LD, Kalsbeek WD and Carey TS: The
rising prevalence of chronic low back pain. Arch Intern Med.
169:251–258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yurube T, Takada T, Suzuki T, Kakutani K,
Maeno K, Doita M, Kurosaka M and Nishida K: Rat tail static
compression model mimics extracellular matrix metabolic imbalances
of matrix metalloproteinases, aggrecanases, and tissue inhibitors
of metalloproteinases in intervertebral disc degeneration.
Arthritis Res Ther. 14:R512012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vernon-Roberts B, Moore RJ and Fraser RD:
The natural history of age-related disc degeneration: The pathology
and sequelae of tears. Spine (Phila Pa 1976). 32:2797–2804. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yasuma T, Arai K and Yamauchi Y: The
histology of lumbar intervertebral disc herniation. The
significance of small blood vessels in the extruded tissue. Spine
(Phila Pa 1976). 18:1761–1765. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karamouzian S, Eskandary H, Faramarzee M,
Saba M, Safizade H, Ghadipasha M, Malekpoor AR and Ohadi A:
Frequency of lumbar intervertebral disc calcification and
angiogenesis, and their correlation with clinical, surgical, and
magnetic resonance imaging findings. Spine (Phila Pa 1976).
35:881–886. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Urban JP, Smith S and Fairbank JC:
Nutrition of the intervertebral disc. Spine (Phila Pa 1976).
29:2700–2709. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martinelli GB, Olivari D, Re Cecconi AD,
Talamini L, Ottoboni L, Lecker SH, Stretch C, Baracos VE, Bathe OF,
Resovi A, et al: Activation of the SDF1/CXCR4 pathway retards
muscle atrophy during cancer cachexia. Oncogene. 35:6212–6222.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Zhang L, Chen L, Li W, Li F and
Chen Q: Stromal cell-derived factor-1 and its receptor CXCR4 are
upregulated expression in degenerated intervertebral discs. Int J
Med Sci. 11:240–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pablos JL, Santiago B, Galindo M, Torres
C, Brehmer MT, Blanco FJ and García-Lázaro FJ: Synoviocyte-derived
CXCL12 is displayed on endothelium and induces angiogenesis in
rheumatoid arthritis. J Immunol. 170:2147–2152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine (Phila Pa 1976).
26:1873–1878. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwon WK, Moon HJ, Kwon TH, Park YK and Kim
JH: Influence of rabbit notochordal cells on symptomatic
intervertebral disc degeneration: Anti-angiogenic capacity on human
endothelial cell proliferation under hypoxia. Osteoarthritis
Cartilage. 25:1738–1746. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian T, Nan KJ, Guo H, Wang WJ, Ruan ZP,
Wang SH, Liang X and Lu CX: PTEN inhibits the migration and
invasion of HepG2 cells by coordinately decreasing MMP expression
via the PI3K/Akt pathway. Oncol Rep. 23:1593–1600. 2010.PubMed/NCBI
|
|
16
|
Rätsep T, Minajeva A and Asser T:
Relationship between neovascularization and degenerative changes in
herniated lumbar intervertebral discs. Eur Spine J. 22:2474–2480.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Freemont AJ, Watkins A, Le Maitre C, Baird
P, Jeziorska M, Knight MTN, Ross ERS, O'Brien JP and Hoyland JA:
Nerve growth factor expression and innervation of the painful
intervertebral disc. J Pathol. 197:286–292. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang H, Wang P, Zhang X, Zhao W, Ren H
and Hu Z: SDF1/CXCR7 signaling axis participates in angiogenesis in
degenerated discs via the PI3K/AKT pathway. DNA Cell Biol.
38:457–467. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun X, Wei L, Chen Q and Terek RM:
CXCR4/SDF1 mediate hypoxia induced chondrosarcoma cell invasion
through ERK signaling and increased MMP1 expression. Mol Cancer.
9:172010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sugiyama T, Kohara H, Noda M and Nagasawa
T: Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4
chemokine signaling in bone marrow stromal cell niches. Immunity.
25:977–988. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Masyuk M and Brand-Saberi B: Recruitment
of skeletal muscle progenitors to secondary sites: A role for
CXCR4/SDF-1 signalling in skeletal muscle development. Results
Probl Cell Differ. 56:1–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sainz J and Sata M: CXCR4, a key modulator
of vascular progenitor cells. Arterioscler Thromb Vasc Bio.
27:263–265. 2007. View Article : Google Scholar
|
|
23
|
Ziegler M, Elvers M, Baumer Y, Leder C,
Ochmann C, Schönberger T, Jürgens T, Geisler T, Schlosshauer B,
Lunov O, et al: The bispecific SDF1-GPVI fusion protein preserves
myocardial function after transient ischemia in mice. Circulation.
125:685–696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Huang J, Li Y and Yang GY: Roles
of chemokine CXCL12 and its receptors in ischemic stroke. Curr Drug
Targets. 13:166–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du LL and Liu P: CXCL12/CXCR4 axis
regulates neovascularization and lymphangiogenesis in sutured
corneas in mice. Mol Med Rep. 13:4987–4994. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kanda S, Mochizuki Y and Kanetake H:
Stromal cell-derived factor-1alpha induces tube-like structure
formation of endothelial cells through phosphoinositide 3-kinase. J
Biol Chem. 278:257–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang BH and Liu LZ: PI3K/PTEN signaling
in angiogenesis and tumorigenesis. Adv Cancer Res. 102:19–65. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hur J, Yoon CH, Lee CS, Kim TY, Oh IY,
Park KW, Kim JH, Lee HS, Kang HJ, Chae IH, et al: Akt is a key
modulator of endothelial progenitor cell trafficking in ischemic
muscle. Stem Cells. 25:1769–1778. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pérez-Ramírez C, Cañadas-Garre M, Molina
MA, Faus-Dáder MJ and Calleja-Hernández MA: PTEN and PI3K/AKT in
non-small-cell lung cancer. Pharmacogenomics. 16:1843–1862. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mao G, Liu Y, Fang X, Liu Y, Fang L, Lin
L, Liu X and Wang N: Tumor-derived microRNA-494 promotes
angiogenesis in non-small cell lung cancer. Angiogenesis.
18:373–382. 2015. View Article : Google Scholar : PubMed/NCBI
|