Introduction
Tissue biobanks support the collection, analysis,
storage and distribution of biospecimens for basic, translational
and clinical research. With the advent of proteomics,
transcriptomics and metabolomics, as well as the development of
bioinformatics, molecular imaging and drug discovery, the demand
for high-quality tissue biospecimens continues to increase
(1,2), large-scale and high-flux research
requirements for high quantity and quality biospecimens have
allowed tissue biobanks to develop from undeveloped, small-scale
storages to complex and multifaceted large-scale storages (3).
Quality assurance and quality control are critical
for tissue biobanks to ensure that biospecimens distributed to
researchers are comparable and produce accurate results; as a rough
estimate, the number of stored tissue biospecimens was >300
million in the US around 2000, with this number increasing at the
rate of 20 million/year (4).
However, according to a survey taken by >700 cancer researchers,
47% of them were of the opinion that sample quality is difficult to
control and 60% questioned the quality of samples (4).
There are various factors influencing the quality of
biospecimens, and pre-analytical variables are crucial (5,6).
Pre-analytical variables are divided into the pre-acquisition and
post-acquisition phases. In the pre-acquisition phase, factors such
as anesthesia (7), surgical trauma
(8,9) and in vivo ischemia (10) might affect the cell integrity and
gene expression profiles of the biospecimen, but they cannot be
controlled very well because of the uncertainty of clinical treat
requirements (11). Rather,
factors such as ex vivo ischemia time, delayed processing
time and processing temperature, which are crucial for bio-specimen
quality (12), can be controlled
and standardized effectively.
To the best of our knowledge, little research has
been conducted of the impact of ex vivo ischemia time and
delayed processing of biospecimens on hypoxic, stress, apoptotic
and autophagic pathways. Therefore, the current study
systematically assessed the impact of ex vivo ischemia time
and delayed processing time on fresh tissue specimens in terms of
the RNA quality and relative mRNA expression of genes involved in
hypoxia, stress, apoptosis and autophagy to provide a theoretical
basis for the standard processing and assessment of tissue
biospecimens.
Materials and methods
Procurement of tissues
A total of 18 carcinoma tissue specimens were
collected from patients who underwent surgery at Peking Union
Medical College Hospital, Beijing, China from April 2017 to June
2017. The clinical characteristics of 18 patients are presented in
Table I. The median age of the
patients at diagnosis was 49.67±12.76 years (range, 29–79 years),
of which 16 patients were female (88.9%) and 2 patients were male
(11.1%). The collected tissues included specimens from 10 patients
with thyroid cancer (55.5%), 3 with ovarian cancer (16.6%), 2 with
breast cancer (11.1%), 1 with pancreatic cancer (5.6%), 1 with
polymorphous adenocarcinoma (5.6%) and 1 with lung cancer (5.6%).
These 18 specimens and 6 types of cancer were included due to their
prevalence and commonality in the clinical and tissue biobank. All
patients provided signed informed consent for the donation of the
specimens. The current study was approved by the Ethics Committee
of the Institutional Review Board of Peking Union Medical College
Hospital.
 | Table I.Clinical characteristics of the
patients (n=18). |
Table I.
Clinical characteristics of the
patients (n=18).
| Clinical
characteristic | Number of patients,
n (%) |
|---|
| Sex |
|
|
Male | 2
(11.1) |
|
Female | 16 (88.9) |
| Age, years |
|
|
≤50 | 8
(44.4) |
|
>50 | 10 (55.6) |
| Type of
carcinoma |
|
|
Thyroid | 10 (55.5) |
|
Breast | 3
(16.6) |
|
Ovary | 2
(11.1) |
|
Pancreas | 1 (5.6) |
|
Polymorphous
adenocarcinoma | 1 (5.6) |
|
Lung | 1 (5.6) |
Following gross examination, the tissues were
resected and washed several times with PBS (pH 7.2) to remove cell
debris and remaining blood. Tissues were then dissected into two
parts, one part of the specimen (1 cm3) was used for
clinical pathology diagnosis, while the other part was divided to 7
smaller specimens (volume, 100 mm3). One of the 7 small
specimens was immediately snap-frozen in liquid nitrogen, thus the
time from tissue resection to storage in liquid nitrogen was ~15
min, which was set as the first point of ex vivo ischemia
time. The other 6 specimens were stored at room temperature for 15,
45, 105, 225, 465 and 1,425 min, and then were stored in liquid
nitrogen, so the ex vivo ischemia time was 30 min, 1, 2, 4,
8 or 24 h, respectively. Following storage, the specimens were
cryopreserved for the subsequent RNA extraction, gene expression
analysis and morphological features assessment, which were reviewed
by two experienced pathologists to confirm final diagnoses.
RNA extraction and integrity
control
Total RNA was extracted from all tissue samples; ~30
mg of 30 mm3 tissue specimen was homogenized using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) with a homogenizer (IKA Werke GmbH & Co. KG) for RNA
extraction, according to the manufacturers' protocols. RNA
concentration was quantified using NanoDrop™ One (Thermo Fisher
Scientific, Inc.) by measuring the extinction coefficient at 260
nm. Additionally, the ratio of optical density (OD) 260/230 and OD
260/280, which indicates RNA purity, was tested using the NanoDrop
One. An Agilent 2100 Bioanalyzer and an Agilent RNA 6000 Nano kit
(Agilent Technologies, Inc.) were used to determine RNA quality,
according to the manufacturers' protocol. RNA integrity numbers
(RINs) were calculated using Agilent Technologies 2100 Bioanalyzer
expert software (version B.02.0×; Agilent Technologies, Inc.), and
were presented as a value (1–10) to
indicate the degree of degradation.
Reverse transcription
Total RNA (1 µg) was diluted to a final volume of 25
µl. A reverse transcription master mix was prepared as follows to
reverse transcribe RNA into cDNA: 5 µl 5X reaction buffer, 1 µl
random primers (cat. no. C1181; Promega Corporation), 1.25 µl dNTPs
(cat. no. U1330; Promega Corporation), 1 µl M-MLV reverse
transcriptase (cat. no. M1701; Promega Corporation), 0.5 µl RNase
inhibitors (Takara Bio, Inc.) and 6.25 µl nuclease-free water (cat.
no. AM9937; Invitrogen; Thermo Fisher Scientific, Inc.). Following
reverse transcription, all samples were diluted to a final volume
of 100 µl.
Reverse transcription-quantitative PCR
(RT-qPCR)
All PCR procedures were set up using a QIAgility
robotic system (Qiagen, Inc.). Mixes were prepared with 5 µl Fast
SYBR1 Green Master mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.; cat. no. 4385612) and 2 µl primer mix (1.67 µM;
Table II). Then, 2 µl cDNA (10
ng/µl) were added for a final volume of 10 µl/well. Gene expression
quantification was performed via RT-qPCR using an Applied
Biosystems® 7500 Real-Time PCR system (Thermo Fisher
Scientific, Inc.). The following thermocycling conditions were
used: 95°C for 20 sec; followed by 40 cycles of 95°C for 3 sec and
60°C for 30 sec. The expression of the housekeeping gene GAPDH was
used for normalization and mRNA levels were quantified using the
2−ΔΔCq method (13,14).
The primers used in the analysis of gene expression were associated
with hypoxia, stress, apoptosis and autophagy: Caspase 8 (CASP8),
Fas cell surface death receptor (FAS), hypoxia-inducible factor 1α
(HIF1α), unc-51 like autophagy activating kinase 1 (ULK1), mTOR,
NF-κB, phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic
subunit β isoform (PI3KCB), AKT serine/threonine kinase 1 (AKT1),
protein kinase AMP-activated catalytic subunit α1 (AMPKα1) and
(Table II).
 | Table II.Primer sequences used for reverse
transcription-quantitative PCR. |
Table II.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Primer sequences
(5′→3′) | Fragment size,
bp |
|---|
| CASP8 | F:
GCCCCCATCTATGAGCTGAC | 100 |
|
| R:
TATCCCCCTGACAAGCCTGA |
|
| FAS | F:
GTGGACCCGCTCAGTACG | 121 |
|
| R:
GGACGATAATCTAGCAACAGACG |
|
| HIF1α | F:@
TTGGCAACCTTGGATTGGATG | 190 |
|
| R:
AAATCTCCGTCCCTCAACCTC |
|
| ULK1 | F:
GTCACACGCCACATAACAG | 90 |
|
| R:
TCTTCTAAGTCCAAGCACAGT |
|
| mTOR | F:
GCTTAGAGGACAGCGGGGAA | 120 |
|
| R:
AAGCATCTTGCCCTGAGGTT |
|
| NF-κB | F:
CCACAAGACAGAAGCTGAAG | 149 |
|
| R:
AGATACTATCTGTAAGTGAACC |
|
| PI3KCB | F:
TGGAACTTGGCACTGGAACT | 114 |
|
| R:
GCTGGGAAACAAGGGGAGAA |
|
| AKT1 | F:
CTGGTCCTGTCTTCCTCATGTT | 190 |
|
| R:
AGGCAGCCCCTTTGACTTCT |
|
| AMPKα1 | F:
TGAAAGAAAAGTGTGTGCTGT | 64 |
|
| R:
TGGGTGAGAAGATGAGGGAAAGA |
|
| GAPDH | F:
GAAGGTGAAGGTCGGAGTC | 226 |
|
| R:
GAAGATGGTGATGGGATTTC |
|
Histological assessment
The other part of all the fresh tissues specimen
were used for the pathological clinical diagnosis. In addition,
after the RNA isolation, the frozen thyroid tissues in liquid
nitrogen with different ex vivo ischemia times were fixed in
10% neutral buffered formalin at room temperature for 24 h.
Specimens were then embedded in paraffin. Sections (thickness, 5
µm) were microtomed and placed on slides. Slides were baked for 1 h
at 60°C in an oven and stained with hematoxylin and eosin, both at
room temperature for 1 h with an autostainer XL (ST5010; Leica
Microsystems GmbH). Histological evaluations were completed by two
experienced pathologists on a light microscope (magnification, ×100
or ×200; Axio Scope.A1; Zeiss GmbH).
Statistical analysis
Statistical analysis was performed using SPSS
(version 19.0; IBM Corp.) and Prism software (version 5; GraphPad
Software, Inc.). One-way ANOVA followed by Tukey's post hoc test
was used to analyse differences between multiple groups. Pearson's
correlation coefficient was used for correlation analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of ex-vivo ischemia time on RNA
integrity
To evaluate the influence of tissue ex vivo
ischemia time points on RNA integrity under controlled conditions,
18 fresh carcinoma tissues from different organs were collected,
separated and stored at room temperature for various ex-vivo
ischemia times prior to being snap-frozen in liquid nitrogen. The
RNA quality was assessed according to integrity and purity. The RNA
integrity data of tissues ex vivo ischemia at 15 min, 2, 8
and 24 h are presented in Fig. 1A.
The distinct ribosomal peaks of 18S and 28S indicated good RNA
integrity at 15 min and 2 h, but not at 8 and 24 h. The RINs of
each group were 8.12±0.576, 7.78±0.546, 7.88±0.621, 7.67±0.523,
7.31±0.589, 7.15±0.844 and 6.63±0.688 for ex vivo ischemia
time points 15, 30 min, 1, 2, 4, 8 and 24 h, respectively (Fig. 1B). SPSS analysis demonstrated that
the RINs at 4, 8 and 24 h were significantly decreased compared
with 15 min (P<0.001). Furthermore, compared with 30 min, RINs
at 8 h (P<0.01) and 24 h (P<0.001) were significantly
decreased. Similarly, compared with 1 h, the RINs at 4 h
(P<0.05), 8 h (P<0.001) and 24 h (P<0.001) were decreased,
and the RINs at 8 h (P<0.05) and 24 h (P<0.001) were
significantly declined compared with 2 h. In addition, the RINs at
8 h were decreased compared with 4 h (P<0.01), and similarly,
the RINs at 24 h were significantly decreased compared with 8 h
(P<0.05). The results indicated that RNA remained intact
following 2 h of ex vivo ischemia at room temperature and
integrity began to gradually decline after 4 h.
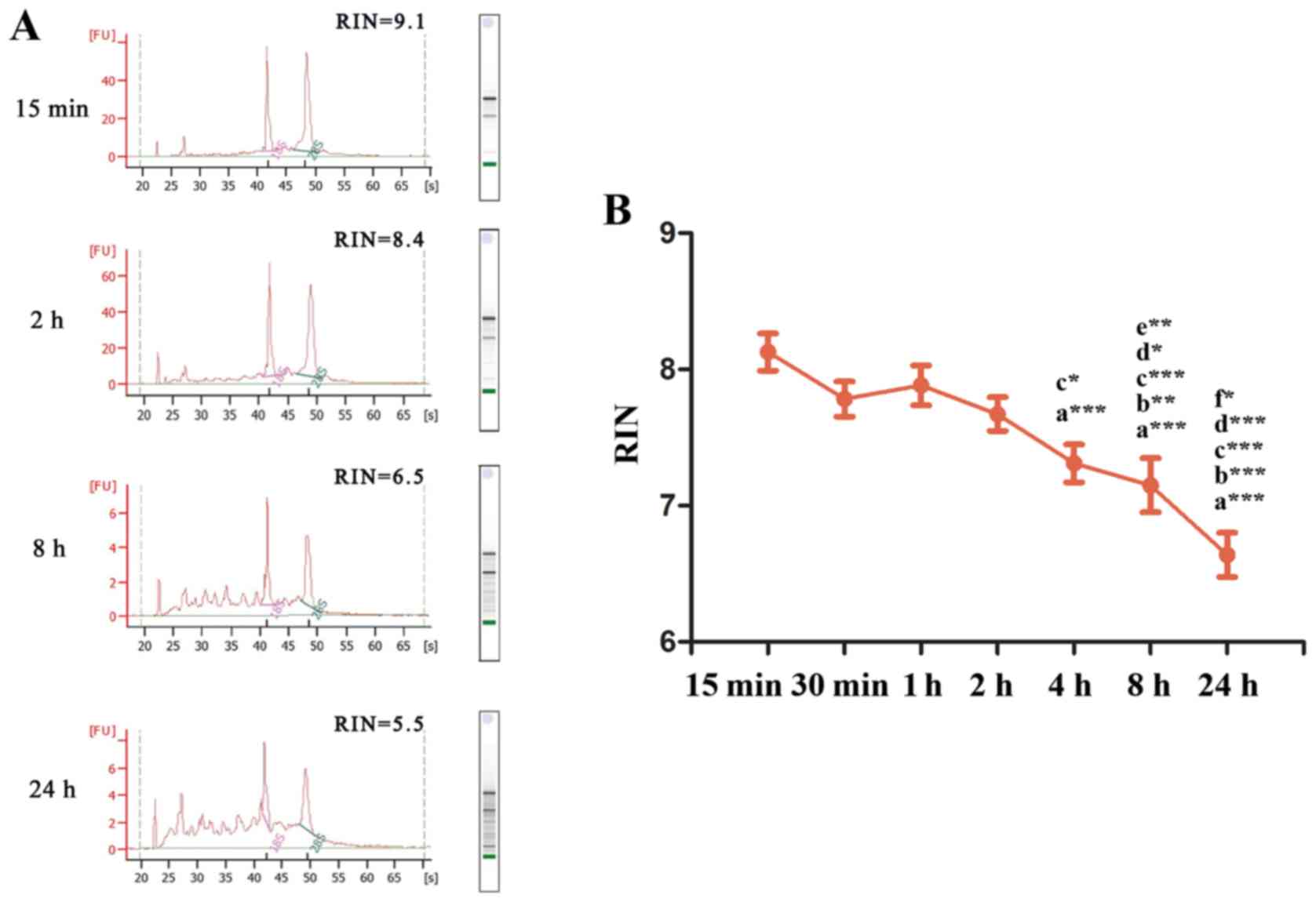 | Figure 1.RNA integrity of tissues for various
ex vivo ischemia time points. (A) mRNA RINs at 15 min, 2, 8
and 24 h of ex vivo ischemia. (B) Changes in RNA integrity
at increasing ex vivo ischemia time points at room
temperature. a, vs. 15 min; b, vs. 30 min; c, vs. 1 h; d, vs. 2 h;
e, vs. 4 h; f, vs. 8 h. *P<0.05, **P<0.01, ***P<0.001.
RINs, RNA integrity numbers. |
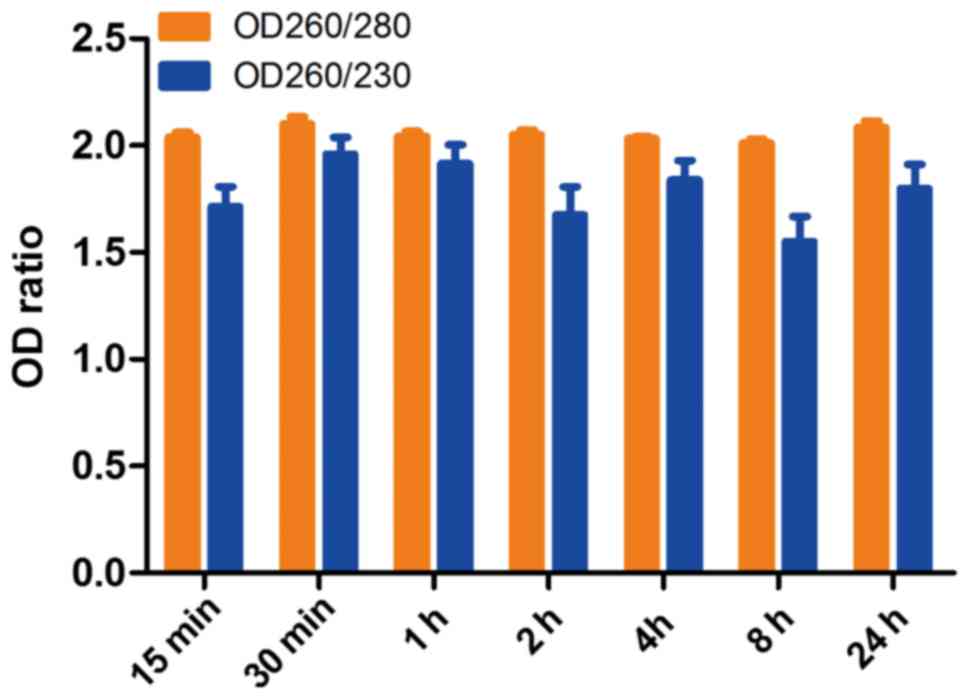
The means of RNA OD260/280 and OD260/230 ratios of 7
time points were 2.05±0.1 and 1.78±0.43, respectively (n=126). The
OD260/280 ratios of each group were 2.028±0.099, 2.101±0.144,
2.043±0.091, 2.051±0.085, 2.031±0.045, 2.011±0.076 and 2.085±0.128
for ex vivo ischemia time points 15, 30 min, 1, 2, 4, 8 and
24 h, respectively (Fig. 2). The
OD260/230 ratios were 1.713±0.398, 1.960±0.330, 1.916±0.367,
1.675±0.561, 1.839±0.391, 1.550±0.500 and 1.799±0.481 for ex
vivo ischemia time points 15, 30 min, 1, 2, 4, 8 and 24 h,
respectively. The ratios of OD260/280 and OD260/230 of all groups
indicated that the degradation of RNA integrity does not affect RNA
purity reflected by absorbance under ultraviolet light.
Effect of ex vivo ischemia time on
gene expression
To explore the influence of ex vivo ischemia
time points on cell functions involving hypoxia, stress, apoptosis
and autophagy, the gene expression of mTOR, HIF1α, PI3KCB, AKT1,
NF-κB, AMPKα1, CASP8, ULK1 and FAS were evaluated using RT-qPCR.
The RINs of the 7 different ex vivo ischemia times indicated
that RNA remained intact following 2 h of ex vivo ischemia
at room temperature and integrity began to gradually decline after
4 h. Thus, the present study mainly focused on ex vivo
ischemia times of 15 min, 2, 8 and 24 h, and more times will be
explored in further study. The results demonstrated that AKT1 was
significantly decreased at 8 h compared with 15 min (P<0.01) and
that PI3KCB, AMPKα1 and CASP8 were significantly decreased at 24 h
compared with 15 min (P<0.05; Fig.
3). However, the expression of mTOR, HIF1α, NF-κB, ULK1 and FAS
were not significantly different between time points.
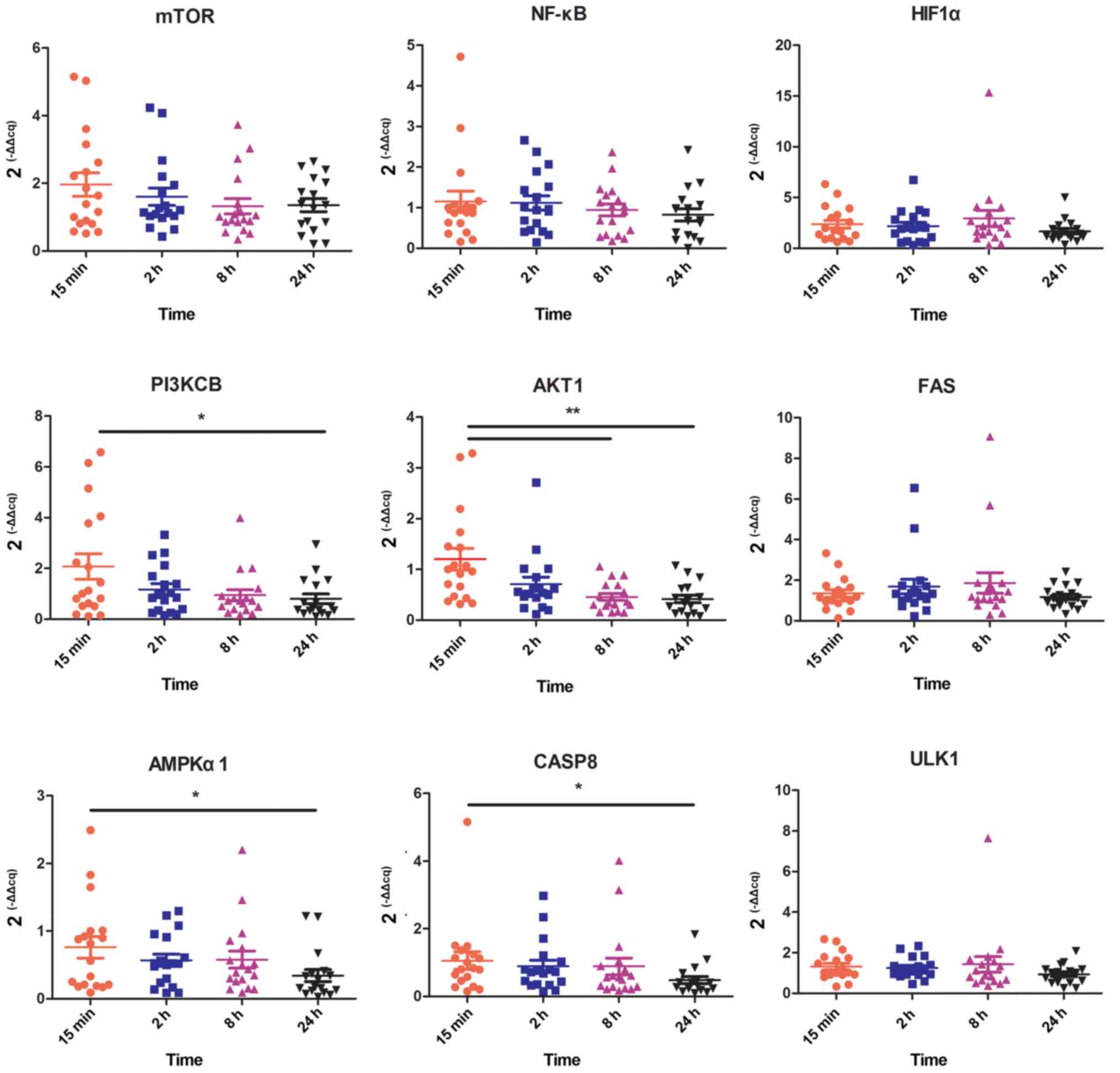 | Figure 3.Gene expression of genes associated
with hypoxic, stress, apoptotic and autophagic pathways for various
ex vivo ischemia time points. HIF1α, hypoxia-inducible
factor 1α; PI3KCB, phosphatidylinositol 4,5-bisphosphate 3-kinase
catalytic subunit β isoform; AKT1, threonine kinase 1; FAS, Fas
cell surface death receptor; AMPKα1, protein kinase AMP-activated
catalytic subunit α1; CASP8, caspase 8; ULK1, unc-51 like autophagy
activating kinase 1. *P<0.05, **P<0.01. |
Further analysis indicated that there was no
significant correlation between RIN and gene expression (Table III). In addition, the interclass
Pearson correlation coefficients among the gene expression levels
were strong, such as rmTOR/PI3KCB=0.721,
rmTOR/AKT1=0.792, rmTOR/NF-κB=0.640,
rmTOR/AMPKα1=0.630, rPI3KCB/AKT1=0.741,
rPI3KCB/NF-κB=0.572, rPI3KCB/AMPKα1=0.835,
rAKT1/NF-κB=0.646 and rAKT1/AMPKα1=0.666,
rAKT1/CASP8=0.508, rCASP8/FAS=0.762,
rCASP8/mTOR=0.607, rHIF1α/ULK1=0.728
(P<0.001) (Table IV). In
addition, HIF1α had slight positive correlation with mTOR, PI3KCB
and AKT1 (r=0.281, r=0.293 and r=0.298, respectively; P<0.05),
and FAS has correlation with NF-κB (r=0.281; P<0.05). In
addition, there were no correlations observed between some gene
expression (P>0.05), such as AKT1 vs. FAS, ULK1 vs. AKT1, AMPKα1
vs. FAS, ULK1 vs. mTOR, ULK1 vs. PI3KCB, ULK1 vs. NF-κB and FAS vs.
PI3KCB. A scatter plot of gene expression, including the
correlation between the expression of these genes is presented in
Fig. 4; the scatterplot trend was
consistent with the correlation coefficients of the gene expression
presented in Table IV.
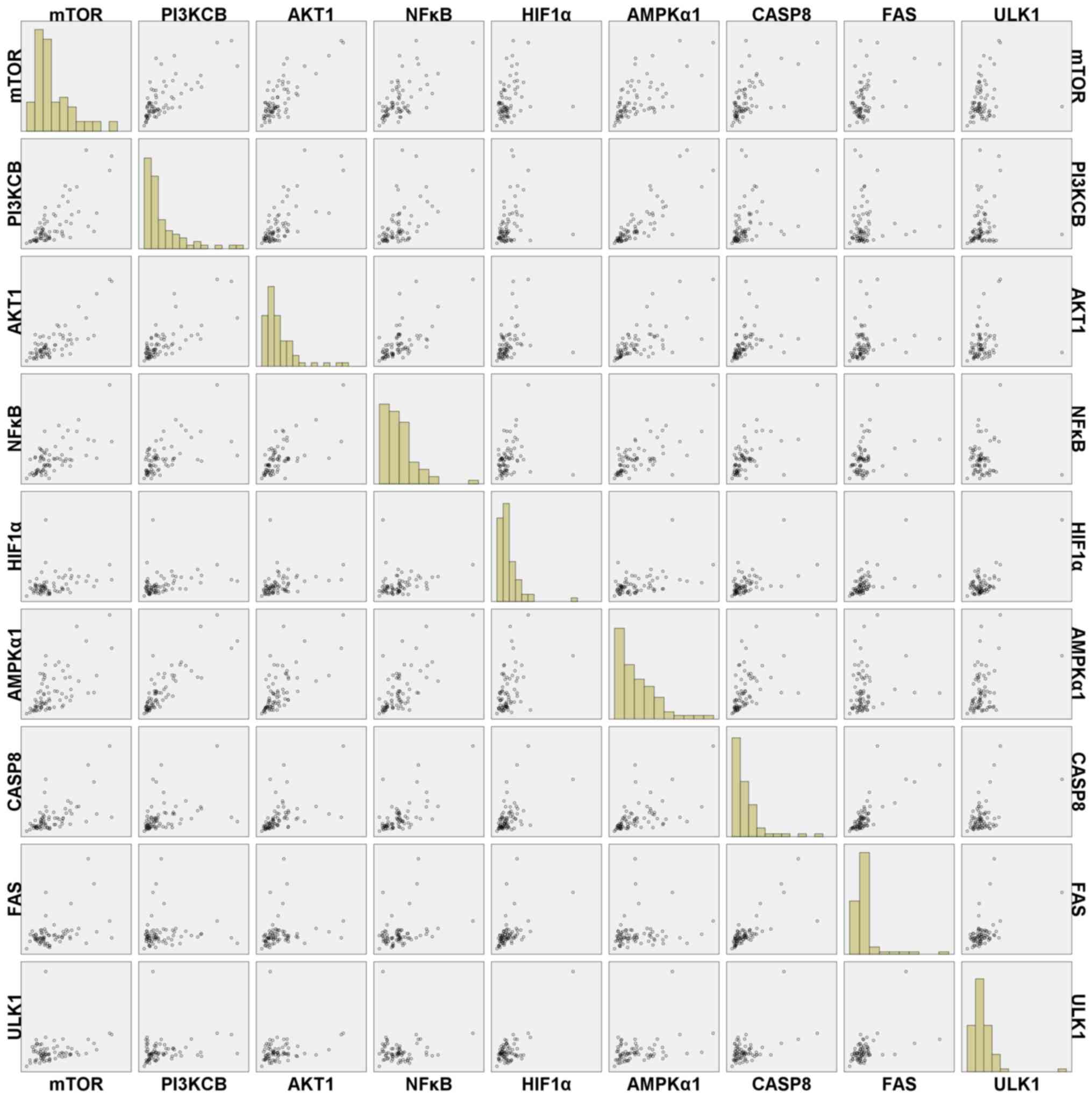 | Figure 4.Scatter plots of the expression of
genes associated with hypoxic, stress, apoptotic and autophagic
pathways for various ex vivo ischemia time points. PI3KCB,
phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit β
isoform; AKT1, threonine kinase 1; HIF1α, hypoxia-inducible factor
1α; AMPKα1, protein kinase AMP-activated catalytic subunit α1;
CASP8, caspase 8; FAS, Fas cell surface death receptor; ULK1,
unc-51 like autophagy activating kinase 1. |
 | Table III.Correlation coefficients between RIN
and gene expression. |
Table III.
Correlation coefficients between RIN
and gene expression.
|
| RIN |
|---|
|
|
|
|---|
| Gene | Pearson correlation
coefficient | Significance
(bilateral) |
|---|
| mTOR | −0.065 | 0.590 |
| PI3KCB | −0.051 | 0.668 |
| AKT1 |
0.172 | 0.150 |
| NF-κB |
0.102 | 0.394 |
| HIF1α |
0.018 | 0.882 |
| AMPKα1 | −0.040 | 0.741 |
| CASP8 |
0.133 | 0.267 |
| FAS |
0.066 | 0.582 |
| ULK1 |
0.024 | 0.840 |
 | Table IV.Pearson's correlation coefficients of
gene expression. |
Table IV.
Pearson's correlation coefficients of
gene expression.
|
| Gene
expression |
|---|
|
|
|
|---|
| Variable | mTOR | PI3KCB | AKT1 | NF-κB | HIF1α | AMPKα1 | CASP8 | FAS | ULK1 |
|---|
| mTOR |
|
|
|
|
|
|
|
|
|
|
r-value | 1 | 0.721c | 0.792c | 0.640c | 0.281a | 0.630c | 0.607c | 0.409c | 0.221 |
|
Significance (bilateral) | N/A | 0.000 | 0.000 | 0.000 | 0.017 | 0.000 | 0.000 | 0.000 | 0.063 |
| N | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 |
| PI3KCB |
|
|
|
|
|
|
|
|
|
|
r-value | 0.721c | 1 | 0.741c | 0.572c | 0.293a | 0.835c | 0.372b | 0.019 | 0.150 |
|
Significance (bilateral) | 0.000 | N/A | 0.000 | 0.000 | 0.013 | 0.000 | 0.001 | 0.876 | 0.208 |
| N | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 |
| AKT1 |
|
|
|
|
|
|
|
|
|
|
r-value | 0.792c | 0.741c | 1 | 0.646c | 0.298a | 0.666c | 0.508c | 0.181 | 0.189 |
|
Significance (bilateral) | 0.000 | 0.000 | N/A | 0.000 | 0.011 | 0.000 | 0.000 | 0.127 | 0.111 |
| N | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 |
| NF-κB |
|
|
|
|
|
|
|
|
|
|
r-value | 0.640c | 0.572c | 0.646c | 1 | 0.226 | 0.674c | 0.658c | 0.281a | 0.021 |
|
Significance (bilateral) | 0.000 | 0.000 | 0.000 | N/A | 0.056 | 0.000 | 0.000 | 0.017 | 0.861 |
| N | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 |
| HIF1α |
|
|
|
|
|
|
|
|
|
|
r-value | 0.281a | 0.293a | 0.298a | 0.226 | 1 | 0.433c | 0.525c | 0.482c | 0.728c |
|
Significance (bilateral) | 0.017 | 0.013 | 0.011 | 0.056 | N/A | 0.000 | 0.000 | 0.000 | 0.000 |
| N | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 |
| AMPKα1 |
|
|
|
|
|
|
|
|
|
|
r-value | 0.630c | 0.835c | 0.666c | 0.674c | 0.433c | 1 | 0.557c | 0.137 | 0.342c |
|
Significance (bilateral) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | N/A | 0.000 | 0.250 | 0.003 |
| N | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 |
| CASP8 |
|
|
|
|
|
|
|
|
|
|
r-value | 0.607c | 0.372c | 0.508c | 0.658c | 0.525c | 0.557c | 1 | 0.762c | 0.467c |
|
Significance (bilateral) | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | N/A | 0.000 | 0.000 |
| N | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 |
| FAS |
|
|
|
|
|
|
|
|
|
|
r-value | 0.409c | 0.019 | 0.181 | 0.281a | 0.482c | 0.137 | 0.762c | 1 | 0.501c |
|
Significance (bilateral) | 0.000 | 0.876 | 0.127 | 0.017 | 0.000 | 0.250 | 0.000 | N/A | 0.000 |
| N | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 |
| ULK1 |
|
|
|
|
|
|
|
|
|
|
r-value | 0.221 | 0.150 | 0.189 | 0.021 | 0.728c | 0.342b | 0.467c | 0.501c | 1 |
|
Significance (bilateral) | 0.063 | 0.208 | 0.111 | 0.861 | 0.000 | 0.003 | 0.000 | 0.000 | N/A |
| N | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 |
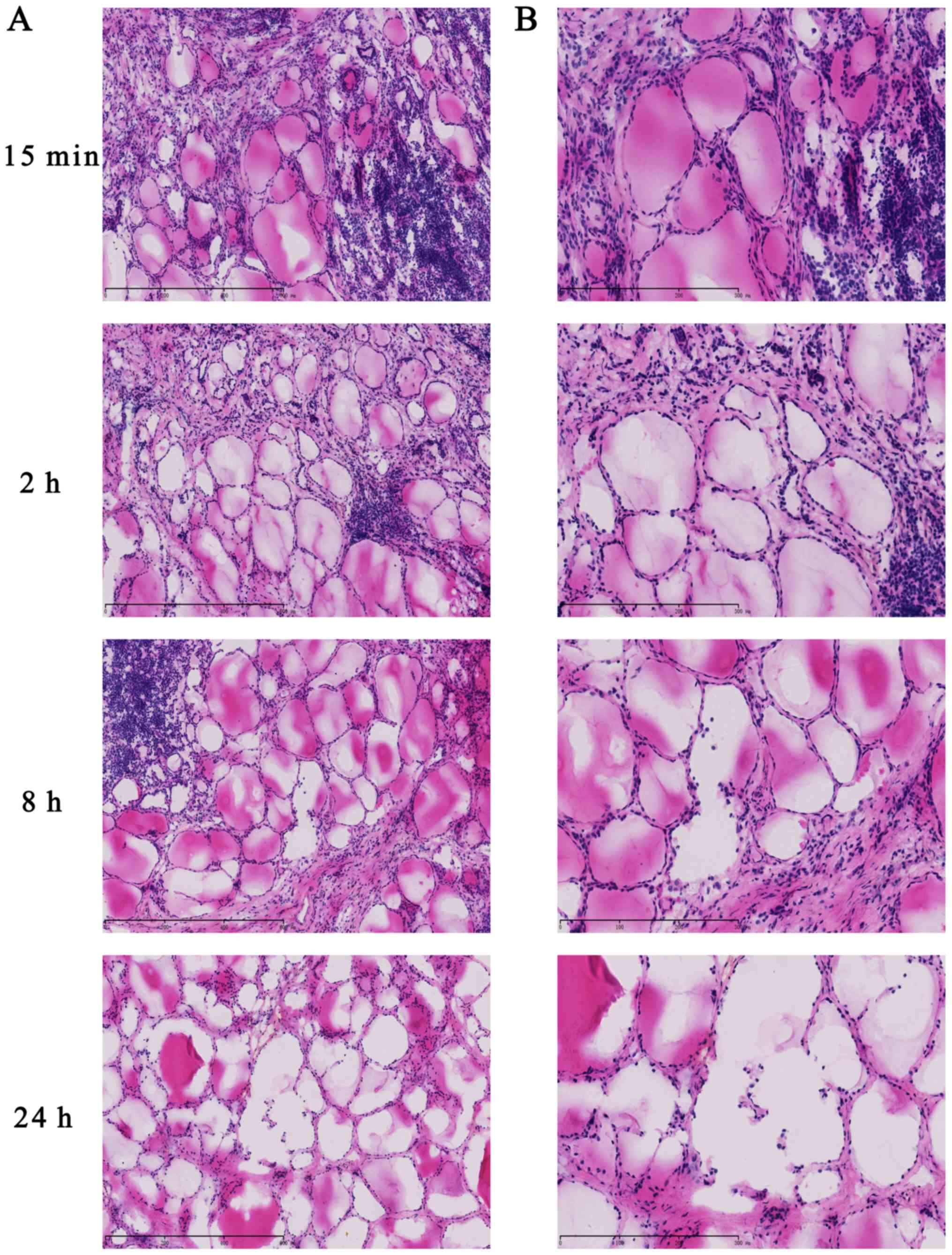
Histological morphology
To evaluate histomorphology at various ex
vivo ischemia time points, frozen tissue specimens were fixed,
sectioned and stained with H&E. The histological morphology
demonstrated that follicular epithelial cells were arranged closely
and maintained integrity at 15 min and 2 h in thyroid tissues
(Fig. 5). However, the
microstructure of the thyroid follicles was destroyed at 8 h and
cells displayed looseness and follicle disintegration at 24 h.
These results demonstrated that ex vivo ischemia and delayed
processing induced morphological changes in thyroid carcinoma,
which was accompanied by RNA degradation.
Discussion
RNA degradation is one of the major issues when
storing tissues in biobanks, and previous studies have investigated
the effect of tissue type, storage buffers and ex vivo
ischemia times on RNA quality (15,16).
Among these factors, ex vivo ischemia and delayed processing
time are crucial pre-analysis challenges for RNA quality (6,12).
The results of the current study demonstrated that RNA integrity
decreased significantly following ex vivo ischemia for 4 h,
indicating that RNA remained intact for 2 h at room
temperature.
Previous research reported that the effects of ex
vivo ischemia time on RNA integrity varied in different types
of tumors. Bao et al (17)
demonstrated that RNA remained stable both at room temperature and
on ice for ≤4 h in colon cancer tissues. Additionally, retained
ileum mucosa was revealed to exhibit high RNA integrity for 1.5 h
at room temperature and 6 h at 4°C (18). In kidney renal cell carcinoma
tissues, RNA degradation was observed at room temperature for 1 h
and degradation was not detected following storage on ice for 4 h
(19,20). Similarly, RIN gradually decreased
in tumor and adjacent normal tissues at 24°C at ischemia time
points 15, 30, 60 and 120 min in hepatocellular carcinoma tissues
(21). Even though our study did
not verify whether the RNA integrity from different tumor tissues
(such as thyroid, ovary, breast, pancreas, jaw and lung) had
different sensitivities to ex vivo ischemia time points, it
could be verified by further studies with more carcinoma
specimens.
In addition, a previous study demonstrated that the
RIN of non-small cell lung cancer tissue was significantly lower
(OR=0.08; P=0.01) in samples that were preserved for >3 h prior
to cryopreservation, and prolonged in vivo (>2 h) and
ex vivo ischemia (>10 h) times were associated with lower
patient-derived xenograft engraftment rates (22). Furthermore, long term ex
vivo ischemia of human brain tumor biopsy tissues affected
ribosomal RNA integrity and increased RNA degradation (23). When snap freezing and multiple
sampling of biopsies were combined, the percentage of specimens
with degraded RNA was reduced by two-fold (23). However, another previous study on
RNA integrity and gene expression in human myocardial tissue
reported that there was no significant correlation between RIN and
a post-mortem interval of 5–24 h at 4°C (24). Furthermore, RNA integrity was not
correlated with 1 h ex vivo ischemia time points in
different types of tumors, including breast, colon, stomach,
oesophageal, gall bladder, ovarian, kidney, liver, lung,
pancreatic, small intestinal, spleen, common bile duct,
retroperitoneum, testis and urinary bladder cancer (24,25).
Additionally, the duration of ex vivo
ischemia affects the related gene expression profile (20). The results of the current study
demonstrated that the gene expression of PI3KCB, AKT1, AMPKα1 and
CASP8 were significantly decreased following ex vivo
ischemia at time points 8–24 h compared with 15 min. PI3KCB, AKT1,
AMPKα1 and CASP8 serve important roles in the hypoxic, stress,
apoptotic and autophagic signaling pathways (26,27).
AMPKα1 is critical for hypoglycemia, hypoxia, ischemia and heat
shock (28), and regulates growth,
metabolism reprogramming, glucose and fatty acid metabolism and
mitochondrial function (29).
Oncogenes AKT1 and PI3KCB are major factors in the regulation of
various cellular functions, including metabolism, growth,
proliferation, survival, transcription and protein synthesis
(30,31). Furthermore, AMPKα1 stimulates
autophagy by inhibiting mTOR, whereas PI3KCB, Ras and AKT1 inhibit
autophagy by activating mTOR (32). Additionally, CASP8 is induced by
endoplasmic reticulum stress and proteotoxic stress (33), which participate in the apoptosis
signal pathway (34).
An increased number of studies have demonstrated
that when the blood supply is cut off, tissues undergo oxygen
deprivation and mammalian cells undergo hypoxemia, autophagy and
endoplasmic reticulum stress response (35). Then, hypoxemia, autophagy,
apoptosis and endoplasmic reticulum stress occur and coordinate
(36,37). Furthermore, a previous study has
reported that exposure to ex vivo ischemia significantly
altered gene expression of G-protein signaling 1 and eukaryotic
translation elongation factor 1 α 1 in normal and colorectal cancer
tissue samples (38).
Dr Carolyn Compton, a former Office of Biorepository
and Biospecimen Research executive, proposed that when biological
samples are excised, cut off from a blood supply and exposed to
abrupt changes in temperature, cellular behavior becomes difficult
to predict (4). Gene expression
and protein phosphorylation fluctuate extensively and cellular
self-destruct pathways may be activated (4). The American Society of Clinical
Oncology, College of American Pathologists (2014), Biorepositories
and Biospecimen Research Branch, National Cancer Institute (2016),
Clinical Laboratory Standards Institute (2011), Clinical Laboratory
Standards Institute (2005), International Standards
Organization/Technical Committee (2016) and European Committee for
Standardization/Technical Committee (2015) demonstrated that ex
vivo ischemia times in tissues should be as short as possible
(optimally <20 min and not >1 h) for the analysis of
biomarkers, molecular analysis, and RNA, DNA and protein isolation
(6).
Although the results of the current study revealed
that the expression of PI3KCB, AKT1, AMPKα1 and CASP8 decreased
with time, there was no correlation between RIN and gene
expression, indicating that the decrease in gene expression was not
induced by RNA degradation. Instead, the decrease in gene
expression was associated with hypoxia, stress, apoptosis and
autophagy induced by ex vivo ischemia and delayed
processing.
Furthermore, the results demonstrated positive
correlations between the expression of mTOR, PI3KCB, AKT1, NF-κB
and AMPKα1, indicating that their expression levels may be
coordinated during ex vivo ischemia and delayed processing.
Certain stress responsive transcription factors, including NF-κB,
serve a major role in autophagic response (39) and orchestrate numerous
transcriptional responses, including autophagy and execution of
cell death (40). Wang et
al (26) reported that
endoplasmic reticulum stress and mitochondrial injury on myocardial
ischemia and reperfusion upregulated phosphorylated PI3KCB and
AKT1. Hypoxia and reperfusion can evoke autophagy and apoptosis via
the AKT1 and mTOR signaling pathways in microglia cells and the
expression of mTOR and AKT1 was not altered under hypoxic
conditions for 6 h and reperfusion injury at 24; however, the
levels of p-AKT1 and mTOR were altered (27). Thus, the results of the current
study indicated that while mRNA expression was altered due to ex
vivo ischemia and delayed processing, whether the expression of
phosphorylated proteins, which is important in cell signal
transduction, may be altered requires further study.
Furthermore, the results demonstrated that there
were fewer positive correlations between HIF1α and mTOR, PI3KCB and
AKT1. A prior study revealed that HIF1α promotes the expression of
hundreds of genes involved in autonomous and non-autonomous
cellular adaptations to hypoxia, which are upregulated at the
protein level via mTOR or at the mRNA level via STAT3 and NF-κB
signaling (41). Although the
current study did not report ULK and/or FAS downregulation at the
mRNA level or an association with the other factors, the activity
of ULK is required for autophagy (42). ULK-mediated Beclin-1
phosphorylation in vivo is crucial for the function of
Beclin-1 in autophagy (42).
Moreover, in addition to RNA degradation, the
results of the present study showed that ex vivo ischemia
and delayed processing induced morphological changes in thyroid
carcinoma tissues, which is another factor to be evaluated
following delayed processing.
In addition to the processing variables, biological
and environmental factors, including donor sex, age (43), drug use (44) and diet, exercise and lifestyle
habits, such as smoking, could affect the quality of biospecimens
(45). Furthermore, various
acquisition methods, such as collecting during the surgical
operation (in situ) or after resection (ex vivo)
(9), and surgical approaches, such
as laparoscopic colectomy or open colectomy (8) could influence gene expression
analysis.
There were certain limitations of the present study.
Firstly, the current study was a preliminary study that could not
verify whether RNA integrity from various tumor tissues had
different sensitivities to ex vivo ischemia time due to the
small sample size. Future studies should include larger sample
sizes and more tumor specimens to comprehensively examine the
effects of various ex vivo ischemia time points and delayed
processing on RNA integrity and tissue quality in different
carcinoma tissues. Secondly, tissue biobanks are the basic
infrastructure that provide biospecimens to various institutions
for basic, translational and clinical research, and their
procedures for the collection, analysis, storage and distribution
of biospecimens may affect the results of downstream studies.
Furthermore, tissue banks should set up individual procedures
according to the requirements by researchers to accommodate
research institutions that require tissues for specific purposes.
Therefore, a future study should investigate the commonality of
more tissues in biobanks, such as breast, colon, stomach,
oesophagus, gall bladder, ovary, kidney, liver, lung and pancreas,
which could be used to provide research institutions with
standardized processing procedures for specimens.
In conclusion, ex vivo ischemia time and
delayed processing induced RNA degradation and altered gene
expression in various carcinoma tissues. Fresh tissues should be
processed within 2 h to avoid RNA degradation to acquire high
quality biospecimens. Furthermore, the gene expression of PI3KCB,
AKT1, AMPKα1 and CASP8 may be considered as markers to evaluate
tissue quality at the gene expression level, providing a method for
the standard processing and assessment of tissue specimens.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Chinese
Academy of Medical Sciences Initiative for Innovative Medicine
(grant nos. 2017-I2M-1-001 and 2017-I2M-2-001) and The National Key
Research and Development Program of China: The Cluster Construction
of Human Genetic Resource Biobank in North China (grant no.
2016YFC1201703).
Availability of data and materials
All data generated or analyzed during the current
study are included in this published article.
Authors' contributions
AW, SZ and DC processed the tissue specimens,
performed the RNA extraction and qPCR. DG and TX designed the
study, contributed to the analysis of the data and wrote the
manuscript. JS performed the histological analysis and interpreted
the patient data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All patients provided signed informed consent for
the donation of the specimens. The current study was approved by
the Ethics Committee of the Institutional Review Board of Peking
Union Medical College Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Watson PH, Wilson-McManus JE, Barnes RO,
Barnes, Giesz SC, Png A, Hegele RG, Brinkman JN, Mackenzie IR,
Huntsman DG, Junker A, et al: Evolutionary concepts in
biobanking-the BC BioLibrary. J Transl Med. 7:952009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nishihara H: Clinical biobank: A novel
system to support cancer clinical sequencing in Japan. Rinsho
Byori. 65:167–172. 2017.(In Japanese). PubMed/NCBI
|
|
3
|
Ginsburg GS, Burke TW and Febbo P:
Centralized biorepositories for genetic and genomic research. JAMA.
299:1359–1361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baker M: Biorepositories: Building better
biobanks. Nature. 486:141–146. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carithers LJ, Agarwal R, Guan P, Odeh H,
Sachs MC, Engel KB, Greytak SR, Barcus M, Soria C, Jason Lih CJ, et
al: The biospecimen preanalytical variables program: A multiassay
comparison of effects of delay to fixation and fixation duration on
nucleic acid quality. Arch Pathol Lab Med. 143:1106–1118. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Compton CC, Robb JA, Anderson MW, Berry
AB, Birdsong GG, Bloom KJ, Branton PA, Crothers JW, Cushman-Vokoun
AM, Hicks DG, et al: Preanalytics and precision pathology:
Pathology practices to ensure molecular integrity of cancer patient
biospecimens for precision medicine. Arch Pathol Lab Med.
143:1346–1363. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kahn N, Riedlinger J, Roessler M, Rabe C,
Lindner M, Koch I, Schott-Hildebrand S, Herth FJ, Schneider MA,
Meister M and Muley TR: Blood-sampling collection prior to surgery
may have a significant influence upon biomarker concentrations
measured. Clin Proteomics. 12:192015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galissier T, Schneider C, Nasri S,
Kanagaratnam L, Fichel C, Coquelet C, Diebold MD, Kianmanesh R,
Bellon G, Dedieu S, et al: Biobanking of fresh-frozen human
adenocarcinomatous and normal colon tissues: Which parameters
influence RNA quality? PLoS One. 11:e01543262016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou JH, Sahin AA and Myers JN: Biobanking
in genomic medicine. Arch Pathol Lab Med. 139:812–818. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olsen J, Kirkeby LT, Eiholm S, Jess P,
Troelsen JT, Gögenür I and Olsen J: Impact of in vivo ischemic time
on RNA quality-experiences from a colon cancer biobank. Biopreserv
Biobank. 13:255–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng XH, Zhang SD, Zhang PF, Li XZ, Hu
YZ, Tian T, Zhu L, Wang RZ and Jia WH: Tumor cell content and RNA
integrity of surgical tissues from different types of tumors and
its correlation with ex vivo and in vivo ischemia. Ann Surg Oncol.
25:3764–3770. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Betsou F, Lehmann S, Ashton G, Barnes M,
Benson EE, Coppola D, DeSouza Y, Eliason J, Glazer B, Guadagni F,
et al: Standard preanalytical coding for biospecimens: Defining the
sample PREanalytical code. Cancer Epidemiol Biomarkers Prev.
19:1004–1011. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mazzei M, Vascellari M, Zanardello C,
Melchiotti E, Vannini S, Forzan M, Marchetti V, Albanese F and
Abramo F: Quantitative real time polymerase chain reaction
(qRT-PCR) and RNAscope in situ hybridization (RNA-ISH) as effective
tools to diagnose feline herpesvirus-1-associated dermatitis. Vet
Dermatol. 30:491–e147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Micke P, Ohshima M, Tahmasebpoor S, Ren
ZP, Ostman A, Pontén F and Botling J: Biobanking of fresh frozen
tissue: RNA is stable in nonfixed surgical specimens. Lab Invest.
86:202–211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rudloff U, Bhanot U, Gerald W, Klimstra
DS, Jarnagin WR, Brennan MF and Allen PJ: Biobanking of human
pancreas cancer tissue: Impact of ex-vivo procurement times on RNA
quality. Ann Surg Oncol. 17:2229–2236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bao WG, Zhang X, Zhang JG, Zhou WJ, Bi TN,
Wang JC, Yan WH and Lin A: Biobanking of fresh-frozen human colon
tissues: Impact of tissue ex-vivo ischemia times and storage
periods on RNA quality. Ann Surg Oncol. 20:1737–1744. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SM, Schelcher C, Thasler R, Schiergens
TS and Thasler WE: Pre-analytical determination of the effect of
extended warm or cold ischaemia on RNA stability in the human ileum
mucosa. PLoS One. 10:e01382142015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun H, Sun R, Hao M, Wang Y, Zhang X, Liu
Y and Cong X: Effect of duration of ex vivo ischemia time and
storage period on RNA quality in biobanked human renal cell
carcinoma tissue. Ann Surg Oncol. 23:297–304. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng H, Tao YP, Chen FQ, Li HF, Zhang ZD,
Zhou XX, Yang Y and Zhou WP: Temporary ischemia time before snap
freezing is important for maintaining high-integrity RNA in
hepatocellular carcinoma tissues. Biopreserv Biobank. 17:425–432.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guerrera F, Tabbo F, Bessone L, Maletta F,
Gaudiano M, Ercole E, Annaratone L, Todaro M, Boita M, Filosso PL,
et al: The influence of tissue ischemia time on RNA integrity and
patient-derived xenografts (PDX) engraftment rate in a non-small
cell lung cancer (NSCLC) biobank. PLoS One. 11:e01451002016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Castells Domingo X, Ferrer-Font L, Davila
M, Candiota AP, Simões RV, Fernández-Coello A, Gabarrós A, Boluda
S, Barceló A, Ariño J and Arús C: Improving ribosomal RNA integrity
in surgically resected human brain tumor biopsies. Biopreserv
Biobank. 14:156–164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gonzalez-Herrera L, Valenzuela A, Marchal
JA, Lorente JA and Villanueva E: Studies on RNA integrity and gene
expression in human myocardial tissue, pericardial fluid and blood,
and its postmortem stability. Forensic Sci Int. 232:218–228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song SY, Jun J, Park M, Park SK, Choi W,
Park K, Jang KT and Lee M: Biobanking of fresh-frozen cancer
tissue: RNA is stable independent of tissue type with less than 1
hour of cold ischemia. Biopreserv Biobank. 16:28–35. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Wang Y, Ye J, Lu X, Cheng Y, Xiang
L, Chen L, Feng W, Shi H, Yu X, et al: bFGF attenuates endoplasmic
reticulum stress and mitochondrial injury on myocardial
ischaemia/reperfusion via activation of PI3K/Akt/ERK1/2 pathway. J
Cell Mol Med. 19:595–607. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen CM, Wu CT, Yang TH, Chang YA, Sheu ML
and Liu SH: Green tea catechin prevents hypoxia/reperfusion-evoked
oxidative stress-regulated autophagy-activated apoptosis and cell
death in microglial cells. J Agric Food Chem. 64:4078–4085. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth, autophagy and
metabolism. Nat Cell Biol. 13:1016–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qi D and Young LH: AMPK: Energy sensor and
survival mechanism in the ischemic heart. Trends Endocrinol Metab.
26:422–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hers I, Vincent EE and Tavare JM: Akt
signalling in health and disease. Cell Signal. 23:1515–1527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Avalos Y, Canales J, Bravo-Sagua R,
Criollo A, Lavandero S and Quest AF: Tumor suppression and
promotion by autophagy. Biomed Res Int. 2014:6039802014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iurlaro R and Munoz-Pinedo C: Cell death
induced by endoplasmic reticulum stress. FEBS J. 283:2640–2652.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saggioro FP, Neder L, Stavale JN,
Paixão-Becker ANP, Malheiros SMF, Soares FA, Pittella JEH, Matias
CCMS, Colli BO, Carlotti CG Jr and Franco M: Fas, FasL, and cleaved
caspases 8 and 3 in glioblastomas: A tissue microarray-based study.
Pathol Res Pract. 210:267–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schito L and Semenza GL: Hypoxia-inducible
factors: Master regulators of cancer progression. Trends Cancer.
2:758–770. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
LaGory EL and Giaccia AJ: The
ever-expanding role of HIF in tumour and stromal biology. Nat Cell
Biol. 18:356–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kurokawa M and Kornbluth S: Caspases and
kinases in a death grip. Cell. 138:838–854. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lange N, Unger FT, Schoppler M, Pursche K,
Juhl H and David KA: Identification and validation of a potential
marker of tissue quality using gene expression analysis of human
colorectal tissue. PLoS One. 10:e01339872015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pietrocola F, Izzo V, Niso-Santano M,
Vacchelli E, Galluzzi L, Maiuri MC and Kroemer G: Regulation of
autophagy by stress-responsive transcription factors. Semin Cancer
Biol. 23:310–322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Verzella D, Pescatore A, Capece D,
Vecchiotti D, Ursini MV, Franzoso G, Alesse E and Zazzeroni F:
Life, death, and autophagy in cancer: NF-κB turns up everywhere.
Cell Death Dis. 11:2102020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4:512011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nazarko VY and Zhong Q: ULK1 targets
beclin-1 in autophagy. Nat Cell Biol. 15:727–728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim SJ, Dix DJ, Thompson KE, Murrell RN,
Schmid JE, Gallagher JE and Rockett JC: Effects of storage, RNA
extraction, genechip type, and donor sex on gene expression
profiling of human whole blood. Clin Chem. 53:1038–1045. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Becker N and Lockwood CM: Pre-analytical
variables in miRNA analysis. Clin Biochem. 46:861–868. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Willemse EA and Teunissen CE: Biobanking
of cerebrospinal fluid for biomarker analysis in neurological
diseases. Adv Exp Med Biol. 864:79–93. 2015. View Article : Google Scholar : PubMed/NCBI
|