Introduction
Diabetic retinopathy (DR) is a microvascular
complication associated with diabetes and is the major cause of
blindness (1,2). The prevalence of DR ranges from 15.3
to 42.4% worldwide, and the risk factors for DR progression include
blood glucose, pressure and lipids (3). Although the pathogenesis of DR is
complicated, it has been reported that nerve degeneration plays a
vital role (4). Müller cells are
the primary glial cells in the retina, which provide structural
support and the energy required for metabolism. Therefore, Müller
cells may have a role in the growth, injury, repair and
regeneration of retinal neurons, thus may be used to investigate DR
in in vitro studies.
Berberine (BBR) is a bioactive constituent extracted
from Rhizoma coptidis that displays effects on congestive
heart failure, as well as blood glucose and lipid levels. BBR also
protects against oxidative stress-induced injuries (5), including the oxidative stress
observed during the early stages of DR development, which is
evidenced by increased reactive oxygen species (ROS) (6). Moreover, BBR ameliorates fatty acid-
and glutamate-induced oxidative stress, and alleviates oxidative
damage-induced apoptosis (7–9).
Therefore, BBR might serve as a therapeutic agent for type 2
diabetes mellitus, hyperlipidemia and hypertension (10).
BBR displays a relieving effect on diabetic
complications. Glial fibrillary acidic protein, a structural
protein, is upregulated in Müller cells in response to retinal
injury or stress. Fu et al (11) reported that BBR inhibits modified
LDL-induced Müller cell injury by activating the AMPK signaling
pathway. In addition, BBR can inhibit leukocyte-mediated killing of
vascular endothelium, decrease antioxidant enzyme activities and
protect against retinal diseases involving oxidative stress
(12,13). The aforementioned effects of BBR
suggest that the compound may display therapeutic effects during
DR; however, the potential mechanism underlying the effect of BBR
in DR is not completely understood.
The present study investigated the effects of BBR on
cell apoptosis and oxidative stress. A rat model of DR was
successfully established, and subsequently, blood glucose levels,
retinal structures, and the inner and outer nuclear layers were
examined in vivo. Müller cells were used to further
investigate oxidative stress and cell apoptosis in vitro.
The results of the present study may provide a novel insight for
the prevention and treatment of DR.
Materials and methods
Animal experiments
The present study was approved by the Ethics
Committee of Xi'an Ninth Hospital and followed the guidelines set
by the Association for Research in Vision and Ophthalmology
Resolution on Treatment of Animals in Research (14,15).
Six-week-old male Sprague-Dawley (SD) rats (weight, 200 g) were
purchased from Liaoning Changsheng Biotechnology Co., Ltd. All SD
rats were housed under standard conditions, with a temperature of
25°C, humidity of 45–55%, light/dark cycle of 12/12 h, and food and
water was freely available. Following adaptive feeding for one
week, the rats were administered 65 mg/kg streptozotocin (STZ; cat.
no. 18883-66-4; Aladdin; prepared in citrate buffer) to establish
the rat model of DR. Control rats were administered 65 mg/kg
citrate buffer. After 3 days, blood glucose levels in blood samples
obtained from the tail vein were measured and a glucose level ≥300
mg/dl was considered as a successful establishment of the rat model
of DR. BBR was prepared in 0.5% carboxymethylcellulose (CMC)-Na. A
total of 18 DR rats were divided into three groups (n=6 per group):
i) The DR group, ii) the DR + BBR-L group and iii) the DR + BBR-H
group. The DR group was treated with CMC-Na by gavage for 8 weeks.
The DR + BBR-L group was treated with 100 mg/kg BBR by gavage every
day for 8 weeks. The DR + BBR-H group was treated with 200 mg/kg
BBR by gavage every day for 8 weeks.
Body weight and blood glucose levels were measured
every 2 weeks for the 8-week treatment period. After an 8-week
treatment, the rats were sacrificed by excessive anesthesia with
200 mg/kg pentobarbital sodium. Death was verified by monitoring
cessation of breathing and heartbeats. Subsequently, the eyes were
isolated and fixed with 4% paraformaldehyde for 48 h at room
temperature.
Cell culture
Müller cells are primary glial retina cells and are
considered to be the perfect in vitro cell model of DR
(16). Müller cells (cat. no.
CP-M117; Procell Life Science & Technology Co., Ltd.) were
cultured in Müller cell complete medium (MCCM; cat. no. CM-M117;
Procell Life Science & Technology Co., Ltd.) at 37°C with 5%
CO2. After 24-h incubation, Müller cells were incubated
for 48 h with the following: MCCM containing 5.5 mM glucose
(control); MCCM containing 33.3 mM glucose [high glucose (HG)];
MCCM containing 33.3 mM glucose and 20 µM BBR (HG + BBR); and MCCM
containing 33.3 mM glucose and 100 µM pyrrolidine dithiocarbamate
(PDTC, an NF-κB inhibitor; cat. no. HY-18738; MedChemExpress; HG +
PDTC). Subsequently, the treated Müller cells were used for further
experiments.
Metabolic analysis
The eye tissues were added to normal saline
(weight:volume=1:9) and homogenized. Total protein was quantified
using a Bicinchoninic Acid Protein assay kit (cat. no. P0009;
Beyotime Institute of Biotechnology), according to the
manufacturer's protocols. Subsequently, the catalase (CAT) and
superoxide dismutase (SOD) activity, as well as the malonaldehyde
(MDA), reactive oxygen species (ROS) and reduced glutathione (GSH)
contents of the tissue samples containing equal amounts of protein
or treated Müller cells were assessed using the CAT (cat. no.
A007-1; Nanjing Jiancheng Bioengineering Institute), MDA (cat. no.
A003-1; Nanjing Jiancheng Bioengineering Institute), ROS (cat. no.
E004; Jiancheng Bioengineering Institute), SOD (cat. no. A001-1;
Jiancheng Bioengineering Institute) and GSH (cat. no. A006-2;
Jiancheng Bioengineering Institute) assay kits according to the
manufacturer's protocol. The samples were detected using a NovoCyte
flow cytometer (ACEA Biosciences, Inc.) and analyzed by
NovoExpress® 1.2.5 (ACEA Biosciences, Inc.).
Mitochondrial membrane potential (ΔΨm)
assay
Loss of membrane potential (ΔΨm) is a
hallmark of cell apoptosis. Following treatment, Müller cells at a
density of 90% were washed with PBS. Subsequently, the
mitochondrial membrane potential was measured using a JC-1
mitochondrial membrane potential detection kit (cat. no. C2006;
Beyotime Institute of Biotechnology), according to the
manufacturer's protocols. The mitochondrial membrane potential was
measured using flow cytometry (NovoCyte; ACEA Biosciences, Inc.)
and analyzed by NovoExpress 1.2.5 (ACEA Biosciences, Inc.).
Cell Counting Kit-8 (CCK-8) assay
Müller cells were seeded (2×103
cells/well) in 96-well plates and incubated for 48 h at 37°C with
nine different mediums: i) MCCM (control); ii) MCCM containing 33.3
mM glucose (HG); iii) MCCM containing 33.3 mM glucose and 1 µM BBR
(HG + 1 µM BBR); iv) MCCM containing 33.3 mM glucose and 2 µM BBR
(HG + 2 µM BBR); v) MCCM containing 33.3 mM glucose and 5 µM BBR
(HG + 5 µM BBR); vi) MCCM containing 33.3 mM glucose and 10 µM BBR
(HG + 10 µM BBR); vii) MCCM containing 33.3 mM glucose and 20 µM
BBR (HG + 20 µM BBR); viii) MCCM containing 33.3 mM glucose and 50
µM BBR (HG + 50 µM BBR). Cell viability was detected by CCK-8 assay
kit (cat. no. 96992; Sigma-Aldrich; Merck KGaA), according to the
manufacturer's protocols. Briefly, 100 µl MCCM and 10 µl CCK-8
reagent (Beyotime Institute of Biotechnology) was added to each
well, and incubated for 1 h at 37°C. The absorbance of each well
was recorded at a wavelength of 450 nm using a microplate reader to
identify the optimal therapeutic concentration of BBR.
Western blotting
Total protein from treated Müller cells and eye
samples was mechanically lysed using RIPA buffer (cat. no. P0013B;
Beyotime Institute of Biotechnology) supplemented with a cocktail
of PMSF (cat. no. ST506; Beyotime Institute of Biotechnology).
Total protein was extracted using the Nuclear and Cytoplasmic
Protein Extraction kit (cat. no. P0028; Beyotime Institute of
Biotechnology) or the Mitochondrial Protein Extraction kit (cat.
no. AR0156; Boster Biological Technology), according to the
manufacturer's protocols. Protein content was measured by the BCA
Protein Assay kit (cat. no. P0009; Beyotime Institute of
Biotechnology), according to the manufacturer's instruction.
Proteins (30 µg per lane) were separated by 10% SDS-PAGE and
transferred onto PVDF membranes (cat. no. LC2005; Thermo Fisher
Scientific, Inc.). After washing with TBS with 0.15% (v/v)
Tween-20, the membranes were blocked with 5% (m/v) bull serum
albumin (cat. no. BS043; Biosharp Life Sciences) for 1 h at room
temperature and incubated overnight at 4°C with primary antibodies
targeted against: NF-κB inhibitor (IκBα; cat. no. 9242; 1:500; Cell
Signaling Technology, Inc.), phosphorylated (p)-IκBα (cat. no.
2859; 1:1,000; Cell Signaling Technology, Inc.), NF-κB p65 (cat.
no. 8242; 1:500; Cell Signaling Technology, Inc.), cleaved
caspase-3 (cat. no. 9654; 1:500; Cell Signaling Technology, Inc.),
cleaved caspase-9 (cat. no. 9507; 1:1,000; Cell Signaling
Technology, Inc.), Bcl-2 (cat. no. 12789-1-AP; 1:500; Proteintech),
Bax (cat. no. 50599-2-Ig; 1:500; Proteintech Group, Inc.),
Cytochrome c (cat. no. 4272; 1:1,000; Cell Signaling Technology,
Inc.), COX IV (cat. no. 11242-1-AP; 1:500; Proteintech Group,
Inc.), Histone H3 (cat. no. 17168-AP; 1:500; Proteintech Group,
Inc.) and β-actin (cat. no. 60008-1-Ig; 1:2,000; Proteintech Group,
Inc.). Subsequently, the membranes were incubated with
HRP-conjugated goat anti-rabbit (cat. no. SA00001-2; 1:10,000;
Proteintech Group, Inc.) and HRP-conjugated goat anti-mouse (cat.
no. SA00001-1; 1:10,000; Proteintech Group, Inc.) secondary
antibodies at room temperature for 40 min. Protein bands were
visualized using an ECL kit (cat. no. E003; 7Sea Biotech),
according to the manufacturer's protocols, and quantified using
Gel-Pro-Analyzer software (version 4; Media Cybernetics, Inc.).
TUNEL assay
Cell apoptosis in eye tissues was determined using a
TUNEL assay with the In Situ Cell Death Detection kit (cat.
no. 11684817910; Merck KGaA), according to the manufacturer's
protocols. Eye tissues were cut into 5 µm thick slices, dewaxed,
dehydrated and washed with PBS. The sections were permeabilized
using 0.1% Triton X-100 for 8 min at room temperature (cat. no.
ST795; Beyotime Institute of Biotechnology), washed with PBS and
incubated with TdT Labeling Buffer for 1 h at 37°C. Subsequently,
the sections were incubated with 50 µl Converter-POD (horseradish
peroxidase-labeled fluorescein antibody), washed with PBS and
developed with DAB (cat. no. DA1010; Beijing Solarbio Science &
Technology Co., Ltd.). After mounting with neutral balsam,
TUNEL-positive cells were observed and counted using a fluorescence
microscope (magnification, ×600) in three random fields.
Hematoxylin & Eosin (H&E)
staining
SD rats were sacrificed and the eye tissues were
isolated. Following dewaxing and dehydration, tissues (5 µm) were
stained with hematoxylin for 5 min at room temperature. Then, the
eye sections were differentiated using 1% hydrochloric acid alcohol
for 3 sec at room temperature. Subsequently, the sections were
washed with ddH2O, stained with eosin for 3 min at room
temperature and dehydrated using an ascending alcohol series for 2
min. The sections were hyalinized using dimethylbenzene, sealed and
observed using a light microscope (magnification, ×200).
Immunofluorescence staining
Müller Cells were fixed with 4% paraformaldehyde for
15 min at room temperature, permeabilized with 0.1% Triton X-100
(cat. no. ST795; Beyotime Institute of Biotechnology) for 30 min at
room temperature, and washed three times with PBS. Subsequently,
the cells were embedded in 5% blocking serum (cat. no. SL038;
Beijing Solarbio Science & Technology Co., Ltd.) for 15 min at
room temperature. Cells were incubated overnight at 4°C with an
anti-NF-κB p65 primary antibody (cat. no. #8242; 1:200; Cell
Signaling Technology, Inc.) and subsequently washed with PBS.
Following primary incubation, cells were incubated with an
FITC-labeled goat anti-rabbit IgG (H+L) secondary antibody (cat.
no. A0562; 1:200; Beyotime Institute of Biotechnology) at room
temperature for 60 min. Subsequently, cells were stained with DAPI
for 5 min at room temperature and observed using a DP73
fluorescence microscope (Olympus Corporation; magnification,
×400).
Hoechst staining
Following treatment, Müller cells were washed twice
with PBS and subsequently stained using the Hoechst Staining kit
(cat. no. C0003; Beyotime Institute of Biotechnology), according to
the manufacturer's protocols. Stained cells were observed using a
DP73 fluorescence microscope (Olympus Corporation; magnification,
×400) in three random fields.
Annexin V-FITC apoptosis
detection
Apoptotic cells were determined using the Annexin
V-FITC Apoptosis Detection kit (cat. no. C1062; Beyotime Institute
of Biotechnology), according to the manufacturer's protocols.
Briefly, Müller cells of each group at a density of 90% were seeded
into a 6-well plate and incubated for 48 h at 37°C. Subsequently,
cells were collected, washed twice with PBS, and stained with 5 µl
Annexin V-FITC and 10 µl propidium iodide for 15 min at room
temperature in the dark. Early apoptotic and necrotic cells were
identified using flow cytometry (NovoCyte; ACEA Biosciences, Inc.)
and analyzed by NovoExpress 1.2.5 (ACEA Biosciences, Inc.).
Statistical analysis
All experiments were repeated at least three times.
Data are presented as the mean ± SD. Statistical analyses were
performed using GraphPad Prism software (version 7.0; GraphPad
Software, Inc.). Data containing 2 groups were analyzed using the
Student's t-test. Data containing >2 groups were analyzed using
one-way ANOVA followed by Bonferroni's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
BBR inhibits retinal ganglion cell
apoptosis in a rat model of DR
In the present study, a rat model of DR was
successfully established by STZ injection. STZ was used to
establish a rat model of DR, characterized by high blood glucose
levels. Firstly, alterations in body weight and blood glucose
levels in response to STZ and BBR were monitored. Body weight
(Table I) and blood glucose levels
(Table II) were significantly
increased in the DR group compared with the control group. Body
weight and blood glucose levels were not significantly altered
during the 8-week treatment period across the four groups. H&E
(Fig. 1A) and TUNEL (Fig. 1B) staining assays suggested that
retinal ganglion cell layer (GCL) apoptosis was decreased by BBR
treatment in the rat model of DR, especially in the BBR-H treatment
group. Retinal MDA, SOD, CAT, GSH and ROS contents were measured as
metabolic indicators of the eyes (Fig.
1C-G). MDA and ROS contents were significantly increased in the
DR group compared with the control group; however, the BBR treated
groups displayed significantly decreased MDA and ROS contents
compared with the DR group (Fig. 1C
and G). Furthermore, GSH levels and antioxidant enzyme
activities were significantly decreased in the DR group compared
with the control group. The BBR treated groups displayed
significantly increased GSH levels and antioxidant enzyme
activities compared with the DR group (Fig. 1D-F). The expression levels of p-IκB
(Ser32), IκB, NF-κB (cytoplasm) and NF-κB (nuclear) were detected
by western blotting. The expression levels of p-IκB (Ser32) and
NF-κB (nuclear) were significantly increased in the DR group
compared with the control group, and the BBR treated groups
displayed significantly decreased expression levels compared with
the DR group (Fig. 1H). However,
the expression levels of IκB and NF-κB (cytoplasm) displayed the
opposite trend (Fig. 1H). The
results suggested that BBR decreased the phosphorylation of IκB at
Ser32 and deactivated the NF-κB signaling pathway in the rat model
of DR.
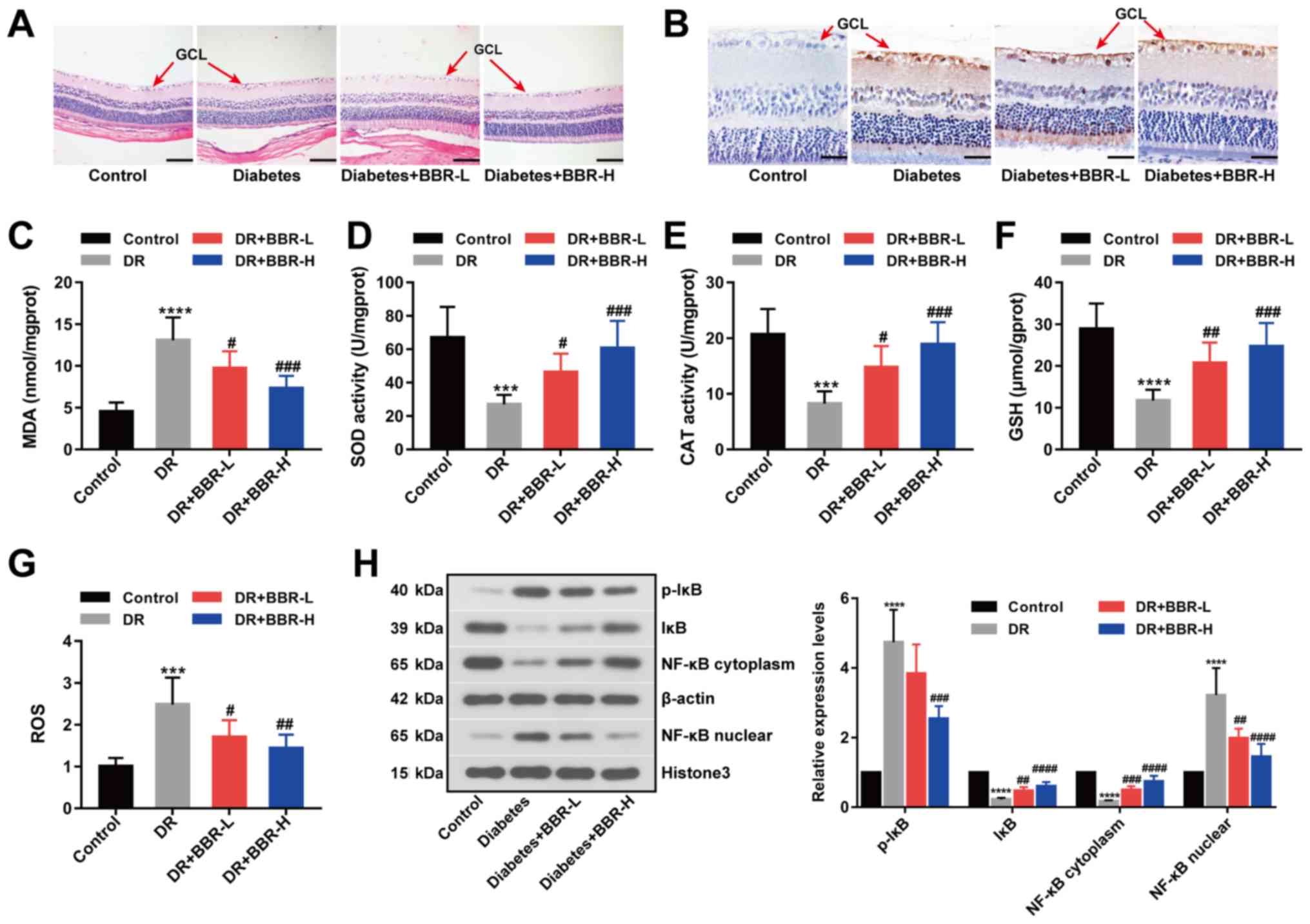 | Figure 1.BBR decreases retinal ganglion cell
apoptosis in a rat model of DR. (A) Hematoxylin and eosin-stained
eye sections (scale bar, 100 µm). (B) Eye sections assessed using
the TUNEL staining assay (scale bar, 33 µm). The (C) MDA, (D) SOD,
(E) CAT, (F) GSH and (G) ROS contents of the eye sections. (H)
Expression levels of p-IκB (Ser32), IκB, NF-κB (nuclear) and NF-κB
(cytoplasm) were examined by western blotting. ***P<0.001 and
****P<0.0001 vs. the control group. #P<0.05,
##P<0.01, ###P<0.001 and
####P<0.0001 vs. the DR group. BBR, berberine; DR,
diabetic retinopathy; MDA, malondialdehyde; SOD, superoxide
dismutase; CAT, catalase; GSH, glutathione; ROS, reactive oxygen
species; p, phosphorylated; IκB, NF-κB inhibitor; GCL, ganglion
cell layer; BBR-L, 100 mg/kg BBR; BBR-H, 200 mg/kg BBR. |
 | Table I.Body weight changes (g) in the groups
of rats. |
Table I.
Body weight changes (g) in the groups
of rats.
| Group | 0 weeks | 2 weeks | 4 weeks | 6 weeks | 8 weeks |
|---|
| Control | 239.00±11.00 | 265.50±14.32 | 277.83±15.03 | 304.67±30.53 | 321.33±33.42 |
| DR |
240.17±10.11a |
218.00±18.35a |
208.33±18.27a |
194.67±17.65a |
180.50±15.08a |
| DR + BBR-L | 237.83±9.00 | 217.50±15.60 | 204.83±15.22 | 188.50±10.37 | 173.83±16.12 |
| DR + BBR-H | 239.33±10.88 | 222.33±19.01 | 207.33±17.84 | 192.00±17.37 | 178.17±20.00 |
 | Table II.Blood glucose level (mg/dl) changes
in the groups of rats. |
Table II.
Blood glucose level (mg/dl) changes
in the groups of rats.
| Group | 0 weeks | 2 weeks | 4 weeks | 6 weeks | 8 weeks |
|---|
| Control | 5.45±0.43 | 5.72±0.72 | 5.75±0.33 | 5.73±0.47 | 5.65±0.33 |
| DR |
18.82±1.45a |
20.37±1.60a |
21.62±1.51a |
20.57±1.93a |
20.42±1.41a |
| DR+BBR-L | 18.75±0.89 | 20.07±1.45 | 20.32±1.28 | 20.73±1.67 | 20.78±1.60 |
| DR+BBR-H | 18.88±1.12 | 20.07±1.35 | 21.18±1.86 | 20.68±0.92 | 21.33±1.10 |
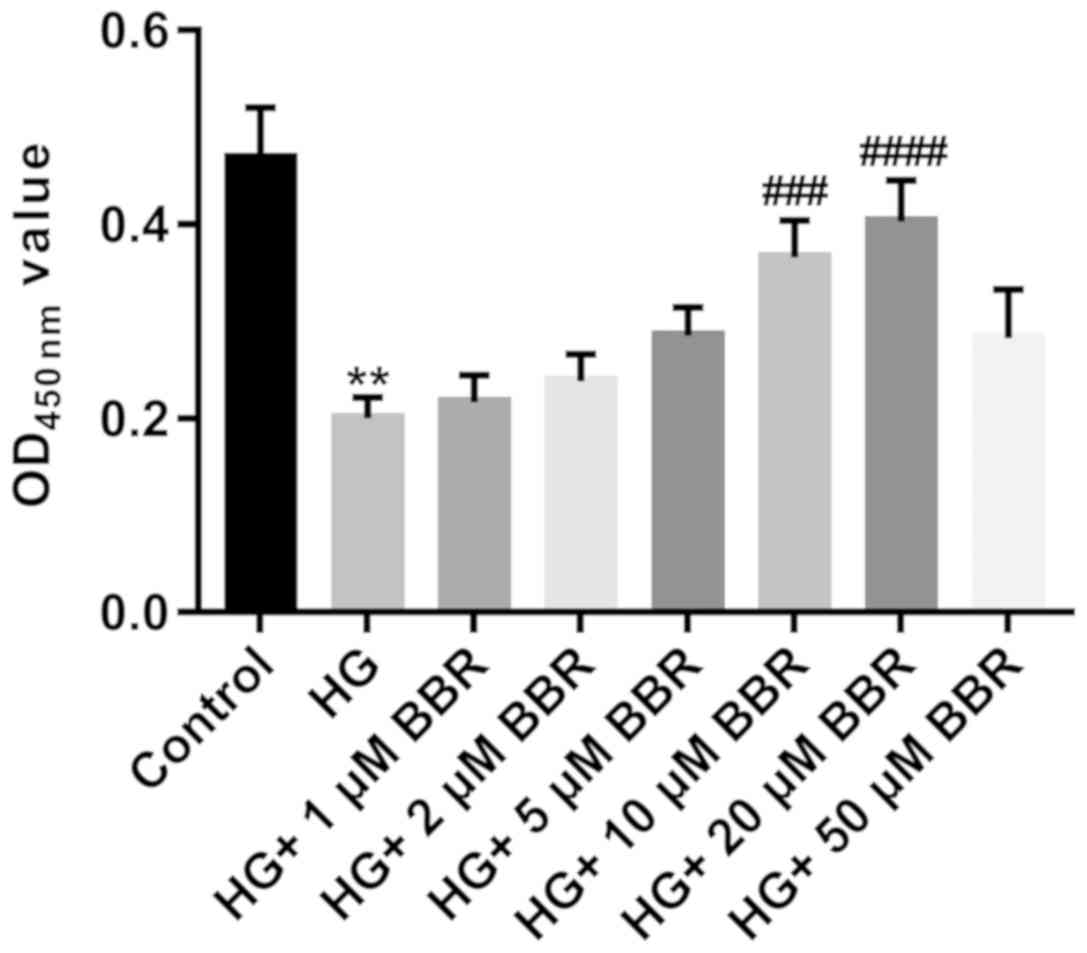
Effect of BBR on the viability of
Müller cells
Müller cells are primary retinal glial cells. The
viability of Müller cells was significantly decreased in the HG
group compared with the control group (Fig. 2). To evaluate the effect of BBR on
the viability of HG-treated Müller cells, HG-treated Müller cells
were incubated with different doses of BBR (1, 2, 5, 10, 20 and 50
µM). BBR treatment increased the viability of HG-treated Müller
cells, especially at a concentration of 20 µM (P<0.0001;
Fig. 2); therefore, 20 µM BBR was
used for subsequent experiments.
BBR decreases HG-induced cell
apoptosis and oxidative stress in Müller cells
To investigate the effect of BBR on DR in
vitro, HG-treated Müller cells were used to model retinal
ganglion cells. Rat models of DR display excessive activation of
the NF-κB signaling pathway; therefore, HG-treated Müller cells
were incubated with PDTC (a NF-κB inhibitor) or BBR. Apoptotic
cells were identified using Annexin V-FITC apoptosis detection. The
rate of apoptosis was significantly increased in the HG group
compared with the control group; however, BBR or PDTC treated cells
displayed significantly decreased rates of apoptosis compared with
the HG group (Fig. 3A). Similar
results were also obtained by the Hoechst staining assay (Fig. 3B). Subsequently, apoptosis-related
proteins were examined by western blotting. Cleaved caspase-3,
cleaved caspase-9 and Bax expression levels were significantly
increased in the HG group compared with the control group.
HG-treated cells incubated with BBR or PDTC displayed significantly
decreased expression levels of cleaved caspase-3, caspase-9 and Bax
compared with the HG group (Fig.
3C). The expression of Bcl-2 displayed the opposite trend
(Fig. 3C). MMP may be decreased
during cell apoptosis; therefore, MMP was evaluated using the JC-1
assay. The results suggested that MMP was decreased in HG-induced
Müller cells compared with the control group, and recovered to
control levels following treatment with BBR or PDTC (Fig. 3D). The expression of cytoplasmic
cytochrome c was increased and the expression of mitochondrial
cytochrome c was decreased in the HG group compared with the
control group. BBR or PDTC treatment reversed HG-induced effects on
cytochrome c expression (Fig. 3E).
The results suggested that cytochrome c was released from the
mitochondria into the cytoplasm following treatment with HG, and
BBR or PDTC treatment could reverse HG-induced effects.
Furthermore, GSH levels and antioxidant enzyme activity were
decreased in the HG group compared with the control group, but
increased in the BBR and PDTC treated groups compared with the HG
group. MDA content displayed the opposite trend (Fig. 3F). ROS levels were detected by flow
cytometry, which suggested that the HG group displayed increased
ROS levels compared with the control group, but BBR or PDTC treated
groups showed decreased ROS levels compared with the HG group
(Fig. 3G). The results suggested
that BBR reversed HG-induced effects on cell apoptosis and
oxidative stress in Müller cells.
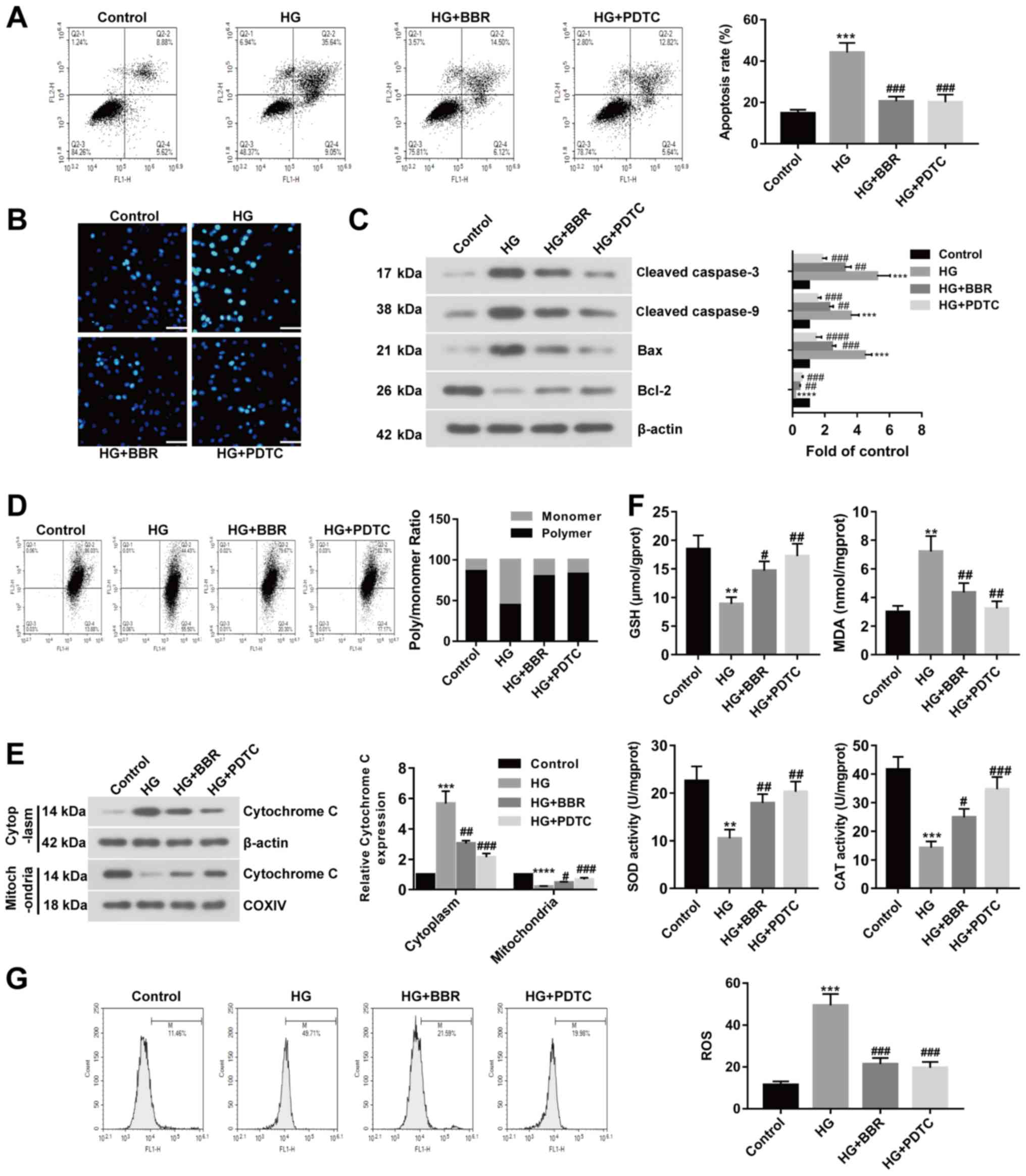 | Figure 3.BBR reverses HG-induced effects on
cell apoptosis and oxidative stress in Müller cells. PDTC is an
NF-κB inhibitor. Müller cells were treated with 5.5 mM glucose;
33.3 mM glucose; 33.3 mM glucose and 20 µM BBR; or 33.3 mM glucose
and 100 µM PDTC. Cell apoptosis was detected using. (A) Annexin
V-FITC apoptosis detection and (B) Hoechst staining (scale bar, 50
µm). (C) Protein expression was determined using western blotting.
(D) MMP was detected using the JC-1 assay. (E) Cytoplasmic and
nuclear expression levels of cytochrome c were detected using
western blotting. (F) GSH, MDA, SOD and CAT contents were measured
in treated Müller cells. (G) ROS levels were measured in treated
Müller cells. **P<0.01, ***P<0.001 and ****P<0.0001 vs.
the control group. #P<0.05, ##P<0.01
and ###P<0.001 vs. the HG group. BBR, berberine; HG,
high glucose; PDTC, pyrrolidine dithiocarbamate; MMP, mitochondrial
membrane potential; GSH, glutathione; MDA, malondialdehyde; SOD,
superoxide dismutase; CAT, catalase; COXIV, mitochondrial
cytochrome c oxidase subunit IV. |
BBR decreases cell apoptosis and
oxidative stress by deactivating the NF-κB signaling pathway
To investigate the association between NF-κB and
BBR, the expression of related proteins, including p-IκB (Ser32),
IκB, NF-κB (nuclear), NF-κB (cytoplasm), were assessed by western
blotting (Fig. 4A). The expression
levels of p-IκB (Ser32) and NF-κB (nuclear) were significantly
upregulated in HG-induced Müller cells compared with control cells.
However, BBR or PDTC treated Müller cells showed significantly
decreased expression levels of p-IκB (Ser32) and NF-κB (nuclear)
compared with the HG group. IκB and NF-κB (cytoplasm) expression
displayed the opposite trend (Fig.
4A). NF-κB translocation was determined using an
immunofluorescence assay, which indicated that BBR or PDTC
treatment inhibited NF-κB translocation and deactivated the NF-κB
signaling pathway in HG-treated cells (Fig. 4B). The results suggested that BBR
decreased cell apoptosis and oxidative stress by deactivating the
NF-κB signaling pathway.
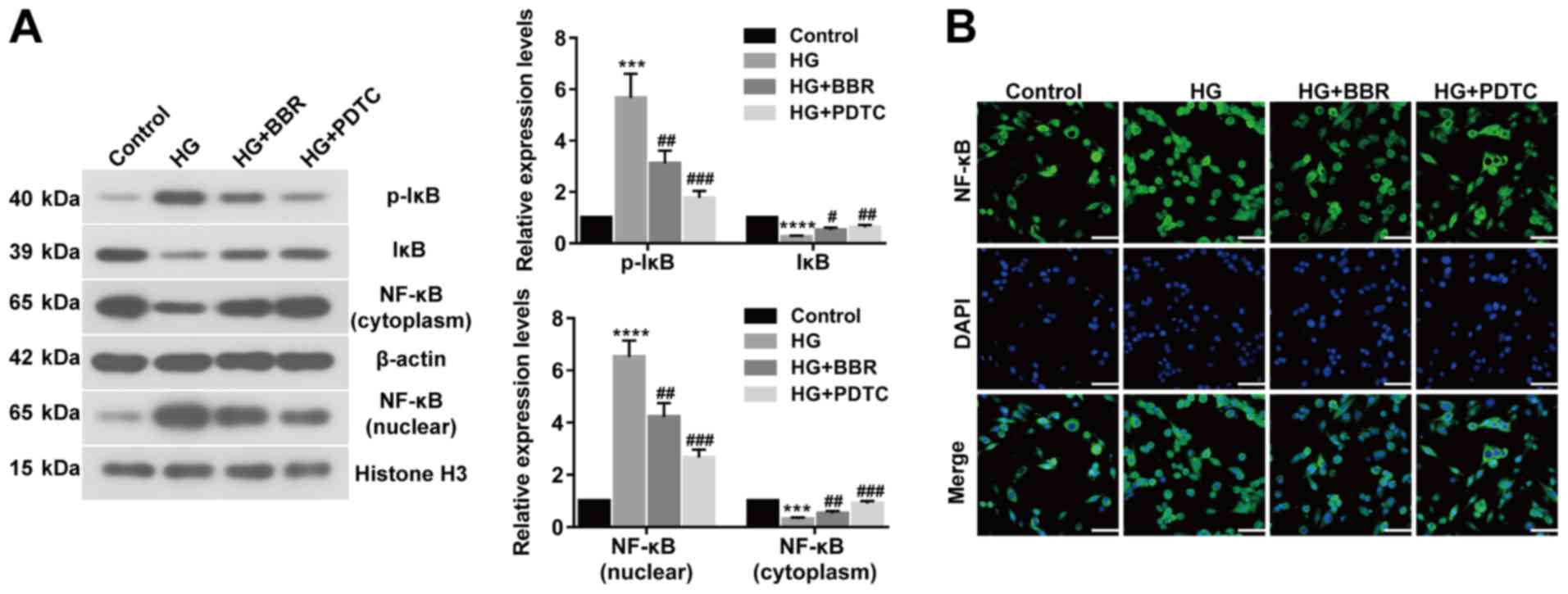 | Figure 4.BBR reduces cell apoptosis and
oxidative stress by deactivating the NF-κB signaling pathway. (A)
Expression levels of p-IκB (Ser32), IκB, NF-κB (nuclear), and NF-κB
(cytoplasm) were determined using western blotting. (B) NF-κB
nuclear translocation in Müller cells was detected using an
immunofluorescence assay (scale bar, 50 µm). ***P<0.001 and
****P<0.0001 vs. the control group. #P<0.05,
##P<0.01 and ###P<0.001 vs. the HG
group. BBR, berberine; p, phosphorylated; IκB, NF-κB inhibitor; HG,
high glucose; PDTC, pyrrolidine dithiocarbamate. |
Discussion
BBR is an extract of Rhizoma Coptidis that
displays therapeutic activities in several diseases, such as fatty
liver, type 2 diabetes and obesity (10,17).
DR is diagnosed in patients with diabetes and can lead to blindness
(1). Müller cell apoptosis affects
the retinal vasculature and neurons, which contributes to retinal
complications of diabetes, including DR (18). In the present study, a rat model of
DR was established and the results suggested that BBR decreased GCL
cell apoptosis, reduced diabetic-induced oxidative stress, and
deactivated the NF-κB signaling pathway. In vitro
experiments indicated that BBR reversed HG-induced effects on
oxidative stress and cell apoptosis in Müller cells by deactivating
the NF-κB signaling pathway. Collectively, in vivo and in
vitro results suggested that BBR protected against DR by
inhibiting cell apoptosis and reducing oxidative stress via
deactivation of the NF-κB signaling pathway.
BBR is a novel organic compound that displays
protective effects against multiple diseases (17). For example, BBR displays
antioxidant and anti-inflammatory properties, which provide
protective effects in neurodegenerative disorders (19). BBR also protects against breast
cancer by reducing EGFR and AKT phosphorylation (20). Liang et al (21) reported that BBR ameliorates acute
lung injury via the eukaryotic translation initiation factor 2α
kinase 3-mediated nuclear factor erythroid 2-like 2/heme oxygenase
1 signaling axis. BBR participates in the pathogenesis of several
diseases by regulating cell apoptosis and oxido-nitrosative stress.
BBR can partially suppress the apoptotic cascade and
oxido-nitrosative stress, reduce ischemia/reperfusion-induced
myocardial apoptosis, and inhibit inflammation and
mitochondria-dependent apoptosis in a rat model of diabetes
(19,22,23).
Furthermore, BBR mitigates HG-induced apoptosis of mouse podocytes
in vitro (24). Müller
cells form part of the macroglia of the retina, and BBR attenuates
apoptosis and injury of HG-induced Müller cells by enhancing
autophagy and the AMPK signaling pathway (11,25).
BBR can also serve as an inducer of apoptosis, which has been
reported in thyroid and hepatocellular carcinoma, as well as
colorectal cancer (26–28). Although BBR is a promising agent
with anti-carcinogenic activity, the pharmacological properties of
BBR also suggest that BBR has a therapeutic effect in diabetes,
nonalcoholic fatty liver disease, rheumatoid arthritis, Alzheimer's
disease and cardiovascular diseases (29). In vitro and in vivo
experiments suggested that BBR may mitigate diabetes-induced retina
cell apoptosis and oxidative stress, and have therapeutic effects
for DR. However, the mechanism underlying how BBR mitigates DR
requires further investigation.
In the present study, BBR alleviated HG-induced cell
apoptosis and oxidative stress in Müller cells by deactivating the
NF-κB signaling pathway. Furthermore, the expression of IκB was
decreased and p-IκB was increased in HG-induced Müller cells
compared with control cells. BBR or PDTC treatment reversed
HG-induced effects. The results suggested that BBR deactivated the
NF-κB signaling pathway by decreasing the phosphorylation of IκB,
resulting in abundant cytoplasmic IκB and inhibition of NF-κB
nuclear translocation. Li et al (28) reported that BBR inhibited the
proliferation of HepG2 cells by promoting apoptosis via the NF-κB
p65 signaling pathway. Collectively, the aforementioned results
suggest that BBR may serve as a potential therapeutic agent for
multiple diseases and different types of cancer; however the
underlying mechanisms of action may differ. Similarly, different
cell lines may be another reason for distinct interactions with the
NF-κB signaling pathway. Therefore, the protective effects of BBR
against diseases and different types of cancer requires further
investigation.
Oxidative stress is a vital factor for the
development of DR (29). Rat
models of DR display damaged retinal mitochondria that accelerate
apoptosis of retinal cells, and the regulation of oxidative stress
protects mitochondrial homeostasis and prevents the development of
DR (30). Similarly,
mitochondria-induced apoptosis and alterations to oxidative stress
were observed in the present study. The oxidation of
H2O2 leads to activation of the NF-κB
signaling pathway by the release of the inhibitory subunit of IκB
(31,32). In the present study, IκB expression
was decreased in HG-induced Müller cells compared with control
cells. BBR or PDTC treatment upregulates IκB expression by
inhibiting the phosphorylation of IκB. Furthermore, NF-κB nuclear
translocation was inhibited and the NF-κB signaling pathway was
deactivated in the DR group. DNA-binding subunits of NF-κB
containing redox-sensitive cysteine residues inhibit oxidation
activity (33). The deactivation
of the NF-κB signaling pathway reduces DNA-binding to NF-κB, which
inhibits oxidation activity and regulates oxidative stress,
resulting in decreased cell apoptosis (33). BBR displayed protective effects
against DR-induced cell apoptosis and oxidative stress; however,
the mechanisms underlying the effects of BBR in other signaling
pathways are not completely understood and require further
investigation.
To conclude, BBR protected against DR by inhibiting
cell apoptosis and oxidative stress via deactivation of the NF-κB
signaling pathway. The results of the present study may provide a
novel therapeutic strategy for DR.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81573746).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL designed the study and revised the manuscript.
JJZ and ZL performed the experiments, analyzed the data and wrote
the manuscript. HZ, LM, ZM, YZ, JZ, ML, LM and XW performed the
experiments and analyzed the data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Xijing Hospital, Air Force Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cheung N, Mitchell P and Wong TY: Diabetic
retinopathy. Lancet. 376:124–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Porojan MD, Cătană A, Popp RA, Dumitrascu
DL and Bala C: The role of NOS2A −954G/C and vascular endothelial
growth factor +936C/T polymorphisms in type 2 diabetes mellitus and
diabetic nonproliferative retinopathy risk management. Ther Clin
Risk Manag. 11:1743–1748. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao C, Wang W, Xu D, Li H, Li M and Wang
F: Insulin and risk of diabetic retinopathy in patients with type 2
diabetes mellitus: Data from a meta-analysis of seven cohort
studies. Diagn Pathol. 9:1302014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toft-Kehler AK, Gurubaran IS, Desler C,
Rasmussen LJ, Skytt DM and Kolko M: Oxidative stress-induced
dysfunction of muller cells during starvation. Invest Ophthalmol
Vis Sci. 57:2721–2728. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He XF, Zhang L, Zhang CH, Zhao CR, Li H,
Zhang LF, Tian GF, Guo MF, Dai Z and Sui FG: Berberine alleviates
oxidative stress in rats with osteoporosis through receptor
activator of NF-kB/receptor activator of NF-kB
ligand/osteoprotegerin (RANK/RANKL/OPG) pathway. Bosn J Basic Med
Sci. 17:295–301. 2017.PubMed/NCBI
|
|
6
|
Kowluru RA, Kowluru A, Veluthakal R,
Mohammad G, Syed I, Santos JM and Mishra M: TIAM1-RAC1 signalling
axis-mediated activation of NADPH oxidase-2 initiates mitochondrial
damage in the development of diabetic retinopathy. Diabetologia.
57:1047–1056. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun Y, Yuan X, Zhang F, Han Y, Chang X, Xu
X, Li Y and Gao X: Berberine ameliorates fatty acid-induced
oxidative stress in human hepatoma cells. Sci Rep. 7:113402017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sadeghnia HR, Kolangikhah M, Asadpour E,
Forouzanfar F and Hosseinzadeh H: Berberine protects against
glutamate-induced oxidative stress and apoptosis in PC12 and N2a
cells. Iran J Basic Med Sci. 20:594–603. 2017.PubMed/NCBI
|
|
9
|
Hsu YY, Chen CS, Wu SN, Jong YJ and Lo YC:
Berberine activates Nrf2 nuclear translocation and protects against
oxidative damage via a phosphatidylinositol 3-kinase/Akt-dependent
mechanism in NSC34 motor neuron-like cells. Eur J Pharm Sci.
46:415–425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lan J, Zhao Y, Dong F, Yan Z, Zheng W, Fan
J and Sun G: Meta-analysis of the effect and safety of berberine in
the treatment of type 2 diabetes mellitus, hyperlipemia and
hypertension. J Ethnopharmacol. 161:69–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fu D, Yu JY, Connell AR, Yang S, Hookham
MB, McLeese R and Lyons TJ: Beneficial effects of berberine on
oxidized LDL-induced cytotoxicity to human retinal muller cells.
Invest Ophthalmol Vis Sci. 57:3369–3379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian P, Ge H, Liu H, Kern TS, Du L, Guan
L, Su S and Liu P: Leukocytes from diabetic patients kill retinal
endothelial cells: Effects of berberine. Mol Vis. 19:2092–2105.
2013.PubMed/NCBI
|
|
13
|
Song D, Song J, Wang C, Li Y and Dunaief
JL: Berberine protects against light-induced photoreceptor
degeneration in the mouse retina. Exp Eye Res. 145:1–9. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kronfeld PC: The association for research
in vision and ophthalmology. Am J Ophthalmol. 76:305–306. 1973.
View Article : Google Scholar
|
|
15
|
National Research Council, . Guide for the
care and use of laboratory animals. (Eighth edition). Publication
No. 85–23(rev.) 327. 963–965. 2010.
|
|
16
|
Coughlin BA, Feenstra DJ and Mohr S:
Müller cells and diabetic retinopathy. Vision Res. 139:93–100.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Y, Xia M, Yan H, Han Y, Zhang F, Hu Z,
Cui A, Ma F, Liu Z, Gong Q, et al: Berberine attenuates hepatic
steatosis and enhances energy expenditure in mice by inducing
autophagy and fibroblast growth factor 21. Br J Pharmacol.
175:374–387. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cuenca N, Fernández-Sánchez L, Campello L,
Maneu V, De la Villa P, Lax P and Pinilla I: Cellular responses
following retinal injuries and therapeutic approaches for
neurodegenerative diseases. Prog Retin Eye Res. 43:17–75. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sadraie S, Kiasalari Z, Razavian M, Azimi
S, Sedighnejad L, Afshin-Majd S, Baluchnejadmojarad T and Roghani
M: Berberine ameliorates lipopolysaccharide-induced learning and
memory deficit in the rat: Insights into underlying molecular
mechanisms. Metab Brain Dis. 34:245–255. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jabbarzadeh Kaboli P, Leong MP, Ismail P
and Ling KH: Antitumor effects of berberine against EGFR, ERK1/2,
P38 and AKT in MDA-MB231 and MCF-7 breast cancer cells using
molecular modelling and in vitro study. Pharmacol Rep. 71:13–23.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang Y, Fan C, Yan X, Lu X, Jiang H, Di
S, Ma Z, Feng Y, Zhang Z, Feng P, et al: Berberine ameliorates
lipopolysaccharide-induced acute lung injury via the PERK-mediated
Nrf2/HO-1 signaling axis. Phytother Res. 33:130–148. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen K, Li G, Geng F, Zhang Z, Li J, Yang
M, Dong L and Gao F: Berberine reduces ischemia/reperfusion-induced
myocardial apoptosis via activating AMPK and PI3K-Akt signaling in
diabetic rats. Apoptosis. 19:946–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu L, Zheng X, Wang Y, Fan Q, Zhang M, Li
R, Ye J, Wu X, Zhao W and Zhang Y: Berberine protects acute liver
failure in mice through inhibiting inflammation and
mitochondria-dependent apoptosis. Eur J Pharmacol. 819:161–168.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin Y, Liu S, Ma Q, Xiao D and Chen L:
Berberine enhances the AMPK activation and autophagy and mitigates
high glucose-induced apoptosis of mouse podocytes. Eur J Pharmacol.
794:106–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen H, Ji Y, Yan X, Su G, Chen L and Xiao
J: Berberine attenuates apoptosis in rat retinal Müller cells
stimulated with high glucose via enhancing autophagy and the
AMPK/mTOR signaling. Biomed Pharmacother. 108:1201–1207. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li L, Wang X, Sharvan R, Gao J and Qu S:
Berberine could inhibit thyroid carcinoma cells by inducing
mitochondrial apoptosis, G0/G1 cell cycle arrest and suppressing
migration via PI3K-AKT and MAPK signaling pathways. Biomed
Pharmacother. 95:1225–1231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dai W, Mu L, Cui Y, Li Y, Chen P, Xie H
and Wang X: Berberine promotes apoptosis of colorectal cancer via
regulation of the long non-coding RNA (lncRNA) cancer
susceptibility candidate 2 (CASC2)/AU-binding factor 1
(AUF1)/B-cell CLL/lymphoma 2 (Bcl-2) axis. Med Sci Monit.
25:730–738. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li M, Zhang M, Zhang ZL, Liu N, Han XY,
Liu QC, Deng WJ and Liao CX: Induction of apoptosis by berberine in
hepatocellular carcinoma HepG2 cells via downregulation of NF-κB.
Oncol Res. 25:233–239. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brownlee M: The pathobiology of diabetic
complications: A unifying mechanism. Diabetes. 54:1615–1625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kowluru RA and Shan Y: Role of oxidative
stress in epigenetic modification of MMP-9 promoter in the
development of diabetic retinopathy. Graefes Arch Clin Exp
Ophthalmol. 255:955–962. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sies H: Hydrogen peroxide as a central
redox signaling molecule in physiological oxidative stress:
Oxidative eustress. Redox Biol. 11:613–619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schreck R, Rieber P and Baeuerle PA:
Reactive oxygen intermediates as apparently widely used messengers
in the activation of the NF-kappa B transcription factor and HIV-1.
EMBO J. 10:2247–2258. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Toledano MB and Leonard WJ: Modulation of
transcription factor NF-kappa B binding activity by
oxidation-reduction in vitro. Proc Natl Acad Sci USA. 88:4328–4332.
1991. View Article : Google Scholar : PubMed/NCBI
|