Introduction
Hypertension, which promotes the occurrence of
cerebral venous dysfunction-mediated brain tissue damage, such as
venous sinus thrombosis, venous cerebral infarction, venous
cerebral hemorrhage, periventricular venous disease, venous
neurocytotoxicity, neurodegeneration and demyelination, is one of
the primary risk factors of cerebrovascular disease (1–4).
Vascular remodeling has been shown to be important for the
progression of hypertension-mediated cerebrovascular disease
(5). Under hypertensive
conditions, blood vessels undergo a series of structural and
functional changes to develop and maintain high blood pressure,
which leads to hemiplegia, epilepsy and dementia, thus seriously
affecting the health of the patient (6). Therefore, understanding the
underlying mechanism of vascular remodeling will aid in the
prevention of hypertension-mediated cerebral venous disease.
Caveolin-1 (cav-1) is a primary structural protein
and a regulatory component of caveolae. It participates in various
physiological processes, such as vesicle transport, membrane
phospholipid metabolism, cholesterol transport and cell signaling
(7). Aberrant expression of cav-1
is closely associated with the occurrence of various diseases,
including cardiovascular diseases, lung injury, tumorigenesis and
infectious diseases (8–11). Cav-1 regulates its activity through
complementary binding with other signal molecules and thus,
modulates physiological processes, such as cell proliferation and
differentiation, via different signaling pathways. Increasing
evidence has indicated that cav-1 may be implicated in the
development of hypertension. It has been reported that, in mice,
cav-1 acted on inflammation and vascular remodeling, independent of
the regulation of blood pressure or cardiac hypertrophy (12). Another study demonstrated that the
upregulation of cav-1 and caveolae increased agonist-induced
production of pulmonary arteries via the modulation of
store-operated Ca(2+) entry and receptor-operated
calcium entry, contributing to the progression of pulmonary
hypertension in rats (13).
However, the detailed mechanism of cav-1 in Ang-II-induced
hypertension remains largely unknown.
The Notch pathway has been shown to play a crucial
role in vascular development (14). The activation of the Notch
signaling pathway is mediated by the binding of cellular surface
receptors (Notch1-4) to their ligands, which results in the
cleavage of the intracellular domain of the receptors.
Subsequently, the intracellular domain enters the nucleus where it
promotes the transcription of downstream effectors (15). Previous studies reported that
Notch1 signaling is implicated in the progression of pulmonary
hypertension, but the function and mechanism of Notch1 in
Angiotensin II (Ang-II)-induced hypertension remains unclear
(16–18).
The Ang-II-induced hypertension model is commonly
used in studies on hypertension (12,19).
In the present study, the Ang-II-induced hypertension model was
successfully established in rats and it was found that the
expression of cav-1 and Notch1 were significantly upregulated in
the brain tissues of hypertensive rats. Moreover, the present data
demonstrated that cav-1 modulated hypertensive vascular remodeling
by regulating the Notch signaling pathway. These findings may
provide novel therapeutic candidate targets for the treatment of
hypertension.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
purchased from the American Type Culture Collection and were
cultured in endothelial cell medium supplemented with 10% fetal
bovine serum (FBS) and 1% penicillin/streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C with 5% CO2. HUVECs
were treated with 5 ng/ml Ang-II (Sigma-Aldrich; Merck KGaA). A
total of 24 h later, cells were harvested and used for further
experiments.
Cell treatment
Cells were treated with 20 µM DAPT
(N-[N-(3,5-difluorophenacetyl)-1-alanyl]- S-phenylglycine t-butyl
ester) (Sigma-Aldrich; Merck KGaA), a Notch pathway inhibitor, for
24 h at 37°C.
Cell Counting Kit-8 (CCK-8) assay
Cells (5×103) were seeded in 96-well
plates. After treatment with Ang-II (5 ng/ml) for 24 h, cell
viability was determined with a CCK-8 assay (Beyotime Institute of
Biotechnology), according to the manufacturer's instructions.
Apoptosis detection
Apoptosis analysis was performed using a
FITC-Annexin V apoptosis detection kit (BD Biosciences). Cells
(1×106) were harvested and washed once with PBS. Then,
cells were incubated with propidium iodide and FITC-Annexin V for
15 min at 4°C. After washing with PBS three times, cells were
suspended in binding buffer and analyzed using a CytoFLEX flow
cytometer (Beckman Coulter, Inc.) and FlowJo software (version 10;
FlowJo LLC). Early + late apoptosis was assessed.
Establishment of hypertension
model
A total of 12 female Sprague-Dawley (SD) rats (age,
6 weeks; weight, 153±12.4 g) were randomly divided into two groups:
Hypertension model group (n=6) and sham operation group (sham
group, n=6). One rat in the hypertension model group died, the
cause of death was cerebral infarction, it was found with symptoms
of hemiparalysis and the dissection was performed after death. The
duration of the animal experiment was 1 month. Animal health and
behavior were monitored once a day post-surgery. All the animals
were kept in the specific-pathogen-free animal laboratory at 24±1°C
with 50±10% humidity, 12-h light/dark cycles, and free access to
food and water. The weight of the SD rats was measured before
surgery. Rats were anesthetized with pentobarbital sodium (30
mg/kg). Under sterile conditions, an incision was made in the mid
scapular region and an osmotic pump containing angiotensin II
(infusion rate, 0.7 mg/kg per day) was implanted. Blood pressure
was measured once before surgery, and 1 week and 3 weeks after
surgery. Sham-operated rats underwent the same surgical procedure
after anesthesia, except that no osmotic pump was implanted. Rats
were euthanized by pentobarbital sodium (150 mg/kg), and the heart
rates were detected to verify death. All experimental protocols
were approved by the Committee of Animal Care and Use at Sun
Yat-sen University (approval no. SYSU-IACUC-2019PGY260K; Guangdong,
China).
Western blotting
Protein from cells or tissues were isolated and
lysed in RIPA lysis buffer (Sigma-Aldrich; Merck KGaA), containing
protease inhibitors (Sigma-Aldrich; Merck KGaA). Protein
concentration was determined by the Braford method (BioRad
Laboratories, Inc.). Proteins (50 µg) were separated via SDS-PAGE
on a 10% gel, and subsequently transferred onto PVDF membranes. The
membrane was blocked with 5% non-fat milk for 1 h at room
temperature, and then incubated overnight at 4°C with anti-collagen
(COL)-I (cat. no. ab260043), anti-matrix metalloprotease (MMP)2
(cat. no. ab92536), anti-MMP9 (cat. no. ab38898), anti-cav-1 (cat.
no. ab18199), anti-Notch1 (cat. no. ab167441) and GAPDH (cat. no.
ab9485) antibodies (all 1:1,000; all purchased from Abcam).
Subsequently, the membrane was incubated with a HRP-conjugated goat
anti-rabbit IgG H&L secondary antibody (1:5,000; cat. no.
ab6721; Abcam) for 2 h at room temperature. The proteins were
detected by ECL chemiluminescence (EMD Millipore) and analyzed
using ImagePro Plus software (version 6.0; Media Cybernetics,
Inc.).
Small interfering (si)RNA
transfection
siRNAs (si-1, si-2, and si-3) targeting cav-1 and
control siRNA were synthesized by Shanghai GenePharma Co., Ltd.
siRNA transfection (50 nmol) was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Then, 48 h post-transfection, cells were
collected and used for further experiments.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA from cells and tissues was isolated using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RNA was reverse-transcribed into cDNA using a RevertAid
First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.).
The following temperature protocol was used for reverse
transcription: 37°C for 2 min, 23°C for 10 min, 55°C for 10 min and
85°C for 10 min. RT-qPCR was conducted on a PRISM 7500 System
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with
SYBR-Green (Tiangen Biotech Co., Ltd.). The following thermocycling
conditions were used for qPCR: 95°C for 5 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 1 min. The expression was
calculated using the 2−ΔΔCq method (20). GAPDH was used as a control. Primers
used are shown in Table I.
 | Table I.Primer sequences used for reverse
transcription- quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription- quantitative PCR.
| Genes | Primer sequences
(5′→3′) |
|---|
| Caveolin-1 | F:
GCTGAGCGAGAAGCAAGTGT |
|
| R:
GGTGAAGCTGGCCTTCCAAA |
| Notch1 | F:
AATGTGGATGCCGCAGTTGT |
|
| R:
TGATGTCCCGGTTGGCAAAG |
| MMP2 | F:
CCCATGAAGCCCTGTTCACC |
|
| R:
CGGTCGTAGTCCTCAGTGGT |
| MMP9 | F:
GACGGCAATGCTGATGGGAA |
|
|
R:GCAGAAGCCGAAGAGCTTGT |
| COL-1 | F:
CCAGTGTGGCCCAGAAGAAC |
|
| R:
GCAGGAAGGTCAGCTGGATG |
| β-actin | F:
CGTAAAGACCTCTATGCCAACA |
|
| R:
CGGACTCATCGTACTCCTGCT |
Immunofluorescence staining
Tissues were fixed with 4% paraformaldehyde
overnight at room temperature. Paraffin-embedded tissue slices
(thickness, 4 µm) were dewaxed and rehydrated. Slices were blocked
with 10% goat serum (Thermo Fisher Scientific, Inc.) for 30 min at
room temperature, and then incubated with anti-cav-1 (1:1,000; cat.
no. ab17052; Abcam) at 4°C overnight. After washing with PBS,
slices were incubated with Alexa 555-conjugated secondary antibody
(1:5,000, cat. no. A28180; Thermo Fisher Scientific, Inc.) for 2 h
at room temperature. Diamidino-phenyl-indole (DAPI) was utilized to
stain the nuclei. Images were obtained using a fluorescence
microscope (Nikon Corporation) and analysed using ImagePro Plus
software (version 6; Media Cybernetics, Inc.).
Transmission electron microscopy
To observe the alteration of the cerebral vein
following the induction of hypertension, rat brain tissues were
sliced into ~1 mm3 and the white-matter region with
small veins were incubated in 2.5% glutaraldehyde for 12 h at 4°C.
After washing with PBS, tissues were fixed with 1% osmic acid for 3
h at 4°C. Tissues were dehydrated and embedded in epoxy resin for 4
h at room temperature. Then, the tissue was cut into 70-nm
sections. After staining with uranyl acetate at room temperature
for 2 h and lead citrate at room temperature for 15 min, tissues
were observed using a transmission electron microscope (Leica
Microsystems, Inc.).
Statistical analysis
Statistical analysis was carried out using SPSS 19.0
software (IBM Corp.). Comparisons were analyzed by one-way ANOVA
and followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Ang-II infusion induces hypertension
in rats
In order to understand the molecular mechanism of
cav-1 in the pathogenesis of hypertension, an Ang-II-induced
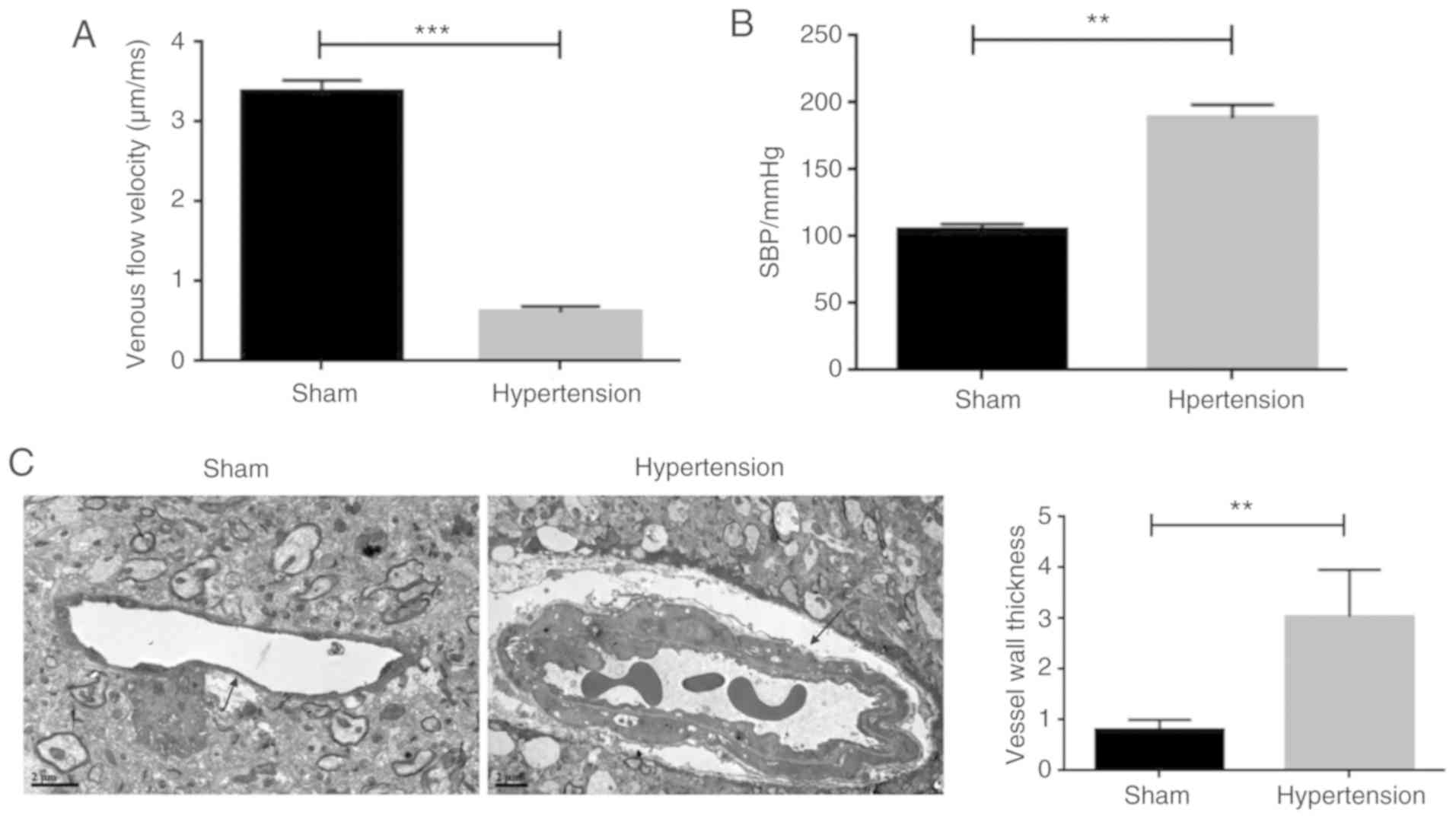
hypertension model was established in rats. As shown in Fig. 1A, rats infused with Ang-II (the
hypertension group) showed a significantly lower venous flow
velocity compared with the sham-operated rats. In contrast, the
blood pressure in the hypertension group was significantly higher
compared with the sham-operated group (Fig. 1B). Moreover, the thickness of the
vessel walls was significantly higher compared with the
sham-operated animals (Fig. 1C).
Together, these results suggested that the rat hypertension model
induced by Ang-II was successfully established.
Cav-1 and Notch1 are upregulated after
Ang-II infusion
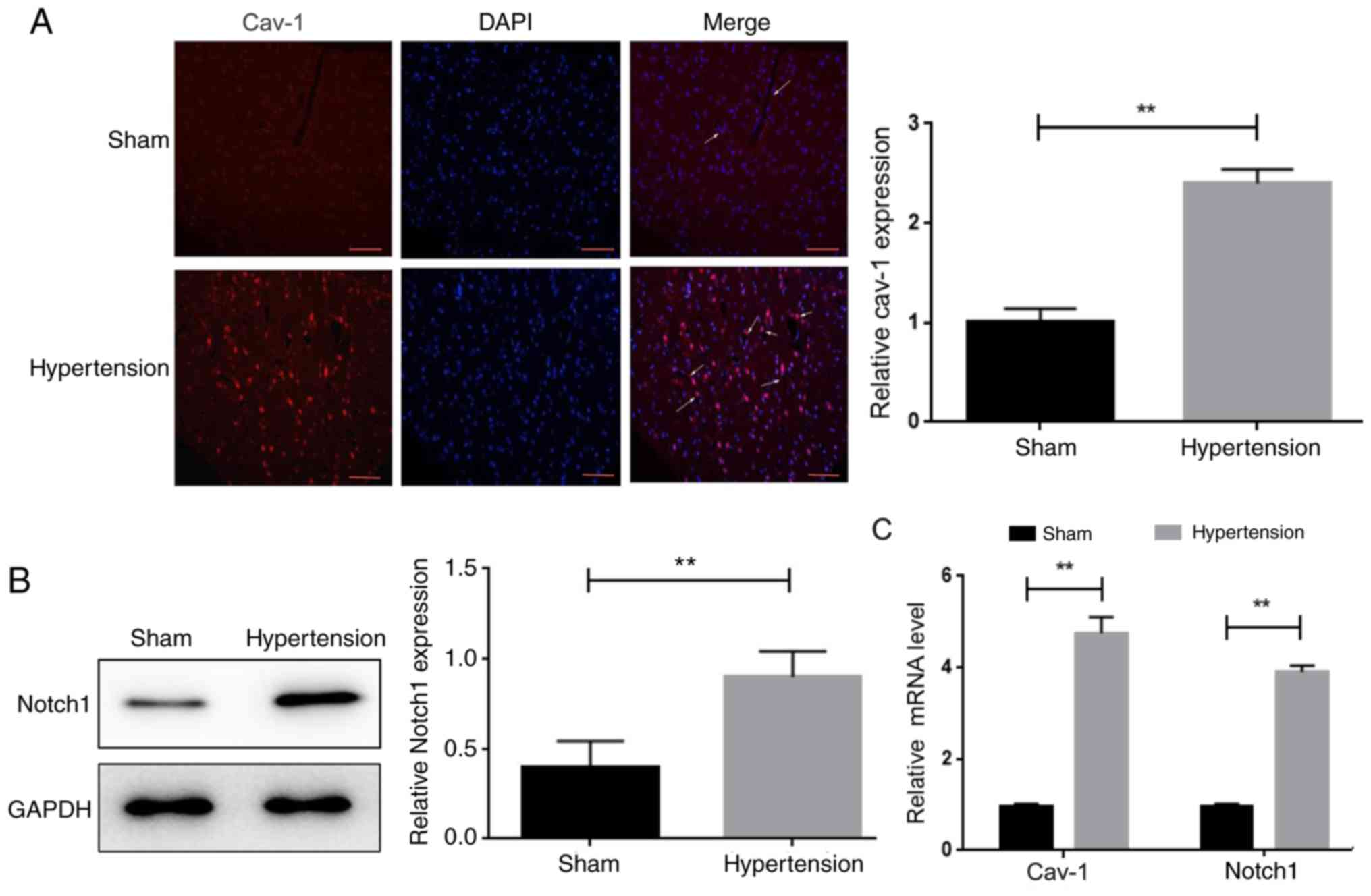
The expression levels of cav-1 and Notch1 were
examined in rat brain tissues. The protein levels of cav-1 and
Notch1 were significantly increased in the brain tissue of the
hypertension group compared with the sham-operated group, as
characterized by immunofluorescence staining and western blotting,
respectively (Fig. 2A and B).
Consistent with these results, RT-qPCR analysis showed that the
mRNA levels of cav-1 and Notch1 in the brain tissues of
hypertensive rats were significantly higher compared with the
sham-operated animals (Fig. 2C).
Also, the mRNA expression of Notch3 was examined in the two groups,
but there was no significant difference between them (Fig. S1).
Ang-II regulates HUVEC viability and
extracellular matrix-related genes
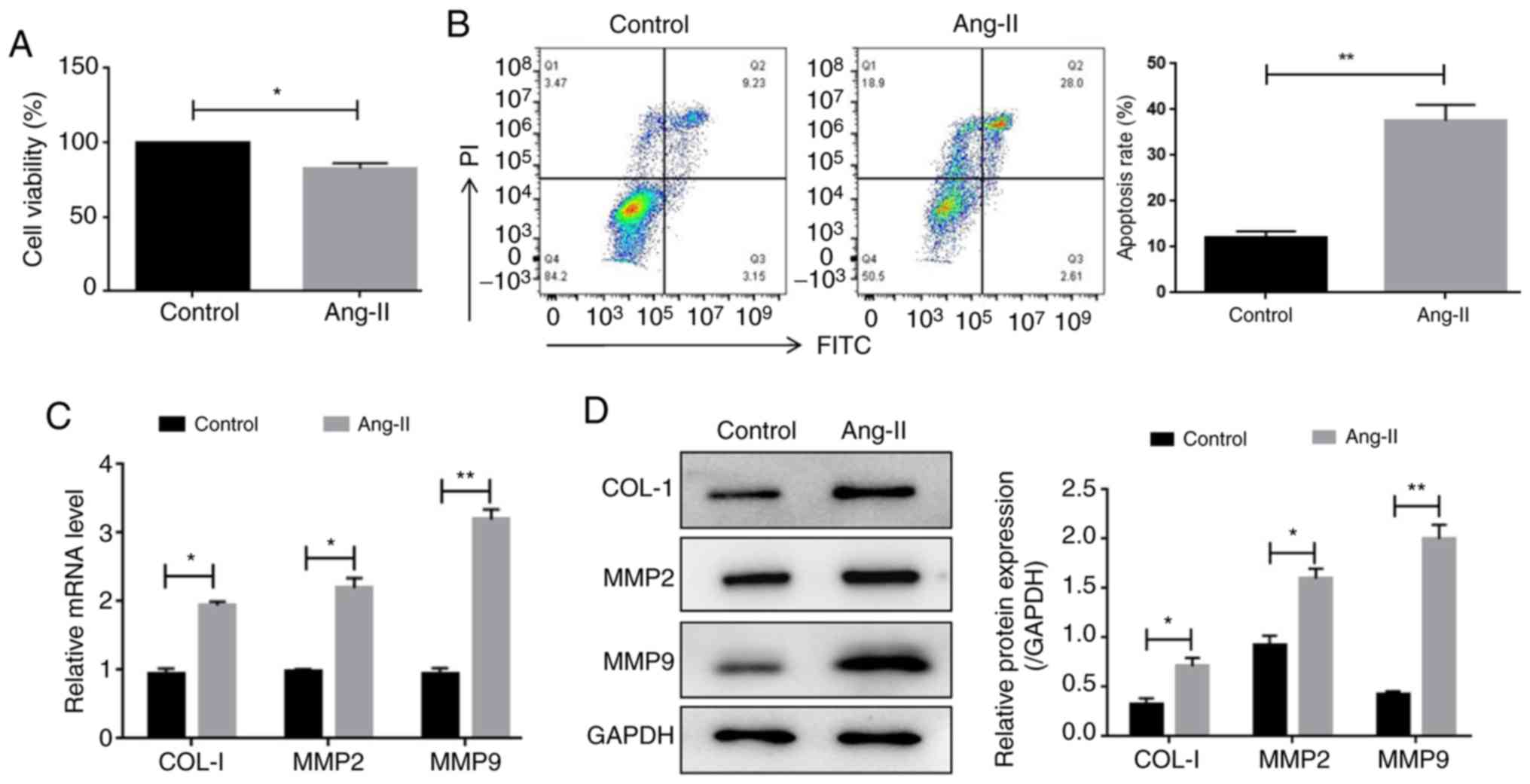
To provide further support for the in vivo
results, cultured HUVECs were utilized. A CCK-8 assay was carried
out to determine the effect of Ang-II on HUVEC viability. The
results showed that the viability of Ang-II-treated HUVECs was
significantly decreased compared with the control cells (Fig. 3A). A flow cytometry assay revealed
that Ang-II exposure significantly increased the apoptotic rate of
HUVECs (Fig. 3B). In addition,
RT-qPCR and western blotting demonstrated that Ang-II treatment
significantly increased the mRNA and protein levels of collagen-1,
MMP2 and MMP9, which are important regulators of the extracellular
matrix (Fig. 3C and D). Therefore,
these results indicated that Ang-II regulated HUVEC viability and
altered extracellular matrix-associated protein expression.
Cav-1 modulates HUVEC viability and
the extracellular matrix
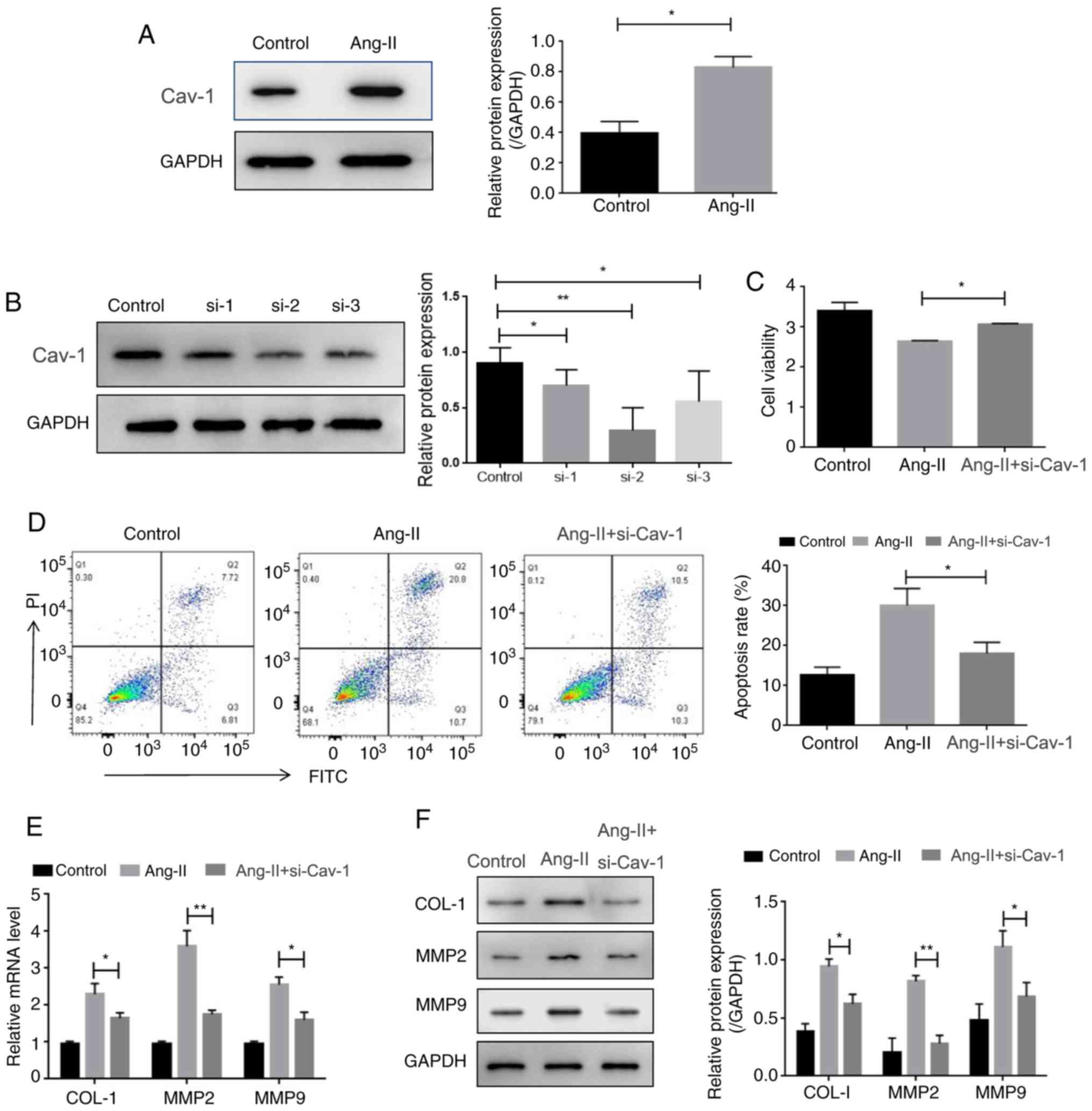
Increased expression of cav-1 was also observed in
HUVECs, following the administration of Ang-II (Fig. 4A). Thus, it was investigated
whether cav-1 might contribute to Ang-II-mediated HUVEC cell
viability and extracellular matrix alteration. To assess this,
cav-1 expression was knocked down by siRNAs. As shown in Fig. 4B, the expression levels of cav-1
were significantly downregulated after transfection with cav-1
siRNAs (si-1, si-2, and si-3), as si-2 was shown to be the most
effective it was selected for further experiments. It was found
that the silencing of cav-1 restored the Ang-II-inhibited viability
of HUVECs (Fig. 4C) Additionally,
the enhancement of apoptosis in HUVECs induced by Ang-II was also
reversed by cav-1 knockdown (Fig.
4D). Moreover, the depletion of cav-1 downregulated the mRNA
and protein levels of collagen-1, MMP2 and MMP9, which were
increased by Ang-II (Fig. 4E and
F). Together, these results suggested that cav-1 reversed
Ang-II-induced viability and extracellular matrix alteration in
HUVECs.
Cav-1 modulates HUVEC viability and
the extracellular matrix via Notch signaling
The upregulation of Notch1 in Ang-II-infused mice
allowed for the investigation of whether cav-1 may regulate
vascular remodeling via Notch signaling. Indeed, consistent with
the in vivo results, treatment with Ang-II increased the
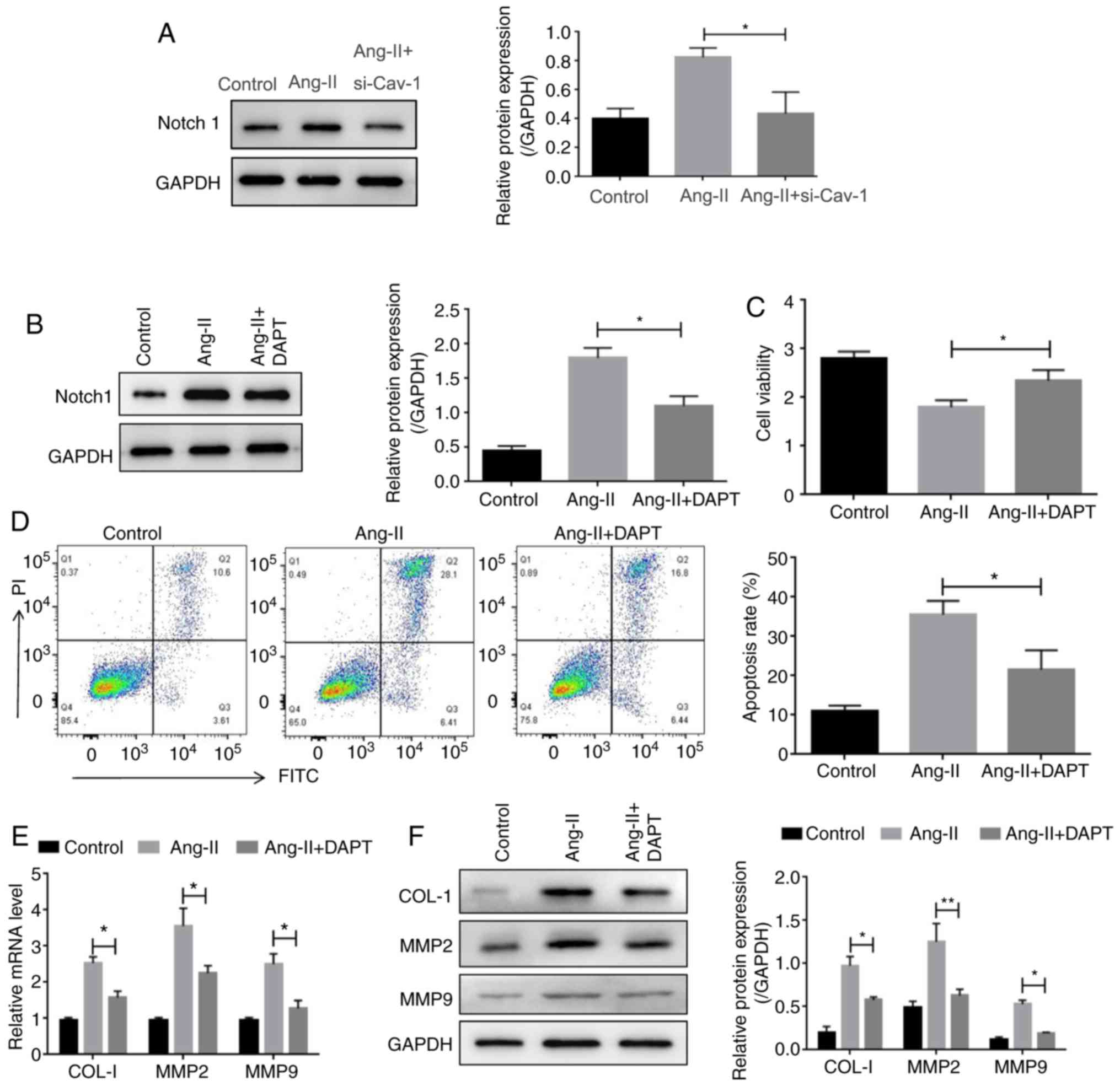
expression of Notch1, whereas knockdown of cav-1 decreased the
protein expression of Notch1 (Fig.
5A). To confirm the involvement of Notch signaling activation
in vascular remodeling, the Notch pathway inhibitor, DAPT
(N-[N-(3,5-difluorophenacetyl)-1-alanyl]-S-phenylglycine t-butyl
ester), was applied, which was demonstrated to decrease the
expression of Notch1 (Fig. 5B). It
was observed that while Ang-II treatment decreased the viability of
HUVECs, DAPT treatment reversed this effect (Fig. 5C). Consistently, the increased
apoptosis observed in Ang-II-treated HUVECs was decreased following
DAPT administration (Fig. 5D).
Moreover, treatment with DAPT reversed the Ang-II-mediated
upregulation of Notch1, collagen-1, MMP2 and MMP9 (Fig. 5E and F). Overall, these results
implied that cav-1/Notch functions in vascular remodeling.
Discussion
In the present study, a hypertension model induced
by infusion of Ang-II was constructed. Using this model, it was
found that cav-1 and Notch1 expression levels were significantly
increased in the brain tissues of hypertensive rats compared with
control animals. In cultured HUVECs, it was found that knockdown of
cav-1 promoted Ang-II-induced HUVEC viability and altered
hypertensive vascular remodeling. Furthermore, the data
demonstrated that cav-1 exerted its function via regulation of the
Notch pathway. Therefore, the present findings revealed a novel
mechanism of cav-1/Notch in hypertension.
Multiple studies have suggested that cav-1 is
implicated in different models of hypertension. For example, it has
been reported that the depletion of C-C motif chemokine 5 rescued
pulmonary vascular dysfunction and reversed pulmonary hypertension
via activation of bone morphogenetic protein receptor type 2 by
cav-1 (21). Fluvastatin protected
against monocrotaline-induced pulmonary arterial hypertension via
the downregulation of cav-1 in rats (22). In addition, cav-1 participated in
the regulation of vascular remodeling. The upregulation of cav-1
decreased cavin-1 expression and promoted the viability and
migration of vascular smooth muscle cells (VSMCs) in balloon
injury-induced neointimal hyperplasia (23). It was also found that cav-1
deficiency prevented Ang II-mediated hypertrophy and inward
remodeling of pial arterioles, which indicated that cav-1 promoted
Ang-II-mediated hypertrophy and vascular remodeling (24). Furthermore, another study reported
that the overexpression of cav-1 in mice enhanced medial
hypertrophy and perivascular fibrosis of the aorta, and its
coronary and renal arteries, and that cav-1 promoted hypertensive
vascular remodeling (12). The
results of the present study were consistent with previous studies.
It was observed that cav-1 was increased in the brain tissue of
Ang-II-infused hypertensive rats. Moreover, cav-1 depletion
reversed the effects of Ang-II on HUVEC viability and apoptosis.
Additionally, the knockdown of cav-1 decreased the expression
levels of collagen-1, MMP2 and MMP9, which are critical factors
involved in promoting vascular remodeling. Therefore, the present
data indicated a crucial role for cav-1 in vascular remodeling,
which may further contribute to hypertension.
Previous studies have reported that, in coronary
microvascular remodeling, the administration of Ang-II antagonists
in rats increased caveolin-1 protein expression, and nitric oxide
synthase (NOS) 3 gene and protein expression, as well as NOS
activity, thereby reducing collagen expression in the vessel walls
and suppressing vascular remodeling (25). The difference in the results of
Ang-II-mediated cav-1 protein expression and the effects on
vascular remodeling among different studies may be the result of
studying different tissues, and the involvement of additional
signaling modules. Thus, further research is required.
The Notch signaling pathway is a critical regulator
in the occurrence and development of the vascular system (26). Notch belongs to the membrane
protein receptor family. Notch1 and Notch4 are expressed in
endothelial cells, whereas Notch1 and Notch3 are expressed in VSMCs
(27,28). Notch activation regulates
angiogenesis by inhibiting vascular endothelial growth factor
receptors (VEGFR) and limiting the amount of vascular sprouting
(28). It has been reported that
treatment with DAPT attenuated intimal hyperplasia by inhibiting
Notch1 signaling (29). Notch
receptors 1 and 3 have been demonstrated to play important roles in
the proliferation and migration of VSMCs, and the secretion of MMP2
and MMP9 (30,31), which have roles in the modulation
of the degradation of the extracellular matrix and the destruction
of vascular wall matrix. Previous studies also suggested that the
Notch pathway is involved in the development of hypertension. It
has been reported that TNF-α triggered pulmonary arterial
hypertension by repressing the bone morphogenetic type-II receptor
and modulating the Notch pathway (32). Notch was revealed to regulate the
Ca2+-sensing receptor, and thus promoted hypoxia-induced
pulmonary hypertension (33). In
the present study, it was found that Notch1 expression was elevated
in the brain tissues of Ang-II-induced hypertensive rats compared
with sham-operated rats. The silencing of cav-1 decreased the
expression of Notch1 induced by Ang-II. Moreover, treatment with
DAPT reversed the effects of Ang-II on cell viability and
apoptosis. Furthermore, DAPT decreased the Ang-II-induced
upregulation of collagen-1, MMP2 and MMP9 expression. These results
indicated that cav-1/Notch signaling contributed to Ang-II-induced
vascular remodeling.
Of note, a limitation of the present study was that
the alteration of cav-1 in hypertensive rats was determined using
brain tissue instead of cerebrovascular tissue. This was because
after the rats were sacrificed, the cerebral veins started
shrinking and thus it was difficult to separate them from the brain
tissue.
In summary, the present data demonstrated an
important role for cav-1/Notch1 signaling in the regulation of
Ang-II-induced hypertension and vascular remodeling. Targeting the
cav-1 or Notch signaling pathways may present promising strategies
for the treatment of hypertension. The process of vascular
remodeling includes degradation of the extracellular matrix, cell
proliferation, apoptosis, vascular inflammation and fibrosis.
Whether additional signaling molecules, such as EPH receptor 4,
VEGF and epidermal growth factor receptors, are involved in the
process, and how important the signaling pathway is in vivo
in animals, will be investigated further in future research.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The study was supported by grants from: National
Natural Science Foundation of China (NSFC) (grant no. 81671153);
the Natural Science Foundation of Guangdong Province, China (grant
no. 2016A030313203); Guangdong Provincial Key Laboratory for
Diagnosis and Treatment of Major Neurological Diseases (grant no.
2017B030314103); The Southern China International Cooperation Base
for Early Intervention and Functional Rehabilitation of
Neurological Diseases (grant no. 2015B050501003); Guangdong
Provincial Engineering Center for Major Neurological Disease
Treatment; and Guangdong Provincial Translational Medicine
Innovation Platform for Diagnosis and Treatment of Major
Neurological Disease.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW designed and performed the experiments, analyzed
the data and wrote the manuscript. ML conducted the experiments. ZX
and MD performed some of the experiments. SG and LL participated in
the experimental design, provided financial support and supervised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Committee of Animal Care and Use at Sun Yat-Sen University
(Guangdong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Ang-II
|
angiotensin II
|
|
DAPT
|
N-[N-(3,5-
difluorophenacetyl)-1-alanyl]-S-phenylglycine t-butyl ester
|
|
VSMCs
|
vascular smooth muscle cells
|
|
VEGFR
|
vascular endothelial growth factor
receptors
|
References
|
1
|
Matsubara T, Ayuzawa S, Aoki T, Ikeda G,
Shiigai M and Matsumura A: Cerebral venous thrombosis after
ventriculoperitoneal shunting: A case report. Neurol Med Chir
(Tokyo). 54:554–557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Binnani P, Bahadur MM and Dalal K: Dural
sinus thrombosis-a rare manifestation of internal jugular venous
occlusion. Saudi J Kidney Dis Transpl. 23:799–803. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Albano B, Gandolfo C and Del Sette M:
Post-coital intra-cerebral venous hemorrhage in a 78-year-old man
with jugular valve incompetence: A case report. J Med Case Rep.
4:2252010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chung SW, Hwang SN, Min BK, Kwon JT, Nam
TK and Lee BH: Unilateral thrombosis of a deep cerebral vein
associated with transient unilateral thalamic edema. J Cerebrovasc
Endovasc Neurosurg. 14:233–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao M, Sun L, Liu YL, Xie JW, Qin L, Xue
J, Wang YT, Guo KM, Ma MM and Li XY: Reduction of glyoxalase 1
(GLO1) aggravates cerebrovascular remodeling via promoting the
proliferation of basilar smooth muscle cells in hypertension.
Biochem Biophys Res Commun. 518:278–285. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mills KT, Stefanescu A and He J: The
global epidemiology of hypertension. Nat Rev Nephrol. 16:223–237.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fernandez-Rojo MA and Ramm GA: Caveolin-1
function in liver physiology and disease. Trends Mol Med.
22:889–904. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haines P, Samuel GH, Cohen H, Trojanowska
M and Bujor AM: Caveolin-1 is a negative regulator of MMP-1 gene
expression in human dermal fibroblasts via inhibition of
Erk1/2/Ets1 signaling pathway. J Dermatol Sci. 64:210–216. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma X, Liu L, Nie W, Li Y, Zhang B, Zhang J
and Zhou R: Prognostic role of caveolin in breast cancer: A
meta-analysis. Breast. 22:462–469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Santibanez JF, Blanco FJ, Garrido-Martin
EM, Sanz-Rodriguez F, del Pozo MA and Bernabeu C: Caveolin-1
interacts and cooperates with the transforming growth factor-beta
type I receptor ALK1 in endothelial caveolae. Cardiovasc Res.
77:791–799. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang JH, Lu L, Lu H, Chen X, Jiang S and
Chen YH: Identification of the HIV-1 gp41 core-binding motif in the
scaffolding domain of caveolin-1. J Biol Chem. 282:6143–6152. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Forrester SJ, Elliott KJ, Kawai T, Obama
T, Boyer MJ, Preston KJ, Yan Z, Eguchi S and Rizzo V: Caveolin-1
deletion prevents hypertensive vascular remodeling induced by
angiotensin II. Hypertension. 69:79–86. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiao HX, Mu YP, Gui LX, Yan FR, Lin DC,
Sham JS and Lin MJ: Increase in caveolae and caveolin-1 expression
modulates agonist-induced contraction and store- and
receptor-operated Ca(2+) entry in pulmonary arteries of pulmonary
hypertensive rats. Vascul Pharmacol. 84:55–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Watson O, Novodvorsky P, Gray C, Rothman
AM, Lawrie A, Crossman DC, Haase A, McMahon K, Gering M, Van Eeden
FJ and Chico TJ: Blood flow suppresses vascular Notch signalling
via dll4 and is required for angiogenesis in response to hypoxic
signalling. Cardiovasc Res. 100:252–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
D'Souza B, Meloty-Kapella L and Weinmaster
G: Canonical and non-canonical notch ligands. Curr Top Dev Biol.
92:73–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiao L, Xie L, Shi K, Zhou T, Hua Y and
Liu H: Notch signaling change in pulmonary vascular remodeling in
rats with pulmonary hypertension and its implication for
therapeutic intervention. PLoS One. 7:e515142012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dabral S, Tian X, Kojonazarov B, Savai R,
Ghofrani HA, Weissmann N, Florio M, Sun J, Jonigk D, Maegel L, et
al: Notch1 signalling regulates endothelial proliferation and
apoptosis in pulmonary arterial hypertension. Eur Respir J.
48:1137–1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiao LN, Xu HB, Shi K, Zhou TF, Hua YM and
Liu HM: Role of notch signal in angiotensin II induced pulmonary
vascular remodeling. Transl Pediatr. 2:5–13. 2013.PubMed/NCBI
|
|
19
|
Atış M, Akcan U, Uğur Yılmaz C, Orhan N,
Düzgün P, Deniz Ceylan U, Arıcan N, Karahüseyinoğlu S, Nur Şahin G,
Ahıshalı B and Kaya M: Effects of methyl-beta-cyclodextrin on
blood-brain barrier permeability in angiotensin II-induced
hypertensive rats. Brain Res. 1715:148–155. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nie X, Tan J, Dai Y, Liu Y, Zou J, Sun J,
Ye S, Shen C, Fan L, Chen J and Bian JS: CCL5 deficiency rescues
pulmonary vascular dysfunction, and reverses pulmonary hypertension
via caveolin-1-dependent BMPR2 activation. J Mol Cell Cardiol.
116:41–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu S, Wang J, Wang X and Zhao J:
Protection against monocrotaline-induced pulmonary arterial
hypertension and caveolin-1 downregulation by fluvastatin in rats.
Mol Med Rep. 17:3944–3950. 2018.PubMed/NCBI
|
|
23
|
Zhou LJ, Chen XY, Liu SP, Zhang LL, Xu YN,
Mu PW, Geng DF and Tan Z: Downregulation of Cavin-1 expression via
increasing Caveolin-1 degradation prompts the proliferation and
migration of vascular smooth muscle cells in balloon injury-induced
neointimal hyperplasia. J Am Heart Assoc. 6:e0057542017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Umesalma S, Houwen FK, Baumbach GL and
Chan SL: Roles of caveolin-1 in angiotensin ii-induced hypertrophy
and inward remodeling of cerebral pial arterioles. Hypertension.
67:623–629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kobayashi N, Mori Y, Nakano S, Tsubokou Y,
Kobayashi T, Shirataki H and Matsuoka H: TCV-116 stimulates eNOS
and caveolin-1cav-1 expression and improves coronary microvascular
remodeling in normotensive and angiotensin II-induced hypertensive
rats. Atherosclerosis. 158:359–368. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rizzo PL, Miele L and Ferrari R: The Notch
pathway: A crossroad between the life and death of the endothelium.
Eur Heart J. 34:2504–2509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hofmann JJ and Iruela-Arispe ML: Notch
signaling in blood vessels: Who is talking to whom about what. Circ
Res. 100:1556–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kume T: Novel insights into the
differential functions of Notch ligands in vascular formation. J
Angiogenes Res. 1:82009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aoyama T, Takeshita K, Kikuchi R, Yamamoto
K, Cheng XW, Liao JK and Murohara T: Gamma-Secretase inhibitor
reduces diet-induced atherosclerosis in apolipoprotein E-deficient
mice. Biochem Biophys Res Commun. 383:216–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou X, Xiao Y, Mao Z, Huang J, Geng Q,
Wang W and Dong P: Soluble Jagged-1 inhibits restenosis of vein
graft by attenuating Notch signaling. Microvasc Res. 100:9–16.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Delbosc S, Glorian M, Le Port AS, Béréziat
G, Andréani M and Limon I: The benefit of docosahexanoic acid on
the migration of vascular smooth muscle cells is partially
dependent on Notch regulation of MMP-2/-9. Am J Pathol.
172:1430–1440. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hurst LA, Dunmore BJ, Long L, Crosby A,
Al-Lamki R, Deighton J, Southwood M, Yang X, Nikolic MZ, Herrera B,
et al: TNFα drives pulmonary arterial hypertension by suppressing
the BMP type-II receptor and altering NOTCH signalling. Nat Commun.
8:140792017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo Q, Xu H, Yang X, Zhao D, Liu S, Sun X
and Huang JA: Notch activation of Ca2+-sensing receptor mediates
hypoxia-induced pulmonary hypertension. Hypertens Res. 40:117–129.
2017. View Article : Google Scholar : PubMed/NCBI
|