Introduction
Sepsis is a systemic inflammatory response syndrome
caused by infection, which leads to an increased risk of multiple
organ injuries and mortality (1).
Acute lung injury (ALI) is one of the most common side effects of
sepsis due to pulmonary susceptibility, and is associated with high
morbidity and mortality worldwide (2,3).
Despite progression in efforts to understand the pathogenesis of
sepsis-induced ALI, current treatment strategies remain ineffective
(4). Thus, it remains critical to
develop novel and effective therapeutic agents for the treatment of
ALI.
Sepsis-induced ALI is generally characterized by an
overwhelming response of innate inflammation, which stimulates
excessive production of inflammatory cytokines, including tumor
necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β and monocyte
chemoattractant protein-1 (MCP-1) (5). This overactivation of the
inflammatory response results in pathological damage of the
alveolar epithelium and vascular endothelium, eventually resulting
in ALI (6). Furthermore, oxidative
stress plays a crucial role in the development of ALI. The
excessive production of reactive oxygen species results in severe
intracellular oxidative damage (7). Since inflammation and oxidative
stress function are key for ALI development, identification of
therapeutic agents with anti-inflammatory and antioxidative
characteristics are required for direct and effective treatment of
sepsis-induced ALI. Increasing evidence has demonstrated that
anesthetic agents can protect against sepsis (8). A recent study reported that
dexmedetomidine mitigates ALI in rats by suppressing caveolin-1
downstream signaling (9).
Sufentanil, a derivative of fentanyl, is considered an opioid, with
a high affinity to opioid receptors. A previous study demonstrated
that sufentanil inhibited the inflammatory response and oxidative
stress in hepatic ischemia-reperfusion injury (10). Previous studies reported that
sufentanil could alleviate ALI induced by hemorrhagic shock in
rabbits (11,12). The STITCH database (http://stitch.embl.de) predicted that the κ-type
opioid receptor (OPRK1) can combine with sufentanil, and the Search
Tool for the Retrieval of Interacting Genes/Proteins (STRING)
database (https://string-db.org) predicted that
kininogen-1 (KNG1) can interact with OPRK1. KNG1 can encode
high-molecular weight kininogen proteins, and increasing evidence
has demonstrated that KNG1 plays a significant role in inflammation
and coagulation (13). A previous
study reported that KNG1 serves as a pro-inflammatory cytokine,
which accelerates the progress of inflammation (14). However, the role of sufentanil in
sepsis-induced ALI via regulation of KNG1 remains unclear.
Thus, the present study investigated the therapeutic
effects of sufentanil on sepsis-induced ALI. A rat sepsis model was
used to determine the underlying molecular mechanisms of sufentanil
on sepsis-induced ALI, in order to evaluate its potential as a
candidate therapeutic agent against ALI.
Materials and methods
Animal study
A total of 40 specific-pathogen-free-grade male
Sprague-Dawley rats (age, 6–8 weeks; weight, 180–200 g) were
provided by the Model Animal Research Center of Nanjing University
(Nanjing, China). All animals were housed in individually
ventilated cages (n=2 in each cage) under a controlled temperature
(20–25°C), humidity (20–30%) and 12-h light/dark cycle conditions
with free access to food and water. Rats were allowed to adapt to
the environment for one week before the experiments. Animal
experimental procedures were performed according to the Guide for
Care and Use of Laboratory Animals (15,16)
and protocols were approved by the Animal Experiment Ethics
Committee of The First People's Hospital (Wuhan, China).
Establishment of septic ALI model
The rat sepsis model was established using the cecal
ligation and puncture (CLP) method, as previously described
(17). Briefly, all rats were
deprived of from food 12 h before surgery, but had free access to
water during this time. Rats were anesthetized with an
intraperitoneal injection of 50 mg/kg sodium pentobarbital.
Following anesthesia, a 2-cm incision was made along the middle of
the abdomen to expose the cecum. Subsequently, the cecum was
ligated with the No. 4 suture below the ileocecal valve and
double-punctured using a sterile 18-gauge needle, which released a
small amount of feces. The intestinal contents within the cecum
were squeezed through the puncture wound and the cecum was
restored, followed by suturing of the abdominal incision, layer by
layer. Rats in the sham groups underwent identical laparotomy and
resuscitation procedures; however, ligation and perforation were
not performed. All rats were allowed free access to food and water
following CLP. The behavior of the rats was observed every 2 h.
There were no abnormal deaths during the experiment. All animals
were euthanized with an intraperitoneal injection of 200 mg/kg
sodium pentobarbital 24 h after CLP surgery, which was in
accordance with previous studies (18,19).
Death was determined when the animals' hearts stopped completely
and pupils dilated. Lung tissues were collected for further
experimentation. If the rats went into shock, or showed decreased
activity, lethargy or dyspnea, the animals were euthanized prior to
the experimental endpoint.
Grouping and drug administration
All rats were randomly allocated into four groups
(10 rats in each group) as follows: i) Sham group; ii) sham +
sufentanil group; iii) CLP group; and iv) CLP + sufentanil group.
Sufentanil was purchased from Yichang Humanwell Pharmaceutical Co.,
Ltd. Rats in the sham + sufentanil and CLP + sufentanil groups were
intravenously injected with sufentanil (3 µg/kg; 1 ml) 30 min prior
to surgery, whereas rats in the sham only and CLP only groups were
intravenously injected with normal saline at the same time.
Test for lung wet/dry (W/D) weight
ratio
In order to assess the magnitude of lung tissue
edema, the upper right lobe of lung tissues was immediately excised
and the surface fluid and blood was absorbed using filter paper.
After weighing, the tissue samples were placed in an incubator at
80°C for 48 h to acquire the dry weight, and the W/D ratio was
subsequently calculated using the following calculation: Lung W/D
weight ratios=wet weight/dry weight.
Histopathological examination
Lung tissues were fixed in 10% buffered formalin for
24 h at 4°C, conventionally dehydrated, cleared and waxed. The
tissue samples were subsequently embedded in paraffin and cut into
5-µm-thick sections. The tissue sections were deparaffinized in
xylene and rehydrated in a descending ethanol series. The sections
were stained with hematoxylin (5 min, room temperature) and eosin
(3 min, room temperature), prior to dehydration in a graded ethanol
series and xylene. The stained slides were observed under a light
microscope (Olympus Corporation; magnification, ×400).
Cell culture and treatment
Alveolar epithelial type II cells (AEC II) A549
cells (cat. no. SCSP-503) were purchased from The Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences and
maintained in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C in 5% CO2.
In order to mimic the process of ALI, AEC II were
treated with 50 µg/ml lipopolysaccharide (LPS; Sigma-Aldrich; Merck
KGaA) for 24 h at 37°C. Cells in the control group were not
manipulated, while cells in the treatment groups were stimulated
with sufentanil (5, 10, 20 and 40 µM) for 2 h prior to treatment
with LPS at 37°C in 5% CO2.
Cell transfection
Cells were seeded into 6-well plates
(1×106 cells/well) and cultivated in a humidified
chamber until they reached 70% confluence. AEC II were transfected
with KNG1 overexpression pcDNA3.1 plasmid (Oe-KNG1-1 or Oe-KNG1-2;
5 µl; 50 ng) or empty vector plasmid (Oe-NC; both from Shanghai
GenePharma Co., Ltd.) using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Cells were harvested and analyzed 48 h
post-transfection. Transfection efficiency was determined via
reverse transcription-quantitative (RT-q) PCR analysis.
Cell viability
Cell viability was detected using Cell Count Kit-8
(CCK-8) assay (Dojindo Molecular Technologies, Inc.) according to
the manufacturer's instructions. After transfection, cells were
seeded in 96-well plates (1×104 cells/well). Cells were
incubated with sufentanil for 2 h followed by treatment with 50
µg/ml LPS for 24 h at room temperature. A total of 10 µl CCK-8
solution was added to each well. Following incubation for 1 h at
room temperature, the absorbance was detected at 450 nm.
Detection of cytokine in
bronchoalveolar lavage fluid (BALF)
The rats were euthanized 24 h after CLP and the left
lungs were washed three times with 0.5 ml saline. Subsequently,
BALF was collected and centrifuged at 850 × g for 10 min at 4°C.
The supernatant was collected and preserved at −80°C. The
concentrations of the inflammatory factors TNF-α (cat. no. F16960),
IL-1β (cat. no. F15810), IL-6 (cat. no. F15870) and MCP-1 (cat. no.
F16120) in BALF were assessed using ELISA kits (Shanghai Xitang
Biotechnology Co., Ltd.), according to the manufacturer's
protocol.
Determination of oxidative stress
The partial lung tissue specimens were collected and
homogenized (10%, w/v) in cold saline. Malondialdehyde (MDA; cat.
no. A003-1-2) content and the activity levels of superoxide
dismutase (SOD; cat. no. A001-1-2), catalase (CAT; cat. no.
A007-1-1) and glutathione peroxidase (GSH-Px; cat. no. A005-1-2)
were determined in the tissue homogenates or AEC II using
colorimetric commercial kits (Nanjing Jiancheng Bioengineering
Institute) according to the manufacturer's protocols.
RT-qPCR
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and cDNA was synthesized using the PrimeScript RT kit (Takara
Bio, Inc.) at 37°C for 15 min and 85°C for 5 sec. The cDNA solution
was stored at −80°C. Subsequently, qPCR was performed using the
iTaq™ Universal SYBR-Green Supermix (Bio-Rad Laboratories, Inc.) on
an ABI 7500 system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) at 95°C for 3 min, 95°C for 30 sec and 58°C for 30 sec.
Relative expression levels were calculated using the
2−ΔΔCq method and normalized to the internal reference
gene GAPDH (20). The sequences of
all primers were as follows: GAPDH: Forward,
5′-CGCCTGGAGAAAGCTGCTA-3′ and reverse, 5′-ACGACCTGGTCCTCGGTGTA-3′;
and KNG1: Forward, 5′-TAATACGACTCACTATAGGG-3′ and reverse,
5′-TAGAAGGCACAGTCGAGG−3′.
Western blot analysis
Total proteins were extracted from lung tissue
samples or AEC II using RIPA lysis buffer (Beyotime Institute of
Biotechnology) and protein concentrations were measured using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). An equal amount of protein sample (40 µg per lane)
was separated via SDS-PAGE on a 10% gel and subsequently
transferred onto nitrocellulose membranes. Membranes were blocked
with 5% non-fat milk at room temperature for 2 h, prior to
incubation with primary antibodies (overnight at 4°C) for the
target proteins. Following incubation with goat anti-rabbit
horseradish peroxidase-conjugated secondary antibody at room
temperature for 1 h (1:10,000; cat. no. sc-2004; Santa Cruz
Biotechnology, Inc.), the protein bands were visualized using an
enhanced chemiluminescence kit (Thermo Fisher Scientific, Inc.) and
detected using ImageJ software (version 1.8.0; National Institutes
of Health). Anti-KNG1 antibody (1:1,000; cat. no. ab175386) was
obtained from Abcam. Anti-nuclear factor erythroid 2-related factor
2 (Nrf2; 1:1,000; cat. no. 12721T), anti-phospho-nuclear factor-κB
(p-NF-κB p65; 1:1,000; cat. no. 3033T), anti-NF-κB p65 (1:1,000;
cat. no. 8242T); anti-heme oxygenase-1 (HO-1; 1:1,000; cat. no.
43966S), anti-Lamin B1 (1:1,000; cat. no. 13435S) and anti-GAPDH
(1:1,000; cat. no. 5174T) antibodies were purchased from Cell
Signaling Technology, Inc. Protein expression was normalized to the
internal reference gene GAPDH or Lamin B1.
Statistical analysis
All results were obtained from at least three
independent experiments. Data are presented as the mean ± standard
deviation. All statistical analysis was performed using GraphPad
Prism version 6.0 (GraphPad Software, Inc.). A Student's t-test was
used to compare differences between two groups, while one-way
ANOVA, followed by Tukey's post hoc test was used to compare
differences between multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
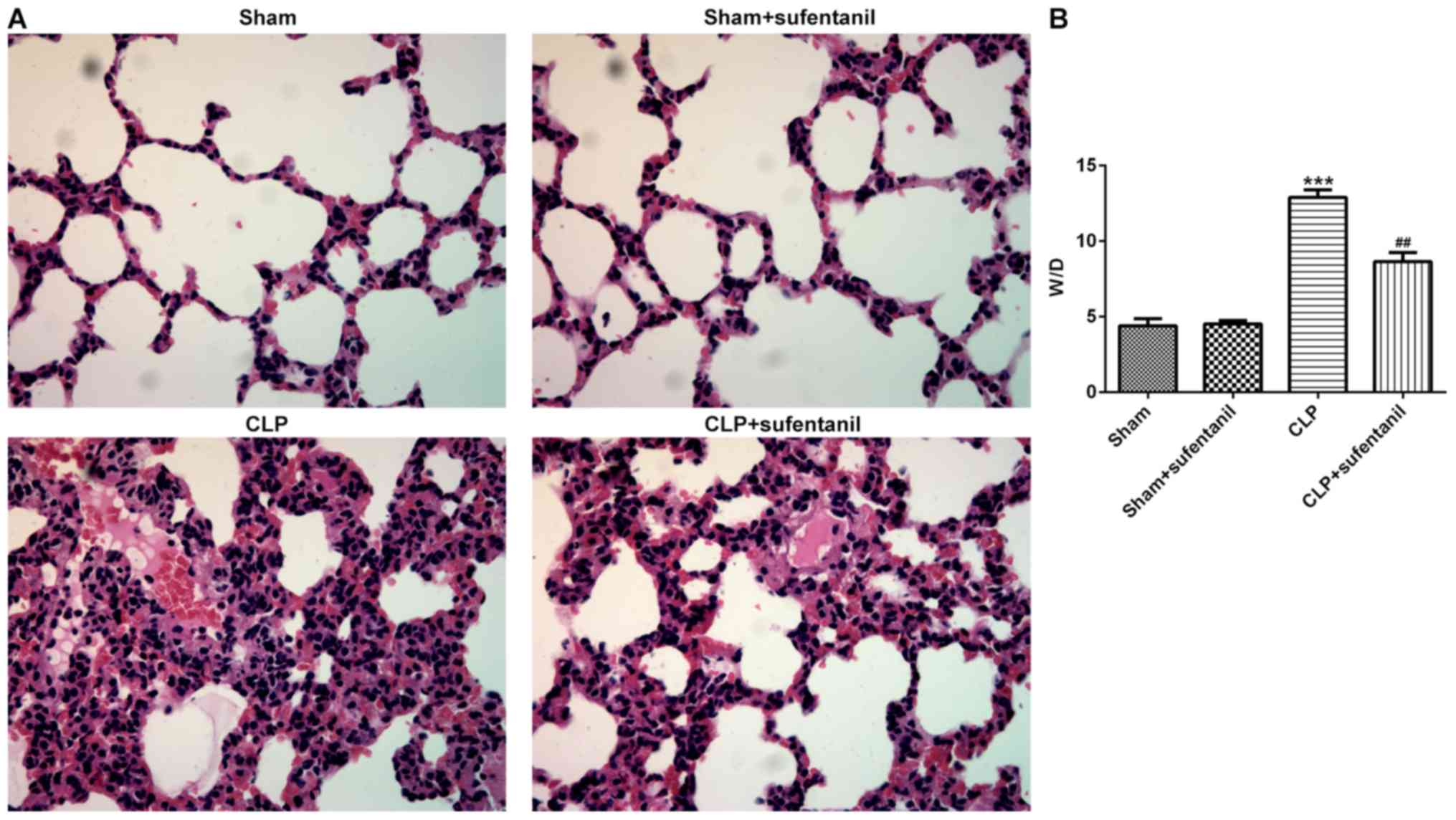
Sufentanil improves lung tissue
pathobiology and edema in CLP-induced ALI rats
H&E staining of lung tissue sections was
performed to determine the pathological changes in each group in
order to investigate the effects of sufentanil in sepsis-induced
ALI. Normal pulmonary alveoli structure was observed in
sham-operated rats, whereas no significant difference in lung
tissue pathology was observed between rats in the sham only and
sham + sufentanil groups (Fig.
1A). However, animals presented with destructive alveolar
structure, thickened alveolar septal walls, visible vascular
congestion and inflammatory cell infiltration following CLP.
Notably, the morphological changes observed in rat lung tissues
following CLP were attenuated after treatment with sufentanil.
Furthermore, the degree of lung tissue edema was calculated using
the W/D ratio. The results demonstrated that the W/D ratio
significantly increased following CLP, whereas treatment with
sufentanil notably attenuated this (Fig. 1B). Taken together, these results
suggested that sufentanil may effectively alleviate lung injury and
edema in sepsis-induced ALI rats.
Sufentanil decreases inflammation and
oxidative stress in CLP-induced ALI rats
The effect of sufentanil on inflammation was
assessed by detecting the concentrations of inflammatory cytokines,
TNF-α, IL-6, IL-1β and MCP-1, in the BALF. The results demonstrated
that the levels of inflammatory cytokines significantly increased
in the CLP group compared with the sham group, whereas treatment
with sufentanil significantly decreased the levels of cytokines in
the CLP group (Fig. 2A-D).
Furthermore, commercial kits were used to assess the levels of
oxidative stress-associated markers. Treatment with sufentanil
significantly decreased MDA content, whereas the activity levels of
the antioxidant enzymes SOD, CAT and GSH-Px increased compared with
the CLP group (Fig. 2E-H).
Collectively, these results suggested that sufentanil may relieve
inflammation and oxidative stress in CLP-induced ALI rats.
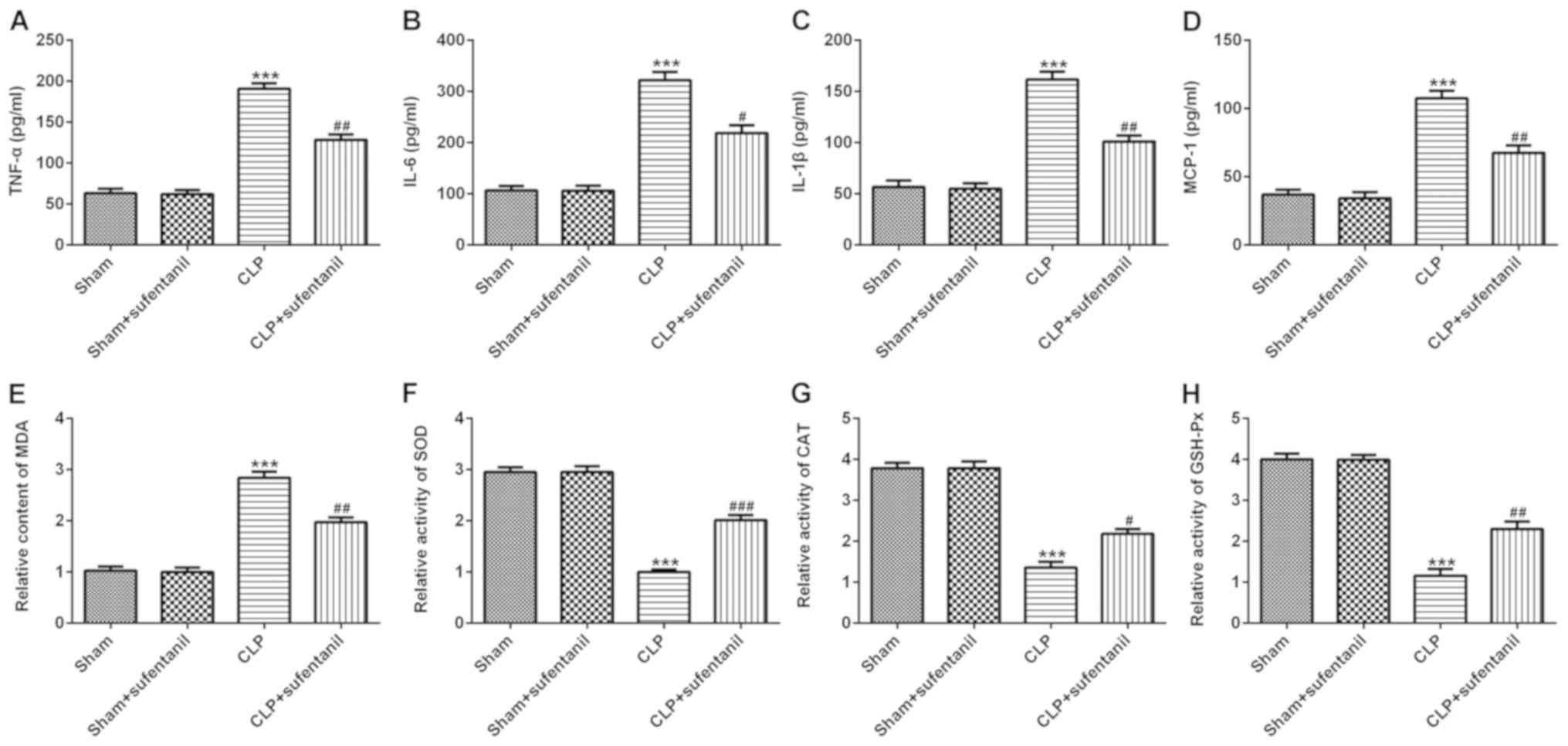 | Figure 2.Sufentanil alleviates inflammation and
oxidative stress in CLP-induced acute lung injury rats. Levels of
(A) TNF-α, (B) IL-6, (C) IL-1β and (D) MCP-1 were determined via
ELISA. (E) MDA content and the activities of (F) SOD, (G) CAT and
(H) GSH-Px were assessed using commercial kits. ***P<0.001 vs.
sham; #P<0.05, ##P<0.01,
###P<0.001 vs. CLP. CLP, cecal ligation and puncture;
TNF-α, tumor necrosis factor-α; IL, interleukin; MCP-1, monocyte
chemoattractant protein-1; MDA, malondialdehyde; SOD, superoxide
dismutase; CAT, catalase; GSH-Px, glutathione peroxidase. |
KNG1 expression is notably
downregulated following treatment with sufentanil
In order to investigate the potential molecular
mechanisms of sufentanil function during ALI, KNG1 expression was
determined in lung tissues via western blotting. No significant
difference in KNG1 expression was observed between the sham and
sham + sufentanil groups (Fig.
3A). However, KNG1 expression was significantly upregulated in
the CLP group, which was reversed following treatment with
sufentanil.
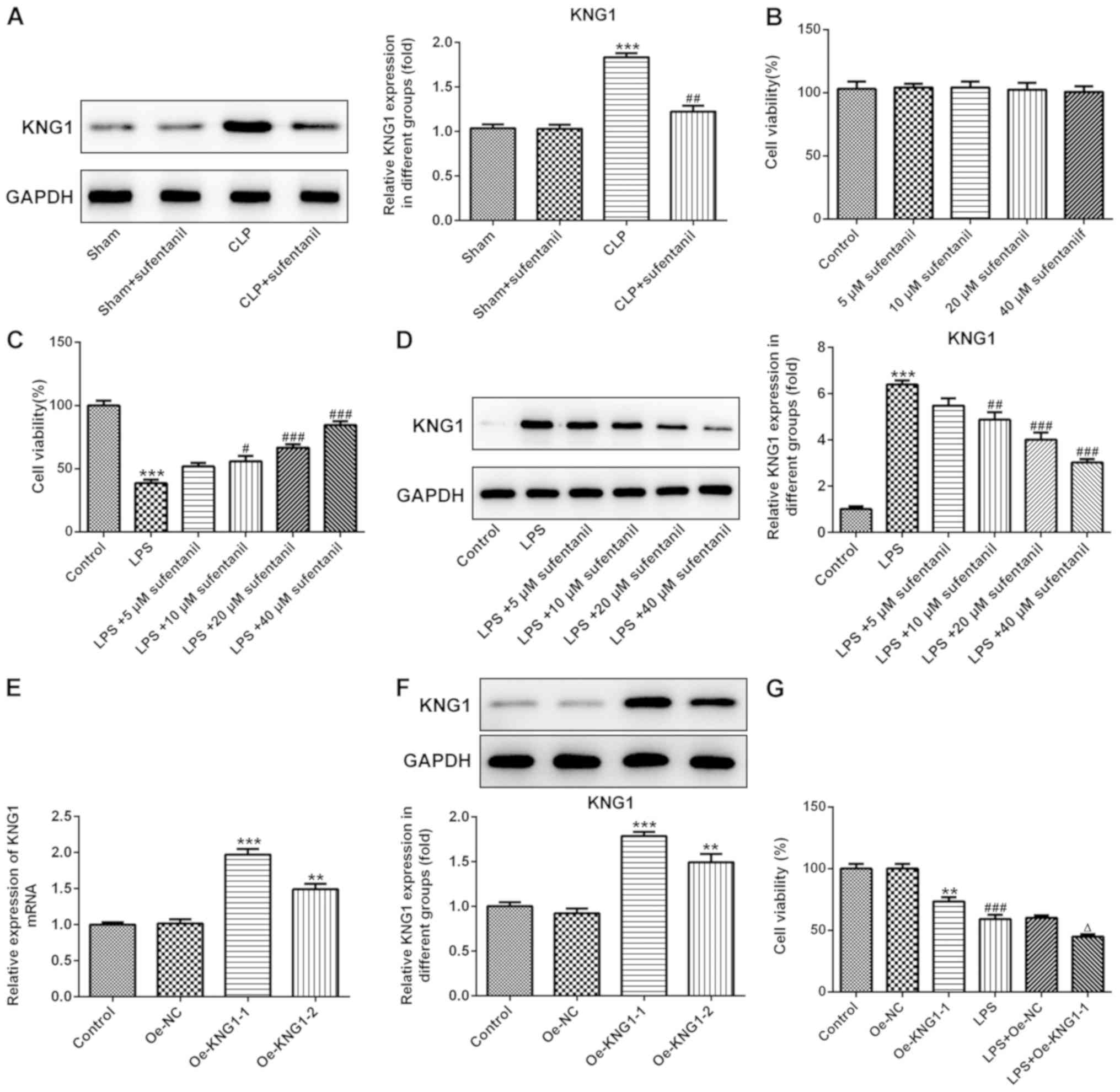 | Figure 3.KNG1 expression is significantly
downregulated following sufentanil treatment. (A) Western blot
analysis was performed to determine KNG1 protein expression in lung
tissues of acute lung injury rats. ***P<0.001 vs. sham;
##P<0.01 vs. CLP. (B) CCK-8 assay was performed to
assess cell viability following treatment with different
concentrations of sufentanil. (C) CCK-8 assay was performed to
assess cell viability and (D) expression of KNG1 was assessed using
western blot analysis in alveolar epithelial type II cells induced
by LPS, following treatment with sufentanil. ***P<0.001 vs.
control; #P<0.05, ##P<0.01,
###P<0.001 vs. LPS. (E) Reverse
transcription-quantitative PCR and (F) western blotting were
performed to determine KNG1 expression following cell transfection.
**P<0.01, ***P<0.001 vs. Oe-NC. (G) Cell viability was
measured using a CCK-8 assay after KNG1 overexpression with and
without LPS. **P<0.01 vs. Oe-NC; ###P<0.001 vs.
control; ΔP<0.05 vs. LPS + Oe-NC. KNG1, kininogen-1;
LPS, lipopolysaccharide; CLP, cecal ligation and puncture; Oe,
overexpression; NC, negative control; CCK-8, Cell Counting
Kit-8. |
AEC II were subsequently treated with different
concentrations of sufentanil (5, 10, 20 and 40 µM) and cell
viability was assessed via CCK-8 assays. The results indicated that
treatment with a series of concentrations of sufentanil had little
effect on the cell viability of AEC II (Fig. 3B). It was also found that cell
viability significantly decreased following treatment of AEC II
with LPS, compared with the control group, while sufentanil
dose-dependently enhanced cell viability (Fig. 3C).
Subsequently, the expression of KNG1 was examined by
western blot analysis. As exhibited in Fig. 3D, KNG1 was significantly
upregulated in AEC II treated with LPS, whereas sufentanil
downregulated KNG1 expression in a dose-dependent manner. Next,
KNG1 was successfully overexpressed by transfection with an
overexpression plasmid (Fig. 3E and
F). Cells transfected with Oe-KNG1-1 were used for all
subsequent experiments. Additionally, cell viability was assessed
using a CCK-8 assay after KNG1 overexpression with and without LPS.
As shown in Fig. 3G,
overexpression of KNG1 and/or LPS treatment significantly reduced
the viability of AEC II, and the lowest viability was observed in
LPS + Oe-KNG1-1 group. Taken together, these results indicated that
sufentanil downregulated KNG1 expression in CLP-induced ALI rats
and AEC II.
Overexpression of KNG1 significantly
reverses the inhibitory effects of sufentanil on inflammation and
oxidative stress
In order to determine whether the inhibitory effects
of sufentanil on inflammation and oxidative stress in ALI were
achieved by regulating KNG1 expression, the levels of inflammatory-
and oxidative stress-associated markers were assessed in AEC II
treated with LPS. As demonstrated in Fig. 4A-D, KNG1 overexpression or LPS
treatment significantly increased the levels of TNF-α, IL-6, IL-1β
and MCP-1, and the highest levels of the aforementioned factors
were found in the LPS + Oe-KNG1-1 group. Sufentanil significantly
decreased the levels of TNF-α, IL-6, IL-1β and MCP-1 in LPS-treated
cells, which was consistent with the results of the CLP-induced ALI
rat model. Overexpression of KNG1 significantly alleviated the
inhibitory effects of sufentanil on the levels of inflammatory
factors. Furthermore, overexpression of KNG1 in LPS-induced AEC II
significantly elevated MDA content, which was accompanied by a
decline in the activity levels of SOD, CAT and GSH-Px, compared
with the control group (Fig.
4E-H). Taken together, these results indicated that
overexpression of KNG1 significantly attenuated the inhibitory
effects of sufentanil on inflammation and oxidative stress.
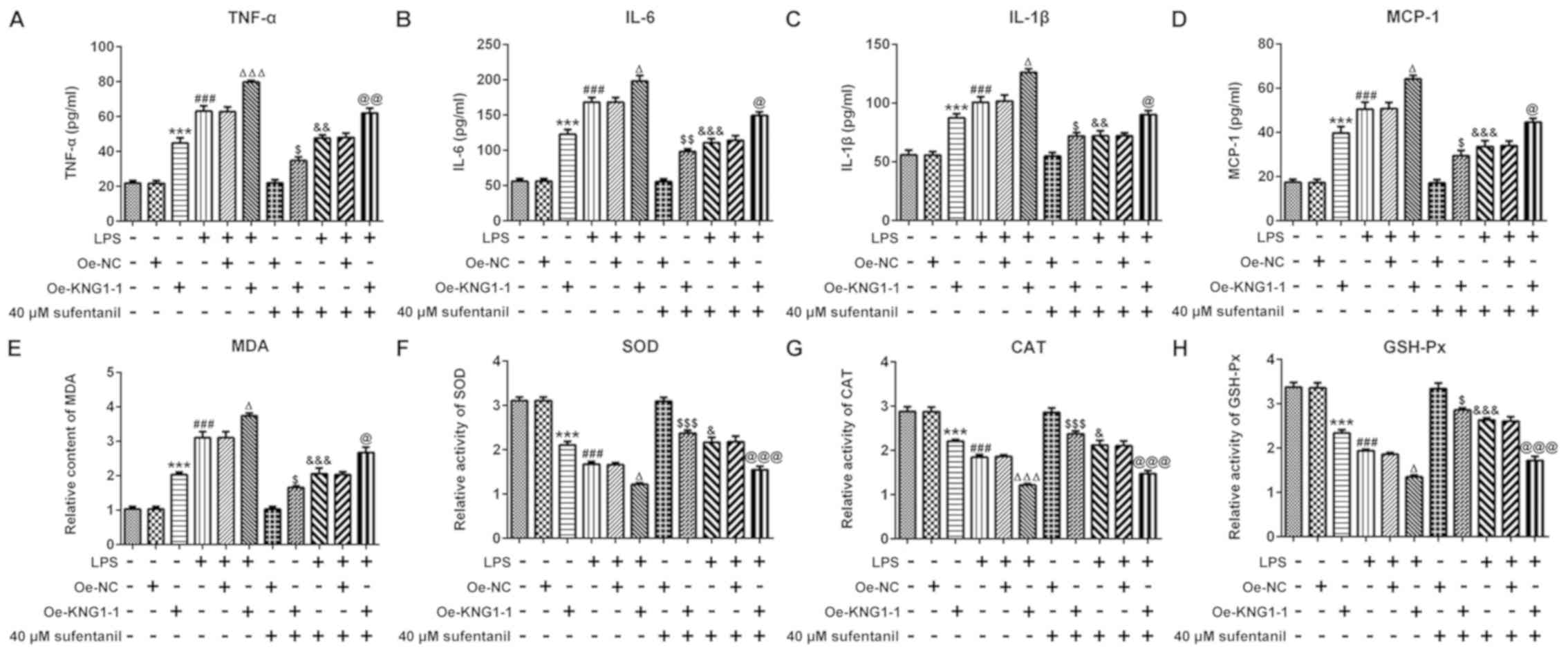 | Figure 4.Overexpression of KNG1 significantly
reverses the inhibitory effects of sufentanil on inflammation and
oxidative stress. Levels of (A) TNF-α, (B) IL-6, (C) IL-1β and (D)
MCP-1 were determined via ELISA. (E) MDA content and the activities
of (F) SOD, (G) CAT and (H) GSH-Px were assessed using commercial
kits. ***P<0.001 vs. Oe-NC; ###P<0.001 vs.
control; ΔP<0.05, ΔΔΔP<0.001 vs. LPS +
Oe-NC; $P<0.05, $$P<0.01,
$$$P<0.001 vs. 40 µM sufentanil + Oe-NC;
&P<0.05, &&P<0.01,
&&&P<0.001 vs. LPS;
@P<0.05, @@P<0.01, @@@P<0.001 vs. LPS + 40 µM sufentanil +
Oe-NC. KNG1, kininogen-1; TNF-α, tumor necrosis factor-α; IL,
interleukin; MCP-1, monocyte chemoattractant protein-1; MDA,
malondialdehyde; SOD, superoxide dismutase; CAT, catalase; GSH-Px,
glutathione peroxidase; Oe, overexpression; NC, negative
control. |
Overexpression of KNG1 markedly
reverses the effects of sufentanil on NF-κB and Nrf2/HO-1
signaling
In order to further investigate the molecular
mechanisms underlying inflammation and oxidative stress regulated
by sufentanil in ALI, the expression levels of NF-κB and Nrf2/HO-1
signaling pathway proteins were measured via western blot analysis,
following overexpression of KNG1 in LPS-induced AEC II. The
expression levels of p-NF-κB p65 significantly increased in the LPS
group, which was decreased following treatment with sufentanil. The
effects of sufentanil treatment were reversed following
overexpression of KNG1 (Fig. 5A).
In addition, expression levels of Nrf2 and HO-1 were significantly
elevated following treatment with LPS + sufentanil compared with
the LPS group (Fig. 5B).
Overexpression of KNG1 significantly reversed the regulatory
effects of sufentanil on the Nrf2/HO-1 signaling pathway. Taken
together, these results suggested that sufentanil relieved
inflammation and oxidative stress in ALI by downregulating KNG1
expression.
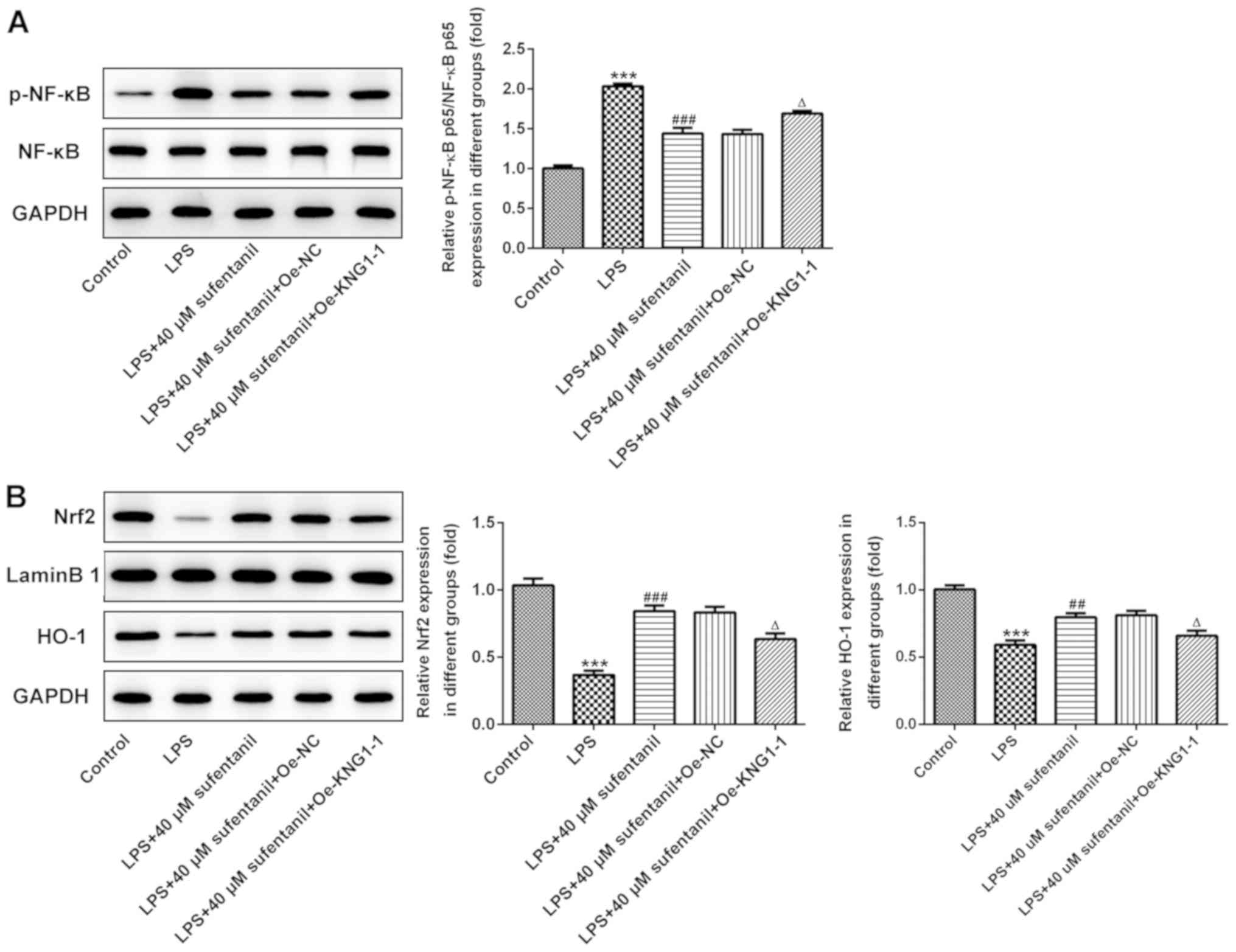 | Figure 5.Overexpression of KNG1 notably
restores the regulatory effects of sufentanil on NF-κB and
Nrf2/HO-1 signaling. Western blot analysis was performed to detect
the protein expression levels of (A) p-NF-κB and NF-κB, and (B)
Nrf2 and HO-1 in LPS-stimulated alveolar epithelial type II cells.
***P<0.001 vs. control; ##P<0.01,
###P<0.001 vs. LPS; ΔP<0.05 vs. LPS +
40 µM sufentanil + Oe-NC. KNG1, kininogen-1; NF-κB, nuclear
factor-κB; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1,
heme oxygenase-1; LPS, lipopolysaccharide; Oe, overexpression; NC,
negative control; p-, phosphorylated. |
Discussion
Sepsis can result in multiple organ failure and is
considered a leading cause of mortality in intensive care unit
patients (21). The lung serves as
the most susceptible target organ in sepsis that can further
develop into life-threating acute respiratory distress syndrome
(ARDS), which is the principle risk factor for mortality (22,23).
The present study demonstrated that sufentanil protected against
sepsis-induced ALI by regulating KNG1-mediated NF-κB and Nrf2/HO-1
signaling.
Increasing evidence has demonstrated that anesthesia
possesses indirect functions to regulate the progression of human
disease. For example, a previous study found that ropivacaine
suppressed lung endothelial hyperpermeability, induced by pressure
in an acute hypertension model (24). Furthermore, etomidate restrained
the expression of NF-κB by downregulating expression of the
glucocorticoid receptor in septic rats (8), whereas desflurane was found to
alleviate ventilator-induced lung injury in rats with ARDS
(25). Sufentanil is an opioid,
with high affinity to opioid receptors (26). It has been reported that µ-opioid
receptor signaling could ameliorate LPS-induced acute ARDS, and AEC
was demonstrated to express opioid receptors (27,28).
To the best of our knowledge, the effects of sufentanil on
sepsis-induced ALI were investigated for the first time in the
present study. H&E staining analysis and the W/D ratio
demonstrated that sufentanil notably improved lung tissue
pathobiology and CLP-induced edema, suggesting the protective
effects of sufentanil on ALI.
The early phase of sepsis is characterized by
excessive inflammation, which is mediated by the sustained
secretion of inflammatory cytokines, including TNF-α, IL-6, IL-1β
and MCP-1 (29). Increasing
evidence has demonstrated that persistently increased
concentrations of the aforementioned inflammatory cytokines in
plasma are highly predicative of mortality in patients with ALI
(30). Thus, inhibiting
inflammation is critical to effectively treat sepsis-induced ALI. A
previous study reported that sufentanil preconditioning can
specifically protect against myocardial ischemic-reperfusion injury
in rabbits (31). Furthermore,
sufentanil has been demonstrated to inhibit inflammatory response
and oxidative stress in hepatic ischemia-reperfusion injury
(10). In the present study,
sufentanil notably decreased the levels of inflammatory factors and
MDA content, whereas the activities of the antioxidant enzymes
(SOD, CAT and GSH-Px) increased. These findings suggested that
sufentanil attenuated inflammation and oxidative stress in
sepsis-induced ALI.
In order to determine the underlying regulatory
mechanisms of sufentanil on ALI, KNG1 expression was detected in
lung tissues following CLP. KNG1 encodes high-molecular weight
kininogen proteins, and increasing evidence has demonstrated that
KNG1 plays a significant role in inflammation and coagulation
(12,32). A previous study reported that the
absence of KNG1 protein decreased thrombosis and inflammation in
ischemic mice (33). Furthermore,
as a pro-inflammatory cytokine, KNG1 has been demonstrated to
accelerate the progress of inflammation (14). An increasing trend of KNG1
expression was found in lung tissues of chronic obstructive
pulmonary disease models and inhibition of KNG1 could relieve
cellular inflammation (34). The
results of the present study demonstrated that KNG1 expression was
significantly upregulated in lung tissues following CLP, and
treatment with sufentanil restored the expression, suggesting an
underlying regulatory association between sufentanil and KNG1. In
order to determine the biological basis of this association, KNG1
was overexpressed in LPS-induced AEC II. The results indicated that
the inhibitory effects of sufentanil on inflammation and oxidative
stress were reversed following overexpression of KNG1. Thus, the
present study demonstrated that sufentanil attenuated
sepsis-induced ALI by downregulating KNG1 expression.
NF-κB signaling is essential for the regulation of
the inflammatory response in sepsis-induced ALI (35), and activation of the Nrf2/HO-1
signaling pathway may relieve sepsis-induced ALI by suppressing
inflammation and oxidative stress (36,37).
Increasing evidence has demonstrated that regulation of the
Nrf2/HO-1 and NF-κB signaling pathways alleviates lung injury
induced by ventilator, by suppressing inflammation and oxidative
stress (38). In order to further
investigate the molecular mechanisms underlying sufentanil in ALI,
the expression levels of NF-κB and Nrf2/HO-1 signaling pathway
proteins were measured via western blot analysis, following
overexpression of KNG1 in LPS-stimulated AEC II. The results
demonstrated that overexpression of KNG1 partially reversed the
regulatory effects of sufentanil on the NF-κB and Nrf2/HO-1
signaling pathways. Taken together, these results suggested that
sufentanil modulated NF-κB and Nrf2/HO-1 signaling in ALI by
downregulating KNG1 expression.
In conclusion, the results of the present study
indicated that sufentanil may protect lung tissues against
sepsis-induced inflammation and oxidative stress damage by
regulating the KNG1-mediated NF-κB and Nrf2/HO-1 signaling
pathways. Thus, sufentanil could be a potential novel therapeutic
agent for effective clinical treatment of sepsis-induced ALI.
However, it remains unknown if AEC II express opioid receptors and
future studies on human lung injury samples are required to further
verify the results of the present study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QH and YY designed the study and performed the
experiments. YY wrote the manuscript. QW and CH performed the
statistical analysis and revised the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Animal Experiment Ethics Committee of The First People's Hospital
(Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bellani G, Laffey JG, Pham T, Fan E,
Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley
DF, et al: Epidemiology, patterns of care, and mortality for
patients with acute respiratory distress syndrome in intensive care
units in 50 countries. JAMA. 315:788–800. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peng LY, Yuan M, Shi HT, Li JH, Song K,
Huang JN, Yi PF, Fu BD and Shen HQ: Protective effect of
piceatannol against acute lung injury through protecting the
integrity of air-blood barrier and modulating the TLR4/NF-κB
signaling pathway activation. Front Pharmacol. 10:16132019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Villar J, Sulemanji D and Kacmarek RM: The
acute respiratory distress syndrome: Incidence and mortality, has
it changed? Current Opin Crit Care. 20:3–9. 2014. View Article : Google Scholar
|
|
4
|
Dong Y, Zhang L, Jiang Y, Dai J, Tang L
and Liu G: Emodin reactivated autophagy and alleviated inflammatory
lung injury in mice with lethal endotoxemia. Exp Anim. 68:559–568.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu B, Miao X, Ye J and Pu X: The
protective effects of protease inhibitor MG-132 on sepsis-induced
acute lung rats and its possible mechanisms. Med Sci Monit.
25:5690–5699. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen L, Lin X, Xiao J, Tian Y, Zheng B and
Teng H: Sonchus oleraceus Linn protects against LPS-induced sepsis
and inhibits inflammatory responses in RAW264.7 cells. J
Ethnopharmacol. 236:63–69. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo K and Jin F: Dipeptidyl peptidase-4
(DPP-4) inhibitor saxagliptin alleviates lipopolysaccharide-induced
acute lung injury via regulating the Nrf-2/HO-1 and NF-κB pathways.
J Invest Surg. 1–8. 2019. View Article : Google Scholar
|
|
8
|
Zhang Y, Li RM, Wang C, Liu N, Lv S and
Xiong JY: Etomidate inhibits nuclear factor-kappaB through
decreased expression of glucocorticoid receptor in septic rats. Mol
Med Rep. 14:5760–5766. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu J, Huang X, Hu S, He H and Meng Z:
Dexmedetomidine attenuates lipopolysaccharide induced acute lung
injury in rats by inhibition of caveolin-1 downstream signaling.
Biomed Pharmacother. 118:1093142019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lian YH, Fang J, Zhou HD, Jiang HF and Xie
KJ: Sufentanil preconditioning protects against hepatic
ischemia-reperfusion injury by suppressing inflammation. Med Sci
Monit. 25:2265–2273. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang CM, Yu JL, Zhang Y, Chen LX, Cao HJ
and Wang YY: The effect of sufentanil on acute lung injury in
rabbits. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 20:108–110.
2008.(In Chinese). PubMed/NCBI
|
|
12
|
Reupke V, Walliser K, Perl T, Kimmina S,
Schraepler A, Quintel M and Kunze-Szikszay N: Total intravenous
anaesthesia using propofol and sufentanil allows controlled
long-term ventilation in rabbits without neuromuscular blocking
agents. Lab Anim. 51:284–291. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Furniss SK, Yao R and Gonzalez G:
Automatic gene prioritization in support of the inflammatory
contribution to Alzheimer's disease. AMIA Jt Summits Transl Sci
Proc. 2014:42–47. 2014.PubMed/NCBI
|
|
14
|
Wu D, Huo M, Chen X, Zhang Y and Qiao Y:
Mechanism of tanshinones and phenolic acids from Danshen in the
treatment of coronary heart disease based on co-expression network.
BMC Complement Med Ther. 20:282020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin S, Wang H, Liu G, Mei H and Chen M:
miR215p ameliorates hyperoxic acute lung injury and decreases
apoptosis of AEC II cells via PTEN/AKT signaling in rats. Mol Med
Rep. 20:4953–4962. 2019.PubMed/NCBI
|
|
16
|
Kata D, Foldesi I, Feher LZ, Hackler L Jr,
Puskas LG and Gulya K: A novel pleiotropic effect of aspirin:
Beneficial regulation of pro- and anti-inflammatory mechanisms in
microglial cells. Brain Res Bull. 132:61–74. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li R, Ren T and Zeng J: Mitochondrial
coenzyme Q protects sepsis-induced acute lung injury by activating
PI3K/Akt/GSK-3β/mTOR pathway in rats. BioMed Res Int.
2019:52408982019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu HY, Shi D, Hu CL, Yuan X, Zhang J and
Sun H: Dexmedetomidine mitigates CLP-stimulated acute lung injury
via restraining the RAGE pathway. Am J Transl Res. 9:5245–5258.
2017.PubMed/NCBI
|
|
19
|
Wu B, Miao X, Ye J and Pu X: The
protective effects of protease inhibitor MG-132 on sepsis-induced
acute lung rats and its possible mechanisms. Med Sci Monitor.
25:5690–5699. 2019. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu C, Guo Z, Zhao C, Zhang X and Wang Z:
Potential mechanism and drug candidates for sepsis-induced acute
lung injury. Exp Ther Med. 15:4689–4696. 2018.PubMed/NCBI
|
|
22
|
Shen N, Cheng A, Qiu M and Zang G: Allicin
improves lung injury induced by sepsis via regulation of the
toll-like receptor 4 (TLR4)/myeloid differentiation primary
response 88 (MYD88)/nuclear factor kappa B (NF-κB) pathway. Med Sci
Monit. 25:2567–2576. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou J, Fu Y, Liu K, Hou L and Zhang W:
miR-206 regulates alveolar type II epithelial cell Cx43 expression
in sepsis-induced acute lung injury. Exp Ther Med. 18:296–304.
2019.PubMed/NCBI
|
|
24
|
Patel M, Chignalia AZ, Isbatan A,
Bommakanti N and Dull RO: Ropivacaine inhibits pressure-induced
lung endothelial hyperpermeability in models of acute hypertension.
Life Sci. 222:22–28. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Strosing KM, Faller S, Gyllenram V,
Engelstaedter H, Buerkle H, Spassov S and Hoetzel A: Inhaled
anesthetics exert different protective properties in a mouse model
of ventilator-induced lung injury. Anesth Analg. 123:143–151. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin X, Ju YN, Gao W, Li DM and Guo CC:
Desflurane attenuates ventilator-induced lung injury in rats with
acute respiratory distress syndrome. Biomed Res Int.
2018:75073142018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ji S and Wang L: µ-Opioid receptor
signalling via PI3K/Akt pathway ameliorates
lipopolysaccharide-induced acute respiratory distress syndrome. Exp
Physiol. 104:1555–1561. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dong S, Liu J, Li L, Wang H, Ma H, Zhao Y
and Zhao J: The HECT ubiquitin E3 ligase Smurf2 degrades µ-opioid
receptor 1 in the ubiquitin-proteasome system in lung epithelial
cells. Am J Physiol Cell Physiol. 316:C632–C640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Q, Liu J, Wang W, Liu S, Yang X, Chen
M, Cheng L, Lu J, Guo T and Huang F: Sini decoction ameliorates
sepsis-induced acute lung injury via regulating ACE2-Ang (1–7)-Mas
axis and inhibiting the MAPK signaling pathway. Biomed
Pharmacother. 115:1089712019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qiu N, Xu X and He Y: LncRNA TUG1
alleviates sepsis-induced acute lung injury by targeting
miR-34b-5p/GAB1. BMC Pulm Med. 20:492020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang XH, Zeng JF, Lin C and Chen SB:
Effects of morphine and sufentanil preconditioning against
myocardial ischemic-reperfusion injury in rabbits. Int J Clin Exp
Med. 8:15692–15699. 2015.PubMed/NCBI
|
|
32
|
Wang H, Tan X, Xu J, Li H, Wang M, Chen S,
Yang X, Liu Y and Wang F: Negative correlation between CSF lactate
levels and MoCA scores in male Chinese subjects. Psychiatry Res.
255:49–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Langhauser F, Göb E, Kraft P, Geis C,
Schmitt J, Brede M, Göbel K, Helluy X, Pham M, Bendszus M, et al:
Kininogen deficiency protects from ischemic neurodegeneration in
mice by reducing thrombosis, blood-brain barrier damage, and
inflammation. Blood. 120:4082–4092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi K, Chen X, Xie B, Yang SS, Liu D, Dai
G and Chen Q: Celastrol alleviates chronic obstructive pulmonary
disease by inhibiting cellular inflammation induced by cigarette
smoke via the Ednrb/Kng1 signaling pathway. Front Pharmacol.
9:12762018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu M, Cao FL, Zhang YF, Shan L, Jiang XL,
An XJ, Xu W, Liu XZ and Wang XY: Tanshinone IIA therapeutically
reduces LPS-induced acute lung injury by inhibiting inflammation
and apoptosis in mice. Acta Pharmacol Sin. 36:179–187. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qing R, Huang Z, Tang Y, Xiang Q and Yang
F: Cordycepin alleviates lipopolysaccharide-induced acute lung
injury via Nrf(2)/HO-1 pathway. Int Immunopharmacol. 60:18–25.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao B, Gao W, Gao X, Leng Y, Liu M, Hou J
and Wu Y: Sulforaphane attenuates acute lung injury by inhibiting
oxidative stress via Nrf2/HO-1 pathway in a rat sepsis model. Int J
Clin Exp Pathol. 10:9021–9028. 2017.PubMed/NCBI
|
|
38
|
Xu J, Li HB, Chen L, Wang YX, Lu S, Li SN,
Cui SN, Xiao HR, Qin L, Hu H, et al: BML-111 accelerates the
resolution of inflammation by modulating the Nrf2/HO-1 and NF-κB
pathways in rats with ventilator-induced lung injury. Int
Immunopharmacol. 69:289–298. 2019. View Article : Google Scholar : PubMed/NCBI
|