Introduction
Axillary osmidrosis (AO) is a distressing disease
that has a detrimental effect on the social lives of affected
patients, due to the associated unpleasant odor and yellowish
staining of clothing, which is more common in Asian countries
(1). Odors may be produced by
bacteria that colonize the initially odorless secretions of sweat
and sebaceous glands (2), or it
may be related to the expression level of apolipoprotein D (ApoD)
in the apocrine sweat glands (3,4).
There are currently no standardized evidence-based clinical
evaluation methods for AO. Traditional measures, such as those
reported by Jung et al (5)
and Wang et al (6), have
been used in a clinical setting to evaluate the degree of malodor.
However, traditional methods have a judgement bias and lack
sensitivity (7). To solve these
problems, the Lu swab method (LSM) was proposed by our laboratory,
which has a higher sensitivity in determining the odor degree of AO
by clinicians.
It is well-known that the composition and activity
of the microbial community of the human axilla has a key role in
the formation of body odor (8).
Microbiological studies based on traditional culture have revealed
that the skin-resident microbiota primarily consists of
Gram-positive bacteria, such as Staphylococcus and
Corynebacterium (2). These
techniques form a sound basis; however, they misrepresent the true
bacterial diversity of a complex community (9). The recent emerging 16S rDNA
sequencing technology provides researchers with the opportunity to
investigate the composition and the skin microbiome in a
high-throughput manner (10). In
addition, bacterial growth on the skin is pH-dependent (11). The skin's pH is normally acidic,
with values ranging from 4 to 6. The physiological role of an
acidic skin surface is to act as a defense barrier against invading
organisms (12).
The present study aimed to identify a sensitive and
convenient method to determine the therapeutic effect of AO in a
clinical setting, to investigate the association between pH value
and disease severity and to further examine the potential
pathogenic bacteria and probiotic pathogens of AO.
Materials and methods
Subjects
The present study was approved by the institutional
review board of the Third Xiangya Hospital, Central South
University (Hunan, China). All patients provided written informed
consent. For participants below the age of 18 years, the legal
guardians provided written consent. There was no compensation
provided for participation. A total of 32 patients (64 axillae) had
bilateral axillary osmidrosis (13). The exclusion criteria were as
follows: i) Any treatment received in the previous 2 weeks, a
serious infection or other disruptive skin disease in the axillary
fossa; ii) current pregnancy or breastfeeding; and iii) severe
systemic diseases. A total of 32 patients (24 females, 8 males; age
range, 11–45 years; mean age, 25.75±7.62 years) and 32 healthy
control subjects who had no axillary odor (17 females, 15 males;
age range, 14–42 years; mean age, 25.84±6.44 years) with no history
of dermatological disorders or other chronic medical disorders and
with no current skin infections were enrolled at the Department of
Dermatology, the Third Xiangya Hospital, Central South University
between December 2017 and December 2018. The family history was
primarily characterized by an unpleasant unilateral or bilateral
smell in the armpits of the parents; 23 patients had a family
history of the disease (Table
I).
 | Table I.Clinical information of the patients
with axillary osmidrosis and healthy volunteers. |
Table I.
Clinical information of the patients
with axillary osmidrosis and healthy volunteers.
| Characteristic | Patients (n=32) | Healthy subjects
(n=32) | P-value |
|---|
| Age (years) | 25.75±7.62 | 25.84±6.44 | 0.9578 |
| Sex, n (%) |
|
| 0.068 |
| Male | 8
(25) | 15 (46.88) |
|
|
Female | 24 (75) | 17 (53.13) |
|
| Family history, n
(%) |
|
| / |
|
Yes | 23 (71.88) | 0 (0) |
|
| No | 9
(28.13) | 32
(100) |
|
| Disease course
(years) | 10.22±7.14 | 0 | / |
Clinical study design
The subjects were instructed not to use any products
in the axillae, such as antiperspirants, talcum powder, perfume or
bath additives, and to avoid eating spicy foods for 2 weeks prior
to the test. Local cleansing was stopped following a shower taken
on the evening prior to the test, and the test was performed in the
morning. The test was performed in a comfortable
temperature-controlled (24–26°C) environment with closed doors and
windows. After 15 min of daily activity (walk), the armpits of the
subjects were exposed to rule out any interference from their
clothes. A total of 2 designated physicians used the following two
methods to simultaneously evaluate and determine the severity of AO
for each patient, when they scored different levels, the mean
scores were recorded. Each subject was examined three times.
Furthermore, the physicians (JL and JZ) are trained to perform
tests and they have ample experience and are involved in the
development of tests in our laboratory.
Traditional method (TM)
The physicians gradually approached the exposed
armpit and rated the abnormal smell, according to the abnormal
smell grading system, using the following scoring system: 0, none;
1, mild; 2, moderate and 3, severe (Table II) (6,14).
 | Table II.Axillae odor analogue scale. |
Table II.
Axillae odor analogue scale.
| Score | Degree | Definition |
|---|
| 0 | None | No odor detected
within 5 cm |
| 1 | Mild | Odor detected
within 15 cm |
| 2 | Moderate | Odor detected
within 15–30 cm |
| 3 | Severe | Odor detected
>30 cm |
LSM
Patients were instructed to fully expose their
armpits and each side was wiped with cotton swabs 10 times by the
physicians. The cotton swabs were then smelt immediately by two
examiners while holding them 5–10 cm under the examiner's nose.
According to the cotton swab odor degree grading system, the
following scoring was used: 0, none; 1, mild; 2, moderate; and 3,
severe (Table III).
 | Table III.Grading scores of odor degree of the
cotton swabs. |
Table III.
Grading scores of odor degree of the
cotton swabs.
| Score | Degree | Definition |
|---|
| 0 | None | No odor
detected |
| 1 | Mild | Faint peculiar
odor |
| 2 | Moderate | Obvious peculiar
odor |
| 3 | Severe | Pungent odor |
Measurement of pH
The pH value of the underarm skin surface was
evaluated on the bilateral axilla following the olfactory
assessment. In each patient, 3 measurements were obtained at the
center of the axilla and the acquired average was used for
subsequent calculations and statistical analysis. The in
vivo skin surface pH value was measured non-invasively using a
PH 30A pH meter (Clean), consisting of a combined flat glass probe
and a reference electrode in a single probe. The pH meter was
calibrated using standard buffers. The probe was rinsed with
distilled water prior to each measurement.
Microbiome sampling
Sterile cotton swabs soaked with sterile saline were
used to collect samples from the axillary sites of each patient
with AO and each healthy subject. Skin samples were collected by
rubbing the skin 10 times perpendicularly.
DNA extraction and sequencing
The DNA collected on the swabs was isolated by BGI
Genomics and stored at −80°C until further experimentation. The V4
region of the 16S rDNA gene was amplified using the following
primers to generate an amplicon library for each sample: Primer 515
forward, 5′-GTGCCAGCMGCCGCGGTAA-3′ and primer 806 reverse,
5′-GGACTACHVGGGTWTCTAAT-3′ (15).
PCR amplification, cloning and sequencing were performed as
previously described (16). The
fragments in the DNA library, of the expected sizes, were selected
using the Agencourt AMPure XP (Beckman Coulter, Inc.) according to
the manufacturer's protocols and then subjected to qualification
using a Bioanalyzer-2100 (Agilent Technologies, Inc.) and pair-end
sequencing on a Hiseq-2500 (Illumina Inc.) (17).
Statistical analysis
Descriptive parameters, such as the mean and
standard deviation for normally distributed continuous data and the
frequencies and percentages for categorical data were calculated.
Student's t-test was used to evaluate continuous variables and 95%
CIs were compared between the 2 groups. For multiple comparisons,
one-way ANOVA was used to analyze differences among groups. Tukey's
method was applied for the post-hoc test. All tests were 2-tailed
and P<0.05 was considered to indicate a statistically
significant difference. SPSS version 21.0 (IBM Corp.) was used to
perform the statistical analyses.
Results
Comparison of scoring methods to
evaluate axillary odor
The TM was used to evaluate the degree of odor and
14 patients were graded as having mild, 8 as moderate and 10 as
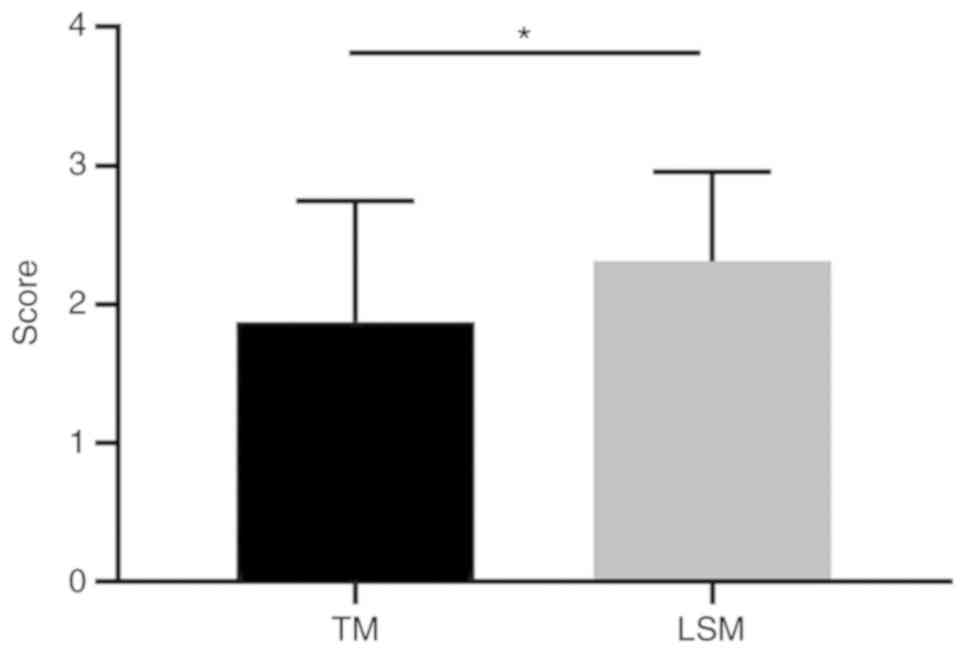
severe axillary odor. The mean TM score was 1.88±0.87. When the LSM
was used to evaluate these patients, 3 were graded as having mild,
16 as moderate and 13 as severe axillary odor, with a mean LSM
score of 2.31±0.64. The difference between the TM and LSM scores
was statistically significant (P=0.03; Fig. 1), demonstrating that the LSM was
more sensitive compared with the TM in evaluating odor severity in
patients with AO.
Comparison of axillary pH
Comparison of the pH values of the left and right
axillae in patients with AO revealed no significant difference
between sites (6.39±0.54 vs. 6.47±0.59; P>0.05). Similarly, the
pH value did not significantly differ between sites in the healthy
volunteers (5.80±0.31 vs. 5.88±0.38; P>0.05; Fig. 2A). The pH value of the ipsilateral
axilla in patients with AO was significantly higher compared with
that in healthy volunteers (P<0.0001; Fig. 2A). This result indicated that an
increased pH in the armpit may be associated with the onset of
AO.
The pH values of the axillae in patients with
different degrees of malodor were also compared and the results
indicated that the axillary pH value in patients graded as having
moderate odor was significantly lower compared with that in
patients graded as having severe odor (6.23±0.29 vs. 6.77±0.70;
P<0.001; Fig. 2B). The pH value
of the axillae in patients graded as having mild odor was also
significantly lower compared with that in patients graded as having
severe odor (6.05±0.07 vs. 6.77±0.70; P<0.0001; Fig. 2B). This suggests that a more severe
condition of the patient was associated with a higher pH of the
armpit.
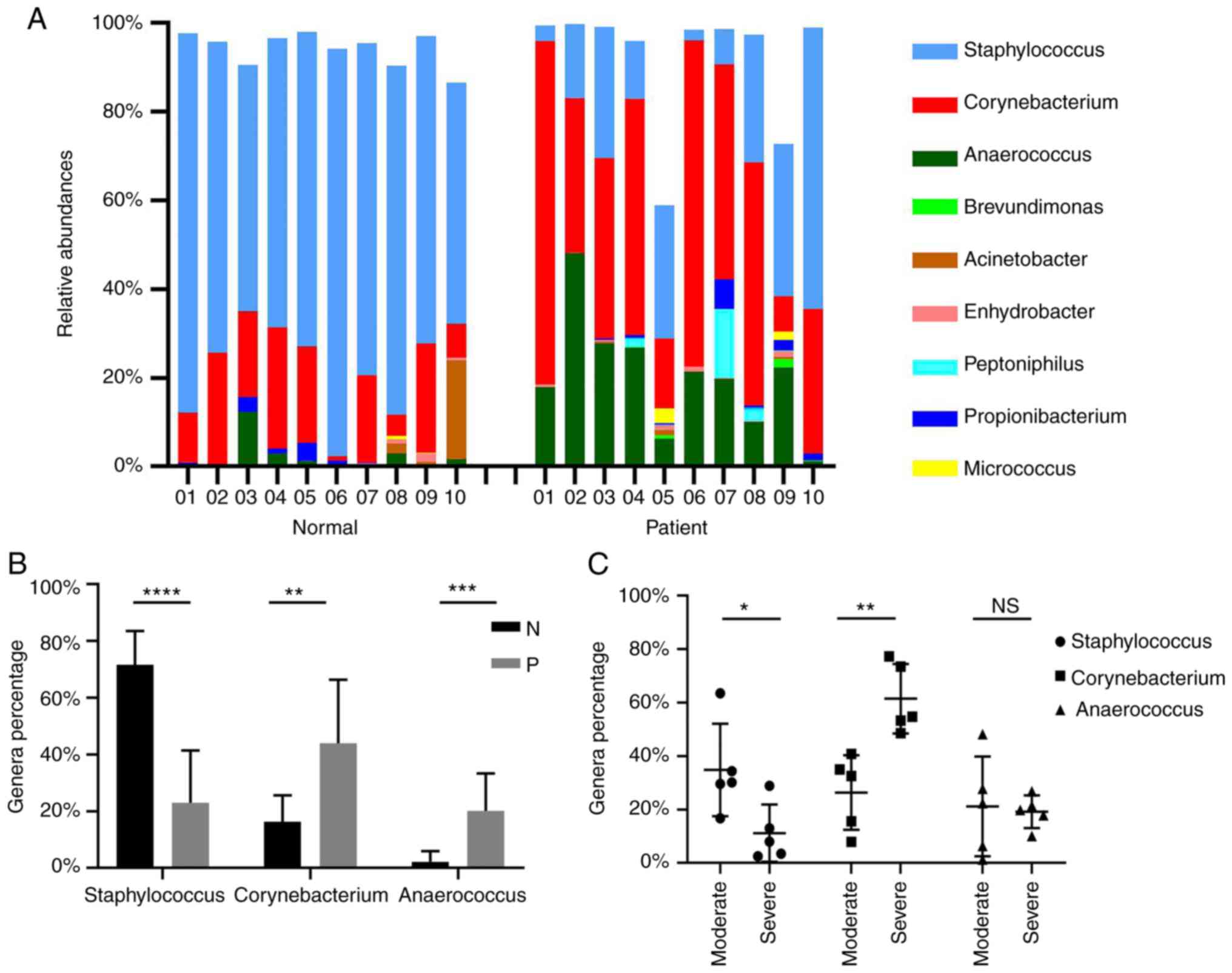
Axillary microecology
As presented in Fig.
3A, there were genus-level changes in the composition of the
microbial community in the case group. The axillary flora was
randomly detected in 10 healthy volunteers and 10 patients with
moderate to severe AO. The major bacteria in the control group were
Staphylococcus and Corynebacterium.
Staphylococcus species accounted for a large proportion of
the bacteria in the armpits (range, 54.42–91.94%; mean, 71.64%),
while the second and third most common bacteria were
Corynebacterium (16.26%) and Anaerococcus (2.20%),
respectively. By contrast, the dominant genus in the malodor group
was Corynebacterium and Anaerococcus (43.94 and
20.17%, respectively). Of note, the proportion of
Staphylococcus species was reduced (23.01%). These results
indicated that AO may be associated with the elevated ratios of
Corynebacterium and Anaerococcus. As the proportion
of Staphylococcus species was decreased in AO, it may be a
useful probiotic for treating AO.
Quantitative comparison of the major flora between
patients and healthy subjects revealed that Staphylococcus
was less prevalent in patients compared with that in healthy
subjects, with an average reduction of 48.63% (23.01±18.45 vs.
71.64±11.89%; P<0.0001), whereas the proportion of
Corynebacterium species was significantly higher in patients
compared with that in the healthy subjects with an average increase
of 27.68% (43.94±22.46 vs. 16.26±9.38; P<0.01). In addition, the
ratio of Anaerococcus species was higher in patients with AO
compared with that in healthy volunteers, with an average increase
of 17.97% (20.17±13.13 vs. 2.20±3.72; P<0.001; Fig. 3B). These results suggested that
Anaerococcus and Corynebacterium species may be
accountable for the production of the strong odor in patients with
AO.
Furthermore, the main flora differences in patients
with various disease severities were also compared (Fig. 3C). The amount of
Corynebacterium species was higher in patients with severe
disease compared with that in those with moderate disease
(61.51±12.99 vs. 26.37±13.94; P<0.01), indicating that
Corynebacterium may also be a strong odor-secreting microbe
(18). There was no significant
difference in the population of Anaerococcus between the
moderate and severe odor groups. The proportion of the
Staphylococcus species exhibited the opposite trend, as it
was reduced in patients with severe odor as compared with that in
patients with moderate odor (11.17±10.72 vs. 34.84±17.33;
P<0.05), suggesting that the proportion may be negatively
associated with the odor degree.
Discussion
In the present study, the LSM was used to determine
odor severity. According to our general experience, using the TM,
moderate odor may be easily misjudged as mild, while the LSM was
more sensitive to distinguish mild from moderate odors compared
with TM. Therefore, it was concluded that LSM was comparatively
more sensitive to evaluate the severity of AO, and it was more
convenient to use in clinical practice.
The pH of the skin surface may also influence the
cutaneous microflora (19).
Impaired acidification of the axillary pH in patients may be a
possible factor for promoting host susceptibility to pathogenic
bacteria. A previous study revealed that patients with diabetes had
a significantly higher axillary pH level compared with that in
healthy controls and candida infection in patients with diabetes
was also associated with the elevated pH value in their armpits
(20). Another study identified an
association between a high skin pH and the growth of microorganisms
that produce malodor (21). In the
present study, the pH value in the axillary fossa of the patients
was significantly higher compared with that in normal controls,
which may be associated with the bacteria that produce odor. The
difference between the pH values of the right and left armpit was
not statistically significant in either group, which was consistent
with previous studies (22,23).
In addition, it was observed that the different odor severities
were significantly associated with different pH values of the
axilla: The pH value in patients with severe AO was significantly
higher compared with that in patients with mild and moderate AO,
indicating that a higher axillary pH value was associated with the
production of a stronger odor.
The production of malodor on the underarm skin is
primarily attributed to the action of the resident microbiota on
odorless natural secretions from the apocrine gland (24). Corynebacterium is one of the
dominant bacterial groups in the armpit microbiota, which has an
important role in malodor generation (25). Furthermore, a previous study
revealed that the Anaerococcus species in sweat contributed
to axillary odor (26). Recently,
an analysis using next-generation sequencing (without culturing)
confirmed that the skin microbiota contains Anaerococcus
(2,27). In the present study, the
proportions of Anaerococcus and Corynebacterium
species in patients with axillary osmidrosis were higher compared
with those in healthy subjects, indicating that Anaerococcus
and Corynebacterium species may contribute to the production
of odor. It was also indicated that different odor severities were
associated with the axillary microbiome composition.
Corynebacterium species were significantly elevated in
patients with severe AO compared with those in patients with
moderate AO, indicating that Corynebacterium was associated
with body odor. The results are also consistent with the data from
a culture-based study (18). On
the contrary, Staphylococcus species were significantly more
prevalent in patients with moderate AO compared with those in
patients with severe AO, in normal subjects, the
Staphylococcus concentration was highest and that they are
the major constituent of a normal armpit flora, indicating that the
Staphylococcus species may be negatively associated with
odor strength and may serve as a potential probiotic. Okamoto et
al (28) determined that at
the genus level, there was no direct association between
Staphylococcus species counts and malodor intensity in the
axilla; however, this may not preclude a role of these
microorganisms in the production of odorant There was no
significant difference in the proportion of Anaerococcus
between patients with moderate disease and those with severe
disease. The possible reasons for this include the following: i)
Anaerococcus grows slowly, so no significant difference may
be observed over a short period of time (29,30);
and ii) the insufficient sample size was unable to reduce variances
due to individual variations in Anaerococcus susceptibility
to environmental factors; and iii) the characteristics of their
sensitivity to oxygen (29).
The technological limitations of 16S rDNA sequencing
allow it to identify only a small proportion of strains at the
species level. Most bacteria may only be authenticated at the genus
level. Furthermore, the small number of physicians performing the
smell scoring may be a limitation of the present study. Additional
further exploratory research is urgently required to increase the
understanding of the association between the microbiome and odor
generation.
In conclusion, the present study demonstrated that
the LSM was more sensitive in detecting odor severity and more
convenient for use in the clinical setting than the TM. The
axillary pH value of patients with AO was higher compared with that
in healthy subjects, and a more severe the condition of AO was
associated with a higher the pH value in the armpit.
Corynebacterium and Anaerococcus may be pathogenic
bacteria of AO, while the role of Staphylococcus species as
a potential probiotic requires confirmation in further clinical
trials.
Acknowledgements
Not applicable.
Funding
The current study was funded by New Xiangya Talent
Project of the Third Xiangya Hospital of Central South University
(grant no. 20170309 to JL).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the SRA repository (https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA646090)
under the accession number PRJNA646090.
Authors' contributions
JL conceived and designed the present study. JL, JZ
and HD performed the experiments. JZ and SD analyzed the data and
prepared the images. LG analyzed the data and discussed the
results. HD and LG drafted the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the institutional
review board of the Third Xiangya Hospital, Central South
University (Hunan, China). All patients provided written informed
consent. For participants below the age of 18 years, the legal
guardians provided written consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Toyoda Y, Gomi T, Nakagawa H, Nagakura M
and Ishikawa T: Diagnosis of human axillary osmidrosis by
genotyping of the human ABCC11 gene: Clinical practice and basic
scientific evidence. Biomed Res Int. 2016:76704832016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
James AG, Austin CJ, Cox DS, Taylor D and
Calvert R: Microbiological and biochemical origins of human
axillary odour. FEMS Microbiol Ecol. 83:527–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wood A and Kelly D: Skin microbiology,
body odor, and methylotrophic bacteria. Handbook Hydrocarbon Lipid
Microbiol. 2010:3204–3213. 2010.
|
|
4
|
Chen H, Yang G, Li Y, Li X and Du J:
Expression of apolipoprotein D and androgen receptor in axillary
osmidrosis and its molecular mechanism. Int J Clin Exp Med.
6:497–503. 2013.PubMed/NCBI
|
|
5
|
Jung SW, Lee SJ and Park HR: Comparison of
outcomes of two methods of axillary osmidrosis surgery: Subdermal
excision versus liposuction combined with diode laser ablation.
Arch Aesthetic Plast Surg. 26:20–27. 2020. View Article : Google Scholar
|
|
6
|
Wang R, Yang J and Sun J: A minimally
invasive procedure for axillary osmidrosis: Subcutaneous curettage
combined with trimming through a small incision. Aesthetic Plast
Surg. 39:106–113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Memarian F, Amani-Tehran M and Latifi M:
Rank ordering and image processing methods aided fabric wrinkle
evaluation. Fibers Polym. 12:8302011. View Article : Google Scholar
|
|
8
|
Egert M, Höhne HM, Weber T, Simmering R,
Banowski B and Breves R: Identification of compounds inhibiting the
C-S lyase activity of a cell extract from a Staphylococcus
sp. isolated from human skin. Lett Appl Microbiol. 57:534–539.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao Z, Tseng CH, Pei Z and Blaser MJ:
Molecular analysis of human forearm superficial skin bacterial
biota. Proc Natl Acad Sci USA. 104:2927–2932. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fredrich E, Barzantny H, Brune I and Tauch
A: Daily battle against body odor: Towards the activity of the
axillary microbiota. Trends Microbiol. 21:305–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tarun J, Susan J, Suria J, Susan VJ and
Criton S: Evaluation of pH of bathing soaps and shampoos for skin
and hair care. Indian J Dermatol. 59:442–444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ali SM and Yosipovitch G: Skin pH: From
basic science to basic skin care. Acta Derm Venereol. 93:261–267.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai Y, Xu AE and He J: A refined surgical
treatment modality for bromhidrosis: Subcutaneous scissor with
micropore. Dermatol Ther. 30:2017. View Article : Google Scholar
|
|
14
|
He J, Wang T and Dong J: A close positive
correlation between malodor and sweating as a marker for the
treatment of axillary bromhidrosis with botulinum toxin A. J
Dermatolog Treat. 23:461–464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ring HC, Thorsen J, Saunte DM, Lilje B,
Bay L, Riis PT, Larsen N, Andersen LO, Nielsen HV, Miller IM, et
al: The follicular skin microbiome in patients with hidradenitis
suppurativa and healthy controls. JAMA Dermatol. 153:897–905. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grice EA, Kong HH, Conlan S, Deming CB,
Davis J, Young AC; NISC Comparative Sequencing Program, ; Bouffard
GG, Blakesley RW, Murray PR, et al: Topographical and temporal
diversity of the human skin microbiome. Science. 324:1190–1192.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fadrosh DW, Ma B, Gajer P, Sengamalay N,
Ott S, Brotman RM and Ravel J: An improved dual-indexing approach
for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq
platform. Microbiome. 2:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Troccaz M, Gaia N, Beccucci S, Schrenzel
J, Cayeux I, Starkenmann C and Lazarevic V: Mapping axillary
microbiota responsible for body odours using a culture-independent
approach. Microbiome. 3:32015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rippke F, Berardesca E and Weber TM: pH
and microbial infections. Curr Probl Dermatol. 54:87–94. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Behm B, Schreml S, Landthaler M and
Babilas P: Skin signs in diabetes mellitus. Eur Acad Dermatol
Venereol. 26:1203–1211. 2012. View Article : Google Scholar
|
|
21
|
Kemper M, Bielfeldt S, Knie U, Wilhelm KP
and Abels C: Significant reduction of body odor in older people
with a pH 4.0 emulsion. Cosmetics. 2:136–145. 2015. View Article : Google Scholar
|
|
22
|
Williams S, Davids M, Reuther T, Kraus D
and Kerscher M: Gender difference of in vivo skin surface pH in the
axilla and the effect of a standardized washing procedure with tap
water. Skin Pharmacol Physiol. 18:247–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stenzaly-Achtert S, Schölermann A,
Schreiber J, Diec KH, Rippke F and Bielfeldt S: Axillary pH and
influence of deodorants. Skin Res Technol. 6:87–91. 2008.
View Article : Google Scholar
|
|
24
|
Ren Y, Liu W, Chen J, Wang J, Wang K, Zhou
J, Cai S, Zheng M, Liu J, Liu L and Xue D: A missense variant of
the ABCC11 gene is associated with axillary osmidrosis
susceptibility and clinical phenotypes in the Chinese han
population. Sci Rep. 7:463352017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li M, Budding AE, van der Lugt-Degen M,
Du-Thumm L, Vandeven M and Fan A: The influence of age, gender and
race/ethnicity on the composition of the human axillary microbiome.
Int J Cosmet Sci. 41:371–377. 2019.PubMed/NCBI
|
|
26
|
Fujii T, Shinozaki J, Kajiura T, Iwasaki K
and Fudou R: A newly discovered Anaerococcus strain
responsible for axillary odor and a new axillary odor inhibitor,
pentagalloyl glucose. FEMS Microbiol Ecol. 89:198–207. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Minhas GS, Bawdon D, Herman R, Rudden M,
Stone AP, James AG, Thomas GH and Newstead S: Structural basis of
malodour precursor transport in the human axilla. Elife.
7:e349952018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okamoto H, Koizumi S, Shimizu H, Cho O and
Sugita T: Characterization of the axillary microbiota of Japanese
male subjects with spicy and milky odor types by pyrosequencing.
Biocontrol Sci. 23:1–5. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murphy EC and Frick IM: Gram-positive
anaerobic cocci-commensals and opportunistic pathogens. FEMS
Microbiol Rev. 37:520–553. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Badri M, Nilson B, Ragnarsson S, Senneby E
and Rasmussen M: Clinical and microbiological features of
bacteraemia with gram-positive anaerobic cocci: A population-based
retrospective study. Clin Microbiol Infect. 25:760.e1–760.e6. 2019.
View Article : Google Scholar
|