Introduction
T-2 toxin
(4β,15-diacetoxy-8α-(3-methylbutyryloxy)-3α-hydroxy-12,
13-epoxytrichothece-9-ene;
C24H34O9) (relative molecular
weight 466.52), a type A trichothecene mycotoxin, is the secondary
metabolite of Fusarium sporotrichioides and F.
Langsethiae (1,2). T-2 toxin is found extensively in
moldy cereals (wheat, maize, barley and oats) and moldy food
(3). It is acknowledged as an
unavoidable contaminant in human foods (4). Exposure to T-2 toxin causes oral
injury (5), liver injury (6), decrease in body weight,
gastrointestinal injury and even mortality (7). The severe tissue damage caused by T-2
toxin is associated with the inhibition of protein and DNA
synthesis, metabolic alteration, cell membrane injury,
immunosuppression, and glycoprotein and collagen synthesis
(8–10), thus resulting in apoptosis. T-2
toxin poses great harm to the health of human beings and
livestock.
Early studies on T-2 toxin only focused on
toxicology and metabolism aspects (11–13).
In the 20th century, it was reported that T-2 toxin has toxic
effects on a number of cancer cell types (14). T-2 toxin can induce apoptosis in
HL-60 promyelotic leukemia cells and hepatocellular carcinoma cells
(15). The mechanism of apoptosis
induced by T-2 toxin is proposed to be linked with oxidative stress
and the activation of caspase 3/9 and the mitochondrial pathway
(16,17). Therefore, the toxin possesses
potential applications in tumor treatment. However, due to the
toxicity of T-2 toxin on normal cells, T-2 by itself does not
exhibit selectivity for tumoral tissues and hence might be
characterized by a low therapeutic index. A search for an
alternative strategy is required.
The use of targeted drugs is a valuable method to
solve the aforementioned selectivity issues (18). Liposomes are phospholipid bilayer
vesicles that possess great potential for application in the
targeted delivery of chemotherapeutics in the treatment of cancer
(19). The use of liposomes as
drug carriers for chemotherapeutic targeting to tumor tissues is
based on their greater advantages compared with other dosage
methods, due to their low systemic toxicity, bioavailability and
the capability to enhance the solubility of a range of
chemotherapeutic agents, in addition to their ability for
encapsulation of hydrophilic and lipophilic drugs (20). Liposomes can reduce drug toxicity
without changing drug efficacy against tumor cells, making them a
highly efficient targeting drug carrier (21). Liposomes enhance the anticancer
drug therapeutic index by increasing the drug concentration in
tumor cells through tumor targeting (22). The most advanced targeting
strategies proposed for treating cancer involve the development of
multifunctional liposomes, with combined targeting mechanisms.
There are a number of types of targeting strategies for liposomes,
such as temperature-, light-, redox reagent- and pH-sensitive
(23–25). Due to their characteristics of
targeting the acidic tumor microenvironment (pH 6.8–6.5),
pH-sensitive liposomes have received much attention recently
(26,27). pH-sensitive liposomes consist of
phosphatidylcholine or dioleoylphosphatidylethanolamine (DOPE),
which are stable at physiological pH (pH 7.4), but undergo
destabilization under acidic conditions (28). Various antitumor drug-containing
pH-sensitive liposomes have been successfully prepared, such as
those for 5-fluorouracil, doxorubicin (DOX) and taxol (29–31).
The objective of the present study was to design and
optimize the preparation process of a novel pH-sensitive liposomal
delivery system containing T-2 toxin (LP-pHS-T2). The particle
size, stability and pH-sensitivity of the liposomes in buffers were
determined. Furthermore, the antitumor activity of pHS-LP-T2 was
evaluated in vitro. This is an exploration of T-2 toxin as a
new antitumor drug.
Materials and methods
Reagents
1,2-Dioleoyl-3-trimethylammonium-propane (DOTAP),
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene
glycol)-2000] (DSPE-mPEG-2000),
1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), dipalmitoyl
phosphatidylcholine (DPPC), DOPE and cholesterol (chol) were
purchased from Shanghai Dongshang Biotechnology Co., Ltd.
3-(4,5-Dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT),
Hoechst 33342, propidium iodide (PI), NaCl, HCl, high performance
liquid chromatography (HPLC) grade methanol and
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) sodium
salt were purchased from Thermo Fisher Scientific, Inc. Dulbecco's
modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were
purchased from Gibco; Thermo Fisher Scientific, Inc.
T-2 toxin was separated and purified from corn
contaminated with Fusarium sporotrichioides by graduated
organic solvent extraction, silica column chromatography and
preparative HPLC, as previously described (32). Purity, >98%, the
three-dimensional HPLC chromatogram of T-2 toxin is shown in
Fig. S1.
The A549, Hep-G2, MKN-45 and K562 cell lines are
human tumor cell lines and were kept at −80°C; L929 cells are mouse
fibroblast cells (normal cells) and were kept at −80°C. All cell
lines were purchased from the American Type Culture Collection. All
cells were incubated in DMEM with 10% FBS and 1% antibiotics in a
humidified atmosphere containing 5% CO2/95% air at 37°C.
The Hep-G2 cells were authenticated as a hepatoma cell line using
short tandem repeats (STR) profiling.
HPLC analysis of T-2 toxin
The concentration of T-2 toxin was determined using
a HPLC system (Waters Corporation) equipped with an Agilent ZORBAX
SB-C3 column (250×4.6 mm, 5 µm; Agilent Technologies Inc.). The
mobile phase was methanol/water (60:40, v/v) driven by a double
pump (Waters 150; Waters Corporation) at a flow rate of 1 ml/min.
The amount of T-2 toxin was detected at absorption wavelengths of
198 nm with an injection volume of 10 µl at 30°C. Each sample was
spiked with 6 ng/ml T-2 toxin as the internal standard. Each run
was performed in triplicate. T-2 toxin limit of detection was
determined by dissolving T-2 toxin at decreasing concentrations in
methanol until the signal-to-noise ratio was equal to 3. According
to the previous methods (33), the
linearity of the standard curves, intraday and interday precision,
and accuracy were determined using six T-2 toxin concentrations of
100, 200, 400, 600, 800 and 1,000 µg/ml.
Preparation of liposomes
Due to the hydrophobicity of T-2 toxin and its high
solubility in methanol, T-2 toxin was wrapped in the lipid bilayer
of the liposome. T-2 toxin-loaded pH-sensitive liposomes were
prepared using the thin-film hydration method (34). In brief, T-2 toxin or
DPPC:DOPE:chol at 1:2:1 (weight:weight:weight) was dissolved in
ethanol, respectively. T-2 toxin and phospholipid mixture were
mixed in a ratio of 1:1, 1:2, 1:3, 2:1, 4:1, 5:1, 6:1 or 10:1 and a
total phospholipid concentration of 5, 10, 15, 20 or 25 mg/ml,
respectively. The ethanol was then removed using a rotary
evaporator at 40°C, until a uniform film was formed at the bottom
of the flask. The film was hydrated with an appropriate volume of
20 mM HEPES buffer solution for 1 h. Liposomes were sonicated with
a 20-kHz frequency probe-type sonicator (Xinhi Biolab Co., Ltd.) at
300 W for 10 min with 5-sec intervals in an ice bath. After
ultrasonication, titanium particles released from the probe were
removed by centrifugation at 2,000 × g for 10 min at room
temperature. Free T-2 toxin was removed by ultrafiltration with a
300 K membrane filter (pore size 0.2 µm; Sartorius Stedim Biotech;
Sartorius AG) at 6,000 × g for 30 min at room temperature. Finally,
the liposomes were filtered through a NanoAble-150 Extruder (PhD
Technology LLC) equipped with a 200-µm pores of polycarbonate
membrane three times. An equal volume of 60% methanol was added to
the liposomes to release T-2 toxin from the inside of the
liposomes. The amount of released T-2 toxin in the liposomes was
determined by HPLC and the entrapment efficiency of LP-pHS-T2 was
calculated according to the following equation: EE (%) =
Wencapsulated/Wtotal ×100, where EE is
entrapment efficiency, Wtotal is the total amount of T-2
toxin initially added in the liposome preparation and
Wencapsulated is the amount of T-2 toxin encapsulated
into the liposomes.
Particle size and ζ potential
The particle diameter, polydispersity index (PDI)
value and ζ potential of LP-pHS-T2 were measured by laser light
scattering using a particle size analyzer according to the
manufacturer's protocol (Zetasizer 3000HSA; Malvern Instruments,
Ltd.) (35). The determination was
repeated three times for each sample.
Morphology
The morphology of the LP-pHS-T2 was observed using a
transmission electron microscope (TEM; FEI; Thermo Fisher
Scientific, Inc.). For TEM studies, the LP-pHS-T2 was two-fold
diluted with deionized water, and the final dilution of 0.25 mg/ml
was placed on the surface of a copper grid. Next, 2% aqueous
solution of sodium phosphotungstate was added for negative staining
at room temperature for 15 min. Following air-drying, the copper
grid was placed in the TEM (magnification, ×2,000) and imaged using
Gatan DigitalMicrograph software version 1.4.3 (Gatan, Inc.)
(35).
Drug release profile in vitro
The release of T-2 toxin from LP-pHS-T2 in
vitro was monitored using a dialysis method (35). LP-pHS-T2 (1 ml) was added into a
dialysis bag with a molecular weight cutoff of 6,000-8,000 Da and
immersed in 20 ml phosphate-buffered solution (pH 7.4) at 37°C for
0, 1, 2, 4, 8, 12, 24, 48 or 72 h. An equal volume of 60% methanol
was added to the liposomes. The amount of T-2 toxin released at
each time point was determined by HPLC.
Stability of LP-pHS-T2 at different pH
values
LP-pHS-T2 (1 ml) was added into a 6,000 to 8,000-Da
molecular weight-cutoff dialysis bag and immersed in 1/15 mol/l
disodium hydrogen phosphate-potassium dihydrogen phosphate buffer
(pH 5) or a series of 0.2 mol/l disodium phosphate-sodium
dihydrogen phosphate buffers (pH 5, 5.5, 6, 6.5 and 7) at 37°C for
30 min. An equal volume of 60% methanol was added to the liposomes.
The amount of T-2 toxin released was determined by HPLC as
aforementioned.
Antitumor activity of LP-pHS-T2
Cytotoxicity assay
MTT assay was used to evaluate the cytotoxicity of
T-2 or LP-pHS-T2 on A549, Hep-G2, MKN-45, K562 and L929 cell lines
(36). The cells were seeded on
96-well culture plates at a density of 5×105 cells/well.
Following incubation overnight, fresh medium containing T-2 toxin
or LP-pHS-T2 at final concentrations of 0.5, 5, 10 and 15 µg/ml was
added to the cells at 37°C. After incubation for 48 h at 37°C, the
plates were washed with PBS and incubated with 5 mg/ml MTT for 4 h
at 37°C in darkness. The supernatant was aspirated and 100 µl DMSO
was added to each well to dissolve the purple formazan crystals.
After continuous agitation for 15 min, the reaction product was
quantified by measuring the absorbance at 495 nm using a
Multi-plate Reader (Model 680; Bio-Rad Laboratories Inc.). The
IC50 values for each cell line were calculated using the
GraphPad prism 7.0 software (GraphPad Software, Inc.) and compared
between T-2 toxin and LP-pHS-T2.
Apoptosis detection using Hoechst
staining
Hep-G2 cells were randomly selected from the four
tumor cell lines and cultured in six-well plates for 24 h at 37°C,
with a density of 5×105 cells/well. The cells were
treated with 10 µg/ml of T-2 or LP-pHS-T2 at 37°C. After 4 h of
incubation, the cells were fixed with 4% paraformaldehyde at 37°C,
followed by staining with Hoechst 33342 to stain the nucleus for 30
min at 37°C in darkness (36).
Cell imaging was performed with a fluorescence inverted microscope
combined with cellSens standard version 1.5 software
(magnification, ×20; IX70; Olympus Corporation). Areas of cells
stained with blue fluorescence were imaged. Each group was images
three times and the picture with the most stained cells was
selected.
Migration assay
The Hep-G2 cells were plated in 6-well plates,
cultured to 100% confluence at 37°C for 24 h, and then scratched
with a p200 pipette tip (diameter, 0.57 mm). The plates were washed
with PBS three times. Fresh serum-free medium containing 10 µg/ml
T-2 toxin or LP-pHS-T2 was added to the cells. After 24-h culture,
the distances of migrating cells were analyzed to evaluate the cell
migratory ability by a fluorescence inverted microscope combined
with cellSens standard version 1.5 software (magnification, ×20;
IX70; Olympus Corporation), which was performed as previously
described (37). The average width
of the wound was measured.
Apoptosis detection via flow
cytometry
The Hep-G2 cells (5×105 cells/sample)
were incubated in 6-well plates at 37°C. T-2 or LP-pHS-T2 (10
µg/ml) was added to the cells, which were incubated at 37°C for 18
h. Apoptosis was measured by a PI/Annexin V-FITC dual-staining kit
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocols, and a BD Accuri™ C6 flow cytometer (BD Biosciences)
combined with cFlow version 1.023.1 software, as previously
described (38). Each assay was
repeated in triplicate.
Statistical analysis
All data were analyzed by Origin 8.0 (OriginLab
Corporation) and are presented as the mean ± standard deviation.
All data were measured in triplicate. The results were in normal
distribution. The statistical significance among multiple groups
was evaluated using one-way ANOVA followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
T-2 toxin detection using HPLC
HPLC was used to determine the concentration of T-2
toxin. The standard curve, the intraday precision, the interday
precision, the accuracy and the limit of detection were
investigated (Table I); the linear
regression equation was y=11393.41×+14937.51 (where × =
concentration of T-2 toxin in µg/ml and y = the peak area) and the
coefficient of determination was R2=0.999, indicating
good linearity. Intraday (n=3) and interday (n=3) precision was not
>10% in any of the assays. The limit of detection was 14.78±0.85
µg/ml.
 | Table I.Establishment of T-2 toxin
detection. |
Table I.
Establishment of T-2 toxin
detection.
| Detection
parameter | Value |
|---|
| RSD, % |
|
|
Intraday precision | 1.42±0.14 |
|
Interday precision | 1.94±0.58 |
|
Accuracy | 2.93±0.15 |
| Limit of detection,
µg/ml | 14.78±0.85 |
Preparation and characterization of
liposomes
Optimization of liposome preparation
process by a single factor experiment
On the basis of preliminary experiments, the effects
of six influencing factors [type of phospholipid, DPPC:DOPE (w/w),
phospholipids:chol (w/w), hydration volume, phospholipid
concentration and drug-lipid ratio] on EE values were investigated
by a single factor experiment. As presented in Fig. 1A, several phospholipids commonly
used in the preparation of pH-sensitive liposomes, including DOTAP,
DOPE, DPPC, DSPE-mPEG-2000 and DOPC, were investigated. DOPE and
DPPC were the most efficient phospholipids for encapsulating T-2
toxins, hence were chosen for the following experiments. Similarly,
the highest EE values was obtained when the T-2 toxin or
DPPC:DOPE:chol=1:2:1 (w/w/w) were mixed at the total phospholipid
concentration of 20 mg/ml and drug-lipid ratio of 2:1 (w/w) and
hydrated with 10 ml of 20 mM HEPES buffer solution as shown in
Fig. 1B-F. Next, after sonication
and extrusion, the maximum EE was 95±2.43%.
 | Figure 1.Factors that influence the EE values
of liposomes. Effects of (A) types of phospholipid, (B) DPPC:DOPE
(w/w), (C) phospholipids:cholesterol (w/w), (D) hydration volume,
(E) phospholipid concentration and (F) drug-lipid ratio on EE
investigated by a single factor experiment. Data are expressed as
mean ± standard deviation (n=3). DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane; DOPE,
dioleoylphosphatidylethanolamine; DPPC, dipalmitoyl
phosphatidylcholine; DSPE-mPEG-2000,
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene
glycol)-2000]; DOPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine; EE,
entrapment efficiency. |
Characterization of LP-pHS-T2
The mean particle size of LP-pHS-T2 was ~267 nm
before extrusion (Fig. 2A) and 100
nm after extrusion (Fig. 2B).
These nanoparticles ranging from 100–150 nm possess advantages in
controllable pore diameter and biocompatibility (39). Data from the particle size analyzer
showed that the ζ potential was −29.3 mV, which demonstrated that
LP-pHS-T2 was stable at room temperature (40). The PDI value was 0.216, which
indicated a moderate dispersion of nanoparticles (data not shown).
The morphology of LP-pHS-T2 was found to be homogeneous and
spherical when visualized under a TEM (Fig. 2C).
Release profile of LP-pHS-T2 at
different time points and pH values
The release profile of T-2 toxin from LP-pHS-T2
in vitro was monitored within 72 h at 37°C (pH 7.4). The
results are shown in Fig. 3A. The
release profile demonstrated a two-phase downward trend, with fast
leakage of T-2 toxin in the first 6 h (~20% released), followed by
sustained release up to 48 h (~46% released). From 48 to 72 h, the
leakage rate increased (~76% released), until reaching a minimum at
72 h. The stability of LP-pHS-T2 at different pH values is shown in
Fig. 3B. The release amount of T-2
toxin was up to 91.2% when the pH was 6.5, which indicated that
LP-pHS-T2 may be structurally unstable under this faintly acid
condition and release T-2 toxin.
Antitumor activity of LP-pHS-T2
LP-pHS-T2 possesses cytotoxicity
effects on tumor cells
MTT assay was employed to test the inhibition rate
of T-2 toxin and LP-pHS-T2 on a series of tumor cells and a normal
cell line, L929. The IC50 values of each group are shown
in Table II. T-2 toxin and
LP-pHS-T2 exhibited good antitumor activity at the same
concentration (P>0.05), which indicated that the proposed
liposomal formulation did not noticeably reduce the therapeutic
index and that T-2 toxin could be released gradually. However,
possibly due to the sustained release of liposomes, only the
IC50 values of LP-pHS-T2 on K562 cells were slightly
higher compared with T-2 toxin alone. In addition, the
IC50 value of LP-pHS-T2 in normal cells (L929 cells) was
significantly higher compared with that of T-2 toxin (P<0.05),
which demonstrated the reduction of T-2 side effects as result of
its encapsulation.
 | Table II.IC50 values of T-2 toxin
and LP-pHS-T2 by
3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide assay
at pH 7.4. |
Table II.
IC50 values of T-2 toxin
and LP-pHS-T2 by
3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide assay
at pH 7.4.
| Cell line | T-2 toxin,
ng/ml | LP-pHS-T2,
ng/ml |
|---|
| A549 | 150.68±0.85 | 174.38±2.46 |
| HepG-2 | 210.41±8.14 | 253.41±5.47 |
| MKN-45 | 213.13±6.48 | 249.36±9.61 |
| K562 | 11.59±1.05 |
43.42±2.87a |
| L929 | 1437.53±20.80 |
1864.24±20.47b |
Apoptosis analysis using Hoechst
staining
The nucleus of apoptotic cells can be stained dense
blue using Hoechst 33342. The results from the present study are
shown in Fig. 4A and they
demonstrate that T-2 toxin and LP-pHS-T2 at dose of 10 µg/ml
markedly induced apoptosis in Hep-G2 cells, as indicated by the
enhanced intensity of blue fluorescence.
Wound healing assay
Certain tumor cells are capable of migration. Thus,
a wound-healing assay was performed to observe the inhibitory
effect of T-2 toxin and LP-pHS-T2 on the migration ability in
Hep-G2 cells. The results are shown in Fig. 4B. After a 24-h incubation, the
wound areas in the control group were almost healed. By contrast,
the migration ability in the Hep-G2 cells was significantly
inhibited after T-2 toxin or LP- pHS-T2 treatment at 10 µg/ml as
shown in Fig. 5 (P<0.05).
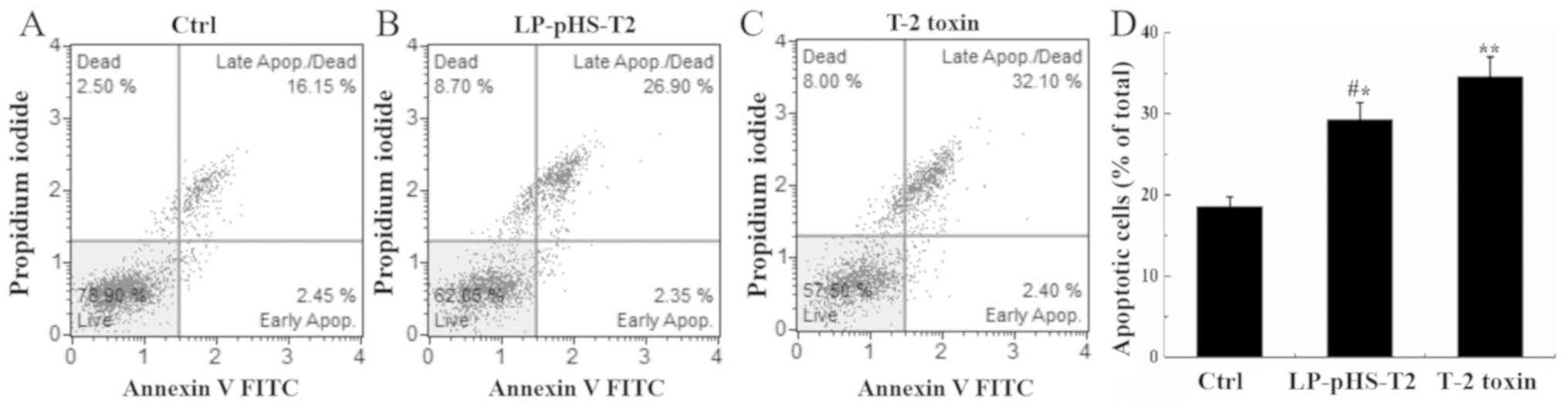
Apoptosis detection via flow
cytometry
The nucleic acid dye PI and Annexin V can
differentiate early apoptosis from late apoptosis and necrotic
cells. As shown in Fig. 6, the
percentage of total apoptotic cells increased nearly two-fold after
T-2 toxin or LP-pHS-T2 treatment in Hep-G2 cells compared with that
in the control group (P<0.05).
Discussion
It is known that T-2 toxin has strong toxicity
(41,42) and thus a great advantage in killing
cancer cells. However, due to the toxic side effects on normal
cells, the use of T-2 toxin in the clinic is limited. Studies on
targeted agents appear to be the only solution. Attempts have been
made to prepare T-2 toxin-conjugated antibody drugs, but no
substantial progress has occurred (43).
As a novel type of nano drug carrier, pH-sensitive
liposomes have been widely studied in tumor therapy for their
advantages of tumor-targeting and sustained release (44). Changes in pH in the tumor
microenvironment can cleave the linkages between liposomes and
drugs, and prompt drug release to the specific tumor tissues
(45). In a previous study, DOX
loaded into pH-sensitive micelles exhibited an enhanced cytotoxic
effect in MCF-7 cancer cells (46). Dextran sulfate-DOX and
alginate-cisplatin polymer-drug complex-loaded liposomes also
exhibit specific receptor-mediated endocytic uptake in cancer cells
(47). In the present study, a
pH-sensitive liposome containing T-2 toxin was prepared. In the
preparation process, the EE value was affected mainly by the type
of biomaterials, the loading method, the hydration volume and the
drug-lipid ratio. The density of T-2 toxin is twice that of
phospholipids. As a fat-soluble small molecule drug, T-2 toxin is
mainly wrapped in the voids of the phospholipid bilayer (48,49).
In the present study, the packaging of T-2 toxin was achieved under
the condition of a higher drug-to-lipid ratio compared with other
reports (25,26). Ultrasound and extrusion steps
showed significant effects on the particle size. Following
ultrasound, the particle size was distributed in a wide range from
100–500 nm, which was improved after extrusion. Following the
optimization of the preparation process, the EE value of LP-pHS-T2
reached 95±2.43%. The morphology of LP-pHS-T2 was a spherical shape
~100 nm in diameter. LP-pHS-T2 was stable at pH 7.4. The release
profile showed a two-phase downward trend starting with a quick
release within 10 h, followed by a slow release until reaching a
minimum EE value at 72 h. In the measurement of sensitivity to pH
of LP-pHS-T2, the remaining T-2 toxin in the sample was immediately
detected after incubation for 30 min. It was identified that
LP-pHS-T2 released the most toxin at pH 6.5 in the first 30 min of
incubation, which indicated that LP-pHS-T2 was extremely sensitive
to this pH value, which may be due to the fusogenic properties of
lipids. When the pH decreased to 6.5, the carboxyl groups of DPPC
and DOPE were sensitively protonated and formed a hexagonal phase
structure, which accelerated the drug release by membrane fusion
(50). By contrast, at pH values
higher or lower than 6.5, only slight amounts of T-2 toxin were
released within 30 min. Based on the natural active targeting
properties of liposomes, it can be reasonably hypothesized that
LP-pHS-T2 may target the pathological tissues, including cancer,
inflammation and infection sites, and ischemic areas, in which the
pH is known to be lower compared with normal tissue (51).
The antitumor effects of LP-pHS-T2 were tested on a
series of tumor cells in vitro by MTT assays, with T-2 toxin
as the control. The data demonstrated that LP-pHS-T2 can inhibit
the proliferation of carcinoma cells. Different types of cells
exhibited different degrees of tolerance; K562 cells were most
sensitive to T-2 toxin compared with A549, Hep-G2, MKN-45 and L929
cell lines. The difference in IC50 values for different
cancer cells was due to the toxic mechanism of T-2 toxin. T-2 toxin
inhibits cell proliferation by inhibiting some key enzymes involved
in protein and nucleic acid synthesis (8). So the greater toxicity of T-2 toxin
might be observed in the more vigorously proliferative cells.
Furthermore, the IC50 value of LP-pHS-T2 on L929 cells
was increased by up to 1.3-fold compared with that of T-2 toxin,
which indicated that the side effects of T-2 toxin were decreased.
However, although these IC50 values were statistically
different, as a potential antitumor drug, the safety of LP-pHS-T2
has not been able to meet clinical requirements due to the lack of
preclinical studies and its toxicity to normal cells. It remains
necessary to modify or further verify its safety through more
experiments. Following LP-pHS-T2 and T-2 toxin treatment, apoptosis
and cell death occurred, and the migration ability of Hep-G2 cells
was significantly inhibited. However, compared to the non-treated
group, T-2 toxin and LP-pHS-T2 only caused a slightly increased
cell cycle arrest at the G0/G1 phase. The
mechanism of apoptosis induced by T-2 toxin may be a non-cell cycle
dependent pathway (data not shown). Moreover, considering that the
pH-sensitive liposomes can be recognized and sequestered by the
phagocytes of the reticulo-endothelial system (RES), the clinical
use of LP-pHS-T2 remains a future prospect. To avoid their uptake
by RES, further reduce the side effects and prolong circulation
time, grafting of the liposomal membranes with pegylated
phospholipids (52), construction
of a programmed nano-selenium overcoat nanoparticles for T-2 toxin
(53), or combination of T-2 toxin
and multiple targeting carriers may be the solution for LP-pHS-T2
targeted therapy (54).
In summary, the present study investigated
LP-pHS-T2, a novel pH-sensitive liposome delivery system containing
T-2 toxin; it not only has a release ability in the tumor
microenvironment, but also has advantageous antitumor activity
in vitro. Additionally, due to the encapsulation of
liposomes, the side effects of T-2 toxin are relatively reduced.
The present study provided a novel approach for the development of
T-2 toxin-based anticancer drugs. However, it is important to note
that the mechanism and the modification of LP-pHS-T2 on tumor cells
require further studies.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Program of
Administration of Traditional Chinese Medicine of Jilin Province,
P.R. China (grant no. 2020132), the Research and Development of
Industrial Technology' Program of Jilin Province, P.R. China (grant
nos. 20180623045TC and 20170204005YY), the Program of Jilin Science
and Technology Bureau, P.R. China (grant nos. 2019001179 and
20200104093) and the Start Funding of Jilin Medical University
(grant no. 2017kyqd001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YD and YL prepared the liposomes. GM performed the
HPLC. JG was responsible for cell culture. MY evaluated the pH
sensitivity and stability of liposomes. HX analyzed the data
regarding the characterization of liposomes. JZ and WZ investigated
the antitumor activity of drugs. ML and YL optimized the
preparation conditions of liposomes. HW analyzed all the data and
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu J, Zhou Y, Yuan Z, Yi J, Chen J, Wang N
and Tian Y: Autophagy and apoptosis interact to modulate T-2
toxin-induced toxicity in liver cells. Toxins (Basel). 11:452019.
View Article : Google Scholar
|
|
2
|
Yang L, Tu D, Wang N, Deng Z, Zhan Y, Liu
W, Hu Y, Liu T, Tan L, Li Y, et al: The protective effects of
DL-selenomethionine against T-2/HT-2 toxins-induced cytotoxicity
and oxidative stress in broiler hepatocytes. Toxicol In Vitro.
54:137–146. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meng-Reiterer J, Varga E, Nathanail AV,
Bueschl C, Rechthaler J, McCormick SP, Michlmayr H, Malachova A,
Fruhmann P, Adam G, et al: Tracing the metabolism of HT-2 toxin and
T-2 toxin in barley by isotope-assisted untargeted screening and
quantitative LC-HRMS analysis. Anal Bioanal Chem. 407:8019–8033.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Zhang L, Peng D, Xie S, Chen D,
Pan Y, Tao Y and Yuan Z: Construction of electrochemical
immunosensor based on gold-nanoparticles/carbon nanotubes/chitosan
for sensitive determination of T-2 toxin in feed and swine meat.
Int J Mol Sci. 19:38952018. View Article : Google Scholar
|
|
5
|
Seeboth J, Solinhac R, Oswald IP and
Guzylack-Piriou L: The fungal T-2 toxin alters the activation of
primary macrophages induced by TLR-agonists resulting in a decrease
of the inflammatory response in the pig. Vet Res. 43:352012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Osselaere A, Li SJ, De Bock L, Devreese M,
Goossens J, Vandenbroucke V, Van Bocxlaer J, Boussery K, Pasmans F,
Martel A, et al: Toxic effects of dietary exposure to T-2 toxin on
intestinal and hepatic biotransformation enzymes and drug
transporter systems in broiler chickens. Food Chem Toxicol.
55:150–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Doi K, Ishigami N and Sehata S: T-2
toxin-induced toxicity in pregnant mice and rats. Int J Mol Sci.
9:2146–2158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fu YT, Lin WG, BaoCheng Z and Quan G: The
effect of T-2 toxin on IL-1beta and IL-6 secretion in human fetal
chondrocytes. Int Orthop. 25:199–201. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen X, Xu J, Liu D, Sun Y, Qian G, Xu S,
Gan F, Pan C and Huang K: The aggravating effect of selenium
deficiency on T-2 toxin-induced damage on primary cardiomyocyte
results from a reduction of protective autophagy. Chem Biol
Interact. 300:27–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang Z, Wang Y, Qiu M, Sun L, Liao J,
Wang R, Sun X, Bi S and Gooneratne R: Effect of T-2 toxin-injected
shrimp muscle extracts on mouse macrophage cells (RAW264.7). Drug
Chem Toxicol. 41:16–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nathanail AV, Varga E, Meng-Reiterer J,
Bueschl C, Michlmayr H, Malachova A, Fruhmann P, Jestoi M, Peltonen
K, Adam G, et al: Metabolism of the fusarium mycotoxins T-2 toxin
and HT-2 toxin in wheat. J Agric Food Chem. 63:7862–7872. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Q, Huang L, Liu Z, Yao M, Wang Y, Dai M
and Yuan Z: A comparison of hepatic in vitro metabolism of T-2
toxin in rats, pigs, chickens, and carp. Xenobiotica. 41:863–873.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Konigs M, Mulac D, Schwerdt G, Gekle M and
Humpf HU: Metabolism and cytotoxic effects of T-2 toxin and its
metabolites on human cells in primary culture. Toxicology.
258:106–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Yang C, Yuan Z, Yi J and Wu J: T-2
toxin exposure induces apoptosis in TM3 cells by inhibiting
mammalian target of rapamycin/serine/threonine protein
kinase(mTORC2/AKT) to promote Ca2+production. Int J Mol
Sci. 19:33602018. View Article : Google Scholar
|
|
15
|
Ueno Y, Umemori K, Niimi E, Tanuma S,
Nagata S, Sugamata M, Ihara T, Sekijima M, Kawai K, Ueno I, et al:
Induction of apoptosis by T-2 toxin and other natural toxins in
HL-60 human promyelotic leukemia cells. Nat Toxins. 3:129–137.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagase M, Alam MM, Tsushima A, Yoshizawa T
and Sakato N: Apoptosis induction by T-2 toxin: Activation of
caspase-9, caspase-3, and DFF-40/CAD through cytosolic release of
cytochrome c in HL-60 cells. Biosci Biotechnol Biochem.
65:1741–1747. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chaudhari M, Jayaraj R, Bhaskar AS and
Lakshmana Rao PV: Oxidative stress induction by T-2 toxin causes
DNA damage and triggers apoptosis via caspase pathway in human
cervical cancer cells. Toxicology. 262:153–161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao C and Zhang Y, Chen J, Wang T, Qian Y,
Yang B, Dong P and Zhang Y: Targeted drug delivery system for
platinum-based anticancer drugs. Mini Rev Med Chem. 16:872–891.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
T S A, Shalumon KT and Chen JP:
Applications of magnetic liposomes in cancer therapies. Curr Pharm
Des. 25:1490–1504. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paliwal SR, Paliwal R and Vyas SP: A
review of mechanistic insight and application of pH-sensitive
liposomes in drug delivery. Drug Deliv. 22:231–242. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shaban N, Abdel-Rahman S, Haggag A, Awad
D, Bassiouny A and Talaat I: Combination between taxol-encapsulated
liposomes and eruca sativa seed extract suppresses mammary tumors
in female rats induced by 7,12 dimethylbenz(α)anthracene. Asian Pac
J Cancer Prev. 17:117–123. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cadinoiu AN, Rata DM, Atanase LI, Daraba
OM, Gherghel D, Vochita G and Popa M: Aptamer-functionalized
liposomes as a potential treatment for basal cell carcinoma.
Polymers (Basel). 11:15152019. View Article : Google Scholar
|
|
23
|
Yang Y, Yang X, Li H, Li C, Ding H, Zhang
M, Guo Y and Sun M: Near-infrared light triggered liposomes
combining photodynamic and chemotherapy for synergistic breast
tumor therapy. Colloids Surf B Biointerfaces. 173:564–570. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sadeghi N, Kok RJ, Bos C, Zandvliet M,
Geerts WJC, Storm G, Moonen CTW, Lammers T and Deckers R:
Hyperthermia-triggered release of hypoxic cell radiosensitizers
from temperature-sensitive liposomes improves radiotherapy efficacy
in vitro. Nanotechnology. 30:2640012019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Odette WL, Payne NA, Khaliullin RZ and
Mauzeroll J: Redox-triggered disassembly of nanosized liposomes
containing ferrocene-appended amphiphiles. Langmuir. 35:5608–5616.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li B, Li B, He D, Feng C, Luo Z and He M:
Preparation, characterization, and in vitro pH-sensitivity
evaluation of superparamagnetic iron oxide nanoparticle-
misonidazole pH-sensitive liposomes. Curr Drug Deliv. 16:254–267.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Du Q, Guo Q, Huang J, Liu L, Shen
X and Peng J: A W/O emulsion mediated film dispersion method for
curcumin encapsulated pH-sensitive liposomes in the colon tumor
treatment. Drug Dev Ind Pharm. 45:282–291. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duan Y, Wei L, Petryk J and Ruddy TD:
Formulation, characterization and tissue distribution of a novel
pH-sensitive long-circulating liposome-based theranostic suitable
for molecular imaging and drug delivery. Int J Nanomedicine.
11:5697–5708. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chiang NJ, Chao TY, Hsieh RK, Wang CH,
Wang YW, Yeh CG and Chen LT: A phase I dose-escalation study of
PEP02 (irinotecan liposome injection) in combination with
5-fluorouracil and leucovorin in advanced solid tumors. BMC Cancer.
16:9072016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rehman AU, Omran Z, Anton H, Mely Y, Akram
S, Vandamme TF and Anton N: Development of doxorubicin
hydrochloride loaded pH-sensitive liposomes: Investigation on the
impact of chemical nature of lipids and liposome composition on
pH-sensitivity. Eur J Pharm Biopharm. 133:331–338. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Riondel J, Jacrot M, Fessi H, Puisieux F
and Potier: Effects of free and liposome-encapsulated taxol on two
brain tumors xenografted into nude mice. In Vivo. 6:23–27.
1992.PubMed/NCBI
|
|
32
|
Rubio DP, Roa LG, Soto DA, Velasquez FJ,
Gregorcic NA, Soto JA, Martinez MC, Kalergis AM and Vasquez AE:
Purification and characterization of saxitoxin from mytilus
chilensis of southern chile. Toxicon. 108:147–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi R, Xu X, Wu J, Wang T, Li Y, Ma B and
Ma Y: Hydrophilic interaction chromatography-tandem mass
spectrometry based on an amide column for the high-throughput
quantification of metformin in rat plasma. RSC Adv.
5:101386–101392. 2015. View Article : Google Scholar
|
|
34
|
Zhang H: Thin-film hydration followed by
extrusion method for liposome preparation. Methods Mol Biol.
1522:17–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu GX, Fang GQ and Xu W: Dual targeting
biomimetic liposomes for paclitaxel/DNA combination cancer
treatment. Int J Mol Sci. 15:15287–15303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dornetshuber-Fleiss R, Heilos D, Mohr T,
Richter L, Sussmuth RD, Zlesak M, Novicky A, Heffeter P,
Lemmens-Gruber R and Berger W: The naturally born fusariotoxin
enniatin B and sorafenib exert synergistic activity against
cervical cancer in vitro and in vivo. Biochem Pharmacol.
93:318–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Song J, Wang Y, Teng M, Zhang S, Yin M, Lu
J, Liu Y, Lee RJ, Wang D and Teng L: Cordyceps militaris induces
tumor cell death via the caspase-dependent mitochondrial pathway in
HepG2 and MCF-7 cells. Mol Med Rep. 13:5132–5140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ovejero S, Ayala P, Malumbres M,
Pimentel-Muinos FX, Bueno A and Sacristan MP: Biochemical analyses
reveal amino acid residues critical for cell cycle-dependent
phosphorylation of human Cdc14A phosphatase by cyclin-dependent
kinase 1. Sci Rep. 8:118712018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Belwal VK and Singh KP:
Nanosilica-supported liposome (protocells) as a drug vehicle for
cancer therapy. Int J Nanomedicine. 13:125–127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Soema PC, Willems GJ, Jiskoot W, Amorij JP
and Kersten GF: Predicting the influence of liposomal lipid
composition on liposome size, zeta potential and liposome-induced
dendritic cell maturation using a design of experiments approach.
Eur J Pharm Biopharm. 94:427–435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang X, Wang Y, Velkov T, Tang S and Dai
C: T-2 toxin-induced toxicity in neuroblastoma-2a cells involves
the generation of reactive oxygen, mitochondrial dysfunction and
inhibition of Nrf2/HO-1 pathway. Food Chem Toxicol. 114:88–97.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu FF, Lin XL, Yang L, Liu H, Wang X, Fang
H, Lammi ZJ and Guo X: Comparison of T-2 toxin and HT-2 toxin
distributed in the skeletal system with that in other tissues of
rats by acute toxicity test. Biomed Environ Sci. 30:851–854.
2017.PubMed/NCBI
|
|
43
|
Kojima S, Nakamura N, Ueno Y, Yamaguchi T
and Takahashi T: Anti-tumor activity of T-2 toxin-conjugated A7
monoclonal antibody (T-2-A7 MoAb) against human colon carcinoma.
Nat Toxins. 1:209–215. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ferreira Ddos S, Lopes SC, Franco MS and
Oliveira MC: pH-sensitive liposomes for drug delivery in cancer
treatment. Ther Deliv. 4:1099–1123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bellat V, Lee HH, Vahdat L and Law B:
Smart nanotransformers with unique enzyme-inducible structural
changes and drug release properties. Biomacromolecules.
17:2040–2049. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yi XQ, Zhang Q, Zhao D, Xu JQ, Zhong ZL,
Zhuoa RX and Li F: Preparation of pH and redox dual-sensitive core
crosslinked micelles for overcoming drug resistance of DOX. Polym
Chem. 7:1719–1729. 2016. View Article : Google Scholar
|
|
47
|
Ruttala HB, Ramasamy T, Gupta B, Choi HG,
Yong CS and Kim JO: Multiple polysaccharide-drug complex-loaded
liposomes: A unique strategy in drug loading and cancer targeting.
Carbohydr Polym. 173:57–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vlassov A, Khvorova A and Yarus M: Binding
and disruption of phospholipid bilayers by supramolecular RNA
complexes. Proc Natl Acad Sci USA. 98:7706–7711. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Falck E, Patra M, Karttunen M, Hyvonen MT
and Vattulainen I: Impact of cholesterol on voids in phospholipid
membranes. J Chem Phys. 121:12676–12689. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bellavance MA, Poirier MB and Fortin D:
Uptake and intracellular release kinetics of liposome formulations
in glioma cells. Int J Pharm. 395:251–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Drummond DC, Zignani M and Leroux J:
Current status of pH-sensitive liposomes in drug delivery. Prog
Lipid Res. 39:409–460. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ramasamy T, Haidar ZS, Tran TH, Choi JY,
Jeong JH, Shin BS, Choi HG, Yong CS and Kim JO: Layer-by-layer
assembly of liposomal nanoparticles with PEGylated polyelectrolytes
enhances systemic delivery of multiple anticancer drugs. Acta
Biomater. 10:5116–5127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ramasamy T, Ruttala HB, Sundaramoorthy P,
Poudel BK, Youn YS, Ku SK, Choi HG, Yong CS and Kim JO: Multimodal
selenium nanoshell-capped Au@mSiO2 nanoplatform for
NIR-responsive chemo-photothermal therapy against metastatic breast
cancer. NPG Asia Mater. 10:197–216. 2018. View Article : Google Scholar
|
|
54
|
Ramasamy T, Ruttala HB, Kaliraj K, Poudel
K, Jin SG, Choi HG, Ku SK, Yong CS and Kim JO: Polypeptide
derivative of metformin with the combined advantage of a gene
carrier and anticancer activity. ACS Biomater Sci Eng. 5:5159–5168.
2019. View Article : Google Scholar
|