Introduction
Hypertrophic scarring (HS), caused by various
cutaneous injuries, including surgery, insect bites, vaccination
and folliculitis, is a highly prevalent clinical condition
(1). For example, HS has been
reported to occur following 70% of burns (2). Patients with HS suffer from pain,
itching and loss of joint mobility (3). Although the exact mechanisms
underpinning HS formation are not yet fully understood, the
excessive collagen deposition at the end of wound healing has been
widely demonstrated to result in HS formation (4). At the end of normal cutaneous wound
healing, dermal fibroblasts stop generating more extracellular
matrix (ECM; mainly collagen) and the excessive ECM is degraded by
collagenases; failure of this process results in overabundance of
ECM and triggers the formation of HS (4). Overexpression of ECM is therefore a
hallmark of hypertrophic scar fibroblasts. Therefore, blockage of
the collagen production from dermal fibroblasts at an appropriate
time point in the healing process may reduce the formation of
HS.
Transforming growth factor-β1 (TGF-β1) has been
demonstrated to serve essential roles in HS formation as TGF-β1
stimulates the proliferation and collagen deposition of dermal
fibroblasts (5). Evidence
indicates that the expression of TGF-β1 is higher in HS tissues
compared with normal skin tissues (6). The Smad protein family serves
important roles in regulating various signaling pathways. Smad2, 3
and 4 are particularly crucial in the TGF-β1/Smad signaling pathway
(7), although there are also
Smad-independent signaling cascade (8). Binding of TGF-β1 and its receptor
(TβR) activates the phosphorylation of Smad2/3 (9). Phosphorylated Smad 2/3 further binds
to Smad4 and this complex eventually translocates into nucleus to
initiate the transcription of collagens (10). It has been reported that the
expression of TβR and Smad3 are increased in HS tissues, indicating
the roles of Smad in HS formation (11). In addition, suppression of the
TGF-β1/Smad signaling pathway has been demonstrated to attenuate
the formation of HS (12).
As human Smad 3 and 4 have been demonstrated to
specifically bind an 8 bp palindromic sequence to activate
transcription (13), we
hypothesized that a double-stranded DNA decoy that binded Smad 3
and 4 would decrease the expression of Smad pathway-associated
genes, including collagen (9), by
sequestering Smad, while not interfering with the non-Smad
signaling pathways of the TGF-β1 pathway. In the present study, a
Smad decoy was generated and its effects on collagen deposition,
collagen I and Smad2/3 protein expression, and the gene expression
of hypertrophic scar-derived human skin fibroblasts (HSF) were
evaluated. In addition, effects of the Smad decoy on the collagen
production and gene expression of TGF-β1-stimulated HSF were also
examined.
Materials and methods
Cell culture
The HSFs (primary cells derived from 3 patients:
106, 107 and 108) were purchased from Cell Research Corporation
(Singapore), with ethical approval obtained from the A STAR
Institute of Medical Biology (IRB: B-16-135E). Primary human
keratinocytes (Kc) were collected from the Asian Skin Bank,
Institute of Medical Biology, A STAR, Singapore following ethical
approval (IRB: B-16-135E). HSF was cultured in Dulbecco's modified
Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.) containing
4.5 g/l D-Glucose and 110 mg/l sodium pyruvate, supplemented with
10% foetal calf serum (FCS; Thermo Fisher Scientific, Inc.) and 1%
v/v penicillin/streptomycin solution (Thermo Fisher Scientific,
Inc.) at 37°C in an incubator with 5% CO2. Kc was
cultured in Green's medium containing 10% FCS, according to a
previously described protocol (14). The cell culture medium was changed
every 2–3 days.
Preparation of the reagents
The Smad decoy was a double-stranded DNA with the
following sequence: GGAGTATGTCTAGACTGACAATGTAC. The
underlined palindromic sequence is the cognate recognition sequence
for Smad3 and 4. The Smad decoy was annealed by adding 100 µM of
the sequence and its reverse complement in water, prior to heating
to 95°C and gradually cooling at 0.5°C per second to 25°C. The
negative control sequence has the same nucleotide balance, but a
scrambled sequence: GATGAAGTTCGAATCTGACATAGTAC. A cell-penetrating
peptide (PepC) was synthesized by Pepscan as the delivery vehicle
for the Smad decoy based on previous studies with modification
(15,16). TGF-β1 was purchased from Merck KGaA
and used at 5 ng/ml for the stimulation of HSF.
Collagen production
Effects of the Smad decoy on HSF (with or without
TGF-β1 stimulation) collagen production were assayed using Sirius
red staining (Sigma-Aldrich; Merck KGaA), as previously described
(17). In brief, HSFs
(4×104) were seeded onto a 24-well culture plate for 24
h. Smad decoy (to reach a final concentration in 500 µl medium of
30, 100 and 300 nM) was mixed with PepC at a mass ratio of 1:1 and
then applied to the cell cultures. Following exposure to the Smad
decoy for 48 and 72 h, Sirius red was added to each well and the
plates were incubated at 37°C for a further 90 min. Following
drying overnight, the staining was dissolved in 0.1 M sodium
hydroxide (NaOH; Sigma-Aldrich; Merck KGaA) and the absorbance was
measured at 540 nm using a SpectraMax M5 Multi-Mode microplate
reader (Molecular Devices, LLC).
Western blotting
Expression of collagen I and Smad 2/3 in HSF
following Smad decoy treatment was detected using western blotting
using secondary antibodies with fluorescence. HSFs were treated
with 100 nM Smad decoy for 24 h and the whole cell lysate was
collected in RIPA buffer (Merck KGaA), containing protease
inhibitor cocktail (PIC; Sigma-Aldrich), 10 mM sodium fluoride
(NaF; Sigma-Aldrich) and 2 mM sodium vanadate
(Na3VO4; Sigma-Aldrich). The protein
concentrations were quantified using Bradford protein assay
(Bio-Rad Laboratories, Inc.). Equal amounts of protein (10 µg) from
each cell lysate were prepared and separated using NuPage 4–12%
gradient Bis-tris protein gel (Thermo Fisher Scientific, Inc.),
prior to being transferred onto nitrocellulose membranes (Bio-Rad
Laboratories, Inc.). The membranes were incubated with primary
antibodies at 4°C overnight in Odyssey blocking buffer (LI-COR
Biosciences). Primary antibodies included rabbit anti-collagen I
(cat. no. 21286; Abcam; dilution 1:1,000), rabbit anti-Smad 2/3
(cat. no. 8685; Cell Signaling Technology, Inc.; dilution 1:1,000)
and mouse anti-GAPDH (cat. no. G8795; Sigma-Aldrich; Merck KgaA;
dilution 1:10,000). Secondary antibodies used were anti-rabbit
Alexa Fluor 680 (cat. no. A-21076; Invitrogen; Thermo Fisher
Scientific, Inc.; dilution 1:10,000), anti-mouse Alexa Fluor 790
(cat. no. A11371; Invitrogen; Thermo Fisher Scientific, Inc.;
dilution 1:10,000) against anti-Smad2/3 and anti-collagen, and
anti-GAPDH respectively. Images were captured and analysed using
the Odyssey Infrared Imaging system and Image Studio Version 5.2
software (LI-COR Biosciences).
Sequencing
HSFs were seeded onto a 12-well plate at
1×105 cells per well. Following a 24-h incubation, the
cells were left untreated or transfected with 100 nM of the Smad
decoy mixed with PepC (Pepscan). Following a 72-h transfection, the
cells were harvested and the total RNA was isolated using DirectZol
kit (Zymo Research Corp.). The barcoded mRNA sequencing library was
subsequently prepared using TruSeq RNA Library Prep v2 (Illumina,
Inc.), according to the manufacturer's protocol. The resultant
libraries were pooled and sequenced on a HiSeq 2000 sequencer
(Illumina, Inc.). Following removal of the adaptor sequences using
Prinseq, the sequences were mapped to the human transcriptome (hg19
genome using TOPHAT (18) and the
aligned BAM file was used to read counts for each gene using HTSeq
(19). Subsequent analysis was
performed using normalised counts and standard deviations derived
from the 3-fold-changes of individual genes between the untreated
and treated samples. Gene ontology analysis was performed by
analysing the 178 most differentially regulated genes using the
DAVID analysis algorithm v6.8 hosted on the website (https://david.ncifcrf.gov) using the GOTERM_CC_DIRECT
(cellular component) annotation (20).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
HSFs or Kc were plated onto a 24-well plate at
5×104 cells per well. Following a 24-h incubation, the
cells were left untreated, treated with the scrambled Smad decoy
oligonucleotide or the Smad decoy mixed with PepC. A total of 48 h
after transfection, RNA from the cells were harvested using
DirectZol kit (Zymo Research Corp.) according to the manufacturer's
protocols, and reverse transcribed using MMLV reverse transcriptase
(Promega Corporation) using random nanomers (Integrated DNA
Technologies, Inc.), according to the manufacturer's protocol. The
amplicons were then assayed by qPCR using Maxima SyBr Green
Mastermix (Thermo Fisher Scientific, Inc.) using the primers listed
in Table I with the following
protocol: 95°C for 5 min, followed by 50 cycles of 95°C for 10 sec,
58°C for 8 sec and 60°C for 30 sec. The samples were quantified
using the StepOnePlus software (Thermo Fisher Scientific, Inc.)
using the relative standard curve method with automatic Ct calling.
The relative expression of each gene was quantified against a
relative standard curve made up of serial dilution of a few of the
samples pooled together and then normalised to GAPDH expression by
dividing the relative expression of a query gene by GAPDH
expression relative expression. The ratios were then normalised to
1 for the untreated control compared between sample groups
(21).
 | Table I.Primers used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward and reverse
primers |
|---|
| COL1A1 | F:
5′-TCTGCGACAACGGCAAGGTG-3′ |
|
| R:
5′-GACGCCGGTGGTTTCTTGGT-3′ |
| COL1A2 | F:
5′-ACACGTCTGGCTAGGAGAAAC-3′ |
|
| R:
5′-GGCATAGTTGGCCAGCAGGC-3′ |
| COL3A1 | F:
5′-ACCAGGAGAGAAGGGATCGC-3′ |
|
| R:
5′-TTCCCCTAGGACCTGGCATG-3′ |
| TAGLN | F:
5′-CCGTGGAGATCCCAACTGG-3′ |
|
| R:
5′-CCATCTGAAGGCCAATGACAT-3′ |
| SPARC | F:
5′-ACATCGCCCTGGATGAGTGG-3′ |
|
| R:
5′-CGGTACTGTGGAAGGAGTGG-3′ |
| MMP1 | F:
5′-GCCGACAGAGATGAAGTCCG-3′ |
|
| R:
5′-CTTGGGGTATCCGTGTAGCAC-3′ |
| MMP3 | F:
5′-CTCCAACCGTGAGGAAAATCG-3′ |
|
| R:
5′-TGGGAAAGCCTGGCTCCATG-3′ |
|
SERPINB2 | F:
5′-TTGCCGATGTGTCCACTGGC-3′ |
|
| R:
5′-GTCTTTGCTGGTCCACTTGTTG-3′ |
| MMP2 | F:
5′-GATGCCGCCTTTAACTGGAGC-3′ |
|
| R:
5′-TCCAGGCATCTGCGATGAGC-3′ |
| GAPDH | F:
5′-AAGGTGAAGGTCGGAGTCAA-3′ |
|
| R:
5′-GAAGATGGTGATGGGATTTC-3′ |
| TIMP1 | F:
5′-TCAACCAGACCACCTTATACC-3′ |
|
| R:
5′-GTAGACGAACCGGATGTCAGC-3′ |
| FN14 | F:
5′-CTGGACAAGTGCATGGACTGC-3′ |
|
| R:
5′-CCAAGGATGGGCCAAAGCAG-3′ |
Statistical analysis
All experiments were performed 3 times using cells
from 3 different patients. The data are expressed as the percentage
of the control group. One-way analysis of variance, followed by
Tukey's post hoc test, were used to analyse the statistical
difference. P<0.05 was considered to indicate a statistically
significant difference.
Results
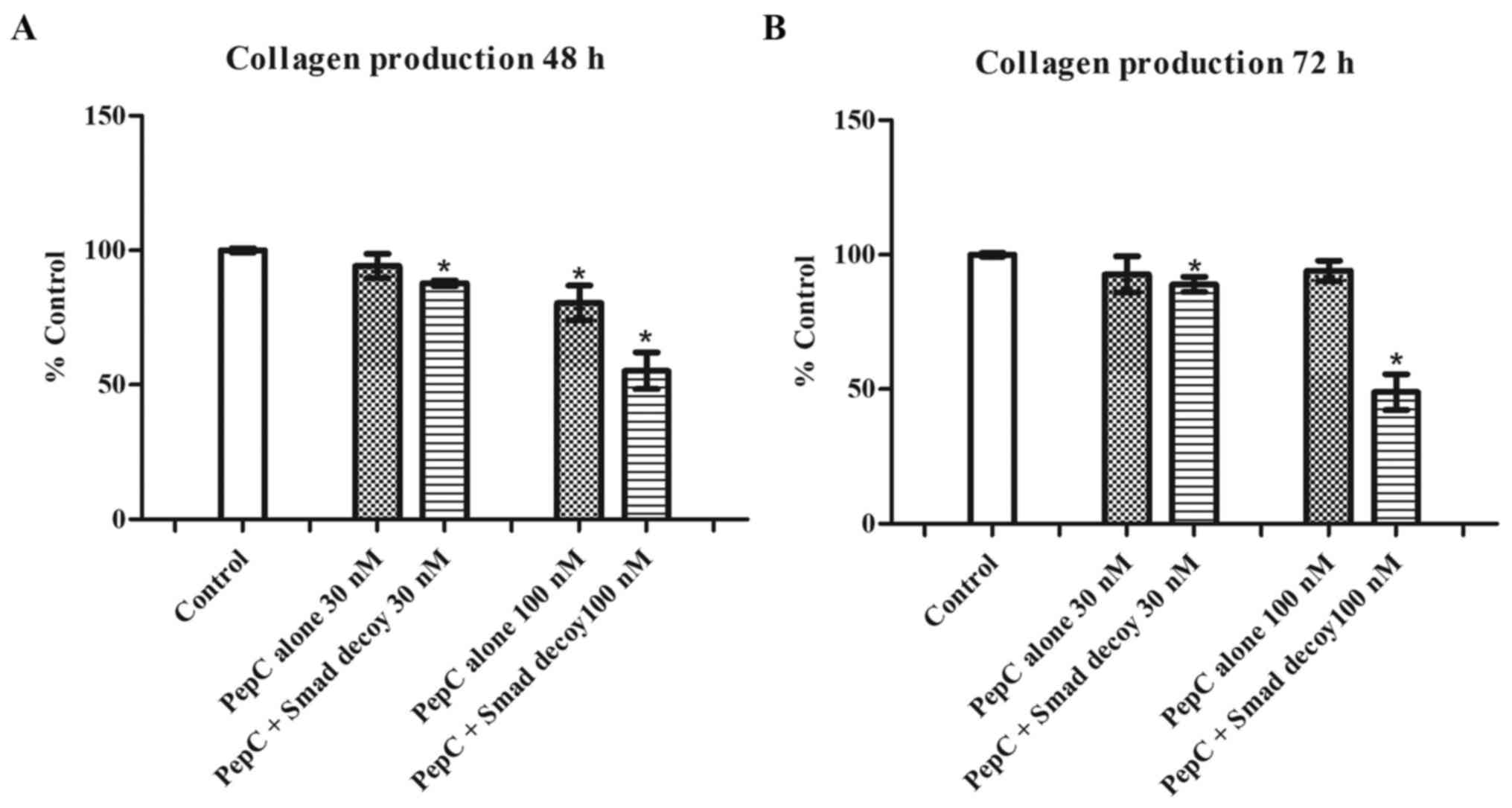
The Smad decoy inhibits the total
amount of collagen produced in HSF
To evaluate the effects of the Smad decoy on the
total amount of collagen produced in HSF, 3 different
concentrations of the Smad decoy mixed with PepC, a novel peptide
transfection reagent, were applied to HSF. The same concentrations
of PepC alone were used as the vehicle control. As demonstrated in
Fig. 1, PepC alone at 30 nM showed
no effects on HSF collagen production at 48 and 72 h. However, the
Smad decoy mixed with PepC at 30 nM significantly decreased HSF
collagen production by 12.2±1.0 and 11.0±2.8% at 48 and 72 h,
respectively, compared with the control (P<0.05). PepC alone at
100 nM decreased HSF collagen production by 19.5±6.5% at 48 h
compared with the control (P<0.05); however, no inhibitory
effects of PepC on HSF collagen production was detected at 72 h.
The Smad decoy mixed with PepC at 100 nM significantly attenuated
HSF collagen production by 44.8±6.8 and 51.0±6.6% at 48 and 72 h,
respectively, compared with the control (P<0.05). The data
suggested that the Smad decoy inhibits HSF collagen production in a
dose-dependent manner. In addition, the Smad decoy mixed with PepC
had no effects on cell viability at 72 h (Fig. S1), suggesting that the
downregulation of collagen induced by the Smad decoy is not simply
due to the cytotoxicity.
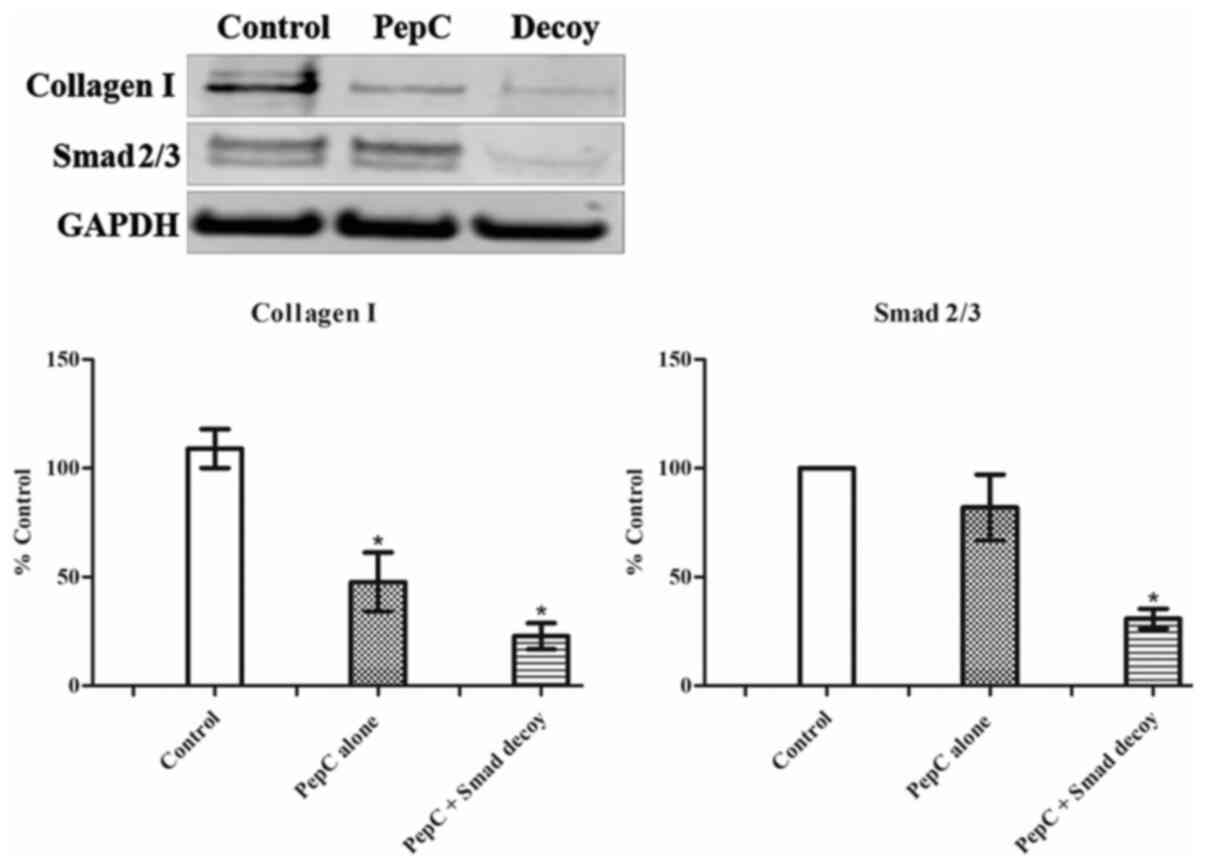
The Smad decoy attenuates the
expression of collagen I and Smad 2/3 in HSF
To further investigate the effects of the Smad decoy
on the TGF-β1/Smad signaling pathway, expression of collagen I and
Smad2/3 in HSF following treatment with the Smad decoy was analysed
using western blotting (Fig. 2).
PepC alone and Smad decoy mixed with PepC at 100 nM attenuated the
expression of collagen I in HSF. Although the western blotting
indicated that the Smad decoy has a greater inhibitory effect on
collagen I expression compared with PepC alone, the difference
between these two groups was not statistically significant. PepC
alone showed no effects on the expression of Smad 2/3 in HSF;
however, the Smad decoy significantly downregulated the expression
of Smad2/3 in HSF compared with the control. These results
suggested that the Smad decoy is capable of regulating Smad2/3
expression, thereby resulting in a decrease in collagen I.
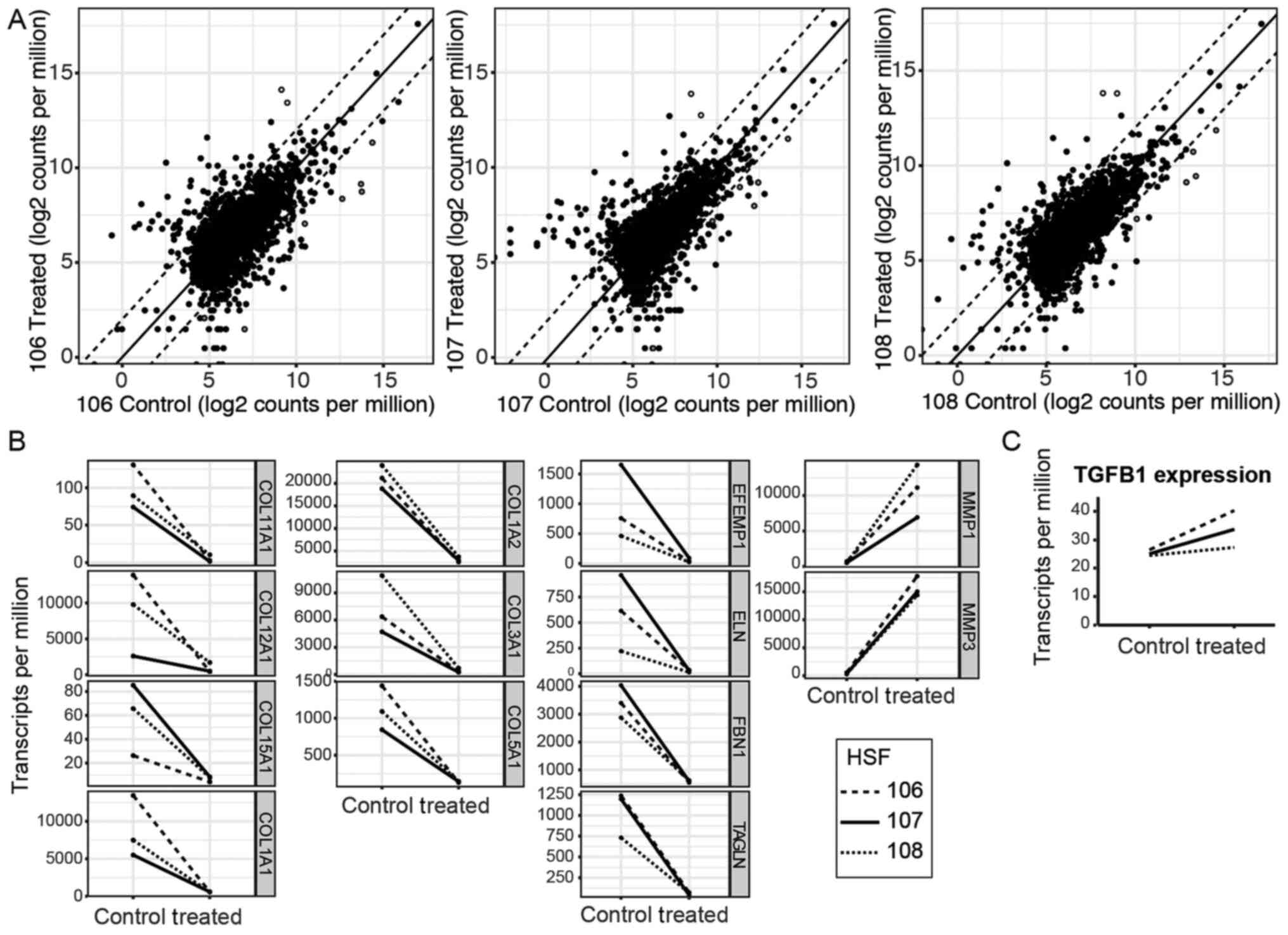
The Smad decoy induces an alteration
of gene expression in HSF and human primary keratinocytes (Kc)
To ascertain the genes affected, HSFs were treated
with the Smad decoy, put through RNA sequencing and compared
individually with untreated matched controls. The top 5,000
expressed genes were then plotted against each other on a
logarithmic plot. The majority of the genes fell within a 4-fold
difference in expression between untreated and treated samples,
with 178 genes exceeding this (Fig.
3A and Table II). The 178
genes were analyzed with cell component gene ontology analysis
using DAVID (20) to determine
which cellular component the most differentially expressed genes
functioned in Table III. The
list suggests that the Smad decoy primarily affects ECM-associated
functions and the present study aimed to highlight the ECM
expression changes with selected upregulated and downregulated
genes (Fig. 3B) corresponding to
the hollow (upregulated) and grey (downregulated) dots in Fig. 3A. Notably, the Smad decoy does not
appear to affect the expression of the TGFB1 gene, which codes for
TGF-β1 (Fig. 3C). Therefore, it is
unlikely that the changes triggered by the Smad decoy are directly
attributable to unintended perturbation of TGF-β1 levels rather
than direct perturbation of the Smad signaling pathway. The effect
on the ECM is similar to the results of a previous study, which
used a microarray of dermal fibroblasts stimulated with TGF-β1
(22). In particular, COL1A2,
COL3A1 and FBN1 were downregulated by inhibition of
Smad, corresponding to upregulation when TGF-β1 was added. However,
several genes upregulated by increase in TGF-β1 levels, including
TIMP1, TIMP3 and COL6A1, were notably not
downregulated by inhibition by the Smad decoy. Furthermore, while
Verrecchia et al (2001) noted upregulation of matrix
metalloproteinase-1 (MMP1) and MMP3 by TGF-β1, the
present study reported marked increases in MMP1 and
MMP3 expression by Smad inhibition (22). The divergence in the results
suggests that, in the present study, it was possible to isolate the
Smad pathway from the other TGF-β1 signaling pathways using the
Smad decoy.
 | Table II.Genes differentially expressed in
treated and untreated HSFs. |
Table II.
Genes differentially expressed in
treated and untreated HSFs.
|
| Treated/untreated,
log2 fold-change |
| Treated/untreated,
log2 fold-change |
| Treated/untreated,
log2 fold-change |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Gene | 106 | 107 | 108 | Gene | 106 | 107 | 108 | Gene | 106 | 107 | 108 |
|---|
| RSAD2 | 7.52 | 6.49 | 5.73 | LIF | 3.75 | 4.01 | 0.24 | AKR1C1 | 3.44 | 2.03 | 2.74 |
| IL24 | 3.71 | 6.91 | 7.14 | IFI27 | 2.19 | 4.14 | 2.98 | DDX60 | 2.7 | 3.16 | 2.52 |
|
SERPINB2 | 6.67 | 6.1 | 6.03 | RND3 | 2.57 | 3.71 | 3.41 | MX2 | 2.75 | 3.31 | 1.89 |
| PTGS2 | 5.1 | 6.81 | 5.68 | OAS2 | 2.55 | 4.12 | 2.44 | IFITM1 | 2.69 | 3.08 | 2.43 |
| TFPI2 | 5.92 | 5.83 | 6.22 | IL11 | 3.7 | 3.27 | 2.54 | MT2A | 2.99 | 2.37 | 2.71 |
| THBD | 5.46 | 6.41 | 5.07 | HSPA1B | 3.07 | 2.45 | 3.79 | STC1 | 2.38 | 2.39 | 3.16 |
| OASL | 6.44 | 5.2 | 3.91 | DUSP10 | 4.23 | 1.72 | 2.39 | PID1 | 2.94 | 2.4 | 2.63 |
| ESM1 | 5.72 | 5.77 | 4.74 | INHBA | 3.89 | 2.65 | 2.54 | SAMD9 | 3.3 | 2 | 2.37 |
| MMP10 | 4.81 | 5.92 | 5.31 | SAT1 | 3.16 | 3.5 | 2.73 | SQSTM1 | 2.82 | 2.35 | 2.58 |
| MMP3 | 4.97 | 5.42 | 5.62 | HMGA2 | 3.19 | 3.53 | 2.63 | ANGPTL4 | 2.22 | 2.81 | 2.69 |
| BMP2 | 5.46 | 5.79 | 4.57 | FAM167A | 2.58 | 3.3 | 3.28 | PITPNC1 | 3.35 | 2.26 | 1.59 |
| MX1 | 3.15 | 6.72 | 3.07 | TNFAIP3 | 2.57 | 3.61 | 2.61 | SLC5A3 | 2.49 | 1.49 | 3.25 |
| MMP12 | 5.08 | 5.52 | 4.44 | ID1 | 3.76 | 2.41 | 2.33 | DDX58 | 2.92 | 2.6 | 2.09 |
| IL1B | 5.65 | 5.2 | 3.5 | TBX3 | 2.7 | 3.51 | 2.14 | HSPA1A | 2.6 | 1.39 | 3.13 |
| CMPK2 | 5.13 | 5.28 | 3.87 | IFI44 | 3.22 | 2.96 | 2.36 | IL6 | 1.48 | 3.21 | 2.3 |
| CXCL8 | 3.86 | 5.52 | 4.58 | SPRY2 | 2.93 | 2.42 | 3.21 | DUSP5 | 2.16 | 2.77 | 2.43 |
| ITGA2 | 5.29 | 4.38 | 4.27 | AKR1C1 | 3.44 | 2.03 | 2.74 | CXCL1 | 1.99 | 3.12 | 1.95 |
| CXCL5 | 4.36 | 5.33 | 2.57 | DDX60 | 2.7 | 3.16 | 2.52 | IER3 | 2.46 | 2.59 | 2.19 |
| OAS1 | 3.74 | 5.33 | 3.49 | MX2 | 2.75 | 3.31 | 1.89 | TNFAIP6 | 1.84 | 2.93 | 2.25 |
| IFIT1 | 3.91 | 5.32 | 3.24 | IFITM1 | 2.69 | 3.08 | 2.43 | GNG11 | 2.58 | 1.89 | 2.46 |
| IFIT2 | 5.03 | 4.47 | 3.21 | MT2A | 2.99 | 2.37 | 2.71 | HMOX1 | 3.14 | 1.71 | 1.61 |
| IFIT3 | 4.59 | 4.84 | 3.5 | STC1 | 2.38 | 2.39 | 3.16 | JARID2 | 2.35 | 2.41 | 2.24 |
| CXCL3 | 3.78 | 5.28 | 3.26 | PID1 | 2.94 | 2.4 | 2.63 | NPC1 | 2.13 | 1.9 | 2.7 |
| MMP1 | 3.95 | 3.71 | 4.82 | SAMD9 | 3.3 | 2 | 2.37 | ABL2 | 1.55 | 2.77 | 2.26 |
| PHLDA1 | 4.03 | 4.35 | 4.06 | SQSTM1 | 2.82 | 2.35 | 2.58 | FOSL1 | 2.28 | 2.1 | 2.4 |
| IFI44L | 3.13 | 5.19 | 2.64 | ANGPTL4 | 2.22 | 2.81 | 2.69 | ITPRIP | 2.43 | 2.52 | 1.67 |
| IFI6 | 1.83 | 5.3 | 2.44 | PITPNC1 | 3.35 | 2.26 | 1.59 | FGF5 | 0.93 | 2.58 | 2.55 |
| DUSP6 | 3.95 | 4.28 | 3.24 | SLC5A3 | 2.49 | 1.49 | 3.25 | NT5E | 2.16 | 2.41 | 1.94 |
| HERC6 | 2.93 | 4.7 | 3.41 | DDX58 | 2.92 | 2.6 | 2.09 | KLHL21 | 2.39 | 1.94 | 2.15 |
| PLIN2 | 3.17 | 3.93 | 4.11 | HSPA1A | 2.6 | 1.39 | 3.13 | PTGS1 | 2.41 | 1.82 | 2.21 |
| NAMPT | 3.99 | 3.55 | 3.76 | IL6 | 1.48 | 3.21 | 2.3 | PARP12 | 2.16 | 2.24 | 2.06 |
| IFIH1 | 3.44 | 4.43 | 2.86 | DUSP5 | 2.16 | 2.77 | 2.43 | DCBLD2 | 2.11 | 2.52 | 1.65 |
| TMEM158 | 3.71 | 4.37 | 2.56 | CXCL1 | 1.99 | 3.12 | 1.95 | PLSCR1 | 2.05 | 2.28 | 1.96 |
| CDCP1 | 3.01 | 4.24 | 3.43 | IER3 | 2.46 | 2.59 | 2.19 | PRDM1 | 2.14 | 2.42 | 1.47 |
| OAS3 | 2.61 | 4.51 | 2.82 | TNFAIP6 | 1.84 | 2.93 | 2.25 | PDGFD | −5.58 | −6.17 | −4.33 |
| DUSP4 | 3.32 | 4.19 | 2.85 | ID1 | 3.76 | 2.41 | 2.33 | SPARC | −3.26 | −3.33 | −2.39 |
| ISG15 | 3.68 | 4.09 | 2.26 | TBX3 | 2.7 | 3.51 | 2.14 | FIBIN | −2.09 | −2.95 | −2.21 |
| PTGES | 4.34 | 2.78 | 2.79 | IFI44 | 3.22 | 2.96 | 2.36 | RCAN2 | −4.34 | −4.49 | −4.9 |
| PODXL | 4.05 | 3.29 | 2.94 | SPRY2 | 2.93 | 2.42 | 3.21 | COL5A1 | −3.43 | −2.54 | −2.89 |
|
ADAMTSL1 | −1.86 | −1.55 | −3.81 | SYNPO2 | −5.52 | −4.89 | −2.84 | WISP1 | −2.33 | −3.34 | −2.97 |
| GAS6 | −2.37 | −2.65 | −2.01 | COL11A1 | −5.12 | −4.97 | −2.99 | NEDD9 | −5.79 | −1.32 | −1.52 |
| ADAM33 | −2.47 | −2.15 | −2.36 | ELN | −4.05 | −4.99 | −3.98 | COL15A1 | −2.4 | −3.2 | −2.93 |
| LRIG3 | −3.76 | −2.29 | −0.88 | DKK2 | −4.05 | −4.56 | −4.03 | DAPK1 | −2.16 | −3.89 | −2.43 |
| INHBB | −3.54 | −1.68 | −1.71 | EFEMP1 | −4.3 | −4.14 | −4.1 | KIF26B | −2.49 | −3.92 | −2.04 |
| ANGPT1 | −2.7 | −2.4 | −1.81 | COL3A1 | −4.27 | −4.22 | −3.97 | COL1A2 | −3.04 | −2.7 | −2.69 |
| LOXL4 | −2.14 | −1.96 | −2.79 | HMCN1 | −4.05 | −3.51 | −4.76 | SORT1 | −4.06 | −2.28 | −2.05 |
| ITIH5 | −3.02 | −2.21 | −1.63 | POSTN | −3.67 | −5 | −3.49 | SGCD | −2.76 | −3.29 | −2.29 |
| SERAC1 | −2.73 | −2.27 | −1.83 | CNN1 | −3.36 | −3.38 | −5.12 | CARMN | −3.43 | −3.1 | −1.78 |
| FBLN2 | −2.25 | −2.5 | −2.05 | WNT2 | −3.72 | −5.31 | −2.52 | CTGF | −4.64 | −2.39 | −1.27 |
| PDE1C | −2.71 | −2.18 | −1.88 | COL1A1 | −4.57 | −3.22 | −3.74 | VCAN | −2.9 | −2.84 | −2.52 |
| TENM2 | −3.97 | −1.08 | −1.71 | TAGLN | −4.05 | −4 | −3.37 | ACTA2 | −3.92 | −2.51 | −1.67 |
| GADD45B | −3.18 | −2.08 | −1.5 | LMOD1 | −3.6 | −4.93 | −2.61 |
STARD4-AS1 | −2.8 | −2.59 | −2.65 |
| SLIT3 | −2.63 | −1.31 | −2.81 | OXTR | −4.21 | −4.5 | −2.1 | PRUNE2 | −2.69 | −3.13 | −2.2 |
| COMP | −2.49 | −1.56 | −2.64 | ITGA11 | −4.43 | −3.34 | −2.95 | PODN | −1.91 | −3.61 | −2.44 |
|
TNFRSF11B | −4 | −1.93 | −0.69 | CHN1 | −3.26 | −4.56 | −2.81 | NTN4 | −4.04 | −3.24 | −0.68 |
| FBLN1 | −2.08 | −1.96 | −2.44 | ITGBL1 | −3 | −4.29 | −3.21 | VSIR | −2.1 | −2.78 | −2.94 |
| PYCR1 | −2.45 | −1.77 | −2.25 | MFAP4 | −2.51 | −5.12 | −2.84 | ALDH1B1 | −3.35 | −2.48 | −1.95 |
| RHOBTB1 | −2.51 | −1.94 | −2.01 | DPT | −2.46 | −4.36 | −3.57 |
TNFRSF19 | −2.31 | −3 | −2.4 |
| CCL2 | −2.26 | −0.9 | −3.22 | MXRA5 | −3.98 | −2.99 | −2.97 | FBN1 | −2.64 | −2.77 | −2.2 |
| MYL9 | −1.47 | −2.96 | −1.92 | COL12A1 | −5.02 | −2.38 | −2.52 | NTN1 | −1.35 | −3.06 | −3.15 |
| CCDC80 | −2.01 | −2.46 | −1.86 | FBN2 | −3.85 | −3.31 | −2.49 | RDH10 | −4.59 | −1.8 | −1.17 |
| DHCR24 | −2.04 | −2.88 | −1.35 | LMCD1 | −4.14 | −3.72 | −1.75 | RNF150 | −2.41 | −2.45 | −2.63 |
| COL8A1 | −2.68 | −1.21 | −2.35 | IGFBP5 | −4.33 | −4.2 | −1.07 | THY1 | −1.92 | −2.78 | −2.79 |
| GLT8D2 | −1.28 | −3.02 | −1.92 | NREP | −3.12 | −4.11 | −2.17 | CNN2 | −2.56 | −2.6 | −2.31 |
|
SERPINH1 | −1.48 | −2.62 | −2.07 | SVEP1 | −3.84 | −2.86 | −2.64 | APCDD1 | −1.96 | −2.29 | −3.16 |
| RN7SL5P | −0.18 | −0.89 | −5.07 | GFRA1 | −3.56 | −3.2 | −2.57 | DIO2 | −0.43 | −4.8 | −2.11 |
| COL5A2 | −2.1 | −2.23 | −1.78 | MFAP5 | −2.4 | −4.27 | −2.34 | ALPK2 | −3.12 | −3.02 | −1.13 |
| TP53I11 | −1.51 | −2.11 | −2.47 |
|
|
|
|
|
|
|
|
 | Table III.Gene classification of differentially
expressed genes. |
Table III.
Gene classification of differentially
expressed genes.
| Gene Ontology
analysis for differentially expressed genes | Count | % of genes out of
178 | P-value |
|---|
| Proteinaceous
extracellular matrix | 30 | 16.9 |
1.7×10−22 |
| Extracellular
region | 61 | 34.3 |
1.7×10−21 |
| Extracellular
space | 52 | 29.2 |
2.3×10−18 |
| Extracellular
matrix | 25 | 14.0 |
8.4×10−16 |
| Endoplasmic
reticulum lumen | 14 | 7.9 |
3.8×10−8 |
| Collagen
trimer | 10 | 5.6 |
2.2×10−7 |
| Basement
membrane | 8 | 4.5 |
1.0×10−5 |
| Microfibril | 4 | 2.2 |
9.6×10−5 |
| Elastic fiber | 3 | 1.7 |
5.3×10−4 |
| Perinuclear region
of cytoplasm | 14 | 7.9 |
6.4×10−3 |
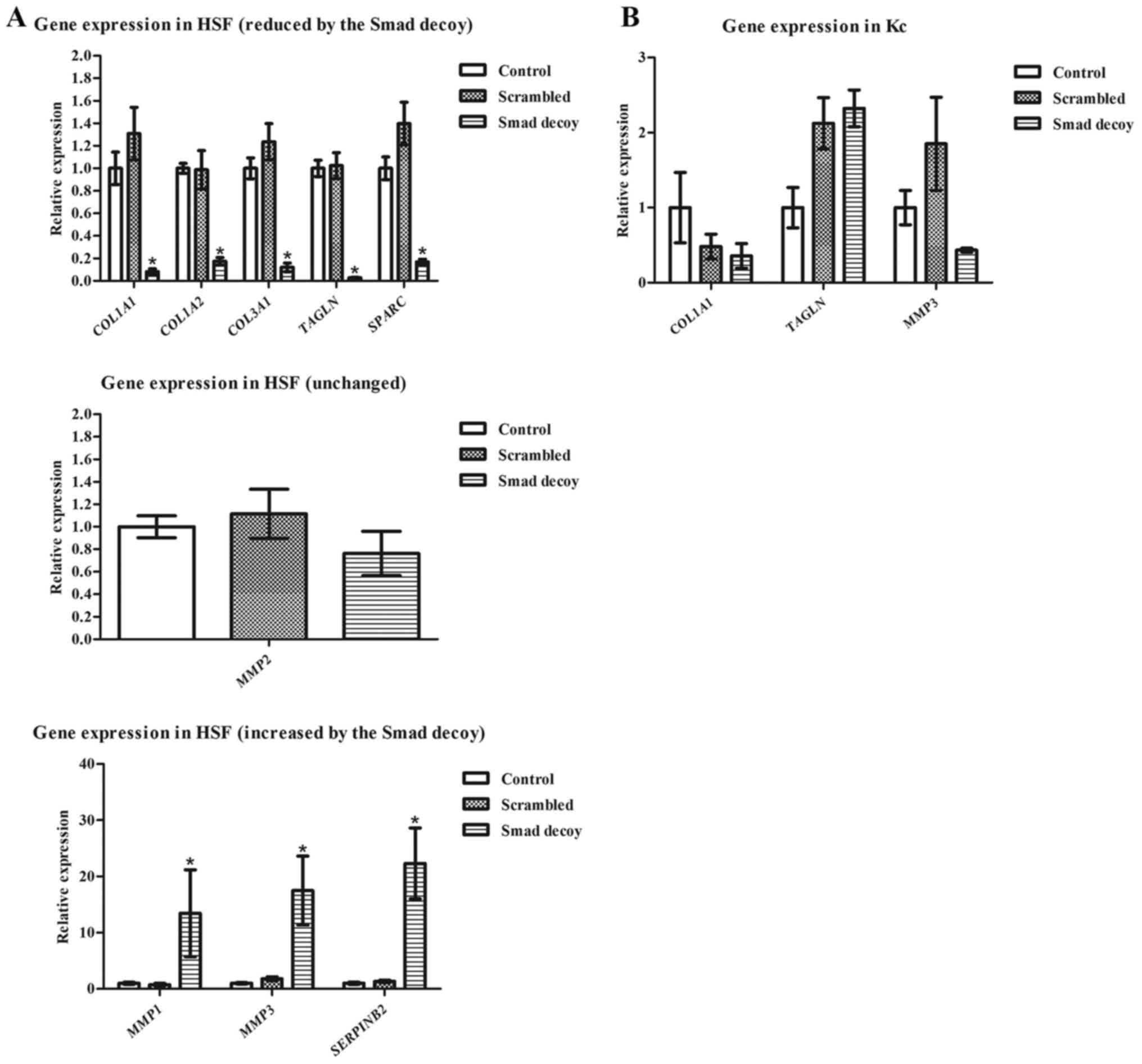
To validate the sequencing results, RT-qPCR analysis
was performed of a number of genes, which are highly associated
with HS formation, on HSF treated for 3 days with the Smad decoy,
using a scrambled decoy as a negative control. Corresponding to the
sequencing data, COL1A1, COL1A2, COL3A1, TAGLN and
SPARC were significantly downregulated following exposure to
the Smad decoy, but not with the scrambled decoy (Fig. 4A). Similarly, MMP1, MMP3 and
SERPINB2 were significantly upregulated in the Smad decoy
treated group compared with the scrambled and control groups. To
ascertain that genes unchanged in the sequencing data were also
unchanged in the independent experiment, MMP2, another
matrix metalloprotease was also assayed using RT-qPCR. Data from
the sequencing assay suggested that MMP2 is not affected by
the Smad decoy (treated/untreated in sequencing=0.93±0.03);
consistently, no significant changes in MMP2 expression were
observed in RT-qPCR. This suggests that the collagen-inhibiting
ability of the Smad decoy relies on its regulation of the
expression of various genes, including COL1A1, COL3A1 and
MMPs.
Additionally, effects of the Smad decoy on the gene
expression of Kc were evaluated as Kc also serves essential roles
in HS formation (23). Application
of the Smad decoy did not appear to affect COL1A1 nor
MMP3 significantly, but it increased TAGLN expression
slightly (Fig. 4B). However, the
effect appears to be associated with PepC-based delivery as the
scrambled oligonucleotide also resulted in increased TAGLN
expression.
Effects of the Smad decoy on
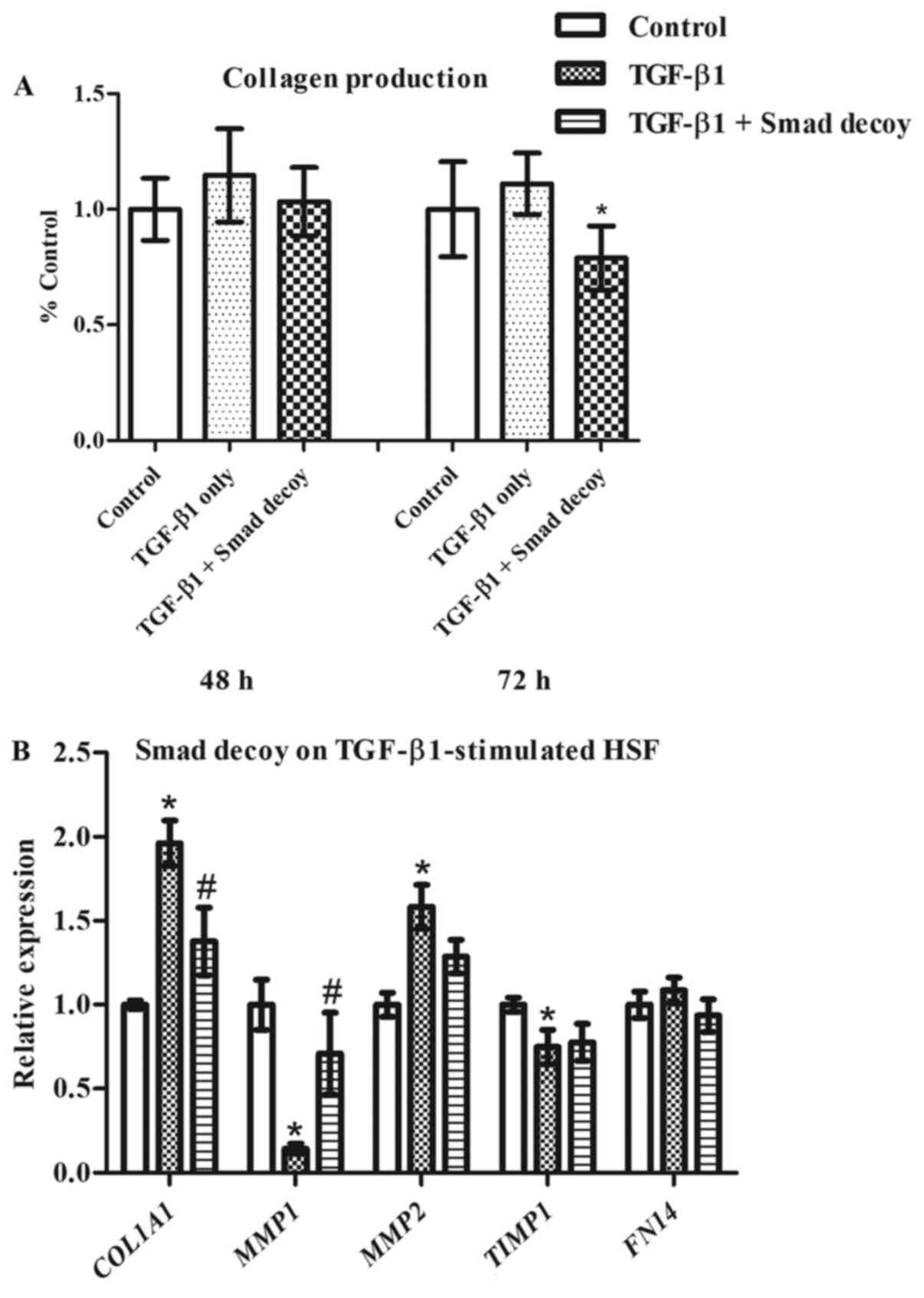
TGF-β1-stimulated HSF
As TGF-β1 has been demonstrated to serve essential
roles in HS formation and overabundant TGF-β1 expression is
frequently observed in HS tissues (6), the present study investigated the
effects of the Smad decoy on TGF-β1-stimulated HSF. The Smad decoy
was initially delivered with PepC into the cells followed by the
addition of TGF-β1 3 h later. Although collagen production was
modestly increased by TGF-β1, Smad decoy inhibition of collagen was
markedly less (Fig. 5A). TGF-β1
has been revealed to significantly increase the expression of
COL1A1 and MMP2, and reduce the expression of
MMP1 and TIMP1 in HSF, as demonstrated in Fig. 5B. The Smad decoy significantly
inhibited TGF-β1-induced upregulation of COL1A1 and
downregulation of MMP1 in HSF. Notably, FN14,
reported to be upregulated by TGF-β1 in dermal fibroblasts
(24), was not affected by the
addition of TGF-β1 in the present study. This demonstrates that the
Smad decoy alters TGF-β1-induced ECM expression in HSF.
Discussion
HS remains a challenging problem for both patients
and clinicians. The underlying mechanisms for HS formation remain
elusive. However, excessive collagen deposition by fibroblasts has
been widely demonstrated to result in HS formation (4). Numerous therapies are available in
clinic, but no single therapy can guarantee the improvement of HS.
For example, silicone dressings have been used to treat HS since
1983 (25), but require frequent
changes and cause skin rashes (26). Laser therapy is becoming more
popular in the clinic for managing HS, but high recurrence is
observed in patients receiving laser therapy (27). Therefore, novel therapies with more
accurate pathological target are of particular relevance and may
present patients with more options. Targeted therapy is becoming
increasingly important in healthcare as many traditional therapies
lack selectivity and specificity (28). One of the major advantages of small
molecule drugs to treat HS is that they are typically cell
permeable, thereby blocking the activities of targeted proteins and
regulating the downstream signaling pathways (29). For example, a TGF-β1 inhibitor has
been reported to prevent HS formation when it is applied during
wound healing (30), as increased
TGF-β1 results in the formation of HS (6). The mechanism underpinning
TGF-β1-induced HS formation is that TGF-β1 activates the
phosphorylation of Smad 2/3, which then initiates collagen
expression (9,10). The aim of the present study was to
investigate if a Smad decoy that sequesters the Smad protein may
inhibit collagen production and deposition.
As human Smad 3 and 4 have been identified to
specifically bind a 8 bp palindromic sequence to activate
transcription (13), a
double-stranded DNA decoy that contains this sequence was designed.
To evaluate the effects of this Smad decoy on HSF, the present
study aimed to make use of a non-toxic reagent for transfection.
Transfection efficiency is limited by the biological barriers of
the cells, including cellular uptake, intracellular trafficking,
metabolic degradation and nuclear entry (31). Lipofectamine is the most frequently
used transfection reagent due to its high transfection efficiency
of DNA and RNA on various cells, including those considered to be
difficult-to-transfect cells (31). However, Lipofectamine is cytotoxic
(16). A novel CPP-based peptide
(PepC), similar to the PF14 peptide that was recently reported to
be more effective in transfecting splice-correcting
oligonucleotides into HeLa pLuc705 cells with significantly lower
cytotoxicity than Lipofectamine (16), was used for delivery of the Smad
decoy. PepC had no effects on the collagen production in HSF at 72
h; by contrast, the Smad decoy delivered by PepC significantly
inhibited HSF collagen production at 72 h. These results not only
demonstrated that PepC is a suitable transfection reagent for HSF,
but also suggested that the Smad decoy may potentially be used as a
novel anti-scarring therapy. As collagen type I is the most
abundant collagen type in humans (32) and is mediated by the TGF-β1/Smad
signaling pathway (33), the
present study also investigated the effects of the Smad decoy on
the expression of collagen I and Smad2/3 in HSF. The results
suggested that the Smad decoy markedly attenuates the expression of
collagen I and Smad2/3, suggesting its roles in regulating the
TGF-β1/Smad signaling pathway. Notably, PepC was revealed to
decrease collagen I expression in HSF based on western blot
analysis, albeit to a lesser extent than the Smad decoy (Fig. 2). This appears to contradict the
results in Fig. 1 in which PepC
does not decrease total collagen. This difference may be partially
explained by the fact that only type I collagen was measured by
western blotting and this was performed 24 h after treatment; while
Sirius red staining in Fig. 1
quantified the total amount of collagen production and was
performed at 72 h post-treatment. The data suggested that PepC may
have short-term inhibitory effects on HSF collagen I deposition,
but the effect size is much less than the combined effect of Smad
and PepC together, particularly after 72 h. Limiting the western
blots to only collagen I and Smad 2/3 provides only a small
snapshot of the full picture. Insight into how the Smad decoy
affects the expression of different types of collagen found in the
extracellular matrix and the precise effect of these changes on
hypertrophic scaring would aid in clarifying the mechanisms of how
the Smad decoy may ameliorate hypertrophic scaring. This, in turn,
could aid in refining a therapeutic plan to improve on the results
reported in the present study.
At the cellular level, the collagen-reducing ability
of the Smad decoy makes it a potential novel HS treatment as
excessive collagen deposition is the hallmark of HS formation.
However, the exact mechanisms of HS formation remain elusive and
numerous other factors contribute toward the formation of HS. For
example, Kc reform a functional epidermis (re-epithelialization) to
protect exposed dermal tissue at the end of wound healing (34) and delayed re-epithelialization has
been revealed to induce HS formation (35). In addition, MMPs are well known for
their roles in regulating tissue remodeling (36). Decreased expression of MMP-1 is
associated with HS formation (37). Therefore, the present study
investigated the effects of the Smad decoy on HSF and Kc at the
transcriptional level using sequencing and RT-qPCR. A total of 178
genes were reported to exceed a 4-fold difference in HSF treated
with the Smad decoy compared with the untreated control. Certain
representative genes were further analyzed (Fig. 3B) and it was revealed that the
expression of these genes has similar trend in cells from 3
separate patient cell lines. Notably, the majority of those genes
are associated with the extracellular matrix and are highly
relevant to HS formation. For example, COL1A1, COL1A2, COL3A1,
COL5A1, COL11A1, COL12A1 and COL15A1, genes encoding
various collagen types, have been found to be downregulated by the
Smad decoy, indicating the mechanism of the Smad decoy-induced
collagen reduction in HSF. In particular, COL1A1, COL1A2 and
COL3A1 are key genes encoding collagen I and collagen III in
HS formation (38). In addition,
EGF-containing fibulin-like extracellular matrix protein 1, encoded
by EFEMP1, has been found to be highly expressed in HS and
keloids (39,40). The results of the present study
suggested that the Smad decoy attenuates the expression of
EFEMP1 in HSF from all 3 individual patients. A previous
study also suggested that elastin (encoded by ELN) and
fibrillin-1 (encoded by FBN1) are differentially expressed
in HS compared with normal tissues, suggesting their roles in HS
formation (41). The results of
the present study showed that the Smad decoy significantly
decreases the expression of ELN and FBN1 in HSF,
compared with the control. Transgelin, encoded by TAGLN, is
an actin-binding protein in smooth muscle and fibroblasts (42). Although the function of transgelin
remains largely unknown, one study reported that transgelin may be
involved in the calcium-independent smooth muscle contraction
(43). The increased fibroblast
contraction has been reported to be associated with the
over-abundant expression of collagen (17), a downregulated TAGLN
expression may therefore improve the reduction in HS. The MMPs are
widely known for their roles in tissue remodeling by degrading
collagen and other ECM during wound healing (44). In particular, MMP-1 is decreased in
HS tissue in vivo and HSF in vitro, compared with
normal skin tissue or dermal fibroblasts, suggesting that the
decreased expression of MMP-1 contributes toward the formation of
HS (37). Similarly to MMP-1,
another important condition for HS formation is the lack of MMP-3
(45), a metalloprotease
responsible for the degradation of collagen. The results of the
present study showed that the Smad decoy significantly enhances the
expression of MMP1 and MMP3 in HSF, offering further
evidence supporting its use as an anti-HS reagent. It may be
worthwhile to investigate the effects of the Smad decoy on other
Smads in future studies as other Smads also participate in the
regulation the TGF/collagen signalling pathway, although Smads have
different sequence specificity (46). For example, Smad1/5 are
anti-fibrotic proteins, which antagonize Smad2/3, and Smad 7 has
been demonstrated to inhibit the collagen synthesis (47).
TGF-β1 is a cytokine known to be involved in cell
proliferation, differentiation and apoptosis on top of its effects
in mediating ECM expression in dermal fibroblasts, as described
previously (5). Although part of
its activity is mediated through Smad3/4, there are numerous
Smad-independent pathways involved. We hypothesized that the Smad
decoy may facilitate the isolation of the TGF-β1-dependent ECM
modulation pathway for wound healing, which was supported by the
Gene Ontology assignment of the differentially expressed genes
(Table III). Therefore, while
TGF-β1 has been proposed to be useful for wound healing
applications, a Smad decoy delivered locally to the HS site may be
a better option in chronic wound applications.
High throughput RNA sequencing revealed the
potential underlying mechanism of the Smad decoy-induced collagen
reduction in HSF. To further investigate the findings, cells were
treated with the Smad decoy or the scrambled oligonucleotide and
the expression of selected genes was measured using RT-qPCR.
Consistent with the sequencing data, the Smad decoy downregulated
the expression of COL1A1, COL1A2, COL3A1 and TAGLN
and upregulated the expression of MMP1 and MMP3 in
HSF. The scrambled oligonucleotide had no effect on the expression
of those genes. In addition, the secreted protein acidic and rich
in cysteine (SPARC) encoded by SPARC is a well-known protein
which serves essential roles in various biological activities,
including wound healing and angiogenesis (48). In particular, increased SPARC has
been reported to upregulate the expression of collagen type I in
fibroblasts (49). The results of
the present study suggested that the expression of SPARC was
significantly attenuated in HSF following the Smad decoy treatment.
Furthermore, although the roles of plasminogen activator
inhibitor-2 (SERPINB2) in wound healing and HS formation are
unknown, there is evidence to suggest that the expression of
SERPINB2 is detected in macrophages and Kc, and it may participate
in regulating the inflammation of wound healing (50). The present study was the first to
demonstrate that SERPINB2 may contribute toward the formation of HS
and that the Smad decoy significantly attenuates the expression of
SERPINB2 in HSF. MMP-2 is a key regulator for cell migration
and re-epithelialization during wound healing, and increased MMP-2
has been reported to induce HS formation (51). Notably, the results of the present
study demonstrated that the Smad decoy has no effects on the
expression of MMP2. In addition to the gene expression in
HSF, effects of the Smad decoy on Kc gene expression were also
investigated as Kc also serves important roles in HS formation
(35). These results indicated
that the expression of COL1A1, TAGLN and MMP3 in Kc
were not affected following exposure to the Smad decoy, indicating
that the Smad decoy has minor effects on Kc compared with those on
HSF. These results further support the use of the Smad decoy for HS
treatment, as delayed re-epithelialization causes HS formation
(35). An ideal HS therapy should
be able to decrease the excessive collagen deposition of
fibroblasts without affecting the function of Kc.
After investigating the effects of the Smad decoy on
HSF and Kc, the present study investigated the effects of the Smad
decoy on TGF-β1-stimulated HSF as overabundant TGF-β1 expression
has been demonstrated to induce HS formation (6). Previous studies have reported that
additional TGF-β1 alters the expression of COL1A1 (17), MMP2 (52), TIMP1 (53) and FN14 (24) in HSF. Although the total amount of
collagen was not increased in HSF following TGF-β1 stimulation, the
Smad decoy was reported to significantly attenuate the collagen
production compared with TGF-β1-stimulated HSF. In addition,
increased COL1A1 and decreased MMP1 were detected in
HSF stimulated with TGF-β1, which is consistent with previous
studies (17). The Smad decoy,
however, significantly decreased TGF-β1-induced upregulation of
COL1A1 and increased TGF-β1-induced downregulation of
MMP1 in HSF, suggesting its ability to inhibit the effects
of TGF-β1 on HSF. Notably, although TGF-β1 has been reported to
significantly increase MMP2 and decrease TIMP1
expression in HSF, no effects of the Smad decoy on the expression
of MMP2 and TIMP1 were detected compared with the
TGF-β1-treated group. Fibroblast growth factor-inducible molecule
14, encoded by FN14, has been reported to be a downstream
target of the TGF-β signaling pathway and serves essential roles in
HS formation (24). Notably,
neither TGF-β1 nor the Smad decoy was revealed to affect
FN14 expression in HSF.
In addition to decreasing collagen production, when
the transcriptomic changes induced by the Smad decoy were
investigated, results from sequencing and RT-qPCR suggested that
the primary effect of the Smad decoy was to decrease ECM and
membrane components and increase their degradation through the
upregulation of MMP1 and MMP3. This limits the side
effects that may be associated TGF-β inhibition beyond the ECM
components. Notably, MMP-2, which had been reported to be regulated
through p38 MAPK signaling rather than Smad (54), is thought to be important in
collagen remodeling, while MMP-1 activity is more biased towards a
reduction of collagen I (55).
Therefore, the ability to maintain TGF-β-induced upregulation of
MMP-2, while decreasing collagen I and increasing MMP-1 through
Smad inhibition may be beneficial to the wound-healing process.
Therefore, the application of Smad inhibitors may be more
beneficial than TGF-β inhibition therapies, including with
anti-TGF-β antibodies (56). An
interesting phenomenon identified in the present study was that the
Smad decoy has effects independent of exogenous addition of TGF-β1
(Fig. 5). This is probably due to
the fact that TGFB1 mRNA is produced in all HSFs from 3
patients and was detected in the top 5,000 genes expressed in these
cells, which suggests that TGF-β1 is autoregulated in these
fibroblasts. In fact, TGF-β1 has been previously demonstrated to be
produced in HS fibroblasts (57);
therefore, it makes sense that the Smad decoys would work despite
the lack of exogenously added TGF-β1.
In conclusion, a novel Smad decoy was designed,
which inhibits the total amount of collagen production, including
collagen type I in HSF, by altering the expression of various
genes. In particular, the Smad decoy has been reported to change
the expression of COL1A1, COL1A2, COL3A1, TAGLN, SPARC, MMP1
and MMP3 in HSF, which is associated with collagen
deposition and HS formation. In addition, the Smad decoy is able to
attenuate TGF-β1-induced upregulation of COL1A1 and increase
TGF-β1-induced downregulation of MMP1 in HSF. The results of
the present study support the use of this Smad decoy as a potential
novel HS therapy. However, this in vitro study is limited in
its scope and further studies are required to establish the safety
of PepC and the decoy in animal models prior to its clinical
application. The efficacy of spray-on local delivery for a nucleic
acid Smad decoy drug is also unknown as no previous studies are
known to have utilized a simple nozzle to deliver complexed nucleic
acid drugs, although nucleic acid drugs have been nebulized. It is
also not known if Smad-specific inhibition of the ECM proteins will
be sufficient for the inhibition of HS formation, particularly
since little is known about HS formation. In conclusion, the Smad
decoy may offer patients another option to combat HS.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was funded by core funding for the
Molecular Engineering Laboratory and the Molecular Therapeutics
Programme grant (grant no. IAF-PP/H17/01/a0/012) and Wound Care
Innovation for the Tropics [grant no. IAF-PP/2017 (HBMS)
H17/01/a0/009].
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author upon reasonable
request.
Author contributions
YS designed the Smad decoy. CF and YS designed the
experimental protocol. SEA and TL provided PepC and engaged in
discussions on the optimal use of PepC. CF, YS and KWK performed
the experiments. CF and YS analysed the data. CF and YS wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The HSF (primary cells derived from 3 patients: 106,
107 and 108) was purchased from Cell Research Corporation
(Singapore), with ethical approval obtained from A STAR Institute
of Medical Biology (IRB: B-16-135E). Primary human keratinocytes
(Kc) were collected from Asian Skin Bank, Institute of Medical
Biology, A STAR, with ethical approval (IRB: B-16-135E).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ogawa R: Keloid and hypertrophic scars are
the result of chronic inflammation in the reticular dermis. Int J
Mol Sci. 18:6062017. View Article : Google Scholar
|
|
2
|
Bombaro KM, Engrav LH, Carrougher GJ,
Wiechman SA, Faucher L, Costa BA, Heimbach DM, Rivara FP and Honari
S: What is the prevalence of hypertrophic scarring following burns?
Burns. 29:299–302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiao Z, Zhang M, Liu Y and Ren L:
Botulinum toxin type a inhibits connective tissue growth factor
expression in fibroblasts derived from hypertrophic scar. Aesthetic
Plast Surg. 35:802–807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Son D and Harijan A: Overview of surgical
scar prevention and management. J Korean Med Sci. 29:751–757. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cowin AJ, Holmes TM, Brosnan P and
Ferguson MW: Expression of TGF-beta and its receptors in murine
fetal and adult dermal wounds. Eur J Dermatol. 11:424–431.
2001.PubMed/NCBI
|
|
6
|
Tredget EE, Yang L, Delehanty M,
Shankowsky H and Scott PG: Polarized Th2 cytokine production in
patients with hypertrophic scar following thermal injury. J
Interferon Cytokine Res. 26:179–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Macias MJ, Martin-Malpartida P and
Massague J: Structural determinants of Smad function in TGF-β
signaling. Trends Biochem Sci. 40:296–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang YE: Non-Smad pathways in TGF-beta
signaling. Cell Res. 19:128–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi Y and Massague J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng XM, Chung AC and Lan HY: Role of the
TGF-β/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond).
124:243–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chin GS, Liu W, Peled Z, Lee TY,
Steinbrech DS, Hsu M and Longaker MT: Differential expression of
transforming growth factor-beta receptors I and II and activation
of Smad 3 in keloid fibroblasts. Plast Reconstr Surg. 108:423–429.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Walraven M, Gouverneur M, Middelkoop E,
Beelen RH and Ulrich MM: Altered TGF-β signaling in fetal
fibroblasts: What is known about the underlying mechanisms? Wound
Repair Regen. 22:3–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zawel L, Dai JL, Buckhaults P, Zhou S,
Kinzler KW, Vogelstein B and Kern SE: Human Smad3 and Smad4 are
sequence-specific transcription activators. Mol Cell. 1:611–617.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rheinwald JG and Green H: Serial
cultivation of strains of human epidermal keratinocytes: The
formation of keratinizing colonies from single cells. Cell.
6:331–343. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andaloussi SE, Lehto T, Mager I,
Rosenthal-Aizman K, Oprea II, Simonson OE, Sork H, Ezzat K,
Copolovici DM, Kurrikoff K, et al: Design of a peptide-based
vector, PepFect6, for efficient delivery of siRNA in cell culture
and systemically in vivo. Nucleic Acids Res. 39:3972–3987. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ezzat K, Andaloussi SE, Zaghloul EM, Lehto
T, Lindberg S, Moreno PM, Viola JR, Magdy T, Abdo R, Guterstam P,
et al: PepFect 14, a novel cell-penetrating peptide for
oligonucleotide delivery in solution and as solid formulation.
Nucleic Acids Res. 39:5284–5298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan C, Dong Y, Xie Y, Su Y, Zhang X,
Leavesley D and Upton Z: Shikonin reduces TGF-β1-induced collagen
production and contraction in hypertrophic scar-derived human skin
fibroblasts. Int J Mol Med. 36:985–991. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trapnell C, Roberts A, Goff L, Pertea G,
Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL and Pachter L:
Differential gene and transcript expression analysis of RNA-seq
experiments with TopHat and Cufflinks. Nat Protoc. 7:562–578. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anders S, Pyl PT and Huber W: HTSeq-a
Python framework to work with high-throughput sequencing data.
Bioinformatics. 31:166–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang DW, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Applied Biosystems: Guide to performing
relative quantitation of gene expression using real-time
quantitative PCR. 2004. simplehttps://assets.thermofisher.com/TFS-Assets/LSG/manuals/cms_042380.pdf
|
|
22
|
Verrecchia F, Chu ML and Mauviel A:
Identification of novel TGF-beta/Smad gene targets in dermal
fibroblasts using a combined cDNA microarray/promoter
transactivation approach. J Biol Chem. 276:17058–17062. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan C, Xie Y, Dong Y, Su Y and Upton Z:
Investigating the potential of Shikonin as a novel hypertrophic
scar treatment. J Biomed Sci. 22:702015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen S, Liu J, Yang M, Lai W, Ye L, Chen
J, Hou X, Ding H, Zhang W, Wu Y, et al: Fn14, a downstream target
of the TGF-β signaling pathway, regulates fibroblast activation.
PLoS One. 10:e01438022015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Perkins K, Davey RB and Wallis KA:
Silicone gel: A new treatment for burn scars and contractures.
Burns Incl Therm Inj. 9:201–204. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Oliveira GV, Nunes TA and Magna LA:
Silicone versus nonsilicone gel dressings: A controlled trial.
Dermatol Surg. 27:721–726. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Niessen FB, Spauwen PH, Schalkwijk J and
Kon M: On the nature of hypertrophic scars and keloids: A review.
Plast Reconstr Surg. 104:1435–1458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Casi G and Neri D: Antibody-drug
conjugates and small molecule-drug conjugates: Opportunities and
challenges for the development of selective anticancer cytotoxic
agents. J Med Chem. 58:8751–8761. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
An S and Fu L: Small-molecule PROTACs: An
emerging and promising approach for the development of targeted
therapy drugs. EBioMedicine. 36:553–562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang L, Yang J, Ran B, Yang X, Zheng W,
Long Y and Jiang X: Small Molecular TGF-β1-inhibitor-loaded
electrospun fibrous scaffolds for preventing hypertrophic scars.
ACS Appl Mater Interfaces. 9:32545–32553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cardarelli F, Digiacomo L, Marchini C,
Amici A, Salomone F, Fiume G, Rossetta A, Gratton E, Pozzi D and
Caracciolo G: The intracellular trafficking mechanism of
Lipofectamine-based transfection reagents and its implication for
gene delivery. Sci Rep. 6:258792016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Di Lullo GA, Sweeney SM, Korkko J,
Ala-Kokko L and San Antonio JD: Mapping the ligand-binding sites
and disease-associated mutations on the most abundant protein in
the human, type I collagen. J Biol Chem. 277:4223–4231. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu Y, Tao H, Jin C, Liu Y, Lu X, Hu X and
Wang X: Transforming growth factor-β1 induces type II collagen and
aggrecan expression via activation of extracellular
signal-regulated kinase 1/2 and Smad2/3 signaling pathways. Mol Med
Rep. 12:5573–5579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Santoro MM and Gaudino G: Cellular and
molecular facets of keratinocyte reepithelization during wound
healing. Exp Cell Res. 304:274–286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brown NJ, Kimble RM, Gramotnev G, Rodger S
and Cuttle L: Predictors of re-epithelialization in pediatric burn.
Burns. 40:751–758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Das S, Mandal M, Chakraborti T, Mandal A
and Chakraborti S: Structure and evolutionary aspects of matrix
metalloproteinases: A brief overview. Mol Cell Biochem. 253:31–40.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eto H, Suga H, Aoi N, Kato H, Doi K, Kuno
S, Tabata Y and Yoshimura K: Therapeutic potential of fibroblast
growth factor-2 for hypertrophic scars: Upregulation of MMP-1 and
HGF expression. Lab Invest. 92:214–223. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu J, Shen JX, Wu HT, Li XL, Wen XF, Du
CW and Zhang GJ: Collagen 1A1 (COL1A1) promotes metastasis of
breast cancer and is a potential therapeutic target. Discov Med.
25:211–223. 2018.PubMed/NCBI
|
|
39
|
Ma L, Gan C, Huang Y, Wang Y, Luo G and Wu
J: Comparative proteomic analysis of extracellular matrix proteins
secreted by hypertrophic scar with normal skin fibroblasts. Burns
Trauma. 2:76–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Seifert O, Bayat A, Geffers R, Dienus K,
Buer J, Löfgren S and Matussek A: Identification of unique gene
expression patterns within different lesional sites of keloids.
Wound Repair Regen. 16:254–265. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Amadeu TP, Braune AS, Porto LC,
Desmouliere A and Costa AM: Fibrillin-1 and elastin are
differentially expressed in hypertrophic scars and keloids. Wound
Repair Regen. 12:169–174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu R, Hossain MM, Chen X and Jin JP:
Mechanoregulation of SM22α/Transgelin. Biochemistry. 56:5526–5538.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Assinder SJ, Stanton JA and Prasad PD:
Transgelin: An actin-binding protein and tumour suppressor. Int J
Biochem Cell Biol. 41:482–486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ulrich D, Ulrich F, Unglaub F, Piatkowski
A and Pallua N: Matrix metalloproteinases and tissue inhibitors of
metalloproteinases in patients with different types of scars and
keloids. J Plast Reconstr Aesthet Surg. 63:1015–1021. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Poormasjedi-Meibod MS, Hartwell R, Kilani
R and Ghahary A: Anti-scarring properties of different tryptophan
derivatives. PLoS One. 9:e919552014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Martin-Malpartida P, Batet M, Kaczmarska
Z, Freier R, Gomes T, Aragón E, Zou Y, Wang Q, Xi Q, Ruiz L, et al:
Structural basis for genome wide recognition of 5-bp GC motifs by
SMAD transcription factors. Nat Commun. 8:20702017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Walton KL, Johnson KE and Harrison CA:
Targeting TGF-β Mediated SMAD signaling for the prevention of
fibrosis. Front Pharmacol. 8:4612017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Basu A, Kligman LH, Samulewicz SJ and Howe
CC: Impaired wound healing in mice deficient in a matricellular
protein SPARC (osteonectin, BM-40). BMC Cell Biol. 2:152001.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chavez-Muñoz C, Hartwell R, Jalili RB,
Jafarnejad SM, Lai A, Nabai L, Ghaffari A, Hojabrpour P, Kanaan N,
Duronio V, et al: SPARC/SFN interaction, suppresses type I collagen
in dermal fibroblasts. J Cell Biochem. 113:2622–2632. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dougherty KM, Pearson JM, Yang AY,
Westrick RJ, Baker MS and Ginsburg D: The plasminogen activator
inhibitor-2 gene is not required for normal murine development or
survival. Proc Natl Acad Sci USA. 96:686–691. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rohani MG and Parks WC: Matrix remodeling
by MMPs during wound repair. Matrix Biol. 44-46:113–121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu Y, Li Y, Li N, Teng W, Wang M, Zhang Y
and Xiao Z: TGF-β1 promotes scar fibroblasts proliferation and
transdifferentiation via up-regulating MicroRNA-21. Sci Rep.
6:322312016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bai X, He T, Liu J, Wang Y, Fan L, Tao K,
Shi J, Tang C, Su L and Hu D: Loureirin B inhibits fibroblast
proliferation and extracellular matrix deposition in hypertrophic
scar via TGF-β/Smad pathway. Exp Dermatol. 24:355–360. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim ES, Kim MS and Moon A:
TGF-beta-induced upregulation of MMP-2 and MMP-9 depends on p38
MAPK, but not ERK signaling in MCF10A human breast epithelial
cells. Int J Oncol. 25:1375–1382. 2004.PubMed/NCBI
|
|
55
|
Gill SE and Parks WC: Metalloproteinases
and their inhibitors: Regulators of wound healing. Int J Biochem
Cell Biol. 40:1334–1347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Qiu SS, Dotor J and Hontanilla B: Effect
of P144® (Anti-TGF-β) in an ‘in vivo’ human hypertrophic
scar model in nude mice. PLoS One. 10:e01444892015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tredget EE, Wang R, Shen Q, Scott PG and
Ghahary A: Transforming growth factor-beta mRNA and protein in
hypertrophic scar tissues and fibroblasts: Antagonism by IFN-alpha
and IFN-gamma in vitro and in vivo. J Interferon Cytokine Res.
20:143–151. 2000. View Article : Google Scholar : PubMed/NCBI
|