Introduction
Ischemia-reperfusion (IR) injury remains a key
medical challenge because it relates to organ transplantation and
alterations in vascular perfusion (1–3).
Liver I/R injury frequently occurs as a result of liver
transplantation, partial hepatectomy, hemorrhagic shock, trauma,
severe infection and metabolic disorder (1,4). I/R
injury contributes to 10% of early graft dysfunction following
organ transplantation and accounts for acute and chronic rejection
(5,6). Much effort has been made to elucidate
the underlying mechanisms of I/R injury, but so far little is
understood (7,8). Oxidative stress, innate and adaptive
immune responses, excessive inflammation, anaerobic metabolism and
acidosis are considered to be the main molecular and cellular
events that contribute to liver injury following I/R (7–9).
However, no approved clinical intervention is currently available
for I/R-related liver injury (10). Our latest study indicated that
thymic stromal lymphopoietin protects against liver I/R injury via
activation of the PI3K/Akt pathway (11). Akt is thought to be a potential
therapeutic target in liver I/R injury (12). Thus, seeking factors that can
activate Akt pathway may be a promising protection strategy for
liver I/R injury.
Hesperidin (HDN) is a bioflavonoid, abundant in
citrus fruits, such as oranges, grapefruits and lemons, that serves
a role as an antioxidant in biological systems (13). It has been confirmed that HDN has
hydrogen radical- and hydrogen peroxide-removal activities
(13,14). Additionally, it has been reported
that HDN has numerous beneficial effects, such as
anti-inflammatory, antibacterial, anticancer and anti-edema
properties (13–16). Thus, HDN has a protective effect on
sterile organ injuries, including ischemic stroke and arthritis,
and infectious diseases such as lipopolysaccharide-induced
inflammation (17–20). A number of studies reveal that the
activation of PI3K/Akt pathway is one of the most important
mechanisms for the biological effects of HDN (16,18,21,22).
Recently, Park et al (23)
observed that HDN ameliorates hepatic injury in Sprague-Dawley
rats. However, the underlying mechanisms remain unknown.
The present study hypothesized that HDN ameliorates
liver I/R injury through the Akt pathway. Wild-type (WT) mice were
subjected to liver I/R and administered HDN. HDN ameliorated liver
I/R injury, but the hepatoprotective effects of HDN were prevented
by an Akt inhibitor. The results of the present study demonstrated
that HDN ameliorated liver oxidative stress, suppressed
inflammatory responses and prevented hepatocyte apoptosis during
liver I/R injury. Thus, HDN administration may be useful to prevent
injury after liver I/R.
Materials and methods
Animals and model
A total of 65 male C57BL/6J WT mice (8–10 weeks old,
22–30 g) were purchased and housed in the Guangxi Medical
University Laboratory Animal Center (Guangxi, China). The mice were
bred in a specific pathogen-free animal facility under controlled
conditions at 19–23°C and 40–60% humidity with a 12-h dark/light
cycle and had free access to food and water. A warm partial liver
I/R injury model with occlusion of 70% segments of the liver blood
supply was established as previously described (24). Briefly, the mice were injected
intraperitoneally (i.p.) with ketamine (100 mg/kg)-xylazine (5
mg/kg). The left and middle hepatic lobe blood supply was occluded
with an atraumatic clamp (Fine Science Tools, Inc.). The clamp was
removed after 60 min of ischemia, and the reperfusion procedure
lasted for 6 h. HDN was administered orally to the mice by gavage
for 3 days before surgery. To ascertain the optimal dose for the
3-day HDN administration, a fixed-dose study was designed with
doses of 100, 200 and 400 mg/kg. LY294002 (0.5 mg/kg, i.p.) was
administered 30 min before surgery. Sham (treated without HDN) and
control (treated with HDN at the dose of 200 mg/kg for 3 days) mice
underwent anesthesia, laparotomy and exposure of the portal triad
but without vasculature occlusion. Mice were euthanized by
exsanguination under anesthesia following reperfusion for 6 h. Mice
were anaesthetized with 1.5% isoflurane, 1 ml of blood was
harvested by cardiac puncture and liver samples were collected and
stored as detailed below. Following blood and tissue collection,
euthanasia was confirmed by bilateral thoracotomy and subsequently
by the absence of any respiratory movement and heartbeat.
The whole blood of the mice was collected in a
covered test tube and allowed to clot by leaving it undisturbed for
20 min at room temperature. The clot was removed by centrifuging at
2,000 × g for 10 min in a refrigerated (4°C) centrifuge. The
resulting supernatant was designated serum and was immediately
transferred into a clean polypropylene tube using a pipette. The
serum was stored at −80°C for further analysis. Part of the liver
samples were embedded in 2% paraformaldehyde for histological
analysis, and the remainder were frozen in liquid nitrogen and
stored at −80°C for further analysis.
The mice were divided into the following groups: i)
Sham group (n=8); ii) control group (n=8); iii) I/R group (n=8);
iv) I/R + HDN group (n=8/group, at three different concentrations:
100, 200 and 400 mg/kg); v) I/R+LY294002 group (n=8); and vi)
I/R+HDN (200 mg/kg) +LY294002 group (n=8).
Reagents
Antibodies for western blotting were as follows: the
GAPDH antibody (cat. no. ab8245) was purchased from Abcam, and the
Bcl-2 (cat. no. 3498), pro-caspase 3 (cat. no. 9662), cleaved
caspase 3 (cat. no. 9661), phosphorylated (p)-Akt (S473) (cat. no.
9271) and Akt antibodies (cat. no. 9272) were purchased from Cell
Signaling Technology, Inc. Goat anti-mouse secondary antibody (cat.
no. 31430) and goat anti-rabbit secondary antibody (cat. no. 31460,
both at 1:20,000 dilution) were from Thermo Fisher Scientific, Inc.
HDN (HPLC>98%; cat. no. XW05202631) was obtained from Sinopharm
Chemical Reagent Co., Ltd. LY294002 (cat. no. L9908) was purchased
from Sigma-Aldrich (Merck KGaA). Malondialdehyde assay kit (MDA;
cat. no. A003-1), superoxide dismutase assay kit (SOD; cat. no.
A001-1), catalase assay kit (CAT; cat. no. A007-2), glutathione
peroxidase assay kit (GPx; cat. no. A006-1) and glutathione assay
kit (GSH; cat. no. A006-1) were purchased from Nanjing Jiancheng
Bioengineering Institute.
Hepatocyte isolation and culture
Hepatocytes (HCs) were isolated as described
previously (25). Mice were
euthanized with 5% isoflurane for 5 minutes in a plexiglass
chamber, and bilateral thoracotomy was performed for a secondary
confirmation of death. The mice were perfused following euthanasia
in vivo by using an in situ collagenase (type VI,
Worthington Biochemical Corporation) technique. Next, HCs were
separated from nonparenchymal cells (NPCs) by two cycles of
differential centrifugation (50 × g for 2 min, 4°C) to ensure that
the purification exceeded 99%. Finally, trypan blue exclusion was
used to test the viability to ensure that it was greater than 95%.
HCs were cultured as described previously (26). Briefly, hepatocytes (1.5×105
cells/ml) were plated on gelatin-coated culture plates with
collagen I (BD Pharmingen; BD Biosciences) in Williams medium E
(Invitrogen; Thermo Fisher Scientific, Inc.) with 10% calf serum
(Thermo Fisher Scientific, Inc.), 15 mM HEPES (Thermo Fisher
Scientific, Inc.), 1 µM insulin (Eli Lilly and Company), 2 mM
L-glutamine (Thermo Fisher Scientific, Inc.), penicillin (100 U/ml)
and streptomycin (100 U/ml; Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C. Cells were allowed to attach to plates overnight,
and the culture media was replaced with fresh culture media before
the cells were treated for experiments.
Immunofluorescence staining
After hepatocytes were cultured for 24 h at 37°C,
cells were washed by phosphate-buffered saline (PBS; pH 7.4) once,
and then were fixed with 4% paraformaldehyde (Rich Joint) for 30
min at 4°C. Cells were then permeabilized by 0.1% Triton-X 100
(Rich Joint) for 15 min at room temperature, washed twice with PBS,
incubated with 5% fetal bovine serum (HyClone; Thermo Fisher
Scientific, Inc.) for 15 min at room temperature and then incubated
with hepatocyte-specific marker Cytokeratin-18 (CK18) primary
antibody (1:1,000 dilution) (cat. no. 10830-1-AP; Wuhan Sanying
Biotechnology) overnight at 4°C. Cells were then washed with PBS
three times (5 min each) and incubated with fluorescein
isothiocyanate (FITC)-conjugated goat anti-rabbit secondary
antibody (1:100 dilution; cat. no. SA00003-2; Wuhan Sanying
Biotechnology) at 37°C for 1 h, followed by Hoechst 33258 (cat. no.
C1017; Beyotime Institute of Biotechnology) for nuclear staining
for 15 min at room temperature. Slides were viewed using an Olympus
IX71 fluorescence microscope (Olympus Corporation).
Serum sample assays
Serum alanine aminotransferase (ALT) levels were
measured by alanine aminotransferase assay kit using standard
spectrophotometric procedures with 5 µl serum according to the
manufacturer's instructions (cat. no. C009-2-1, Nanjing Jiancheng
Institute of Biotechnology). The levels of ALT were expressed as
units/L serum (U/L).
Histological analysis
Liver tissues were harvested as described in the
Animals and model subsection, embedded in 2% paraformaldehyde for 2
h at 4°C and then removed for preservation at 4°C with 70% ethanol.
Liver sections (5 µm) were stained with hematoxylin and eosin
(H&E) to assess histopathology. Images of five randomly
selected fields were acquired with a light microscope
(magnification, ×10). Necrotic areas were analyzed by ImageJ
software (version 1.51w, National Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
The ischemic lobe of the liver was frozen in liquid
nitrogen and stored at −80°C for comparative PCR analysis. Total
RNA was extracted with the RNeasy Mini Kit (Qiagen China Co., Ltd.)
according to the manufacturer's instructions. cDNA was synthesized
using 1 µg of RNA and with 2 µM oligodT primers (Qiagen China Co.,
Ltd.), 4 U Omniscript™ reverse transcriptase (Qiagen China Co.,
Ltd.), 4 µl of 5X RT buffer, and 16 µl RNA in RNase-free water. The
synthesis of cDNA was performed at 37°C for 60 min. SYBR Green PCR
Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) was
used to prepare the PCR reaction mixes. qPCR was performed with
sense and antisense primer pre-validated and specific for β-actin,
IL-1β, IL-6, and TNF-α (Qiagen China Co., Ltd.) with the following
thermocycling parameters: Initial denaturation at 94°C for 5 min;
followed by 40 cycles of 94°C for 15 sec, 60°C for 20 sec and 72°C
for 30 sec. The reaction cocktail used for test contained 10 µl
iTaq SYBR Green PCR Master Mix, 8 µl RNase-free water, 0.5 µl sense
primer, 0.5 µl antisense primer and 1 µl cDNA. All samples were
assayed in duplicate and normalized to β-actin mRNA abundance. Gene
expression levels were quantified using the 2−ΔΔCq
method (27). The primers used for
qPCR were as follows: β-actin, forward
5′-TGTGATGGTGGGAATGGGTCAG-3′, reverse 5′-TTTGATGTCACGCACGATTTCC-3′;
IL-1β, forward 5′-CCAGCTTCAAATCTCACAGCAG-3′, reverse
5′-CTTCTTTGGGTATTGCTTGGGATC-3′; IL-6, forward
5′-TCCAGTTGCCTTCTTGGGAC-3′, reverse 5′-GTACTCCAGAAGACCAGAGG-3′; and
TNF-α, forward 5′-CACAGAAAGCATGATCCGCGACGT-3′, reverse
5′-CGGCAGAGAGGAGGTTGACTTTCT-3′.
Western blotting analysis
Protein expression was determined in frozen liver
tissues using a previously described western blotting protocol
(11). Briefly, snap-frozen liver
was homogenized in cell lysis buffer (Cell Signaling Technology,
Inc.) and centrifuged (16,000 × g for 15 min, 4°C), after which the
supernatant was collected. Protein concentrations were determined
using a BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.).
Loading buffer was added to the samples (30 µg/well), which were
then resolved by 10 or 15% SDS-PAGE. Samples were then transferred
to a polyvinylidene difluoride membrane at 250 mA for 2 h. The
membrane was blocked in 5% milk for 1 h at room temperature and
then incubated with primary antibody (1:1,000 dilution) in 1% milk
overnight at 4°C. Membranes were washed in Tris-buffered
saline-0.05% Tween-20 (TBST) for 10 min, incubated with horseradish
peroxidase-conjugated secondary antibody (1:20,000 dilution) for 1
h and then washed for 1 h in TBST at room temperature, before being
developed by an enhanced chemiluminescence kit (Thermo Fisher
Scientific Inc.). The signal was acquired and quantified with a
ChemiDoc™ MP Imaging System (Bio-Rad Laboratories, Inc.).
Cell death detection assay
Cell death was detected as described previously
(25). Briefly, liver tissues were
incubated with the In-Situ Cell Death Detection TMR Red kit
(cat. no. 12156792910; Roche Diagnostics) according to the
manufacturer's instructions. Images (magnification, ×40) were
captured with a Nikon A1 confocal microscope (Nikon Instruments)
from at least six randomly selected fields/sections. Dead cells
were quantified by NIS Elements (version 4.1, Nikon
Instruments).
Statistical analysis
All experiments were performed in triplicate. All
data are presented as the mean ± standard error of the mean.
Two-tailed unpaired Student's t-test was performed to compare the
differences between two experimental groups. One-way ANOVA followed
by a Bonferroni post hoc test was performed to compare the
differences between multiple groups. GraphPad Prism (version 8.0.2;
GraphPad Software, Inc.) was used to perform the data analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Hesperidin ameliorates liver I/R
injury
To determine the role of HDN in liver I/R, WT mice
were subjected to warm partial liver I/R injury with or without HDN
treatment. Liver injury was assessed by detecting serum ALT levels
and morphological indexes (H&E staining). Compared with that of
the sham group the ALT levels indicated that liver damage was
markedly increased after liver I/R (Fig. 1A). There was no effect on ALT
levels in control group but without liver I/R (Fig. 1A). However, HDN significantly
ameliorated liver I/R injury in a dose-dependent way (Fig. 1A). H&E staining also indicated
that the necrotic areas of the ischemic hepatic lobe were
significantly attenuated by HDN treatment (Fig. 1B). In order to explore the
underlying mechanisms, the median concentration of HDN (200 mg/kg)
was used for the following experiments.
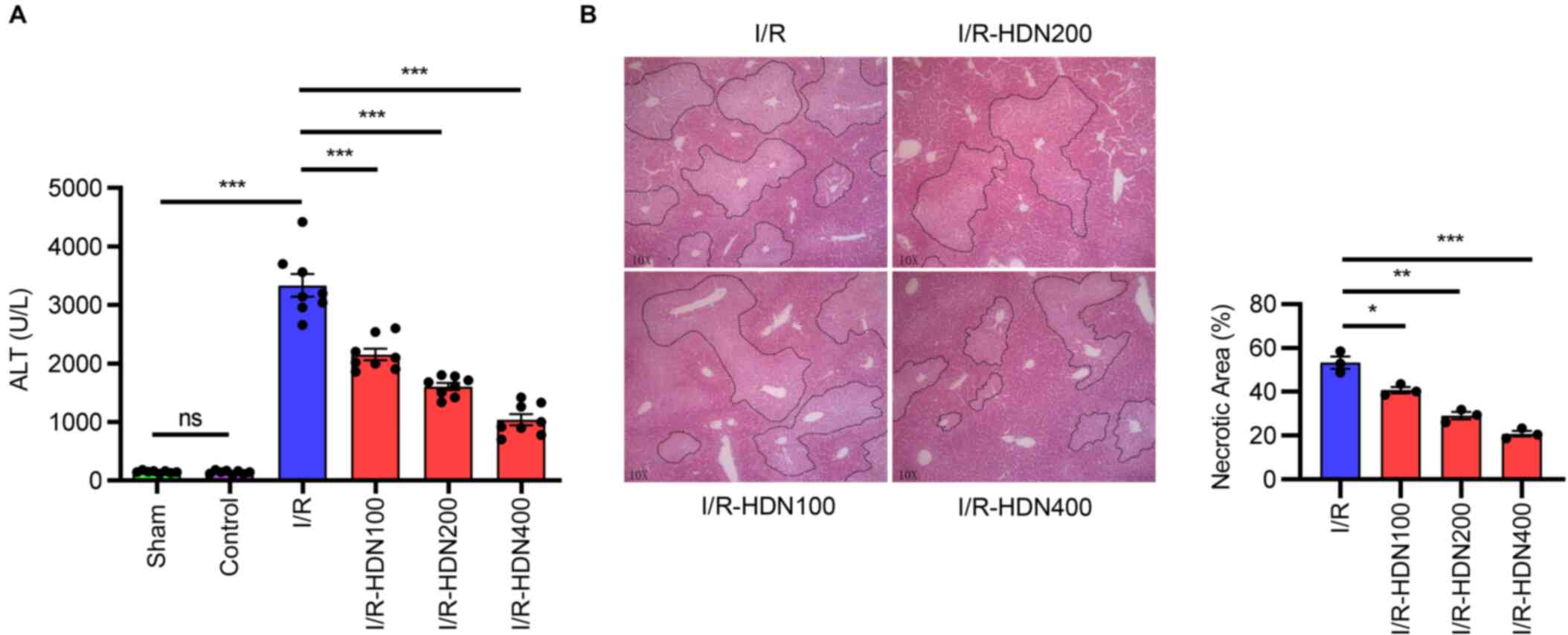 | Figure 1.HDN ameliorates liver I/R injury. (A)
Serum ALT levels in the livers of mice after sham surgery, control,
I/R injury or I/R injury with 100, 200 or 400 mg/kg HDN treatment;
n=8 mice/group. (B) Representative hematoxylin and eosin staining
images and necrotic areas of ischemic liver lobes of mice at 6 h
post-reperfusion with or without HDN treatment; magnification, ×10.
Dotted lines indicate measured areas of necrosis, quantified on bar
graph. n=8 mice/group. *P<0.05, **P<0.01 and ***P<0.001.
ALT, alanine aminotransferase; HDN, hesperidin; I/R,
ischemia/reperfusion; ns, not significant. |
Hesperidin ameliorates liver oxidative
stress in liver I/R injury
Oxidative stress is considered to serve a key role
in liver I/R injury (9,28). It has been reported that HDN
eliminates oxidative stress in a number of diseases, such as
toxin-induced tissue damage and inflammation (13,14).
To ascertain whether HDN ameliorates oxidative stress during liver
I/R, the levels of MDA, SOD, CAT, GPx and GSH in ischemic liver
lobes were measured using commercial kits. Notably, compared with
that of the sham group MDA content increased significantly after
liver I/R, and this was significantly reversed by HDN treatment
(Fig. 2A). In addition, compared
with that of the sham group the antioxidant activities of SOD, CAT,
GPx and GSH, decreased significantly after liver I/R, and these
effects were reversed by HDN treatment (Fig. 2B-E).
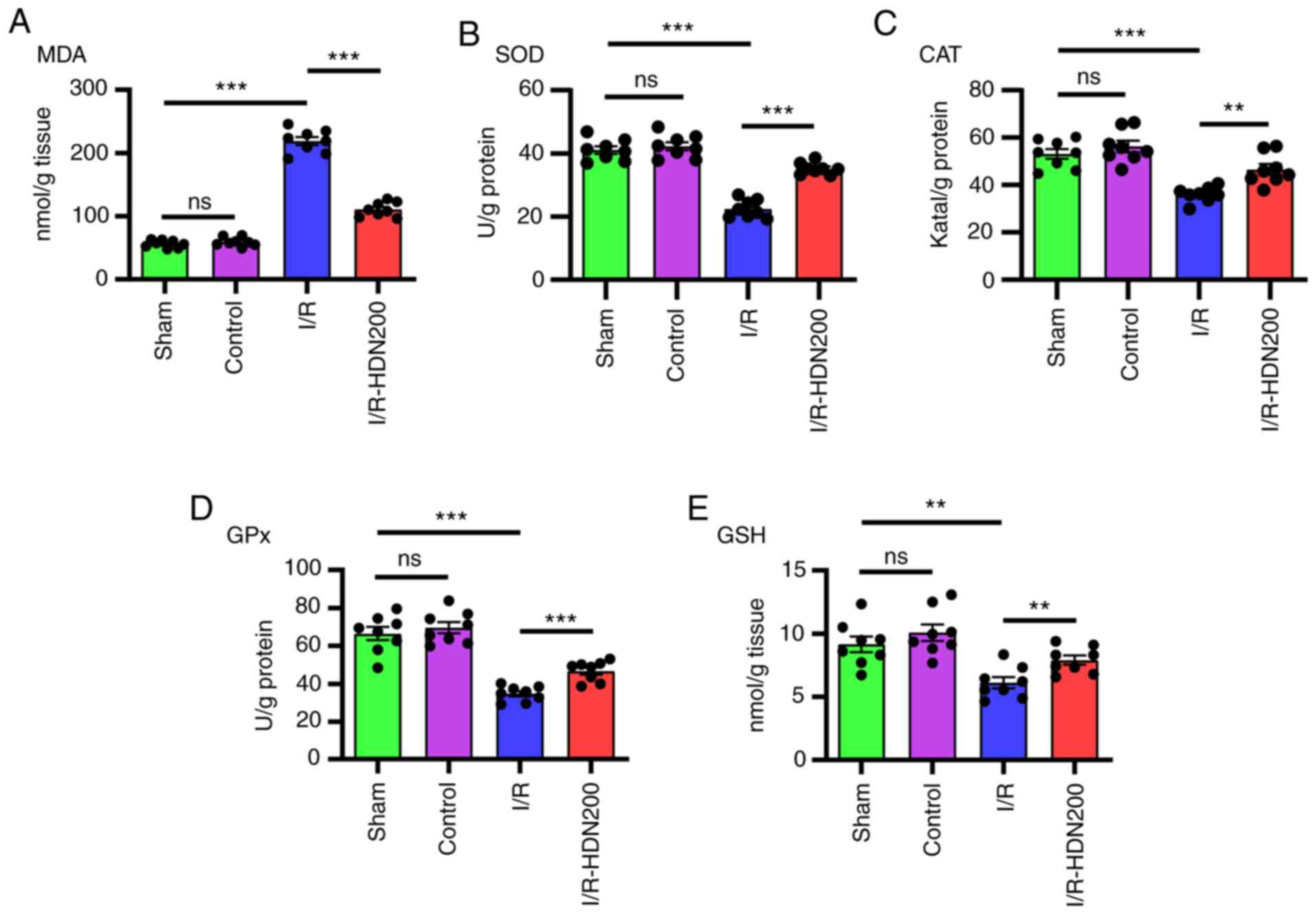 | Figure 2.HDN ameliorates liver oxidative
stress in liver I/R injury. The levels of (A) MDA, (B) SOD, (C)
CAT, (D) GPx and (E) GSH of liver lobes in sham, control, I/R or
I/R with HDN treatment (200 mg/kg) groups. n=8 mice/group;
**P<0.01 and ***P<0.001. CAT, catalase; GPx, glutathione
peroxidase; GSH, glutathione; HDN, hesperidin; I/R,
ischemia/reperfusion; MDA, malondialdehyde; ns, not significant;
SOD, superoxide dismutase. |
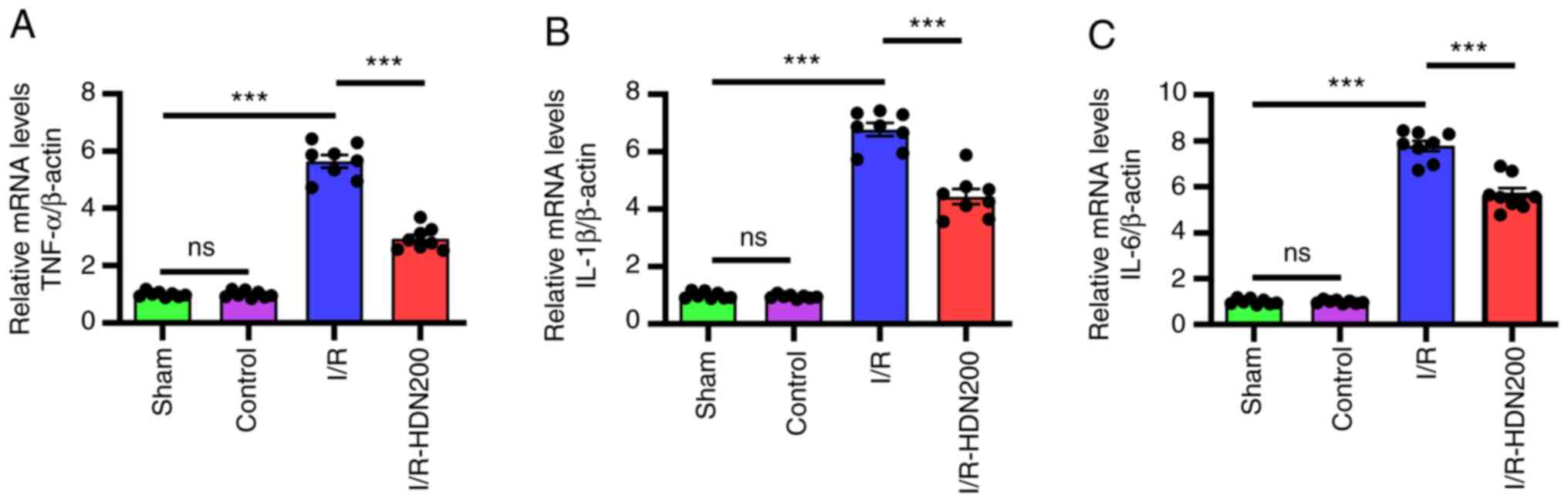
Hesperidin suppresses inflammation
during liver I/R injury
Inflammation serves important roles in liver I/R
injury, and numerous cytokines are involved in liver injury
(7,29). To determine the relationship
between HDN and inflammation during liver I/R injury, the mRNA
levels of TNF-α, IL-6 and IL-1β in ischemic liver lobes were
assessed by RT-qPCR. Consistent with a previous study (5), the results demonstrated that compared
with those of the sham group the mRNA levels of TNF-α, IL-6 and
IL-1β in liver tissues increased significantly after I/R and HDN
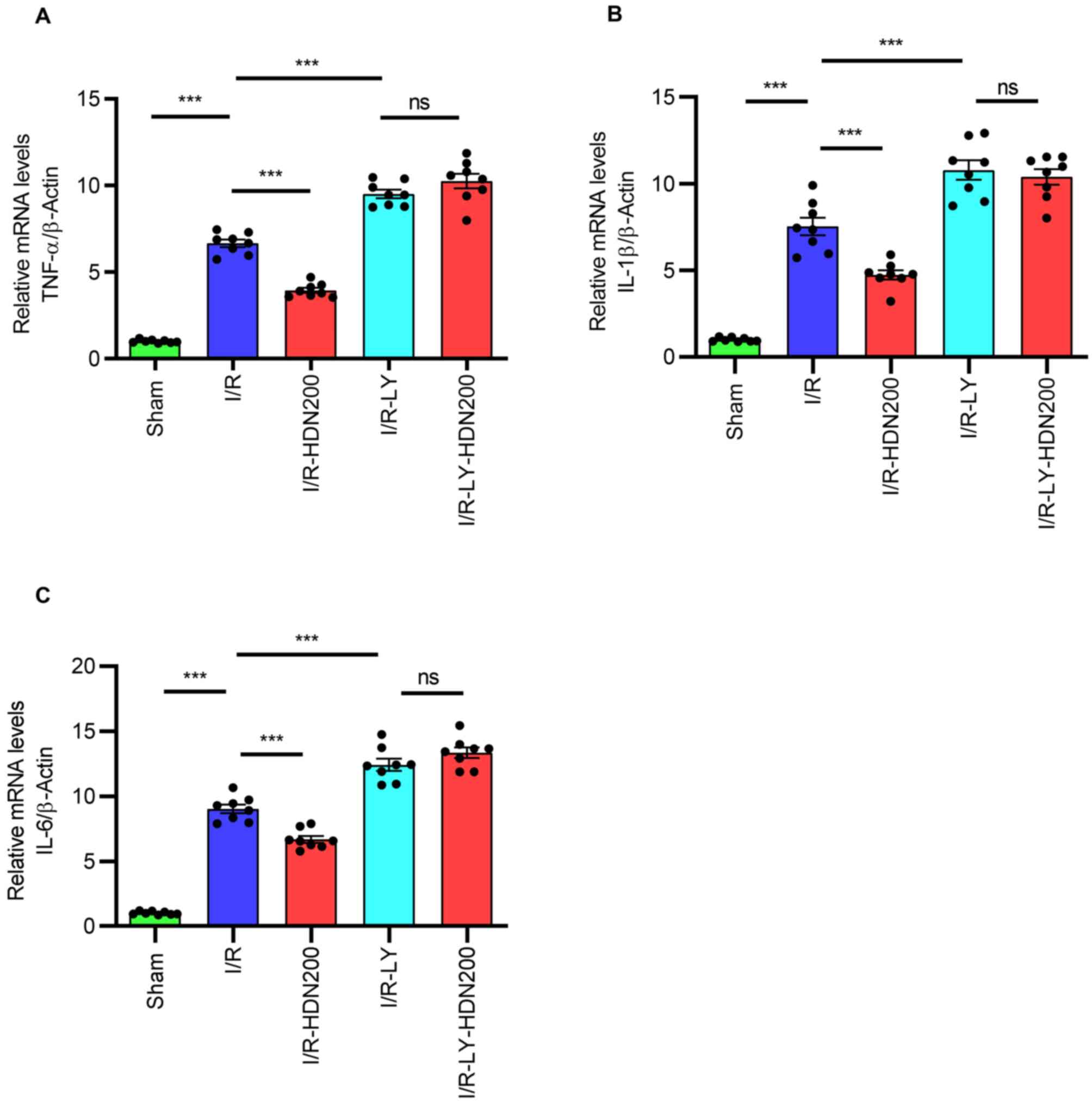
significantly attenuated these effects (Fig. 3A-C).
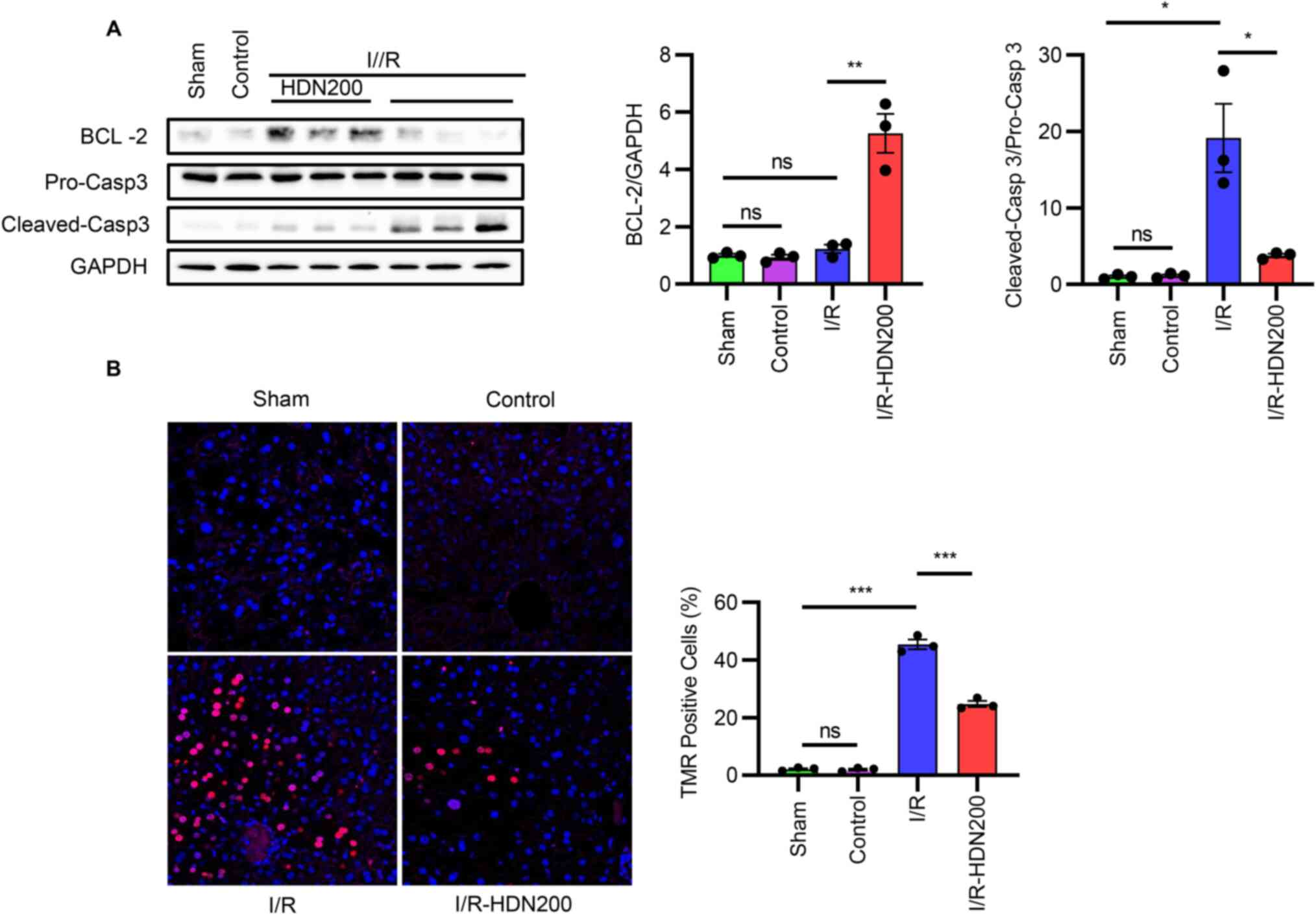
Hesperidin protects the liver against
apoptosis following I/R injury
Inhibition of apoptosis has been reported to have a
modest protective effect on liver I/R injury (30). Thus, apoptotic markers in the
livers of WT mice with or without HDN treatment after liver I/R
injury was assessed. Western blotting results demonstrated that the
expression levels of cleaved-caspase 3 increased markedly after
liver I/R and were significantly reversed after HDN treatment
(Fig. 4A). By contrast, no
significant differences were observed in the levels of Bcl-2 in the
liver after I/R compared with those of the sham group, but HDN
treatment significantly increased the levels of Bcl-2 compared with
those of the I/R group (Fig. 4A).
In addition, TMR assay results demonstrated that there were
significantly fewer TMR-positive apoptotic cells in livers in the
I/R group treated with HDN compared with the number of apoptotic
cells in the untreated I/R group (Fig.
4B).
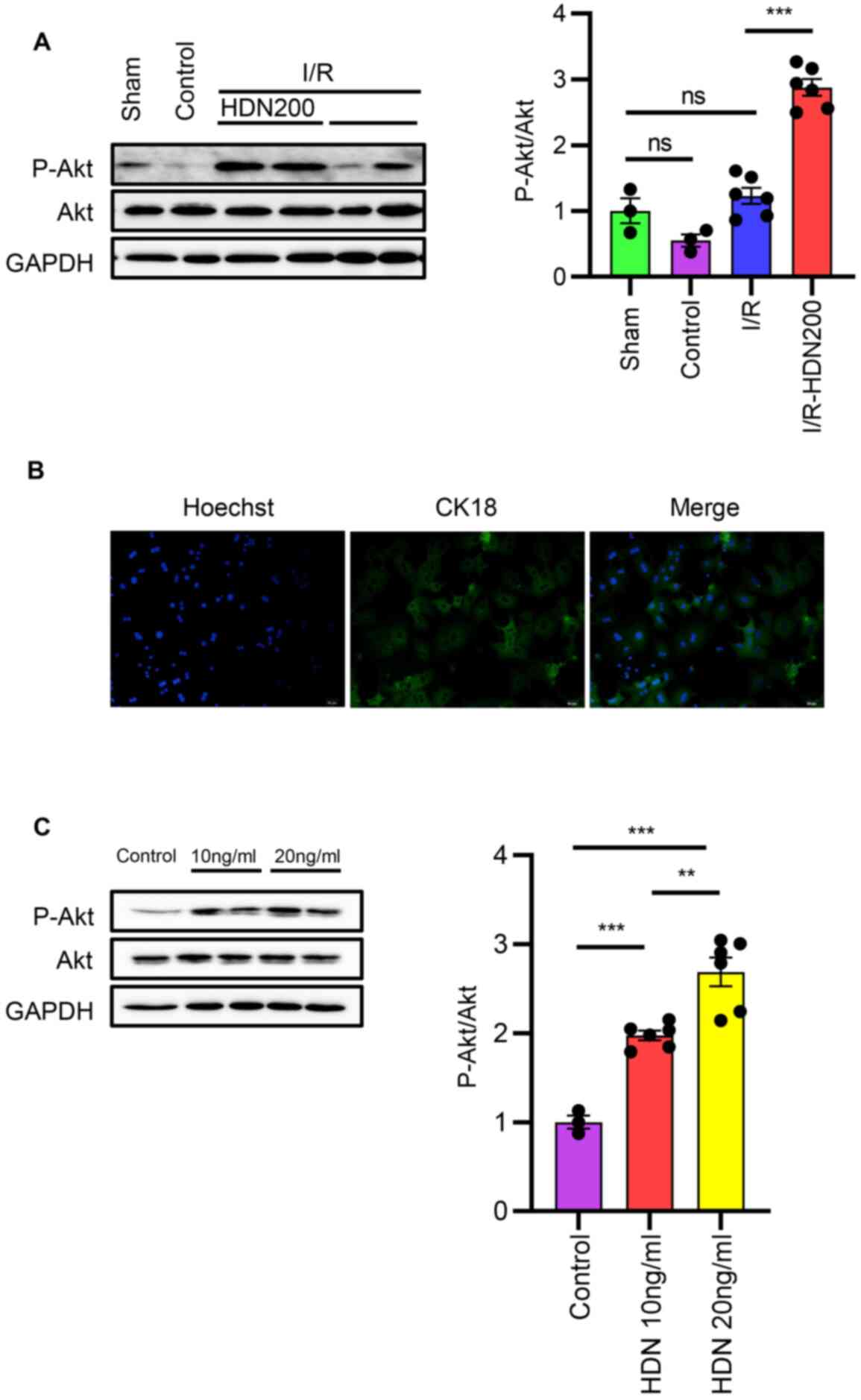
Hesperidin activates the Akt pathway
in liver I/R injury in vivo and in hepatocytes in vitro
Having determined that HDN ameliorates liver I/R
injury, the mechanisms underlying the modulation of cellular
functions by HDN were next examined. Akt serves a key role in
protecting against liver I/R injury (11,12),
and several previous studies indicated that HDN induces Akt
phosphorylation and influences apoptosis (16,18,21,22).
Thus, the relationship between HDN and the Akt pathway in liver I/R
injury was investigated. No significant differences were observed
in the levels of p-Akt in the liver after I/R compared with those
of the sham group, but HDN increased the expression levels of p-Akt
compared with those of the untreated I/R group (Fig. 5A). To ascertain the role of HDN in
phosphorylating Akt in HCs, HCs were isolated and cultured in
vitro (Fig. 5B). Cultured HCs
were exposed to HDN for 6 h, and the results demonstrated that 10
and 20 ng/ml HDN increased the levels of phosphorylated Akt in HCs
(Fig. 5C).
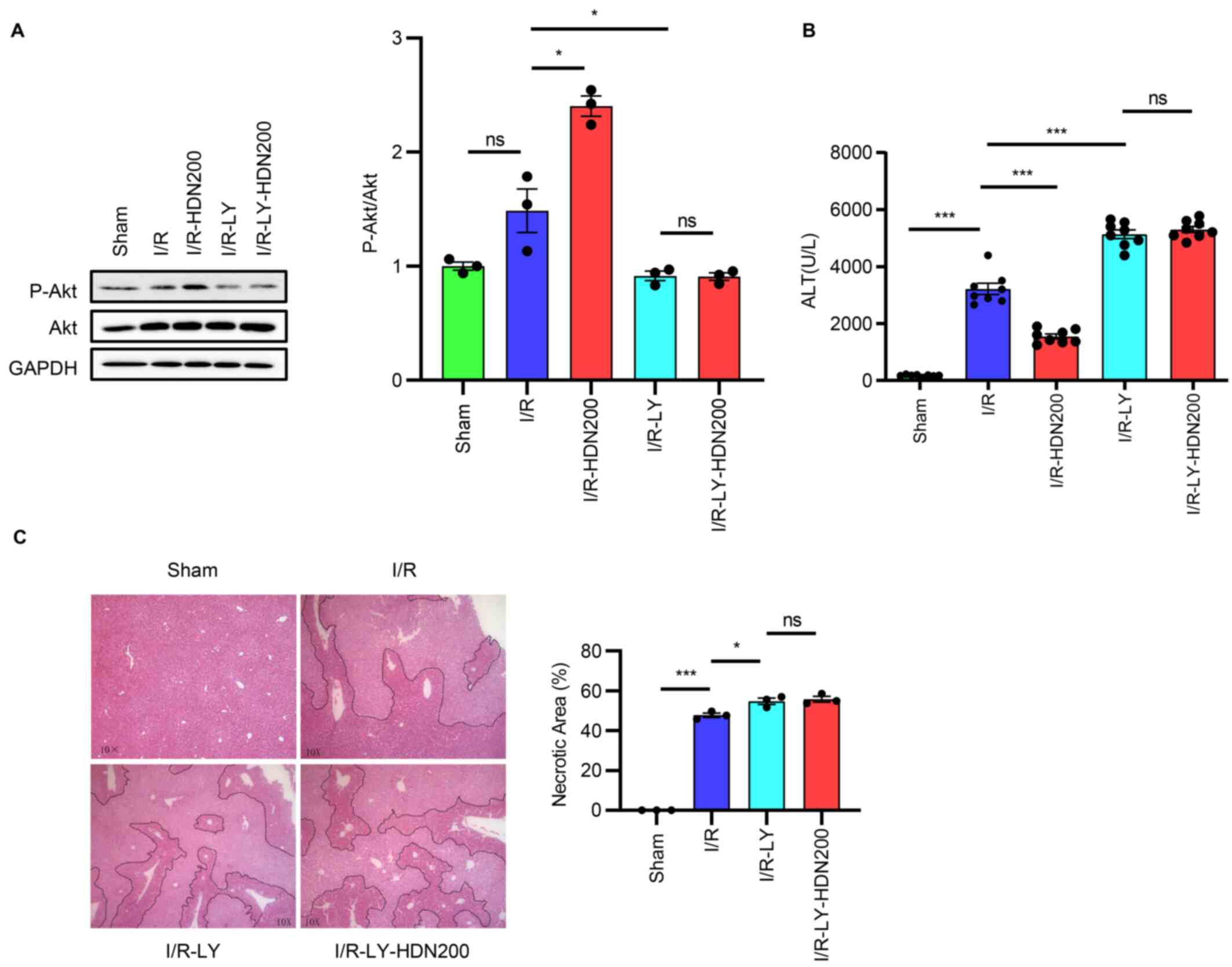
Hesperidin ameliorates liver I/R
injury through the Akt pathway
To elucidate whether activation of the Akt pathway
is necessary for HDN to ameliorate liver I/R injury, LY294002,
which inhibits Akt activation and exacerbates liver injury after
I/R, was used (11,31). The results demonstrated that
compared with those of the I/R group the expression levels of p-Akt
were significantly decreased after administration of LY294002
(Fig. 6A). Notably, these effects
were not rescued by the administration of HDN during liver I/R
(Fig. 6A). In addition, consistent
with the results of p-Akt, the levels of serum ALT increased
significantly after administration of LY294002 compared with those
of the I/R group (Fig. 6B).
Furthermore, the protective effect of HDN was eliminated after
administration of LY294002 (Fig.
6B). The elimination of the protective effect of HDN in the
presence of LY294002 was also confirmed by the H&E staining
index (Fig. 6C).
Having determined that HDN ameliorated liver
oxidative stress during liver I/R injury, whether the
hepatoprotective effects were eliminated by the Akt inhibitor
LY294002 was investigated. The results demonstrated that LY294002
significantly increased MDA levels and significantly decreased the
levels of SOD, CAT, GPx and GSH compared with those of the
respective I/R group, and these effects were unaffected by HDN in
the presence of LY294002 (Fig.
7).
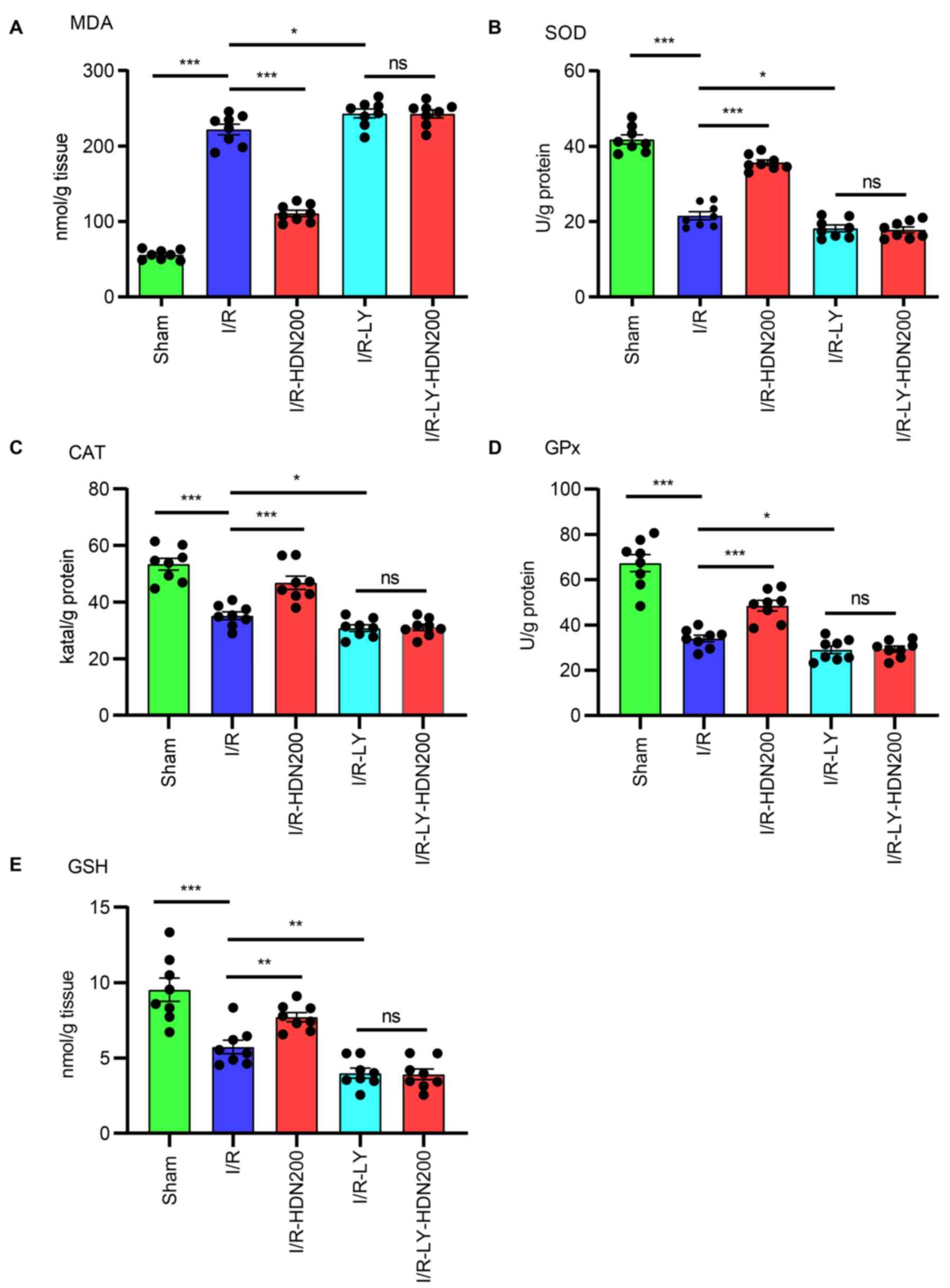 | Figure 7.HDN ameliorates liver oxidative
stress during I/R injury through the Akt signaling pathway. The
levels of (A) MDA, (B) SOD, (C) CAT, (D) GPx and (E) GSH of livers
in sham, I/R, I/R with HDN treatment (200 mg/kg), I/R with Akt
inhibitor (LY294002) treatment or I/R with LY294002 plus HDN
treatment groups. n=8 mice/group; *P<0.05, **P<0.01 and
***P<0.001. CAT, catalase; GPx, glutathione peroxidase; GSH,
glutathione; HDN, hesperidin; I/R, ischemia/reperfusion; LY,
LY294002; MDA, malondialdehyde; ns, not significant; SOD,
superoxide dismutase. |
Having determined that the inflammatory response
increased significantly after liver I/R and decreased markedly with
HDN treatment, whether these effects were regulated by the Akt
pathway was investigated. Cytokine expression levels after LY294002
administration were assessed; the results demonstrated that
LY294002 significantly increased the mRNA expression levels of
TNF-α, IL-6 and IL-1β compared with those of the untreated I/R
group and that these effects were unaffected by co-treatment with
HDN (Fig. 8).
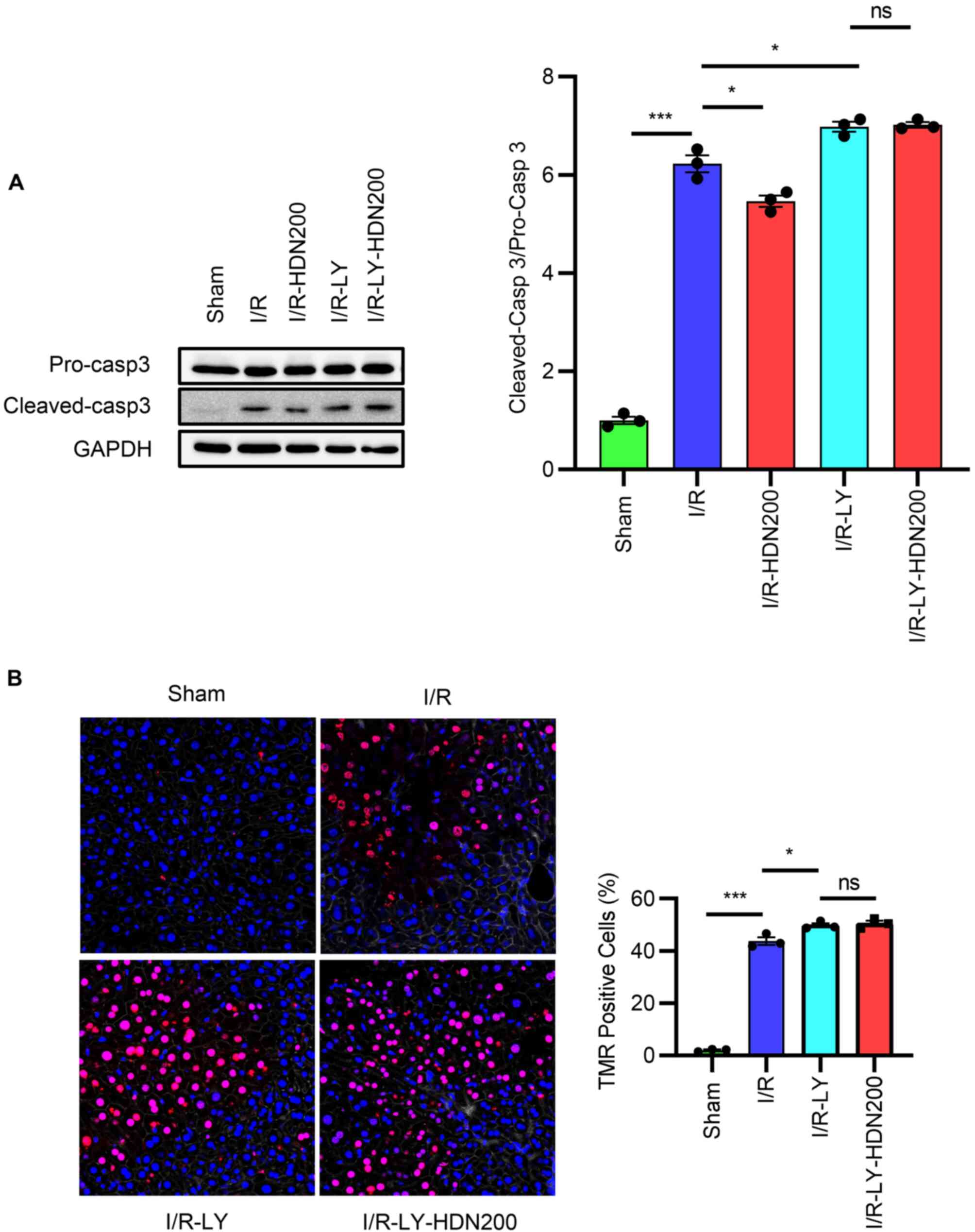
It has been reported that the Akt inhibitor LY294002
promotes apoptosis during liver I/R (12,32);
thus, the effects of HDN and the Akt pathway in liver I/R were
investigated. The results demonstrated that compared with those of
the I/R group the levels of cleaved-caspase 3 significantly
increased after treatment with LY294002 and that these effects were
unaffected by HDN treatment in the presence of LY294002 (Fig. 9A). In addition, TMR assays
demonstrated that there were a similar number of TMR-positive
apoptotic cells in livers co-treated with LY294002 and HDN or
LY294002 alone (Fig. 9B).
Discussion
HDN is a compound that is extracted from citrus
fruits (13), that has been
demonstrated to have potent pharmacological effects against
oxidative stress, inflammation, microbes and cancer (13–16).
However, the exact role and mechanisms of HDN in liver I/R injury
remains unknown. The results of the present study demonstrated that
HDN ameliorated liver I/R injury by decreasing liver oxidative
stress, suppressing inflammatory responses and preventing
hepatocyte apoptosis through the activation of the Akt pathway.
Thus, HDN may be a useful factor for protection against liver
injury. These findings raise the possibility that HDN may be used
to ameliorate liver injury during I/R.
During the ischemia phase, blood flow is blocked and
hepatocytes suffer from anaerobic metabolism and oxidative stress,
which are considered to be the initiation of ischemic injury
(9). Factors involved in oxidative
stress, such as reactive oxygen species and nitric oxide, have been
reported to have important roles in liver I/R injury (9). Oxygen radicals cause lipid
peroxidation of cellular membranes, resulting in inflammatory cell
infiltration, neutrophil activation and hepatic cell injury
(7–9,28).
MDA, which is an oxidative damage indicator, is an essential
product of lipid peroxidation (33). SOD, CAT, GPx and GSH, which can
eliminate free radicals, are antioxidant indicators (34,35).
To determine the oxidative stress in liver I/R, the oxidative
damage and antioxidant parameters were evaluated in the present
study. The results demonstrated that MDA content increased markedly
after liver I/R and was reversed by HDN treatment. The antioxidant
indicators, SOD, CAT, GPx and GSH, decreased significantly after
liver I/R; however, these levels were increased by HDN treatment.
These data suggested that HDN may ameliorate liver oxidative stress
during liver I/R injury and were consistent with previous studies
(36–39). In a sepsis-induced lung injury
model, Yuan et al (37)
reported that oxidative stress was attenuated by HDN. In a sodium
arsenite-induced nephrotoxicity and hepatotoxicity model, Turk
et al (38) observed that
HDN reduced oxidative stress and oxidative DNA damage depending on
the dose. Similar results were observed in bilateral common carotid
artery occlusion reperfusion injury (36) and in skeletal muscle I/R injury
(39). However, how HDN
ameliorates oxidative stress remains unknown.
Oxidative stress and nutrient deprivation initiate
liver damage, which releases damage-associated molecular patterns
(DAMPs), such as high mobility group box 1 and histone/DNA
complexes (8). DAMPs induce
inflammatory cell infiltration and activate innate immune
responses, which leads to the release of pro-inflammatory cytokines
and chemokines (8,40,41).
In the subsequent reperfusion phase, the inflammatory responses
become more extensive and cause more inflammatory mediator release
(8,40,41).
The pro-inflammatory milieu recruits and activates more innate and
adaptive immune cells into the inflamed liver and causes more
hepatocyte death (8,40,41).
Thus, the inflammatory response is both the result from and cause
of liver damage. The results of the present study demonstrated that
the mRNA expression levels of TNF-α, IL-6 and IL-1β in liver
tissues increased significantly after I/R, and that HDN
significantly attenuated these expression levels after liver I/R.
These results suggested that HDN may attenuate inflammation in I/R.
In a previous study, HDN was reported to reduce the secretion of
inflammatory cytokines, differentiation and proliferation of
cardiac fibroblasts by TGF-β through the Notch 1 signaling pathway
(42). In another infectious
disease study, Ye et al (43) reported that HDN pretreatment
significantly attenuated lipopolysaccharide-induced pulmonary
injury and total protein concentration and markedly decreased the
number of neutrophils and the levels of the inflammatory cytokines
TNF-α and IL-6 in an acute lung injury model in vivo and
in vitro. Additionally, they also found that HDN binds
directly with myeloid differentiation 2 (MD2) and inhibits MAPK
activation, regulates Iκβ degradation, and blocks the interaction
of MD2 and its co-receptor Toll-like receptor 4 TLR4 (43). Thus, HDN attenuates inflammation in
liver I/R. However, the underlying mechanisms need to be further
explored.
Several previous studies have indicated that
apoptosis serves a key role in liver I/R (5,30,44)
and that inhibiting apoptosis attenuates liver injury (30). Additionally, it is reported that
HDN ameliorates apoptosis in many situations (22,45–47).
The results of the present study demonstrated that HDN suppressed
apoptosis in liver I/R injury, as indicated by increased Bcl-2 and
decreased cleaved-caspase 3 protein expression levels. In addition,
HDN induced decreases in apoptotic cells were confirmed by TMR
assays. Taken together, these data suggested that HDN suppressed
apoptosis in liver I/R injury. These results corroborate with the
effects of HDN in other I/R models. For example, Li et al
(22) observed that short-term
pretreatment with HDN protected against myocardial I/R injury by
suppressing myocardial apoptosis. He et al (46) also observed that hesperetin (an
active metabolite of HDN) post-treatment significantly inhibited
apoptosis by increasing the expression of Bcl-2 and decreasing the
expression of Bax and cleaved-caspase 3, which diminished the
apoptotic cardiomyocyte ratio in an in vitro cardiomyocyte
model of hypoxia/reoxygenation injury. HDN also attenuated cerebral
I/R injury through inhibiting the apoptotic pathway (47). However, studies have also reported
that HDN induces apoptosis to reduce cancer cell proliferation
(15,48). These differences may be due to the
acute nature of I/R injury compared with the chronic induction of
carcinoma.
The PI3K/Akt pathway is a protective pathway in
liver I/R injury (11,12,32,44,49).
Akt is considered to be a potential therapeutic target for liver
I/R (12). HDN activates the Akt
pathway to protect cardiomyocytes in vivo and in
vitro (22,46,50).
Consistent with these previous studies (22,46,50),
the present study demonstrated that HDN increased the levels of
p-Akt after liver I/R injury and that treatment with the Akt
inhibitor LY294002 diminished the protective effects of HDN in
liver I/R injury. Thus, these results suggested that HDN supports
Akt signaling to protect against liver I/R injury.
In conclusion, the present study revealed that HDN
may protect against liver I/R injury by ameliorating oxidative
stress, suppressing inflammatory responses and decreasing apoptosis
through activating the Akt pathway. HDN is a potential therapeutic
factor for treating liver I/R injury.
Acknowledgements
Not applicable.
Funding
This study was supported by The Young Teachers'
Basic Ability Improvement in Guangxi University Project (grant no.
2019KY0108), The National Natural Science Foundation of China
(grant no. 81960358), Talents Sub-Highland of Emergency and Medical
Rescue of Guangxi Province in China (grant no. GXJZ201405), Health
Commission of Guangxi (grant no. Z2016289) and The Medical
Excellence Award funded by the Creative Research Development Grant
from the First Affiliated Hospital of Guangxi Medical
University.
Availability of data and materials
The datasets used and /or analyzed during the
current study are available from the corresponding authors on
reasonable request.
Authors' contributions
SL designed and performed the majority of
experiments, established the model mice, collected and analyzed the
experimental data, and drafted the manuscript. QQ and DL performed
the serum analysis and the H&E staining. WP and YW isolated and
cultured hepatocytes, and performed the immunofluorescence
staining. YX performed the RT-qPCR and western blot analysis. JZ
and LS supervised the project, provided technical advice, conducted
analyses of the raw data, and reviewed and edited the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal protocols were approved by The Animal Care
and Use Committee of The First Affiliated Hospital of Guangxi
Medical University (Nanjing, China), and the experiments were
performed in adherence to the National Institutes of Health
guidelines for the use of laboratory animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Black CK, Termanini KM, Aguirre O,
Hawksworth JS and Sosin M: Solid organ transplantation in the 21st
century. Ann Transl Med. 6:4092018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Czigany Z, Lurje I, Schmelzle M, Schöning
W, Öllinger R, Raschzok N, Sauer IM, Tacke F, Strnad P, Trautwein
C, et al: Ischemia-reperfusion injury in marginal liver grafts and
the role of hypothermic machine perfusion: Molecular mechanisms and
clinical implications. J Clin Med. 9:8462020. View Article : Google Scholar
|
|
3
|
Kadono K, Gruszynski M, Azari K and
Kupiec-Weglinski JW: Vascularized composite allotransplantation
versus solid organ transplantation: Innate-adaptive immune
interphase. Curr Opin Organ Transplant. 24:714–720. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nastos C, Kalimeris K, Papoutsidakis N,
Tasoulis MK, Lykoudis PM, Theodoraki K, Nastou D, Smyrniotis V and
Arkadopoulos N: Global consequences of liver ischemia/reperfusion
injury. Oxid Med Cell Longev. 2014:9069652014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun P, Zhang P, Wang PX, Zhu LH, Du Y,
Tian S, Zhu X and Li H: Mindin deficiency protects the liver
against ischemia/reperfusion injury. J Hepatol. 63:1198–1211. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Riel WG, van Golen RF, Reiniers MJ,
Heger M and van Gulik TM: How much ischemia can the liver tolerate
during resection? Hepatobiliary Surg Nutr. 5:58–71. 2016.PubMed/NCBI
|
|
7
|
Peralta C, Jiménez-Castro MB and
Gracia-Sancho J: Hepatic ischemia and reperfusion injury: Effects
on the liver sinusoidal milieu. J Hepatol. 59:1094–1106. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu L, Zhou H, Ni M, Wang X, Busuttil R,
Kupiec-Weglinski J and Zhai Y: Innate immune regulations and liver
ischemia-reperfusion injury. Transplantation. 100:2601–2610. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guan LY, Fu PY, Li PD, Li ZN, Liu HY, Xin
MG and Li W: Mechanisms of hepatic ischemia-reperfusion injury and
protective effects of nitric oxide. World J Gastrointest Surg.
6:122–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhai Y, Petrowsky H, Hong JC, Busuttil RW
and Kupiec-Weglinski JW: Ischaemia-reperfusion injury in liver
transplantation--from bench to bedside. Nat Rev Gastroenterol
Hepatol. 10:79–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li S, Yi Z, Deng M, Scott MJ, Yang C, Li
W, Lei Z, Santerre NM, Loughran P and Billiar TR: TSLP protects
against liver I/R injury via activation of the PI3K/Akt pathway.
JCI Insight. 4:1290132019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Covington SM, Bauler LD and Toledo-Pereyra
LH: Akt: A therapeutic target in hepatic ischemia-reperfusion
injury. J Invest Surg. 30:47–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Parhiz H, Roohbakhsh A, Soltani F, Rezaee
R and Iranshahi M: Antioxidant and anti-inflammatory properties of
the citrus flavonoids hesperidin and hesperetin: An updated review
of their molecular mechanisms and experimental models. Phytother
Res. 29:323–331. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elhelaly AE, AlBasher G, Alfarraj S,
Almeer R, Bahbah EI, Fouda MMA, Bungău SG, Aleya L and Abdel-Daim
MM: Protective effects of hesperidin and diosmin against
acrylamide-induced liver, kidney, and brain oxidative damage in
rats. Environ Sci Pollut Res Int. 26:35151–35162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pandey P, Sayyed U, Tiwari RK, Siddiqui
MH, Pathak N and Bajpai P: Hesperidin induces ROS-mediated
apoptosis along with cell cycle arrest at G2/M phase in human gall
bladder carcinoma. Nutr Cancer. 71:676–687. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mo'men YS, Hussein RM and Kandeil MA:
Involvement of PI3K/Akt pathway in the protective effect of
hesperidin against a chemically induced liver cancer in rats. J
Biochem Mol Toxicol. 33:e223052019.PubMed/NCBI
|
|
17
|
Qin Z, Chen L, Liu M, Tan H and Zheng L:
Hesperidin reduces adverse symptomatic intracerebral hemorrhage by
promoting TGF-β1 for treating ischemic stroke using tissue
plasminogen activator. Neurol Sci. 41:139–147. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qi W, Lin C, Fan K, Chen Z, Liu L, Feng X,
Zhang H, Shao Y, Fang H, Zhao C, et al: Hesperidin inhibits
synovial cell inflammation and macrophage polarization through
suppression of the PI3K/AKT pathway in complete Freund's
adjuvant-induced arthritis in mice. Chem Biol Interact. 306:19–28.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Selim NM, Elgazar AA, Abdel-Hamid NM,
El-Magd MRA, Yasri A, Hefnawy HME and Sobeh M: Chrysophanol,
physcion, hesperidin and curcumin modulate the gene expression of
pro-inflammatory mediators induced by LPS in HepG2: In silico and
molecular studies. Antioxidants. 8:3712019. View Article : Google Scholar
|
|
20
|
Jo SH, Kim ME, Cho JH, Lee Y, Lee J, Park
YD and Lee JS: Hesperetin inhibits neuroinflammation on microglia
by suppressing inflammatory cytokines and MAPK pathways. Arch Pharm
Res. 42:695–703. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Justin-Thenmozhi A, Dhivya Bharathi M,
Kiruthika R, Manivasagam T, Borah A and Essa MM: Attenuation of
aluminum chloride-induced neuroinflammation and caspase activation
through the AKT/GSK-3β pathway by hesperidin in wistar rats.
Neurotox Res. 34:463–476. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Hu X, Wang J, Xu W, Yi C, Ma R and
Jiang H: Short-term hesperidin pretreatment attenuates rat
myocardial ischemia/reperfusion injury by inhibiting high mobility
group box 1 protein expression via the PI3K/Akt pathway. Cell
Physiol Biochem. 39:1850–1862. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park HK, Kang SW and Park MS: Hesperidin
ameliorates hepatic ischemia-reperfusion injury in Sprague-Dawley
rats. Transplant Proc. 51:2828–2832. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yazdani HO, Chen HW, Tohme S, Tai S, van
der Windt DJ, Loughran P, Rosborough BR, Sud V, Beer-Stolz D,
Turnquist HR, et al: IL-33 exacerbates liver sterile inflammation
by amplifying neutrophil extracellular trap formation. J Hepatol.
68:130–139. 2017. View Article : Google Scholar
|
|
25
|
Lei Z, Deng M, Yi Z, Sun Q, Shapiro RA, Xu
H, Li T, Loughran PA, Griepentrog JE, Huang H, et al: cGAS-mediated
autophagy protects the liver from ischemia-reperfusion injury
independently of STING. Am J Physiol Gastrointest Liver Physiol.
314:G655–G667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun Q, Loughran P, Shapiro R, Shrivastava
IH, Antoine DJ, Li T, Yan Z, Fan J, Billiar TR and Scott MJ:
Redox-dependent regulation of hepatocyte absent in melanoma 2
inflammasome activation in sterile liver injury in mice.
Hepatology. 65:253–268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Konishi T and Lentsch AB: Hepatic
ischemia/reperfusion: Mechanisms of tissue injury, repair, and
regeneration. Gene Expr. 17:277–287. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abu-Amara M, Yang SY, Tapuria N, Fuller B,
Davidson B and Seifalian A: Liver ischemia/reperfusion injury:
Processes in inflammatory networks--a review. Liver Transpl.
16:1016–1032. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bral M, Pawlick R, Marfil-Garza B,
Dadheech N, Hefler J, Thiesen A and Shapiro AMJ: Pan-caspase
inhibitor F573 mitigates liver ischemia reperfusion injury in a
murine model. PLoS One. 14:e02245672019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li H, Chen O, Ye Z, Zhang R, Hu H, Zhang
N, Huang J, Liu W and Sun X: Inhalation of high concentrations of
hydrogen ameliorates liver ischemia/reperfusion injury through A2A
receptor mediated PI3K-Akt pathway. Biochem Pharmacol. 130:83–92.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang R, Zhang L, Manaenko A, Ye Z, Liu W
and Sun X: Helium preconditioning protects mouse liver against
ischemia and reperfusion injury through the PI3K/Akt pathway. J
Hepatol. 61:1048–1055. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ayala A, Muñoz MF and Argüelles S: Lipid
peroxidation: Production, metabolism, and signaling mechanisms of
malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev.
2014:3604382014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kurutas EB: The importance of antioxidants
which play the role in cellular response against
oxidative/nitrosative stress: Current state. Nutr J. 15:712016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kuyumcu F and Aycan A: Evaluation of
oxidative stress levels and antioxidant enzyme activities in burst
fractures. Med Sci Monit. 24:225–234. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Praveen Kumar P, Sunil Kumar KT, Kavya
Nainita M, Sai Tarun A, Raghu Ramudu BG, Deepika K, Pramoda A and
Yasmeen C: Cerebroprotective potential of hesperidin nanoparticles
against bilateral common carotid artery occlusion reperfusion
injury in rats and in silico approaches. Neurotox Res. 37:264–274.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yuan X, Zhu J, Kang Q, He X and Guo D:
Protective effect of hesperidin against sepsis-induced lung injury
by inducing the heat-stable protein 70 (Hsp70)/Toll-like receptor 4
(TLR4)/ Myeloid differentiation primary response 88 (MyD88)
pathway. Med Sci Monit. 25:107–114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Turk E, Kandemir FM, Yildirim S, Caglayan
C, Kucukler S and Kuzu M: Protective effect of hesperidin on sodium
arsenite-induced nephrotoxicity and hepatotoxicity in rats. Biol
Trace Elem Res. 189:95–108. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ekinci Akdemir FN, Gülçin İ, Karagöz B,
Soslu R and Alwasel SH: A comparative study on the antioxidant
effects of hesperidin and ellagic acid against skeletal muscle
ischemia/reperfusion injury. J Enzyme Inhib Med Chem. 31
(sup4):114–118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhai Y, Busuttil RW and Kupiec-Weglinski
JW: Liver ischemia and reperfusion injury: New insights into
mechanisms of innate-adaptive immune-mediated tissue inflammation.
Am J Transplant. 11:1563–1569. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang H, Tohme S, Al-Khafaji AB, Tai S,
Loughran P, Chen L, Wang S, Kim J, Billiar T, Wang Y, et al:
Damage-associated molecular pattern-activated neutrophil
extracellular trap exacerbates sterile inflammatory liver injury.
Hepatology. 62:600–614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu XH, Wang YF, Dai FY, Zhao JH and Li P:
The protective effects of berberine and hesperidin on inflammatory
factor-stimulating cardiac fibroblasts. Eur Rev Med Pharmacol Sci.
23:5468–5476. 2019.PubMed/NCBI
|
|
43
|
Ye J, Guan M, Lu Y, Zhang D, Li C, Li Y
and Zhou C: Protective effects of hesperidin on
lipopolysaccharide-induced acute lung injury by targeting MD2. Eur
J Pharmacol. 852:151–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li Y, Tong L, Zhang J, Zhang Y and Zhang
F: Galangin alleviates liver ischemia-reperfusion injury in a rat
model by mediating the PI3K/AKT pathway. Cell Physiol Biochem.
51:1354–1363. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hanchang W, Khamchan A, Wongmanee N and
Seedadee C: Hesperidin ameliorates pancreatic β-cell dysfunction
and apoptosis in streptozotocin-induced diabetic rat model. Life
Sci. 235:1168582019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
He S, Wang X, Zhong Y, Tang L, Zhang Y,
Ling Y, Tan Z, Yang P and Chen A: Hesperetin post-treatment
prevents rat cardiomyocytes from hypoxia/reoxygenation injury in
vitro via activating PI3K/Akt signaling pathway. Biomed
Pharmacother. 91:1106–1112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang JJ and Cui P: Neohesperidin
attenuates cerebral ischemia-reperfusion injury via inhibiting the
apoptotic pathway and activating the Akt/Nrf2/HO-1 pathway. J Asian
Nat Prod Res. 15:1023–1037. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Naz H, Tarique M, Ahamad S, Alajmi MF,
Hussain A, Rehman MT, Luqman S and Hassan MI: Hesperidin-CAMKIV
interaction and its impact on cell proliferation and apoptosis in
the human hepatic carcinoma and neuroblastoma cells. J Cell
Biochem. 120:15119–15130. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xiao Q, Ye Q, Wang W, Xiao J, Fu B, Xia Z,
Zhang X, Liu Z and Zeng X: Mild hypothermia pretreatment protects
against liver ischemia reperfusion injury via the PI3K/AKT/FOXO3a
pathway. Mol Med Rep. 16:7520–7526. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li X, Hu X, Wang J, Xu W, Yi C, Ma R and
Jiang H: Inhibition of autophagy via activation of PI3K/Akt/mTOR
pathway contributes to the protection of hesperidin against
myocardial ischemia/reperfusion injury. Int J Mol Med.
42:1917–1924. 2018.PubMed/NCBI
|