Introduction
Pelvic organ prolapse (POP) is a common and
persistent gynecological benign disease among the elderly female
population, which reduced quality of life and sexual well-being of
those affected (1). Currently,
surgery remains the most common treatment for patients with severe
POP (2). However, the risk of
reoperation remains relatively high (~29.2%) due to increased
recurrence (3,4). Several factors, including but not
limited to chronic constipation, vaginal birth, chronic cough,
obesity and hormones, have been recognized for their involvement in
the development of POP (1,5). However, the molecular mechanisms
underlying their development remain to be elucidated. Thus, further
studies are required to investigate the progression of POP.
Recent studies have focused on the abnormal
structure and organization of pelvic floor connective tissue, and
the molecular alterations in uterosacral ligaments (USLs) (6–8).
Notably, the breakdown of the extracellular matrix (ECM) is
commonly reported (9,10). This process has been demonstrated
to attenuate the strength of supportive structures and contribute
to the pathogenesis of POP (11,12).
However, the exact molecular mechanisms underlying the breakdown of
the ECM are not yet fully understood.
Collagen (Col) is an important component of the ECM
that supports the stability and plasticity of the pelvic floor.
Col-I predominantly forms the coarse fibers and provides mechanical
tension of the tissue (13). By
contrast, Col-III forms the fine fibers to improve organ
flexibility (13). Col is
synthesized and secreted by fibroblasts in pelvic connective tissue
(14). Col can be degraded by
matrix metalloproteinases (MMPs), including MMP-2 and MMP-9
(15). However, tissue
metalloproteinase inhibitors (TIMPs), including TIMP1 endogenously
inhibit MMPs, inducing degradation of the ECM (16).
Transforming growth factor β (TGF-β) is a
multifunctional cytokine that affects several functions at the
cellular and biological level, including immune regulation, embryo
development, tumorigenesis, injury repair, cell proliferation,
differentiation and migration, and ECM deposition (17). Homeobox11 (HOXA11) is a
transcriptional regulator that has been reported to maintain the
plasticity of the uterus during the menstrual cycle and in
pregnancy (18,19). Connell et al (20) demonstrated that HOXA11 is involved
in the development and maintenance of USLs, and is deficient in
POP. However, whether HOXA11 is involved in the development of USLs
remains to be elucidated.
Thus, the present study aimed to investigate the
expression levels of HOXA11 and TGF-β1 in the USLs of women with
and without POP. In addition, the effects of knockdown and
overexpression of HOXA11 and TGF-β1 were investigated in cultured
L929 fibroblasts to determine their association with Col and
MMPs.
Materials and methods
Patients
A total of 10 USLs of patients with POP and 6 USLs
without (40–60 years old) POP were collected between April 2019 and
December 2019 at the Renmin Hospital of Wuhan University (Wuhan,
China). Written informed consent was provided by all patients prior
to the commencement of the study. The present study was approved by
the Institutional Ethics Committee of Renmin Hospital of Wuhan
University (approval no. 2018017). A pelvic examination was
performed to evaluate for the presence of POP. Women with stage II
POP or higher were assigned to the POP group. Women presenting the
following criteria were excluded from the study: i) connective
tissue diseases or collagen depleted-associated diseases; ii)
pathologically confirmed endometriosis, or estrogen-associated
ovarian tumors; or iii) undergoing surgery in the uterosacral
ligamental site or a history of estrogen application within the
previous three months.
Tissue collection and
immunohistochemistry (IHC)
Tissues were obtained from 6 non-POP patients, who
underwent USLs resection surgery excluding the presence of POP, and
10 patients with POP who underwent hysterectomy. USLs were fixed
with 4% paraformaldehyde for 12 h at room temperature.
Paraffin-embedded USLs were cut into 4-µm-thick sections. Briefly,
the tissue sections were blocked with 3% hydrogen peroxide solution
and 5% BSA (cat. no. A8010; Beijing Solarbio Science &
Technology Co., Ltd.) for 20 min at room temperature. The sections
were incubated with rabbit polyclonal primary antibodies against
HOXA11 (1:200 dilution; cat. no. NBP1-83233; Novus Biologicals,
Ltd.) and TGF-β1 (1:100 dilution; cat. no. ab92486; Abcam) at 4°C
overnight. After washing with PBS, the sections were incubated with
a polyclonal goat anti-rabbit horseradish peroxidase-conjugated
secondary antibody (1:5,000 dilution; cat. no. ab6721; Abcam) at
37°C for 2 h. DAB solution was used for staining for 5 min at room
temperature and the tissue sections were subsequently
counterstained with hematoxylin for 1 min at room temperature. The
stained slides were assessed by two pathologists independent of the
present study under a light microscope (magnification, ×400; Zeiss
AG). A total of 5 fields of view were selected from uniformly dyed
areas of the tissue sections.
Cell transfection
The L929 murine fibroblast cell line was purchased
from The National Centre for Cell Science. Cells were maintained in
RPMI-1640 medium (Hyclone; Cytiva) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin, at 37°C in 5% CO2. Cells were
seeded (1×106 cells/well) into 6-well plates and
transfected with 75 pmol small interfering (si) RNA (HOXA11 or
TGF-β1), siRNA-negative control (NC) or plasmid vectors (all
purchased from Wuhan Sanying Biotechnology) using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Plasmid
vectors were extracted using the endotoxin-free plasmid extraction
kit (cat. no. DP117; Tiangen Biotech Co., Ltd.). Cells transfected
with pcDNA3.1 were used as the negative control. Subsequent
experimentation was performed 48 h post-transfection.
The HOXA11-targeting siRNA expression system
included the following sequences: siRNA-NC,
5′-GCCAAACTCTCTGTTGGTT-3′; siRNA-1, 5′-GCCTGAAACTCTCTTGGTT-3′;
siRNA-2, 5′-GGGTGTGGTCACTGGAGAT-3′; and siRNA-3,
5′-CCATCTCAGAGCTGACTAT-3′. The TGF-β1-targeting siRNA expression
system included the following sequences: siRNA-NC,
5′-GCAACAATTCCTGGCGTTA-3′; siRNA-1, 5′-GCAACAATTCCTGGCGTTA-3′;
siRNA-2, 5′-GGAGAGCCCTGGATACCAA-3′; and siRNA-3,
5′-GGAAGGACCTGGGTTGGAA-3′.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from L929 cells
(2×106) using TRIzol® (Thermo Fisher
Scientific, Inc.) and reverse transcribed into cDNA using the M-MLV
Reverse Transcriptase kit [cat. no. EQ002; ELK (Wuhan)
Biotechnology Co., Ltd.]. The following temperature protocol was
used for reverse transcription: 42°C for 50 min for the reverse
transcription reaction; 99°C for 5 min to inactivate the reverse
transcriptase; and 4°C to save the reverse transcription product.
qPCR was subsequently performed using the SYBR-Green PCR SuperMix
kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions used for qPCR were as follows: Initial
denaturation at 95°C for 10 min; followed by 40 cycles of 95°C for
15 sec and 60°C for 1 min. The primers sequences used for qPCR are
listed in Table I. Relative
expression levels were calculated using the 2−ΔΔCq
method (21) and normalized to the
internal reference gene GAPDH.
 | Table I.Reverse transcription-quantitative
PCR primers. |
Table I.
Reverse transcription-quantitative
PCR primers.
| Gene | Primer | Primer
sequences | Annealing
temperature (°C) |
|---|
| GAPDH | Forward |
5′-TGAAGGGTGGAGCCAAAAG-3′ | 58.3 |
|
| Reverse |
5′-AGTCTTCTGGGTGGCAGTGAT-3′ | 58.4 |
| HOXA11 | Forward |
5′-CAATCTGGCCCACTGCTACTC-3′ | 59.7 |
|
| Reverse |
5′-GTGGGGTGGTGGTAGACGTT-3′ | 59.2 |
| TGF-β1 | Forward |
5′-AGAGCCCTGGATACCAACTATTG-3′ | 59.5 |
|
| Reverse |
5′-TGCGACCCACGTAGTAGACG-3′ | 59.4 |
| COL1A1 | Forward |
5′-CTGACTGGAAGAGCGGAGAG-3′ | 57.2 |
|
| Reverse |
5′-CGGCTGAGTAGGGAACACAC-3′ | 57.8 |
| COL3A1 | Forward |
5′-CTCAAGAGTGGAGAATACTGGGTT-3′ | 58.8 |
|
| Reverse |
5′-CTCAAGAGTGGAGAATACTGGGTT-3′ | 58.1 |
| MMP-2 | Forward |
5′-GAATGCCATCCCTGATAACCT-3′ | 58.2 |
|
| Reverse |
5′-GCTTCCAAACTTCACGCTCTT-3′ | 59 |
| MMP-9 | Forward |
5′-AAGGGTACAGCCTGTTCCTGGT-3′ | 61.7 |
|
| Reverse |
5′-CTGGATGCCGTCTATGTCGTCT-3′ | 61.6 |
| TIMP1 | Forward |
5′-CCAGAAATCATCGAGACCACC-3′ | 59.2 |
|
| Reverse |
5′-ATTTCCGTTCCTTAAACGGC-3′ | 58.8 |
Western blotting
Cells were washed three times with pre-cooled PBS,
seeded into 6-well plates and collected via scraping. Total protein
was extracted using RIPA lysis buffer supplemented with 1%
phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology), and quantified using the bicinchoninic acid assay
kit (cat. no. AS1086; Wuhan Aspen Biotechnology Co., Ltd.). Equal
amounts of protein (40 µg per lane) were separated ovia 10%
SDS-PAGE, transferred onto polyvinylidene difluoride membranes,
which were blocked with 5% skimmed milk for 1 h at room
temperature. The membranes were incubated with primary antibodies
against Col-I (1:300 dilution; cat. ab34710; Abcam), Col-III (1:200
dilution; cat. ab23445; Abcam), HOXA11 (1:200 dilution; cat. no.
NBP1-83233; Novus Biologicals, Ltd.), TGF-β1 (1:100 dilution; cat.
no. ab92486; Abcam), MMP-2 (1:300 dilution; cat. no. ab97779;
Abcam), MMP-9 (1:300 dilution; cat. no. ab38898; Abcam) and TIMP1
(1:300 dilution; cat. no. ab12684; Abcam), overnight at 4°C. The
membranes were washed three times with PBS-0.1% Tween-20 (PBST) for
10 min each time. Following the primary incubation, membranes were
incubated with a polyclonal goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (1:5,000 dilution; cat.
no. ab6721; Abcam) for 1 h at room temperature. Protein bands were
visualized using the Developer and Fixed kit (cat. no. P0020;
Beyotime Institute of Biotechnology) and analyzed using Odyssey
infrared laser scanning imaging (LI-COR Biosciences). Protein
expression was semi-quantified using ImageJ software (version 6.0;
National Institutes of Health) with GAPDH as the loading
control.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software (version 6; GraphPad Software, Inc.). All
experiments were performed in triplicate and data are presented as
the mean ± standard deviation. Student's independent t-test was
used to compare two groups, multiple groups were statistically
analyzed using one-way analysis of variance followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Molecular expression in POP
tissues
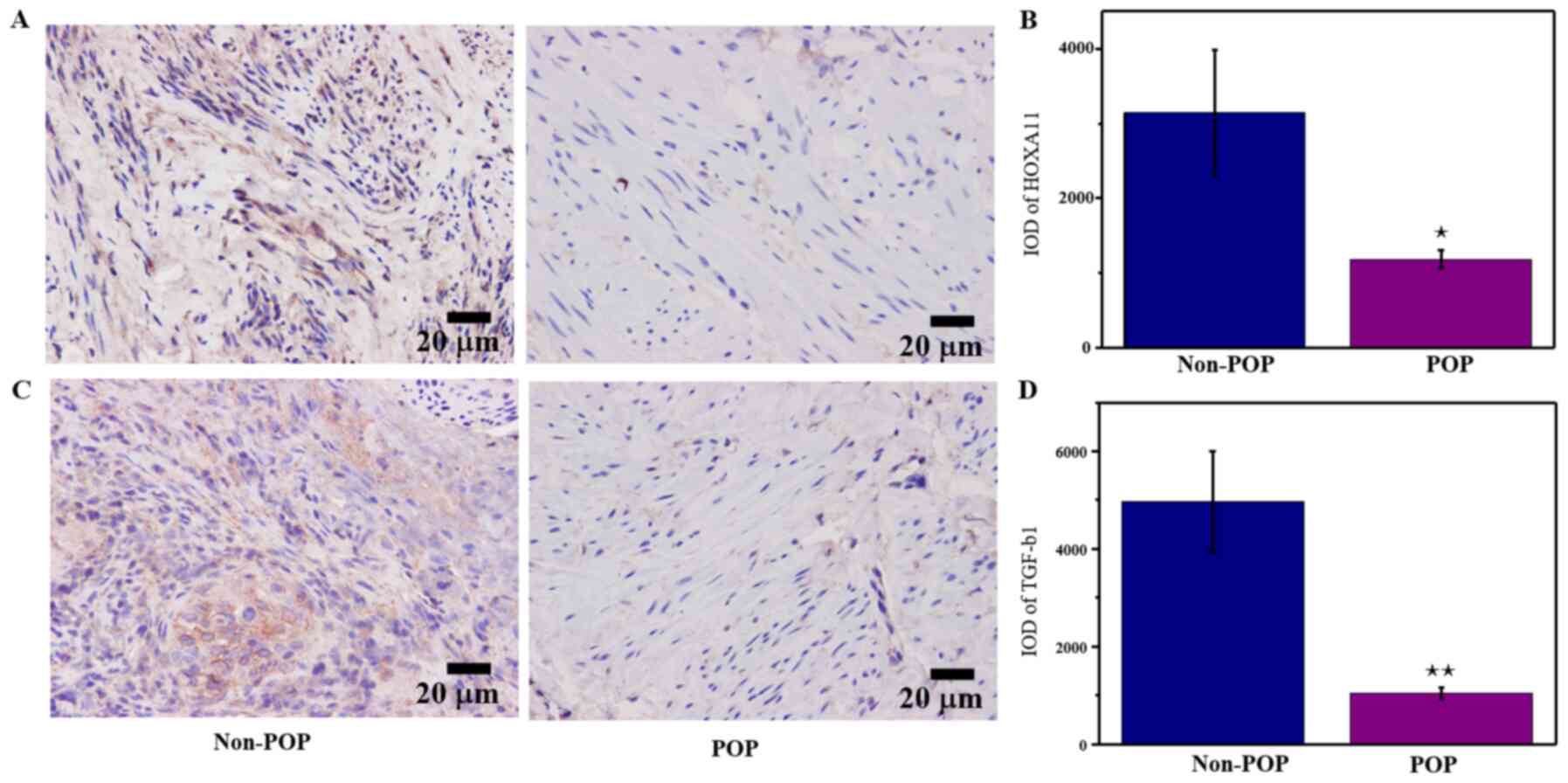
IHC was performed to determine whether HOXA11 and
TGF-β1 were differentially expressed between POP tissues and normal
tissues. The results demonstrated that HOXA11 and TGF-β1 levels
were significantly lower in patients with POP compared with those
without POP (Fig. 1). These
results suggested that downregulation of HOXA11 and TGF-β1
expression may be involved in the development of POP.
Knockdown of HOXA11 and TGF-β1
HOXA11 is a key structural component of pelvic
organs and an essential gene for the development of USLs (20,22).
TGF-β1 is a multifunctional cytokine that serves a key role in ECM
metabolism. TGF-β1 expression is reported to be downregulated in
women with stress urinary incontinence and its expression is
negatively associated with POP (23,24).
In order to determine whether HOXA11 and TGF-β1 influenced ECM
expression, and the combined effect of the two on ECM expression,
the present study successfully established HOXA11 and TGF-β1
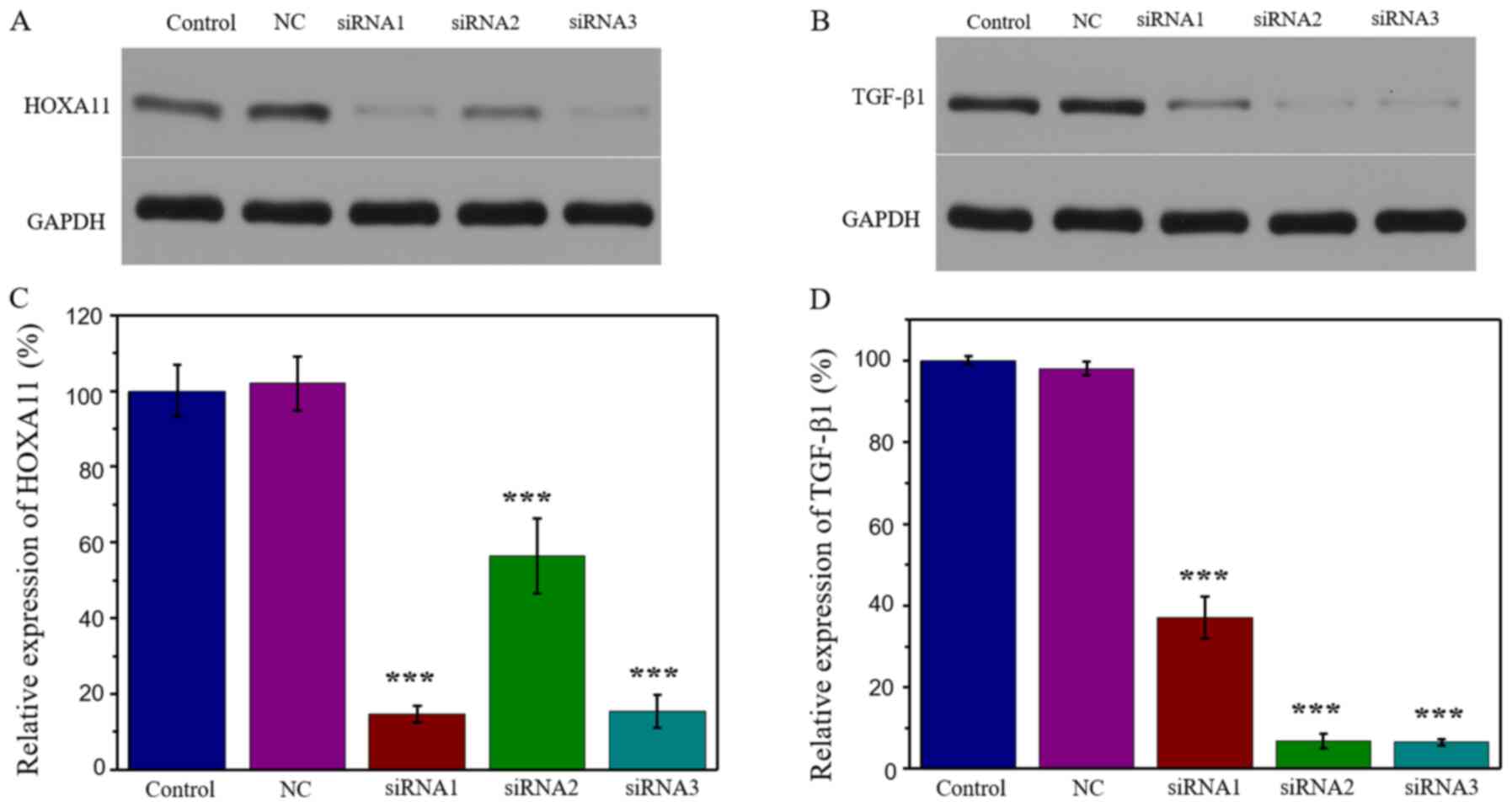
knockdown models. The results demonstrated that transfection with
HOXA11-siRNA1 exhibited the strongest suppression efficiency,
whereby HOXA11 protein expression was 14.70% (Fig. 2A and C). Similarly, transfection
with TGF-β1-siRNA3 exhibited the strongest suppression efficiency
(6.55%; Fig. 2B and D). Thus,
HOXA11-siRNA1 and TGF-β1-siRNA3 were selected for further
experimentation.
Col and MMP expression are altered
when HOXA11 and TGF-β1 are downregulated
HOXA11 mRNA expression was significantly
downregulated in L929 cells transfected with HOXA11-siRNA1. In
addition, mRNA expression levels of Col-I, Col-III and TIMP1
significantly decreased, whereas the levels of MMP-2 and MMP-9
significantly increased (Fig. 3A).
Transfection with TGF-β1-siRNA3 exhibited the same effects as
transfection with HOXA11-siRNA1 (Fig.
3B). The protein expression levels of Cols and MMPs were
further investigated. The results demonstrated that the knockdown
of HOXA11 could suppress Col-I, Col-III and TIMP1 expression, and
elevate MMP-2 and MMP-9 expression (Fig. 4A). Transfection with TGF-β1-siRNA
had the same effect as HOXA11 knockdown (Fig. 4B). In addition, HOXA11 knockdown
could inhibit TGF-β1 expression, and TGF-β1 could alter HOXA11
expression. Taken together, these results suggested that HOXA11 and
TGF-β1 exert mutual functions.
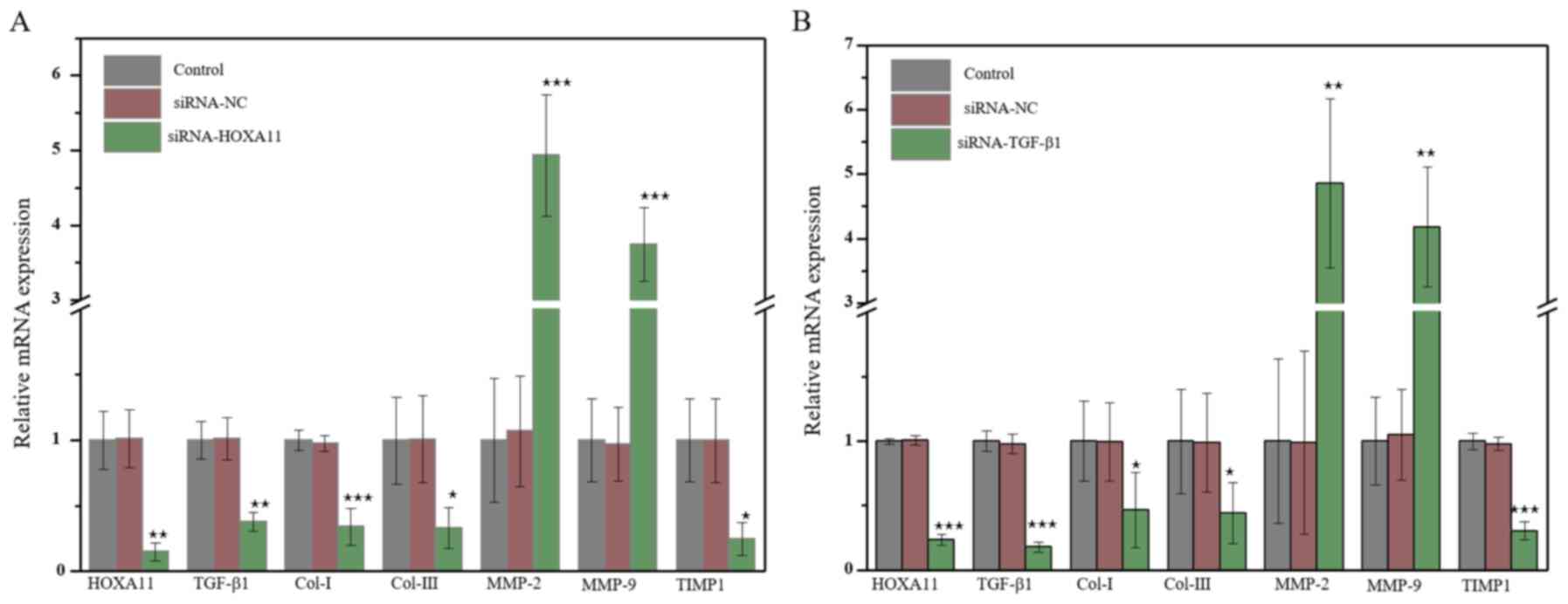 | Figure 3.The molecular mRNA expression when
HOXA11 or TGF-β1 knocked down. mRNA expression of Col-I, Col-III,
HOXA11, TGF-β1, MMP-2, MMP-9 and TIMP1 in (A) HOXA11-siRNA group
and (B) TGF-β1-siRNA group. *P<0.05, **P<0.01 and
***P<0.001 vs. siRNA-NC. Col, collagen; HOXA11, Homeobox11;
TGF-β1, transforming growth factor β; MMP, matrix
metalloproteinases; TIMP1, tissue metalloproteinase inhibitor 1;
siRNA, small interfering RNA; NC, negative control. |
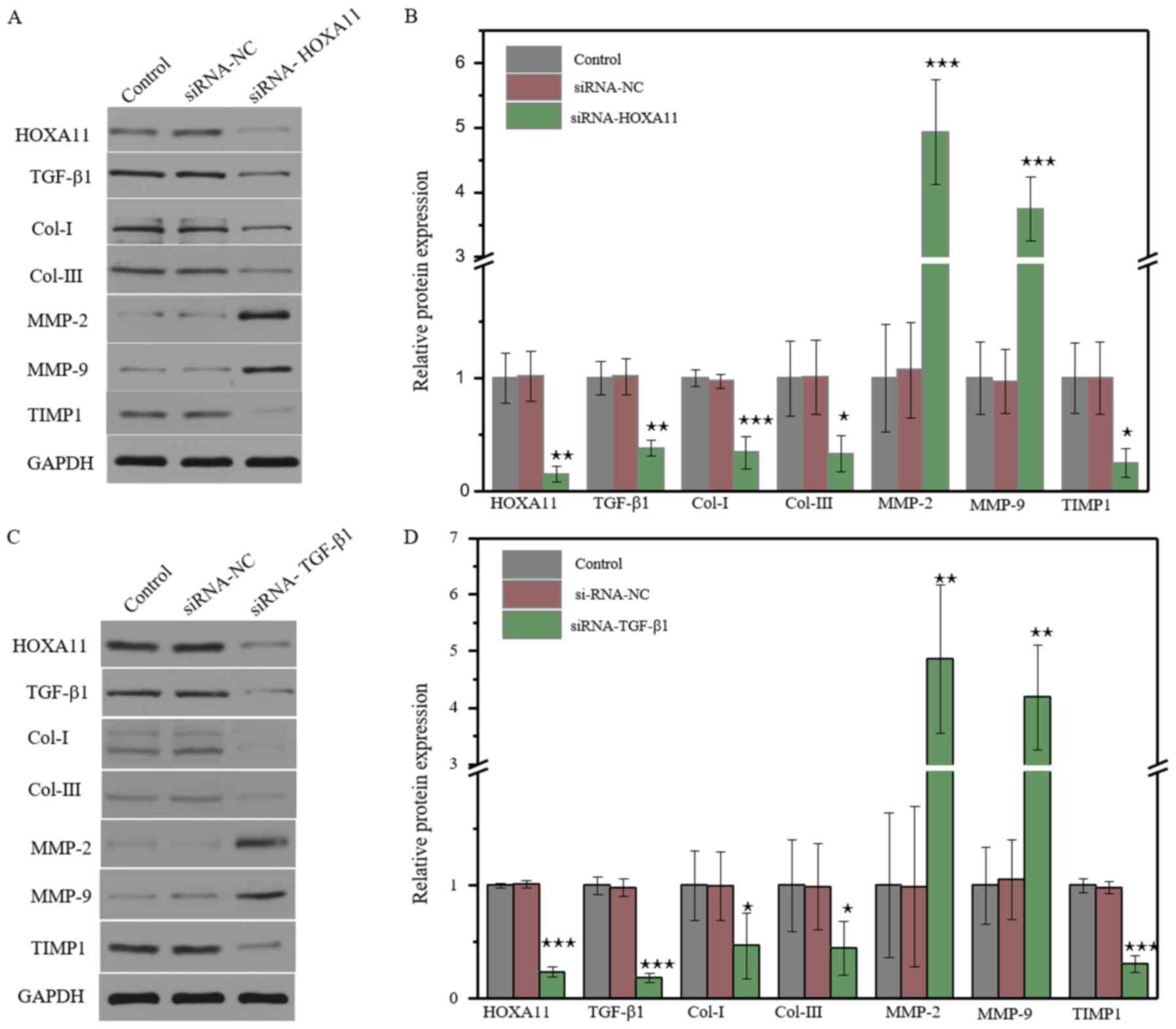 | Figure 4.Molecular protein expression levels
following HOXA11 or TGF-β1 knockdown. The protein expression of
Col-I, Col-III, HOXA11, TGF-β1, MMP-2, MMP-9 and TIMP1 in the (A)
HOXA11-siRNA group and (C) TGF-β1-siRNA group. Semi-quantitative
analysis of protein expression in (B) HOXA11-siRNA group and (D)
TGF-β1-siRNA group. *P<0.05, **P<0.01, ***P<0.001 vs. NC.
Col, collagen; HOXA11, Homeobox11; TGF-β1, transforming growth
factor β; MMP, matrix metalloproteinases; TIMP1, tissue
metalloproteinase inhibitor 1; siRNA, small interfering RNA; NC,
negative control |
Col and MMP expression following
overexpression of HOXA11 and TGF-β1
L929 cells were transfected with HOXA11 and TGF-β1
overexpression plasmids to verify their effects on the expression
of Col and MMPs. As expected, HOXA11 and TGF-β1 mRNA expression
levels significantly increased in cells transfected with HOXA11 and
TGF-β1 vectors. In addition, the mRNA expression levels of Col-I,
Col-III and TIMP1 markedly increased, whereas the levels of MMP-2
and MMP-9 significantly decreased. Col and MMPs expression levels
were notably higher in the co-overexpression group compared with
the single overexpression group (Fig.
5A). In addition, HOXA11 and TGF-β1 possessed a synergistic
effect on the expression of Col and MMPs. The effect of HOXA11 and
TGF-β1 on Cols and MMPs were further assessed at the protein level
(Fig. 5B and C). The results
demonstrated that HOXA11 and TGF-β1 expression significantly
increased following transfection with HOXA11 and TGF-β1 mimics.
Notably, the mRNA expression levels of Col-I, Col-III and TIMP1
significantly increased, whereas the levels of MMP-2 and MMP-9
decreased following co-overexpression.
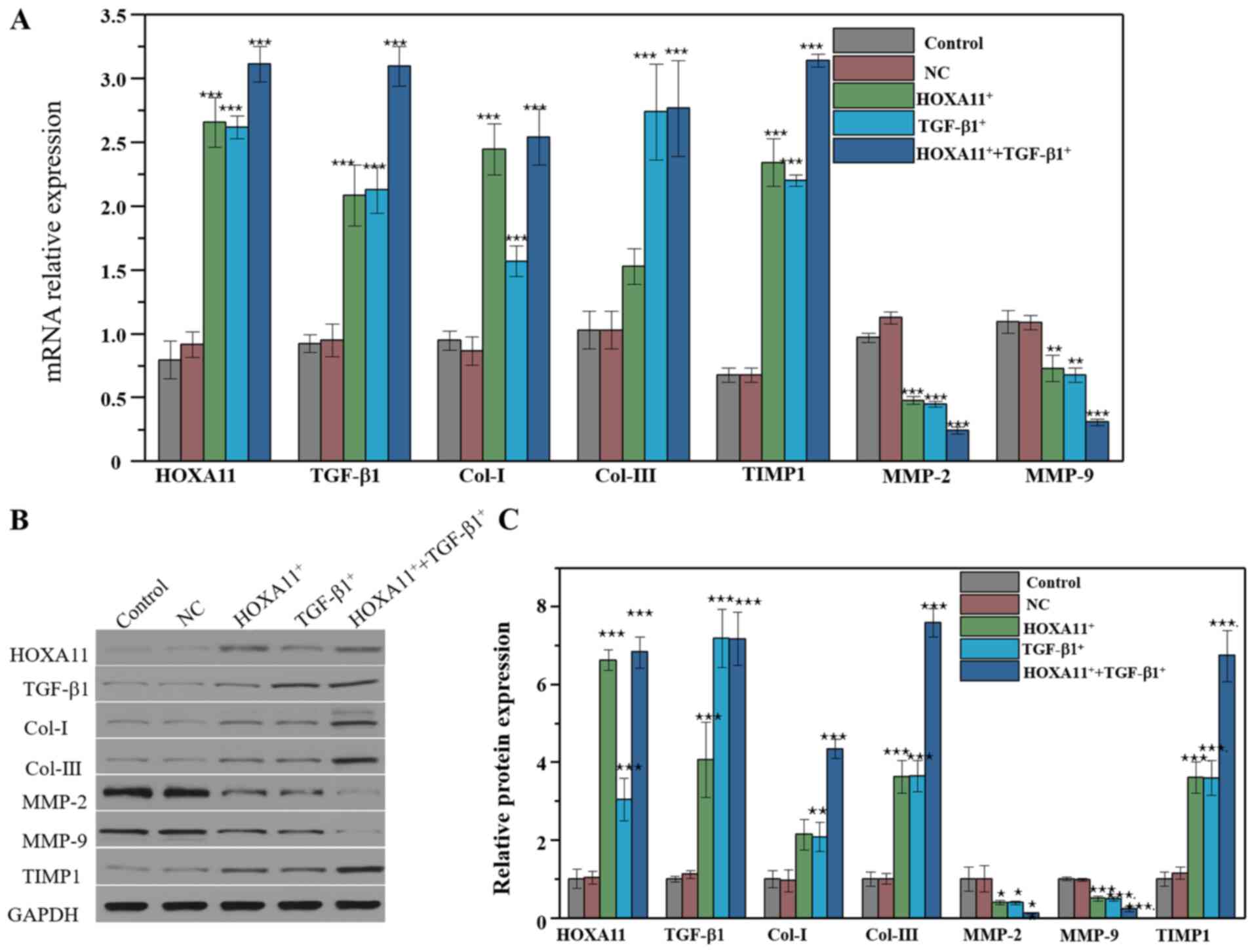 | Figure 5.Molecular protein expression levels
following HOXA11 or TGF-β1 overexpression. (A) mRNA expression of
Col-I, Col-III, HOXA11, TGF-β1, MMP-2, MMP-9 and TIMP1 in HOXA11
and TGF-β1 overexpression groups. (B) Protein expression and (C)
the semi-quantitative analysis of Col-I, Col-III, HOXA11, TGF-β1,
MMP-2, MMP-9 and TIMP1 in HOXA11 and TGF-β1 overexpression groups.
*P<0.05, **P<0.01 and ***P<0.001 vs. NC. Col, collagen;
HOXA11, Homeobox11; TGF-β1, transforming growth factor β; MMP,
matrix metalloproteinases; TIMP1, tissue metalloproteinase
inhibitor 1; NC, negative control. |
Discussion
POP is a prevalent reproductive disease among
menopausal women, which causes a major medical and financial burden
due to the bladder and bowel dysfunction, incontinence and coital
problems (25,26). Several factors such as vaginal
birth, forceps delivery, obesity and aging are considered high risk
factors of POP (1,27). However, the underlying
pathophysiology mechanism of POP remains to be elucidated.
Increasing evidence suggests that alterations to the
connective tissue induce structural damage to the pelvic floor
(28,29). The balance between the synthesis
and degradation of Cols, including Col-I and Col-III, is considered
the basis for the continuous remodeling of ECM (26). The degradation of Col is
predominantly mediated by MMPs, while Col synthesis is regulated by
TIMPs (30). Previous studies have
demonstrated that the levels of Col-I and Col-III are downregulated
in POP tissues (31,32). However, the molecular mechanism
underlying downregulation of Col in POP remains to be
elucidated.
HOXA11 is a transcription factor that regulates
female fertility (33). HOXA11
expression is enhanced during the development of the human
reproductive tract, and is critical for the development of
ligaments (34). TGF-β1 has been
reported to regulate cellular proliferation, differentiation, and
ECM deposition (35). However,
another study indicated that TGF-β1 expression is decreased in the
vaginal wall of women with POP (24). The molecular mechanism of TGF-β1
remains unclear. The results of the present study demonstrated that
the expression levels of HOXA11 and TGF-β1 were significantly
downregulated in the USLs of patients with POP, which was
consistent with previous studies (20,36).
In addition, HOXA11 expression was indicated to influence TGF-β1
expression, and vice versa.
L929 murine fibroblasts have been extensively used
to assess the ECM (37). In order
to further investigate the function of HOXA11 and TGF-β1, the
expression levels of HOXA11 and TGF-β1 were knocked down. Notably,
downregulation of HOXA11 and TGF-β1 significantly decreased ECM
expression. Overexpression of HOXA11 and TGF-β1 in L929 cells
confirmed that they can promote the synthesis of Col while
suppressing its degradation. In addition, co-overexpression of
HOXA11 and TGF-β1 markedly increased the expression levels of Col-I
and Col-III and inhibited the levels of MMPs. Collectively, these
results suggested that HOXA11 and TGF-β1 exerted a synergistic
effect on the expression of Cols, something that has rarely been
reported in previous studies. As expected, overexpression of HOXA11
promoted TGF-β1 expression and overexpression of TGF-β1 similarly
enhanced HOXA11 expression. A previous study reported that HOXA11
regulates the development and progression of non-small cell lung
cancer via the TGF-β1 signaling pathway (38). However, the association between
HOXA11 and TGF-β1 requires further study and the relationship
between HOXA11, TGF-β1 and clinicopathological grading of patients
with POP should be further investigated.
Overall, the results of the present study
demonstrated that the expression levels of HOXA11 and TGF-β1 were
downregulated in the USLs of patients with POP compared with those
without POP. In addition, HOXA11 and TGF-β1 were indicated to
mediate POP by regulating the expression levels of Cols and MMPs.
The results demonstrated that HOXA11 and TGF-β1 exerted a
synergistic effect on the expression levels of Cols and MMPs. In
addition, HOXA11 downregulated TGF-β1 expression, and vice versa.
Together, the results of the present study confirmed that the
reduction of ECM caused by the loss of HOXA11 and TGF-β1 is a
critical factor in the occurrence of POP. The present study may aid
our understanding of the underlying molecular mechanisms and the
development of POP, and provide novel insights into the effective
diagnosis and targeted therapy of patients with POP.
Acknowledgements
Not applicable.
Funding
This study was supported by the Independent
scientific research project of Wuhan University (grant no.
413000117), the Chinese Medical Association Clinical Research
Fund-Reproductive Medicine Young Physicians Research and
Development Project (grant no. 17020310700) and the National
Natural Science Foundation of China NSFC (grant no. 81860276).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ, FD, YC and XY conceived and designed the study.
LZ, FD, SX, YZ, ZD and LZ conducted the experiment. YW, MY, DY, SL
and GC analyzed the data and prepared the diagrams. LZ and GC
drafted the manuscript and FD revised the article. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Renmin Hospital of Wuhan University (approval no.
2018017). Written informed consent was provided by all patients
prior to the commencement of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
POP
|
pelvic organ prolapses
|
|
ECM
|
extracellular matrix
|
|
USLs
|
uterosacral ligaments
|
|
Col
|
collagen
|
|
MMPs
|
matrix metalloproteinases
|
|
TIMPs
|
tissue metalloproteinase
inhibitors
|
|
TGF-β
|
transforming growth factor β
|
|
HOXA11
|
Homeobox11
|
|
IHC
|
immunohistochemistry
|
References
|
1
|
Vergeldt TF, Weemhoff M, IntHout J and
Kluivers KB: Risk factors for pelvic organ prolapse and its
recurrence: A systematic review. Int Urogynecol J. 26:1559–1573.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Altman D, Zetterstrom J, Schultz I,
Nordenstam J, Hjern F, Lopez A and Mellgren A: Pelvic organ
prolapse and urinary incontinence in women with surgically managed
rectal prolapse: A population-based case-control study. Dis Colon
Rectum. 49:28–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clark AL, Gregory T, Smith VJ and Edwards
R: Epidemiologic evaluation of reoperation for surgically treated
pelvic organ prolapse and urinary incontinence. Am J Obstet
Gynecol. 189:1261–1267. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olsen AL, Smith VJ, Bergstrom JO, Colling
JC and Clark AL: Epidemiology of surgically managed pelvic organ
prolapse and urinary incontinence. Obstet Gynecol. 89:501–506.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Delancey JO, Kane Low L, Miller JM, Patel
DA and Tumbarello JA: Graphic integration of causal factors of
pelvic floor disorders: An integrated life span model. Am J Obstet
Gynecol. 199:610.e1–e5. 2008. View Article : Google Scholar
|
|
6
|
Zhao X, Ma C, Li R, Xue J, Liu L and Liu
P: Hypoxia induces apoptosis through HIF-1α signaling pathway in
human uterosacral ligaments of pelvic organ prolapse. Biomed Res
Int. 2017:83160942017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun MJ, Cheng YS, Liu CS and Sun R:
Changes in the PGC-1α and mtDNA copy number may play a role in the
development of pelvic organ prolapse in pre-menopausal patients.
Taiwan J Obstet Gynecol. 58:526–530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Zheng P, Duan A, Hao Y, Lu C and
Lu D: [Corrigendum] Genomewide DNA methylation analysis of
uterosacral ligaments in women with pelvic organ prolapse. Mol Med
Rep. 19:24582019.PubMed/NCBI
|
|
9
|
Tyagi T, Alarab M, Leong Y, Lye S and
Shynlova O: Local oestrogen therapy modulates extracellular matrix
and immune response in the vaginal tissue of post-menopausal women
with severe pelvic organ prolapse. J Cell Mol Med. 23:2907–2919.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alarab M, Kufaishi H, Lye S, Drutz H and
Shynlova O: Expression of extracellular matrix-remodeling proteins
is altered in vaginal tissue of premenopausal women with severe
pelvic organ prolapse. Reprod Sci. 21:704–715. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Landsheere L, Blacher S, Munaut C,
Nusgens B, Rubod C, Noel A, Foidart JM, Cosson M and Nisolle M:
Changes in elastin density in different locations of the vaginal
wall in women with pelvic organ prolapse. Int Urogynecol J.
25:1673–1681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Strinic T, Vulic M, Tomic S, Capkun V,
Stipic I and Alujevic I: Increased expression of matrix
metalloproteinase-1 in uterosacral ligament tissue from women with
pelvic organ prolapse. Acta Obstet Gynecol Scand. 89:832–834. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Budatha M, Roshanravan S, Zheng Q,
Weislander C, Chapman SL, Davis EC, Starcher B, Word RA and
Yanagisawa H: Extracellular matrix proteases contribute to
progression of pelvic organ prolapse in mice and humans. J Clin
Invest. 121:2048–2059. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Woodley DT, Krueger GG, Jorgensen CM,
Fairley JA, Atha T, Huang Y, Chan L, Keene DR and Chen M: Normal
and gene-corrected dystrophic epidermolysis bullosa fibroblasts
alone can produce type VII collagen at the basement membrane zone.
J Invest Dermatol. 121:1021–1028. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Piperi C and Papavassiliou AG: Molecular
mechanisms regulating matrix metalloproteinases. Curr Top Med Chem.
12:1095–1112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai W, Liang Z, Liu H, Zhao G and Ju C:
Lunasin abrogates the expression of matrix metalloproteinases and
reduction of type II collagen. Artif Cells Nanomed Biotechnol.
47:3259–3264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun B, Zhou L, Wen Y, Wang C, Baer TM,
Pera RR and Chen B: Proliferative behavior of vaginal fibroblasts
from women with pelvic organ prolapse. Eur J Obstet Gynecol Reprod
Biol. 183:1–4. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taylor HS, Vanden Heuvel GB and Igarashi
P: A conserved Hox axis in the mouse and human female reproductive
system: Late establishment and persistent adult expression of the
Hoxa cluster genes. Biol Reprod. 57:1338–1345. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taylor HS, Igarashi P, Olive DL and Arici
A: Sex steroids mediate HOXA11 expression in the human
peri-implantation endometrium. J Clin Endocrinol Metab.
84:1129–1135. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Connell KA, Guess MK, Chen H, Andikyan V,
Bercik R and Taylor HS: HOXA11 is critical for development and
maintenance of uterosacral ligaments and deficient in pelvic
prolapse. J Clin Invest. 118:1050–1055. 2008.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma Y, Guess M, Datar A, Hennessey A,
Cardenas I, Johnson J and Connell KA: Knockdown of Hoxa11 in vivo
in the uterosacral ligament and uterus of mice results in altered
collagen and matrix metalloproteinase activity. Biol Reprod.
86:1002012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wen Y, Polan ML and Chen B: Do
extracellular matrix protein expressions change with cyclic
reproductive hormones in pelvic connective tissue from women with
stress urinary incontinence? Hum Reprod. 21:1266–1273. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meijerink AM, van Rijssel RH and van der
Linden PJ: Tissue composition of the vaginal wall in women with
pelvic organ prolapse. Gynecol Obstet Invest. 75:21–27. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Emmerson S, Mukherjee S, Melendez-Munoz J,
Cousins F, Edwards SL, Karjalainen P, Ng M, Tan KS, Darzi S, Bhakoo
K, et al: Composite mesh design for delivery of autologous
mesenchymal stem cells influences mesh integration, exposure and
biocompatibility in an ovine model of pelvic organ prolapse.
Biomaterials. 225:1194952019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elneil S: Complex pelvic floor failure and
associated problems. Best Pract Res Clin Gastroenterol. 23:555–573.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee UJ, Kerkhof MH, van Leijsen SA and
Heesakkers JP: Obesity and pelvic organ prolapse. Curr Opin Urol.
27:428–434. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qiu J, Qin M, Fan B and Chen X: Klotho
protein reduced the expression of matrix metalloproteinase-1
(MMP-1) and matrix metalloproteinase-3 (MMP-3) in fibroblasts from
patients with pelvic organ prolapse (POP) by Down-regulating the
phosphorylation of ERK1/2. Med Sci Monit. 25:3815–3824. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeng C, Liu J, Wang H, Zhou Y, Wu J and
Yan G: Correlation between autophagy and collagen deposition in
patients with pelvic organ prolapse. Female Pelvic Med Reconstr
Surg. 24:213–221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lachowski D, Cortes E, Rice A, Pinato D,
Rombouts K and Del Rio Hernandez A: Matrix stiffness modulates the
activity of MMP-9 and TIMP-1 in hepatic stellate cells to
perpetuate fibrosis. Sci Rep. 9:72992019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dökmeci F, Tekşen F, Çetinkaya ŞE, Özkan
T, Kaplan F and Köse K: Expressions of homeobox, collagen and
estrogen genes in women with uterine prolapse. Eur J Obstet Gynecol
Reprod Biol. 26–29. 2019. View Article : Google Scholar
|
|
32
|
Wang S, Lu D, Zhang Z, Jia X and Yang L:
Effects of mechanical stretching on the morphology of extracellular
polymers and the mRNA expression of collagens and small
leucine-rich repeat proteoglycans in vaginal fibroblasts from women
with pelvic organ prolapse. PLoS One. 13:e01934562018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Makker A, Goel MM, Nigam D, Bhatia V,
Mahdi AA, Das V and Pandey A: Endometrial expression of homeobox
genes and cell adhesion molecules in infertile women with
intramural fibroids during window of implantation. Reprod Sci.
24:435–444. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cunha GR, Robboy SJ, Kurita T, Isaacson D,
Shen J, Cao M and Baskin LS: Development of the human female
reproductive tract. Differentiation. 103:46–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nigdelioglu R, Hamanaka RB, Meliton AY,
O'Leary E, Witt LJ, Cho T, Sun K, Bonham C, Wu D, Woods PS, et al:
Transforming growth factor (TGF)-β promotes de novo serine
synthesis for collagen production. J Biol Chem. 291:27239–27251.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leegant A, Zuckerwise LC, Downing K,
Brouwer-Visser J, Zhu C, Cossio MJ, Strube F, Xie X, Banks E and
Huang GS: Transforming growth factor β1 and extracellular matrix
protease expression in the uterosacral ligaments of patients with
and without pelvic organ prolapse. Female Pelvic Med Reconstr Surg.
21:53–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ventura RD, Padalhin AR, Park CM and Lee
BT: Enhanced decellularization technique of porcine dermal ECM for
tissue engineering applications. Mater Sci Eng C Mater Biol Appl.
104:1098412019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Y, He RQ, Dang YW, Zhang XL, Wang X,
Huang SN, Huang WT, Jiang MT, Gan XN, Xie Y, et al: Comprehensive
analysis of the long noncoding RNA HOXA11-AS gene interaction
regulatory network in NSCLC cells. Cancer Cell Int. 16:892016.
View Article : Google Scholar : PubMed/NCBI
|