Introduction
Affecting ~350 million individuals worldwide in
2015, asthma is currently a major global health concern, with the
global prevalence of asthma increasing by 12.6% (95% CI, 9.0–16.4%)
from 1990 to 2015 (1). Asthma is a
complex inflammatory disorder of the airways and airway
hyperresponsiveness (AHR) is a hallmark feature of asthma (2). Asthma had been considered to be a
single disease for decades; however, previous studies have
recognized distinct phenotypes among patients with asthma (3,4).
Allergic asthma with high T helper 2 (Th2) inflammation is the
primary phenotype among patients with asthma, and increasing novel
biological agents have been developed that target Th2-associated
cytokines, including IL-5 and IL4/13 (5). Dendritic cells (DCs) serve a pivotal
role in determining T cell development after encountering an
antigen, and a number of studies have demonstrated that DCs were
crucial in the pathogenesis of asthma via inducing Th2 inflammation
in response to allergens (6,7).
Atractylodis rhizoma (also known as Cangzhu),
a traditional herbal medicine used to treat digestive disorders,
rheumatic disease and influenza, consists of a number of essential
components, including sesquiterpenes, phenolic acids and polyethene
alkynes (8). Atractylodin (ATL), a
polyethene alkyne extract, was discovered to ameliorate intestinal
inflammation via regulating MAPK activation and alleviate acute
lung injury via suppressing nucleotide-binding domain-like receptor
protein 3 inflammasome and toll-like receptor 4 activation
(9–12). Our recent study further
demonstrated that ATL ameliorated mouse collagen-induced arthritis
via regulating DC maturation (13).
Therefore, the present study aimed to investigate
the impact of ALT on asthma, as DCs have been identified to serve
an essential role in Th2 inflammation in asthma. An ovalbumin
(OVA)-induced asthma mouse model was used to characterize the
effect of ATL on AHR, as well as the production of Th2-associated
cytokines and OVA-specific IgE. The model was also used to evaluate
the impact of ATL on the cellular proliferation and cytokine
production of OVA-specific T cells, and to determine how ATL
affected the maturation of DCs. The results provided evidence of
the therapeutic effect of the i.p. injection of ATL on the
maturation of DCs and downstream Th2 inflammation in asthma, and
indicated a potential application of ATL for treating patients with
asthma and high Th2 inflammatory levels.
Materials and methods
Animals and experimental design
A total of 24 six-week-old male BALB/c mice (weight,
18–22 g), were obtained from the National Laboratory Animal Center.
The mice were placed in sterile cages under a regulated temperature
(22±3°C), humidity (55±5%) and 12-h day/night cycle conditions and
ad libitum access to sterilized mouse chow and water.
Ethical approval for the present study was obtained from the
Institutional Animal Care and Use Committee (IACUC) of National
Chung-Hsing University (Taichung, Taiwan; approval no. IACUC
108–072). Experiments were performed in accordance with relevant
guidelines for Guide for the Care and Use of Laboratory Animals,
8th edition (14). In order to
investigate the protective effects of ATL on asthma in mice, the 24
male BALB/c mice were randomly divided into four groups (n=6 per
group): i) normal control (NC; vehicle), containing mice which were
not challenged with OVA (Thermo Fisher Scientific, Inc.) and
received daily intraperitoneal injection (i.p.) injection equal
volumes of vehicle (10% DMSO (ChemCruz; Santa Cruz Biotechnology,
Inc.) and 90% glyceryl trioctanoate (Sigma-Aldrich; Merck KGaA);
ii) OVA group, in which mice were stimulated with OVA and received
equal volumes of vehicle; iii) OVA/ATL 20 group, in which mice were
stimulated with OVA and received 20 mg/kg ATL (National Institute
for Food and Drug Control); and iv) OVA/ATL 40 group, in which mice
were stimulated with OVA and received 40 mg/kg ATL. The single
daily dose of ATL was administered via i.p. injection for 16 days
starting on day 15 of primary immunization (Fig. 1A). The dosage and administration
frequency were implemented based on previous studies (12,13,15).
The OVA-immunized mice received 20 µg OVA (i.p.) and 2% alum
adjuvant (InvivoGen) dissolved in saline (final volume, 200 µl) on
days 1, 7 and 14. These mice were then challenged with 5% OVA in
0.9% NaCl by inhalation for 30 min daily for 5 consecutive days
(days 27–31). On day 32, the AHR of each mouse was measured as
described below. Finally, on day 33, mice were sacrificed using
carbon dioxide (air displacement rate, 30% of the chamber
volume/min). Mice were exposed to 50% CO2 until they
were unconscious and experienced cardiac arrest.
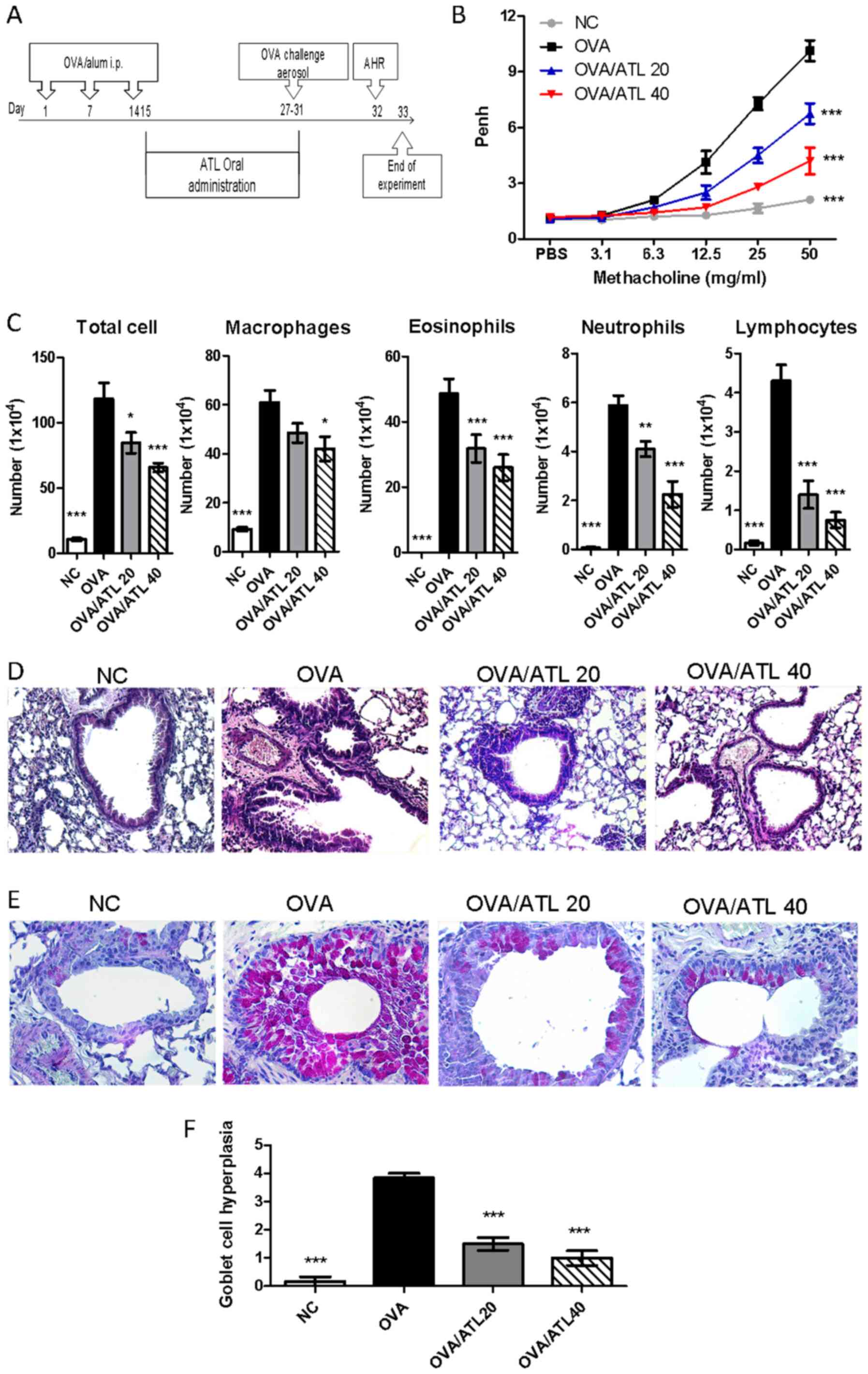 | Figure 1.Effect of ATL on AHR and airway
inflammation. (A) Experimental procedure. BALB/c mice were
randomized into four groups (n=6 mice per group). NC mice were not
sensitized with OVA or treated with ATL. On days 1, 7 and 14, OVA,
OVA/ATL 20 and OVA/ATL 40 groups were sensitized via i.p. injection
of OVA allergen. OVA/ATL 20 and OVA/ATL 40 mice were i.p. injected
daily with 20 and 40 mg/kg ATL, respectively, on days 15–31.
Finally, mice in all 4 groups were exposed to OVA aerosols on day
27–31, and AHR was measured on day 32. BALF, serum, lung and spleen
tissue were collected. (B) Effect of ATL on AHR levels. Mice
inhaled increasing doses of methacholine (3.125–50 mg/ml). Penh
levels represent the degree of AHR. ***P<0.001 vs. OVA. (C) Mice
treated with ATL exhibited decreased cell counts in the BALF. Total
cells, macrophages, eosinophils, lymphocytes and neutrophils were
counted following Diff-Quik staining. Histological examination of
lung sections stained with (D) Hematoxylin and eosin to examine
inflammatory cell infiltration and (E) periodic acid-Schiff
staining to examine goblet cell hyperplasia and mucus secretion
(magnification, ×400 for both). (F) For the semi-quantification of
goblet cell hyperplasia, slides were examined in a double-blind
setting using a semi-quantitative scoring system. A scored scale
from grade 0 to 4 was implemented depending on the percentage of
goblet cells in the epithelium: grade 0 (no goblet cells); grade 1
(<25%), grade 2 (25–50%), grade 3 (51–75%) and grade 4
(>75%). The mean goblet cell hyperplasia score was then
calculated for each mouse. Data are presented as the mean ± SEM of
six mice per group. *P<0.05, **P<0.01, ***P<0.001 vs. OVA.
ATL, atractylodin; AHR, airway hyperresponsiveness; NC, normal
control; OVA, ovalbumin; BALF, bronchoalveolar lavage fluid; Penh,
enhanced pause. |
Measurement of AHR via unrestrained
whole-body plethysmography
On day 32, AHR was measured via methacholine (Mch;
Sigma-Aldrich; Merck KGaA)-induced airflow obstruction in conscious
unrestrained mice placed in a whole-body plethysmograph (Buxco;
Data Sciences International; Harvard Bioscience, Inc.). Pulmonary
resistance was evaluated and expressed as enhanced pause. Briefly,
mice were first exposed to aerosolized 1X PBS [0.8% NaCl, 0.02%
KH2PO4, 0.115% Na2HPO4,
0.02% KCl [pH 7.4]), then challenged with a series of aerosolized
Mch (3.125–50 mg/ml) with an ultrasonic nebulizer over the
recording time period. Each nebulization lasted for 3 min and
records were taken for 3 min after nebulization. Every aerosol was
separated by a 15-min recovery period in order to allow airway Penh
to return to the baseline level.
Collection and analysis of
bronchoalveolar lavage fluid (BALF)
On day 33, mice were sacrificed and placed in a
supine position. The trachea was surgically exposed and cannulated
with catheters pointing towards the lungs. The BALF was obtained by
instilling two 1-ml aliquots of PBS via the catheter followed by 2
aspirations of BALF into the 1 ml syringe. The obtained BALF was
centrifuged at 3,420 × g at 4°C for 5 min and the supernatants were
stored at −80°C for further experiments.
The levels of cytokines in the BALF supernatant,
including IL-4 (cat. no. 88-7044-77), IL-5 (cat. no. 88-7054-22),
IL-13 (cat. no. 88-7137-88) and TNF-α (cat. no. 88-7324-22), were
analyzed using ELISAs (eBioscience; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions.
Furthermore, cell pellets from the BALF were
resuspended in 200 µl saline. Then, 100 µl cell suspension was
mixed with 0.4% trypan blue solution for ~3 min at room temperature
to determine total cell counts using a hemocytometer
(Neubauer-improved; Paul Marienfeld GmbH & Co. KG).
The remaining 100 µl BALF was used to prepare
CytospinR slides (Thermo Shandon Inc.). Briefly, samples were
centrifuged at 30 × g for 6 min at 4°C and the deposited cells were
fixed to the microscope slides with 95% alcohol for 30 sec at room
temperature, and then stained with 1X Diff-Quik solution (cat. no.
38721; Sysmex) for 30 sec at room temperature and visualized under
a light microscope (magnification, ×200). In each case, 10 randomly
chosen high-power fields were selected from the CytospinR slides.
Then, 200 cells were counted, and the percentage of each type of
cell was calculated. White blood cells were classified as
eosinophils, neutrophils, macrophages and lymphocytes based on
cellular staining and morphological characteristics.
Lung histology study
Following lavage via trachea with sterile saline,
lungs were immediately removed and fixed in 10% v/v buffered
formalin (diluted in 0.01 mol/l PBS; pH 7.2) for 15 min at room
temperature. Pulmonary tissues were subsequently sliced, embedded
in paraffin and cut into 5-µm-thick sections. These paraffin
sections were stained with hematoxylin for 10 min and eosin
(H&E; Merck Millipore) for 5 min at room temperature or 0.5%
periodic acid-Schiff stain (ScyTek Laboratories, Inc.) for 5 min at
room temperature to evaluate inflammatory cell infiltration and
goblet cell hyperplasia, respectively. Light microscopy (×200
magnification) was used for histopathological assessment. For the
semi-quantification of goblet cell hyperplasia, slides were
examined in a double-blind setting using a semi-quantitative
scoring system as previously described (16,17)
with certain modifications. Briefly, pathological changes were
evaluated according to the modified 5-point scoring system (grade
0–4) and were expressed by score according to the percentage of the
goblet cells in the epithelium: 0 (no goblet cells), 1 (<25%), 2
(25–50%), 3 (51–75%) and 4 (>75%). The mean goblet cell
hyperplasia score was then calculated for each mouse in each
group.
Detection of serum OVA-specific IgE
and IgG1
A total of 700–1,000 µl mouse blood were collected
from the submandibular vein on days 1, 21 and 33, which were
allowed to coagulate for 1 h at room temperature. Following
centrifugation at 1,000 × g for 10 min at 4°C, the serum samples
were stored at −80°C until further analysis; all samples were
thawed no more than twice. OVA-specific IgE and IgG1 levels in the
mouse serum were analyzed via ELISAs. Briefly, 200 µg OVA diluted
in 0.1 M NaHCO3 (pH 9.6) was used to coat the 96-well
microplates at 4°C overnight. After washing with 1X PBS and
blocking with 1% BSA (Sigma-Aldrich; Merck KGaA) in 1X PBS, diluted
sera (1:5 for IgE and 1:2,000 for IgG1) in blocking buffer were
added to the wells for overnight incubation at 4°C. The following
day, the plates were washed and incubated with horseradish
peroxidase-conjugated rat anti-mouse IgE (1:5,000; clone no.
LO-ME-3; cat. no. GTX761169; GeneTex, Inc.) or goat anti-mouse IgG1
(1:5,000; cat. no. ab97240; Abcam) antibodies at 4°C overnight.
After washing the well six times with 1X PBS to remove the unbound
antibody. Then, 3,3′,5,5′-tetramethylbenzidine (cat. no. 00-4201;
Sigma-Aldrich; Merck KGaA) was added and incubated at room
temperature for 15 min, and the absorbance at 450 nm was measured
using an ELISA reader (Sunrise; Tecan Group Ltd.).
OVA-specific splenocyte responses
Mouse spleens from each treated group were collected
immediately following sacrifice on day 33, and a single-cell
suspension of splenocytes for OVA-specific cell proliferation and
cytokine tests were obtained via repeatedly pressing the spleens
with sterile 50-mesh stainless meshes and plungers as previously
described (18). Then, the cells
(2×106) were stimulated with 100 µg/ml OVA in complete
RPMI-1640 medium (Gibco; Thermo Fisher Scientific) for 72 h in
humidified incubator at 37°C with 5% CO2. The
OVA-specific cell proliferation responses were quantified via
incorporation of radiolabeled [3H] thymidine with specific
activities of 1Ci (3.7 GBq) per well in humidified incubator at
37°C with 5% CO2 for 18 h. After labelling, cells were
precipitated twice with ice-cold 10% trichloroacetic acid (TCA) 30
min at RT each, to remove acid soluble materials and then
solubilized overnight in 1.0 M NaOH. The sample was then
transferred into a scintillation vial and the radioactivity was
measured via liquid scintillation counting β-Counter (Beckman
Coulter, Inc.). The readout is radiation counts per minute (cpm)
per well.
Additionally, centrifugation was performed at 1,000
× g for 15 min at 4°C, the supernatant from the culture system of
cells was collected to measure cytokine concentrations, including
mouse IL-4, IL-5 and IL-13 levels, via standard sandwich ELISAs
(eBioscience; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol.
Flow cytometric analysis of DC surface
markers in vivo
On day 33, splenocytes from each treated group were
extracted and fixed in 100 µl 2% formaldehyde (Sigma-Aldrich; Merck
KGaA) in PBS buffer (pH 7.4) for 15 min at 4°C and washed twice
with 1 ml PBS followed by centrifugation of 400 × g for 5 min at
4°C. Fixed cells were then blocked in 2% BSA/PBS for 15 min at 4°C
and resuspended in 50 µl staining buffer (PBS containing 2.00% FCS
and 0.05% sodium azide). Subsequently, the cells were surface
stained with FITC-conjugated mouse anti-CD11c (1:100; cat. no.
117306, clone no. N418; BioLegend, Inc.), phycoerythrin
(PE)-anti-CD40 (1:100; cat. no. 124610, clone no. 3/23; BioLegend,
Inc.) and PE-CD80 antibodies (1:100; cat. no. 104708, clone no.
16-10A1; BioLegend, Inc.) for 30 min at 4°C. Isotype-matched, PE
Rat IgG2a, λ Isotype Ctrl Antibody (1:100 dilution, cat. no.
402304, clone no. G0C3C12; BioLegend, Inc.) and PE Armenian Hamster
IgG Isotype Ctrl Antibody (1:100 dilution, cat. no. 400908, clone
no. HTK888; BioLegend, Inc.) were stained for 30 min at 4°C and
used for negative staining. CD40 and CD80 fluorescence intensity
was measured using an BD Accuri™ 5 flow cytometer (BD Biosciences).
Events were acquired with a forward side scatter threshold of
80,000 and a live gate on CD11c-positive events. The mean
fluorescence intensity was calculated using BD Accuri™ C6 system
software (CFlow version 1.0.264.15; BD Biosciences).
Isolation of CD11c(+) DCs
On day 33, splenocytes were collected. CD11c(+) DCs
were positively selected using mouse CD11c MicroBeads UltraPure
(cat. no. 130-125-835) and LS separation columns (both Miltenyi
Biotec, Inc.), according to the manufacturer's instructions.
Purified CD11c cells were determined via forward and side scatter
and gated according to the size and granular characteristics of
DCs. Based on flow cytometry staining with FITC-conjugated mouse
anti-CD11c, CD11c(+) DCs were found to be >80% pure (Fig. S1).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from purified CD11c(+) DCs
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), as previously described (19). RNA concentration was measured
spectrophotometrically at 260 nm (A260) and 2 µg RNA was reverse
transcribed into cDNA using an Applied Biosystems 2720 Thermal
cycler (Thermo Fisher Scientific, Inc.) along with the following
reagents: Moloney murine leukemia virus (MMLV) reverse
transcriptase, 5X reaction buffer, dNTPs, RNAasin (RNase inhibitor)
and oligo (dT) 15 primers (Promega Corporation). Briefly, primer
annealing was initiated at 70°C for 5 min followed by the addition
of MMLV reverse transcriptase at 37°C for 60 min. The reverse
transcription reaction was terminated following heating to 72°C for
10 min.
qPCR was subsequently performed using a Fast SYBR™
Green Master Mix (Applied Biosystems; Thermo Fisher Scientific,
Inc.) on an ABI 7500 Fast Real-Time system (Applied Biosystems;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The following thermocycling conditions were used for the
qPCR: Initial denaturation at 95°C for 10 min; annealing and
extension, 95°C for 10 sec and 60°C for 30 sec for 40 cycles. The
following primers sequences were used (Tri-I Biotech, Inc.): IL-4
forward, 5′-TTTGAACGAGGTCACAGGAGAAG-3′ and reverse,
5′-AGGACGTTTGGCACATCCA-3′; IL-12 forward,
5′-GCCAGTACACCTGCCACAAA-3′ and reverse,
5′-TGTGGAGCAGCAGATGTGAGT-3′; and hypoxanthine guanine
phosphoribosyl transferase 1 (HPRT) forward,
5′-GTTGGATAAGGCCAGACTTTGTTG-3′ and reverse,
5′-GATTCAACTTGCGCCATCTTAGGC-3′. Quantification of the expression
levels of the IL-4 and IL-12 genes in the samples was accomplished
by measuring the fractional cycle numbers at which the expression
levels reached a fixed threshold (Cq). The relative gene expression
levels were calculated via the 2−∆∆Cq method (20), using the constitutively expressed
gene, HPRT, as a control for normalization. Data are expressed as
n-fold relative to the NC group.
In vitro DC functional assays
In order to measure antigen-specific CD4+
T cell proliferation and Th2 cytokine production, CD4+ T
cells were used to assay the antigen presenting capacity of
CD11c(+) DCs. Briefly, the EasySep™ Mouse CD4 Positive Selection
kit (Stemcell Technologies, Inc.) is used to isolate CD4(+) cells
from single-cell suspensions of spleen, according to the
manufacturer's protocol. Enriched CD4+ T cells were
co-cultured with enriched CD11c(+) DCs generated from the spleens
of different groups of mice at a 2:1 DC: CD4+ T cell
ratio in RPMI-1640 medium supplemented with 10 % fetal bovine
serum, 100 U/ml penicillin-streptomycin (both Gibco; Thermo Fisher
Scientific, Inc.) and 50 µM β-mercaptoethanol (Sigma-Aldrich; Merck
KGaA) for 96 h in humidified incubator at 37°C with 5%
CO2, and cell proliferation was assayed via
3HTdR incorporation as aforementioned.
In addition, DC/CD4+ T cell culture
supernatants were collected after 96 h by centrifugation of 400 × g
for 5 min at 4°C, and IL-4, IL-5 and IL-13 production levels were
measured via ELISA kits (eBioscience; Thermo Fisher Scientific,
Inc.).
Statistical analysis
The data are expressed as the mean ± SEM of three
independent experiments. A Kruskal Wallis and Dunn's post hoc test,
or one- or two-way ANOVAs and a Tukey's post hoc test were used to
compare multiple experimental groups with GraphPad Prism software
(version 5.0; GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
ATL decreases OVA-induced AHR and
airway inflammation
The anti-allergenic effect of ATL was investigated
in an OVA-induced asthmatic animal model. The experimental
procedure for the treatment is presented in Fig. 1A. To investigate the therapeutic
effect of ATL on asthma, AHR and inflammatory cell infiltration in
the lungs were analyzed. Following the exposure to increasing
concentrations of Mch (3.125–50 mg/ml), the degree of AHR was
significantly increased in the OVA group compared with the NC mice,
whereas treatment with 20 and 40 mg/kg ATL significantly decreased
the response to Mch in a dose-dependent manner compared with the
OVA group (Fig. 1B). In addition,
there was a significantly increased number of inflammatory cells
(including total cells, macrophages, eosinophils, neutrophils and
lymphocytes) in the BALF from the OVA group compared with the NC
group (Figs. 1C and S2). However, the influx of inflammatory
cells in the OVA/ATL 20 and OVA/40 groups was significantly
decreased compared with the OVA group (Figs. 1C and S2). Next, the effect of ATL on
inflammatory cell influx was determined using H&E staining. The
degree of inflammatory cells around the bronchioles was notably
elevated in OVA-exposed mice compared with the NC group (Fig. 1D). However, in mice receiving 20
and 40 mg/kg ATL, the level of inflammatory infiltration was
decreased (Fig. 1D). Moreover, a
significant increase was observed in the number of goblet cells and
mucus overproduction, which was indicated by a violet color, in the
bronchial airways of the OVA group compared with the NC group
(Fig. 1E and F). The ATL-treated
groups did not exhibit notable changes in mucus production compared
with the NC group (Fig. 1E and F).
However, a significant reduction in the number of goblet cells was
observed in both the OVA/ATL 20 and OVA/ATL 40 group compared with
the OVA group. Collectively, these data indicated that ATL may
attenuate OVA-induced allergic airway inflammation in mice.
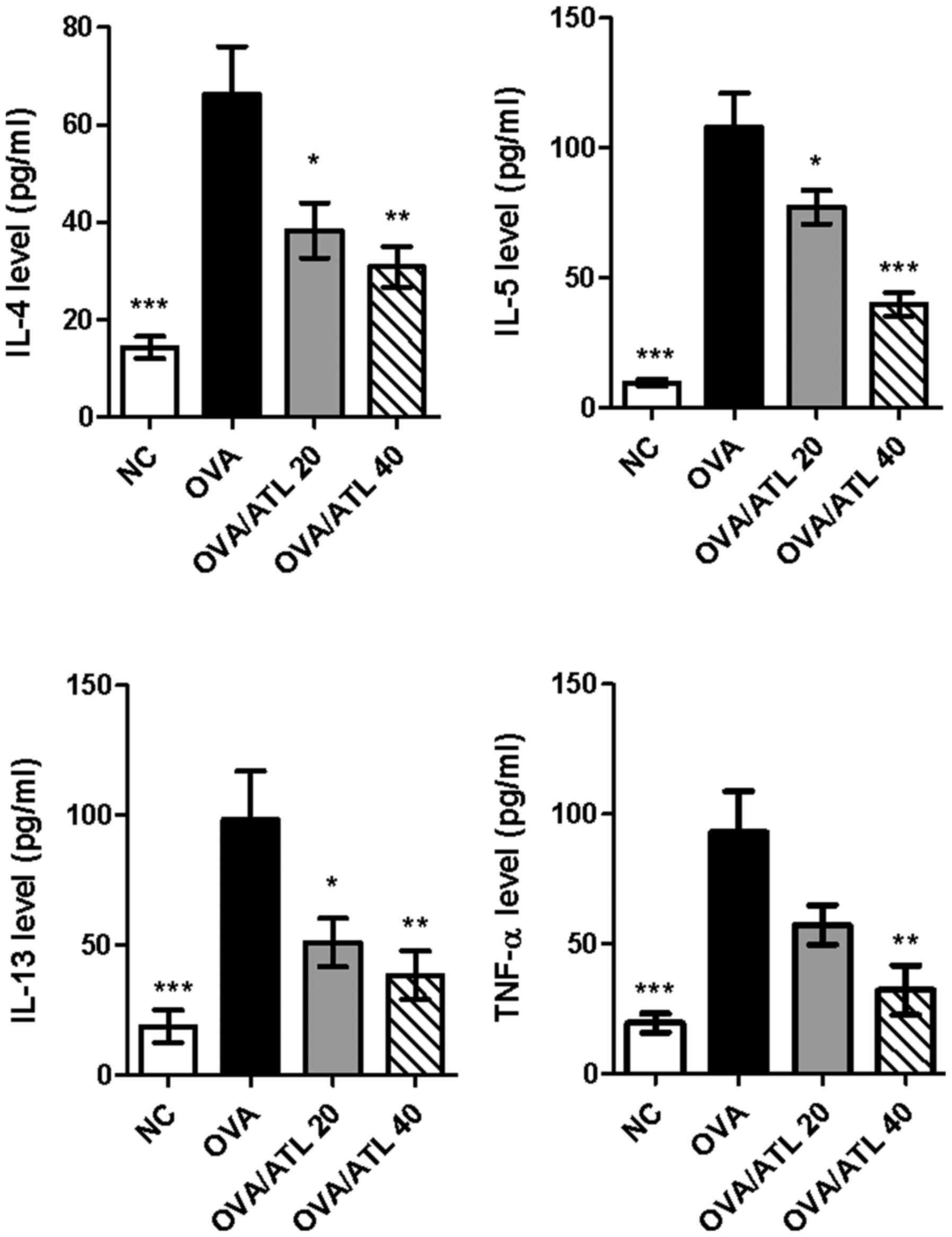
ATL suppresses the production of
OVA-induced Th2 cytokines in the BALF
BALF from the OVA group exhibited significantly
elevated levels of typical Th2 cytokines, including IL-4, IL-5 and
IL-13, and the proinflammatory cytokine, TNF-α, compared with the
NC group (Fig. 2). However, IL-4,
IL-5 and IL-13 levels in the BALF of OVA-sensitized mice were
significantly decreased following the treatment with 20 or 40 mg/kg
ATL, while TNF-α levels were only significantly decreased following
the treatment with 40 mg/kg ATL. Collectively, these findings
indicated that ATL modulated the magnitude of Th2-mediated cytokine
expression levels of BALF during the development of OVA-induced
allergic asthma.
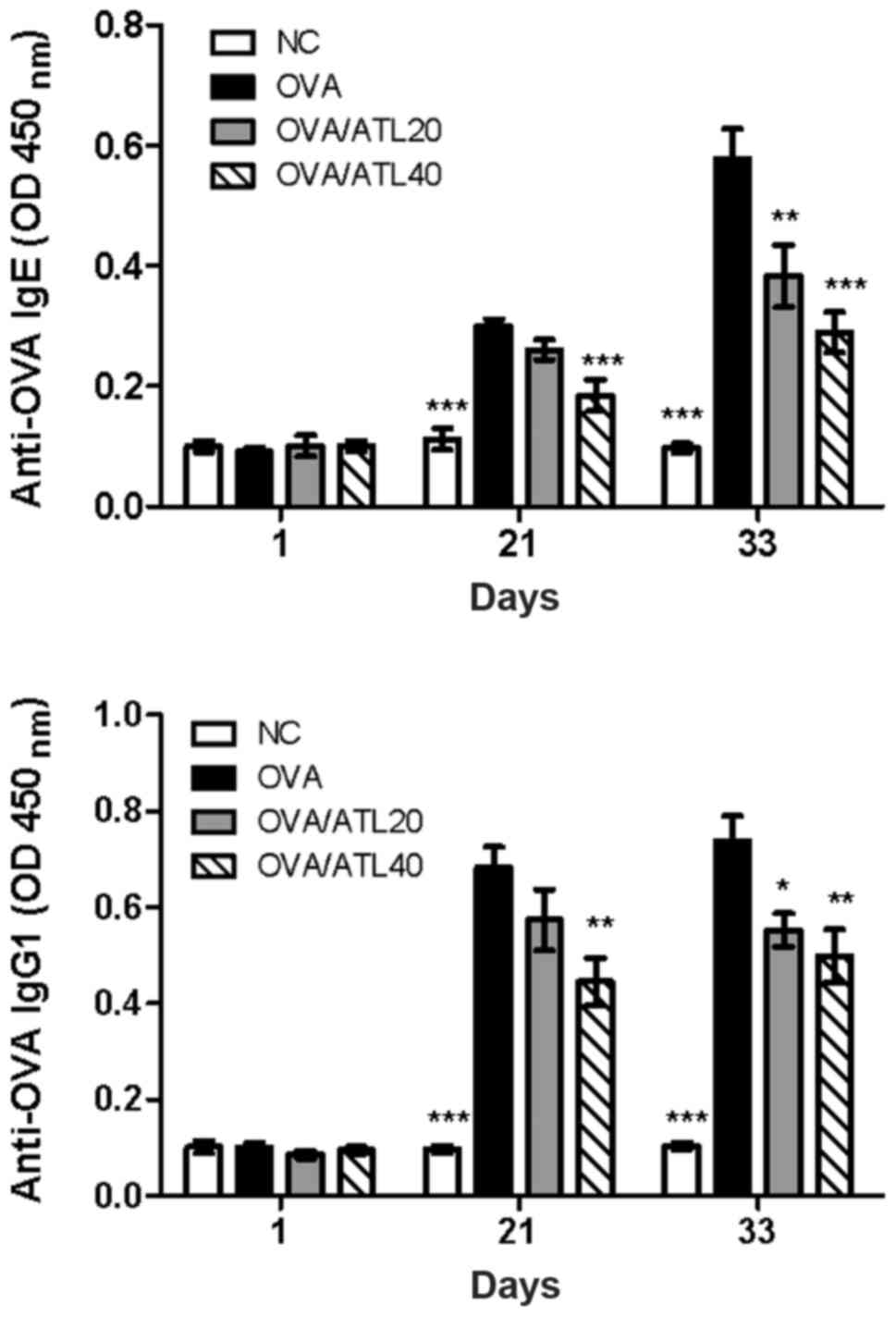
ATL decreases OVA-specific antibody
levels in the serum and T cell expansion in the spleen
Allergic asthma is recognized as a Th2-dependent
immune response with increased serum antigen-specific IgE and IgG1
production (21). In order to
investigate the potential mechanism of ATL-induced inhibition of
airway inflammation, the effect of ATL on the changes of serum IgE
and IgG1 levels was evaluated. OVA challenge in sensitized mice
induced a significant increase in serum OVA-specific IgE and IgG1
levels on days 21 and 33 (Fig. 3)
compared with the NC group. However, 40 mg/kg ATL significantly
inhibited Th2-dependent IgE and IgG1 expression levels compared
with OVA group on day 21 (Fig. 3).
In addition, both 20 and 40 mg/kg ATL significantly suppressed IgE
and IgG1 levels on day 33. The high dose of ATL (OVA/ATL 40)
exhibited a greater suppressive effect than the medium dose
(OVA/ATL 20) on both IgE and IgG1 levels (Fig. 3).
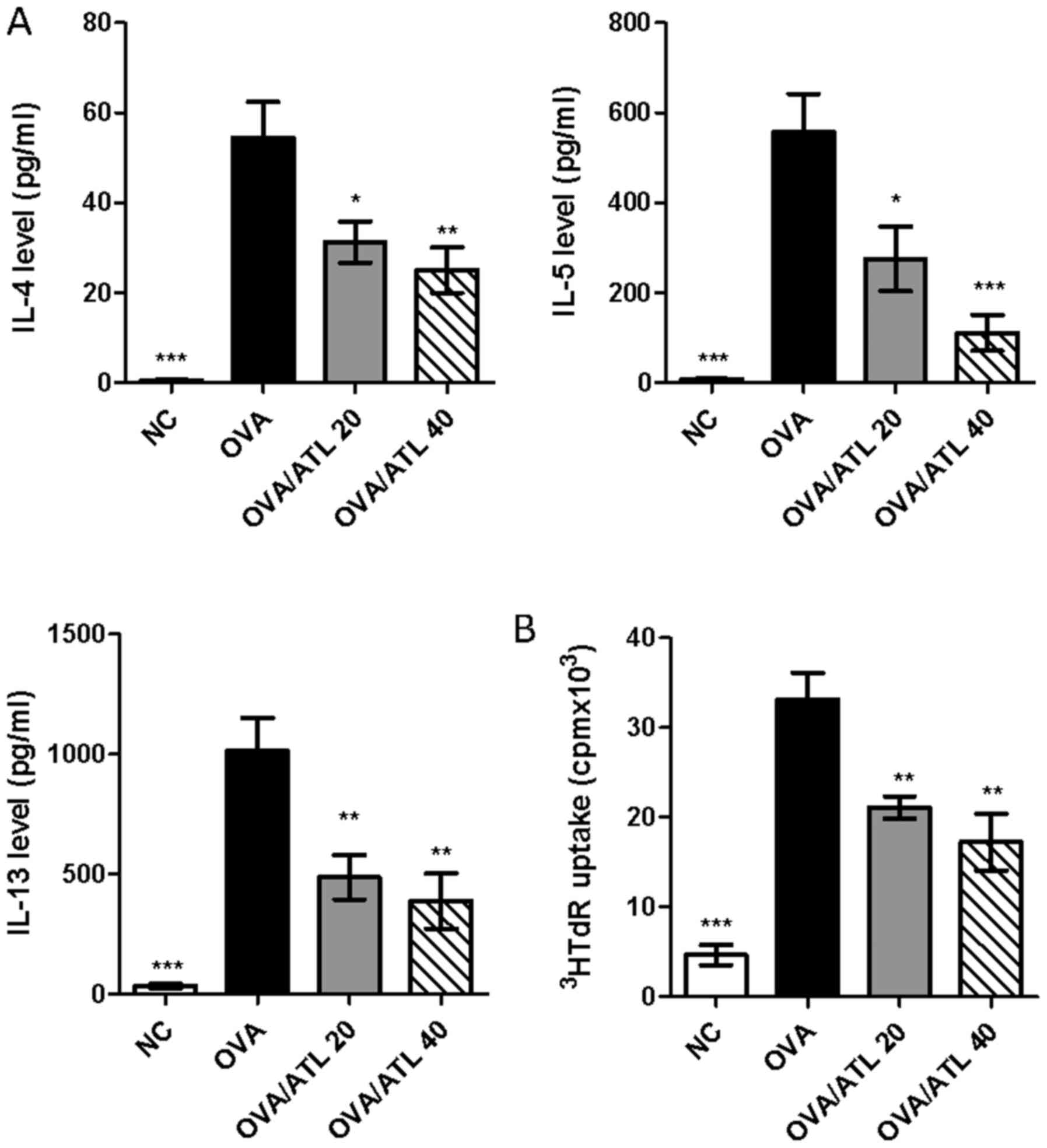
To further investigate the effect of ATL on Th2 cell
responses, splenocytes from the different groups of mice were
stimulated ex vivo with OVA protein for 72 h. Following
stimulation with OVA, ELISAs demonstrated that the splenocyte
culture supernatants from OVA/ATL 20 and OVA/ATL 40 groups
exhibited a significantly decreased production of Th2 cytokines,
including IL-4, IL-5 and IL-13, compared with the splenocytes from
the OVA group (Fig. 4A). In
addition, decreased OVA-stimulated splenocyte proliferation was
also found in OVA/ATL 20 and OVA/ATL 40 groups compared with the
OVA group (Fig. 4B). Taken
together, these results indicated the potential specific inhibition
of the antigen specific Th2 response by ATL in a murine asthmatic
model.
ATL decreases the capacity of
spleen-enriched DCs to stimulate OVA-specific T cell activation in
vitro
Antigen presenting cells, such as DCs, previously
served an important role in the regulation of T cell-mediated
immune responses in an OVA-induced asthma model (6,7). Our
previous study also demonstrated that ATL modulated the
lipopolysaccharide-induced maturation of mouse bone marrow DCs and
suppressed the capacity of DCs to stimulate the T cell response
(13). Thus, to further elucidate
the cellular mechanism of ATL treatment on OVA-induced allergic
asthma in mice, the phenotype and function of DCs obtained from the
spleens were characterized. The treatment with 40 mg/kg ATL
resulted in the sigsup (Fig. 5A and
B). Consistent with the activated phenotype, OVA challenge in
sensitized mice induced a significant increase in IL-4 expression
levels of DCs compared with the NC group. However, DCs from the
spleens of mice in the OVA/ATL 40 group had significantly
downregulated expression levels of IL-4 (Fig. 5C) compared with OVA group, which
serves a critical role in the induction of the Th2 immune response
(5). However, no significant
differences in Th1 cytokine IL-12 expression levels were noted
between groups.
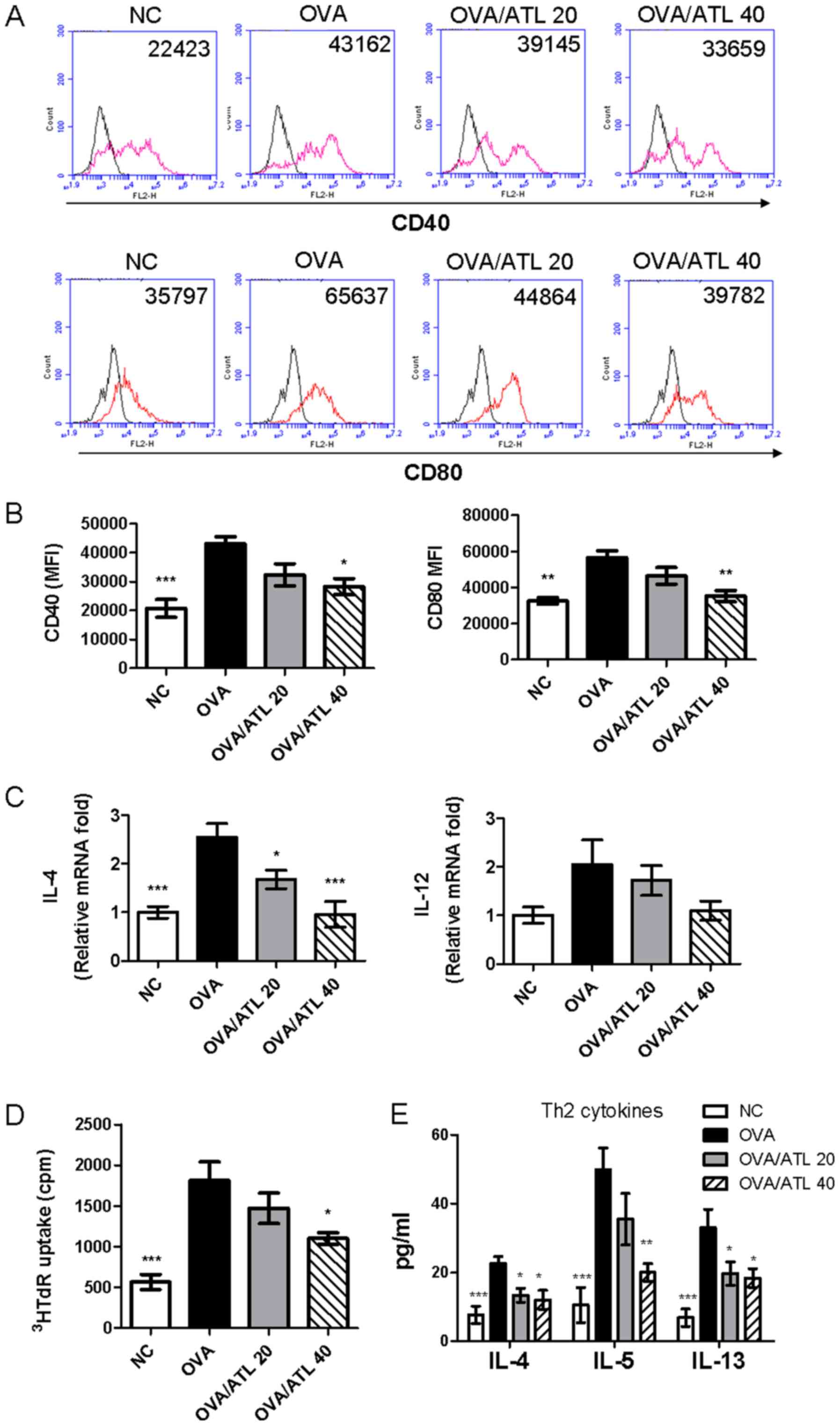 | Figure 5.Treatment with ATL inhibits the
expression levels of costimulatory molecules on splenic DCs. (A)
CD40 and CD80 expression levels on splenic DCs were analyzed by
flow cytometry. (B) Expression levels of activation markers are
presented as MFI. (C) IL-4 and IL-12 mRNA expression levels were
detected in purified splenic CD11c(+) DCs via reverse
transcription-quantitative PCR. Data were normalized to
hypoxanthine guanine phosphoribosyl transferase 1 expression
levels. To determine the effect of splenic CD11c(+) DCs on the OVA
antigen-stimulated T cell response, purified CD11c(+) DCs were
co-cultured with CD4+ T cells from OVA-immunized mice
and the (D) proliferative capacity and (E) Th2 cytokine (IL-4, IL-5
and IL-13) production were measured. Data are presented as the mean
± SEM (n=6 mice per group) The absorbance was measured at 450 nm
with a microplate reader. *P<0.05, **P< 0.01, ***P<0.001
vs. OVA group. ATL, atractylodin; DC, dendritic cell; MFI, mean
fluorescence intensity; OVA, ovalbumin; NC, normal control;
3HRdR, [3H]-thymidine; cpm, counts per minute; Th2, T
helper 2 cell. |
The potency of purified splenic CD11c(+)-enriched
cells isolated to stimulate allogeneic OVA-stimulated T cell
proliferation and cytokine production was compared between the
different groups. CD11c(+) DCs from mice treated with 40 mg/kg ATL
significantly decreased CD4+ T cell proliferation
(Fig. 5D) and decreased Th2
responses (IL-4, IL-5, IL-13; Fig.
5E) compared with CD11c(+) cells obtained from OVA mice. The
OVA/ATL 20 group exhibited significantly inhibited IL-4 and IL-13
expression levels (Fig. 5E) but T
cell proliferation (Fig. 5D) and
the IL-5 expression levels (Fig.
5E) were not significantly altered. These results indicated
that high dose of ATL (OVA/ATL 40) modulation of DC function in
vivo may serve an important role in the suppression of
antigen-specific Th2 responses in an OVA-induced allergic asthma
model.
Discussion
In the present study, an OVA-induced mouse model,
characterized by OVA- induced AHR and allergic airway inflammation,
was used to demonstrate the therapeutic potential of ATL in the
management of asthma. The results showed that ATL may ameliorate
OVA-induced AHR and Th2 inflammation via regulating the maturation
of DCs in OVA-induced allergic asthma. ATL alleviated AHR,
decreased the levels of Th2-associated cytokines, including IL-4,
IL-5 and IL-13, in the lung and decreased the production of
OVA-specific IgG1 and IgE. OVA-stimulated splenocytes were used to
demonstrate that ATL decreased OVA-specific T cell proliferation
and cytokine production. Furthermore, splenic DCs from
OVA-stimulated mice were isolated to determine the regulatory
effect of ATL on the expression levels of costimulatory molecules
and OVA-specific T cells were co-cultured with splenic DCs to
demonstrate the regulatory effect of ATL on DCs. The present
results indicated the potential application of the i.p. injection
of ATL in patients with asthma with high Th2 inflammatory
levels.
Phytochemicals possess numerous pharmacological
activities, including antioxidative, anti-inflammatory,
antirheumatic, antimicrobial and anticancer effects (22,23).
ATL, a phytochemical extracted from Atractylodis rhizoma,
has exhibited anti-inflammatory effects in a number of diseases,
including intestinal inflammation, rheumatic disease and influenza
(9,10). Our previous study reported the
effect of the i.p. injection of ATL on arthritis using a mouse
collagen-induced arthritis model; ATL alleviated the severity of
disease progression, including paw swelling, clinical arthritis
scores and pathological changes in joint tissues, and suppressed
both the Th1 and Th17 pathways (13). In addition, ATL downregulated CD40,
CD80 and CD86 expression levels in splenic DCs (13). The present study further
demonstrated that the i.p. injection of ATL effectively suppressed
DC maturation and downstream Th2 inflammation in asthma.
Collectively, these results highlighted the potential role of ATL
as an anti-inflammatory medication in diseases, including arthritis
and asthma.
Asthma is a complex inflammatory disease, and the
heterogeneity of airway inflammation indicates that distinct
mechanisms may be involved (3).
Among the increasing number of methods used for the identification
of phenotypes among asthmatics, phenotyping via examining
inflammatory cells in induced sputum is currently a practical
method (4,24). It is estimated that ~30% of
asthmatics have high levels of sputum neutrophils instead of
eosinophilia, and the control of asthma in patients with
neutrophilic airway inflammation is poor under standard inhaled
corticosteroid treatment (4).
Using endobronchial tissue gene expression levels analysis in
patients with asthma, Choy et al (25) reported that the Th17 pathway was
implicated in neutrophilic airway inflammation in asthma. However,
there are heterogeneous phenotypes in patients with asthma, and the
majority of current mouse models of asthma mimic eosinophilic
asthma using numerous allergens, including OVA and house dust mite
and cockroach allergens (26). As
only OVA-induced asthma was used in the present study, the impact
of ATL on pathways other than Th2 inflammation remains to be
elucidated by other models mimicking neutrophilic asthma (27). However, our previous study reported
that ATL affected the Th17 pathway in a collagen-induced arthritis
mouse model.
In the past decade, a number of novel biological
agents have been developed in addition to use of a monoclonal
antibody targeting IgE for the management of patients with asthma
and other atopic diseases, including atopic dermatitis and allergic
rhinitis; these biological agents primarily target downstream
cytokines, including IL-5 and IL-4/13 (5). The immunological pathways of IL-5,
IL-4 and IL-13 are associated with DCs during the inflammatory
reaction in the immunological networks of asthma: IL-4 enhances the
capacity of DCs to stimulate the T cell secretion of Th2 cytokines
IL-5 is implicated in the maturation of DCs, and IL-13 enhances the
capacity of DCs to regulate the T cell secretion of IFN-γ (28,29).
Furthermore, IL-4/IL-13 may increase the antigen uptake of DCs and
cell migration into lymph nodes, wherein DCs prime the
differentiation of naive T cells into Th2 cells (30). Additionally, IL-4 drives B cell
class switching to the production of IgE (31). As illustrated in vivo and
ex vivo in the present study, ATL regulated the upstream
antigen presenting function of DCs; therefore, IL-4, IL-5, IL-13,
IgG1 and IgE levels were also discovered to be decreased following
the administration of ATL in the asthma model. Notably, in the
ex vivo study, OVA-specific T cells treated with splenic DCs
were used to specifically clarify the regulatory effect of ATL on
DCs (Fig. 4B). However, an i.p.
injection of ATL was used in the present study; further studies are
warranted to demonstrate the future applications via i.v. or oral
administration of ATL.
As DCs serve an important role in the pathogenesis
of asthma, therapeutic approaches targeting DCs have been suggested
(6). Inhaled corticosteroids, the
cornerstone of asthma management, were discovered to decrease the
number of DCs in the bronchial mucosa of patients with allergic
asthma (32). Using small
interfering RNA to knockdown CD80 and CD86 in an OVA-induced asthma
murine model, Li et al (33) reported that the suppression of
CD80/CD86, markers of DCs, decreased the production of IL-4, which
is consistent with the results of CD80 in the present study. These
findings provided evidence to support the development of medication
targeting DCs in allergic diseases, including asthma.
The number of neutrophils in the NC group was
abnormally low. While the percentage of neutrophils accounts for
>1/2 of total cells in the peripheral blood from normal
subjects, it constitutes <1% in BALF under non-inflammatory
conditions, which was the primary sample source in the present
study (Fig. 1C). In line with the
findings of the present study, Heron et al (34) reported that the percentages of
neutrophils in human peripheral blood mononuclear cells and
bronchoalveolar lavage from healthy individuals were ~54.6 and
0.2%, respectively. Similarly, Van Hoecke et al (35) described the proportions of murine
immune cells in naïve and inflammatory mice and demonstrated that
neutrophils in the BALF of naïve mice accounted for <0.1%,
whereas this was raised to >80% following lipopolysaccharide
stimulation. As a result, it is reasonable to expect a low number
of neutrophils in the NC group in the present study.
Based on our previous study (13), it was hypothesized that the
mechanisms exerted by ATL on DCs may be associated with the direct
suppression of inflammatory cytokines from DCs and the synergistic
decrease in the activation of T cells via the downregulation of the
MAPK signaling pathway (9,11,13).
Additionally, the decrease in inducible nitric oxide synthase was
involved in the mechanisms of ATL (36). However, despite the potent
bioactivity of ATL, the cytotoxicity induced by ATL in immune
cells, particularly DCs, was limited, as evaluated with Cell
Counting Kit-8 cell viability assays. Specifically, bone
marrow-derived DCs treated with <100 µM ATL exhibited marginal
cytotoxicity. The same conclusions were drawn from an animal model
experiment in which 40 mg/kg ATL (the same concentration as applied
in the present study) was injected into mice, and no significant
weight loss was observed (13. Therefore, ATL may be a safe
therapeutic option in the management of asthma.
In conclusion, the present study demonstrated that
ATL regulated DC maturation, and subsequent Th2 inflammation and
AHR in a mouse model of asthma. These results provided evidence
that ATL may be a potential therapeutic option for the management
of asthma and other allergic diseases involving Th2
inflammation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Changhua
Christian Hospital (grant no. 108-CCH-IRP-067) and Animal
Biotechnology Center from the Feature Areas Research Center Program
of Taiwan Ministry of Education (grant no. MOE-107-S-0023-E).
Availability of data and materials
The datasets generated and/or analyzed in the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
YCL, WCC and CCL analyzed the data and drafted the
manuscript. CCY and TCH contributed to the interpretation of data
and revised the manuscript. YCL, WCC and CCL conceived and designed
the study. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Ethical approval for the present study was obtained
from the IACUC of National Chung-Hsing University (Taichung,
Taiwan; approval no. IACUC 108-072).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Collaborators GBDCRD; GBD 2015 Chronic
Respiratory Disease Collaborators, : Global, regional, and national
deaths, prevalence, disability-adjusted life years, and years lived
with disability for chronic obstructive pulmonary disease and
asthma, 1990–2015: A systematic analysis for the Global Burden of
Disease Study 2015. Lancet Respir Med. 5:691–706. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brannan JD: Bronchial hyperresponsiveness
in the assessment of asthma control: Airway hyperresponsiveness in
asthma: its measurement and clinical significance. Chest. 138
(Suppl):11S–17S. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wenzel SE: Asthma phenotypes: The
evolution from clinical to molecular approaches. Nat Med.
18:716–725. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simpson JL, Scott R, Boyle MJ and Gibson
PG: Inflammatory subtypes in asthma: Assessment and identification
using induced sputum. Respirology. 11:54–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cosmi L, Maggi L, Mazzoni A, Liotta F and
Annunziato F: Biologicals targeting type 2 immunity: Lessons
learned from asthma, chronic urticaria and atopic dermatitis. Eur J
Immunol. 49:1334–1343. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lambrecht BN and Hammad H: The role of
dendritic and epithelial cells as master regulators of allergic
airway inflammation. Lancet. 376:835–843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lambrecht BN and Hammad H: Biology of lung
dendritic cells at the origin of asthma. Immunity. 31:412–424.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xia YG, Yang BY, Wang QH, Liang J, Wang D
and Kuang HX: Species classification and quality assessment of
cangzhu (Atractylodis rhizoma) by high-performance liquid
chromatography and chemometric methods. J Anal Methods Chem.
2013:4975322013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jun X, Fu P, Lei Y and Cheng P:
Pharmacological effects of medicinal components of Atractylodes
lancea (Thunb.) DC. Chin Med. 13:592018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu C, Xiong Y, Chen D, Li Y, Xu B, Lin Y,
Tang Z, Jiang C and Wang L: Ameliorative effects of atractylodin on
intestinal inflammation and co-occurring dysmotility in both
constipation and diarrhea prominent rats. Korean J Physiol
Pharmacol. 21:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chae HS, Kim YM and Chin YW: Atractylodin
inhibits interleukin-6 by blocking NPM-ALK activation and MAPKs in
HMC-1. Molecules. 21:11692016. View Article : Google Scholar
|
|
12
|
Tang F, Fan K, Wang K and Bian C:
Atractylodin attenuates lipopolysaccharide-induced acute lung
injury by inhibiting NLRP3 inflammasome and TLR4 pathways. J
Pharmacol Sci. 136:203–211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chuang CH, Cheng YC, Lin SC, Lehman CW,
Wang SP, Chen DY, Tsai SW and Lin CC: Atractylodin suppresses
dendritic cell maturation and ameliorates collagen-induced
arthritis in a mouse model. J Agric Food Chem. 67:6773–6784. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals; Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
15
|
Lyu Z, Ji X, Chen G and An B: Atractylodin
ameliorates lipopolysaccharide and d-galactosamine-induced acute
liver failure via the suppression of inflammation and oxidative
stress. Int Immunopharmacol. 72:348–357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SJ, Kim CH, Ahn JH, Kim MS, Kim SC,
Lee SY, Kwon SS, Kim YK, Lim KH, Moon HS, et al: Time sequence of
airway remodeling in a mouse model of chronic asthma: the relation
with airway hyperresponsiveness. J Korean Med Sci. 22:183–191.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Padrid P, Snook S, Finucane T, Shiue P,
Cozzi P, Solway J and Leff AR: Persistent airway
hyperresponsiveness and histologic alterations after chronic
antigen challenge in cats. Am J Respir Crit Care Med. 151:184–193.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiang CY, Lee CC, Fan CK, Huang HM,
Chiang BL and Lee YL: Osthole treatment ameliorates Th2-mediated
allergic asthma and exerts immunomodulatory effects on dendritic
cell maturation and function. Cell Mol Immunol. 14:935–947. 2017.
View Article : Google Scholar
|
|
19
|
Weng TY, Li CJ, Li CY, Hung YH, Yen MC,
Chang YW, Chen YH, Chen YL, Hsu HP, Chang JY, et al: Skin delivery
of Clec4a small hairpin RNA elicited an effective antitumor
response by enhancing CD8+ immunity in vivo. Mol Ther Nucleic
Acids. 9:419–427. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kubo M: T follicular helper and TH2 cells
in allergic responses. Allergol Int. 66:377–381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kumar H, Kim IS, More SV, Kim BW and Choi
DK: Natural product-derived pharmacological modulators of Nrf2/ARE
pathway for chronic diseases. Nat Prod Rep. 31:109–139. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu F, Du B and Xu B: Anti-inflammatory
effects of phytochemicals from fruits, vegetables, and food
legumes: A review. Crit Rev Food Sci Nutr. 58:1260–1270. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schatz M and Rosenwasser L: The allergic
asthma phenotype. J Allergy Clin Immunol Pract. 2:645–648, quiz
649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choy DF, Hart KM, Borthwick LA, Shikotra
A, Nagarkar DR, Siddiqui S, Jia G, Ohri CM, Doran E, Vannella KM,
et al: TH2 and TH17 inflammatory pathways are reciprocally
regulated in asthma. Sci Transl Med. 7:301ra1292015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nials AT and Uddin S: Mouse models of
allergic asthma: Acute and chronic allergen challenge. Dis Model
Mech. 1:213–220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu QL and Chen Z: Establishment of
different experimental asthma models in mice. Exp Ther Med.
15:2492–2498. 2018.PubMed/NCBI
|
|
28
|
Webb DC, Cai Y, Matthaei KI and Foster PS:
Comparative roles of IL-4, IL-13, and IL-4Ralpha in dendritic cell
maturation and CD4+ Th2 cell function. J Immunol. 178:219–227.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yi H, Zhang L, Zhen Y, He X and Zhao Y:
Dendritic cells induced in the presence of GM-CSF and IL-5.
Cytokine. 37:35–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahn JS and Agrawal B: IL-4 is more
effective than IL-13 for in vitro differentiation of dendritic
cells from peripheral blood mononuclear cells. Int Immunol.
17:1337–1346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roper RL, Conrad DH, Brown DM, Warner GL
and Phipps RP: Prostaglandin E2 promotes IL-4-induced IgE and IgG1
synthesis. J Immunol. 145:2644–2651. 1990.PubMed/NCBI
|
|
32
|
Möller GM, Overbeek SE, Van
Helden-Meeuwsen CG, Van Haarst JM, Prens EP, Mulder PG, Postma DS
and Hoogsteden HC: Increased numbers of dendritic cells in the
bronchial mucosa of atopic asthmatic patients: Downregulation by
inhaled corticosteroids. Clin Exp Allergy. 26:517–524. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li JG, Du YM, Yan ZD, Yan J, Zhuansun YX,
Chen R, Zhang W, Feng SL and Ran PX: CD80 and CD86 knockdown in
dendritic cells regulates Th1/Th2 cytokine production in asthmatic
mice. Exp Ther Med. 11:878–884. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Heron M, Grutters JC, ten Dam-Molenkamp
KM, Hijdra D, van Heugten-Roeling A, Claessen AM, Ruven HJ, van den
Bosch JM and van Velzen-Blad H: Bronchoalveolar lavage cell pattern
from healthy human lung. Clin Exp Immunol. 167:523–531. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Van Hoecke L, Job ER, Saelens X and Roose
K: Bronchoalveolar lavage of murine lungs to analyze inflammatory
cell infiltration. J Vis Exp. 4:e553982017.
|
|
36
|
Ishii T, Okuyama T, Noguchi N, Nishidono
Y, Okumura T, Kaibori M, Tanaka K, Terabayashi S, Ikeya Y and
Nishizawa M: Antiinflammatory constituents of Atractylodes
chinensis rhizome improve glomerular lesions in immunoglobulin
A nephropathy model mice. J Nat Med. 74:51–64. 2020. View Article : Google Scholar : PubMed/NCBI
|