Introduction
Lupus nephritis (LN) is a chronic and complex kidney
disease (1) that is a frequent
complication of systemic lupus erythematosus (2) and is usually associated with
inflammatory cell infiltration and immune complex deposition in
renal tissues (3). LN is
clinically evident in ~50% of patients with systemic lupus
erythematosus (4). In LN, ICs
initiate the synthesis of various proinflammatory cytokines, such
as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6,
resulting in cellular infiltration and renal injury (5). The involvement of LN significantly
increases patient morbidity and mortality rates (6). Patients with LN have a higher
standardized mortality ratio (6-6.8 vs. 2.4) and lower survival
rates compared with patients with systemic lupus erythematosus who
do not have LN (7). LN
pathogenesis is complex, and some patients with LN may develop
end-stage renal disease (8).
Therefore, it is essential to explore new approaches for the
treatment of LN.
Complement component 1q (C1q) is a subcomponent of
the C1 complex, which participates in the classical pathway of
complement activation (9). C1q has
numerous functions, including recognition of ICs and activation of
the complement system (10).
Previous studies have examined the relationship between LN and
anti-C1q. For example, a significantly negative correlation between
C1q and anti-C1q was found in patients with LN (11). Anti-C1q was also associated with
proteinuria and renal activity score in patients with LN, and could
serve as a potential biomarker of LN (12). In addition, high anti-C1q antibody
titers are present in the blood of patients with LN, and the level
of anti-C1q is related with disease progression (13). Anti-C1q has adequate specificity
and sensitivity for LN diagnosis and can be used to evaluate renal
activity (14); however, the
underlying mechanism of C1q in the regulation of LN remains poorly
understood.
Nuclear factor (NF)-κB not only participates in
innate and adaptive immunity (15), but also is considered to be a
proinflammatory transcription factor (16). The NF-κB pathway is related to
several pathological processes in the kidneys, including immune
response, inflammation and mesangial cell (MC) proliferation
(17–19). Deletion of NF-κB p65 was found to
alleviate LN in mice (20). In LN,
inhibition of the NF-κB pathway suppressed the inflammatory
response (21), and blocking this
pathway may decrease macrophage chemotaxis and MC proliferation
(22). Nevertheless, the potential
regulatory mechanism of C1q in relation to the NF-κB pathway in LN
is still unknown.
In the present study, C1q expression in LN mice was
evaluated and the regulatory effects of C1q on renal injury,
inflammation, macrophage infiltration and MC proliferation was
explored. The function of C1q in regulating NF-κB pathway activity
in LN mice was also explored. These results may suggest a potential
therapeutic target for LN.
Materials and methods
Animals
In total, 60 male MRL/lpr mice (used as an LN mouse
model) and 15 C57BL/6 mice (age, 3 months; body weight, 18–22 g)
were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. Mice
were maintained at 23–25°C and 50–55% relative humidity, and kept
under a 12 h/12 h light/dark cycle with ad libitum access to
food and water. The present study was performed with the approval
of The Animal Ethics Committee of Linyi Central Hospital (Linyi,
China).
Experimental design
pcDNA-C1q and pcDNA-negative control (NC) were
obtained from Sangon Biotech Co., Ltd. After one week of
adjustment, LN mice were divided into pc-NC, pcDNA-C1q and Sham
groups, which were injected intraperitoneally with 1 mg/kg pc-NC, 1
mg/kg pcDNA-C1q or an equivalent quantity of normal saline,
respectively (15 mice in each group). C57BL/6 mice without
treatment acted as the BLANK group. In addition, LN mice in the C1q
+ Phorbol 12-myristate 13-acetate (PMA) group were injected
intraperitoneally with 1 mg/kg pcDNA-C1q and treated with PMA (25
nM; Sigma-Aldrich; Merck KGaA).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from renal tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and reverse transcribed into cDNA using the PrimeScript RT
reagent kit (Takara Bio, Inc.). The reaction mixtures were
incubated at 37°C for 60 min, 95°C for 5 min and then held at 4°C.
miScript SYBR Green PCR kit (Qiagen, Inc.) was used to conduct
qPCR. The qPCR reaction was performed on the ABI 7500HT Fast
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with the following conditions: 95°C for 3 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 30 sec, and a final
extension step at 72°C for 10 min. Relative expression was
calculated using the 2−ΔΔCq method (23). GAPDH was used for normalization.
Primer sequences are shown in Table
I.
 | Table I.Primer sequences used in reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used in reverse
transcription-quantitative PCR.
| Primer | Sequences
(5′→3′) |
|---|
| Complement 1q | F:
GAAACAATGGGAACAATGGAG |
|
| R:
TGCTGAAGGTGAAGAAATACA |
| GAPDH | F:
ACACCTTCTACAATGAGCTG |
|
| R:
CTGCTTGCTGATCCACATCT |
Evaluation of renal function
The 24-h urine protein excretion was measured once
every 2 weeks using Multistix 10SG reagent strips (Siemens
Healthineers). After 4 weeks continuous treatment, mice were
anesthetized by intraperitoneal administration of 50 mg/kg
pentobarbital sodium and sacrificed by cervical dislocation. Blood
samples and renal tissues were collected for future experiments.
Blood urea nitrogen (BUN) levels were measured using an automatic
biochemical analyzer (Hitachi, Ltd.).
H&E staining
Renal tissues were fixed in 4% paraformaldehyde for
24 h at 37°C, embedded in paraffin, cut into 4-µm thick sections,
dewaxed in xylene and rehydrated with 90% ethanol at 37°C. Sections
were then stained with hematoxylin for 2 min and eosin for 2 min at
37°C. Using light microscopy, the degree of histological damage in
renal tissues was observed (magnification, ×400). The histological
damage index of glomerulus was graded on a scale of 0–3 as
previously described by Muraoka et al (24), where 0 = normal, 1 = mild (cell
proliferation and/or cell infiltration), 2 = moderate (cell
proliferation and/or cell infiltration with membrane proliferation)
and 3 = severe (cell proliferation and/or cell infiltration,
membrane proliferation and crescent formation and/or
hyalinosis).
Western blotting
Renal tissues were lysed using ice-cold RIPA lysis
buffer (Beyotime Institute of Biotechnology) to obtain total
protein. The concentration of total protein was detected using a
bicinchoninic acid protein concentration assay kit (Cell Signaling
Technology, Inc.). Total protein (60 µg/lane) was separated using
sodium dodecyl sulphate polyacrylamide gel electrophoresis (10%
separating gum and 5% concentrating gum), and subsequently
transferred onto a polyvinylidene fluoride membrane. The membranes
were blocked with 5% skimmed milk for 1 h at 37°C. Then, membranes
were incubated with primary antibodies overnight at 4°C. The
antibodies used were as follows: Anti-IκBα (1:1,000; cat. no.
9242), anti-phosphorylated (p)-IκBα (1:1,000; cat. no. 2859),
anti-NF-κB p65 (1:1,000; cat. no. 8242) and anti-p-NF-κB p65
(1:1,000; cat. no. 8214) (all CST Biological Reagents Co., Ltd.).
Next, membranes were incubated with HRP-labelled goat anti-rabbit
IgG (1:2,000; cat. no. I5006MSDS) and HRP-labelled goat anti-mouse
IgG secondary antibodies (1:4,000; cat. no. 12-349; both purchased
from Sigma-Aldrich; Merck KGaA) for 1 h at 25°C. Finally, protein
bands were visualized using enhanced chemiluminescence exposure
solution (Invitrogen; Thermo Fisher Scientific, Inc.) and
semi-quantified using Quantity One 1-D software (version 4.62;
Bio-Rad Laboratories, Inc.). GAPDH (1:1,000; cat. no. 100242-MM05;
Sino Biological) was used as a loading control.
ELISA
Renal tissue homogenate from each group was
centrifuged at 3,000 × g at 4°C for 10 min and the resulting
supernatant was collected. Then, the levels of TNF-α (cat. no.
ab236712), IL-1β (cat. no. ab197742), IL-6 (cat. no. ab100713) and
anti-C1q (cat. no. ab170246) were measured using ELISA kits (all
purchased from Abcam). The level of anti-dsDNA was measured by the
automated Alegria® ELISA reader (Orgentec Diagnostika
GmbH), according to the manufacturers instructions. The absorbance
of each well was measured at 450 nm using an enzyme mark instrument
(Thermo Fisher Scientific, Inc.).
Immunofluorescence staining
Frozen glomeruli sections (5 µm) were dried for 15
min at 25°C. After rinsing three times, sections were blocked in
PBS containing 10% goat serum (cat. no. 16210064; Thermo Fisher
Scientific, Inc.) for 1 h at 25°C. Glomeruli sections were
incubated with anti-CD68 antibody (1 µg/ml; cat. no. ab201340) or
anti-Ki67 antibody (1 µg/ml; cat. no. ab15580; both Abcam)
overnight at 4°C, followed by incubation with Alexa Fluor
488-conjugated goat anti-rabbit IgG (1:500; cat. no. ab150077;
Abcam) for 1 h at 25°C. Glomeruli sections were counterstained with
DAPI (2.5 ng/µl) at 25°C for 1 h, and the percentages of CD68- and
Ki67-positivity were detected using immunofluorescence microscopy
(magnification, ×400; Olympus Corporation). The CellProfiler 4.0
software (www.cellprofiler.org) was used to
quantify the DAPI intensity at the peri-nucleolar region of ~100
individual cells.
Statistical analysis
Statistical analysis was performed with SPSS 23.0
(IBM Corp.). Data were presented as the mean ± SD. All experiments
were repeated three times. Differences among multiple groups were
analyzed using one-way ANOVA followed by Tukeys multiple
comparisons test. Data of two groups were assessed using unpaired
Students t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Overexpression of C1q alleviates renal
injury in LN mice
Analysis using RT-qPCR indicated that C1q mRNA
expression was decreased in MRL/lpr mice (LN mice) compared with
C57BL/6 mice. C1q-overexpression was induced by transfecting
pcDNA-C1q (Fig. 1A), and the urine
protein and BUN levels were examined. Compared with the BLANK
group, urine protein and BUN levels were significantly increased in
the Sham group, whereas transfection of pcDNA-C1q significantly
decreased these levels (P<0.01; Fig. 1B and C). ELISA analysis showed that
the levels of serum anti-C1q and anti-dsDNA were significantly
elevated in the Sham group compared with the BLANK group.
Overexpression of C1q reduced the levels of serum anti-C1q and
anti-dsDNA compared with the pc-NC group (Fig. 1D and E). Compared with the BLANK
group, H&E staining showed a higher histological damage index
of glomeruli in the Sham group. Additionally, the histological
damage index was lower in the pcDNA-C1q group compared with that in
the pc-NC group (Fig. 1F). These
results suggested that C1q overexpression may attenuate renal
injury in LN mice.
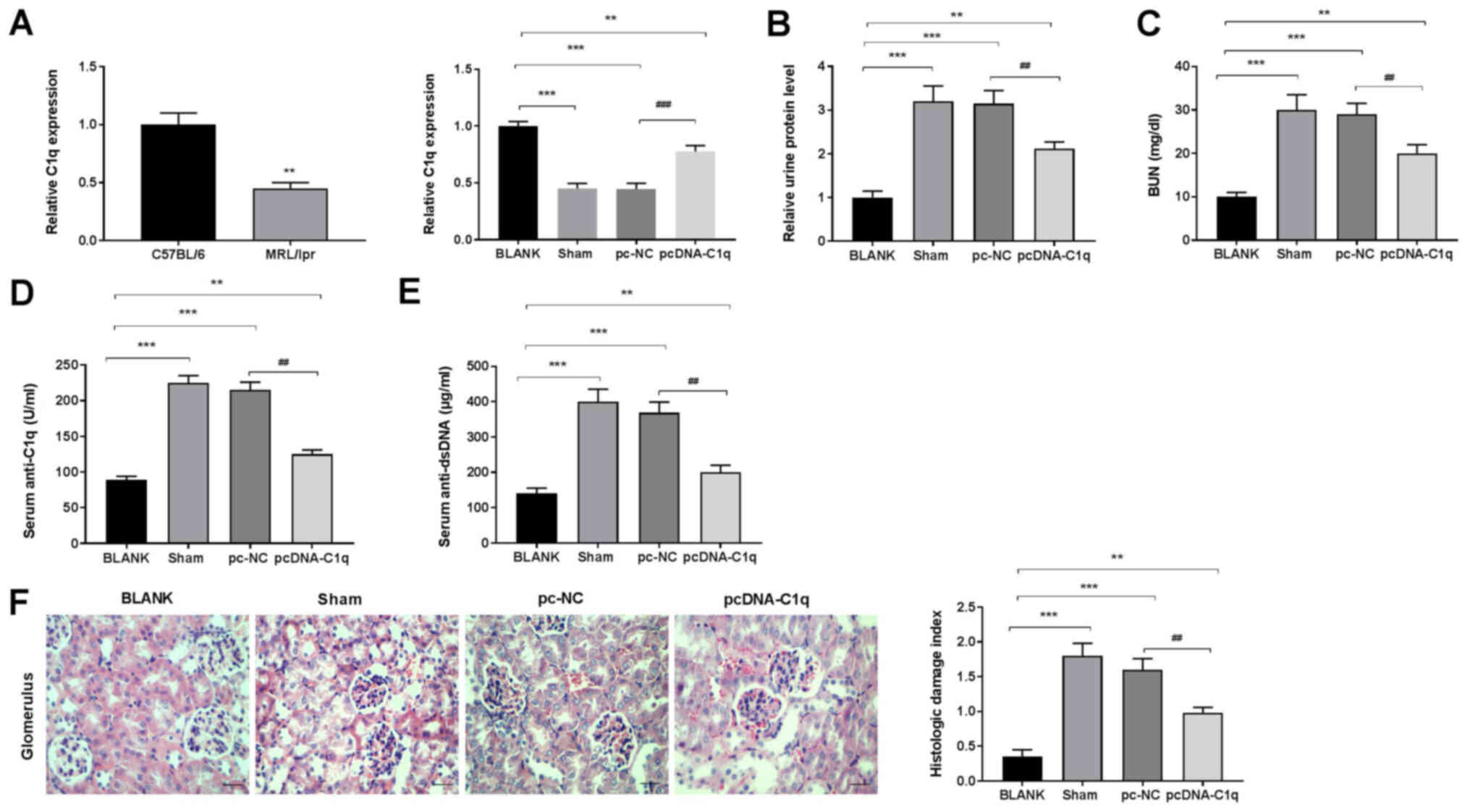 | Figure 1.Overexpression of C1q alleviates
renal injury in LN mice. (A) mRNA expression of C1q in renal
tissues was detected using reverse transcription-quantitative PCR.
(B) Relative level of urine protein at 24 h. (C) Levels of serum
BUN. Levels of serum (D) anti-C1q and (E) anti-dsDNA were measured
using ELISA. (F) The histological damage index of glomerulus was
determined using H&E staining. There were 15 mice in each group
and the experiments were repeated three times. **P<0.01,
***P<0.001 vs. C57BL/6 or BLANK; ##P<0.01,
###P<0.001 vs. pc-NC. C1q, complement component 1q;
LN, lupus nephritis; BUN, blood urea nitrogen; NC, negative
control; dsDNA, double stranded DNA; BLANK, C57BL/6 mice without
treatment; Sham, LN mice injected intraperitoneally with normal
saline; pc-NC, LN mice injected intraperitoneally with pc-NC;
pcDNA-C1q, LN mice injected intraperitoneally with pcDNA-C1q. |
Overexpression of C1q attenuates renal
inflammation in LN mice
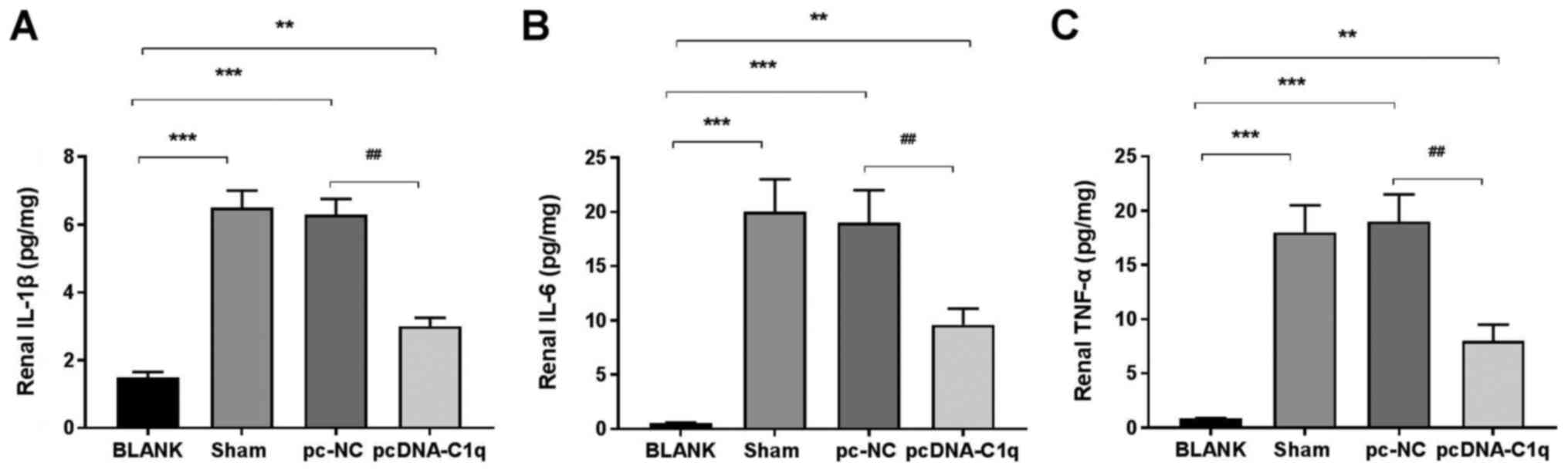
ELISA analysis revealed that the levels of TNF-α,
IL-6 and IL-1β in renal tissues in the Sham group were markedly
increased compared with those in the BLANK group. Overexpression of
C1q significantly reduced the levels of TNF-α, IL-6 and IL-1β in
renal tissues in the pcDNA-C1q compared with the pc-NC group
(Fig. 2A-C). Overall, these
results indicated that C1q overexpression may alleviate the renal
inflammation in LN mice.
Overexpression of C1q reduces
macrophage infiltration and MC proliferation in renal tissues of LN
mice
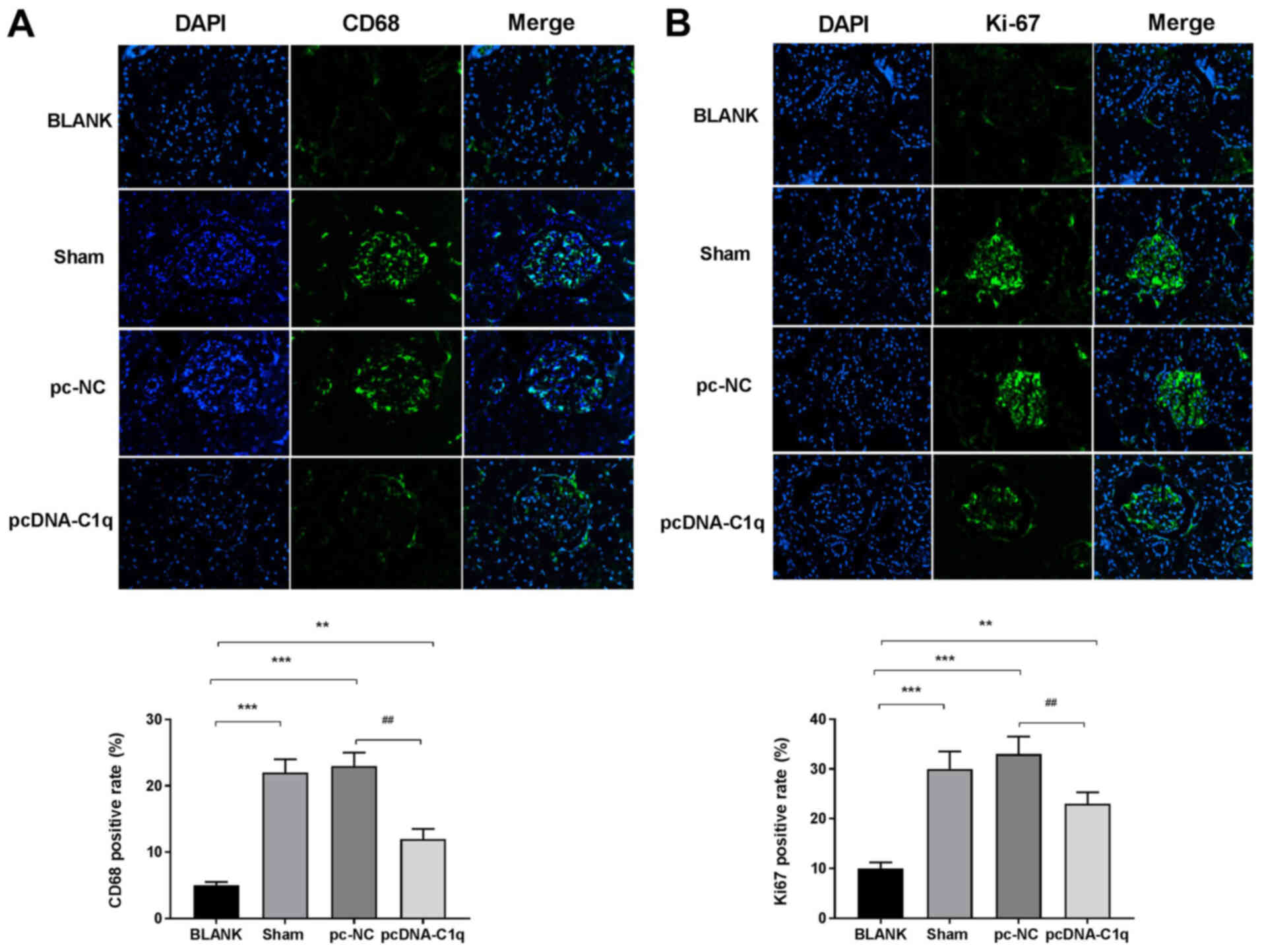
To investigate the effect of C1q on macrophage
infiltration and the proliferation of MCs in the renal tissues of
LN mice, the percentages of CD68- and Ki67-positivity were measured
using immunofluorescence staining. The percentage of
CD68-positivity in Sham group renal tissues was higher compared
with that of the BLANK group. Furthermore, compared with the Sham
group, this percentage was decreased in the pcDNA-C1q group
(Fig. 3A). In addition, with the
BLANK group, the percentage of Ki67-positivity in renal tissues was
increased in the Sham group and was decreased by C1q-overexpression
(Fig. 3B). Taken together, C1q
overexpression may decrease macrophage infiltration and MC
proliferation in renal tissues of LN mice.
Overexpression of C1q inhibits the
NF-κB pathway in the renal tissues of LN mice
To evaluate the effect of C1q-overexpression on the
NF-κB pathway in LN mouse renal tissues, the expression of
NF-κB-related proteins was measured via western blotting. Compared
with the BLANK group, the protein expression of p-IκBα/IκBα and
p-NF-κB p65/NF-κB p65 in renal tissues was significantly increased
in the Sham group. These levels in renal tissues were significantly
decreased in the pcDNA-C1q group compared with the Sham group. PMA,
an activator of the NF-κB pathway (25), was injected into the
pcDNA-C1q-treated LN mice. Consequently, the inhibitory effect of
C1q on the NF-κB pathway was attenuated by PMA compared with the
pcDNA-C1q group (Fig. 4). These
results demonstrated that C1q overexpression may inhibit the NF-κB
pathway in the renal tissues of LN mice.
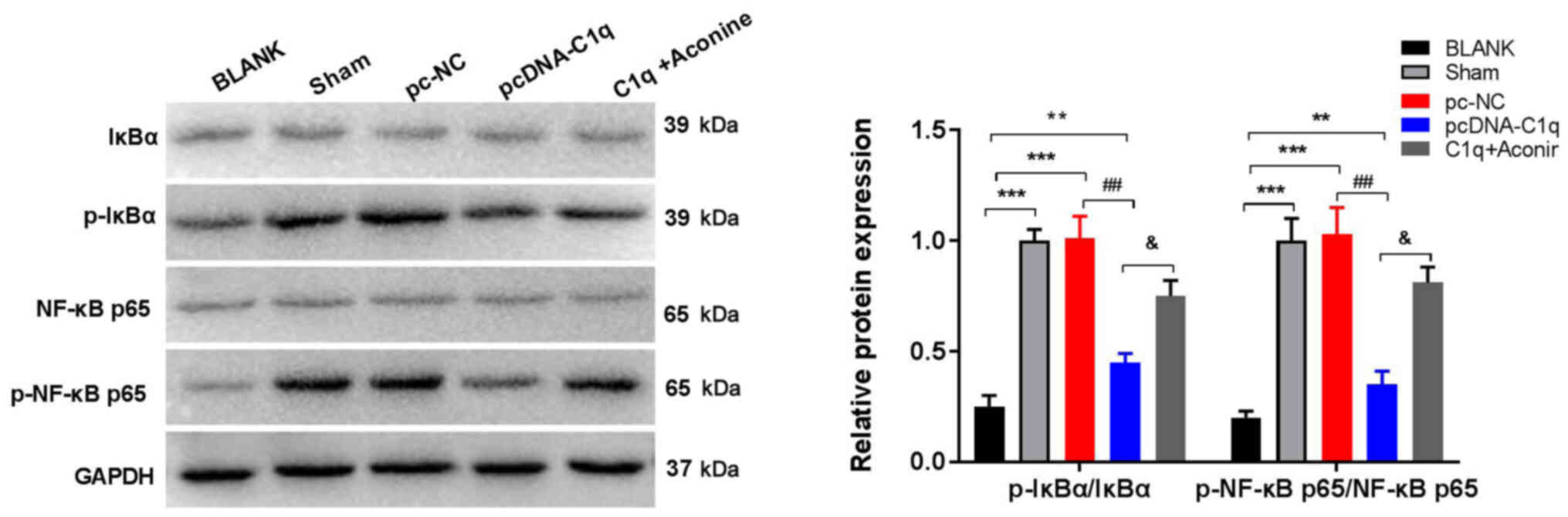 | Figure 4.Overexpression of C1q inhibits the
NF-κB pathway in renal tissues of LN mice. Protein expression of
p-IκBα/IκBα and p-NF-κB p65/NF-κB p65 was measured using western
blot analysis. There were 15 mice in each group and the experiments
were repeated three times. **P<0.01,***P<0.001 vs. BLANK;
##P<0.01 vs. pc-NC; &P<0.05 vs.
pcDNA-C1q. C1q, complement component 1q; LN, lupus nephritis; p,
phosphorylated; PMA, phorbol 12-myristate 13-acetate; BLANK,
C57BL/6 mice without treatment; Sham, LN mice injected
intraperitoneally with normal saline; pc-NC, LN mice injected
intraperitoneally with pc-NC; pcDNA-C1q, LN mice injected
intraperitoneally with pcDNA-C1q; C1q + PMA, LN mice were injected
intraperitoneally with pcDNA-C1q and treated with PMA. |
Overexpression of C1q ameliorates
renal injury in LN mice via inhibiting the NF-κB pathway
PMA was administered to LN mice, and the levels of
renal inflammatory factors were measured. The results showed that
overexpression of C1q significantly decreased the levels of TNF-α,
IL-1β and IL-6 in the renal tissues of LN mice (Fig. 5A). In addition, immunofluorescence
staining demonstrated that the percentages of CD68- and
Ki67-positivity in renal tissues were decreased in the pcDNA-C1q
group compared with the pc-NC group (Fig. 5B and C). Moreover, PMA attenuated
the effects of C1q on inflammatory factors and percentages of CD68-
and Ki67-positivity in LN mice renal tissues (Fig. 5A-C). Overall this indicated that
C1q overexpression may ameliorate renal injury by inhibiting the
NF-κB pathway in LN mice.
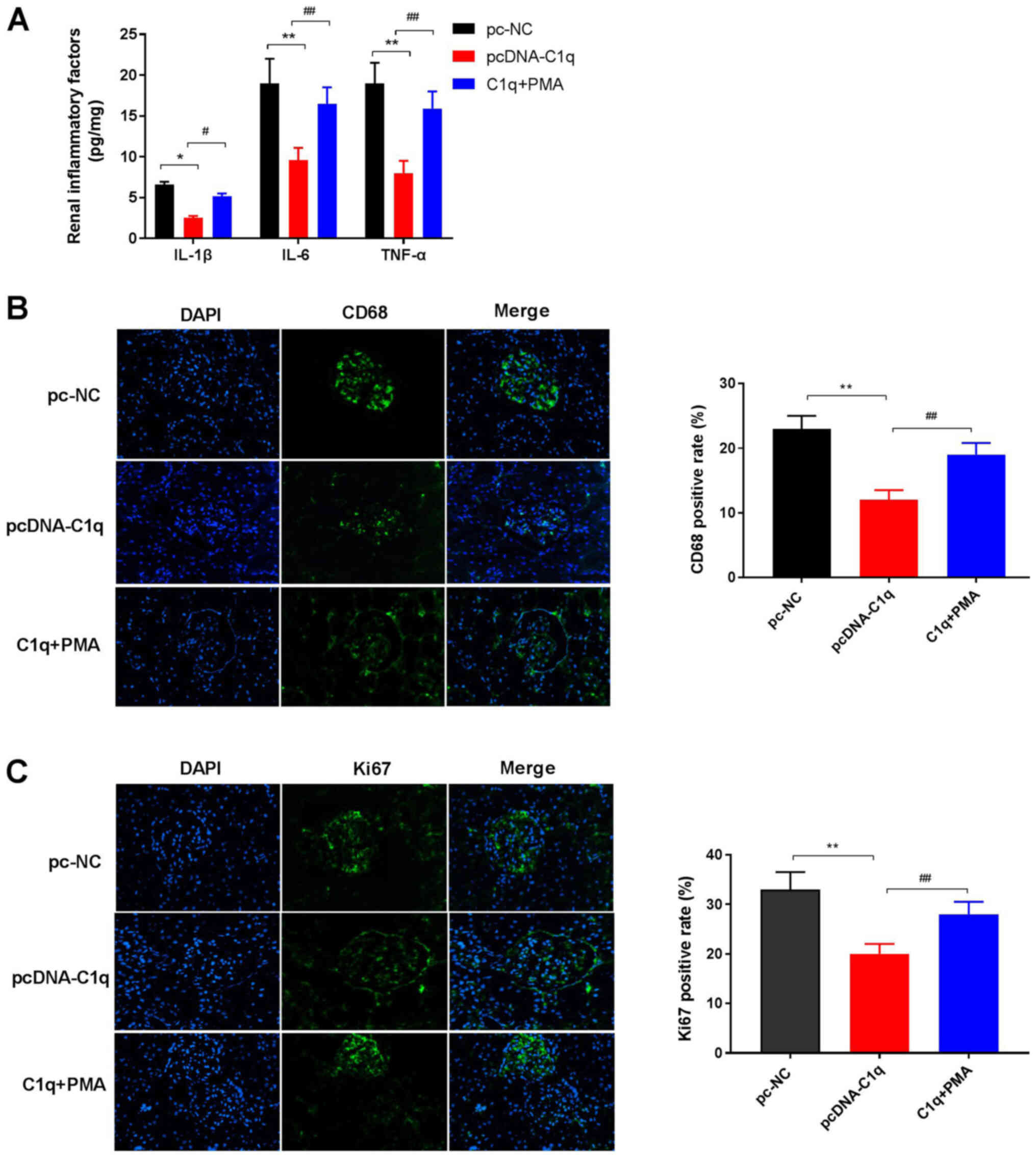 | Figure 5.Overexpression of C1q ameliorates
renal injury in LN mice via inhibiting the NF-κB pathway. (A)
Levels of TNF-α, IL-6 and IL-1β in renal tissues were measured
using ELISA. (B) CD68- (C) Ki67-positivity were detected using
immunofluorescence staining (magnification, ×400). There were 15
mice in each group and the experiments were repeated three times.
*P<0.05, **P<0.01 vs. pc-NC; #P<0.05,
##P<0.01 vs. pcDNA-C1q. C1q, complement component 1q;
LN, lupus nephritis; IL, interleukin; PMA, phorbol 12-myristate
13-acetate; pc-NC, LN mice injected intraperitoneally with pc-NC;
pcDNA-C1q, LN mice injected intraperitoneally with pcDNA-C1q; C1q +
PMA, LN mice were injected intraperitoneally with pcDNA-C1q and
treated with PMA. |
Discussion
The MRL/lpr mouse model is frequently recognized as
a suitable model of human LN (26,27).
In the present study, C1q expression was decreased in LN mice. The
24-h urine protein and BUN levels are reliable measures of renal
function in patients with LN (28,29),
and, in the present study, these levels were decreased by
C1q-overexpression in LN mice. Anti-C1q and anti-dsDNA are valuable
biological markers for the prediction of human LN (30,31).
It was shown in the present study that transfection of pcDNA-C1q
significantly reduced the levels of serum anti-C1q and anti-dsDNA
in LN mice. In addition, overexpression of C1q also decreased the
histological damage index of glomeruli in LN mice. Taken together,
these results suggested that C1q alleviates renal injury in LN mice
through improving renal function and attenuating histological
damage.
Proinflammatory cytokines play critical roles in the
occurrence and developmental process of LN (32,33).
For example, nucleotide-binding oligomerization domain-containing
protein 2 was found to participate in LN pathogenesis through
promoting the release of proinflammatory cytokines (34). Upregulation of microRNA-146a
alleviated LN in patients via suppressing the gene expression of
TNF-α, IL-1β and IL-6 (35). In
the present study, overexpression of C1q significantly decreased
the levels of proinflammatory cytokines in the renal tissues of LN
mice. CD68-positivity is a macrophage marker in renal diseases
(36). Macrophage infiltration in
renal tissues induced by T cells is related to podocyte injury in
patients with LN (37). Ki67 is a
nuclear protein associated with the cell cycle and is used as a
marker for MC proliferation in glomeruli (38). Normal MC proliferation is involved
in maintaining glomerular function and structure (39). The results of the present study
demonstrated that C1q-overexpression significantly reduced
macrophage infiltration and MC proliferation in the renal tissues
of LN mice. Together, these data indicated that C1q protected
against LN by decreasing inflammation, macrophage infiltration and
MC proliferation in renal tissues.
Previous research found that the NF-κB pathway
participates in LN progression (40,41).
In the present study, the expression of NF-κB-related proteins in
the renal tissues of LN mice was significantly decreased by
treatment with pcDNA-C1q, indicating that C1q inhibited the NF-κB
pathway in these tissues. The NF-κB pathway was found to play a
role in LN inflammation, and was ameliorated by demethylzeylasteral
through attenuating the NF-κB pathway (42). NF-κB-related proteins are also
related to the macrophage infiltration and MC proliferation in
renal diseases. Curcumin alleviated macrophage infiltration of
diabetic nephropathy by inhibiting NF-κB activation (43). Resveratrol reduced renal MC
proliferation in diabetic nephropathy via downregulating the
expression of NF-κB-related proteins (44). In the present study, it was found
that overexpression of C1q decreased inflammation, macrophage
infiltration and MC proliferation through inhibiting the NF-κB
pathway in the renal tissues of LN mice. To further confirm this
result, LN mice were treated with PMA, an NF-κB pathway activator
(45). The results showed that PMA
effectively reversed the inhibitory effect of C1q on inflammation,
macrophage infiltration and MC proliferation in the renal tissues
of LN mice. Taken together, it was demonstrated that C1q may
protect against LN through inhibiting the NF-κB pathway.
The present study has some limitations. Firstly,
only glomerular nephritis in mice was analyzed. Interstitial
nephritis, another type of renal inflammation in LN mice (46), may more comprehensively reflect the
histopathological changes in LN. Secondly, IFN-γ, a key regulator
in renal tissues, was not detected. IFN-γ plays a role in the
perpetuation of local inflammatory processes in the kidney by the
activation of monocytes, macrophages or renal resident cells. The
expression of IFN-γ in renal tissues may better reflect the
inflammatory changes in LN. Finally, the detailed mechanism of
action of C1q in LN remains to be studied. Further in vitro
experiments are needed to identify the regulatory mechanism of C1q
in the development of LN.
In summary, C1q expression was decreased in the
renal tissues of LN mice. Overexpression of C1q alleviated
inflammation and macrophage infiltration and inhibited MC
proliferation in these tissues. The protective effect of C1q on LN
was closely associated with the suppression of the NF-κB pathway.
Therefore, C1q may be a promising therapeutic target for LN.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
JS conceived and designed the study, performed the
data analyses and wrote the manuscript. SG, FN and DL contributed
to the conception of the study. JS, SG, FN and DL performed the
experiments. YZ contributed to analysis and manuscript preparation.
All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Linyi Central Hospital (approval no. 2020013; Linyi,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Davidson A, Aranow C and Mackay M: Lupus
Nephritis: Challenges and progress. Curr Opin Rheumatol.
31:682–688. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cameron JS: Lupus Nephritis. J Am Soc
Nephrol. 10:413–424. 1999.PubMed/NCBI
|
|
3
|
Faurschou M, Dreyer L, Kamper AL,
Starklint H and Jacobsen S: Long-term mortality and renal outcome
in a cohort of 100 patients with lupus nephritis. Arthritis Care
Res (Hoboken). 62:873–880. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parikh SV, Almaani S, Brodsky S and Rovin
BH: Update on lupus nephritis: Core curriculum 2020. Am J Kidney
Dis. 76:265–281. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inoue A, Hasegawa H, Kohno M, Ito MR,
Terada M, Imai T, Yoshie O, Nose M and Fujita S: Antagonist of
fractalkine (CX3CL1) delays the initiation and ameliorates the
progression of lupus nephritis in MRL/lpr mice. Arthritis Rheum.
52:1522–1533. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mok CC, Kwok RC and Yip PS: Effect of
renal disease on the standardized mortality ratio and life
expectancy of patients with systemic lupus erythematosus. Arthritis
Rheum. 65:2154–2160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Almaani S, Meara A and Rovin BH: Update on
lupus nephritis. Clin J Am Soc Nephrol. 12:825–835. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Houssiau FA and Ginzler EM: Current
treatment of lupus nephritis. Lupus. 17:426–430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kishore U and Reid KBM: C1q: Structure,
function, and receptors. Immunopharmacology. 49:159–170. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu G, Pang Y, Liu X and Li QW: Structure,
distribution, classification, and function of C1q protein family: A
review. Yi Chuan. 35:1072–1080. 2013.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang FC, Zhou B and Dong Y: The roles of
complement 1q and anti-C1q autoantibodies in pathogenesis of lupus
nephritis. Zhonghua Yi Xue Za Zhi. 85:955–959. 2005.(In Chinese).
PubMed/NCBI
|
|
12
|
Akhter E, Burlingame RW, Seaman AL, Magder
L and Petri M: Anti-C1q antibodies have higher correlation with
flares of lupus nephritis than other serum markers. Lupus.
20:1267–1274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sinico RA, Radice A, Ikehata M,
Giammarresi G, Corace C, Arrigo G, Bollini B and Li Vecchi M:
Anti-C1q autoantibodies in lupus nephritis: Prevalence and clinical
significance. Ann N Y Acad Sci. 1050:193–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin Y, Wu X, Shan G and Zhang X:
Diagnostic value of serum anti-C1q antibodies in patients with
lupus nephritis: A meta-analysis. Lupus. 21:1088–1097. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mankan AK, Lawless MW, Gray SG, Kelleher D
and McManus R: NF-κB regulation: The nuclear response. J Cell Mol
Med. 13:631–643. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruan Q and Chen YH: Nuclear factor-κB in
immunity and inflammation: The Treg and Th17 connection. Adv Exp
Med Biol. 946:207–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong J, Shi QQ, Zhu MM, Shen J, Wang HH,
Ma D and Miao CH: MFHAS1 is associated with sepsis and stimulates
TLR2/NF-κB signaling pathway following negative regulation. PLoS
One. 10:e01436622015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou Y, Fang L, Jiang L, Wen P, Cao H, He
W, Dai C and Yang J: Uric acid induces renal inflammation via
activating tubular NF-κB signaling pathway. PLoS One. 7:e397382012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu F, Wang Y, Cui W, Yuan H, Sun J, Wu M,
Guo Q, Kong L, Wu H and Miao L: Resveratrol prevention of diabetic
nephropathy is associated with the suppression of renal
inflammation and mesangial cell proliferation: Possible roles of
Akt/NF-B Pathway. Int J Endocrinol. 2014:1–9. 2014. View Article : Google Scholar
|
|
20
|
Jiang X, Zhao X, Luo H and Zhu K:
Therapeutic effect of polysaccharide of large yellow croaker swim
bladder on lupus nephritis of mice. Nutrients. 6:1223–1235. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang T, Tian F, Zheng H, Whitman SA, Lin
Y, Zhang Z, Zhang N and Zhang DD: Nrf2 suppresses lupus nephritis
through inhibition of oxidative injury and the NF-κB-mediated
inflammatory response. Kidney Int. 85:333–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun F, Teng J, Yu P, Li W, Chang J and Xu
H: Involvement of TWEAK and the NF-κB signaling pathway in lupus
nephritis. Exp Ther Med. 15:2611–2619. 2018.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Muraoka M, Hasegawa H, Kohno M, Inoue A,
Miyazaki T, Terada M, Nose M and Yasukawa M: IK cytokine
ameliorates the progression of lupus nephritis in MRL/lpr mice.
Arthritis Rheum. 54:3591–3600. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kong R, Kang OH, Seo YS, Zhou T, Kim SA,
Shin DW and Kwon DY: MAPKs and NF-κB pathway inhibitory effect of
bisdemethoxycurcumin on phorbol 12 myristate 13 acetate and A23187
induced inflammation in human mast cells. Mol Med Rep. 17:630–635.
2018.PubMed/NCBI
|
|
26
|
Wang YY, Li HT, Lu Y, Jia XY, Li YL, Chen
S, Chai JX, Zhang JJ, Liu D and Xie CH: Protective effects of
glycyrrhizic acid against lupus nephritis in MRL/lpr mice. Nan Fang
Yi Ke Da Xue Xue Bao. 37:957–961. 2017.(In Chinese). PubMed/NCBI
|
|
27
|
Liu Z, Xue L, Liu Z, Huang J, Wen J, Hu J,
Bo L and Yang R: Tumor necrosis factor-like weak inducer of
apoptosis accelerates the progression of renal fibrosis in lupus
nephritis by activating SMAD and p38 MAPK in TGF-β1 signaling
pathway. Mediators Inflamm. 2016:1–13. 2016. View Article : Google Scholar
|
|
28
|
Christopher-Stine L, Petri M, Astor BC and
Fine D: Urine protein-to-creatinine ratio is a reliable measure of
proteinuria in lupus nephritis. J Rheumatol. 31:1557–1559.
2004.PubMed/NCBI
|
|
29
|
Wang Q, Sun P, Wang R and Zhao X:
Therapeutic effect of dendrobium candidum on lupus nephritis in
mice. Pharmacogn Mag. 13:129–135. 2017.PubMed/NCBI
|
|
30
|
Fenton K, Fismen S, Hedberg A, Seredkina
N, Fenton C, Mortensen ES and Rekvig OP: Anti-dsDNA antibodies
promote initiation, and acquired loss of renal Dnase1 promotes
progression of lupus nephritis in autoimmune (NZBxNZW)F1 mice. PLoS
One. 4:e84742009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Z, Wang GS, Wang GH and Li XP:
Anti-C1q antibody is a valuable biological marker for prediction of
renal pathological characteristics in lupus nephritis. Clin
Rheumatol. 31:1323–1329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li H and Ding G: Elevated serum
inflammatory cytokines in lupus nephritis patients, in association
with promoted hsa-miR-125a. Clin Lab. 62:631–638. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Q, Sun P, Wang R and Zhao X:
Therapeutic effect of Dendrobium candidum on lupus nephritis
in mice. Pharmacogn Mag. 13:129–135. 2017.PubMed/NCBI
|
|
34
|
Jin O, Hou CC, Li XQ, Zhang X, Qiu M, Lin
D, Fang L, Guo X, Lin Z, Liao Z, et al: THU0274 upregulation of
NOD2 involved in the inflammatory response by activation of MAPK
signaling pathway in lupus nephritis. Annals of the Rheumatic
Diseases. 75:282–286. 2016. View Article : Google Scholar
|
|
35
|
Zheng CZ, Shu YB, Luo YL and Luo J: The
role of miR-146a in modulating TRAF6-induced inflammation during
lupus nephritis. Eur Rev Med Pharmacol Sci. 21:1041–1048.
2017.PubMed/NCBI
|
|
36
|
Saitoh A, Ikoma M, Kamiyama C, Doi K and
Koitabashi Y: Investigation of CD68 positive monocytes/
macrophage(CD68+ Mo/M phi) in urine and infiltrated tissue of
various kidney diseases in children. Nihon Jinzo Gakkai Shi.
44:798–805. 2002.(In Japanese). PubMed/NCBI
|
|
37
|
Ma R, Jiang W, Li Z, Sun Y and Wei Z:
Intrarenal macrophage infiltration induced by T cells is associated
with podocyte injury in lupus nephritis patients. Lupus.
25:1577–1586. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wong CY, Cheong SK, Mok PL and Leong CF:
Differentiation of human mesenchymal stem cells into mesangial
cells in post-glomerular injury murine model. Pathology. 40:52–57.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schlöndorff D and Banas B: The mesangial
cell revisited: No cell is an island. J Am Soc Nephrol.
20:1179–1187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu J, Zhu L, Xie GL, Bao JF and Yu Q:
Let-7 miRNAs modulate the activation of NF-κB by targeting TNFAIP3
and are involved in the pathogenesis of lupus nephritis. PLoS One.
10:e01212562015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang F, Zhang RY and Song L: Beneficial
effect of magnolol on lupus nephritis in MRL/lpr mice by
attenuating the NLRP3 inflammasome and NF-κB signaling pathway: A
mechanistic analysis. Mol Med Rep. 16:4817–4822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Geng C, Li J, Ding F, Wu G, Yang Q, Sun Y,
Zhang Z, Dong T and Tian X: Curcumin suppresses 4-hydroxytamoxifen
resistance in breast cancer cells by targeting SLUG/Hexokinase 2
pathway. Biochem Biophys Res Commun. 473:147–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Soetikno V, Sari FR, Veeraveedu PT,
Thandavarayan RA, Harima M, Sukumaran V, Lakshmanan AP, Suzuki K,
Kawachi H and Watanabe K: Curcumin ameliorates macrophage
infiltration by inhibiting NF-κB activation and proinflammatory
cytokines in streptozotocin induced-diabetic nephropathy. Nutr
Metab (Lond). 8:35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang L, Pang S, Deng B, Qian L, Chen J,
Zou J, Zheng J, Yang L, Zhang C, Chen X, et al: High glucose
induces renal mesangial cell proliferation and fibronectin
expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is
inhibited by resveratrol. Int J Biochem Cell Biol. 44:629–638.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Korashy HM and El-Kadi AOS: The role of
redox-sensitive transcription factors NF-κB and AP-1 in the
modulation of the Cyp1a1 gene by mercury, lead, and copper. Free
Radic Biol Med. 44:795–806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wilson PC, Kashgarian M and Moeckel G:
Interstitial inflammation and interstitial fibrosis and tubular
atrophy predict renal survival in lupus nephritis. Clin Kidney J.
11:207–218. 2018. View Article : Google Scholar : PubMed/NCBI
|