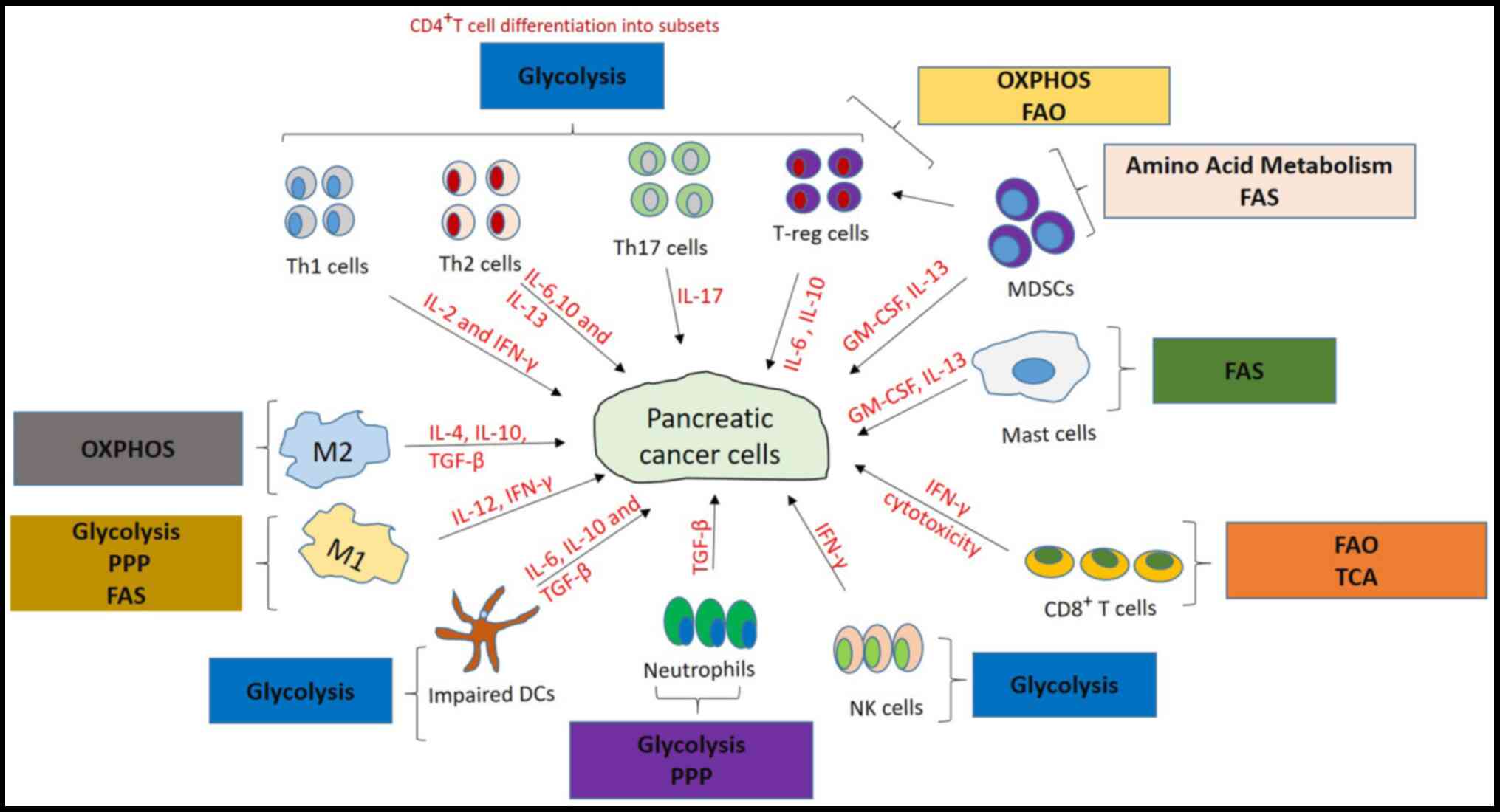
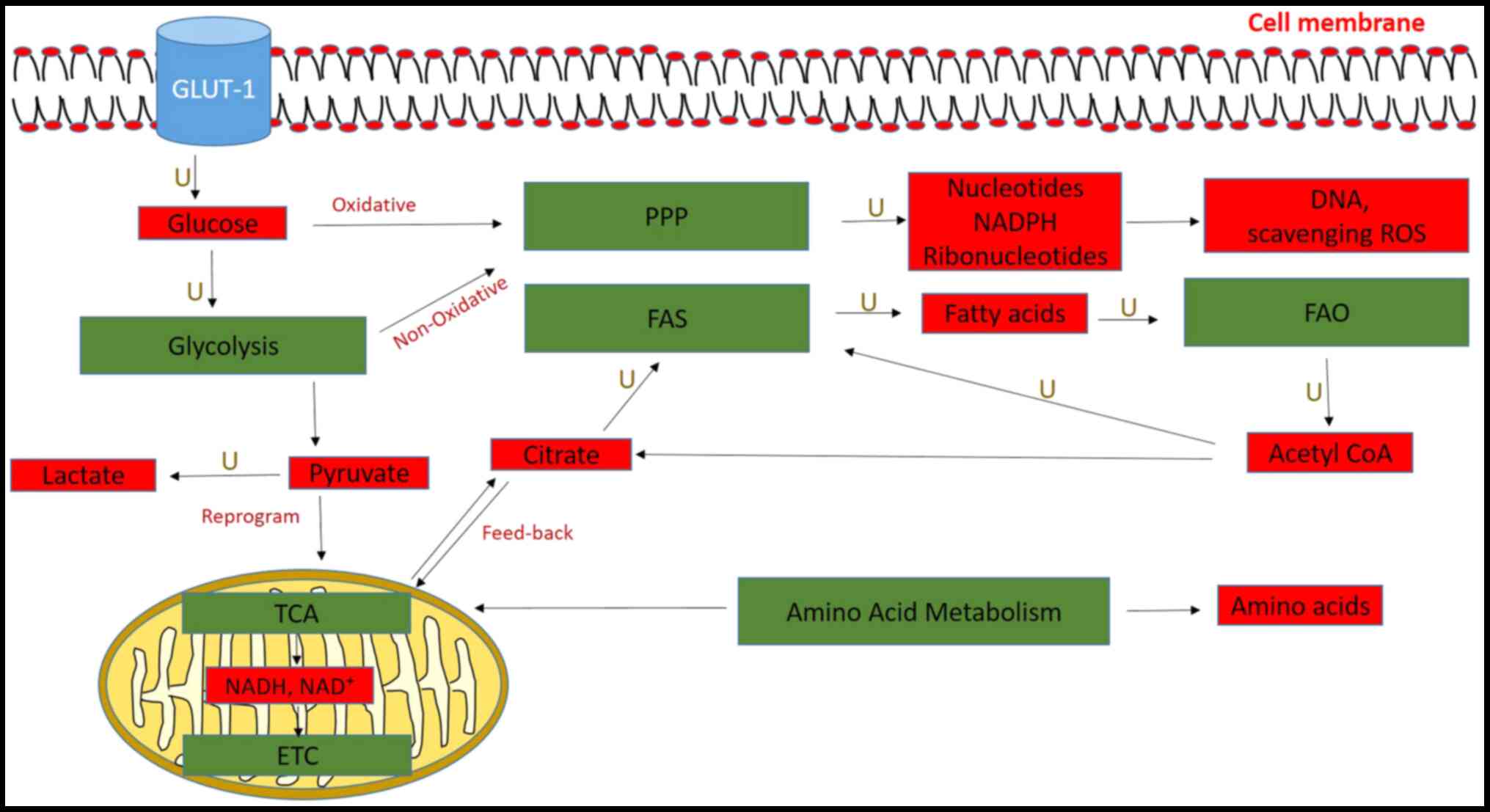
|
1
|
Rawla P, Sunkara T and Gaduputi V:
Epidemiology of pancreatic cancer: Global trends, etiology and risk
factors. World J Oncol. 10:10–27. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:1039–1049. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Polireddy K and Chen Q: Cancer of the
pancreas: Molecular pathways and current advancement in treatment.
J Cancer. 7:1497–1514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yachida S, Jones S, Bozic I, Antal T,
Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al:
Distant metastasis occurs late during the genetic evolution of
pancreatic cancer. Nature. 467:1114–1147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sarantis P, Koustas E, Papadimitropoulou
A, Papavassiliou AG and Karamouzis MV: Pancreatic ductal
adenocarcinoma: Treatment hurdles, tumor microenvironment and
immunotherapy. World J Gastrointest Oncol. 12:173–181. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghesquière B, Wong BW, Kuchnio A and
Carmeliet P: Metabolism of stromal and immune cells in health and
disease. Nature. 511:167–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hato T and Dagher PC: How the innate
immune system senses trouble and causes trouble. Clin J Am Soc
Nephrol. 10:1459–1469. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Inman KS, Francis AA and Murray NR:
Complex role for the immune system in initiation and progression of
pancreatic cancer. World J Gastroenterol. 20:11160–11181. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pearce EL and Pearce EJ: Metabolic
pathways in immune cell activation and quiescence. Immunity.
38:633–643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Odegaard JI and Chawla A: The immune
system as a sensor of the metabolic state. Immunity. 38:644–654.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
von Ahrens D, Bhagat TD, Nagrath D, Maitra
A and Verma A: The role of stromal cancer-associated fibroblasts in
pancreatic cancer. J Hematol Oncol. 10:762017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fukunaga A, Miyamoto M, Cho Y, Murakami S,
Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y,
et al: CD8+ tumor-infiltrating lymphocytes together with
CD4+ tumor-infiltrating lymphocytes and dendritic cells
improve the prognosis of patients with pancreatic adenocarcinoma.
Pancreas. 28:e26–e31. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tjomsland V, Sandström P, Spångeus A,
Messmer D, Emilsson J, Falkmer U, Falkmer S, Magnusson KE, Borch K
and Larsson M: Pancreatic adenocarcinoma exerts systemic effects on
the peripheral blood myeloid and plasmacytoid dendritic cells: An
indicator of disease severity? BMC Cancer. 10:872010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Sanctis F, Solito S, Ugel S, Molon B,
Bronte V and Marigo I: MDSCs in cancer: Conceiving new prognostic
and therapeutic targets. Biochim Biophys Acta. 1865:35–48.
2016.PubMed/NCBI
|
|
15
|
Bayne LJ, Beatty GL, Jhala N, Clark CE,
Rhim AD, Stanger BZ and Vonderheide RH: Tumor-derived
granulocyte-macrophage colony-stimulating factor regulates myeloid
inflammation and T cell immunity in pancreatic cancer. Cancer Cell.
21:822–835. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Padoan A, Plebani M and Basso D:
Inflammation and pancreatic cancer: Focus on metabolism, cytokines,
and immunity. Int J Mol Sci. 20:6762019. View Article : Google Scholar
|
|
17
|
Pergamo M and Miller G: Myeloid-derived
suppressor cells and their role in pancreatic cancer. Cancer Gene
Therapy. 24:100–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gabitass RF, Annels NE, Stocken DD, Pandha
HA and Middleton GW: Elevated myeloid-derived suppressor cells in
pancreatic, esophageal and gastric cancer are an independent
prognostic factor and are associated with significant elevation of
the Th2 cytokine interleukin-13. Cancer Immunol Immunother.
60:1419–1430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dietl K, Renner K, Dettmer K, Timischl B,
Eberhart K, Dorn C, Hellerbrand C, Kastenberger M, Kunz-Schughart
LA, Oefner PJ, et al: Lactic acid and acidification inhibit TNF
secretion and glycolysis of human monocytes. J Immunol.
184:1200–1209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kurahara H, Shinchi H, Mataki Y, Maemura
K, Noma H, Kubo F, Sakoda M, Ueno S, Natsugoe S and Takao S:
Significance of M2-polarized tumor-associated macrophage in
pancreatic cancer. J Surg Res. 167:e211–e219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mielgo A and Schmid MC: Impact of tumour
associated macrophages in pancreatic cancer. BMB Rep. 46:131–138.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Esposito I, Menicagli M, Funel N, Bergmann
F, Boggi U, Mosca F, Bevilacqua G and Campani D: Inflammatory cells
contribute to the generation of an angiogenic phenotype in
pancreatic ductal adenocarcinoma. J Clin Pathol. 57:630–636. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lesina M, Kurkowski MU, Ludes K, Rose-John
S, Treiber M, Klöppel G, Yoshimura A, Reindl W, Sipos B, Akira S,
et al: Stat3/Socs3 activation by IL-6 transsignaling promotes
progression of pancreatic intraepithelial neoplasia and development
of pancreatic cancer. Cancer Cell. 19:456–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JS, Park YS, Kim JY, Kim YG, Kim YJ,
Lee HK, Kim HS, Hong JT, Kim Y and Han SB: Inhibition of human
pancreatic tumor growth by cytokine-induced killer cells in nude
mouse xenograft model. Immune Netw. 12:247–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu L, Zhao G, Wu W, Rong Y, Jin D, Wang
D, Lou W and Qin X: Low intratumoral regulatory T cells and high
peritumoral CD8(+) T cells relate to long-term survival in patients
with pancreatic ductal adenocarcinoma after pancreatectomy. Cancer
Immunol Immunother. 65:73–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kurts C: Th17 cells: A third subset of
CD4+ T effector cells involved in organ-specific
autoimmunity. Nephrol Dial Transplant. 23:816–819. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamamoto K, Venida A, Yano J, Biancur DE,
Kakiuchi M, Gupta S, Sohn ASW, Mukhopadhyay S, Lin EY, Parker SJ,
et al: Autophagy promotes immune evasion of pancreatic cancer by
degrading MHC-I. Nature. 581:100–105. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Herber DL, Cao W, Nefedova Y, Novitskiy
SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B, et
al: Lipid accumulation and dendritic cell dysfunction in cancer.
Nat Med. 16:880–886. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ochi A, Nguyen AH, Bedrosian AS, Mushlin
HM, Zarbakhsh S, Barilla R, Zambirinis CP, Fallon NC, Rehman A,
Pylayeva-Gupta Y, et al: MyD88 inhibition amplifies dendritic cell
capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp
Med. 209:1671–1687. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki
M, Kosuge T, Kanai Y and Hiraoka N: Immune cell infiltration as an
indicator of the immune microenvironment of pancreatic cancer. Br J
Cancer. 108:914–923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hiraoka N, Onozato K, Kosuge T and
Hirohashi S: Prevalence of FOXP3+ regulatory T cells
increases during the progression of pancreatic ductal
adenocarcinoma and its premalignant lesions. Clin Cancer Res.
12:5423–5434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Knutson KL and Disis ML: Tumor
antigen-specific T helper cells in cancer immunity and
immunotherapy. Cancer Immunol Immunother. 54:721–728. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wörmann SM, Diakopoulos KN, Lesina M and
Algül H: The immune network in pancreatic cancer development and
progression. Oncogene. 33:29562013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zou W and Restifo NP: TH17 cells in tumour
immunity and immunotherapy. Nat Rev Immunol. 10:248–256. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gnerlich JL, Mitchem JB, Weir JS, Sankpal
NV, Kashiwagi H, Belt BA, Porembka MR, Herndon JM, Eberlein TJ,
Goedegebuure P and Linehan DC: Induction of Th17 cells in the tumor
microenvironment improves survival in a murine model of pancreatic
cancer. J Immunol. 185:4063–4071. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He Q, Luo X, Huang Y and Sheikh MS:
Apo2L/TRAIL differentially modulates the apoptotic effects of
sulindac and a COX-2 selective non-steroidal anti-inflammatory
agent in Bax-deficient cells. Oncogene. 21:6032–6040. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He S, Fei M, Wu Y, Zheng D, Wan D, Wang L
and Li D: Distribution and clinical significance of Th17 cells in
the tumor microenvironment and peripheral blood of pancreatic
cancer patients. Int J Mol Sci. 12:7424–7437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
De Monte L, Reni M, Tassi E, Clavenna D,
Papa I, Recalde H, Braga M, Carlo VD, Doglioni C and Protti MP:
Intratumor T helper type 2 cell infiltrate correlates with
cancer-associated fibroblast thymic stromal lymphopoietin
production and reduced survival in pancreatic cancer. J Exp Med.
208:469–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bellone G, Turletti A, Artusio E, Mareschi
K, Carbone A, Tibaudi D, Robecchi A, Emanuelli G and Rodeck U:
Tumor-associated transforming growth factor-β and interleukin-10
contribute to a systemic Th2 immune phenotype in pancreatic
carcinoma patients. Am J Pathol. 155:537–547. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Macintyre AN, Gerriets VA, Nichols AG,
Michalek RD, Rudolph MC, Deoliveira D, Anderson SM, Abel ED, Chen
BJ, Hale LP and Rathmell JC: The glucose transporter Glut1 is
selectively essential for CD4 T cell activation and effector
function. Cell Metab. 20:61–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cham CM, Driessens G, O'Keefe JP and
Gajewski TF: Glucose deprivation inhibits multiple key gene
expression events and effector functions in CD8+ T
cells. Eur J Immunol. 38:2438–2450. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sakaguchi S, Miyara M, Costantino CM and
Hafler DA: FOXP3+ regulatory T cells in the human immune
system. Nat Rev Immunol. 10:490–500. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Walker LS and Sansom DM: The emerging role
of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev
Immunol. 11:852–863. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yamamoto T, Yanagimoto H, Satoi S,
Toyokawa H, Hirooka S, Yamaki S, Yui R, Yamao J, Kim S and Kwon AH:
Circulating CD4+CD25+ regulatory T cells in
patients with pancreatic cancer. Pancreas. 41:409–415. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chang DZ, Ma Y, Ji B, Wang H, Deng D, Liu
Y, Abbruzzese JL, Liu YJ, Logsdon CD and Hwu P: Mast cells in tumor
microenvironment promotes the in vivo growth of pancreatic ductal
adenocarcinoma. Clin Cancer Res. 17:7015–7023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ito T, Amakawa R, Inaba M, Ikehara S,
Inaba K and Fukuhara S: Differential regulation of human blood
dendritic cell subsets by IFNs. J Immunol. 166:2961–2969. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Duan X, Deng L, Chen X, Lu Y, Zhang Q,
Zhang K, Hu Y, Zeng J and Sun W: Clinical significance of the
immunostimulatory MHC class I chain-related molecule A and NKG2D
receptor on NK cells in pancreatic cancer. Med Oncol. 28:466–474.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cai SW, Yang SZ, Gao J, Pan K, Chen JY,
Wang YL, Wei LX and Dong JH: Prognostic significance of mast cell
count following curative resection for pancreatic ductal
adenocarcinoma. Surgery. 149:576–584. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Strouch MJ, Cheon EC, Salabat MR, Krantz
SB, Gounaris E, Melstrom LG, Dangi-Garimella S, Wang E, Munshi HG,
Khazaie K and Bentrem DJ: Crosstalk between mast cells and
pancreatic cancer cells contributes to pancreatic tumor
progression. Clin Cancer Res. 16:2257–2265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Feig C, Gopinathan A, Neesse A, Chan DS,
Cook N and Tuveson DA: The pancreas cancer microenvironment. Clin
Cancer Res. 18:4266–4276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Stopa BK, Kusiak AA, Szopa DM, Ferdek EP
and Jakubowska AM: Pancreatic cancer and its
microenvironment-recent advances and current controversies. Int J
Mol Sci. 21:32182020. View Article : Google Scholar
|
|
52
|
Li KY, Yuan JL, Trafton D, Wang JX, Niu N,
Yuan CH, Liu XB and Zheng L: Pancreatic ductal adenocarcinoma
immune microenvironment and immunotherapy prospects. Chronic Dis
Transl Med. 6:6–17. 2020.PubMed/NCBI
|
|
53
|
Li X, Wenes M, Romero P, Huang SC, Fendt
SM and Ho PC: Navigating metabolic pathways to enhance antitumour
immunity and immunotherapy. Nat Rev Clin Oncol. 16:425–441. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yao W, Maitra A and Ying H: Recent
insights into the biology of pancreatic cancer. EBioMedicine.
53:1026552020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
O'Neill LAJ, Kishton RJ and Rathmell J: A
guide to immunometabolism for immunologists. Nat Rev Immunol.
16:553–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Palmer CS, Ostrowski M, Balderson B,
Christian N and Crowe SM: Glucose metabolism regulates T cell
activation, differentiation, and functions. Front Immunol. 6:12015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chung JC, Oh MJ, Choi SH and Bae CD:
Proteomic analysis to identify biomarker proteins in pancreatic
ductal adenocarcinoma. ANZ J Surg. 78:245–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yoon DY, Buchler P, Saarikoski ST, Hines
OJ, Reber HA and Hankinson O: Identification of genes
differentially induced by hypoxia in pancreatic cancer cells.
Biochem Biophys Res Commun. 288:882–886. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Natsuizaka M, Ozasa M, Darmanin S,
Miyamoto M, Kondo S, Kamada S, Shindoh M, Higashino F, Suhara W,
Koide H, et al: Synergistic up-regulation of Hexokinase-2, glucose
transporters and angiogenic factors in pancreatic cancer cells by
glucose deprivation and hypoxia. Exp Cell Res. 313:3337–3348. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cameron ME, Yakovenko A and Trevino JG:
Glucose and lactate transport in pancreatic cancer: Glycolytic
metabolism revisited. J Oncol. 2018:62148382018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Warburg O, Wind F and Negelein E: The
metabolism of tumors in the body. J Gen Physiol. 8:519–530. 1927.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Colegio OR, Chu NQ, Szabo AL, Chu T,
Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC,
Phillips GM, et al: Functional polarization of tumour-associated
macrophages by tumour-derived lactic acid. Nature. 513:559–563.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Choi SYC, Collins CC, Gout PW and Wang Y:
Cancer-generated lactic acid: A regulatory, immunosuppressive
metabolite? J Pathol. 230:350–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mills EL and O'Neill LA: Reprogramming
mitochondrial metabolism in macrophages as an anti-inflammatory
signal. Eur J Immunol. 46:13–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rodríguez-Prados JC, Través PG, Cuenca J,
Rico D, Aragonés J, Martín-Sanz P, Cascante M and Boscá L:
Substrate fate in activated macrophages: A comparison between
innate, classic, and alternative activation. J Immunol.
185:605–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Murray PJ, Allen JE, Biswas SK, Fisher EA,
Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence
T, et al: Macrophage activation and polarization: Nomenclature and
experimental guidelines. Immunity. 41:14–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li Y and Zhu B: Editorial: Metabolism of
cancer cells and immune cells in the tumor microenvironment. Front
Immunol. 9:30802018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Michalek RD, Gerriets VA, Jacobs SR,
Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG and
Rathmell JC: Cutting edge: Distinct glycolytic and lipid oxidative
metabolic programs are essential for effector and regulatory
CD4+ T cell subsets. J Immunol. 186:3299–3303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
MacIver NJ, Michalek RD and Rathmell JC:
Metabolic regulation of T lymphocytes. Annu Rev Immunol.
31:259–283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Waickman AT and Powell JD: mTOR,
metabolism, and the regulation of T-cell differentiation and
function. Immunol Rev. 249:43–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Mao Z and Zhang W: Role of mTOR in glucose
and lipid metabolism. Int J Mol Sci. 19:20432018. View Article : Google Scholar
|
|
73
|
Ersahin T, Tuncbag N and Cetin-Atalay R:
The PI3K/AKT/mTOR interactive pathway. Mol Biosyst. 11:1946–1954.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Doughty CA, Bleiman BF, Wagner DJ, Dufort
FJ, Mataraza JM, Roberts MF and Chiles TC: Antigen
receptor-mediated changes in glucose metabolism in B lymphocytes:
Role of phosphatidylinositol 3-kinase signaling in the glycolytic
control of growth. Blood. 107:4458–4465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Rodríguez-Espinosa O, Rojas-Espinosa O,
Moreno-Altamirano MMB, López-Villegas EO and Sánchez-García FJ:
Metabolic requirements for neutrophil extracellular traps
formation. Immunology. 145:213–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Dallal RM, Christakos P, Lee K, Egawa S,
Son YI and Lotze MT: Paucity of dendritic cells in pancreatic
cancer. Surgery. 131:135–138. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Krawczyk CM, Holowka T, Sun J, Blagih J,
Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG
and Pearce EJ: Toll-like receptor-induced changes in glycolytic
metabolism regulate dendritic cell activation. Blood.
115:4742–4749. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Everts B, Amiel E, Huang SC, Smith AM,
Chang CH, Lam WY, Redmann V, Freitas TC, Blagih J, van der Windt
GJ, et al: TLR-driven early glycolytic reprogramming via the
kinases TBK1-IKKε supports the anabolic demands of dendritic cell
activation. Nat Immunol. 15:323–332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Martínez-Reyes I and Chandel NS:
Mitochondrial TCA cycle metabolites control physiology and disease.
Nat Commun. 11:1022020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Reyes-Castellanos G, Masoud R and Carrier
A: Mitochondrial metabolism in PDAC: From better knowledge to new
targeting strategies. Biomedicines. 8:2702020. View Article : Google Scholar
|
|
82
|
Laurenti G and Tennant DA: Isocitrate
dehydrogenase (IDH), succinate dehydrogenase (SDH), fumarate
hydratase (FH): Three players for one phenotype in cancer? Biochem
Soc Trans. 44:1111–1116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Waitkus MS, Diplas BH and Yan H:
Biological role and therapeutic potential of IDH mutations in
cancer. Cancer Cell. 34:186–195. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Nogueira V and Hay N: Molecular pathways:
Reactive oxygen species homeostasis in cancer cells and
implications for cancer therapy. Clin Cancer Res. 19:4309–4314.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Schlichtholz B, Turyn J, Goyke E,
Biernacki M, Jaskiewicz K, Sledzinski Z and Swierczynski J:
Enhanced citrate synthase activity in human pancreatic cancer.
Pancreas. 30:99–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Swierczynski J, Hebanowska A and
Sledzinski T: Role of abnormal lipid metabolism in development,
progression, diagnosis and therapy of pancreatic cancer. World J
Gastroenterol. 20:2279–2303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Halabe Bucay A: Hypothesis proved...citric
acid (citrate) does improve cancer: A case of a patient suffering
from medullary thyroid cancer. Med Hypotheses. 73:2712009.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Amedei A, Niccolai E and Prisco D:
Pancreatic cancer: Role of the immune system in cancer progression
and vaccine-based immunotherapy. Hum Vaccin Immunother.
10:3354–3368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Patra KC and Hay N: The pentose phosphate
pathway and cancer. Trends Biochem Sci. 39:347–354. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu H, Huang D, McArthur DL, Boros LG,
Nissen N and Heaney AP: Fructose induces transketolase flux to
promote pancreatic cancer growth. Cancer Res. 70:6368–6376. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Shukla SK, Purohit V, Mehla K, Gunda V,
Chaika NV, Vernucci E, King RJ, Abrego J, Goode GD, Dasgupta A, et
al: MUC1 and HIF-1alpha signaling crosstalk induces anabolic
glucose metabolism to impart gemcitabine resistance to pancreatic
cancer. Cancer Cell. 32:71–87.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Haschemi A, Kosma P, Gille L, Evans CR,
Burant CF, Starkl P, Knapp B, Haas R, Schmid JA, Jandl C, et al:
The sedoheptulose kinase CARKL directs macrophage polarization
through control of glucose metabolism. Cell Metab. 15:813–826.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Palsson-McDermott EM, Curtis AM, Goel G,
Lauterbach MA, Sheedy FJ, Gleeson LE, van den Bosch MW, Quinn SR,
Domingo-Fernandez R, Johnston DG, et al: Pyruvate kinase M2
regulates Hif-1α activity and IL-1β induction and is a critical
determinant of the warburg effect in LPS-activated macrophages.
Cell Metab. 21:65–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Fernandez-Zapico M, Kim DW, Philip P,
Vandell A, Eckard J, Korn R, Del Priore G and Simeone D: Abstract
B15: Therapeutic potential of targeting amino acid metabolism in
pancreatic cancer. Cancer Res. 79:B152019.
|
|
95
|
Altan B, Kaira K, Watanabe A, Kubo N, Bao
P, Dolgormaa G, Bilguun EO, Araki K, Kanai Y, Yokobori T, et al:
Relationship between LAT1 expression and resistance to chemotherapy
in pancreatic ductal adenocarcinoma. Cancer Chemother Pharmacol.
81:141–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Commisso C, Davidson SM, Soydaner-Azeloglu
RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin
JA, Thompson CB, et al: Macropinocytosis of protein is an amino
acid supply route in Ras-transformed cells. Nature. 497:633–637.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ananieva EA and Wilkinson AC:
Branched-chain amino acid metabolism in cancer. Curr Opin Clin Nutr
Metab Care. 21:64–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhao H, Yang L, Baddour J, Achreja A,
Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA,
et al: Tumor microenvironment derived exosomes pleiotropically
modulate cancer cell metabolism. Elife. 5:e102502016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kamphorst JJ, Nofal M, Commisso C, Hackett
SR, Lu W, Grabocka E, Vander Heiden MG, Miller G, Drebin JA,
Bar-Sagi D, et al: Human pancreatic cancer tumors are nutrient poor
and tumor cells actively scavenge extracellular protein. Cancer
Res. 75:544–553. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Son J, Lyssiotis CA, Ying H, Wang X, Hua
S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, et
al: Glutamine supports pancreatic cancer growth through a
KRAS-regulated metabolic pathway. Nature. 496:101–105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Singer K, Cheng WC, Kreutz M, Ho PC and
Siska PJ: Immunometabolism in cancer at a glance. Dis Models Mech.
11:dmm0342722018. View Article : Google Scholar
|
|
102
|
Sinclair LV, Rolf J, Emslie E, Shi Y-B,
Taylor PM and Cantrell DA: Control of amino-acid transport by
antigen receptors coordinates the metabolic reprogramming essential
for T cell differentiation. Nat Immunol. 14:500–508. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Pilotte L, Larrieu P, Stroobant V, Colau
D, Dolusic E, Frédérick R, De Plaen E, Uyttenhove C, Wouters J,
Masereel B and Van den Eynde BJ: Reversal of tumoral immune
resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl
Acad Sci USA. 109:24972012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Cluntun AA, Lukey MJ, Cerione RA and
Locasale JW: Glutamine metabolism in cancer: Understanding the
heterogeneity. Trends Cancer. 3:169–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Nzeako UC and Gores GJ: Increased
expression of cyclooxygenase-2 in human pancreatic neoplasms and
potential for chemoprevention by cyclooxygenase inhibitors. Cancer.
94:1903–1904. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Asano T, Shoda J, Ueda T, Kawamoto T,
Todoroki T, Shimonishi M, Tanabe T, Sugimoto Y, Ichikawa A, Mutoh
M, et al: Expressions of cyclooxygenase-2 and prostaglandin
E-receptors in carcinoma of the gallbladder: Crucial role of
arachidonate metabolism in tumor growth and progression. Clin
Cancer Res. 8:1157–1167. 2002.PubMed/NCBI
|
|
107
|
Molina MA, Sitja-Arnau M, Lemoine MG,
Frazier ML and Sinicrope FA: Increased cyclooxygenase-2 expression
in human pancreatic carcinomas and cell lines. Cancer Res.
59:4356–4362. 1999.PubMed/NCBI
|
|
108
|
DuBois RN, Awad J, Morrow J, Roberts LJ II
and Bishop PR: Regulation of eicosanoid production and mitogenesis
in rat intestinal epithelial cells by transforming growth
factor-alpha and phorbol ester. J Clin Invest. 93:493–498. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Sato T, Nakajima H, Fujio K and Mori Y:
Enhancement of prostaglandin E2 production by epidermal growth
factor requires the coordinate activation of cytosolic
phospholipase A2 and cyclooxygenase 2 in human squamous carcinoma
A431 cells. Prostaglandins. 53:355–369. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
O'Sullivan D, van der Windt GJW, Huang SC,
Curtis JD, Chang CH, Buck MD, Qiu J, Smith AM, Lam WY, DiPlato LM,
et al: Memory CD8(+) T cells use cell-intrinsic lipolysis to
support the metabolic programming necessary for development.
Immunity. 41:75–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zhang A, Sun H, Wang P, Han Y and Wang X:
Modern analytical techniques in metabolomics analysis. Analyst.
137:293–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Hatzivassiliou G, Zhao F, Bauer DE,
Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA and
Thompson CB: ATP citrate lyase inhibition can suppress tumor cell
growth. Cancer Cell. 8:311–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Tadros S, Shukla SK, King RJ, Gunda V,
Vernucci E, Abrego J, Chaika NV, Yu F, Lazenby AJ, Berim L, et al:
De novo lipid synthesis facilitates gemcitabine resistance through
endoplasmic reticulum stress in pancreatic cancer. Cancer Res.
77:5503–5517. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Walter K, Hong SM, Nyhan S, Canto M,
Fedarko N, Klein A, Griffith M, Omura N, Medghalchi S, Kuhajda F
and Goggins M: Serum fatty acid synthase as a marker of pancreatic
neoplasia. Cancer Epidemiol Biomarkers Prev. 18:2380–2385. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Coleman RA, Lewin TM, Van Horn CG and
Gonzalez-Baró MR: Do long-chain acyl-CoA synthetases regulate fatty
acid entry into synthetic versus degradative pathways? J Nutr.
132:2123–2126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Macášek J, Vecka M, Žák A, Urbánek M,
Krechler T, Petruželka L, Staňková B and Zeman M: Plasma fatty acid
composition in patients with pancreatic cancer: Correlations to
clinical parameters. Nutr Cancer. 64:946–955. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chung YT, Matkowskyj KA, Li H, Bai H,
Zhang W, Tsao MS, Liao J and Yang GY: Overexpression and oncogenic
function of aldo-keto reductase family 1B10 (AKR1B10) in pancreatic
carcinoma. Mod Pathol. 25:758–766. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Guillaumond F, Bidaut G, Ouaissi M,
Servais S, Gouirand V, Olivares O, Lac S, Borge L, Roques J, Gayet
O, et al: Cholesterol uptake disruption, in association with
chemotherapy, is a promising combined metabolic therapy for
pancreatic adenocarcinoma. Proc Natl Acad Sci USA. 112:2473–2478.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Alistar AT, Morris B, Harrison L,
Bickenbach K, Starker L, Ginder N, McIlwain L, Luther S, Pardee TS
and Alpert J: A single-arm, open-label, phase I study of CPI-613
(Devimistat) in combination with gemcitabine and nab-paclitaxel for
patients with locally advanced or metastatic pancreatic
adenocarcinoma. J Clin Oncol. 38:4635. 2020. View Article : Google Scholar
|
|
120
|
Philip PA, Buyse ME, Alistar AT, Rocha
Lima CMSP, Luther S, Pardee TS and Van Cutsem E: Avenger 500, a
phase III open-label randomized trial of the combination of CPI-613
with modified FOLFIRINOX (mFFX) versus FOLFIRINOX (FFX) in patients
with metastatic adenocarcinoma of the pancreas. J Clin Oncol.
37:TPS4792019. View Article : Google Scholar
|
|
121
|
O'Donnell JS, Massi D, Teng MWL and
Mandala M: PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux.
Semin Cancer Biol. 48:91–103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Jin J and Zhao Q: Emerging role of mTOR in
tumor immune contexture: Impact on chemokine-related immune cells
migration. Theranostics. 10:6231–6244. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Allard B, Longhi MS, Robson SC and Stagg
J: The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor
targets. Immunol Rev. 276:121–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Arina A and Bronte V: Myeloid-derived
suppressor cell impact on endogenous and adoptively transferred T
cells. Curr Opin Immunol. 33:120–125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Brand A, Singer K, Koehl GE, Kolitzus M,
Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et
al: LDHA-associated lactic acid production blunts tumor
immunosurveillance by T and NK cells. Cell Metab. 24:657–671. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Deaglio S, Dwyer KM, Gao W, Friedman D,
Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al:
Adenosine generation catalyzed by CD39 and CD73 expressed on
regulatory T cells mediates immune suppression. J Exp Med.
204:1257–1265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Hiraoka N, Toue S, Okamoto C, Kikuchi S,
Ino Y, Yamazaki-Itoh R, Esaki M, Nara S, Kishi Y, Imaizumi A, et
al: Tissue amino acid profiles are characteristic of tumor type,
malignant phenotype, and tumor progression in pancreatic tumors.
Sci Rep. 9:98162019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Hossain F, Al-Khami AA, Wyczechowska D,
Hernandez C, Zheng L, Reiss K, Valle LD, Trillo-Tinoco J, Maj T,
Zou W, et al: Inhibition of fatty acid oxidation modulates
immunosuppressive functions of myeloid-derived suppressor cells and
enhances cancer therapies. Cancer Immunol Res. 3:1236–1247. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kalinski P: Regulation of immune responses
by prostaglandin E2. J Immunol. 188:21–28. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Korangath P, Teo WW, Sadik H, Han L, Mori
N, Huijts CM, Wildes F, Bharti S, Zhang Z, Santa-Maria CA, et al:
Targeting glutamine metabolism in breast cancer with
aminooxyacetate. Clin Cancer Res. 21:3263–3273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Leone RD and Emens LA: Targeting adenosine
for cancer immunotherapy. J Immunother Cancer. 6:572018. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Li M, Tan SY and Wang XF: Paeonol exerts
an anticancer effect on human colorectal cancer cells through
inhibition of PGE2 synthesis and COX-2 expression. Oncol
Rep. 32:2845–2853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Liu WR, Tian MX, Yang LX, Lin YL, Jin L,
Ding ZB, Shen YH, Peng YF, Gao DM, Zhou J, et al: PKM2 promotes
metastasis by recruiting myeloid-derived suppressor cells and
indicates poor prognosis for hepatocellular carcinoma. Oncotarget.
6:846–861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Mohammad GH, Olde Damink SW, Malago M,
Dhar DK and Pereira SP: Pyruvate kinase M2 and lactate
dehydrogenase A are overexpressed in pancreatic cancer and
correlate with poor outcome. PLoS One. 11:e01516352016. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Patsoukis N, Bardhan K, Chatterjee P, Sari
D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P, et al:
PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis
and promoting lipolysis and fatty acid oxidation. Nat Commun.
6:66922015. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Yu CP, Fu SF, Chen X, Ye J, Ye Y, Kong LD
and Zhu Z: The clinicopathological and prognostic significance of
IDO1 expression in human solid tumors: evidence from a systematic
review and meta-analysis. Cell Physiol Biochem. 49:134–143. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Biswas SK: Metabolic reprogramming of
immune cells in cancer progression. Immunity. 43:435–449. 2015.
View Article : Google Scholar : PubMed/NCBI
|