Pancreatic cancer is the 7th most common cause of
cancer-related deaths in developed countries and the 3rd most
common in the USA, with >250,000 deaths worldwide annually
(1). Pancreatic ductal
adenocarcinoma (PDAC) is the most common type of pancreatic
neoplasm and accounts for >85% of pancreatic cancer cases
globally (2). It originates in the
head region of the pancreas and exhibits a glandular pattern,
structurally similar to that of the ductal epithelial cells
(3). Despite the high mortality
rate in pancreatic cancer, the disease shows no early warning
signs; therefore, pancreatic tumorigenesis and progression may
remain undetected in a process that takes up to 20 years (4). The lack of early diagnostic markers
has led to the delayed detection and late presentation of
pancreatic cancer, which is usually at a locally advanced or
metastatic stage at the time of diagnosis, thus making the disease
fatal (5).
The immune system, which comprises the innate and
the adaptive immune system, protects the host from foreign
pathogens, including cancer cells (6). The innate immune system includes
antigen-presenting cells, which phagocytose invading pathogens and
present antigenic determinants with the major histocompatibility
complex proteins (MHC)-II to CD4+ T cells. Granulocytes,
mast cells, dendritic cells (DCs), macrophages and natural killers
(NK) cells are also innate immune cells (7). The adaptive immune system is
regulated and comprised mainly of B and T cells, which are usually
activated when the innate immune system cannot eliminate the
pathogens to provide long-lasting immunity (8).
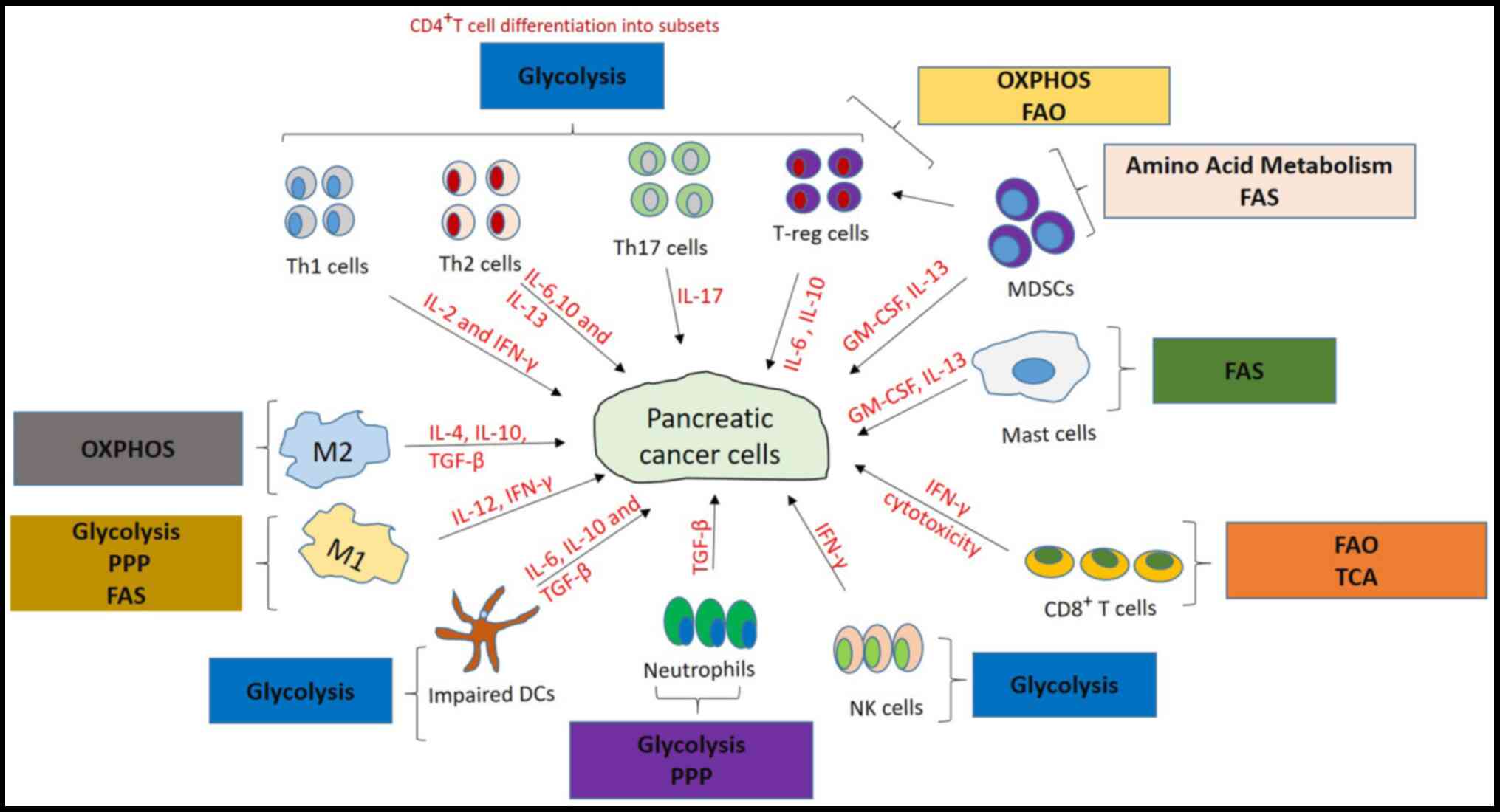
The dysfunctional immune system in pancreatic cancer
has been discovered to promote tumor growth. The pancreatic cancer
microenvironment was identified to serve a vital role in tumor
growth and the therapeutic response (8). Pancreatic cancer cells are rich in
stroma, which comprises both cellular and acellular components,
including the extracellular matrix, fibroblasts, myofibroblasts,
growth factors, cytokines, pancreatic stellate cells and immune
cells (11). DCs, NK cells,
CD8+ and CD4+ T cells are some of the immune
cells discovered to be activated to inhibit tumor growth and
progression in PDAC (12,13). Regulatory T cells (T-regs), tumor
associated macrophages (TAM), myeloid-derived suppressor cells
(MDSCs) and tumor-associated neutrophils have also been reported to
promote tumor growth and progression, and also to suppress
antitumoral responses (8,11).
MDSCs suppress immunity by inhibiting T cell
activation via the sequestration of cysteine, thereby reducing the
availability of the amino acids tryptophan and arginine, which are
required for protein translation by T cells and reactive oxygen
species (ROS) production (14). A
previous study illustrated that the growth factor
granulocyte-macrophage colony-stimulating factor, secreted by
pancreatic tumors, promoted the early recruitment of MDSCs
(15). MDSCs inhibit the immune
functions of effector T (T-eff) cells and NK cells, while the
function of T-regs is promoted (16,17).
Elevated MDSCs levels in pancreatic cancer were discovered to be a
poor prognostic factor associated with elevated T-regs and Th2
cytokines levels (18). Using
peripheral blood mononuclear cells harvested from blood samples
collected from 131 patients with cancer, flow cytometric analysis
revealed that both MDSC and T-reg levels were significantly
elevated in patients with pancreatic cancer (18).
Macrophages switch their differentiation from M1
(proinflammatory) to M2 (anti-inflammatory) phenotypes in the
presence of stimuli, such as the cytokines IL-10, IL-4, and TGF-β,
which are secreted from the PDAC microenvironment (19,20).
TAMs are macrophages initially recruited to the site of tumor
formation in response to the chemotactic factors released by
pancreatic cancer cells, and they promote tumor progression by
suppressing antitumor immune responses and stimulating the
vascularization and metastasis of cancer cells (21). This has been demonstrated using
immunohistochemistry to identify inflammatory cells by evaluating
the expression of proangiogenic and prolymphangiogenic molecules,
VEGFA and VEGFC, produced by macrophages in pancreatic cancer
(22). Furthermore, TAMs were
discovered to regulate pancreatic intraepithelial neoplasia
progression by being the main source of IL-6 in
KrasG12D-mutations, thereby promoting cancer development
(23). Elevated levels of TAMs and
M2 polarized TAMs were also identified to be associated with a
worse prognosis in pancreatic cancer by promoting lymphatic
metastasis by lymphangiogenesis (20).
The role of Th17 cells in pancreatic cancer is not
completely understood because it depends on the cancer type, tumor
stage and location (34). Previous
studies have revealed that an increased level of Th17 cells in
murine pancreatic cancer inhibited tumorigenesis, leading to
improved survival (35), while in
another study, elevated levels of Th17 cells in human pancreatic
cancer promoted cancer progression and were associated with a poor
survival (36,37). Th2 cells exhibit a tumor-promoting
function in pancreatic cancer, and this was suggested to be the
result of the upregulated levels of Th2 cytokines, such as IL-13,
IL-10 and IL-6, found in the plasma of patients with pancreatic
cancer (38,39). T-regs depend on oxidative
phosphorylation and fatty acid oxidation (FAO) for ATP upon
activation (40); this permits
T-regs to survive under tumor conditions in contrast to T-eff
cells, which are impaired due to insufficient glucose production
(41). T-reg suppress immune
responses via the secretion of IL-10 and TGF-β to produce an
immunosuppressive environment (42) and, in pancreatic cancer, they were
discovered to be involved in the early infiltration of preinvasive
lesions, promoting tumor growth and progression (31).
Cytotoxic lymphocyte-associated antigen-4 is a
receptor found on T-regs that produces inhibitory signals upon
interaction with its ligands, CD80 and CD86, on antigen-presenting
cells, thereby inhibiting the formation of the immune synapse
between CD4+ T cells and the antigen-presenting cells,
which normally promotes the release of cytokines for cancer cell
destruction (43). In patients
with pancreatic cancer, a large number of T-regs in the circulation
was associated with the advancement of the disease (44). The chances of successful surgical
resection and survival rate post-resection were also discovered to
be associated with the decreased levels of T-regs in patients with
pancreatic cancer (44).
Additionally, increased numbers of mast cells were identified to be
associated with metastasis and reduced survival in human pancreatic
cancer (45).
DCs control immune responses by regulating the
polarization of T cells into Th1, Th2 or Th3 subtypes depending on
the stimulation by certain cytokines (46). The pancreatic cancer
microenvironment releases tumor-derived factors, such as IL-6 and
VEGF, which promote DC impairment by reprogramming the immune cell
response from a Th1 type to a Th2 type, thereby promoting cancer
development (28,29). One previous study reported that the
elevated levels of DCs and NK cells in PDAC were associated with a
prolonged or improved survival rate (44).
The immune system uses NK cells to target cancer
cells, which inhibits their growth, and a decreased level of NK
cells was suggested to be associated with the advancement of
pancreatic cancer. These findings were observed by determining the
serum levels of soluble MHC class 1 chain-related molecule A
(sMICA) and NK group 2-member D (NKG2D) in the NK cells
of patients with pancreatic cancer using immunohistochemistry.
Elevated levels of sMICA were found in patients with advanced
pancreatic cancer and were correlated with the downregulation of
NKG2D expression, implying decreased levels of NK cells
(47). Mast cells are commonly
known for their role in allergies; however, in PDAC, elevated
levels of mast cells were also discovered to be associated with
tumor progression (48,49).
The excessive growth of the extracellular matrix in
pancreatic cancer as a result of the dense stroma was discovered to
lead to the formation of barriers against the immune system, drug
delivery, oxygen and nutrients (50). Hence, the cells develop mechanisms
that alter the typical metabolic pathways to supply nutrients to
survive (51). Immunotherapy
promotes antitumor activities by reprogramming and enhancing the
immune response (34,52), and the function and differentiation
of immune cells are greatly influenced by metabolism, hence a
combination of both could be a more effective treatment option
(53). Metabolic pathways can
either promote or inhibit immune cell functions, which could be
essential in further understanding the immune response and in
identifying novel therapeutic options to treat immune-dysfunction
in numerous types of disease, including cancer (Figs. 1 and 2).
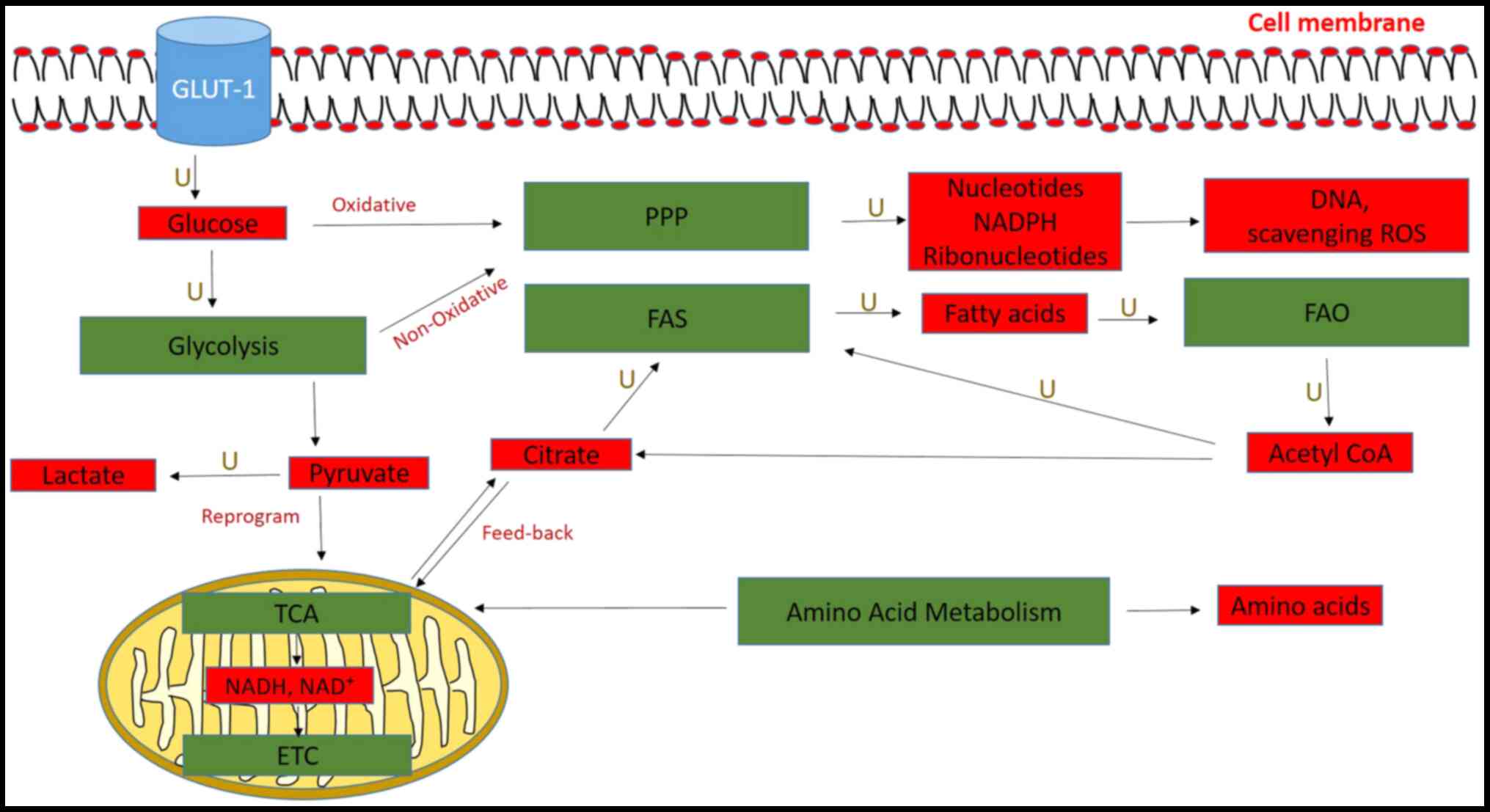
Glucose uptake and glycolysis are activated in
pancreatic cancer cells, and their intermediates are fed into other
biosynthetic pathways, such as the pentose phosphate pathway (PPP)
(54). Glycolysis involves a
series of enzymatic steps, whereby glucose is metabolized to
pyruvate and then finally to lactate to yield ATP and other
substrates for other metabolic pathways (55,56).
Glycolytic enzymes, such as hexokinase, enolase and
phosphoglycerate kinase, among others, were found to be
overexpressed in pancreatic cancer, promoting tumor growth and
metastasis (57,58). The expression of hypoxia-inducible
genes in pancreatic cancer cell lines (MiaPaca-2 and Pcl-43) under
different conditions was investigated, and hexokinase was
identified to be upregulated (59). Glycolysis in pancreatic cancer was
revealed to promote lactate production, tumor growth and protein
glycosylation (53,55,60).
Although glycolysis is less energy efficient compared with the
tricarboxylic acid (TCA) cycle, it is preferred by cancer cells as
it produces ATP faster, occurs independently of mitochondrial
function and conserves nutrients for lipids, amino acids and
nucleic acid biosynthesis (55).
This phenomenon is known as the Warburg effect (61,62)
and, in PDAC, leads to increased lactate production, which alters
the tumor stroma interface, thereby increasing invasiveness
(63). Elevated lactic acid levels
were identified to lead to a decreased pH in the tumor
microenvironment, which inhibited cytotoxic T cell function and
promoted tumor growth and progression (64).
M1 macrophages are characterized by enhanced
glycolysis, while M2 macrophages exhibit decreased levels of
glycolysis (65). M1-polarized
macrophages are highly glycolytic due to the increased stimulation
of the fructose-2,6-biphosphatase enzyme (66), which produces nitric oxide and
TNF-α (67), and exhibit IL-12 and
IL-23 phenotypes, while M2-polarised macrophages exhibit an IL-10
phenotype (19).
T cells require large amounts of glucose and
glutamine catabolism for nucleotide and lipid synthesis, which are
essential for cell growth. However, in their resting state (naïve
state), they require small amounts of glucose, amino acids and
fatty acids for the sustenance and maintenance of energy (68). Glycolysis is necessary for
differentiating CD4+ T cells into its effector subsets,
as well as maintaining a proper balance between protective and
suppressive immunity (69).
Glycolysis is essential for T-eff cell activation and function,
because T-eff cells require high metabolic flux (70). T-eff cells are activated by the
mTOR signaling pathway and hypoxia-inducible factor-α transcription
factors, which promote glycolysis and amino acid metabolism, but
uses FAO for ATP production (71).
The mTOR signaling pathway is highly involved in metabolism,
altering the expression of key pathways such as glycolysis
(72,73).
T-eff subsets, such as Th17, Th1 and Th2, require
elevated levels of glycolysis following activation (69). Macintyre et al (40) demonstrated that glucose transporter
(GLUT)-1 was essential for CD4+ T cell activation and
effector function by examining the GLUT transporter family to
determine their roles in glucose uptake and metabolism in T cells.
The study also revealed that the levels of T-eff cells were
elevated in GLUT-1 transgenic mice, which depend solely on glucose
metabolism (69). Increased levels
of glycolysis were also found to be required in order for activated
B cells to contribute to the immune response (74). In addition, activated neutrophils
were identified to depend on glucose for ATP production via
glycolysis (75).
DCs are usually found in tissues that are in contact
with external environment systems (76). They process and present antigens on
the cell surfaces for T cells to respond to (77). In addition, DCs regulate the immune
response by regulating the polarization of T cells to Th1, Th2 or
Th3 subtypes following the stimulation by cytokines (46). Enhanced glycolysis occurs in DCs,
which enables them to generate sufficient ATP and intermediates to
perform the immune system functions. Krawczyk et al
(78) demonstrated that DCs
undergo maturation by Toll-like receptor signaling, and this
occurred by the metabolic conversion from oxidative phosphorylation
to aerobic glycolysis following the upregulation of fatty acid
synthesis (FAS). The rapid induction of glycolysis was also
discovered to be essential for the activation and function of DCs
(79).
The TCA cycle is a series of reactions that occur in
the matrix of the mitochondria and involves the oxidation of Acetyl
CoA to generate NADH and FADH2, which is then converted
to ATP via the electron transport chain (Fig. 2) (80). The TCA cycle was discovered to be
dysregulated in PDAC, in which increased levels of pyruvate from
glycolysis were reduced to lactate and fed the TCA cycle to
generate citrate for FAS (81).
Metabolites such as fumarate, succinate and D2-hydroxyglutarate
were reportedly upregulated in cancer cells as a result of the
dysfunction of the enzymes, fumarate dehydrogenase, succinate
dehydrogenase and isocitrate dehydrogenase (82). Elevated levels of these metabolites
have been shown to increase ROS levels which, in turn, activated
signaling pathways, such as P13K/AKT/mTOR, which promote
carcinogenesis (83,84). Macrophages are proinflammatory when
there is a shift towards glycolysis and FAS, promoting the
production of IL-β and TGF-β. Conversely, macrophages are polarized
towards the anti-inflammatory state when there is a shift towards
the Krebs cycle and FAO (66).
Increased citrate synthase activity was observed in PDAC upon
measuring the activity in the tissues of patients with pancreatic
cancer (85); citrate synthase
catalyzes the reaction between Acetyl CoA and oxaloacetate to
produce citrate, which is a substrate for membrane lipid synthesis
(86). Although pancreatic cancer
has been associated with elevated citrate synthase levels,
increased citrate production inhibits phosphofructokinase (PFK)2
(87). PFK2 is a promoter of PFK1,
an enzyme that catalyzes the conversion of fructose-6-phosphate to
fructose-1,6-biphosphate in the presence of ATP, thereby
controlling glycolysis in cancer cells. M2 macrophages, which are
observed in PDAC, utilize oxidative phosphorylation to support
their metabolic demands and have an uninterrupted Krebs cycle
(55,88).
The PPP consists of two phases, oxidative and
non-oxidative, both of which were revealed to be upregulated in
pancreatic cancer (86). The major
products of the oxidative phase of PPP are nucleotides and NADPH,
while the non-oxidative phase generates ribonucleotides for DNA
synthesis, which is mediated by transketolase and transaldolase
enzymes (89). The mRNA expression
levels of transketolase were reported to be upregulated in the
pancreatic cancer cell lines, Panc-1, MiaPaca-2 and CaPan-1
(90). In addition, a previous
study revealed that the activation of the non-oxidative phase of
the PPP in pancreatic cancer promoted resistance to gemcitabine
treatment (91).
Macrophages are polarized towards an M2 phenotype
when the PPP is inhibited, thus indicating the importance of the
PPP in the pro- and anti-inflammatory response of macrophages, as
shown in Fig. 1. Screening of 199
human kinases for their potential roles in immunoregulation
revealed that the sedoheptulose kinase (SHK) enzyme, which limits
the PPP, served an important role in macrophage polarization
(92). In addition, the results
proved that SHK enzyme downregulation was essential for the M1
reprogramming in macrophages. The PPP was found to be highly
activated in lipopolysaccharide-activated macrophages due to the
induction of the pyruvate kinase isoenzyme M2, which is an enzyme
that diverts glycolytic intermediates to other biosynthetic
pathways (93).
L-type amino acid transporter (LAT-1) transports
large amino acids, such as tryptophan, valine, phenylalanine,
tyrosine and histidine, among others (94). LAT-1 was discovered to be
overexpressed in PDAC and was linked to angiogenesis and tumor cell
proliferation (95). The breakdown
of tissue proteins to branched-chained amino acids was revealed to
be one of the early consequences of pancreatic cancer, thus, it may
be used as a potential biomarker (96). For example, leucine, isoleucine and
valine are branched-chain essential amino acids (97), which were reported to be elevated
in pancreatic cancer because they are an alternative source of
organic molecules that can fuel the TCA cycle. Exosomes derived
from the tumor microenvironment were also identified to enhance the
proliferation of pancreatic cancer cells by supplying metabolites,
such as proteins, nucleic acids and amino acids (54,98).
Due to poor vascularization, pancreatic tumors do not have a
sufficient supply of glutamine; instead, they use micropinocytosis
to engulf extracellular proteins, which are subsequently degraded
in lysosomes to release glutamine and other amino acids (99). PDAC is characterized by a low
expression of glutamate dehydrogenase and the overexpression of
glutamic oxaloacetic transaminase for the conversion of
glutamine-derived aspartate to oxaloacetate in the cytoplasm, which
is then further converted to malate and finally into pyruvate
(100). Glutamine regulates the
balance between T-eff cells and T-regs; however, in the PDAC
microenvironment, the transporter protein, alanine-serine-cysteine
transporter 2, was found to be deficient, leading to the diminished
generation and function of Th17 and Th1 cells (101), hence promoting T-reg
formation.
Upon activation, T cells consume a large amount of
arginine and tryptophan to generate memory T cells by switching
from glycolysis to oxidative phosphorylation, which activates
antitumor activities (102).
Amino acids produce derivatives that support cancer growth and
progression; for example, in the PDAC microenvironment, the
overexpression of indoleamine-2,3-dioxygenase (IDO) and arginase
depleted tryptophan and arginine, thereby suppressing T cell
proliferation and activating T-reg differentiation (103). Glutamate conversion into
α-ketoglutarate by glutamate dehydrogenase was discovered to
promote cancer growth, because it served as an anaplerotic
intermediate for the TCA cycle and provided nitrogen for
non-essential amino acid biosynthesis (104).
FAO is an alternative source of Acetyl CoA that
enters the TCA cycle for ATP production and energy (Fig. 2) (55). Pancreatic tumors and cell lines
have been shown to overexpress the cyclooxygenase (COX)2 enzyme,
which was identified to be associated with the invasiveness and
metastasis of the disease (105).
COX catalyzes the rate-limiting step in arachidonate metabolism to
produce prostaglandin (106). The
PDAC microenvironment was discovered to release endothelial growth
factors, tumor promoters and cytokines, which induced COX2
expression (107). Dubois et
al (108) showed that
transforming growth factor-α and tumor promoter tetradecanoyl
phorbol acetate stimulate the production of eicosanoids, such as
COX2, in rat intestinal epithelial cell culture. Increased
expression of COX2 plays a major role in the overproduction of
prostaglandins E2, which inhibits immune response in
malignant tissues (109). FAO was
also indicated to serve an important role in regulating the balance
between T-eff cells and suppressive T-regs, as it was observed to
promote the generation of T-regs, while inhibiting T-eff cell
polarization (69). FAO also
enhanced the activation and maintenance of memory CD8+ T
cells (110).
Products derived from other cell metabolic pathways,
such as glycolysis, the TCA cycle and PPP are used by cells to
generate lipids for cellular growth in the FAS pathway (55). In pancreatic cancer, FAS involves
the upregulation of ATP citrate lyase, Acetyl CoA carboxylase,
fatty acid synthase (FASN), Acetyl CoA synthetase, stearoyl CoA
desaturase, polyunsaturated fatty acids, monounsaturated fatty
acids (MUFA) and saturated fatty acids (86). Some plasma lipids, such as
very-low-density lipoproteins, were also discovered to be elevated,
while low-density lipoprotein, high-density lipoprotein and
3-hydroxybutyrate were decreased in patients with PDAC (111). ATP-citrate lyase, an enzyme that
converts citrate to Acetyl CoA which is a precursor for FAS, was
also revealed to be upregulated in PDAC (112).
Deregulated FAS in PDAC, such as the biogenesis of
fatty acids due to the overexpression of FASN, reportedly promoted
cancer progression via resistance to chemotherapy (113). Following resection, FASN levels
are decreased in the majority of patients with PDAC, suggesting
that elevated levels of FASN may be associated with a poor survival
(114). Another enzyme that
serves an essential role in FAS is acyl CoA synthetase, which
converts long-chain fatty acids to acyl CoA, a critical step in
phospholipid and triglycerol biosynthesis (115). Stearoyl-CoA desaturase-1 was
demonstrated to exert an important role in the pathogenesis of PDAC
by regulating the production of MUFAs (116). Alcohol and tobacco-related
carcinomas, such as PDAC, have also been shown to overexpress the
aldo-keto reductase family 1B10 (AKR1B10) enzyme, which catalyzes
the production of aldehyde and NADPH from alcohol and
NADP+ (117). AKR1B10
is essential in FAS by regulating the stability of Acetyl CoA
carboxylase, which is able to catalyze the biosynthesis of malonyl
CoA, a FASN substrate. Fatty acids are esterified to phospholipids,
which are required for membrane formation, and this pathway was
indicated to be the most abundant in advanced pancreatic cancer
(86). Cholesterol uptake was also
identified to be elevated in PDAC, as pancreatic cancer cells are
highly dependent on cholesterol (118).
A summary of the discussed metabolic pathways and
how they influence immune cells is presented in Table I.
Recent treatment developments involving TCA
inhibition in PDAC include phase II and III clinical trials
investigating a TCA cycle inhibitor devimistat, also known as
CPI-613® (ClinicalTrials.gov.nos. NCT03435289 and
NCT03504423). The combination of CPI-613 with gemcitabine, nab
paclitaxel and FOLFIRINOX have been explored for unresectable,
locally advanced and metastatic PDAC (119,120). In addition, the P13K-AKT-mTOR
signaling pathway controls cell cycle, survival, metabolism and
motility in cancer (121),
therefore, studies targeting mTOR inhibition include phase I and II
clinical trials (ClinicalTrials.gov.no. NCT03362412) investigating
Sirolimus, an mTOR kinase inhibitor, for the treatment of patients
with advanced pancreatic cancer (122). A combination of metabolic
regulation and chemotherapy could be more effective than the use of
chemotherapy alone.
The aggressive and unresponsive nature of pancreatic
cancer highlights a requirement for an improved understanding of
the mechanism of progression to provide effective therapeutic
targets. Immunotherapy is a growing and promising treatment
strategy in cancer; however, in pancreatic cancer, it is clear that
further studies are required to investigate the effectiveness. The
dysregulated interaction between the immune system and metabolic
pathways in pancreatic cancer could provide greater insight into
this disease. Furthermore, understanding these interactions may
enable the development of effective therapeutic options that might
increase the survival rate of patients. Targeting these pathways to
enhance or elicit an immune response would be beneficial. Several
studies have investigated the potential of targeting metabolic
pathways and the effect on immune response in carcinogenesis
(123–137), which are summarized in Table II. Future studies to determine
these effects in pancreatic cancer and discover new targets may
prove favorable.
Not applicable.
This study was funded by a grant from the South
African Medical Research Council, which was awarded to the Wits
Common Epithelial Cancer Research group. GPC was funded by the
Cancer Association of South Africa (CANSA).
Not applicable.
NE and EEN conducted the literature search. NE, PF,
JOJ, GPC and EEN drafted the manuscript and critically revised the
manuscript. EEN conceptualized the review article. All authors read
and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Rawla P, Sunkara T and Gaduputi V:
Epidemiology of pancreatic cancer: Global trends, etiology and risk
factors. World J Oncol. 10:10–27. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:1039–1049. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Polireddy K and Chen Q: Cancer of the
pancreas: Molecular pathways and current advancement in treatment.
J Cancer. 7:1497–1514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yachida S, Jones S, Bozic I, Antal T,
Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al:
Distant metastasis occurs late during the genetic evolution of
pancreatic cancer. Nature. 467:1114–1147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sarantis P, Koustas E, Papadimitropoulou
A, Papavassiliou AG and Karamouzis MV: Pancreatic ductal
adenocarcinoma: Treatment hurdles, tumor microenvironment and
immunotherapy. World J Gastrointest Oncol. 12:173–181. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghesquière B, Wong BW, Kuchnio A and
Carmeliet P: Metabolism of stromal and immune cells in health and
disease. Nature. 511:167–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hato T and Dagher PC: How the innate
immune system senses trouble and causes trouble. Clin J Am Soc
Nephrol. 10:1459–1469. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Inman KS, Francis AA and Murray NR:
Complex role for the immune system in initiation and progression of
pancreatic cancer. World J Gastroenterol. 20:11160–11181. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pearce EL and Pearce EJ: Metabolic
pathways in immune cell activation and quiescence. Immunity.
38:633–643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Odegaard JI and Chawla A: The immune
system as a sensor of the metabolic state. Immunity. 38:644–654.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
von Ahrens D, Bhagat TD, Nagrath D, Maitra
A and Verma A: The role of stromal cancer-associated fibroblasts in
pancreatic cancer. J Hematol Oncol. 10:762017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fukunaga A, Miyamoto M, Cho Y, Murakami S,
Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y,
et al: CD8+ tumor-infiltrating lymphocytes together with
CD4+ tumor-infiltrating lymphocytes and dendritic cells
improve the prognosis of patients with pancreatic adenocarcinoma.
Pancreas. 28:e26–e31. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tjomsland V, Sandström P, Spångeus A,
Messmer D, Emilsson J, Falkmer U, Falkmer S, Magnusson KE, Borch K
and Larsson M: Pancreatic adenocarcinoma exerts systemic effects on
the peripheral blood myeloid and plasmacytoid dendritic cells: An
indicator of disease severity? BMC Cancer. 10:872010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Sanctis F, Solito S, Ugel S, Molon B,
Bronte V and Marigo I: MDSCs in cancer: Conceiving new prognostic
and therapeutic targets. Biochim Biophys Acta. 1865:35–48.
2016.PubMed/NCBI
|
|
15
|
Bayne LJ, Beatty GL, Jhala N, Clark CE,
Rhim AD, Stanger BZ and Vonderheide RH: Tumor-derived
granulocyte-macrophage colony-stimulating factor regulates myeloid
inflammation and T cell immunity in pancreatic cancer. Cancer Cell.
21:822–835. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Padoan A, Plebani M and Basso D:
Inflammation and pancreatic cancer: Focus on metabolism, cytokines,
and immunity. Int J Mol Sci. 20:6762019. View Article : Google Scholar
|
|
17
|
Pergamo M and Miller G: Myeloid-derived
suppressor cells and their role in pancreatic cancer. Cancer Gene
Therapy. 24:100–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gabitass RF, Annels NE, Stocken DD, Pandha
HA and Middleton GW: Elevated myeloid-derived suppressor cells in
pancreatic, esophageal and gastric cancer are an independent
prognostic factor and are associated with significant elevation of
the Th2 cytokine interleukin-13. Cancer Immunol Immunother.
60:1419–1430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dietl K, Renner K, Dettmer K, Timischl B,
Eberhart K, Dorn C, Hellerbrand C, Kastenberger M, Kunz-Schughart
LA, Oefner PJ, et al: Lactic acid and acidification inhibit TNF
secretion and glycolysis of human monocytes. J Immunol.
184:1200–1209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kurahara H, Shinchi H, Mataki Y, Maemura
K, Noma H, Kubo F, Sakoda M, Ueno S, Natsugoe S and Takao S:
Significance of M2-polarized tumor-associated macrophage in
pancreatic cancer. J Surg Res. 167:e211–e219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mielgo A and Schmid MC: Impact of tumour
associated macrophages in pancreatic cancer. BMB Rep. 46:131–138.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Esposito I, Menicagli M, Funel N, Bergmann
F, Boggi U, Mosca F, Bevilacqua G and Campani D: Inflammatory cells
contribute to the generation of an angiogenic phenotype in
pancreatic ductal adenocarcinoma. J Clin Pathol. 57:630–636. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lesina M, Kurkowski MU, Ludes K, Rose-John
S, Treiber M, Klöppel G, Yoshimura A, Reindl W, Sipos B, Akira S,
et al: Stat3/Socs3 activation by IL-6 transsignaling promotes
progression of pancreatic intraepithelial neoplasia and development
of pancreatic cancer. Cancer Cell. 19:456–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JS, Park YS, Kim JY, Kim YG, Kim YJ,
Lee HK, Kim HS, Hong JT, Kim Y and Han SB: Inhibition of human
pancreatic tumor growth by cytokine-induced killer cells in nude
mouse xenograft model. Immune Netw. 12:247–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu L, Zhao G, Wu W, Rong Y, Jin D, Wang
D, Lou W and Qin X: Low intratumoral regulatory T cells and high
peritumoral CD8(+) T cells relate to long-term survival in patients
with pancreatic ductal adenocarcinoma after pancreatectomy. Cancer
Immunol Immunother. 65:73–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kurts C: Th17 cells: A third subset of
CD4+ T effector cells involved in organ-specific
autoimmunity. Nephrol Dial Transplant. 23:816–819. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamamoto K, Venida A, Yano J, Biancur DE,
Kakiuchi M, Gupta S, Sohn ASW, Mukhopadhyay S, Lin EY, Parker SJ,
et al: Autophagy promotes immune evasion of pancreatic cancer by
degrading MHC-I. Nature. 581:100–105. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Herber DL, Cao W, Nefedova Y, Novitskiy
SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B, et
al: Lipid accumulation and dendritic cell dysfunction in cancer.
Nat Med. 16:880–886. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ochi A, Nguyen AH, Bedrosian AS, Mushlin
HM, Zarbakhsh S, Barilla R, Zambirinis CP, Fallon NC, Rehman A,
Pylayeva-Gupta Y, et al: MyD88 inhibition amplifies dendritic cell
capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp
Med. 209:1671–1687. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki
M, Kosuge T, Kanai Y and Hiraoka N: Immune cell infiltration as an
indicator of the immune microenvironment of pancreatic cancer. Br J
Cancer. 108:914–923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hiraoka N, Onozato K, Kosuge T and
Hirohashi S: Prevalence of FOXP3+ regulatory T cells
increases during the progression of pancreatic ductal
adenocarcinoma and its premalignant lesions. Clin Cancer Res.
12:5423–5434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Knutson KL and Disis ML: Tumor
antigen-specific T helper cells in cancer immunity and
immunotherapy. Cancer Immunol Immunother. 54:721–728. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wörmann SM, Diakopoulos KN, Lesina M and
Algül H: The immune network in pancreatic cancer development and
progression. Oncogene. 33:29562013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zou W and Restifo NP: TH17 cells in tumour
immunity and immunotherapy. Nat Rev Immunol. 10:248–256. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gnerlich JL, Mitchem JB, Weir JS, Sankpal
NV, Kashiwagi H, Belt BA, Porembka MR, Herndon JM, Eberlein TJ,
Goedegebuure P and Linehan DC: Induction of Th17 cells in the tumor
microenvironment improves survival in a murine model of pancreatic
cancer. J Immunol. 185:4063–4071. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He Q, Luo X, Huang Y and Sheikh MS:
Apo2L/TRAIL differentially modulates the apoptotic effects of
sulindac and a COX-2 selective non-steroidal anti-inflammatory
agent in Bax-deficient cells. Oncogene. 21:6032–6040. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He S, Fei M, Wu Y, Zheng D, Wan D, Wang L
and Li D: Distribution and clinical significance of Th17 cells in
the tumor microenvironment and peripheral blood of pancreatic
cancer patients. Int J Mol Sci. 12:7424–7437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
De Monte L, Reni M, Tassi E, Clavenna D,
Papa I, Recalde H, Braga M, Carlo VD, Doglioni C and Protti MP:
Intratumor T helper type 2 cell infiltrate correlates with
cancer-associated fibroblast thymic stromal lymphopoietin
production and reduced survival in pancreatic cancer. J Exp Med.
208:469–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bellone G, Turletti A, Artusio E, Mareschi
K, Carbone A, Tibaudi D, Robecchi A, Emanuelli G and Rodeck U:
Tumor-associated transforming growth factor-β and interleukin-10
contribute to a systemic Th2 immune phenotype in pancreatic
carcinoma patients. Am J Pathol. 155:537–547. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Macintyre AN, Gerriets VA, Nichols AG,
Michalek RD, Rudolph MC, Deoliveira D, Anderson SM, Abel ED, Chen
BJ, Hale LP and Rathmell JC: The glucose transporter Glut1 is
selectively essential for CD4 T cell activation and effector
function. Cell Metab. 20:61–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cham CM, Driessens G, O'Keefe JP and
Gajewski TF: Glucose deprivation inhibits multiple key gene
expression events and effector functions in CD8+ T
cells. Eur J Immunol. 38:2438–2450. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sakaguchi S, Miyara M, Costantino CM and
Hafler DA: FOXP3+ regulatory T cells in the human immune
system. Nat Rev Immunol. 10:490–500. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Walker LS and Sansom DM: The emerging role
of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev
Immunol. 11:852–863. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yamamoto T, Yanagimoto H, Satoi S,
Toyokawa H, Hirooka S, Yamaki S, Yui R, Yamao J, Kim S and Kwon AH:
Circulating CD4+CD25+ regulatory T cells in
patients with pancreatic cancer. Pancreas. 41:409–415. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chang DZ, Ma Y, Ji B, Wang H, Deng D, Liu
Y, Abbruzzese JL, Liu YJ, Logsdon CD and Hwu P: Mast cells in tumor
microenvironment promotes the in vivo growth of pancreatic ductal
adenocarcinoma. Clin Cancer Res. 17:7015–7023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ito T, Amakawa R, Inaba M, Ikehara S,
Inaba K and Fukuhara S: Differential regulation of human blood
dendritic cell subsets by IFNs. J Immunol. 166:2961–2969. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Duan X, Deng L, Chen X, Lu Y, Zhang Q,
Zhang K, Hu Y, Zeng J and Sun W: Clinical significance of the
immunostimulatory MHC class I chain-related molecule A and NKG2D
receptor on NK cells in pancreatic cancer. Med Oncol. 28:466–474.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cai SW, Yang SZ, Gao J, Pan K, Chen JY,
Wang YL, Wei LX and Dong JH: Prognostic significance of mast cell
count following curative resection for pancreatic ductal
adenocarcinoma. Surgery. 149:576–584. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Strouch MJ, Cheon EC, Salabat MR, Krantz
SB, Gounaris E, Melstrom LG, Dangi-Garimella S, Wang E, Munshi HG,
Khazaie K and Bentrem DJ: Crosstalk between mast cells and
pancreatic cancer cells contributes to pancreatic tumor
progression. Clin Cancer Res. 16:2257–2265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Feig C, Gopinathan A, Neesse A, Chan DS,
Cook N and Tuveson DA: The pancreas cancer microenvironment. Clin
Cancer Res. 18:4266–4276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Stopa BK, Kusiak AA, Szopa DM, Ferdek EP
and Jakubowska AM: Pancreatic cancer and its
microenvironment-recent advances and current controversies. Int J
Mol Sci. 21:32182020. View Article : Google Scholar
|
|
52
|
Li KY, Yuan JL, Trafton D, Wang JX, Niu N,
Yuan CH, Liu XB and Zheng L: Pancreatic ductal adenocarcinoma
immune microenvironment and immunotherapy prospects. Chronic Dis
Transl Med. 6:6–17. 2020.PubMed/NCBI
|
|
53
|
Li X, Wenes M, Romero P, Huang SC, Fendt
SM and Ho PC: Navigating metabolic pathways to enhance antitumour
immunity and immunotherapy. Nat Rev Clin Oncol. 16:425–441. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yao W, Maitra A and Ying H: Recent
insights into the biology of pancreatic cancer. EBioMedicine.
53:1026552020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
O'Neill LAJ, Kishton RJ and Rathmell J: A
guide to immunometabolism for immunologists. Nat Rev Immunol.
16:553–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Palmer CS, Ostrowski M, Balderson B,
Christian N and Crowe SM: Glucose metabolism regulates T cell
activation, differentiation, and functions. Front Immunol. 6:12015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chung JC, Oh MJ, Choi SH and Bae CD:
Proteomic analysis to identify biomarker proteins in pancreatic
ductal adenocarcinoma. ANZ J Surg. 78:245–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yoon DY, Buchler P, Saarikoski ST, Hines
OJ, Reber HA and Hankinson O: Identification of genes
differentially induced by hypoxia in pancreatic cancer cells.
Biochem Biophys Res Commun. 288:882–886. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Natsuizaka M, Ozasa M, Darmanin S,
Miyamoto M, Kondo S, Kamada S, Shindoh M, Higashino F, Suhara W,
Koide H, et al: Synergistic up-regulation of Hexokinase-2, glucose
transporters and angiogenic factors in pancreatic cancer cells by
glucose deprivation and hypoxia. Exp Cell Res. 313:3337–3348. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cameron ME, Yakovenko A and Trevino JG:
Glucose and lactate transport in pancreatic cancer: Glycolytic
metabolism revisited. J Oncol. 2018:62148382018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Warburg O, Wind F and Negelein E: The
metabolism of tumors in the body. J Gen Physiol. 8:519–530. 1927.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Colegio OR, Chu NQ, Szabo AL, Chu T,
Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC,
Phillips GM, et al: Functional polarization of tumour-associated
macrophages by tumour-derived lactic acid. Nature. 513:559–563.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Choi SYC, Collins CC, Gout PW and Wang Y:
Cancer-generated lactic acid: A regulatory, immunosuppressive
metabolite? J Pathol. 230:350–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mills EL and O'Neill LA: Reprogramming
mitochondrial metabolism in macrophages as an anti-inflammatory
signal. Eur J Immunol. 46:13–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rodríguez-Prados JC, Través PG, Cuenca J,
Rico D, Aragonés J, Martín-Sanz P, Cascante M and Boscá L:
Substrate fate in activated macrophages: A comparison between
innate, classic, and alternative activation. J Immunol.
185:605–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Murray PJ, Allen JE, Biswas SK, Fisher EA,
Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence
T, et al: Macrophage activation and polarization: Nomenclature and
experimental guidelines. Immunity. 41:14–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li Y and Zhu B: Editorial: Metabolism of
cancer cells and immune cells in the tumor microenvironment. Front
Immunol. 9:30802018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Michalek RD, Gerriets VA, Jacobs SR,
Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG and
Rathmell JC: Cutting edge: Distinct glycolytic and lipid oxidative
metabolic programs are essential for effector and regulatory
CD4+ T cell subsets. J Immunol. 186:3299–3303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
MacIver NJ, Michalek RD and Rathmell JC:
Metabolic regulation of T lymphocytes. Annu Rev Immunol.
31:259–283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Waickman AT and Powell JD: mTOR,
metabolism, and the regulation of T-cell differentiation and
function. Immunol Rev. 249:43–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Mao Z and Zhang W: Role of mTOR in glucose
and lipid metabolism. Int J Mol Sci. 19:20432018. View Article : Google Scholar
|
|
73
|
Ersahin T, Tuncbag N and Cetin-Atalay R:
The PI3K/AKT/mTOR interactive pathway. Mol Biosyst. 11:1946–1954.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Doughty CA, Bleiman BF, Wagner DJ, Dufort
FJ, Mataraza JM, Roberts MF and Chiles TC: Antigen
receptor-mediated changes in glucose metabolism in B lymphocytes:
Role of phosphatidylinositol 3-kinase signaling in the glycolytic
control of growth. Blood. 107:4458–4465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Rodríguez-Espinosa O, Rojas-Espinosa O,
Moreno-Altamirano MMB, López-Villegas EO and Sánchez-García FJ:
Metabolic requirements for neutrophil extracellular traps
formation. Immunology. 145:213–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Dallal RM, Christakos P, Lee K, Egawa S,
Son YI and Lotze MT: Paucity of dendritic cells in pancreatic
cancer. Surgery. 131:135–138. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Krawczyk CM, Holowka T, Sun J, Blagih J,
Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG
and Pearce EJ: Toll-like receptor-induced changes in glycolytic
metabolism regulate dendritic cell activation. Blood.
115:4742–4749. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Everts B, Amiel E, Huang SC, Smith AM,
Chang CH, Lam WY, Redmann V, Freitas TC, Blagih J, van der Windt
GJ, et al: TLR-driven early glycolytic reprogramming via the
kinases TBK1-IKKε supports the anabolic demands of dendritic cell
activation. Nat Immunol. 15:323–332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Martínez-Reyes I and Chandel NS:
Mitochondrial TCA cycle metabolites control physiology and disease.
Nat Commun. 11:1022020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Reyes-Castellanos G, Masoud R and Carrier
A: Mitochondrial metabolism in PDAC: From better knowledge to new
targeting strategies. Biomedicines. 8:2702020. View Article : Google Scholar
|
|
82
|
Laurenti G and Tennant DA: Isocitrate
dehydrogenase (IDH), succinate dehydrogenase (SDH), fumarate
hydratase (FH): Three players for one phenotype in cancer? Biochem
Soc Trans. 44:1111–1116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Waitkus MS, Diplas BH and Yan H:
Biological role and therapeutic potential of IDH mutations in
cancer. Cancer Cell. 34:186–195. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Nogueira V and Hay N: Molecular pathways:
Reactive oxygen species homeostasis in cancer cells and
implications for cancer therapy. Clin Cancer Res. 19:4309–4314.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Schlichtholz B, Turyn J, Goyke E,
Biernacki M, Jaskiewicz K, Sledzinski Z and Swierczynski J:
Enhanced citrate synthase activity in human pancreatic cancer.
Pancreas. 30:99–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Swierczynski J, Hebanowska A and
Sledzinski T: Role of abnormal lipid metabolism in development,
progression, diagnosis and therapy of pancreatic cancer. World J
Gastroenterol. 20:2279–2303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Halabe Bucay A: Hypothesis proved...citric
acid (citrate) does improve cancer: A case of a patient suffering
from medullary thyroid cancer. Med Hypotheses. 73:2712009.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Amedei A, Niccolai E and Prisco D:
Pancreatic cancer: Role of the immune system in cancer progression
and vaccine-based immunotherapy. Hum Vaccin Immunother.
10:3354–3368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Patra KC and Hay N: The pentose phosphate
pathway and cancer. Trends Biochem Sci. 39:347–354. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu H, Huang D, McArthur DL, Boros LG,
Nissen N and Heaney AP: Fructose induces transketolase flux to
promote pancreatic cancer growth. Cancer Res. 70:6368–6376. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Shukla SK, Purohit V, Mehla K, Gunda V,
Chaika NV, Vernucci E, King RJ, Abrego J, Goode GD, Dasgupta A, et
al: MUC1 and HIF-1alpha signaling crosstalk induces anabolic
glucose metabolism to impart gemcitabine resistance to pancreatic
cancer. Cancer Cell. 32:71–87.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Haschemi A, Kosma P, Gille L, Evans CR,
Burant CF, Starkl P, Knapp B, Haas R, Schmid JA, Jandl C, et al:
The sedoheptulose kinase CARKL directs macrophage polarization
through control of glucose metabolism. Cell Metab. 15:813–826.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Palsson-McDermott EM, Curtis AM, Goel G,
Lauterbach MA, Sheedy FJ, Gleeson LE, van den Bosch MW, Quinn SR,
Domingo-Fernandez R, Johnston DG, et al: Pyruvate kinase M2
regulates Hif-1α activity and IL-1β induction and is a critical
determinant of the warburg effect in LPS-activated macrophages.
Cell Metab. 21:65–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Fernandez-Zapico M, Kim DW, Philip P,
Vandell A, Eckard J, Korn R, Del Priore G and Simeone D: Abstract
B15: Therapeutic potential of targeting amino acid metabolism in
pancreatic cancer. Cancer Res. 79:B152019.
|
|
95
|
Altan B, Kaira K, Watanabe A, Kubo N, Bao
P, Dolgormaa G, Bilguun EO, Araki K, Kanai Y, Yokobori T, et al:
Relationship between LAT1 expression and resistance to chemotherapy
in pancreatic ductal adenocarcinoma. Cancer Chemother Pharmacol.
81:141–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Commisso C, Davidson SM, Soydaner-Azeloglu
RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin
JA, Thompson CB, et al: Macropinocytosis of protein is an amino
acid supply route in Ras-transformed cells. Nature. 497:633–637.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ananieva EA and Wilkinson AC:
Branched-chain amino acid metabolism in cancer. Curr Opin Clin Nutr
Metab Care. 21:64–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhao H, Yang L, Baddour J, Achreja A,
Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA,
et al: Tumor microenvironment derived exosomes pleiotropically
modulate cancer cell metabolism. Elife. 5:e102502016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kamphorst JJ, Nofal M, Commisso C, Hackett
SR, Lu W, Grabocka E, Vander Heiden MG, Miller G, Drebin JA,
Bar-Sagi D, et al: Human pancreatic cancer tumors are nutrient poor
and tumor cells actively scavenge extracellular protein. Cancer
Res. 75:544–553. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Son J, Lyssiotis CA, Ying H, Wang X, Hua
S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, et
al: Glutamine supports pancreatic cancer growth through a
KRAS-regulated metabolic pathway. Nature. 496:101–105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Singer K, Cheng WC, Kreutz M, Ho PC and
Siska PJ: Immunometabolism in cancer at a glance. Dis Models Mech.
11:dmm0342722018. View Article : Google Scholar
|
|
102
|
Sinclair LV, Rolf J, Emslie E, Shi Y-B,
Taylor PM and Cantrell DA: Control of amino-acid transport by
antigen receptors coordinates the metabolic reprogramming essential
for T cell differentiation. Nat Immunol. 14:500–508. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Pilotte L, Larrieu P, Stroobant V, Colau
D, Dolusic E, Frédérick R, De Plaen E, Uyttenhove C, Wouters J,
Masereel B and Van den Eynde BJ: Reversal of tumoral immune
resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl
Acad Sci USA. 109:24972012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Cluntun AA, Lukey MJ, Cerione RA and
Locasale JW: Glutamine metabolism in cancer: Understanding the
heterogeneity. Trends Cancer. 3:169–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Nzeako UC and Gores GJ: Increased
expression of cyclooxygenase-2 in human pancreatic neoplasms and
potential for chemoprevention by cyclooxygenase inhibitors. Cancer.
94:1903–1904. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Asano T, Shoda J, Ueda T, Kawamoto T,
Todoroki T, Shimonishi M, Tanabe T, Sugimoto Y, Ichikawa A, Mutoh
M, et al: Expressions of cyclooxygenase-2 and prostaglandin
E-receptors in carcinoma of the gallbladder: Crucial role of
arachidonate metabolism in tumor growth and progression. Clin
Cancer Res. 8:1157–1167. 2002.PubMed/NCBI
|
|
107
|
Molina MA, Sitja-Arnau M, Lemoine MG,
Frazier ML and Sinicrope FA: Increased cyclooxygenase-2 expression
in human pancreatic carcinomas and cell lines. Cancer Res.
59:4356–4362. 1999.PubMed/NCBI
|
|
108
|
DuBois RN, Awad J, Morrow J, Roberts LJ II
and Bishop PR: Regulation of eicosanoid production and mitogenesis
in rat intestinal epithelial cells by transforming growth
factor-alpha and phorbol ester. J Clin Invest. 93:493–498. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Sato T, Nakajima H, Fujio K and Mori Y:
Enhancement of prostaglandin E2 production by epidermal growth
factor requires the coordinate activation of cytosolic
phospholipase A2 and cyclooxygenase 2 in human squamous carcinoma
A431 cells. Prostaglandins. 53:355–369. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
O'Sullivan D, van der Windt GJW, Huang SC,
Curtis JD, Chang CH, Buck MD, Qiu J, Smith AM, Lam WY, DiPlato LM,
et al: Memory CD8(+) T cells use cell-intrinsic lipolysis to
support the metabolic programming necessary for development.
Immunity. 41:75–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zhang A, Sun H, Wang P, Han Y and Wang X:
Modern analytical techniques in metabolomics analysis. Analyst.
137:293–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Hatzivassiliou G, Zhao F, Bauer DE,
Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA and
Thompson CB: ATP citrate lyase inhibition can suppress tumor cell
growth. Cancer Cell. 8:311–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Tadros S, Shukla SK, King RJ, Gunda V,
Vernucci E, Abrego J, Chaika NV, Yu F, Lazenby AJ, Berim L, et al:
De novo lipid synthesis facilitates gemcitabine resistance through
endoplasmic reticulum stress in pancreatic cancer. Cancer Res.
77:5503–5517. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Walter K, Hong SM, Nyhan S, Canto M,
Fedarko N, Klein A, Griffith M, Omura N, Medghalchi S, Kuhajda F
and Goggins M: Serum fatty acid synthase as a marker of pancreatic
neoplasia. Cancer Epidemiol Biomarkers Prev. 18:2380–2385. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Coleman RA, Lewin TM, Van Horn CG and
Gonzalez-Baró MR: Do long-chain acyl-CoA synthetases regulate fatty
acid entry into synthetic versus degradative pathways? J Nutr.
132:2123–2126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Macášek J, Vecka M, Žák A, Urbánek M,
Krechler T, Petruželka L, Staňková B and Zeman M: Plasma fatty acid
composition in patients with pancreatic cancer: Correlations to
clinical parameters. Nutr Cancer. 64:946–955. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chung YT, Matkowskyj KA, Li H, Bai H,
Zhang W, Tsao MS, Liao J and Yang GY: Overexpression and oncogenic
function of aldo-keto reductase family 1B10 (AKR1B10) in pancreatic
carcinoma. Mod Pathol. 25:758–766. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Guillaumond F, Bidaut G, Ouaissi M,
Servais S, Gouirand V, Olivares O, Lac S, Borge L, Roques J, Gayet
O, et al: Cholesterol uptake disruption, in association with
chemotherapy, is a promising combined metabolic therapy for
pancreatic adenocarcinoma. Proc Natl Acad Sci USA. 112:2473–2478.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Alistar AT, Morris B, Harrison L,
Bickenbach K, Starker L, Ginder N, McIlwain L, Luther S, Pardee TS
and Alpert J: A single-arm, open-label, phase I study of CPI-613
(Devimistat) in combination with gemcitabine and nab-paclitaxel for
patients with locally advanced or metastatic pancreatic
adenocarcinoma. J Clin Oncol. 38:4635. 2020. View Article : Google Scholar
|
|
120
|
Philip PA, Buyse ME, Alistar AT, Rocha
Lima CMSP, Luther S, Pardee TS and Van Cutsem E: Avenger 500, a
phase III open-label randomized trial of the combination of CPI-613
with modified FOLFIRINOX (mFFX) versus FOLFIRINOX (FFX) in patients
with metastatic adenocarcinoma of the pancreas. J Clin Oncol.
37:TPS4792019. View Article : Google Scholar
|
|
121
|
O'Donnell JS, Massi D, Teng MWL and
Mandala M: PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux.
Semin Cancer Biol. 48:91–103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Jin J and Zhao Q: Emerging role of mTOR in
tumor immune contexture: Impact on chemokine-related immune cells
migration. Theranostics. 10:6231–6244. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Allard B, Longhi MS, Robson SC and Stagg
J: The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor
targets. Immunol Rev. 276:121–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Arina A and Bronte V: Myeloid-derived
suppressor cell impact on endogenous and adoptively transferred T
cells. Curr Opin Immunol. 33:120–125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Brand A, Singer K, Koehl GE, Kolitzus M,
Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et
al: LDHA-associated lactic acid production blunts tumor
immunosurveillance by T and NK cells. Cell Metab. 24:657–671. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Deaglio S, Dwyer KM, Gao W, Friedman D,
Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al:
Adenosine generation catalyzed by CD39 and CD73 expressed on
regulatory T cells mediates immune suppression. J Exp Med.
204:1257–1265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Hiraoka N, Toue S, Okamoto C, Kikuchi S,
Ino Y, Yamazaki-Itoh R, Esaki M, Nara S, Kishi Y, Imaizumi A, et
al: Tissue amino acid profiles are characteristic of tumor type,
malignant phenotype, and tumor progression in pancreatic tumors.
Sci Rep. 9:98162019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Hossain F, Al-Khami AA, Wyczechowska D,
Hernandez C, Zheng L, Reiss K, Valle LD, Trillo-Tinoco J, Maj T,
Zou W, et al: Inhibition of fatty acid oxidation modulates
immunosuppressive functions of myeloid-derived suppressor cells and
enhances cancer therapies. Cancer Immunol Res. 3:1236–1247. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kalinski P: Regulation of immune responses
by prostaglandin E2. J Immunol. 188:21–28. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Korangath P, Teo WW, Sadik H, Han L, Mori
N, Huijts CM, Wildes F, Bharti S, Zhang Z, Santa-Maria CA, et al:
Targeting glutamine metabolism in breast cancer with
aminooxyacetate. Clin Cancer Res. 21:3263–3273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Leone RD and Emens LA: Targeting adenosine
for cancer immunotherapy. J Immunother Cancer. 6:572018. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Li M, Tan SY and Wang XF: Paeonol exerts
an anticancer effect on human colorectal cancer cells through
inhibition of PGE2 synthesis and COX-2 expression. Oncol
Rep. 32:2845–2853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Liu WR, Tian MX, Yang LX, Lin YL, Jin L,
Ding ZB, Shen YH, Peng YF, Gao DM, Zhou J, et al: PKM2 promotes
metastasis by recruiting myeloid-derived suppressor cells and
indicates poor prognosis for hepatocellular carcinoma. Oncotarget.
6:846–861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Mohammad GH, Olde Damink SW, Malago M,
Dhar DK and Pereira SP: Pyruvate kinase M2 and lactate
dehydrogenase A are overexpressed in pancreatic cancer and
correlate with poor outcome. PLoS One. 11:e01516352016. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Patsoukis N, Bardhan K, Chatterjee P, Sari
D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P, et al:
PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis
and promoting lipolysis and fatty acid oxidation. Nat Commun.
6:66922015. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Yu CP, Fu SF, Chen X, Ye J, Ye Y, Kong LD
and Zhu Z: The clinicopathological and prognostic significance of
IDO1 expression in human solid tumors: evidence from a systematic
review and meta-analysis. Cell Physiol Biochem. 49:134–143. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Biswas SK: Metabolic reprogramming of
immune cells in cancer progression. Immunity. 43:435–449. 2015.
View Article : Google Scholar : PubMed/NCBI
|