Introduction
Viral stromal keratitis is an inflammatory disease
of the corneal stroma caused by viruses, of which herpes simplex
virus type 1 (HSV-1) is the leading cause (1). HSV-1 keratitis can lead to visual
impairment and blindness, as a result of corneal scarring,
thinning, opacity and neovascularization (1). An epidemiological study in 2012
revealed that the global prevalence of HSV-1 keratitis was
estimated to be 1.5 million cases, of which nearly 40,000 new cases
of severe monocular visual impairment or blindness occur every year
(2). Viral stromal keratitis is
not only a simple infectious disease, but a chronic immune
disorder, that adversely affects quality of life, even when the
infection is not active (3). It is
characterized by complex interactions between infiltrating immune
cells and corneal intrinsic cells. These interactions induce and
sustain an inflammatory response that ultimately lead to corneal
damage (4). Current treatment for
viral stromal keratitis predominantly consists of the
administration of antiviral drugs and topical steroids to control
inflammation (5). However, the
choice of anti-inflammatory agents is relatively limited.
Furthermore, topical glucocorticoids can potentially lead to
various complications, such as glaucoma, cataracts and delayed
healing (6). The development of
reliable anti-inflammatory drugs with fewer side effects is thus, a
clinical priority.
The stroma accounts for over 90% of the corneal
thickness, and largely consists of resident keratocytes embedded in
a matrix of type I collagen (7).
The parallel arrangement of collagen fibers in the extracellular
matrix of the corneal stroma is a key determinant of corneal
transparency (7). These collagen
fibers are produced largely by keratocytes (7). Activated keratocytes, also called
corneal fibroblasts, are highly involved in regulating local
inflammatory and immune responses to viral infections, through the
production of various inflammatory factors, such as IL-6, IL-8 and
chemoattractant protein-1 (MCP-1) (8,9).
Polyinosinic-polycytidylic acid, or poly(I:C), is structurally
similar to viral double-stranded RNA (dsRNA) and has been applied
experimentally to investigate the effects of viral infection on
various cell types, including microglia, human mucosal epithelial
cells and airway epithelial cells (10–12).
The interaction of poly(I:C) with Toll-like receptor 3 (TLR3)
results in the activation of nuclear factor (NF)-κB and
mitogen-activated protein kinases (MAPKs) (13). These mediate pivotal intracellular
signaling pathways underlie the regulation of cell proliferation,
differentiation, migration, senescence and apoptosis in response to
a variety of extracellular stimuli, including viruses,
lipopolysaccharide and certain proinflammatory cytokines, such as
IL-1 and TNF-α (14,15). These effects of poly(I:C) on
intracellular signaling trigger the release of various
proinflammatory factors (13) and
matrix metalloproteinases (MMPs) (16) in human corneal fibroblasts (HCFs).
The proinflammatory factors, including IL-8, MCP-1 and IL-6, have
vital roles in immune cell infiltration and the inflammatory
response (17–19). An inflammatory response contributes
to the removal of pathogens; however, an excessive infiltration of
inflammatory cells and sustained release of cytokines and
chemokines can lead to tissue damage, as evidenced by the
destruction of corneal structure in viral stromal keratitis
(20). The release of MMPs,
particularly MMP-1 and MMP-3, has been significantly associated
with degradation of the corneal stromal matrix and corneal
ulceration in viral stromal keratitis (21).
Sulforaphane (1-isothiocyanate-4-methanesulfonyl
butane; SFN) is a natural plant compound found in cruciferous
plants, particularly in broccoli. SFN is derived from the
hydrolysis of its precursor, glucosinolate (a reaction catalyzed by
the enzyme myrosinase) (22). It
is well-known that SFN is a natural antioxidant, and has
anti-cancer (23) and
anti-inflammatory (24,25) effects. It has been shown that SFN
inhibits the proinflammatory action of lipopolysaccharides on
microglia through the downregulation of the NF-κB and MAPK
signaling pathways (26).
Furthermore, in vitro and in vivo studies have shown
that SFN can suppress the occurrence and progression of ocular
diseases, such as cataracts (27),
age-related macular degeneration (28) and Fuchs' corneal endothelial
dystrophy (29), which were
associated with its potential antioxidant effects. However, the
possible effect of SFN on ocular inflammatory diseases is unclear.
The present study investigated the potential anti-inflammatory
effects of SFN on the release of cytokines, chemokines, and MMPs in
HCFs stimulated by poly(I:C).
Materials and methods
Materials
Fetal bovine serum (FBS), trypsin-EDTA and Eagle's
minimum essential medium (MEM) were purchased from Gibco (Thermo
Fisher Scientific, Inc.). Cell culture dishes, 24-well plates and
flasks were obtained from Corning Inc. Sigma-Aldrich (Merck KGaA)
supplied the SFN; and poly(I:C) was provided by InvivoGen. ELISA
kits for IL-8 (cat. no. Q8000B), MCP-1 (cat. no. DCP00) and IL-6
(cat. no. Q6000B), were obtained from R&D Systems, Inc., while
the kits for MMP-1 (cat. no. ab215083) and MMP-3 (cat. no.
ab269371) were obtained from Abcam. The following antibodies were
obtained from Cell Signaling Technology, Inc.: Anti-JNK (cat. no.
9252), phosphorylated (p)JNK (cat. no. 9251), IκB-α (cat. no.
9242), p-IκB-α (cat. no. 2859), ERK (cat. no. 9102), p-ERK (cat.
no. 9106), c-Jun (cat. no. 9165), p-c-Jun (cat. no. 9164), p38 MAPK
(cat. no. 9212), p-p38 MAPK (cat. no. 9211), Akt (cat. no. 9272)
and p-Akt (cat. no. 9271). The antibody against GAPDH (cat. no.
60004-1-Ig) was purchased from ProteinTech Group, Inc. Goat
anti-mouse horseradish peroxidase (HRP)-conjugated antibody (cat.
no. A0216), rabbit immunoglobulin G (cat. no. A0208), bovine serum
albumin and enhanced chemiluminescence (ECL) reagents were
purchased from Beyotime Institute of Biotechnology. The primary
antibody against NF-κB p65 (cat. no. sc-8008) was purchased from
Santa Cruz Biotechnology, Inc. Molecular Probes (Thermo Fisher
Scientific, Inc.) supplied the 4′,6-diamidino-2-phenylindole (DAPI)
and Alexa Fluor 488-labeled donkey antibodies to mouse
immunoglobulin G (cat. no. A-21202). The RNAprep pure kit (cat. no.
DP430) was supplied by Tiangen Biotech Co., Ltd. A non-radioactive
cytotoxicity assay kit for lactate dehydrogenase (LDH) was
purchased from Promega Corporation. Endotoxin minimization was
performed for all media and reagents used for cell culture.
Cell culture and treatment with
SFN
Human keratocytes (cat. no. 6520) were purchased
from ScienCell Research Laboratories, Inc. The cultured keratocytes
(activated keratocytes), also called corneal fibroblasts, were
maintained in MEM supplemented with 10% FBS at 37°C in a humidified
incubator with 5% CO2. After four to seven passages,
HCFs used for subsequent experimentation were harvested at the
subconfluent stage, and further seeded into 24-well plates
(3×104 cells/well) or 60-mm culture dishes
(5×105 cells/dish). When the HCFs obtained confluence,
the previous media was discarded, and serum-free culture media was
added for an additional day. Poly(I:C) (3 µg/ml) was added to the
medium as a mimic of viral dsRNA, alongside various concentrations
of SFN (1, 2, 5 or 10 µM) as interventions.
Assays of IL-8, MCP-1, IL-6, MMP-1 and
MMP-3
Serum-deprived corneal fibroblasts were incubated in
the serum-free MEM with or without SFN (10 µM) for 24 h, and then
maintained in the same buffer containing poly(I:C) (3 µg/ml) for
another 12, 24, 36 and 48 h. Cell supernatant fluid was obtained
following centrifugation at 120 × g for 5 min at 4°C, and frozen at
−80°C for subsequent assessments of IL-8, MCP-1, IL-6, MMP-1 and
MMP-3 with ELISA kits. Following exposure to trypsin-EDTA, the
cells were isolated from the culture plates, stained with trypan
blue for 3 min at room temperature and further counted using a
hemocytometer. As counting of the cells and morphology were not
influenced by exposure to SFN or poly(I:C), the measurements of
these proteins in the culture supernatants were normalized by cell
number.
Western blot analysis
The protein expression levels of Akt, c-Jun, MAPKs
and IκB-α in the HCFs were detected using western blot analysis.
During the first 24 h, cells were maintained in MEM with 0.5% FBS,
and then cultured in serum-free medium for another 24 h. The
serum-deprived cells were further incubated in serum-free MEM with
or without SFN (10 µM) for 24 h, and were incubated in the same
solution with or without poly(I:C) (3 µg/ml), as previously
described (16) for an additional
30 min (for Akt) or 90 min (for MAPKs, IκB-α and c-Jun). The cells
were washed twice with ice-cold PBS and lysed in a solution
containing the following: 1% protease inhibitor cocktail; 100 mM
NaCl; 1% Nonidet P-40; 10 mM MgCl2; 1 mM dithiothreitol;
50 mM Tris-HCl (pH 7.4); and 1 mM phenylmethylsulfonyl fluoride.
Following centrifugation (120 × g, for 10 min at 4°C), the cell
lysates were collected to measure the protein concentration using
the Bradford method. The total protein (10 µg) was first stacked
with 10% SDS-PAGE gels and then resolved with 6% gels.
Subsequently, the proteins were transferred onto polyvinylidene
difluoride membranes (0.45 µm), then blocked in a mixture
containing 5% skimmed milk in TBS-Tween-20, following which the
membranes were incubated overnight at 4°C, with the primary
antibodies (all at 1:1,000) diluted in the blocking buffer. The
following day, the membranes were washed using a mixture of 20 mM
Tris-HCl (pH 7.4) and 0.1% Tween-20, and incubated with the
secondary antibody conjugated to HRP (dilution 1:3,000) at room
temperature for 1 h. The proteins were imaged immediately with a
Tanon-5200 Multi-imaging System following incubation with ECL
solutions (Tanon Science and Technology Co., Ltd.).
Immunofluorescence staining
HCFs were maintained for 24 h in MEM containing 0.5%
FBS, then serum-free media for an additional 24 h. This was
followed by another 24 h with or without SFN (10 µM) in serum-free
MEM, and an additional 90 min in the same solution with or without
poly(I:C) (3 µg/ml). The cells were fixed with 4% paraformaldehyde
at room temperature for 15 min before being permeabilized with 0.2%
Triton X-100 for 15 min at room temperature. Between each step, the
cells were washed with PBS. Non-specific adsorption of antibodies
was blocked by adding 3% bovine serum albumin for 15 min at room
temperature. The cells were then incubated with a mouse monoclonal
antibody (anti-NF-κB; 1:50) at room temperature for 1 h.
Subsequently, the cells were further incubated for 1 h at room
temperature with Alexa Fluor 488-conjugated secondary antibodies
(diluted 1:500) and DAPI. Finally, the images were obtained using a
fluorescence microscope (Zeiss AG).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Serum-deprived HCFs were incubated first for 24 h
with or without SFN (10 µM) in serum-free MEM, and then for an
additional 4 h with or without poly(I:C) (3 µg/ml) in the same
medium. Total RNA was isolated from the cells using the RNAprep
pure kit and subjected to RT-qPCR analysis, as previously described
(30). The sequences of the PCR
primers were as follows (30):
TLR3 sense, 5′-CGCCAACTTCACAAGGTA-3′ and antisense,
5′-GGAAGCCAAGCAAAGGAA−3′; hypoxanthine phosphoribosyltransferase 1
(HPRT1) sense, 5′-AGATGGTCAAGGTCGCAAGC-3′; and antisense,
5′-CATATCCTACAACAAACTTGTCTGGAA−3′. PCR was performed with an ABI
Prism 7900 Sequence Detection system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The following thermocycling conditions
were used: Denaturation at 95°C for 15 sec, annealing at 60°C for
15 sec followed by elongation at 60°C for 15 sec. The qPCR results,
recorded as threshold cycle numbers (Cq), were calculated using the
2−ΔΔCq method (31)
with normalization against HPRT1 mRNA as an internal control.
LDH (cytotoxicity) assay
A non-radioactive cytotoxicity assay was used to
assess the production of LDH by cultured corneal fibroblasts.
Portions (50 µl) of the same culture supernatants, used for the
assessment of IL-8, MCP-1 and IL-8, were transferred to a 96-well
flat-bottom plate, then mixed with 50 µl CytoTox reagent. The plate
was covered with an opaque box to prevent light exposure for 30 min
at room temperature, then stop solution was added to each well (50
µl). The LDH assay was performed at an optical density of 490 nm
using a microplate reader (Bio-Rad Laboratories, Inc.). As a
positive control, cells were lysed prior to the assay with a lysis
solution containing detergent provided with the assay kit.
Statistical analysis
Data for each group were derived from at least three
independent samples, and all sampling was repeated three times in
the experimental groups. All statistical analyses were performed
using SPSS software (version 20.0; IBM Corp.). Descriptive results
are expressed as the mean ± standard deviation and were compared
with an unpaired two-tailed t-test or one-way analysis of variance,
followed by a Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Cytotoxicity of SFN in HCFs
To examine whether SFN is cytotoxic to HCFs, the
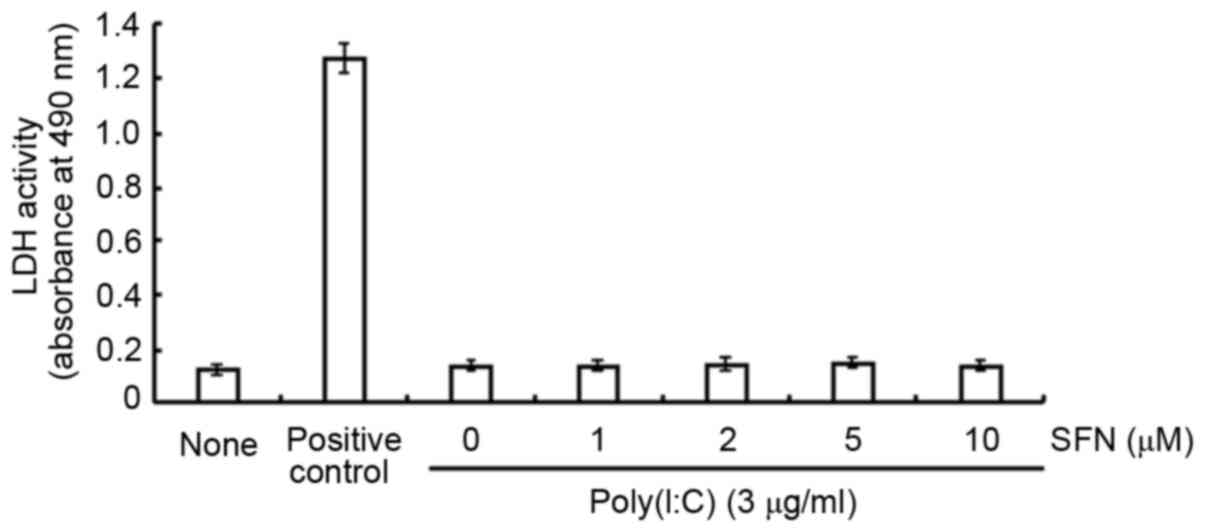
effect of SFN on the release of LDH was investigated. SFN at
concentrations of 1, 2, 5 or 10 µM had no significant effect on LDH
release in the presence of poly(I:C) at 3 µg/ml (Fig. 1), which suggests a lack of
cytotoxicity.
Effects of SFN on the
poly(I:C)-induced release of IL-8, MCP-1 and IL-6 by HCFs
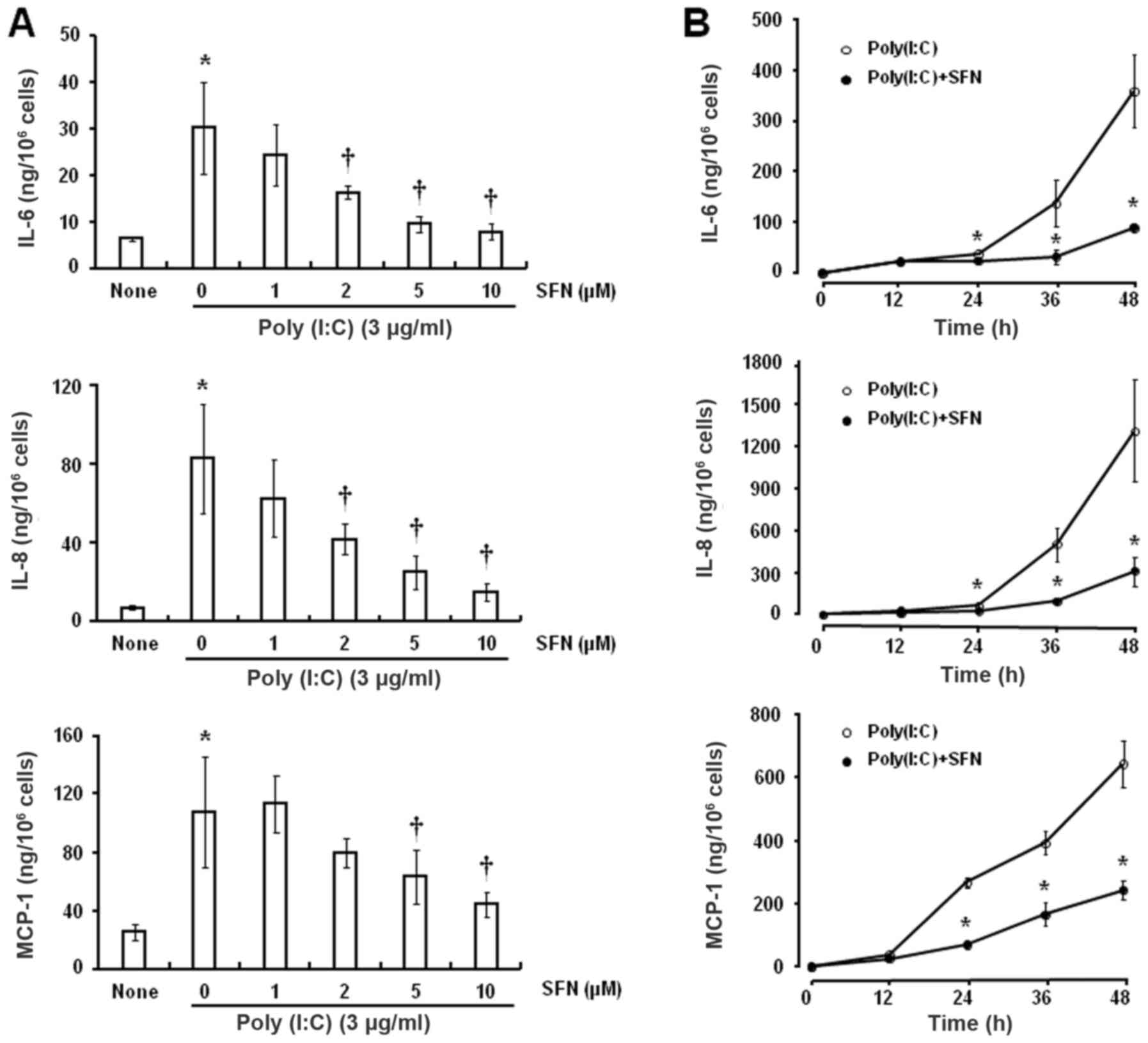
Exposure of HCFs to various concentrations of SFN
for 24 h prior to incubation with poly(I:C) (3 µg/ml) for 24 h
attenuated the secretion of IL-6 and IL-8 in a dose-dependent
manner and that of MCP-1 at 5 and 10 µM SFN (Fig. 2A). The inhibitory effects of SFN
were statistically significant at concentrations of ≥2 µM for IL-6
and IL-8 and at ≥5 µM for MCP-1 compared with 0 µM SFN.
Furthermore, SFN (10 µM) attenuated the poly(I:C)-induced release
of these inflammatory mediators in a time-dependent manner
(Fig. 2B), with each inhibitory
effect being statistically significant following exposure to
poly(I:C) for ≥24 h compared with the respective times in the group
without SFN treatment.
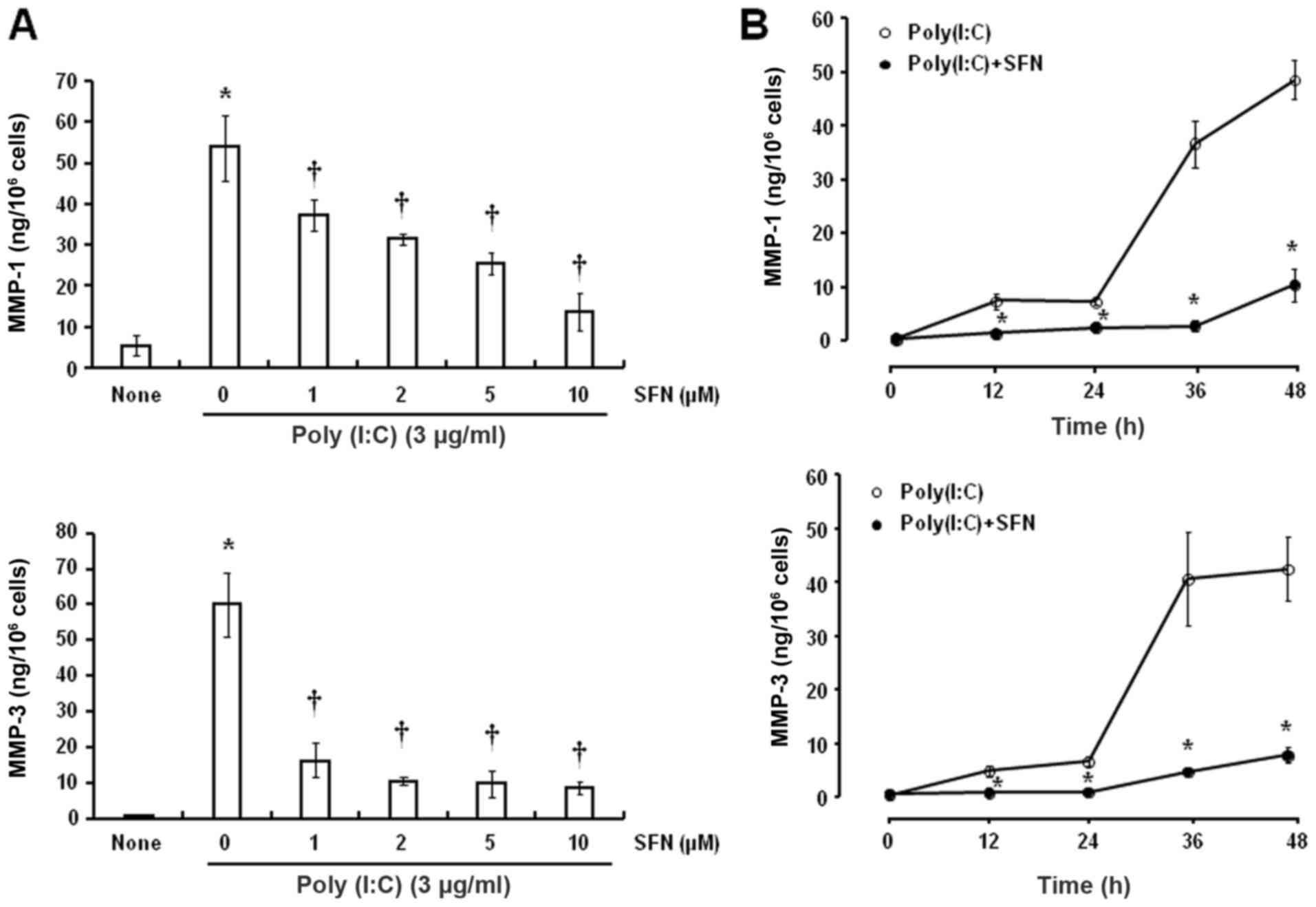
Effects of SFN on poly(I:C)-induced
changes in MMP production by HCFs
Poly(I:C) increased the release of MMP-1 and MMP-3
by HCFs, which was sensitive to inhibition by SFN, in a
dose-dependent manner (Fig. 3A).
The inhibitory effects of SFN were statistically significant at
concentrations ≥1 µM compared with 0 µM SFN. In addition, SFN (10
µM) significantly attenuated the poly(I:C)-induced release of MMP-1
and MMP-3 after exposure to poly(I:C) for 36 and 48 h compared with
the respective times in the group without SFN treatment (Fig. 3B).
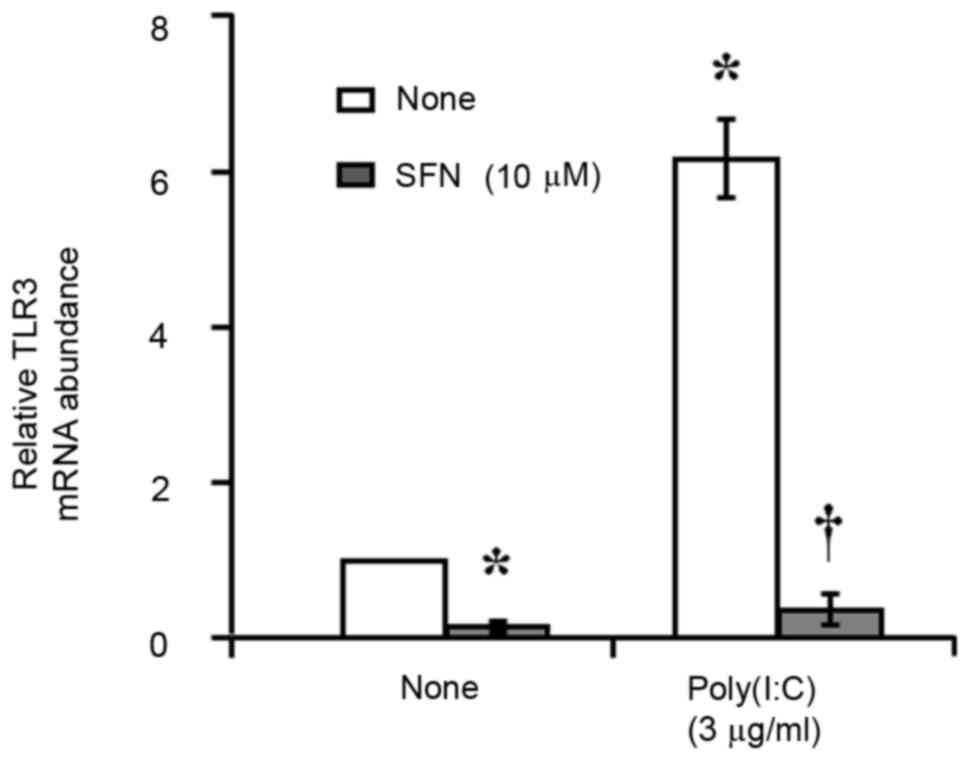
Effect of SFN on poly(I:C)-induced
expression of TLR3 in HCFs
RT-qPCR analysis revealed that incubation of
serum-deprived HCFs with poly(I:C) (3 µg/ml) for 4 h induced
significant upregulation of TLR3 mRNA expression level (Fig. 4). Furthermore, prior exposure of
the cells to SFN (10 µM) for 24 h prevented this effect of
poly(I:C). SFN also reduced the basal abundance of TLR3 mRNA in the
cells (Fig. 4).
Effects of SFN on poly(I:C)-associated
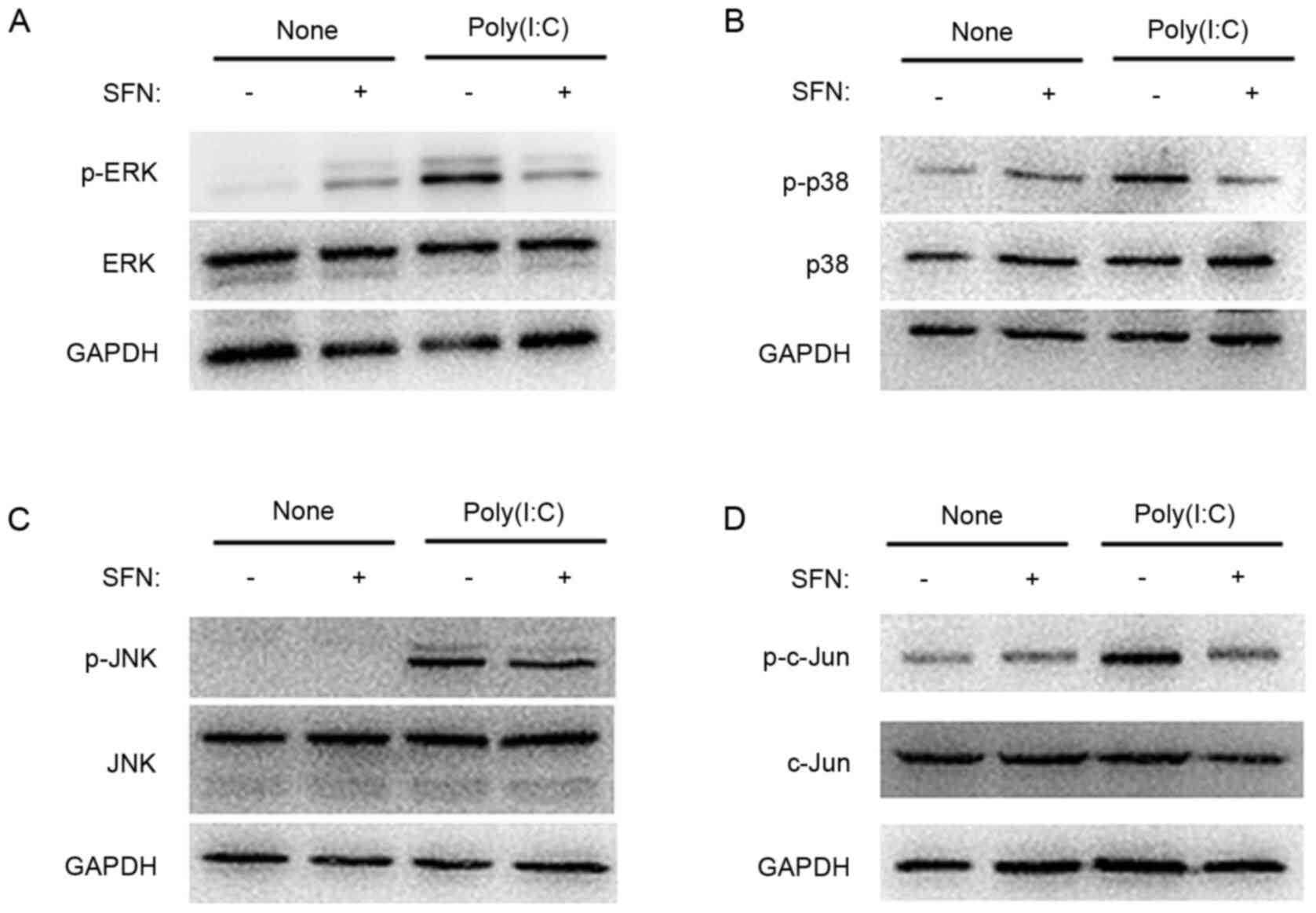
activation of MAPK and AP-1 protein expression level by HCFs
Western blot analysis showed that exposure of HCFs
to poly(I:C) for 90 min increased the expression level of the
phosphorylated forms of ERK, p38, c-Jun NH2-terminal
kinase (JNK) and c-Jun; however, there was no marked effect on the
total amounts of these proteins (Fig.
5), which indicated that poly(I:C) activated the MAPK and AP-1
signal pathways. SFN (10 µM) inhibited the poly(I:C)-induced
phosphorylation of ERK (Fig. 5A),
p38 (Fig. 5B) and c-Jun (Fig. 5D), however, there was no marked
effect on that of JNK (Fig.
5C).
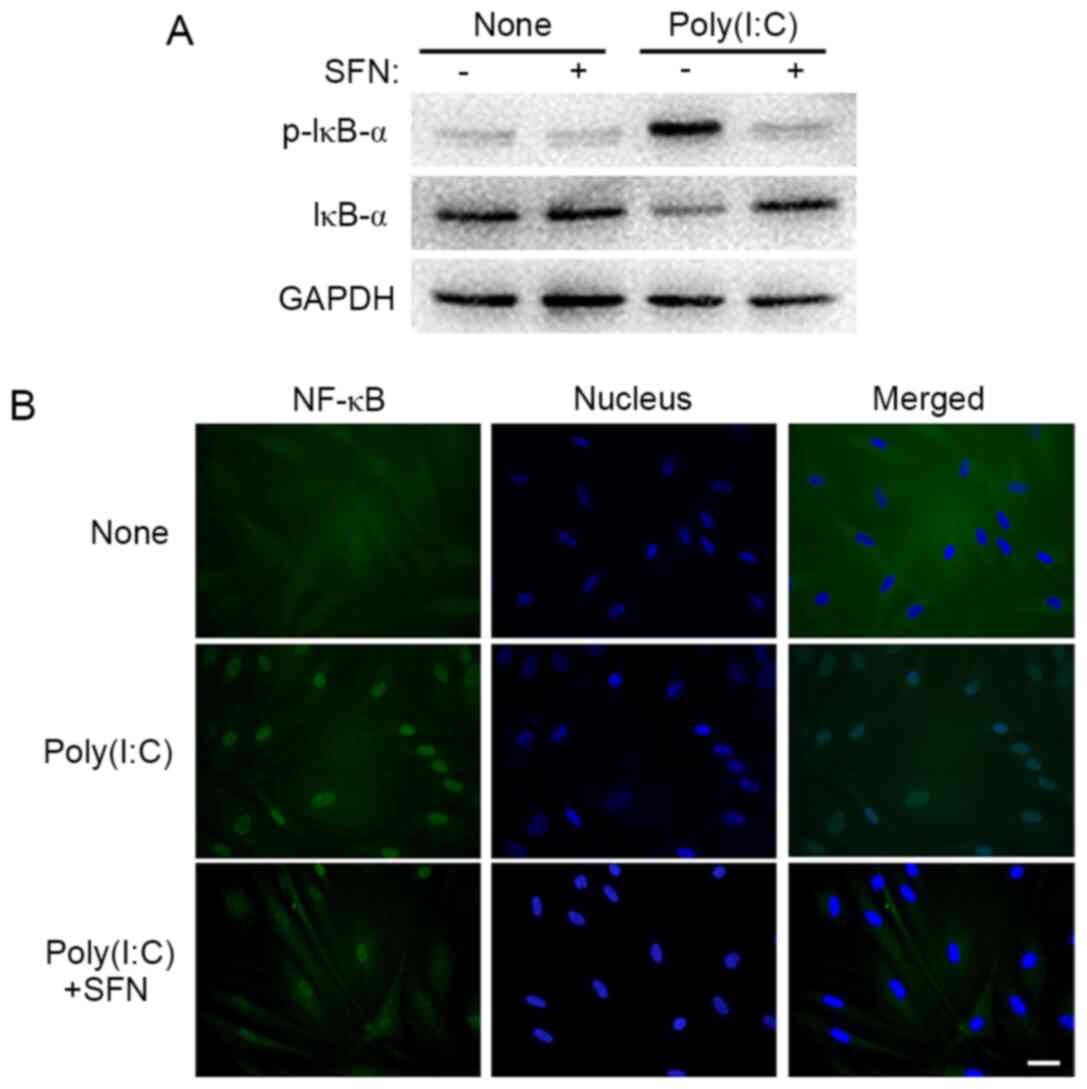
Effects of SFN on poly(I:C)-mediated
activation of IκB-α and NF-κB by HCFs
Western blot analysis revealed that SFN (10 µM)
suppressed the poly(I:C)-induced phosphorylation of the NF-κB
inhibitor IκB-α in HCFs (Fig. 6A).
Furthermore, the immunofluorescence analysis showed that, whereas
the p65 subunit of NF-κB was localized predominantly in the
cytoplasm of HCFs under control conditions, it was localized to the
nucleus following exposure of the cells to poly(I:C) for 90 min,
and the effect of poly(I:C) was partially prevented by SFN
(Fig. 6B). Thus, these results
indicated that SFN attenuated the activation of NF-κB signaling
induced by poly(I:C) in HCFs.
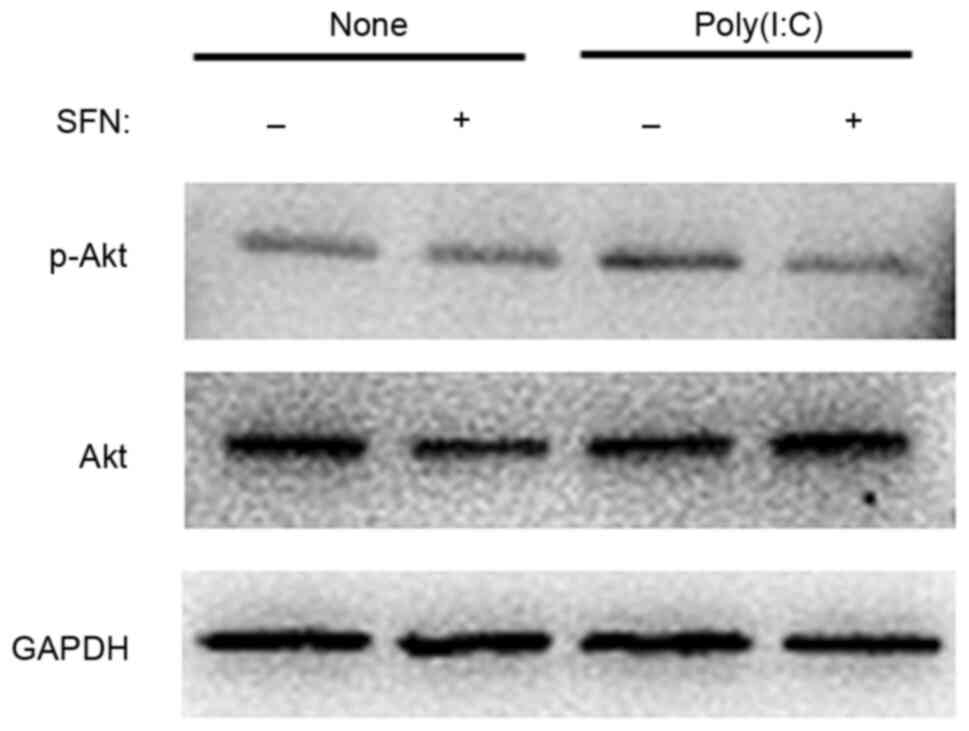
Effect of SFN on the activation of AKT
associated with poly(I:C) in HCFs
Finally, the effect of SFN on the activation of the
phosphoinositide 3-kinase (PI3K)-Akt signaling pathway was
investigated with poly(I:C) in HCFs. Western blot analysis showed
that the phosphorylation of Akt induced by exposure of HCFs to
poly(I:C) for 30 min was inhibited by SFN (10 µM; Fig. 7).
Discussion
Viral stromal keratitis is not only an infectious
disease but also a chronic immunopathological condition (1). Viral stromal keratitis was found to
upregulate the mRNA expression of TLR3 and subsequent production of
various cytokines and chemokines, such as IL-6, IL-8 and MCP-1, in
corneal fibroblasts (32).
Poly(I:C), an analogue of the viral dsRNA, was found to activate
TLR3 and has been adopted as an experimental tool to model the
effects of HSV-1 infection (10,12,13).
The cytokines, IL-8 and IL-6 and the chemokine, MCP-1 have key
roles in the inflammatory response, by attracting neutrophils,
monocytes and macrophages (17–19).
SFN reportedly inhibited the release of IL-6 induced by
lipopolysaccharide in microglial cells (26) in a rat model of endometriosis
(24), and in a mouse model of
acute lung cancer (25). The
present study found that SFN inhibited the poly(I:C)-induced
production of IL-6, IL-8 and MCP-1 by HCFs. This suggested that SFN
could be a promising drug candidate for therapeutic modulation of
the inflammatory response in viral stromal keratitis.
Viral stromal keratitis has been associated with the
proteolytic degradation of stromal collagen, which can lead to
corneal ulceration and ultimately, to the loss of corneal
transparency (21). MMPs are
zinc-dependent enzymes that degrade extracellular matrix proteins,
of which MMP-1 and MMP-3 are two major types and significantly
contribute to corneal ulcers (21). Upregulation of MMP-1 or MMP-3
expression has been associated with varicella zoster virus
(33) and cytomegalovirus
(34) infection. Expression of
MMP-3 was also found elevated in the brain stem of HSV-1-infected
mice (35). The protein expression
of MMPs was induced in viral keratitis (36,37)
and contributed to tissue infiltration by polymorphonuclear
leukocytes (38). These various
observations indicated the important role of MMPs in the
pathogenesis of disease associated with viral keratitis. Consistent
with a previous study (39), the
present study found that poly(I:C) induced the concentration of
MMP-1 and MMP-3 in corneal fibroblasts. This suggested that cells
associated with lesions of viral keratitis may contribute to
remodeling of the extracellular matrix and consequently corneal
ulceration by producing MMPs. In previous studies, SFN was shown to
prevent the upregulation of MMP-1 induced by ultraviolet
irradiation in the skin of mice (40), and inhibited the production of
MMP-1 and MMP-3 stimulated by IL-1β in synovial fibroblasts
associated with rheumatoid arthritis (41). In the present study, SFN was found
to suppress the poly(I:C)-associated release of these two MMPs by
HCFs. Further studies are required to determine the effect of SFN
on viral corneal ulceration; however, these results indicated that
this agent may prove an effective treatment for this condition.
TLRs contribute to the initiation and modulation of
inflammation in the eye (30).
Upregulation of TLR3 mRNA expression in the human cornea has been
association with HSV-1 infection (15). TLR3 is a specific receptor for
dsRNA and would not be expected to detect DNA derived from a DNA
virus, such as HSV-1 (15).
However, dsRNA is produced by most viruses during their replication
cycle and is considered a molecular marker of viral infection
(42). As a synthetic analog of
viral dsRNA, poly(I:C) is recognized by TLR3 (43). The present study found that
poly(I:C) upregulated the TLR3 mRNA expression level in HCFs and
that SFN attenuated this effect. Thus, the inhibitory effects of
SFN on cytokine, chemokine and MMP expression in HCFs, exposed to
poly(I:C) may be mediated by attenuation of the TLR3 signaling
pathway.
Stimulation of TLR3 initiates a cascade of
intracellular signaling, including that mediated by MAPKs and
PI3K-Akt, and results in the activation of NF-κB or AP-1 (14). All of these signaling pathways have
been associated with inflammation, including that of the cornea
(13,16,44,45).
NF-κB, a transcription factor, mediates the mRNA expression level
of inflammation-related genes, including cytokines, chemokines and
adhesion molecules (46). Under
basal conditions, NF-κB is bound to the inhibitor, IκB in the
cytoplasm (47). The
phosphorylation of IκB, induced by inflammatory stimuli, triggers
its degradation and consequently transfers the active NF-κB to the
nucleus, where NF-κB, in turn, activates the mRNA expression level
of cytokines, chemokines and MMP-related genes (13,16).
The JNK, p38 MAPKs and ERK are important pathways in the regulation
of various cell activities, such as cell proliferation,
differentiation and migration, and are significantly associated
with inflammation, innate immunity and apoptosis (48,49).
The activation of TLRs by components of pathogens induces the
phosphorylation of MAPKs, which can then lead to IκB
phosphorylation and activation of NF-κB (50). The transcription factor, AP-1, also
mediates the mRNA expression level of inflammatory genes (51). Activation of the AP-1 component
c-Jun triggers the release of inflammatory mediators, and MMPs in
human synoviocytes (45). It has
been shown that the activation of the PI3K-Akt signaling pathway
was associated with the regulation of MCP-1 mRNA and protein
expression in human retinal pigment epithelial cells (52). Poly(I:C) was confirmed to induce
the activation of Akt in HCFs (44). In the present study, SFN was
demonstrated to inhibit the poly(I:C)-induced activation of ERK,
p38, c-Jun and AKT, and the degradation of IκB-α and the nuclear
translocation of NF-κB in HCFs. This suggested that the
anti-inflammatory effects of SFN in these cells may be mediated by
the attenuation of signaling by the MAPK, AP-1, PI3K-Akt and NF-κB
pathways.
In conclusion, the present study showed that SFN
inhibited the poly(I:C)-associated release of proinflammatory
cytokines, chemokines and MMPs in HCFs, potentially through
suppression of the TLR3, MAPK (ERK and p38), NF-κB, AP-1 and Akt
signaling pathways. SFN may be a potential treatment for corneal
viral infection by limiting immune cell infiltration. Further
research is warranted to investigate the potential efficacy of SFN
for the treatment of viral stromal keratitis.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Science
Foundation of China (grant no. 81770889), the Natural Science
Foundation of Guangdong Province (grant no. 2017A030313774), and
from the Natural Science Foundation of Guangdong Province (grant
no. 2018 A030313428).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YaL, YeL and XZha contributed to the study design.
PL, HZ and LC performed the experiments. PL, XZhe and XY analyzed
the data. XZhe and YaL wrote the first draft of the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rowe AM, St Leger AJ, Jeon S, Dhaliwal DK,
Knickelbein JE and Hendricks RL: Herpes keratitis. Prog Retin Eye
Res. 32:88–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Farooq AV and Shukla D: Herpes simplex
epithelial and stromal keratitis: An epidemiologic update. Surv
Ophthalmol. 57:448–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reynaud C, Rousseau A, Kaswin G, M'Garrech
M, Barreau E and Labetoulle M: Persistent impairment of quality of
life in patients with herpes simplex keratitis. Ophthalmology.
124:160–169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Biswas PS and Rouse BT: Early events in
HSV keratitis-setting the stage for a blinding disease. Microbes
Infect. 7:799–810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Knickelbein JE, Hendricks RL and
Charukamnoetkanok P: Management of herpes simplex virus stromal
keratitis: An evidence-based review. Surv Ophthalmol. 54:226–234.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Urtti A: Challenges and obstacles of
ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev.
58:1131–1135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
DelMonte DW and Kim T: Anatomy and
physiology of the cornea. J Cataract Refract Surg. 37:588–598.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith RS, Smith TJ, Blieden TM and Phipps
RP: Fibroblasts as sentinel cells. Synthesis of chemokines and
regulation of inflammation. Am J Pathol. 151:317–322.
1997.PubMed/NCBI
|
|
9
|
Xi X, McMillan DH, Lehmann GM, Sime PJ,
Libby RT, Huxlin KR, Feldon SE and Phipps RP: Ocular fibroblast
diversity: Implications for inflammation and ocular wound healing.
Invest Ophthalmol Vis Sci. 52:4859–4865. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee G, Park JS, Lee EJ, Ahn JH and Kim HS:
Anti-inflammatory and antioxidant mechanisms of urolithin B in
activated microglia. Phytomedicine. 55:50–57. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guan X, Zhang M, Fu M, Luo S and Hu Q:
Herpes simplex virus type 2 immediate early protein ICP27 inhibits
IFN-beta production in mucosal epithelial cells by antagonizing
IRF3 activation. Front Immunol. 10:2902019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herbert C, Zeng QX, Shanmugasundaram R,
Garthwaite L, Oliver BG and Kumar RK: Response of airway epithelial
cells to double-stranded RNA in an allergic environment. Transl
Respir Med. 2:112014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Kimura K, Yanai R, Chikama T and
Nishida T: Cytokine, chemokine, and adhesion molecule expression
mediated by MAPKs in human corneal fibroblasts exposed to
poly(I:C). Invest Ophthalmol Vis Sci. 49:3336–3344. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garrington TP and Johnson GL: Organization
and regulation of mitogen-activated protein kinase signaling
pathways. Curr Opin Cell Biol. 11:211–218. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsumoto M, Funami K, Oshiumi H and Seya
T: Toll-like receptor 3: A link between toll-like receptor,
interferon and viruses. Microbiol Immunol. 48:147–154. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kimura K, Orita T, Kondo Y, Zhou H and
Nishida T: Upregulation of matrix metalloproteinase expression by
poly(I:C) in corneal fibroblasts: Role of NF-κB and interleukin-1β.
Invest Ophthalmol Vis Sci. 51:5012–5018. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bazzoni F, Cassatella MA, Rossi F, Ceska
M, Dewald B and Baggiolini M: Phagocytosing neutrophils produce and
release high amounts of the neutrophil-activating peptide
1/interleukin 8. J Exp Med. 173:771–774. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bianconi V, Sahebkar A, Atkin SL and Pirro
M: The regulation and importance of monocyte chemoattractant
protein-1. Curr Opin Hematol. 25:44–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scheller J, Chalaris A, Schmidt-Arras D
and Rose-John S: The pro- and anti-inflammatory properties of the
cytokine interleukin-6. Biochim Biophys Acta. 1813:878–888. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thomas J, Kanangat S and Rouse BT: Herpes
simplex virus replication-induced expression of chemokines and
proinflammatory cytokines in the eye: Implications in herpetic
stromal keratitis. J Interferon Cytokine Res. 18:681–690. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fini ME, Cook JR and Mohan R: Proteolytic
mechanisms in corneal ulceration and repair. Arch Dermatol Res. 290
(Suppl):S12–S23. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matusheski NV, Juvik JA and Jeffery EH:
Heating decreases epithiospecifier protein activity and increases
sulforaphane formation in broccoli. Phytochemistry. 65:1273–1281.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lenzi M, Fimognari C and Hrelia P:
Sulforaphane as a promising molecule for fighting cancer. Cancer
Treat Res. 159:207–223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou A, Hong Y and Lv Y: Sulforaphane
attenuates endometriosis in rat models through inhibiting PI3K/Akt
signaling pathway. Dose Response. 17:15593258198555382019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qi T, Xu F, Yan X, Li S and Li H:
Sulforaphane exerts anti-inflammatory effects against
lipopolysaccharide-induced acute lung injury in mice through the
Nrf2/ARE pathway. Int J Mol Med. 37:182–188. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qin S, Yang C, Huang W, Du S, Mai H, Xiao
J and Lü T: Sulforaphane attenuates microglia-mediated neuronal
necroptosis through down-regulation of MAPK/NF-κB signaling
pathways in LPS-activated BV-2 microglia. Pharmacol Res.
133:218–235. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu H, Smith AJO, Lott MC, Bao Y, Bowater
RP, Reddan JR and Wormstone IM: Sulforaphane can protect lens cells
against oxidative stress: Implications for cataract prevention.
Invest Ophthalmol Vis Sci. 54:5236–5248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dulull NK, Dias DA, Thrimawithana TR and
Kwa FAA: L-sulforaphane confers protection against oxidative stress
in an in vitro model of age-related macular degeneration. Curr Mol
Pharmacol. 11:237–253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ziaei A, Schmedt T, Chen Y and Jurkunas
UV: Sulforaphane decreases endothelial cell apoptosis in fuchs
endothelial corneal dystrophy: A novel treatment. Invest Ophthalmol
Vis Sci. 54:6724–6734. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Erdinest N, Aviel G, Moallem E, Anteby I,
Yahalom C, Mechoulam H, Ovadia H and Solomon A: Expression and
activation of toll-like receptor 3 and toll-like receptor 4 on
human corneal epithelial and conjunctival fibroblasts. J Inflamm
(Lond). 11:32014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin X, Qin Q, Chen W and Qu J: Expression
of toll-like receptors in the healthy and herpes simplex
virus-infected cornea. Cornea. 26:847–852. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nagel MA, Choe A, Rempel A, Wyborny A,
Stenmark K and Gilden D: Differential regulation of matrix
metalloproteinases in varicella zoster virus-infected human brain
vascular adventitial fibroblasts. J Neurol Sci. 358:444–446. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prochnau D, Lehmann M, Straube E, Figulla
HR and Rödel J: Human cytomegalovirus induces MMP-1 and MMP-3
expression in aortic smooth muscle cells. Acta Microbiol Immunol
Hung. 58:303–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Caignard G, Leiva-Torres GA, Leney-Greene
M, Charbonneau B, Dumaine A, Fodil-Cornu N, Pyzik M, Cingolani P,
Schwartzentruber J, Dupaul-Chicoine J, et al: Genome-wide mouse
mutagenesis reveals CD45-mediated T cell function as critical in
protective immunity to HSV-1. PLoS Pathog. 9:e10036372013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang YN, Bauer D, Wasmuth S, Steuhl KP and
Heiligenhaus A: Matrix metalloproteinases (MMP-2 and 9) and tissue
inhibitors of matrix metalloproteinases (TIMP-1 and 2) during the
course of experimental necrotizing herpetic keratitis. Exp Eye Res.
77:227–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Heiligenhaus A, Li HF, Yang Y, Wasmuth S,
Steuhl KP and Bauer D: Transplantation of amniotic membrane in
murine herpes stromal keratitis modulates matrix metalloproteinases
in the cornea. Invest Ophthalmol Vis Sci. 46:4079–4085. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wiegand C, Schönfelder U, Abel M, Ruth P,
Kaatz M and Hipler UC: Protease and pro-inflammatory cytokine
concentrations are elevated in chronic compared to acute wounds and
can be modulated by collagen type I in vitro. Arch Dermatol Res.
302:419–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kimura K, Nomi N, Yan ZH, Orita T and
Nishida T: Inhibition of poly(I:C)-induced matrix metalloproteinase
expression in human corneal fibroblasts by triptolide. Mol Vis.
17:526–532. 2011.PubMed/NCBI
|
|
40
|
Chaiprasongsuk A, Lohakul J, Soontrapa K,
Sampattavanich S, Akarasereenont P and Panich U: Activation of Nrf2
reduces UVA-mediated MMP-1 upregulation via MAPK/AP-1 signaling
cascades: The photoprotective effects of sulforaphane and
hispidulin. J Pharmacol Exp Ther. 360:388–398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Choi YJ, Lee WS, Lee EG, Sung MS and Yoo
WH: Sulforaphane inhibits IL-1β-induced proliferation of rheumatoid
arthritis synovial fibroblasts and the production of MMPs, COX-2,
and PGE2. Inflammation. 37:1496–1503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meylan E and Tschopp J: Toll-like
receptors and RNA helicases: Two parallel ways to trigger antiviral
responses. Mol Cell. 22:561–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takeuchi O and Akira S: Toll-like
receptors; their physiological role and signal transduction system.
Int Immunopharmacol. 1:625–635. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Orita T, Kimura K, Zhou HY and Nishida T:
Poly(I:C)-induced adhesion molecule expression mediated by
NF-{kappa}B and phosphoinositide 3-kinase-Akt signaling pathways in
human corneal fibroblasts. Invest Ophthalmol Vis Sci. 51:5556–5560.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sweeney SE, Kimbler TB and Firestein GS:
Synoviocyte innate immune responses: II. Pivotal role of IFN
regulatory factor 3. J Immunol. 184:7162–7168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Arthur JS and Ley SC: Mitogen-activated
protein kinases in innate immunity. Nat Rev Immunol. 13:679–692.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Trop-Steinberg S and Azar Y: AP-1
expression and its clinical relevance in immune disorders and
cancer. Am J Med Sci. 353:474–483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bian ZM, Elner SG, Yoshida A and Elner VM:
Differential involvement of phosphoinositide 3-kinase/Akt in human
RPE MCP-1 and IL-8 expression. Invest Ophthalmol Vis Sci.
45:1887–1896. 2004. View Article : Google Scholar : PubMed/NCBI
|