Introduction
Chronic kidney disease (CKD), including diabetic
nephropathy (DN), is frequently accompanied by dyslipidemia, which
in turn promotes the occurrence and progression of CKD (1–4).
Dyslipidemia is mainly manifested as decreased high density
lipoprotein-C levels, and high levels of serum triglycerides and
long-chain free fatty acids (FFAs) (1). Lipid accumulation, including the
presence of saturated FFAs in the glomeruli and proximal tubules,
may induce endoplasmic reticulum (ER) stress and generation of
reactive oxygen species (ROS), ultimately leading to cellular
lipotoxicity, apoptosis and inflammation, which serve an important
role in advanced CKD (5).
As terminal differentiated cells, podocyte injury
has been considered a vital event in the pathological mechanism of
CKD. Changes in podocyte structure and podocytopenia can cause
proteinuria and renal dysfunction, and can eventually contribute to
the development of DN (6,7). During the onset and development of DN,
lipid accumulation in podocytes enhanced by high glucose has been
reported to have a key role in podocyte injury (8). Oxidative stress, ER stress and the
development of inflammation may be the main mechanisms underlying
podocyte damage caused by excess lipids (9,10).
Furthermore, palmitic acid (PA), which is a type of saturated FFA,
is abundant in human plasma, and increased levels of FFAs have been
demonstrated to be detrimental to various cell types, including
podocytes (11). Previous studies
have revealed that PA-induced podocyte apoptosis may be ameliorated
by reducing ER stress (10,12). In our previous study, oxidative
stress was demonstrated to be involved in fatty acid-induced
podocyte apoptosis (13).
Berberine (BBR) is a Chinese medicine originally
extracted from Coptis root and Phellodendron, which
has been widely used as a clinical medicine to treat diarrhea in
China (14,15). Previous studies reported the
therapeutic effects of BBR on hypertension and hyperlipidemia, with
no severe side effects (16,17).
BBR has been used in DN treatment because it inhibits oxidative
stress and aldose reductase (18).
In addition, it has been shown that BBR may clearly attenuate the
progression of nonalcoholic fatty liver disease by targeting ER
stress in vivo and in vitro (19). Our previous study also revealed that
BBR may ameliorate Aldo-induced podocyte injury by inhibiting
oxidative stress and ER stress (20). However, it is not clear whether BBR
has a similar effect on PA-induced podocyte apoptosis. Therefore,
the specific mechanism underlying the protective effects of BBR on
PA-induced podocyte apoptosis was explored in the present
study.
Materials and methods
Cell culture and treatment
The conditionally immortalized mouse podocyte cell
line MPC5 was kindly donated by Dr Ruan (Centre for Nephrology,
Royal Free and University College Medical School, London, UK) and
was cultured as previously described (21). Briefly, the cells were cultured in
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) containing 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.),
penicillin (100 U/ml), streptomycin (100 µg/ml) and 10 U/ml
interferon-γ (IFN-γ; PeproTech, Inc.) at 33°C in an atmosphere
containing 5% CO2. Once cell confluence reached 70–80%,
cells were cultured in RPMI-1640 complete medium without 10 U/ml
IFN-γ at 37°C in an atmosphere containing 5% CO2 for
10–14 days to induce podocyte differentiation. The differentiated
podocytes which were polygonal with foot-like structures were
subsequently treated with PA (150 µmol/l) for 24 h, following
pretreatment with BBR (8 µmol/l), 4-phenylbutyric acid (4-PBA; 10
µmol/l) or N-acetylcysteine (NAC; 150 µmol/l; all from
Sigma-Aldrich; Merck KGaA) for 2 h at room temperature.
Western blot analysis
After treatment with the aforementioned reagents,
floating cells were collected in an Eppendorf tube, centrifuged at
800 × g for 3 min at room temperature and the supernatant was
removed. Cells in the Eppendorf tube and cultured cells remaining
in the petri dish were washed twice with 1X PBS and lysed in RIPA
lysis buffer (Beyotime Institute of Biotechnology) containing
protease inhibitors on ice. Subsequently, the cells were sonicated
(20–25 KHz at 4°C) for 15 sec and centrifuged at 12,000 × g for 12
min at 4°C to obtain the total protein samples. Protein samples
were then heated at 100°C for 10 min after determination of protein
concentration using a bicinchoninic acid protein assay kit
(Beyotime Institute of Biotechnology), and were mixed with loading
buffer. Subsequently, proteins (1–3 mg/ml) were separated by
SDS-PAGE on 10% gels, transferred onto PVDF membranes (EMD
Millipore) and blocked with 5% non-fat milk for 3 h at room
temperature. The PVDF membranes were then incubated overnight at
4°C with antibodies against β-actin (1:5,000; cat. no. KM9001T;
Tiangin Sungene Biotech Co., Ltd.), binding immunoglobulin protein
(BIP; 1:1,000; cat. no. 3177; Cell Signaling Technology, Inc.),
protein kinase RNA-like ER kinase (PERK; 1:1,000; cat. no. WL03378;
Wanleibio Co., Ltd.), activating transcription factor (ATF)4
(1:1,000; cat. no. 11815; Cell Signaling Technology, Inc.), C/EBP
homologous protein (CHOP; 1:1,000; cat. no. 5554; Cell Signaling
Technology, Inc.), ATF6 (1:1,000; cat. no. 65880; Cell Signaling
Technology, Inc.), inositol-requiring enzyme 1α (IRE1α; 1:1,000;
cat. no. 3294; Cell Signaling Technology, Inc.), caspase 12
(1:1,000; cat. no. 2202; Cell Signaling Technology, Inc.), and
cleaved-caspase 3 (1:1,000; cat. no. 9664; Cell Signaling
Technology, Inc.). Subsequently, the membranes were washed and
incubated with horseradish peroxidase-conjugated secondary
antibodies [mouse; 1:5,000; cat. no. GAM007; and rabbit; 1:5,000;
cat. no. GAR007; Multisciences (Lianke) Biotech Co., Ltd.] for 1 h
at room temperature. Blots were then incubated with a
chemiluminescence staining reagent kit (Advansta, Inc.), and the
proteins were detected using a chemiluminescence system (Fusion
FX5; Vilber Lourmat). Protein intensity was semi-quantified with
Fusion analysis software (FX5; Vilber Lourmat).
Immunofluorescence
Podocytes grown on coverslips to 70–80% confluence
were washed with 1X PBS, fixed in 4% paraformaldehyde for 30 min,
permeabilized with 0.2% Triton X-100 for 10 min and blocked with 5%
BSA (Sigma-Aldrich; Merck KGaA) for 1 h, all at room temperature.
The cells were then incubated with rabbit anti-BIP antibody (1:200;
Cell Signaling Technology, Inc.) at 4°C overnight, followed by
incubation with a green-fluorescence Alexa Fluor® 488
goat anti-rabbit IgG antibody (1:400; cat. no. A11008, Invitrogen;
Thermo Fisher Scientific, Inc.) for 1 h at 37°C, and
counterstaining with DAPI for 5 min at room temperature. Finally,
images were observed under a fluorescence microscope.
Measurement of ROS
Podocytes were cultured in 12-well plates to 70–80%
confluence, washed with 1X PBS after treatment with the
aforementioned reagents and incubated with 10 µmol/l
2′,7′-dichlorofluorescein diacetate (DCFH-DA; Sigma-Aldrich; Merck
KGaA) for 30 min at 37°C. Cell fluorescence was detected using a
fluorescence microscope, with an excitation wavelength of 488 nm
and an emission wavelength of 525 nm.
Flow cytometric analysis
An Annexin V-FITC/propidium iodide (PI) apoptosis
assay kit (Tiangin Sungene Biotech Co., Ltd.) was applied to detect
cell apoptosis, according to the manufacturer's protocol. Briefly,
cells at 70–80% confluence were harvested and washed with cold 1X
PBS twice. Subsequently, the cells were centrifuged at 1,000 × g at
4°C for 5 min, resuspended in 300 µl binding buffer, and then
incubated with 5 µl Annexin V-FITC and 5 µl PI for 15 min at room
temperature in the dark. Subsequently, 200 µl binding buffer was
added to each sample tube and the cells were analyzed using a BD
FACSVantage SE cytometer (BD Biosciences). Annexin
V-positive/PI-negative podocytes were considered to indicate the
early stages of apoptosis, whereas Annexin V-positive/PI-positive
podocytes were considered to indicate late apoptotic or necrotic
cells. The apoptotic rate of podocytes was calculated as the sum of
early and late apoptosis cells.
Cell counting Kit-8 (CCK-8)
CCK-8 (Dojindo Molecular Technologies, Inc.) was
used to determine cell death and viability, according to the
manufacturer's instructions. Briefly, the cells were cultured in
96-well plates (3.0×103 cells/well). Podocytes were
treated with PA (150 µmol/l) for 24 h after pretreatment with
different concentrations of BBR (2, 4, 8 and 16 µmol/l) for 2 h,
and then incubated at 37°C for 2 h after the addition of 10 µl
CCK-8 working reagent and 90 µl RMPI-1640 medium to each well. The
absorbance was measured at 450 mm with a spectrophotometer (Thermo
Fisher Scientific, Inc.). The cell death rate was calculated by
formula: [1-A450 (experimental)/A450 (control)] ×100.
Statistical analysis
Statistical analyses were performed with Graph Pad
Prism 6.0 (GraphPad Software, Inc.). All analytical data were
obtained from three independent experimental repeats. Unpaired
Student's t-test was used to compare the difference between two
groups, whereas comparisons among multiple groups were analyzed
using one-way ANOVA followed by Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
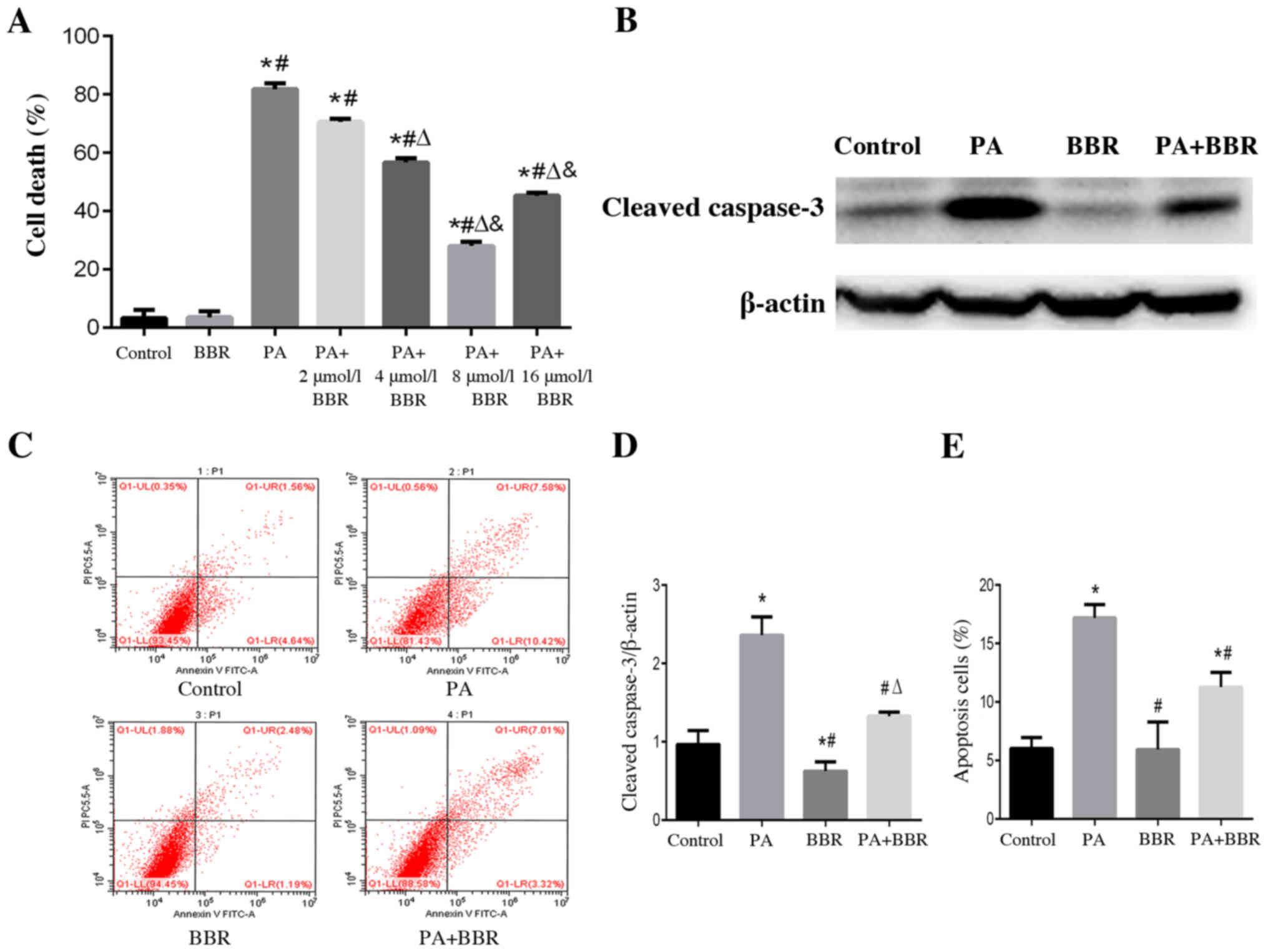
BBR alleviates PA-induced podocyte
death and apoptosis
According to our previous study, PA at a
concentration of 150 µmol/l induced marked lipid accumulation and
apoptosis of podocytes (22);
therefore, 150 µmol/l PA was selected in the present study to treat
podocytes. To evaluate the effects of BBR on PA-induced cell death,
podocytes were treated with PA and different concentrations of BBR
for 24 h. Using the CCK-8 cell viability assay, a significant
increase in podocyte death was observed in the PA group compared
with in the control group, whereas a significant amelioration of
PA-induced cell death was observed when cells were pretreated with
BBR at a concentration of 4, 8 and 16 µmol/l (Fig. 1A). Podocytes pretreated with 8
µmol/l BBR had a higher survival rate than those in the other
treatment groups. Therefore, the suitable concentration of BBR to
treat podocytes in further experiments was considered to be 8
µmol/l (Fig. 1A). Cleaved-caspase 3
is an important marker of apoptosis, which is one of the main types
of cell death (22). The expression
levels of cleaved-caspase 3 were detected using western blotting
(Fig. 1B and D) and apoptotic rate
was assessed by flow cytometry (Fig. 1C
and E); the results confirmed that PA induced podocyte
apoptosis and BBR alleviated PA-induced podocyte apoptosis.
PA induces ER stress in podocytes
When ER stress occurs, the ER aims to maintain a
stable cellular environment by increasing the ER capacity and
activating the ER-associated protein degradation pathway (23). The ER chaperone BIP, also known as
glucose-regulated protein 78, has an important role in protein
folding. Dissociation of BIP from ER-bound sensors, such as PERK,
IRE1α and ATF6, to bind unfolded or misfolded proteins is the
consequence of ER stress, which can regulate the transcription of
BIP to increase the ER capacity (24,25).
To explore whether ER stress may be involved in PA-induced podocyte
apoptosis, western blotting was used to detect the protein
expression levels of BIP. The results revealed that PA treatment
increased the expression of BIP in a concentration-dependent manner
(Fig. 2A and B). A similar finding
regarding BIP expression in podocytes following PA treatment was
confirmed by immunofluorescent staining (Fig. 2H and I). Furthermore, the protein
expression levels of PERK, ATF4, CHOP, IRE1α and ATF6, which are
involved in the ER stress-mediated pathway, were also increased
(Fig. 2A and C-G). These findings
indicated that ER stress and its three downstream signaling
pathways (PERK, IRE1α and ATF6) may be activated by PA treatment in
podocytes.
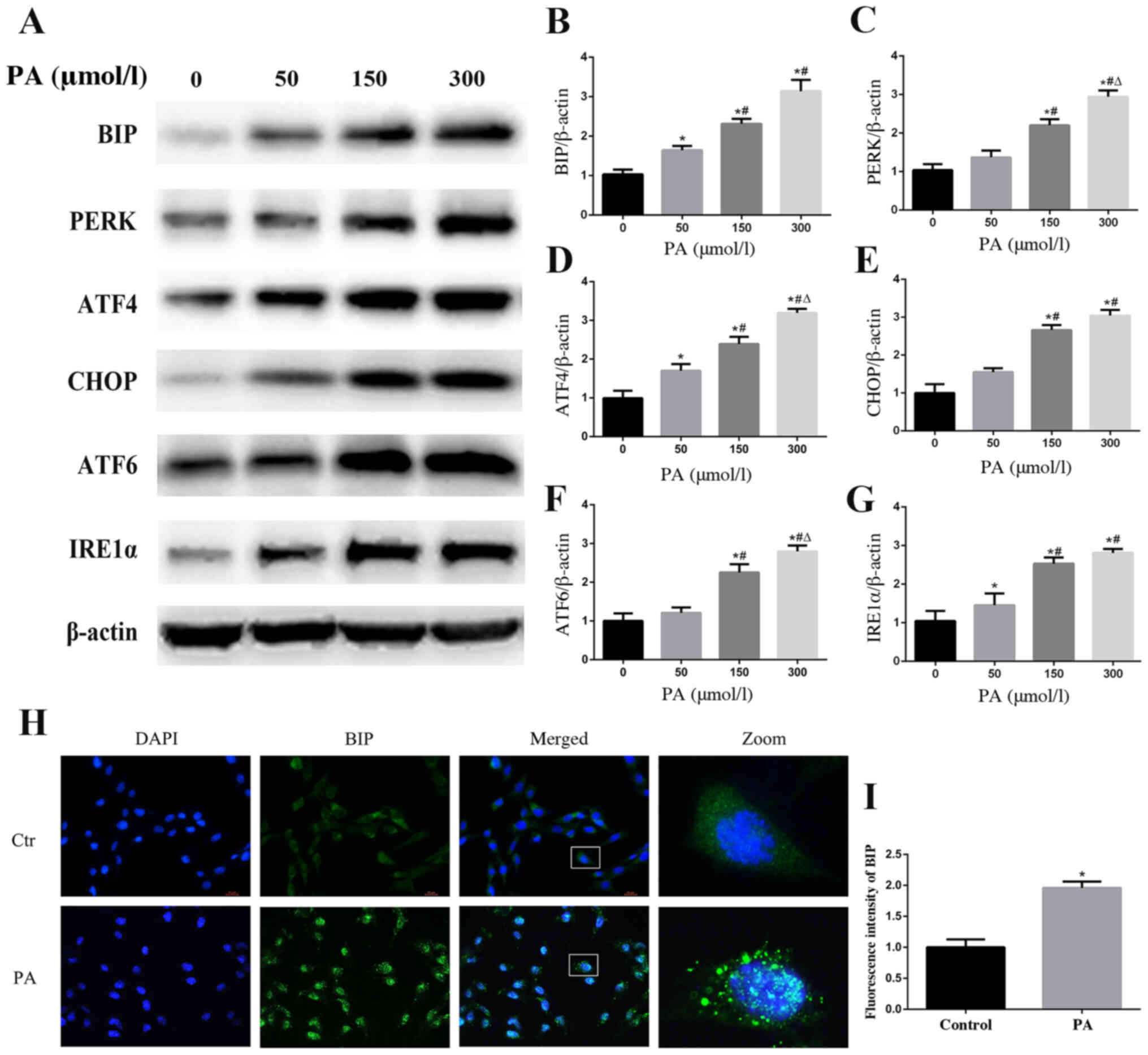 | Figure 2.PA induces endoplasmic reticulum
stress in podocytes. (A) Representative images of BIP, PERK, ATF4,
CHOP, ATF6 and IRE1α expression, as detected by western blot
analysis after treatment with different concentrations of PA.
Densitometric analysis of the results shown in (A) for (B) BIP, (C)
PERK, (D) ATF4, (E) CHOP, (F) ATF6 and (G) IRE1α. Data are
presented as the mean ± SEM (n=3). *P<0.05 vs. 0 µmol/l group,
#P<0.05 vs. 50 µmol/l group, ΔP<0.05
vs. 150 µmol/l group. (H) BIP expression was detected by
fluorescence microscopy after the cells had been treated with PA
(150 µmol/l) for 24 h (magnification, ×400). The rightmost panels
show the enlarged (×7) views of areas in the left panels. (I)
Fluorescence intensities of five random fields per group, as shown
in (H), were determined and analyzed. Data are presented as the
mean ± SEM (n=3). *P<0.05 vs. control group. PA, palmitic acid;
BIP, binding immunoglobulin protein; PERK, protein kinase RNA-like
endoplasmic reticulum kinase; ATF, activating transcription factor;
CHOP, C/EBP homologous protein; IRE1α, inositol-requiring enzyme
1α; SEM, standard error of the mean. |
ER stress is involved in PA-induced
apoptosis of podocytes
To further validate the role of ER stress in
PA-induced apoptosis, podocytes were treated with 10 µmol/l 4-PBA,
an ER stress inhibitor, with or without PA (150 µmol/l) for 24 h.
The results of western blotting revealed that treatment with 4-PBA
ameliorated PA-induced expression of proteins associated with the
three downstream signaling pathways (Fig. 3A-G). In addition, decreased signal
intensity of BIP in podocytes treated with 4-PBA was observed
compared with that exhibited following treatment with PA only, as
determined using immunofluorescent staining (Fig. 3I and J). These results suggested
that 4-PBA may alleviate ER stress induced by PA in podocytes.
Furthermore, an ER stress-associated apoptosis indicator, caspase
12, was significantly downregulated by 4-PBA in PA-treated
podocytes (Fig. 3A and H).
Furthermore, the expression of cleaved-caspase 3 (Fig. 3K and L) and PA-induced podocyte
apoptosis was significantly decreased (Fig. 3M and N) when cells were treated with
4-PBA. These results suggested that ER stress may participate in
PA-induced podocyte apoptosis.
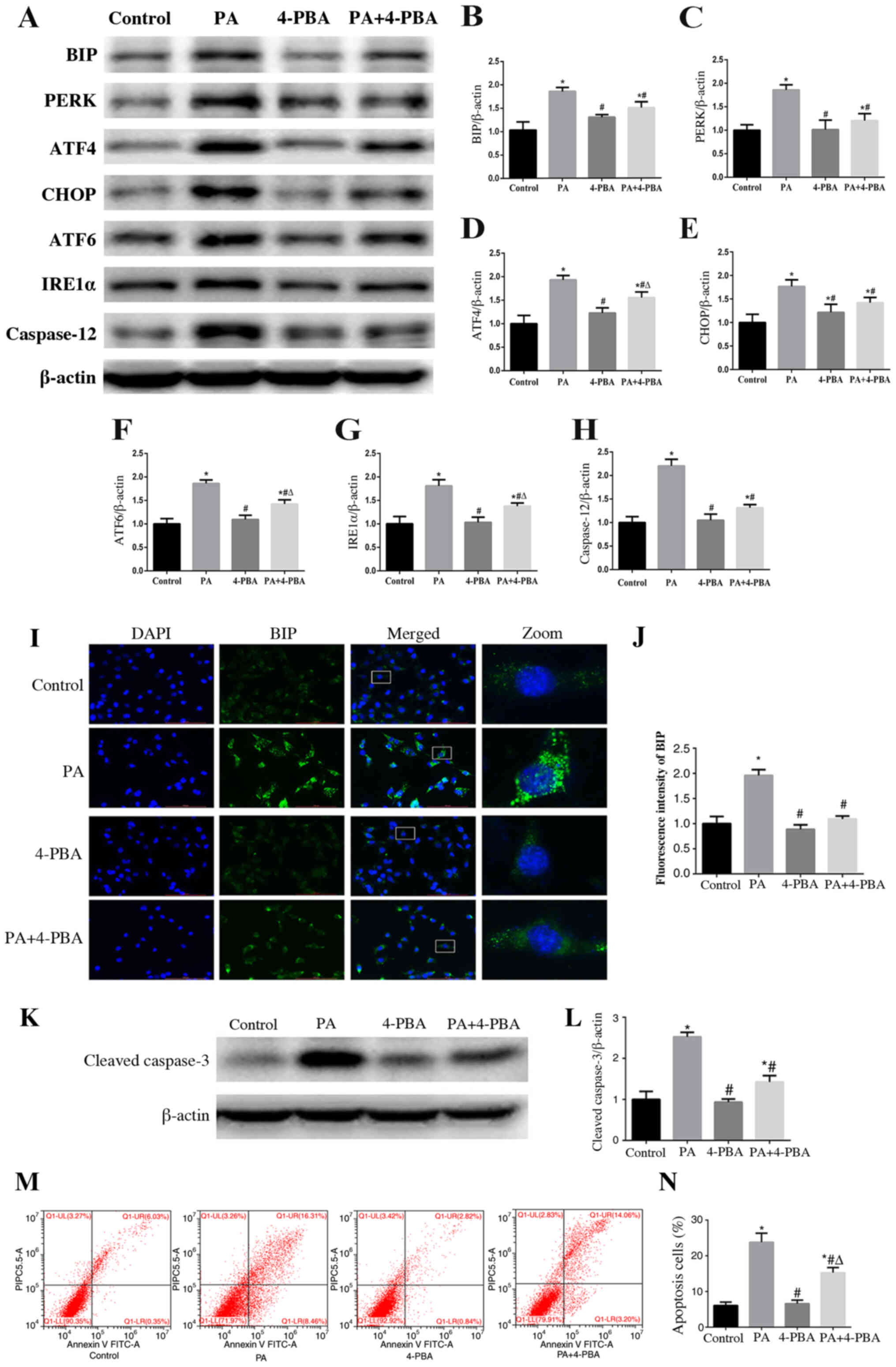 | Figure 3.Endoplasmic reticulum stress is
involved in PA-induced apoptosis of podocytes. (A) Representative
images of BIP, PERK, ATF4, CHOP, ATF6, IRE1α and caspase 12
expression, as detected by western blot analysis after treatment
with PA (150 µmol/l) and/or 4-PBA (10 µmol/l). β-actin was used as
a loading control. Densitometric analysis of the data shown in (A)
for (B) BIP, (C) PERK, (D) ATF4, (E) CHOP, (F) ATF6, (G) IRE1α and
(H) caspase 12. Data are presented as the mean ± SEM (n=3).
*P<0.05 vs. control group, #P<0.05 vs. PA group,
ΔP<0.05 vs. 4-PBA group. (I) Cells were treated with
PA (150 µmol/l) for 24 h after pretreatment with or without 4-PBA
(10 µmol/l) for 2 h and BIP expression was measured by fluorescence
microscopy (magnification, ×400). The rightmost panels show the
enlarged views (×7) of areas in the left panels. (J) Fluorescence
intensities of five randomly selected fields per group, as shown in
(I), were determined and analyzed. Data are presented as the mean ±
SEM (n=3). *P<0.05 vs. control group, #P<0.05 vs.
PA group. (K) Representative images of cleaved-caspase 3
expression, as measured by western blot analysis. (L) Densitometric
analysis of the cleaved-caspase 3 expression shown in (K). (M)
Representative plots of apoptosis determined by flow cytometry. (N)
Quantitative analysis of the results shown in (M). Data are
presented as the mean ± SEM (n=3). *P<0.05 vs. control group,
#P<0.05 vs. PA group, ΔP<0.05 vs. 4-PBA
group. PA, palmitic acid; 4-PBA, 4-phenyl butyric acid; BIP,
binding immunoglobulin protein; PERK, protein kinase RNA-like
endoplasmic reticulum kinase; ATF, activating transcription factor;
CHOP, C/EBP homologous protein; IRE1α, inositol-requiring enzyme
1α; SEM, standard error of the mean. |
Role of oxidative stress in PA-induced
ER stress and apoptosis in podocytes
Using DCFH-DA staining, a sensitive fluorescent
probe to detect cellular ROS, it was confirmed that PA enhanced ROS
generation in a concentration-dependent manner (Fig. 4A and B). However, treatment with
NAC, a ROS scavenger, significantly reduced the ROS production
induced by PA in podocytes (Fig. 4C and
D). To further clarify the role of oxidative stress in
PA-induced ER stress, the expression levels of ER stress-associated
proteins were detected by western blotting in podocytes following
treatment with PA in the presence or absence of NAC. The results
revealed that the expression levels of BIP, PERK, ATF4, CHOP,
IRE1α, ATF6 and caspase-12 induced by PA were suppressed by NAC
(Fig. 4E-L), which indicated that
PA-induced ER stress may be dependent on oxidative stress.
Moreover, treatment with NAC alleviated PA-induced podocyte
apoptosis (Fig. 4M-P), which
suggested that oxidative stress served a crucial role in PA-induced
podocyte apoptosis.
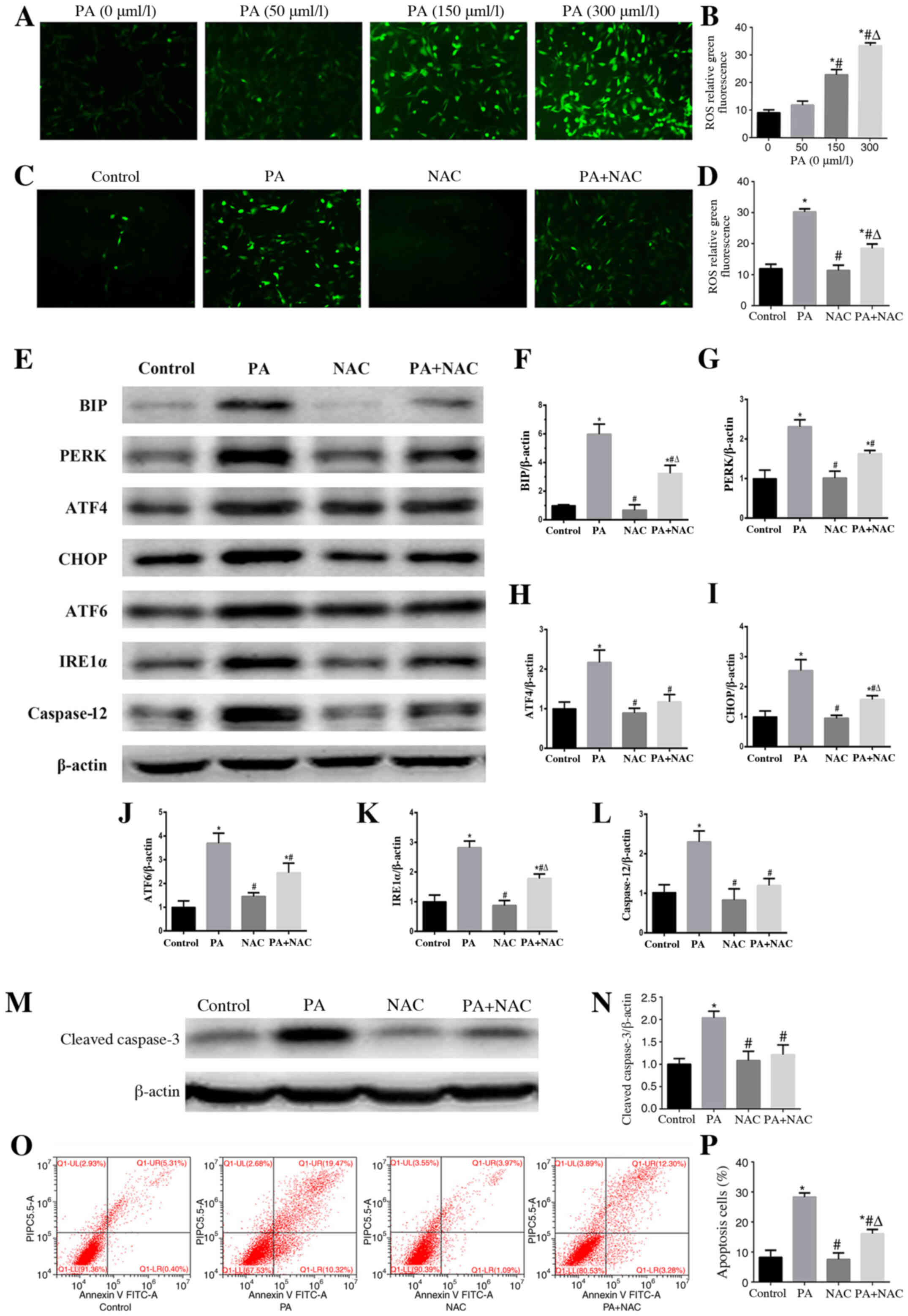 | Figure 4.Role of oxidative stress in
PA-induced endoplasmic reticulum stress and apoptosis in podocytes.
(A and C) Representative immunofluorescence images of intracellular
ROS in podocytes (magnification, ×200) following treatment with
different concentrations of PA, or PA (150 µmol/l) for 24 h after
pretreatment with or without NAC (150 µmol/l) for 2 h. (B) Mean
fluorescence intensities of ROS shown in (A). Data are presented as
the mean ± SEM (n=3). *P<0.05 vs. PA (0 µmol/l) group,
#P<0.05 vs. PA (50 µmol/l) group,
ΔP<0.05 vs. PA (150 µmol/l) group. (D) Mean
fluorescence intensities of ROS shown in (C). Data are presented as
the mean ± SEM (n=3). *P<0.05 vs. control group,
#P<0.05 vs. PA group, ΔP<0.05 vs. NAC
group. (E and M) Representative images of BIP, PERK, ATF4, CHOP,
ATF6, IRE1α, caspase 12 and cleaved-caspase 3 expression, as
detected by western blotting after treatment with PA (150 µmol/l)
or NAC (150 µmol/l). β-actin was used as a loading control.
Densitometric analysis of the data shown in (E and M) for (F) BIP,
(G) PERK, (H) ATF4, (I) CHOP, (J) ATF6, (K) IRE1α, (L) caspase 12
and (N) cleaved-caspase 3. Data are presented as the mean ± SEM
(n=3). *P<0.05 vs. control group, #P<0.05 vs. PA
group, ΔP<0.05 vs. NAC group. (O) Representative
plots of podocyte apoptosis evaluated by flow cytometry after
treatment with PA (150 µmol/l) and NAC (150 µmol/l) for 24 h. (P)
Quantification of podocyte apoptosis shown in (O). Data are
presented as the mean ± SEM (n=3). *P<0.05 vs. control group,
#P<0.05 vs. PA group, ΔP<0.05 vs. NAC
group. PA, palmitic acid; ROS, reactive oxygen species; NAC,
N-acetylcysteine; BIP, binding immunoglobulin protein; PERK,
protein kinase RNA-like endoplasmic reticulum kinase; ATF,
activating transcription factor; CHOP, C/EBP homologous protein;
IRE1α, inositol-requiring enzyme 1α; SEM, standard error of the
mean. |
BBR improves PA-induced ER stress and
oxidative stress in podocytes
To investigate the role of BBR in PA-induced ER
stress and oxidative stress, podocytes were treated with PA (150
µmol/l) and were pretreated with BBR (8 µmol/l). The results
demonstrated that BBR suppressed PA-induced ER stress in podocytes
(Fig. 5A-H). Immunofluorescence
staining also revealed that BIP expression (Fig. 5I and J) and intracellular ROS
production (Fig. 5K and L) were
decreased in podocytes following treatment with BBR compared with
in the PA group. These findings suggested that BBR may alleviate
the ROS-dependent ER stress induced by PA in podocytes.
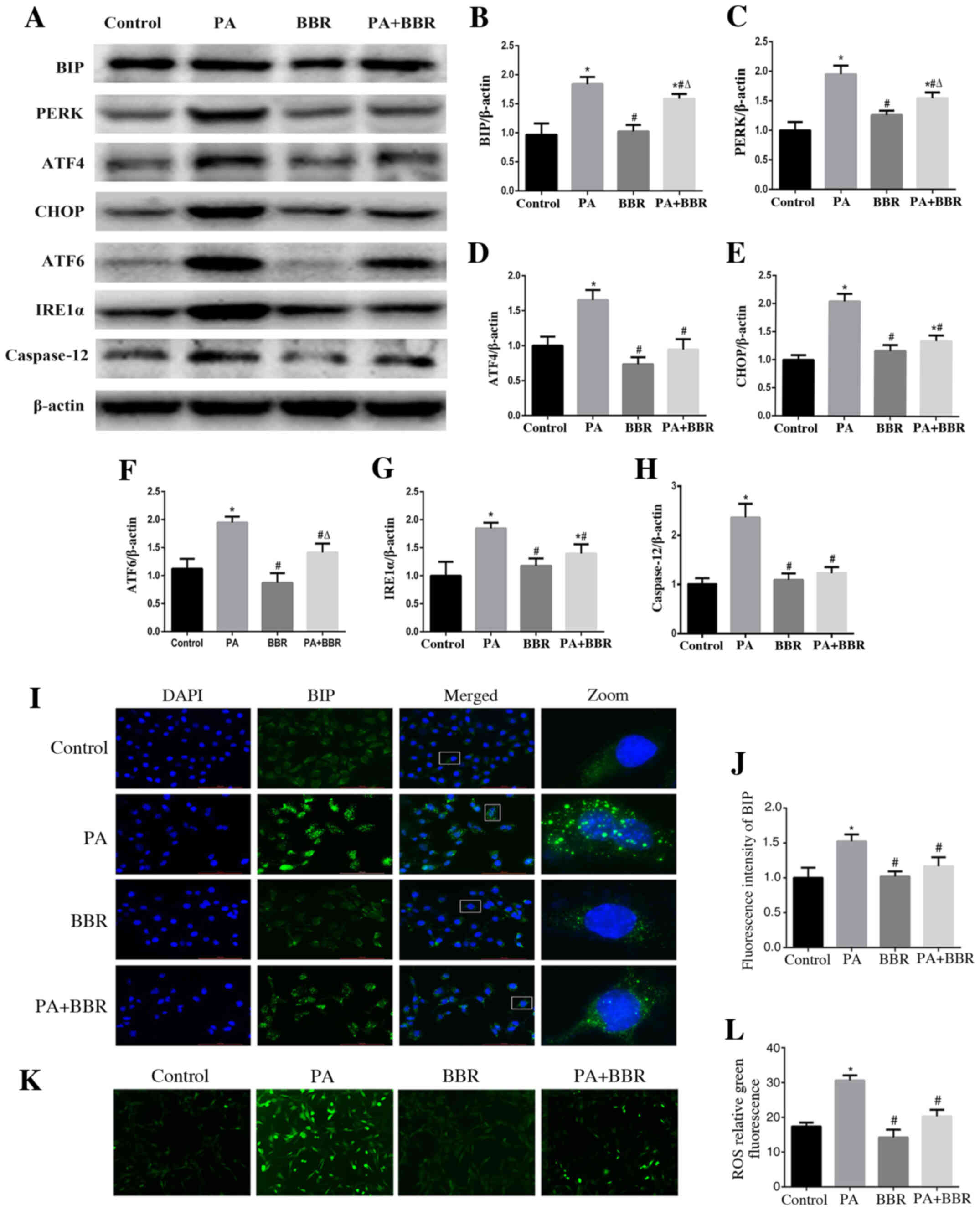 | Figure 5.BBR improves PA-induced endoplasmic
reticulum stress and oxidative stress in podocytes. (A)
Representative images of BIP, PERK, ATF4, CHOP, ATF6, IRE1α and
caspase 12 expression, as detected by western blot analysis after
treatment with PA (150 µmol/l) for 24 h after pretreated with BBR
(8 µmol/l) for 2 h. β-actin was expressed as an internal control.
Densitometric analysis of the results shown in (A) for (B) BIP, (C)
PERK, (D) ATF4, (E) CHOP, (F) ATF6, (G) IRE1α and (H) caspase 12.
Data are presented as the mean ± SEM (n=3). *P<0.05 vs. control
group, #P<0.05 vs. PA group, ΔP<0.05
vs. BBR group. (I) BIP expression was verified by fluorescence
microscopy following treatment of cells with PA (150 µmol/l) for 24
h after pretreatment with or without BBR (8 µmol/l) for 2 h
(magnification, ×400). The rightmost panels show the enlarged views
(×7) of areas in the left panels. (J) Fluorescence intensities of
five randomly selected fields in each group (as shown in panel I)
were determined and analyzed. Data are presented as the mean ± SEM
(n=3). *P<0.05 vs. control group, #P<0.05 vs. PA
group. (K) Representative immunofluorescence images of
intracellular ROS in podocytes by fluorescence microscopy
(magnification, ×200) following treatment with PA (150 µmol/l) for
24 h in the presence or absence of BBR (8 µmol/l) for 2 h. (L) Mean
fluorescence intensities of the ROS shown in (K). Data are
expressed as the mean ± SEM (n=3). *P<0.05 vs. control group,
#P<0.05 vs. PA group. BBR, berberine; BIP, binding
immunoglobulin protein; PERK, protein kinase RNA-like endoplasmic
reticulum kinase; ATF, activating transcription factor; CHOP, C/EBP
homologous protein; IRE1α, inositol-requiring enzyme 1α. |
Discussion
Patients with CKD often present with dyslipidemia at
both the early stages of renal dysfunction and at end-stage renal
disease. Lipid abnormalities in turn accelerate CKD progression and
the development of associated comorbidities (1,26,27).
Hence, lipid-lowering therapy has become one of the beneficial
therapeutic strategies for CKD (28). Podocytes are important components of
the kidney glomerulus and are crucial to maintain the integrity of
kidney filtration; however, podocytes are highly susceptible to
FFAs or an altered lipid environment. Podocyte dysfunction and
apoptosis caused by altered dyslipidemia has been reported to
contribute to the initiation and progression of CKD (29,30).
The present study confirmed that PA may induce podocyte cell death
and apoptosis.
BBR is a traditional treatment applied in
gastrointestinal diseases, such as diarrhea, which has numerous
pharmacological effects, including anti-inflammatory (31), antioxidant (32) and anticancer (31,33)
effects. Numerous studies have assessed the effects of BBR on
hyperglycemia and dyslipidemia (17,34).
Previous studies demonstrated that BBR inhibited kidney damage in
high-fat diet-fed rats (35) and DN
in the clinic (18). In addition,
it has been reported that BBR may exert a protective effect on
hepatocytes and the liver in high-fat diet-fed mice by alleviating
oxidative stress and ER stress (19,36). A
recent study revealed that BBR could protect podocytes from injury
and apoptosis induced by FFAs by regulating Drp1-mediated
mitochondrial function (37). The
present findings also indicated that BBR had a robust protective
effect against PA-induced podocyte apoptosis. Notably, it was
observed that in PA-stimulated podocytes pretreated with 8 µmol/l
BBR there was a higher cell survival ratio than in those pretreated
with 16 µmol/l BBR. These data indicated that the protective effect
of BBR may be related to its dosage, and it could potentially
damage podocytes when the dosage reaches a high concentration.
However, the underlying protective mechanism is complex and further
research is required to clarify it.
The ER is an extensive, interconnected series of
membranous sacs present in eukaryotic cells. The majority of
secreted and transmembrane proteins are translocated into the lumen
of the ER to undergo protein modification and folding. An
accumulation of unfolded proteins or misfolded proteins in the ER
leads to ER stress (38). To
mitigate such circumstances, BIP, an ER-resident molecular
chaperon, detaches from three transmembrane ER stress sensors,
IREI, PERK and ATF6, and activates a homeostatic intracellular
signaling network to alleviate ER stress, which is known as the
unfolded protein response (UPR) (23,38,39).
Among these proteins, PERK can phosphorylate the eukaryotic
translation initiation factor eIF2α to prevent proteins from
entering the ER, encoding the transcription factor ATF4 to activate
downstream UPR target genes and CHOP, which are involved in
apoptosis (40,41). A moderate UPR can help to recover ER
homeostasis; however, severe or prolonged stress ultimately leads
to cell apoptosis (23,41). It has been reported that ER stress
can be stimulated in adipose tissue in high-fat diet-induced obese
mice (42), and ER stress
participates in neurodegenerative, endocrine and various renal
diseases (43–45). In the current study, PA increased
cell apoptosis and upregulated ER stress-related proteins,
including activation of caspase 12, which is one of the two main
signaling proteins of ER stress-induced apoptosis (46). Conversely, 4-PBA, an ER stress
inhibitor, downregulated ER stress, and inhibited PA-induced
expression of caspase 12 and podocyte apoptosis. Therefore, these
findings suggested that ER stress participated in PA-induced
podocyte apoptosis.
Oxidative stress, which is mediated by ROS, has been
reported to serve a role in the pathophysiology of renal impairment
and in the progression of CKD (47). Excessive FFAs can cause podocyte
injury by boosting the production of ROS and lipid peroxidation,
which contribute to glomerular lesions (48). ROS and ER stress often occur
together, and ROS overproduction may lead to ER stress (48,49).
Exogenous ROS can disturb ER protein folding, activate some aspects
of the UPR and induce ER stress (49–51).
On the other hand, oxidative stress can be strengthened by protein
misfolding in the ER (51). In the
present study, PA induced an increase in intracellular ROS, whereas
NAC, an antioxidant reagent, reduced ROS production, and attenuated
ER stress and podocyte apoptosis induced by PA, thus demonstrating
that the ROS-ER stress signaling pathway may be a key mechanism in
podocyte apoptosis induced by PA. Furthermore, it was observed that
treatment with BBR reduced ROS generation and inhibited the ER
stress in PA-treated podocytes. Similarly, it was previously
reported that BBR could alleviate ER stress induced by
hypoxia/reoxygenation injury in HK-2 cells (52). Moreover, Zhu et al (53) demonstrated that BBR ameliorated the
development of DN via inactivating the TLR4/NF-κB pathway. A recent
review elaborated that the efficacy of BBR for treating multiple
diseases was mediated by its multi-target pharmacological profile;
its molecular targets include AMPK, PTP1B, SIRT1, PCSK9, LDLR, PPAR
and NF-κB, and that modulation of gut microbiota was involved in
the metabolic effects of BBR (54).
However, the direct molecular target remains unclear. Taken
together, the present findings indicated that BBR alleviated
podocyte apoptosis via inhibition of the ROS-mediated ER stress
pathway. However, the mechanism by which BBR specifically regulates
ROS mediated-ERS and the direct molecular target of BBR require
further investigation in vivo.
In conclusion, the present study demonstrated that
ER stress and increased production of ROS may be the key mechanism
underlying PA-induced podocyte apoptosis. BBR may protect against
PA-induced podocyte apoptosis by suppressing ROS-dependent ER
stress. However, further studies, such as using reverse
transcription-quantitative PCR, are required to confirm the
mechanism of regulation of BBR. In addition, this was an in
vitro study, and further studies using an in vivo model
are needed to support these results.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81370816) and the Natural
Science Foundation of Chongqing Science and Technology Commission
of China (grant no. cstc2012jjA10136).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XYX and TL performed the experiments, analyzed the
data and wrote the manuscript. XMC and YW participated in study
design and data interpretation, and were responsible for the
overall direction of this work. JLH and XSJ contributed to
analyzing and interpreting the data, drafting the manuscript and
revising it critically for important intellectual content. XGD
analyzed the results, supervised the project and gave final
approval of the version to be published. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BIP
|
binding immunoglobulin protein
|
|
PERK
|
protein kinase RNA-like endoplasmic
reticulum kinase
|
|
ATF4
|
activating transcription factor 4
|
|
CHOP
|
C/EBP homologous protein
|
|
ATF6
|
activating transcription factor 6
|
|
IRE1α
|
inositol-requiring enzyme 1α
|
References
|
1
|
Hager MR, Narla AD and Tannock LR:
Dyslipidemia in patients with chronic kidney disease. Rev Endocr
Metab Disord. 18:29–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Keane WF, Tomassini JE and Neff DR: Lipid
abnormalities in patients with chronic kidney disease: Implications
for the pathophysiology of atherosclerosis. J Atheroscler Thromb.
20:123–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kasiske BL and Wheeler DC: The management
of dyslipidemia in CKD: New analyses of an expanding dataset. Am J
Kidney Dis. 61:371–374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kowalski A, Krikorian A and Lerma EV:
Dyslipidemia in chronic kidney disease. Dis Mon. 61:396–402. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Izquierdo-Lahuerta A, Martinez-Garcia C
and Medina-Gómez G: Lipotoxicity as a trigger factor of renal
disease. J Nephrol. 29:603–610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dai H, Liu Q and Liu B: Research progress
on mechanism of podocyte depletion in diabetic nephropathy. J
Diabetes Res. 2017:26152862017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dalla Vestra M, Masiero A, Roiter AM,
Saller A, Crepaldi G and Fioretto P: Is podocyte injury relevant in
diabetic nephropathy? Studies in patients with type 2 diabetes.
Diabetes. 52:1031–1035. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Ma KL, Liu J, Wu Y, Hu ZB, Liu L
and Liu BC: Dysregulation of low-density lipoprotein receptor
contributes to podocyte injuries in diabetic nephropathy. Am J
Physiol Endocrinol Metab. 308:E1140–E1148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szeto HH, Liu S, Soong Y, Alam N, Prusky
GT and Seshan SV: Protection of mitochondria prevents high-fat
diet-induced glomerulopathy and proximal tubular injury. Kidney
Int. 90:997–1011. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martinez-Garcia C, Izquierdo-Lahuerta A,
Vivas Y, Velasco I, Yeo TK, Chen S and Medina-Gomez G: Renal
lipotoxicity-associated inflammation and insulin resistance affects
actin cytoskeleton organization in podocytes. PLoS One.
10:e01422912015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu S, Nam SM, Kim JH, Das R, Choi SK,
Nguyen TT, Quan X, Choi SJ, Chung CH, Lee EY, et al: Palmitate
induces ER calcium depletion and apoptosis in mouse podocytes
subsequent to mitochondrial oxidative stress. Cell Death Dis.
6:e19762015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tao JL, Wen YB, Shi BY, Zhang H, Ruan XZ,
Li H, Li XM, Dong WJ and Li XW: Endoplasmic reticulum stress is
involved in podocyte apoptosis induced by saturated fatty acid
palmitate. Chin Med J (Engl). 125:3137–3142. 2012.PubMed/NCBI
|
|
13
|
Hua W, Huang HZ, Tan LT, Wan JM, Gui HB,
Zhao L, Ruan XZ, Chen XM and Du XG: CD36 mediated fatty
acid-induced podocyte apoptosis via oxidative stress. PLoS One.
10:e01275072015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rabbani GH, Butler T, Knight J, Sanyal SC
and Alam K: Randomized controlled trial of berberine sulfate
therapy for diarrhea due to enterotoxigenic Escherichia coli
and Vibrio cholerae. J Infect Dis. 155:979–984. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taylor CE and Greenough WB III: Control of
diarrheal diseases. Annu Rev Public Health. 10:221–244. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lan J, Zhao Y, Dong F, Yan Z, Zheng W, Fan
J and Sun G: Meta-analysis of the effect and safety of berberine in
the treatment of type 2 diabetes mellitus, hyperlipemia and
hypertension. J Ethnopharmacol. 161:69–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kong W, Wei J, Abidi P, Lin M, Inaba S, Li
C, Wang Y, Wang Z, Si S, Pan H, et al: Berberine is a novel
cholesterol-lowering drug working through a unique mechanism
distinct from statins. Nat Med. 10:1344–1351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ni WJ, Ding HH and Tang LQ: Berberine as a
promising anti-diabetic nephropathy drug: An analysis of its
effects and mechanisms. Eur J Pharmacol. 760:103–112. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Z, Li B, Meng X, Yao S, Jin L, Yang
J, Wang J, Zhang H, Zhang Z, Cai D, et al: Berberine prevents
progression from hepatic steatosis to steatohepatitis and fibrosis
by reducing endoplasmic reticulum stress. Sci Rep. 6:208482016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang B, Xu X, He X, Wang Z and Yang M:
Berberine improved aldo-induced podocyte injury via inhibiting
oxidative stress and endoplasmic reticulum stress pathways both in
vivo and in vitro. Cell Physiol Biochem. 39:217–228. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang XS, Chen XM, Wan JM, Gui HB, Ruan XZ
and Du XG: Autophagy protects against palmitic acid-induced
apoptosis in podocytes in vitro. Sci Rep. 7:427642017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu T, Chen XM, Sun JY, Jiang XS, Wu Y,
Yang S, Huang HZ, Ruan XZ and Du XG: Palmitic acid-induced podocyte
apoptosis via the reactive oxygen species-dependent mitochondrial
pathway. Kidney Blood Press Res. 43:206–219. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rashid HO, Yadav RK, Kim HR and Chae HJ:
ER stress: Autophagy induction, inhibition and selection.
Autophagy. 11:1956–1977. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lukas J, Pospech J, Oppermann C, Hund C,
Iwanov K, Pantoom S, Petters J, Frech M, Seemann S, Thiel FG, et
al: Role of endoplasmic reticulum stress and protein misfolding in
disorders of the liver and pancreas. Adv Med Sci. 64:315–323. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maamoun H, Abdelsalam SS, Zeidan A,
Korashy HM and Agouni A: Endoplasmic reticulum stress: A critical
molecular driver of endothelial dysfunction and cardiovascular
disturbances associated with diabetes. Int J Mol Sci. 20:16582019.
View Article : Google Scholar
|
|
26
|
Vaziri ND: HDL abnormalities in nephrotic
syndrome and chronic kidney disease. Nat Rev Nephrol. 12:37–47.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reiss AB, Voloshyna I, De Leon J, Miyawaki
N and Mattana J: Cholesterol metabolism in CKD. Am J Kidney Dis.
66:1071–1082. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ferro CJ, Mark PB, Kanbay M, Sarafidis P,
Heine GH, Rossignol P, Massy ZA, Mallamaci F, Valdivielso JM,
Malyszko J, et al: Lipid management in patients with chronic kidney
disease. Nat Rev Nephrol. 14:727–749. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reiser J and Sever S: Podocyte biology and
pathogenesis of kidney disease. Annu Rev Med. 64:357–366. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sieber J and Jehle AW: Free fatty acids
and their metabolism affect function and survival of podocytes.
Front Endocrinol (Lausanne). 5:1862014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zou K, Li Z, Zhang Y, Zhang HY, Li B, Zhu
WL, Shi JY, Jia Q and Li YM: Advances in the study of berberine and
its derivatives: A focus on anti-inflammatory and anti-tumor
effects in the digestive system. Acta Pharmacol Sin. 38:157–167.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng F, Wang Y, Li J, Su C, Wu F, Xia WH,
Yang Z, Yu BB, Qiu YX and Tao J: Berberine improves endothelial
function by reducing endothelial microparticles-mediated oxidative
stress in humans. Int J Cardiol. 167:936–942. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saha SK and Khuda-Bukhsh AR: Berberine
alters epigenetic modifications, disrupts microtubule network, and
modulates HPV-18 E6-E7 oncoproteins by targeting p53 in cervical
cancer cell HeLa: A mechanistic study including molecular docking.
Eur J Pharmacol. 744:132–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Li X, Zou D, Liu W, Yang J, Zhu
N, Huo L, Wang M, Hong J, Wu P, et al: Treatment of type 2 diabetes
and dyslipidemia with the natural plant alkaloid berberine. J Clin
Endocrinol Metab. 93:2559–2565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu U, Cha Y, Huang X, Liu J, Chen Z, Wang
F, Xu J, Sheng L and Ding H: Protective effects of berberine on
high fat-induced kidney damage by increasing serum adiponectin and
promoting insulin sensitivity. Int J Clin Exp Pathol.
8:14486–14492. 2015.PubMed/NCBI
|
|
36
|
Sun Y, Yuan X, Zhang F, Han Y, Chang X, Xu
X, Li Y and Gao X: Berberine ameliorates fatty acid-induced
oxidative stress in human hepatoma cells. Sci Rep. 7:113402017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qin X, Zhao Y, Gong J, Huang W, Su H, Yuan
F, Fang K, Wang D, Li J, Zou X, et al: Berberine protects
glomerular podocytes via inhibiting Drp1-mediated mitochondrial
fission and dysfunction. Theranostics. 9:1698–1713. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kaufman RJ: Stress signaling from the
lumen of the endoplasmic reticulum: Coordination of gene
transcriptional and translational controls. Genes Dev.
13:1211–1233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Begum G, Harvey L, Dixon CE and Sun D: ER
stress and effects of DHA as an ER stress inhibitor. Transl Stroke
Res. 4:635–642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gardner BM, Pincus D, Gotthardt K,
Gallagher CM and Walter P: Endoplasmic reticulum stress sensing in
the unfolded protein response. Cold Spring Harb Perspect Biol.
5:a0131692013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Verfaillie T, Garg AD and Agostinis P:
Targeting ER stress induced apoptosis and inflammation in cancer.
Cancer Lett. 332:249–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen Y, Wu Z, Zhao S and Xiang R: Chemical
chaperones reduce ER stress and adipose tissue inflammation in high
fat diet-induced mouse model of obesity. Sci Rep. 6:274862016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cabral-Miranda F and Hetz C: ER stress and
neurodegenerative disease: A cause or effect relationship? Curr Top
Microbiol Immunol. 414:131–157. 2018.PubMed/NCBI
|
|
44
|
Ariyasu D, Yoshida H and Hasegawa Y:
Endoplasmic reticulum (ER) stress and endocrine disorders. Int J
Mol Sci. 18:3822017. View Article : Google Scholar
|
|
45
|
Cybulsky AV: Endoplasmic reticulum stress,
the unfolded protein response and autophagy in kidney diseases. Nat
Rev Nephrol. 13:681–696. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Szegezdi E, Fitzgerald U and Samali A:
Caspase-12 and ER-stress-mediated apoptosis: The story so far. Ann
N Y Acad Sci. 1010:186–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Coppolino G, Leonardi G, Andreucci M and
Bolignano D: Oxidative stress and kidney function: A brief update.
Curr Pharm Des. 24:4794–4799. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gai Z, Wang T, Visentin M, Kullak-Ublick
GA, Fu X and Wang Z: Lipid accumulation and chronic kidney disease.
Nutrients. 11:7222019. View Article : Google Scholar
|
|
49
|
Seervi M, Rani A, Sharma AK and Santhosh
Kumar TR: ROS mediated ER stress induces Bax-Bak dependent and
independent apoptosis in response to Thioridazine. Biomed
Pharmacother. 106:200–209. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Malhotra JD and Kaufman RJ: Endoplasmic
reticulum stress and oxidative stress: A vicious cycle or a
double-edged sword? Antioxid Redox Signal. 9:2277–2293. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cao SS and Kaufman RJ: Endoplasmic
reticulum stress and oxidative stress in cell fate decision and
human disease. Antioxid Redox Signal. 21:396–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yu W, Sheng M, Xu R, Yu J, Cui K, Tong J,
Shi L, Ren H and Du H: Berberine protects human renal proximal
tubular cells from hypoxia/reoxygenation injury via inhibiting
endoplasmic reticulum and mitochondrial stress pathways. J Transl
Med. 11:242013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhu L, Han J, Yuan R, Xue L and Pang W:
Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-κB
pathway. Biol Res. 51:92018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Feng X, Sureda A, Jafari S, Memariani Z,
Tewari D, Annunziata G, Barrea L, Hassan STS, Šmejkal K, Malaník M,
et al: Berberine in cardiovascular and metabolic diseases: From
mechanisms to therapeutics. Theranostics. 9:1923–1951. 2019.
View Article : Google Scholar : PubMed/NCBI
|