Introduction
At present, the treatment of leukemia is dependent
upon hematopoietic stem cell (HSC) transplantation (1), as HSCs are multipotent cells which can
reconstitute the immune and hematological systems following
irradiation (2–4). However, relatively few HSCs can be
obtained from donor cord blood or bone marrow samples, and as such,
the numbers obtained are insufficient to meet clinical demands
(5,6). To date, numerous studies have reported
that signaling through the p38MAPK, mTOR, glycogen synthase kinase
(GSK)3β and histone deacetylases (HDACs) altered HSCs functionality
in ways that were disadvantageous, impairing their ability to
proliferate and driving the HSCs to undergo differentiation,
thereby impairing the normal hematopoietic restoration of blood
cells (7–9).
p38MAPK is an important MAPK, and the activation of
p38MAPK signaling has been demonstrated to promote necrosis and
differentiation, in addition to impairing the proliferation of HSCs
(7,10,11).
It was previously reported that the inhibition of p38MAPK rescued
the regenerative defects of HSCs caused by reactive oxygen species
production, prolonging the life of HSCs and maintaining their
self-renewal (12). In addition, it
was discovered that the inhibition of p38MAPK induced the homing of
HSCs into the bone marrow in response to chemotactic factors
(13), thereby improving the
proliferation of leukemia cells or lymphocytes (14). Notably, p38MAPK signaling was also
observed to be associated with chemotherapy resistance in patients
with leukemia (15).
While first studied in the context of glycogen
metabolism in the liver, GSK3β has also been identified as an
important regulator of HSC homeostasis (16,17).
In a previous study, GSK3β activation was reported to drive HSC
apoptosis and differentiation through p53 downregulation (18). Moreover, the inhibition of
microRNA-126 regulated the PI3K/AKT/GSK3β signaling pathway and
promoted the proliferation of HSCs (19). It was also illustrated that GSK3β
promoted the migration of hematopoietic stem progenitor cells by
regulating cytoskeletal rearrangement (20).
HDACs are able to induce histone lysine
deacetylation, thereby modulating the progression of a wide range
of diseases and biological processes (21). For example, the HDAC inhibitor
valproic acid sodium salt (VPA) was discovered to enhance HSC
proliferation, and as such, it is currently used in clinical
contexts to treat myelodysplastic syndrome (22–25)
and acute myeloid leukemia (AML) (26). Meanwhile, HDAC8 was discovered to
aberrantly deacetylate p53 and promote leukemic stem cells(LSC)
transformation and maintenance. Conversely, HDAC8 inhibition
induced LSC apoptosis and restored p53 activity, suggesting the
inhibition of HDAC8 as a promising approach to selectively target
LSCs (27). mTOR signaling serves
as a mechanism through which cells integrate inputs from numerous
different factors, including oxygen, nutrients and cytokines, to
induce appropriate growth, proliferation or survival responses
(28).
At steady-state in vivo, the majority of HSCs
remain dormant, with only a small fraction of progenitor cells
serving to mediate blood cell production (29). However, under conditions of stress
or inflammation, these HSCs can become activated and differentiate
into additional progenitor cells, thereby enhancing the rate of
blood cell replenishment (30). The
rate of hematopoietic reconstitution in patients was discovered to
be influenced by a wide range of variables, including the source of
the donor HSCs, how many were transplanted and whether or not the
recipient patients had undergone prior chemotherapy treatment
(31). In a highly complex
intracellular signaling network, the appropriate regulation of a
signaling pathway is strongly dependent on the communication with
other signaling molecules, resulting in synergistic or antagonistic
relationships and a variety of biological effects (32). Thus, understanding the effects of
inhibitors of different signaling pathways on the in vitro
proliferation of HSCs is a promising strategy to promote the
clinical application of HSCs.
Small molecule compounds that hold the potential to
expand HSCs are of great promise in the stem cell transplantation
field. Notably, current available small molecule compounds
primarily affect several important signaling pathways, such as
p38MAPK, mTOR, GSK3β and HDAC (31,33–35).
Therefore, strategies to regulate these crucial signaling pathways
may be of importance for effective HSC expansion in vitro.
To effectively amplify available HSCs, the bone marrow
microenvironment must be effectively mimicked in vitro
without adversely impacting HSC activity (36). However, such mimicry is complex, as
a wide range of mechanical and cytological stimuli work in concert
in the bone marrow to modulate signaling pathway activation within
these HSCs, thereby governing their ultimate functionality.
At present, research into expanding HSCs has
predominantly focused on the following aspects: Promoting
self-renewal, inhibiting differentiation, inhibiting apoptosis and
promoting homing (13,37–39).
HSCs are contained in the LSK cell population; phenotypically, LSK
cells express stem cell antigen (Sca)-1 and c-Kit, but lack the
lineage (Lin) markers expressed on mature myeloid and lymphoid
cells (40). The present study
aimed to investigate the efficacy of small molecule inhibitors on
the manipulation of HSCs, especially the expansion of HSCs in
vitro. Different combinations of inhibitors of the p38MAPK,
mTOR, GSK3β and HDACs were used to treat HSC cultures at a range of
concentrations, in order to explore which signaling pathways could
be targeted to expansion HSCs more effectively in vitro.
SB203580 was the first reported inhibitor of p38 (41,42),
which was discovered to permeate cells and inhibit the activation
of MAPK activated protein kinase (MAPKAPK)-2 and MAPKAPK-3, which
subsequently inhibits the partial signal transduction induced by
certain inflammatory factors, such as IL-1β and TNF-α. SB216763 is
an effective, selective GSK3β inhibitor (43), which was discovered to activate
glycogen synthase, stimulates glycogen synthesis in human
hepatocytes, induces the expression of reporter genes regulated by
β-catenin and promotes the accumulation of β-catenin, in which
β-catenin is an important downstream effector of the Wnt signaling
transduction pathway (44).
Similarly, CHIR99021 is a GSK3α and GSK3β inhibitor (45), which was illustrated to promote HSC
self-renewal, maintain colony morphology and regulate epigenetics
by activating the Wnt signaling pathway (46,47).
The results of the present study demonstrated that suppressing
p38MAPK and/or the GSK3β signaling pathway effectively amplified
HSCs in vitro.
Materials and methods
Materials
The Cyan ADP flow cytometer, Moflo XDP flow
cytometer and cryogenic high-speed centrifuge were all purchased
from Beckman Coulter, Inc. The cell counter was obtained from
Countstar. In addition, 10X Red Blood Cell Lysis buffer was
acquired from eBioscience, 0.4% Trypan blue dye was provided by
Sigma-Aldrich; Merck KGaA and FCS, SB203580, CHIR99021, rapamycin,
VPA and SB216763 were purchased from Selleck Chemicals. The
EasySeo™ Mouse SCA1 Positive Selection kit (cat. no. 18756) was
provided by Stemcell Technologies, Inc.
Animal studies
Female C57BL6/J mice (age, 6–8 weeks, weight,
20–25g) were purchased from the Shanghai SLAC Laboratory Animal
Co., Ltd. All mice were housed under a 12-h light/dark cycle in
microisolator cages contained within a laminar flow ventilation
system in the animal facility at the experimental Animal Center,
Shanghai Normal University under specific-pathogen-free (SPF)
conditions and used as donors to collect bone marrow HSCs. The mice
were sacrificed by cervical dislocation (n=4 per group). All animal
studies were approved by the Institutional Animal Care and Use
Committee of Shanghai Normal University.
Cell culture and flow cytometry
Bone marrow cells were isolated from murine femur
and tibia bone marrow as previously described (48). Briefly, the bones were dissected and
crushed three times with a pestle and, then the cells were
collected in 6 ml dissociation solution (PBS with 2% FCS and 145
U/ml type-4 collagenase; Gibco; Thermo Fisher Scientific, Inc.) and
incubated at 37°C for 30 min. Samples were subsequently filtered
using a 40-µm Nylon cell strainer to obtain single cell
suspensions. The EasySep™ Mouse SCA1 Positive Selection kit was
then used to isolate Sca1-PE positive cells.
Subsequently, the pre-prepared primary antibodies
mixture (2% FCS-PBS solution) containing anti-CD11b-biotin (clone
no. M1/7; eBioscience; cat. no. 13-0112-85; dilution, 1:100),
anti-B220-biotin (clone. no. RA3-6B2; eBioscience; cat. no.
13-0452-85; dilution, 1:100), anti-CD3e-biotin (clone no. 145-2C11;
eBioscience; cat. no. 13-0031-85; dilution, 1:100),
anti-Gr-1-biotin (clone. no. RB6-8C5; dilution, 1:100),
anti-Ter119-biotin (clone no. TER-119; eBioscience; cat. no.
13-5921-85; eBioscience; cat. no. 13-5931-85; dilution, 1:100),
anti-Sca-1-PE (clone no. D7; eBioscience; cat. no. 12-5981-83;
dilution, 1:100) and anti-c-Kit-APC (clone no. 2B8; eBioscience;
cat. no. 17-1171-83; dilution, 1:100) was added to the cell
suspension (1-2ⅹ106 cells/100 µl) and incubated at room
temperature in the dark for 15 min. After washing the cells with 2%
FCS-PBS three times, the cells labelled with biotinylated
antibodies were incubated with a streptavidin-FITC secondary
antibody (eBioscience; cat. no. 11-4317-87; dilution, 1:100) at
room temperature in the dark for 15 min. The cells were
subsequently washed three times with PBS and resuspended in 2%
FCS-PBS, and the LSK hematopoietic progenitor cells were
subsequently sorted by flow cytometry on a Moflo XDP cell
sorter.
The sorted cells were then cultured in DMEM
(Sigma-Aldrich; Merck KGaA), supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific), penicillin-streptomycin and gentamicin
in humidified incubators with anti-vibration platform at 37°C with
5% CO2. The medium also contained 20 ng/ml TPO (cat. no.
315-14-10), 20 ng/ml SCF (cat. no. 250-03-50), 20 ng/ml Flt3-ligand
(cat. no. 250-31L-10) (all from PeproTech, Inc.), 10 µg/ml heparin
and 0.1 mM β-mercaptoethanol.
For treatment, the cells were treated with
combinations of SB203580, CHIR99021, VPA, SB216763 and rapamycin at
the indicated concentrations or DMSO (Ctr) at 37°C with 5%
CO2 for 9 days, followed by flow cytometric
analysis.
The optimal inhibitory concentration of these five
compounds had been obtained (SB216763, 1 µM; rapamycin, 15 nM;
CHIR99021, 500 nM; VPA, 1 mM), cells were treated with different
combinations of these inhibitors (Table
I) at 37°C with 5% CO2, then examined with under a
Leica light microscope (magnification, ×40).
 | Table I.Inhibitor combinations and
corresponding abbreviations. |
Table I.
Inhibitor combinations and
corresponding abbreviations.
| Group | Abbreviation |
|---|
| Freshly
isolated | A |
| DMSO | B |
| VPA | C |
| SB203580 | D |
| Rapamycin | E |
| SB216763 | F |
| SB203580 +
SB216763 | G |
| SB203580 + VPA | H |
| SB216763 + VPA | I |
| Rapamycin +
SB216763 | J |
| SB203580 +
rapamycin | K |
| Rapamycin +
VPA | L |
| SB203580 +
rapamycin + SB216763 | M |
| SB203580 +
rapamycin + VPA | N |
| SB203580 + VPA +
SB216763 | O |
| Rapamycin +
SB216763 + VPA | P |
| VPA, valproic
acid | sodium salt |
Statistical analysis
Data are presented as the mean ± SEM of three
independent experiments. Statistical differences were determined
using a one-way ANOVA, followed by Bonferroni correction, while the
statistical differences between the groups presented in Fig. S6 were analyzed using an unpaired
Student's t-test with GraphPad prism 6.02 (GraphPad
Software, Inc.) software. P<0.05 was considered to indicate a
statistically significant difference.
Results
SB203580, an inhibitor of the p38MAPK
signaling pathway, enhances in vitro HSC functionality
To determine how p38MAPK signaling influences HSC
expansion in vitro, LSK cells were treated over a 9-day
period using a specific inhibitor of this pathway, SB203580. The
treatment with this inhibitor increased the number compared with
the DMSO control (Fig. 1A and B).
Significant changes were observed in the LSK cell counts in a
dose-dependent manner. At 5–20 µM, the number of cells began to
decline, such that following the treatment with 1 µM SB203580, the
number of LSK cells was significantly increased, while the
frequency of Lin− cells at this concentration did not
significantly increase, compared with the DMSO control (Fig. 1C-G). In addition, at 1 µM, the
absolute number of Lin− cells (Fig. 1C) and the frequency and absolute of
LSK (Fig. 1F and G) significantly
increased, compared with DMSO. To improve on the accuracy of these
results, whole bone marrow cells should be used for future
experiments. These results suggested that inhibiting p38MAPK
signaling may alter HSC differentiation and expansion in
vitro.
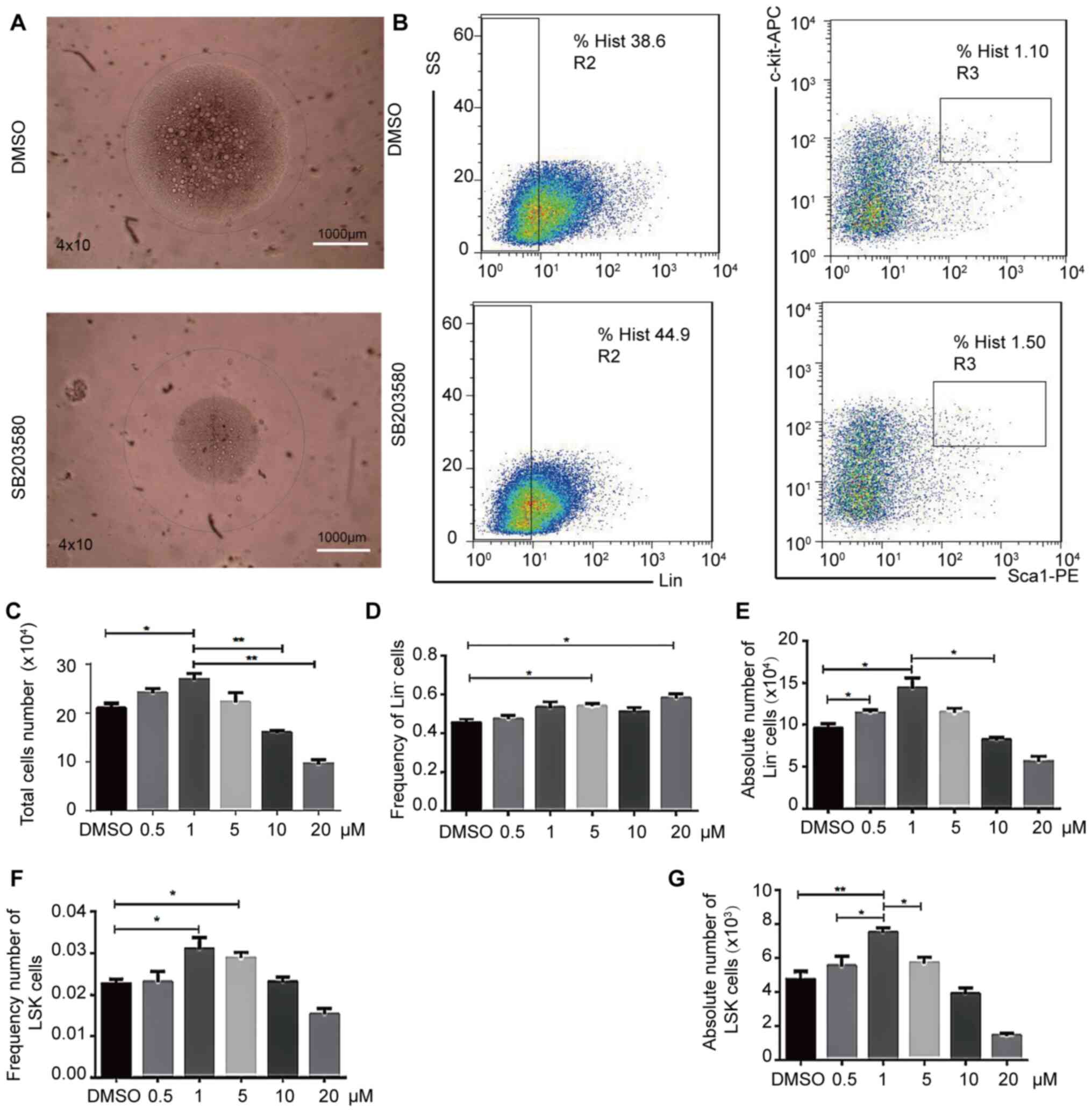 | Figure 1.Effects of p38MAPK inhibition on
hematopoietic stem cell expansion in vitro. LSK cells
cultured in vitro for 9 days were first microscopically
observed and photographed, and then were collected, stained with
antibodies against Gr1, CD11b, Ter119, CD3ε, B220, Sca-1, and
c-Kit, then analyzed by flow cytometry. (A) LSK cell morphology. n
= 4. Scale bar, 1,000-µm. (B) Flow cytometric analysis of LSK cells
treated with 1 µM SB203580 or equal volume DMSO. n =3. (C) Total
cell number. Magnification, ×40. Total images were obtained using
the confocal Leica DM RXA microscope. (D) Relative and (E) absolute
Lin− cell numbers. (F) Relative and (G) absolute LSK
cell numbers. n = 4; *P<0.05, **P<0.01. Lin, lineage; APC,
allophycocyanin; PE, phycoerythrin; SS, side scatter; Sca-1, stem
cell antigen-1. |
Inhibition of GSK3β signaling
significantly enhances HSC expansion in vitro
Given the complexities of the in vivo bone
marrow microenvironment and the role of GSK3β as a regulator of HSC
functionality (8), HSCs were
treated with SB216763, a specific inhibitor of this pathway. At 2
µM, treatment with SB216763 led to changes in morphology and
increased proliferation (Fig. 2A and
B). In addition, an increase in the number of total cells
(Fig. 2C), number of
Lin− cells (Fig. 2D),
LSK cell proportion (Fig. 2E) and
LSK cells absolute number (Fig. 2F)
was also observed, compared with CHIR99021 treatment (Figs. 2 and S1). Although the increase amplitude of
CHIR99021 was higher than that of SB216763 draft at 1 µM, the
increase of LSK was not obvious at this concentration (Fig. 2C). By comparison, SB216763 was
identified to more effectively enhance HSC proliferation, compared
with CHIR99021 (Figs. 2, S2 and S3).
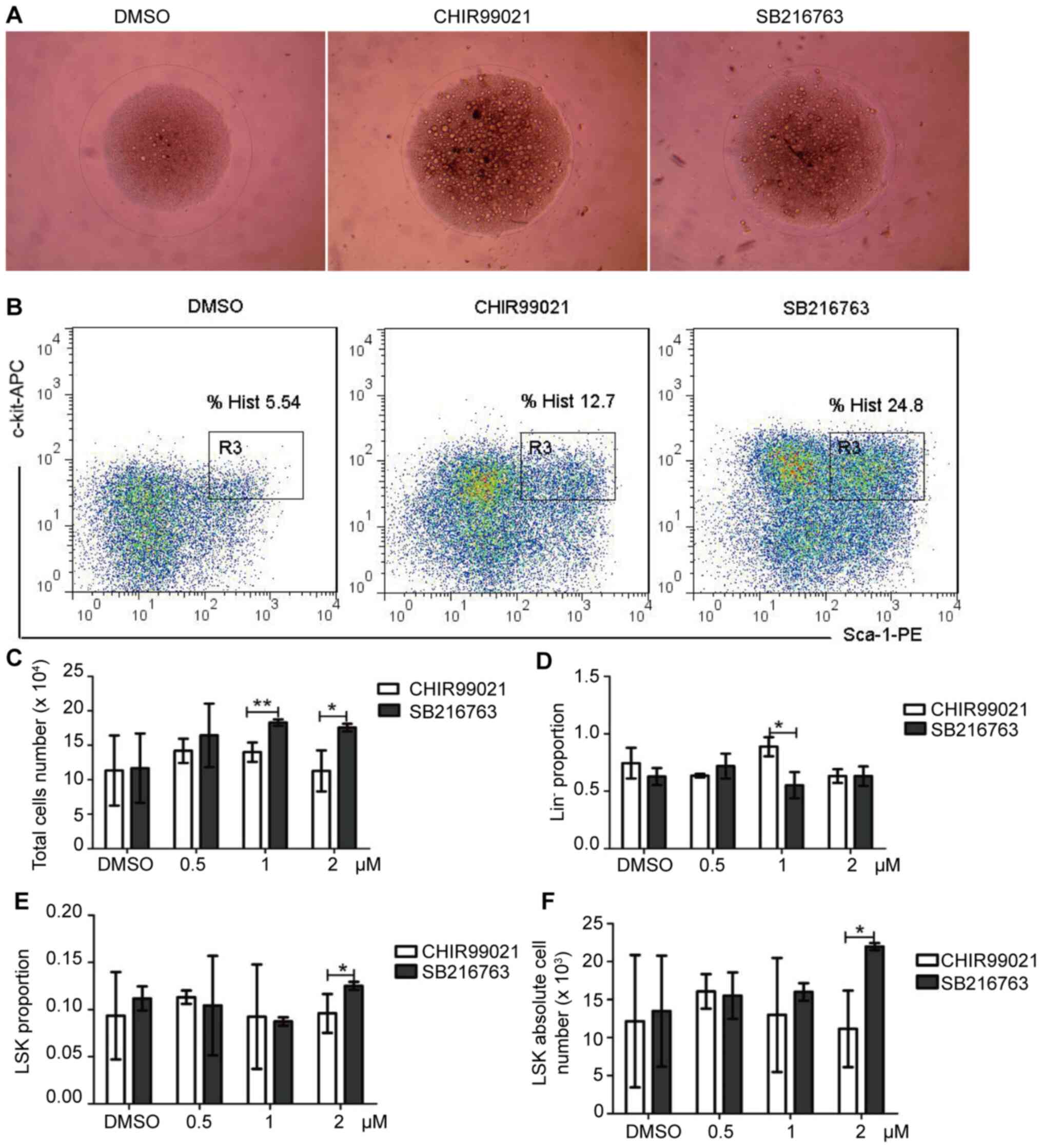 | Figure 2.GSK3β inhibition alters hematopoietic
stem cell expansion in vitro. The GSK3β inhibitors CHIR99021
(0.5, 1, 2 µM) or SB216763 (0.5, 1, 2 µM) were used to treat cells
in media supplemented with cytokines for 9 days. (A) Cell
morphology. Magnification, ×40; scale bar, 1,000 µm. Images were
obtained using the confocal Leica DM RXA microscope. (B) After
cultured in vitro for 9 days, cells were analyzed via flow
cytometry to assess the percentage/number of LSK cells. (C) Total
number of cell numbers following a 9-day culture. (D) Relative
number of Lin− cells. (E) Relative and (F) absolute LSK
cell numbers. n=4; *P<0.05, **P<0.01. APC, allophycocyanin;
PE, phycoerythrin; Lin, lineage; GSK3β, glycogen synthase kinase
3β; Sca-1, stem cell antigen-1. |
Based on these findings, it was hypothesized that
the combined inhibition of p38MAPK and GSK3β signaling pathways may
more effectively expand HSCs. Therefore, excluding the cytotoxic
effect of DMSO on the cells (Fig.
S4), the combination of SB203580 and SB216763 treatment was
used to observe the expansion of HSCs; it was identified that the
proportion of Lin− and LSK cells were not significantly
different, compared with 1 µM SB203580 treatment alone (Fig. S5). However, compared with the DMSO
group, the total number of cells, the frequency and absolute of
Lin- cells, the frequency and absolute of LSK cells of G group was
significantly increased (Fig. S6
and Table I), suggesting that
p38MAPK and GSK3β inhibitors may exert a synergistic effect in
promoting HSCs expansion.
HDAC signaling inhibitor VPA alters
HSC expansion in vitro
The highest total cell number (Fig. 3B), as well as relative and absolute
Lin− and LSK cell numbers (Fig. 3C, E and G) were achieved at the 0.25
mM dose of VPA (HDAC inhibitor). However, it was found that the
frequency of Lin− and LSK cells increased linearly with
increasing VPA concentration (Fig. 3D
and F). Meanwhile, when the VPA concentration was 1 mM, the
number of cells was significantly reduced and were transparent and
uniform, in a very good state under the microscope (Fig. 3A) and maintained the cells in an
undifferentiated state. This 1 mM dose was therefore used in the
following experiments. At the same time, the number of
Lin− cells was discovered to significantly increase in
the H and I groups compared with B, while the combination of VPA
and other inhibitors exerted a minimal effect on stem cell
expansion, demonstrating population frequencies similar to the DMSO
control sample (Fig. S6 and
Table I). Several groups of cells
were seen to be highly differentiated, especially group B. The
number of differentiated cells was much higher than the other
experimental groups, while groups C, H, I, N, O and P could
maintain an even transparent morphology, but the cells barely grew
after all four inhibitors were added, so we did not count this
group when we collected the data, i.e., group Q above was invalid
(Fig. S6 and Table I).
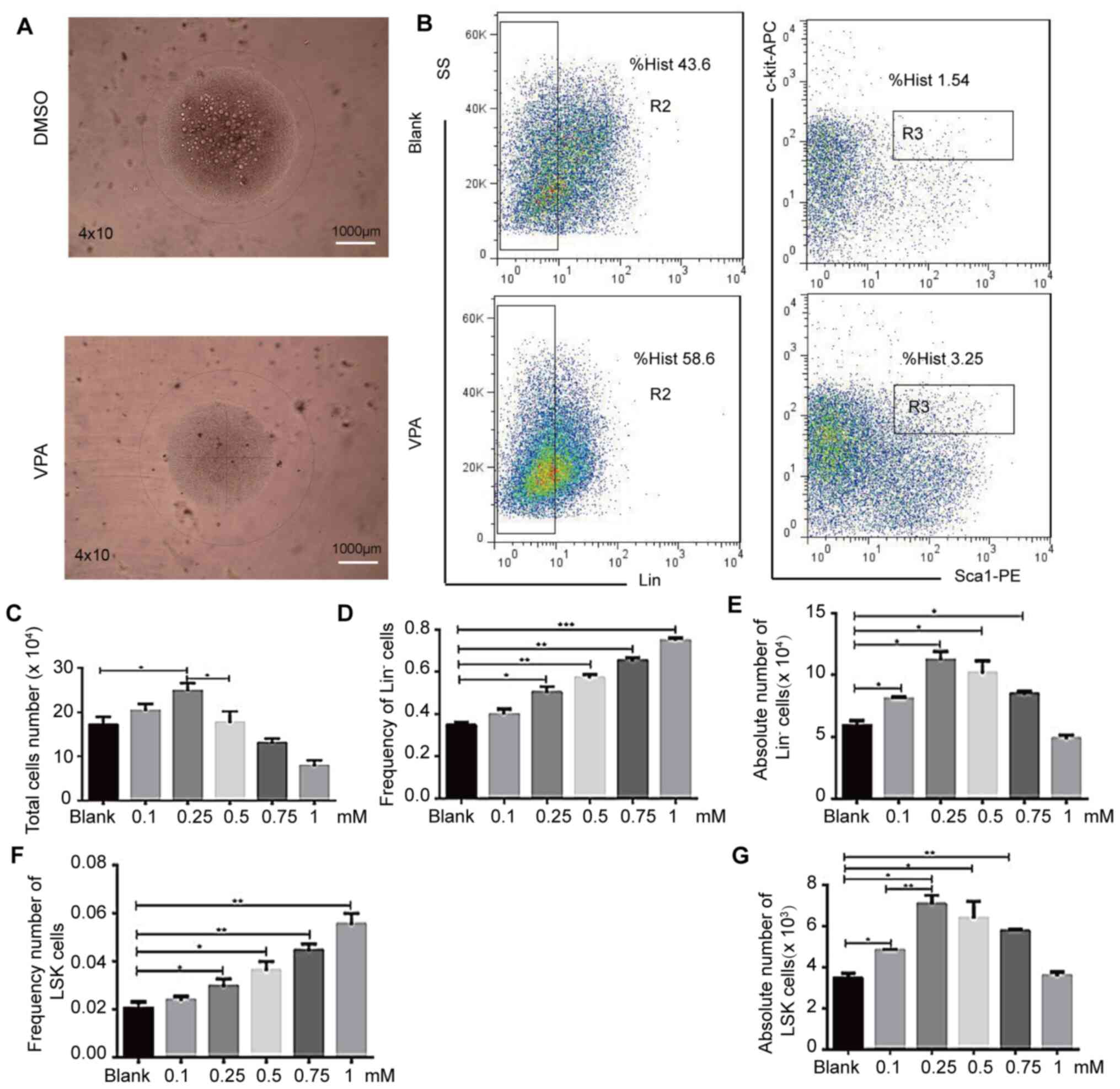 | Figure 3.VPA affects hematopoietic stem cell
expansion in vitro. LSK cells treated with various
concentrations of VPA (0.1, 0.25, 0.5, 0.75, 1 mM) were analyzed by
flow cytometry after a 9-day culture period. Cells were stained for
the expression of Gr1, CD11b, Ter119, CD3ε, B220, Sca-1 and c-Kit.
(A) LSK cell morphology was analyzed using a confocal Leica DM RXA
microscope. Magnification, ×40; scale bar, 1,000 µm. (B) LSK cells
were analyzed via flow cytometry. (C) Total cell number. (D)
Lin− cell frequency. (E) Lin− cell number.
(F) LSK cell frequency. (G) LSK cell number. n = 4; *P<0.05,
**P<0.01, ***P<0.001. APC, allophycocyanin; PE,
phycoerythrin; Lin, lineage; SS, side scatter; VPA, valproic acid
sodium salt; Sca-1, stem cell antigen-1. |
mTOR signaling inhibition alters in
vitro HSC expansion
To determine how mTOR inhibition affects HSC
functionality in vitro, cells were cultured with (0, 10, 15,
20, 25 or 30 nM) rapamycin for nine days, adding additional
rapamycin (0, 10, 15, 20, 25 or 30 nM) every two days during
culture to overcome metabolism of rapamycin. Following 9 days of
rapamycin treatment, the cells were discovered to be healthy, with
very few differentiated cells under the microscope, a lower number
of cells overall and uniform cellular morphology (Fig. 4B), suggesting a rapamycin-dependent
inhibition of cell growth. The cells were further analyzed via flow
cytometry, which revealed that in response to rapamycin treatment,
there was a significant increase in the Lin− cell ratio
(Fig. 4D and E), as well as in the
frequency and absolute of LSK cells (Fig. 4F and G), while total cell numbers
declined (Fig. 4C) which may be
because rapamycin inhibits cell proliferation, thus maintaining
cell stemness and inhibiting cell differentiation. Thus, 15 nM was
determined as a suitable inhibitory concentration for the mTOR
signaling pathway inhibitor rapamycin. Similarly, the proportion of
LSK cells in group K and N was increased compared with the other
combinations in the random combination of inhibitors (Fig. S6 and Table I). These findings suggested that
rapamycin may slow HSC growth, maintaining cells in a relatively
undifferentiated and stem-like state.
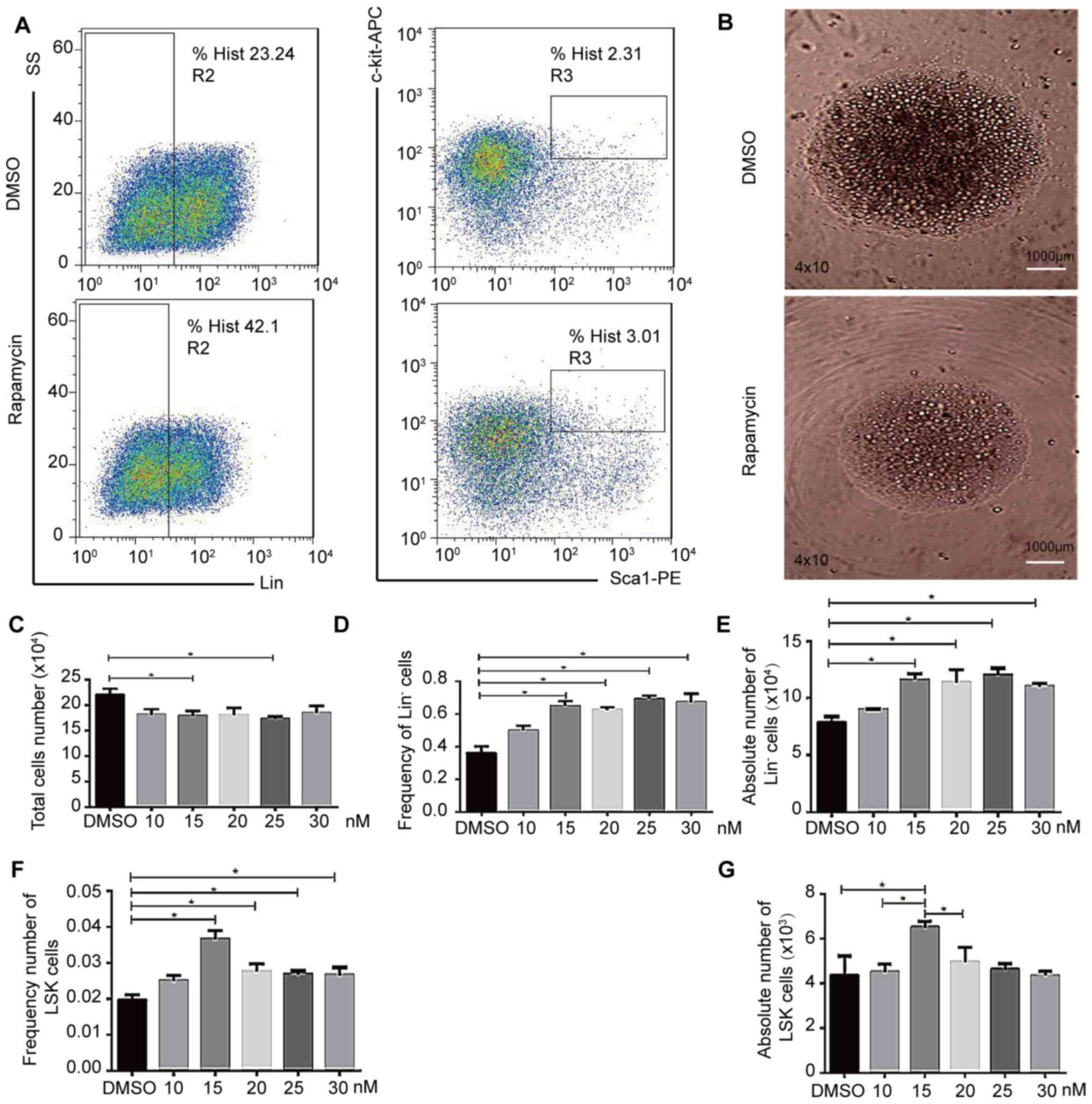 | Figure 4.mTOR inhibition affects hematopoietic
stem cell expansion in vitro. LSK cells were treated with
rapamycin (10, 15, 20, 25 and 30 nM) or DMSO. (A) LSK cells were
analyzed via flow cytometry. (B) Cell morphology following
rapamycin or DMSO treatment. Magnification, ×40; scale bar,
1,000-µm. Cells were visualized using the confocal Leica DM RXA
microscope. (C) Total cell number following rapamycin (10, 15, 20,
25 and 30 nM) or equal volume DMSO treatment. (D) Lin−
cell frequency following rapamycin or equal volume DMSO treatment.
(E) Lin− cell number following rapamycin or DMSO
treatment. (F) LSK cell frequency following rapamycin (10, 15, 20,
25 and 30 nM) or equal volume DMSO treatment. (G) LSK cell number
following rapamycin (10, 15, 20, 25 and 30 nM) or equal volume DMSO
treatment. n = 4. *P<0.05, APC, allophycocyanin; PE,
phycoerythrin; Lin, lineage; SS, side scatter; Sca-1, stem cell
antigen-1. |
Discussion
AML is a very common type of cancer; however,
patients with AML often still have a poor prognosis (49). The majority of patients currently
receive stem cell transplantation therapy as a means of improving
patient outcomes and prolonging survival (50). For such HSC transplantation (HCT)
procedures, donor stem cells are typically derived either from bone
marrow, peripheral blood or umbilical cord blood (51). As the number of HSCs available from
these donor tissues, and particularly in cord blood samples, can
often be very limited, this can lead to very long recovery periods,
making it a priority to identify novel means of enhancing HSC
expansion in vitro (52–54).
However, HSC differentiation and homeostatic regulation in
vivo depends upon a wide array of complex microenvironmental
inputs that can be difficult to replicate in vitro (55,56).
While there have been numerous efforts made to date
to promote ex vivo HSC expansion to improve engraftment
rates in the clinical setting (57,58),
there still remains a significant unmet need in the field of HCT,
and as such novel, ex vivo expansion strategies are still
required. Numerous previous studies revealed that p38MAPK, mTOR,
GSK3β and HDAC signaling were all important for regulating the
proliferation, differentiation, apoptosis and necrosis of HSCs
(7,59–63).
Therefore, the present study hypothesized that the simultaneous
inhibition of multiple of these pathways may promote the
synergistic expansion of HSCs in vitro, thereby representing
a potentially viable strategy for improving HCT outcomes in
patients with leukemia.
In the present study, the expansion of cells using a
single molecule inhibitor revealed that the HSC expansion
efficiency was significantly increased when SB203580 and SB216763
were used alone. HDAC signaling was also discovered to be a crucial
regulator of HSC homeostasis (64).
It was therefore investigated how the suppression of HDAC signaling
may alter HSC functionality in an in vitro experimental
system. High VPA concentrations failed to enhance HSC
proliferation, which may have been potentially due to the cytotoxic
effects of high concentrations of this inhibitor, which induced
apoptotic signaling, and HDAC inhibition was sufficient to maintain
HSCs in an undifferentiated state in vitro, enhancing their
expansion (65,66). Our studies demonstrated that HDAC
inhibitors induced HSC expansion, maintained undifferentiated cells
in vitro. mTOR signaling regulates the growth and metabolism
of cells, in addition to regulating HSC homing (62,67,68).
mTOR activation has also been illustrated to disrupt HSC quiescence
and drive HSC exhaustion, whereas inhibiting this signaling pathway
restored HSC self-renewal (61,69).
In the present study, inhibiting the mTOR pathway led to decreased
cell growth, but also helped maintain the cells in a more stem-like
undifferentiated state. These results suggested that the mTOR
signaling pathway may serve an important role in regulating the
self-renewal of HSCs in vitro. Thus, inhibiting the mTOR
pathway using rapamycin may represent a novel approach to promote
HSC expansion in vitro to improve HCT outcomes.
Next, to investigate how combinations of these
signaling pathway inhibitors affect HSC expansion and
differentiation in vitro, cells were treated with
combinations of the optimal concentrations of these
pathway-specific inhibitors. Consistent with our hypothesis, it was
discovered that the combination of SB203580 and SB216763
significantly increased HSC differentiation in vivo compared
with the other treatment groups. The combined inhibition of p38MAPK
and mTOR was also able to amplify HSCs in vitro, although
not as effectively as the combination of p38MAPK and GSK-3β
inhibition. In contrast, the treatment of cells with HDAC
inhibitors was associated with an almost complete loss of
differentiation (70), suggesting
that HDAC inhibitors may damage cellular responses in this context.
Among all the important signaling pathways, using these various
combinations, the results indicated that through combining the
p38MAPK and GSK3β inhibitors, HSC expansion was significantly
enhanced in vitro.
In conclusion, the findings of the present study may
provide a novel approach to expand HSCs ex vivo via
inhibiting p38MAPK and GSK3β, either alone or in combination.
However, the specific molecular mechanisms through which p38MAPK
and GSK3β signaling altered quiescent HSC expansion and
proliferation remain unclear and warrant further investigations.
Taken together, the aim of future studies is to obtain a series of
small-molecule compounds that inhibit p38MAPK and GSK3β signaling
pathways that can expand human HSCs ex vivo.
Supplementary Material
Supporting Data
Acknowledgements
The authors thank the staff of the Department of
Biology, College of Life and Sciences Shanghai Normal University
for animal care services.
Funding
The present study was supported by the Leukemia
Research Foundation New Investigator Award to JL (grant no.
81670151) and the National Natural Science Foundation of China
(grant no. 81700141).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJ performed the experiments, analyzed and extracted
the data, generated the figures and wrote the manuscript. ZX, NM
and LY analyzed the data; CH conceived the project and reviewed the
paper. JL conceived the project, designed and performed the
experiments, and analyzed and interpreted results. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All animal studies were approved by the
Institutional Animal Care and Use Committee of Shanghai Normal
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shlush LI, Zandi S, Mitchell A, Chen WC,
Brandwein JM, Gupta V, Kennedy JA, Schimmer AD, Schuh AC, Yee KW,
et al: Identification of pre-leukaemic haematopoietic stem cells in
acute leukaemia. Nature. 506:328–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tijaro-Ovalle NM, Karantanos T, Wang HT
and Boussiotis VA: Metabolic targets for improvement of allogeneic
hematopoietic stem cell transplantation and graft-vs.-host disease.
Front Immunol. 10:2952019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cui P, Zhang Y, Cui M, Li Z, Ma G, Wang R,
Wang N, Huang S and Gao J: Leukemia cells impair normal
hematopoiesis and induce functionally loss of hematopoietic stem
cells through immune cells and inflammation. Leuk Res. 65:49–54.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clements WK and Traver D: Signalling
pathways that control vertebrate haematopoietic stem cell
specification. Nat Rev Immunol. 13:336–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harada K, Fuji S, Seo S, Kanda J, Ueki T,
Kimura F, Kato K, Uchida N, Ikegame K, Onizuka M, et al: Comparison
of the outcomes after haploidentical and cord blood salvage
transplantations for graft failure following allogeneic
hematopoietic stem cell transplantation. Bone Marrow Transplant.
55:1784–1795. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ozdemir ZN and Civriz Bozdag S: Graft
failure after allogeneic hematopoietic stem cell transplantation.
Transfus Apher Sci. 57:163–167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karigane D, Kobayashi H, Morikawa T,
Ootomo Y, Sakai M, Nagamatsu G, Kubota Y, Goda N, Matsumoto M,
Nishimura EK, et al: p38α activates purine metabolism to initiate
hematopoietic stem/progenitor cell cycling in response to stress.
Cell stem cell. 19:192–204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guezguez B, Almakadi M, Benoit YD,
Shapovalova Z, Rahmig S, Fiebig-Comyn A, Casado FL, Tanasijevic B,
Bresolin S, Masetti R, et al: GSK3 deficiencies in hematopoietic
stem cells initiate pre-neoplastic state that is predictive of
clinical outcomes of human acute leukemia. Cancer Cell. 29:61–74.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou F, Li X, Wang W, Zhu P, Zhou J, He W,
Ding M, Xiong F, Zheng X, Li Z, et al: Tracing haematopoietic stem
cell formation at single-cell resolution. Nature. 533:487–492.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hinge A, Xu J, Javier J, Mose E, Kumar S,
Kapur R, Srour EF, Malik P, Aronow BJ and Filippi MD: p190-B RhoGAP
and intracellular cytokine signals balance hematopoietic stem and
progenitor cell self-renewal and differentiation. Nat Commun.
8:143822017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kwon B: p38α-mediated purine metabolism is
linked to exit from quiescence of hematopoietic stem cells. Stem
Cell Investig. 3:692016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ito K, Hirao A, Arai F, Takubo K, Matsuoka
S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y and Suda T:
Reactive oxygen species act through p38 MAPK to limit the lifespan
of hematopoietic stem cells. Nat Med. 12:446–451. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ratajczak MZ and Suszynska M: Emerging
strategies to enhance homing and engraftment of hematopoietic stem
cells. Stem Cell Rev Rep. 12:121–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abdelbaset-Ismail A, Borkowska-Rzeszotek
S, Kubis E, Bujko K, Brzeźniakiewicz-Janus K, Bolkun L, Kloczko J,
Moniuszko M, Basak GW, Wiktor-Jedrzejczak W and Ratajczak MZ:
Activation of the complement cascade enhances motility of leukemic
cells by downregulating expression of heme oxygenase 1 (HO-1).
Leukemia. 31:446–458. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao F and Liu WJ: Advance in the study on
p38 MAPK mediated drug resistance in leukemia. Eur Rev Med
Pharmacol Sci. 20:1064–1070. 2016.PubMed/NCBI
|
|
16
|
Ko KH, Holmes T, Palladinetti P, Song E,
Nordon R, O'Brien TA and Dolnikov A: GSK-3β inhibition promotes
engraftment of ex vivo-expanded hematopoietic stem cells and
modulates gene expression. Stem Cells. 29:108–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang J, Zhang Y, Bersenev A, O'Brien WT,
Tong W, Emerson SG and Klein PS: Pivotal role for glycogen synthase
kinase-3 in hematopoietic stem cell homeostasis in mice. J Clin
Invest. 119:3519–3529. 2009.PubMed/NCBI
|
|
18
|
Li H, Feng J, Zhang Y, Feng J, Wang Q,
Zhao S, Meng P and Li J: Mst1 deletion attenuates renal
ischaemia-reperfusion injury: The role of microtubule cytoskeleton
dynamics, mitochondrial fission and the GSK3β-p53 signalling
pathway. Redox Biol. 20:261–274. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lechman ER, Gentner B, van Galen P,
Giustacchini A, Saini M, Boccalatte FE, Hiramatsu H, Restuccia U,
Bachi A, Voisin V, et al: Attenuation of miR-126 activity expands
HSC in vivo without exhaustion. Cell Stem Cell. 11:799–811. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lapid K, Itkin T, D'Uva G, Ovadya Y, Ludin
A, Caglio G, Kalinkovich A, Golan K, Porat Z, Zollo M and Lapidot
T: GSK3β regulates physiological migration of stem/progenitor cells
via cytoskeletal rearrangement. J Clin Invest. 123:1705–1717. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gatla HR, Muniraj N, Thevkar P, Yavvari S,
Sukhavasi S and Makena MR: Regulation of chemokines and cytokines
by histone deacetylases and an update on histone decetylase
inhibitors in human diseases. Int J Mol Sci. 20:11102019.
View Article : Google Scholar
|
|
22
|
Stahl M, Gore SD, Vey N and Prebet T: Lost
in translation? Ten years of development of histone deacetylase
inhibitors in acute myeloid leukemia and myelodysplastic syndromes.
Expert Opin Investig Drugs. 25:307–317. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jabbour E and Garcia-Manero G: Deacetylase
inhibitors for the treatment of myelodysplastic syndromes. Leuk
Lymphoma. 56:1205–1212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Z, Ding K, Li L, Liu H, Wang Y, Liu C
and Fu R: A novel histone deacetylase inhibitor Chidamide induces
G0/G1 arrest and apoptosis in myelodysplastic syndromes. Biomed
Pharmacother. 83:1032–1037. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heuser M, Yun H and Thol F: Epigenetics in
myelodysplastic syndromes. Semin Cancer Biol. 51:170–179. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mao J, Li S, Zhao H, Zhu Y, Hong M, Zhu H,
Qian S and Li J: Effects of chidamide and its combination with
decitabine on proliferation and apoptosis of leukemia cell lines.
Am J Transl Res. 10:2567–2578. 2018.PubMed/NCBI
|
|
27
|
Qi J, Singh S, Hua WK, Cai Q, Chao SW, Li
L, Liu H, Ho Y, McDonald T, Lin A, et al: HDAC8 inhibition
specifically targets Inv (16) acute myeloid leukemic stem cells by
restoring p53 acetylation. Cell Stem Cell. 17:597–610. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen C, Liu Y, Liu Y and Zheng P: mTOR
regulation and therapeutic rejuvenation of aging hematopoietic stem
cells. Sci Signal. 2:ra752009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao D, Song J, Ling S, Niu S, Lu L, Cui Z,
Li Y, Hao S, Zhong G, Qi Z, et al: Hematopoietic stem cells and
lineage cells undergo dynamic alterations under microgravity and
recovery conditions. FASEB J. 33:6904–6918. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kassim AA and Savani BN: Hematopoietic
stem cell transplantation for acute myeloid leukemia: A review.
Hematol Oncol Stem Cell Ther. 10:245–251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chhabra A, Ring AM, Weiskopf K, Schnorr
PJ, Gordon S, Le AC, Kwon HS, Ring NG, Volkmer J, Ho PY, et al:
Hematopoietic stem cell transplantation in immunocompetent hosts
without radiation or chemotherapy. Sci Transl Med. 8:351ra1052016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
De Meyts P: The Insulin Receptor and Its
Signal Transduction Network. Endotext. Feingold KR, Anawalt B,
Boyce A, et al: MDText.com, Inc.; South Dartmouth, MA: 2000
|
|
33
|
Bari S, Zhong Q, Fan X, Poon Z, Lim AST,
Lim TH, Dighe N, Li S, Chiu GNC, Chai CLL and Hwang WYK: Ex vivo
expansion of CD34+ CD90+ CD49f+
hematopoietic stem and progenitor cells from non-enriched umbilical
cord blood with azole compounds. Stem Cells Transl Med. 7:376–393.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cao R, Li L, Ying Z, Cao Z, Ma Y, Mao X,
Li J, Qi X, Zhang Z and Wang X: A small molecule protects
mitochondrial integrity by inhibiting mTOR activity. Proc Natl Acad
Sci USA. 116:23332–23338. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dong G, Chen W, Wang X, Yang X, Xu T, Wang
P, Zhang W, Rao Y, Miao C and Sheng C: Small molecule inhibitors
simultaneously targeting cancer metabolism and epigenetics:
Discovery of novel nicotinamide phosphoribosyltransferase (NAMPT)
and histone deacetylase (HDAC) dual inhibitors. J Med Chem.
60:7965–7983. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu VW and Scadden DT: Hematopoietic stem
cell and its bone marrow niche. Curr Top Dev Biol. 118:21–44. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wilkinson AC, Ishida R, Kikuchi M, Sudo K,
Morita M, Crisostomo RV, Yamamoto R, Loh KM, Nakamura Y, Watanabe
M, et al: Long-term ex vivo haematopoietic-stem-cell expansion
allows nonconditioned transplantation. Nature. 571:117–121. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Höfer T and Rodewald HR:
Differentiation-based model of hematopoietic stem cell functions
and lineage pathways. Blood. 132:1106–1113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu ZY, Chai S, Zhang XQ, Cao Y, Lian CQ,
Wu WJ and Li YY: Influence of BMP4 on regulation of cell cycle and
apoptosis of hematopoietic stem cells/progenitor cells and its
mechanism in chemotherapy-induced myelosuppression. Zhongguo Shi
Yan Xue Ye Xue Za Zhi. 27:1265–1271. 2019.(in Chinese). PubMed/NCBI
|
|
40
|
Morcos MNF, Schoedel KB, Hoppe A, Behrendt
R, Basak O, Clevers HC, Roers A and Gerbaulet A: SCA-1 expression
level identifies quiescent hematopoietic stem and progenitor cells.
Stem Cell Rep. 8:1472–1478. 2017. View Article : Google Scholar
|
|
41
|
Wang Y, Kellner J, Liu L and Zhou D:
Inhibition of p38 mitogen-activated protein kinase promotes ex vivo
hematopoietic stem cell expansion. Stem Cells Dev. 20:1143–1152.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cirillo PF, Pargellis C and Regan J: The
non-diaryl heterocycle classes of p38 MAP kinase inhibitors. Curr
Top Med Chem. 2:1021–1035. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gao LY, Zhao MY, Li P, Kong J, Liu Z, Chen
Y, Huang R, Chu J, Quan J and Zeng R: Glycogen synthase kinase 3
(GSK3)-inhibitor SB216763 promotes the conversion of human
umbilical cord mesenchymal stem cells into neural precursors in
adherent culture. Hum Cell. 30:11–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zeng J, Liu X, Li X, Zheng Y, Liu B and
Xiao Y: Daucosterol inhibits the proliferation, migration, and
invasion of hepatocellular carcinoma cells via Wnt/β-catenin
signaling. Molecules. 22:8622017. View Article : Google Scholar
|
|
45
|
Cheng T, Zhai K, Chang Y, Yao G, He J,
Wang F, Kong H, Xin H, Wang H, Jin M, et al: CHIR99021 combined
with retinoic acid promotes the differentiation of primordial germ
cells from human embryonic stem cells. Oncotarget. 8:7814–7826.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dobzanski A, Khalil SM and Lane AP: Nasal
polyp fibroblasts modulate epithelial characteristics via Wnt
signaling. Int Forum Allergy Rhinol. 8:1412–1420. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu Y, Ai Z, Yao K, Cao L, Du J, Shi X, Guo
Z and Zhang Y: CHIR99021 promotes self-renewal of mouse embryonic
stem cells by modulation of protein-encoding gene and long
intergenic non-coding RNA expression. Exp Cell Res. 319:2684–2699.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mahajan MM, Cheng B, Beyer AI, Mulvaney
US, Wilkinson MB, Fomin ME and Muench MO: A quantitative assessment
of the content of hematopoietic stem cells in mouse and human
endosteal-bone marrow: A simple and rapid method for the isolation
of mouse central bone marrow. BMC Hematol. 15:92015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
DiNardo CD and Cortes JE: Mutations in
AML: Prognostic and therapeutic implications. Hematology Am Soc
Hematol Educ Program. 2016:348–355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cornelissen JJ and Blaise D: Hematopoietic
stem cell transplantation for patients with AML in first complete
remission. Blood. 127:62–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Richter J, Traver D and Willert K: The
role of Wnt signaling in hematopoietic stem cell development. Crit
Rev Biochem Mol Biol. 52:414–424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
de Jamblinne Y, Baudoux E, Delo C and
Coppieters Y: Influence of obstetric factors on characteristics of
umbilical cord blood transplants. Gynecol Obstet Fertil Senol.
46:639–644. 2018.(In French). PubMed/NCBI
|
|
53
|
Lou X, Zhao C and Chen H: Unrelated donor
umbilical cord blood transplant versus unrelated hematopoietic stem
cell transplant in patients with acute leukemia: A meta-analysis
and systematic review. Blood Rev. 32:192–202. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tong J, Xuan L, Sun Y, Huang D, Liu H,
Zheng C, Zhu X, Tang B, Song K, Zhang X, et al: Umbilical cord
blood transplantation without antithymocyte globulin results in
similar survival but better quality of life compared with unrelated
peripheral blood stem cell transplantation for the treatment of
acute leukemia-a retrospective study in China. Biol Blood Marrow
Transplant. 23:1541–1548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kaushansky K and Zhan H: The regulation of
normal and neoplastic hematopoiesis is dependent on
microenvironmental cells. Adv Biol Regul. 69:11–15. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lucas D: The bone marrow microenvironment
for hematopoietic stem cells. Adv Exp Med Biol. 1041:5–18. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dzierzak E and Bigas A: Blood development:
Hematopoietic stem cell dependence and independence. Cell Stem
Cell. 22:639–651. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Derakhshani M, Abbaszadeh H, Movassaghpour
AA, Mehdizadeh A, Ebrahimi-Warkiani M and Yousefi M: Strategies for
elevating hematopoietic stem cells expansion and engraftment
capacity. Life Sci. 232:1165982019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hua Y, Wang C, Jiang H, Wang Y, Liu C, Li
L, Liu H, Shao Z and Fu R: Iron overload may promote alteration of
NK cells and hematopoietic stem/progenitor cells by JNK and p38
pathway in myelodysplastic syndromes. Int J Hematol. 106:248–257.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tesio M, Tang Y, Mudder K, Saini M, von
Paleske L, Macintyre E, Pasparakis M, Waisman A and Trumpp A:
Hematopoietic stem cell quiescence and function are controlled by
the CYLD-TRAF2-p38MAPK pathway. J Exp Med. 212:525–538. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Peng H, Kasada A, Ueno M, Hoshii T,
Tadokoro Y, Nomura N, Ito C, Takase Y, Vu HT, Kobayashi M, et al:
Distinct roles of Rheb and Raptor in activating mTOR complex 1 for
the self-renewal of hematopoietic stem cells. Biochem Biophys Res
Commun. 495:1129–1135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Oburoglu L, Romano M, Taylor N and Kinet
S: Metabolic regulation of hematopoietic stem cell commitment and
erythroid differentiation. Curr Opin Hematol. 23:198–205. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Trowbridge JJ, Xenocostas A, Moon RT and
Bhatia M: Glycogen synthase kinase-3 is an in vivo regulator of
hematopoietic stem cell repopulation. Nature Med. 12:89–98. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dhoke NR, Kalabathula E, Kaushik K,
Geesala R, Sravani B and Das A: Histone deacetylases differentially
regulate the proliferative phenotype of mouse bone marrow stromal
and hematopoietic stem/progenitor cells. Stem Cell Res. 17:170–180.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Papa L, Djedaini M and Hoffman R: Ex vivo
expansion of hematopoietic stem cells from human umbilical cord
blood-derived CD34+ cells using valproic acid. J Vis
Exp. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
66
|
Aztopal N, Erkisa M, Erturk E, Ulukaya E,
Tokullugil AH and Ari F: Valproic acid, a histone deacetylase
inhibitor, induces apoptosis in breast cancer stem cells. Chem Biol
Interact. 280:51–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang M, Liu F, Zhou P, Wang Q, Xu C, Li
Y, Bian L, Liu Y, Zhou J, Wang F, et al: The mTOR signaling pathway
regulates macrophage differentiation from mouse myeloid progenitors
by inhibiting autophagy. Autophagy. 15:1150–1162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Mossmann D, Park S and Hall MN: mTOR
signalling and cellular metabolism are mutual determinants in
cancer. Nat Rev Cancer. 18:744–757. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Luo Y, Li L, Zou P, Wang J, Shao L, Zhou D
and Liu L: Rapamycin enhances long-term hematopoietic
reconstitution of ex vivo expanded mouse hematopoietic stem cells
by inhibiting senescence. Transplantation. 97:20–29. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Anastas JN, Zee BM, Kalin JH, Kim M, Guo
R, Alexandrescu S, Blanco MA, Giera S, Gillespie SM, Das J, et al:
Re-programing chromatin with a bifunctional LSD1/HDAC inhibitor
induces therapeutic differentiation in DIPG. Cancer Cell.
36:528–544.e10. 2019. View Article : Google Scholar : PubMed/NCBI
|


















