Introduction
Osteoporosis is a type of bone disease that
decreases bone mass and density, leading to an increased risk of
fractures (1,2). It is the most common reason for a
broken bone among elderly individuals. Until a break occurs, there
are typically no symptoms, and as the disease progresses, the bones
may weaken to such a degree that a break may occur spontaneously or
as a result of only minor stress. The underlying mechanism of
osteoporosis is the imbalance between osteoclast-mediated bone
resorption and osteoblast-mediated bone formation. Osteoclasts are
primarily regulated by transcription factor PU.1, and they degrade
the bone matrix, whereas osteoblasts participate in rebuilding the
bone matrix. Excess degradation results in low bone mass density.
Therefore, how to re-establish the balance between osteogenesis and
bone degradation in patients with osteoporosis is a clinical focus
for researchers.
Numerous cellular signalling pathways are involved
in the process of differentiation of bone marrow-derived
mesenchymal stem cells (BMSCs) into osteocytes in osteogenesis
(3–5). A number of signal transduction
proteins, such as NF-κb (6),
SMAD1/5/8 (7), bone morphogenetic
protein (BMP)/TGF-β (8) and
Wnt/β-catenin (9), have been
previously reported to participate in osteogenesis.
Previous studies have revealed that enhanced
β-catenin activity promotes human osteogenic differentiation,
whereas phosphorylation of the Ser 9 site of glycogen synthase
kinase (GSK)3β decreases its own activity, which can result in
decreased expression levels of β-catenin and increased
differentiation of BMSCs (10–12).
Moreover, osteogenic differentiation of BMSCs can be potentiated
via a GSK3β inhibitor (GI). In addition, evidence has demonstrated
that metformin, a drug used to control blood glucose levels, can
induce human BMSCs to differentiate into osteoblasts via inhibiting
GSK3β and promoting its phosphorylation at Ser9 (13).
To the best of our knowledge, the function of
astragaloside (AST) in fracture healing has rarely been studied.
Studies have demonstrated that the AST monomer enhances osteogenic
differentiation (14–16). Cheng et al (14) revealed that AST-I activated the
Wnt/β-catenin signalling pathway to stimulate osteogenic
differentiation. Kong et al (15) demonstrated that AST-II regulated the
BMP-1/MAPK and SMAD1/5/8 signalling pathways to induce osteogenic
activation of osteoblasts. Li et al (17) reported that AST-IV inhibited
receptor activator of NF-κB ligand-mediated breakdown of bone
tissue by osteoclasts. However, whether AST-IV promotes BMSC
osteogenesis has not yet been reported and therefore warrants
further investigation. Furthermore, Yin et al (18) demonstrated that AST exhibited a
therapeutic effect on local ischaemia-reperfusion injury and served
a neuroprotective role by producing neuronal growth factor
(NGF)/TrkA. Previous studies have demonstrated that NGF can
phosphorylate Ser9 of GSK3β (19,20).
Based on the results of these studies, it was hypothesized that
AST-IV may affect the GSK3β/β-catenin signalling pathway via
regulating NGF and consequently induce osteogenic differentiation
of BMSCs.
The present study investigated the potential
functions of AST-IV during osteogenic differentiation of BMSCs, and
demonstrated that AST-IV promotes osteoblast differentiation of
BMSCs via the GSK3β/β-catenin signalling pathway. Additionally,
differentiation of BMSCs induced by AST-IV was revealed to be an
NGF-dependent process.
Materials and methods
BMSC culture
Human BMSCs (Cyagen Biosciences, Inc.) were seeded
and cultured in T25 culture flasks (Thermo Fisher Scientific,
Inc.). Cells were cultured in high glucose DMEM supplemented with
10% foetal bovine serum (both Gibco; Thermo Fisher Scientific,
Inc.), 2 mM L-glutamine (Sigma-Aldrich; Merck KGaA) and antibiotics
[100 U/ml penicillin G and 100 µg/ml streptomycin (Gibco; Thermo
Fisher Scientific, Inc.)]. Cells were maintained in an incubator at
37°C with 5% CO2.
MTT assay
BMSCs were plated at a concentration of
1×104 cells/ml in 96-well plates. Cells were incubated
with 200 µl DMEM per well at 37°C to allow cells to adhere to the
bottoms of the wells. After 24 h, 20 µl MTT solution (5 mg/ml)
(Beyotime Institute of Biotechnology) was added to each well. The
medium in each well was discarded after 4 h; then, 150 µl DMSO was
added, and samples were agitated at a speed of 50 oscillations/min
for 10 min at 25°C. The optical density of each well was measured
via a Universal Microplate Reader (Bio-Tek Instruments, Inc.) at
490 nm. Wells containing media without cells were used as internal
blank controls.
Alizarin red staining (ARS) and
alkaline phosphatase (ALP) activity assay
For ARS, BMSCs were added to 96-well plates at a
density of 2×105 cells/well, and following 21 days of
AST-IV treatment, cells were cultured and fixed with 70% ethyl
alcohol for 1 h at 25°C. Then, 2% alizarin red (Sigma-Aldrich;
Merck KGaA) (pH 8.3) reagent was added to each well for staining at
37°C for 30 min. Next, 200 µl hexadecylpyridinium chloride solution
(0.28 M hexadecylpyridinium chloride, 11.7 mM
NaH2PO4 in distilled water) was added to
wells and incubated for 15 min at room temperature to remove the
calcium-bound alizarin red S. Mineralized calcium nodules were
observed with a light microscope (magnification, ×200).
ALP activity assays were performed following 7 days
of AST-IV treatment. BMSCs were washed twice with PBS, and then
treated with lysis buffer [1% Triton X-100, 20 mM Tris-HCl (pH,
7.5) and 150 mM NaCl] on ice for 10 min. The ALP activity was
detected by ALP Assay kit (Beyotime Institute of Biotechnology)
according to the manufacturer's instructions. The activity was
detected by measuring the absorbance at 405/650 nm on an absorbance
microplate reader (BioTek Instruments, Inc.).
Western blotting
For total protein extraction by RIPA Lysis Buffer
(Beyotime Institute of Biotechnology), cells were washed three
times with PBS and then incubated for 30 min on ice with cell
lysate. Samples were then centrifuged at 4°C and 11,500 × g for 10
min. The supernatant was subsequently collected and stored in a
−80°C freezer. Then, the protein concentration was measured via a
bicinchoninic acid kit. Electrophoresis was performed on 20 µg
protein sample on 10% SDS-PAGE. Then, the protein was transferred
onto a PVDF membrane. A 5% skimmed non-fat milk solution in
tris-buffered saline with 0.1% Tween-20 (TBST) solution was used
for blocking the membrane for 1 h at 25°C. Primary antibodies were
incubated with the membrane at 4°C overnight, and after being
washed by TBST three times, the membrane was incubated with
secondary antibodies at room temperature for 1 h. The following
primary antibodies were used: β-catenin (1:1,000; cat. no. 8480;
Cell Signaling Technology, Inc.), Osteocalcin (OCN; 1:1,000; cat.
no. ab133612; Abcam), Osteopontin (OPN; 1:1,000; cat. no. ab216406;
Abcam); Runt-related transcription factor 2 (Runx2; 1:1,000; cat.
no. 8486; Cell Signaling Technology, Inc.), osterix (OSX; 1:1,000;
cat. no. ab229258; Abcam), β-actin (1:1,000; cat. no. A5316;
Sigma-Aldrich; Merck KGaA), p-S9-GSK3β (1:10,000; cat. no. ab75814;
Abcam), GSK3β (1:10,000; cat. no. ab75814; Abcam) and NGF (1:1,000;
cat. no. ab68151; Abcam). After probing for 1 h at room temperature
with the corresponding secondary antibodies [horseradish
peroxidase-conjugated goat anti-rabbit and donkey anti-goat IgG
(both 1:5,000; cat. nos. ab205718 and ab97110, respectively; both
Abcam)], the protein bands were detected with an ECL kit using a
ChemiDoc XRS imaging system. ImageJ software (version 1.52v;
National Institutes of Health) was used for densitometric
analysis.
Reverse transcription-quantitative
(RT-q)PCR
BMSCs were lysed in 1 ml TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), and RNA was extracted using RNeasy
Mini kits (Qiagen GmbH). Isolation steps were performed according
to the manufacturer's instructions. In order to synthesize cDNA, a
PrimeScript™ First Strand cDNA Synthesis kit (Takara Bio, Inc.) was
used according to the manufacturer's instructions. Then, qPCR was
performed using FastStart Universal Probe Master Mix (Roche Applied
Science). The amplification conditions used were as follows: 95°C
for 2 min for denaturation, 30 cycles of denaturation at 95°C for
30 sec, 55°C for 30 sec for annealing and 72°C for 30 sec for
extension. Using GAPDH as an endogenous control. The following
primers were used: OPN forward, 5′-GATGGCCGAGGTGATAGTGT-3′ and
reverse, 5′-GTGGGTTTCAGCACTCTGGT-3′; OCN forward,
5′-GGCAGCGAGGTAGTGAAGAG-3′ and reverse, 5′-CTAGACCGGGCCGTAGAAG-3′;
Runx2 forward, 5′-CGGAATGCCTCTGCTGTTAT-3′ and reverse,
5′-TTCCCGAGGTCCATCTACTG-3′; NGF forward, 5′-ATACAGGCGGAACCACACTC-3′
and reverse, 5′-AGCCTGGGGTCCACAGTAAT-3′; OSX forward,
5′-GGTCCCCAGTCGAGGAT-3′ and reverse, 5′-CTAGAGCCGCCAAATTTGCT-3′;
and GAPDH forward, 5′-CCAGGTGGTCTCCTCTGA−3′ and reverse,
5′-GCTGTAGCCAAATCGTTGT-3′.
Plasmid transfection and reagents
GSK3β clone plasmid and human anti-NGF antibody were
purchased from TsingKe Biotech (Beijing) Co., Ltd.
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to transfect cells according to the
manufacturer's instructions. The ratio of gene/Lipofectamine was
1:3. After 48 h transfection, the cells were collected for
subsequent experiments. The GSK3β inhibitor (GI) SB216367 and
AST-IV were purchased from Sigma-Aldrich (Merck KGaA) Trading Co.
Ltd. Anti-NGF-H0, which does not bind to NGF, was used as a
negative control for the anti-NGF antibody. The same dose of DMSO
(10 µl) was used as a control for the GI group.
Statistical analysis
Data are expressed as the mean ± standard deviation
of three experimental repeats Statistical analysis was performed
using unpaired Student's t-test or one-way ANOVA followed by
Tukey's post hoc test. Linear association was demonstrated by
scatter plots and line of best fit-based linear regression models.
P<0.05 was considered to indicate a statistically significant
difference.
Results
AST-IV promotes osteogenic
differentiation but not proliferation of BMSCs
In order to examine whether AST-IV promotes the
proliferation of BMSCs, BMSCs were incubated with BMSC gradient
concentrations of AST-IV (10, 20 and 40 µmol/l) and the number of
cells was counted. The cell viability of BMSCs are summarized in
Fig. 1A, and no significant
differences were observed between the AST-IV and control groups.
However, ALP activity (day 7) and ARS (day 21) of BMSCs indicated
that AST-IV significantly increased BMSC osteogenic differentiation
in a dose-dependent manner (Fig.
1B-D). In order to confirm the function of AST-IV in
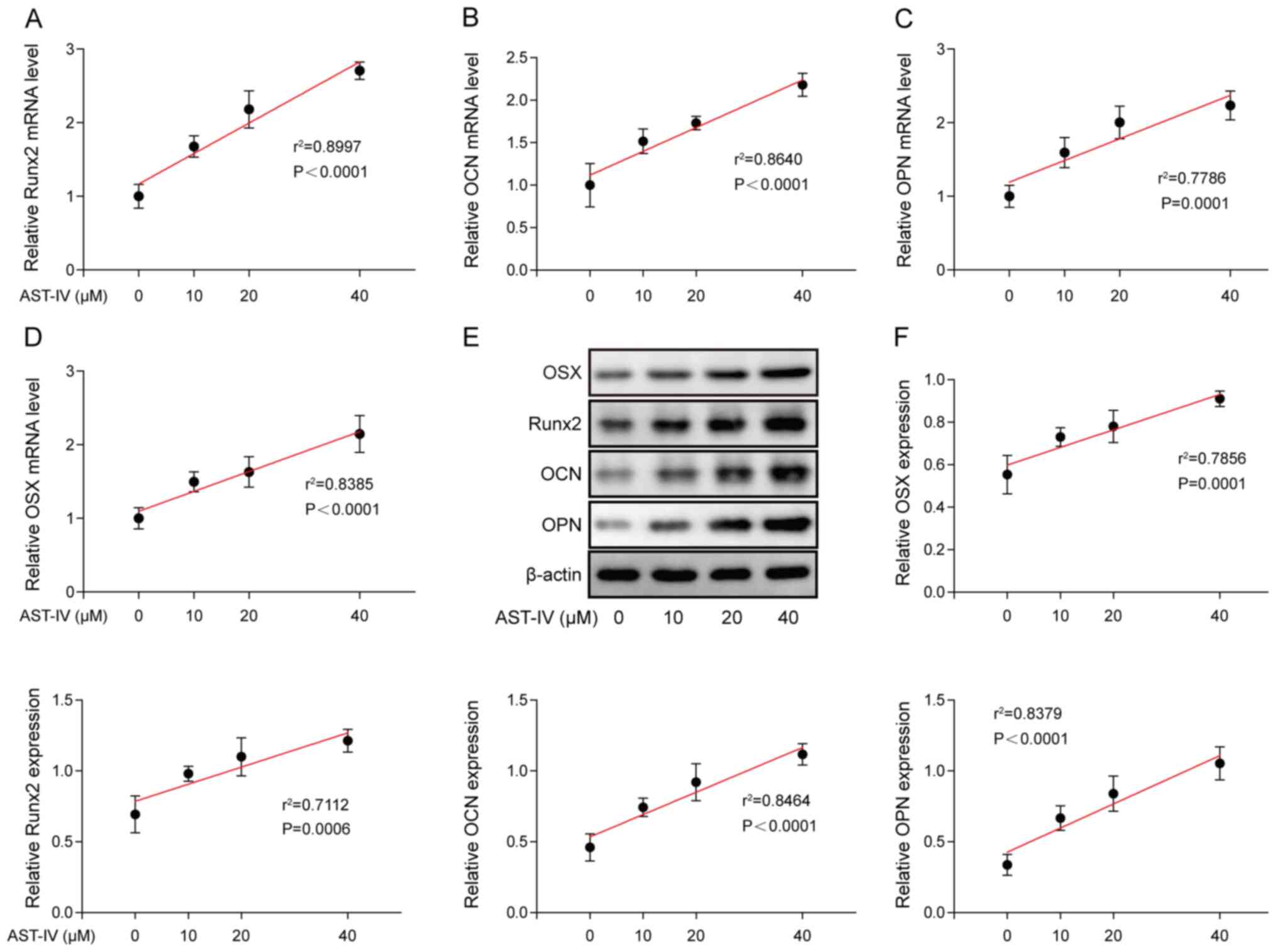
accelerating osteoblastic differentiation of BMSCs, mRNA and
protein expression levels of Runx2, OCN, OPN and OSX were
determined via RT-qPCR and western blotting following incubation
with AST-IV for 7 days (Fig. 2A-F).
The results demonstrated that both the mRNA and protein levels of
Runx2, OCN, OPN and OSX were significantly increased following
treatment, and this increase in expression levels was associated
with the concentration of AST-IV.
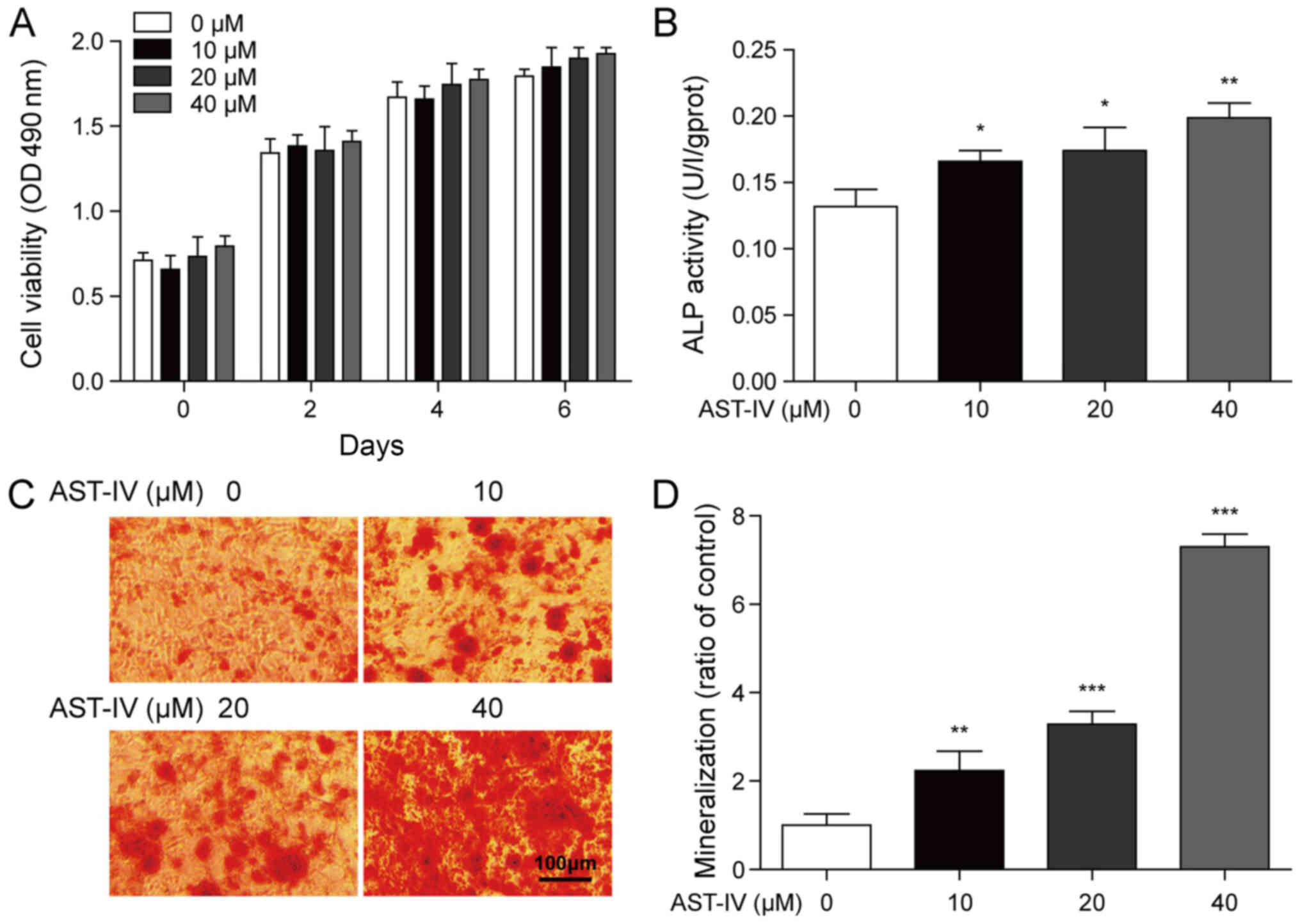 | Figure 1.Proliferation and differentiation
properties of BMSCs incubated with AST-IV. (A) BMSCs were incubated
with gradient concentrations of AST-IV (0, 10, 20 and 40 µM), and
cell proliferation was detected at days 0, 2, 4 and 6. (B)
Following AST-IV treatment, ALP activity of BMSCs was measured at
day 7. (C) Following 21-day treatment with AST-IV (0, 10, 20 and 40
µM), alizarin red S staining was used to detect mineralized nodules
in BMSCs (magnification, ×200). Scale bar, 100 mm. (D) as a ratio
of the Control. n=3; *P<0.05, **P<0.01 and ***P<0.001.
BMSCs, bone marrow-derived mesenchymal stem cells; AST-IV,
astragaloside-IV; ALP, alkaline phosphatase. |
AST-IV stimulates osteogenic
differentiation of BMSCs via GSK3β/β-catenin signalling
AST-IV incubation significantly increased the ratio
of p-S9-GSK3β/total GSK3β and protein expression levels of
β-catenin in BMSCs (Fig. 3A and B).
In order to investigate the function of GSK3β during osteogenic
differentiation, a GSK3β-overexpressing vector was transfected into
BMSCs. Compared with the empty vector control, the protein
expression levels of β-catenin were decreased in AST-IV-treated
BMSCs following transfection with the GSK3β overexpression vector
(Fig. 3C and D). Overexpression of
GSK3β in BMSCs also counteracted the promotion of osteogenic
differentiation by AST-IV. ALP activity and mineralization of cells
were also decreased in GSK3β-overexpressing BMSCs (Fig. 3E-G). Moreover, GSK3β overexpression
decreased the expression levels of Runx2, OCN, OPN and OSX,
providing evidence to support the hypothesis that GSK3β signalling
affects osteoblastic differentiation of BMSCs (Fig. 3H-J).
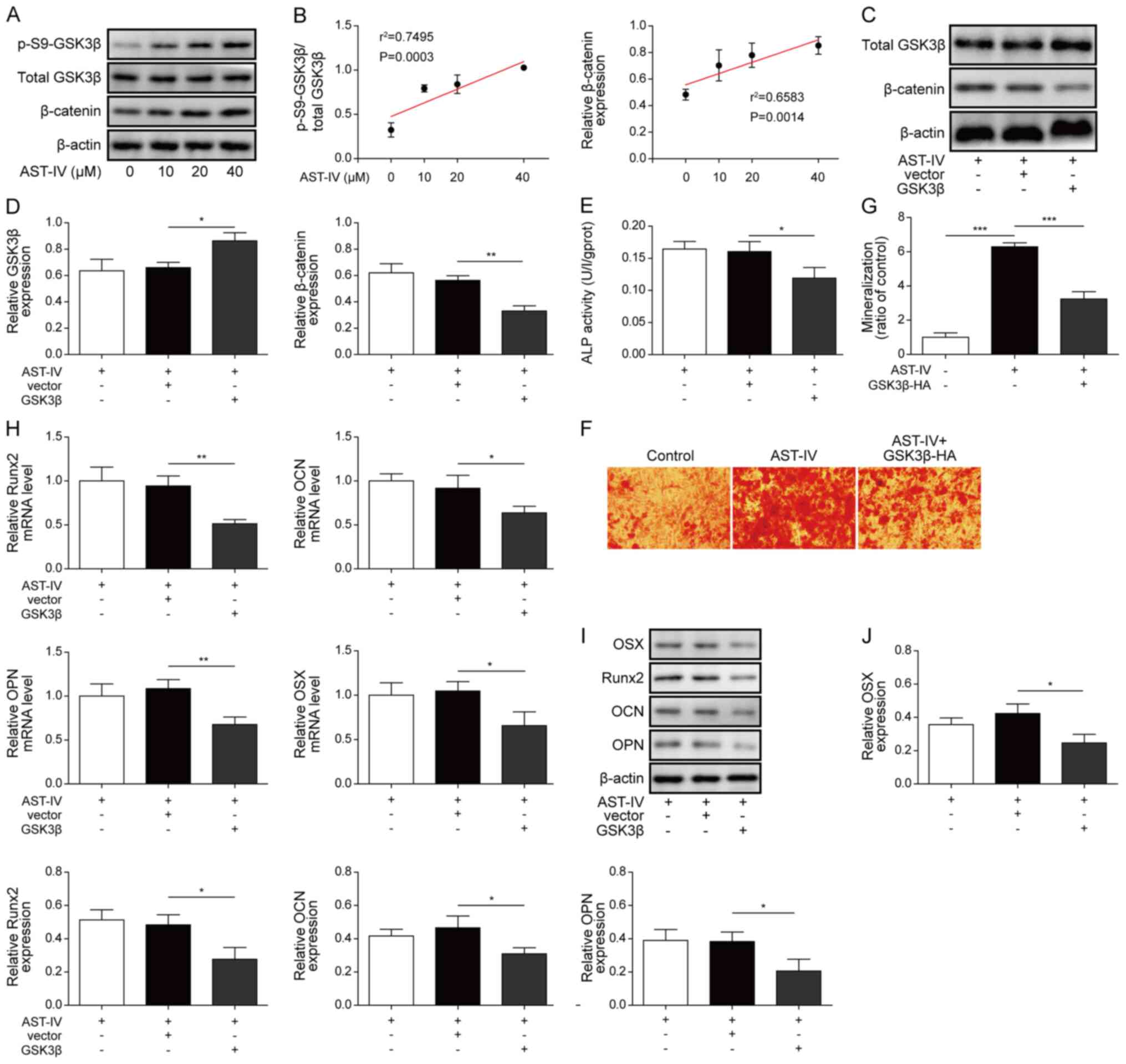 | Figure 3.AST-IV stimulates osteogenic
differentiation of BMSCs via GSK3β/β-catenin signalling. (A)
Western blotting was used to determine the expression levels of
p-S9-GSK3β, total GSK3β and β-catenin in untreated BMSCs and BMSCs
treated with AST-IV (0, 10, 20 and 40 µM) and (B) linear
association was determined. (C) Western blotting was used to detect
and (D) quantify the total GSK3β and β-catenin protein changes in
BMSCs overexpressing GSK3β or treated with AST-IV (40 µM). β-actin
was used as an internal control. (E) ALP activity of
GSK3β-overexpressing BMSCs following treatment with AST-IV on day
7. (F) Alizarin red S staining of mineralization conditions in
GSK3β-overexpressing BMSCs with AST-IV incubation on day 21 are (G)
expressed as a ratio of the Control (magnification, ×200). Scale
bar, 100 µm. (H) Expression levels of Runx2, OCN, OPN and OSX mRNA
following GSK3β overexpression in BMSCs. (I) Runx2, OCN, OPN and
OSX protein expression levels were detected via western blotting,
with β-actin acting as the endogenous control and (J) quantified.
n=3; *P<0.05, **P<0.01 and ***P<0.001. AST-IV,
astragaloside-IV; BMSCs, bone marrow-derived mesenchymal stem
cells; GSK3β, glycogen synthase kinase 3β; ALP, alkaline
phosphatase. |
AST-IV-mediated osteogenic
differentiation is dependent on NGF expression levels
Compared with the control group, there was an
increase in both mRNA and protein levels of NGF in BMSCs following
treatment with different concentrations of AST-IV (Fig. 4A-C). In order to investigate the
role of NGF in BMSC osteogenic differentiation, an anti-NGF
antibody was added to AST-IV-treated cells and as anticipated it
suppressed NGF protein expression levels (Fig. 4D and E). The levels of p-GSK3β and
β-catenin were consequently decreased (Fig. 4F and G); the expression levels of
Runx2, OCN, OPN and OSX were also decreased in BMSCs following
treatment with the anti-NGF antibody (Fig. 4J and L), indicating that NGF
directly participated in AST-IV-stimulated BMSC osteogenic
differentiation. BMSCs were treated with GI; β-catenin and p-GSK3β
levels were increased in the GI group compared with the control
group (Fig. 4H and I). Then,
cultured BMSCs were co-treated with the anti-NGF antibody, AST-IV
and GI. During AST-IV treatment, the mRNA expression levels of
Runx2, OCN, OPN and OSX were decreased by treatment with the
anti-NGF antibody, while co-treatment with anti-NGF antibody and GI
significantly reversed the inhibitory effects of treatment with
anti-NGF-alone (Fig. 4J). The
protein expression levels of Runx2, OCN, OPN and OSX in the
anti-NGF group were decreased compared with those in the
anti-NGF-HO group. The protein expression levels of Runx2, OCN, OPN
and OSX were increased in the anti-NGF+GI group compared with those
of the anti-NGF group (Fig. 4K and
L). This indicated that NGF-promoted osteogenic differentiation
was dependent on GSK3β/β-catenin signalling.
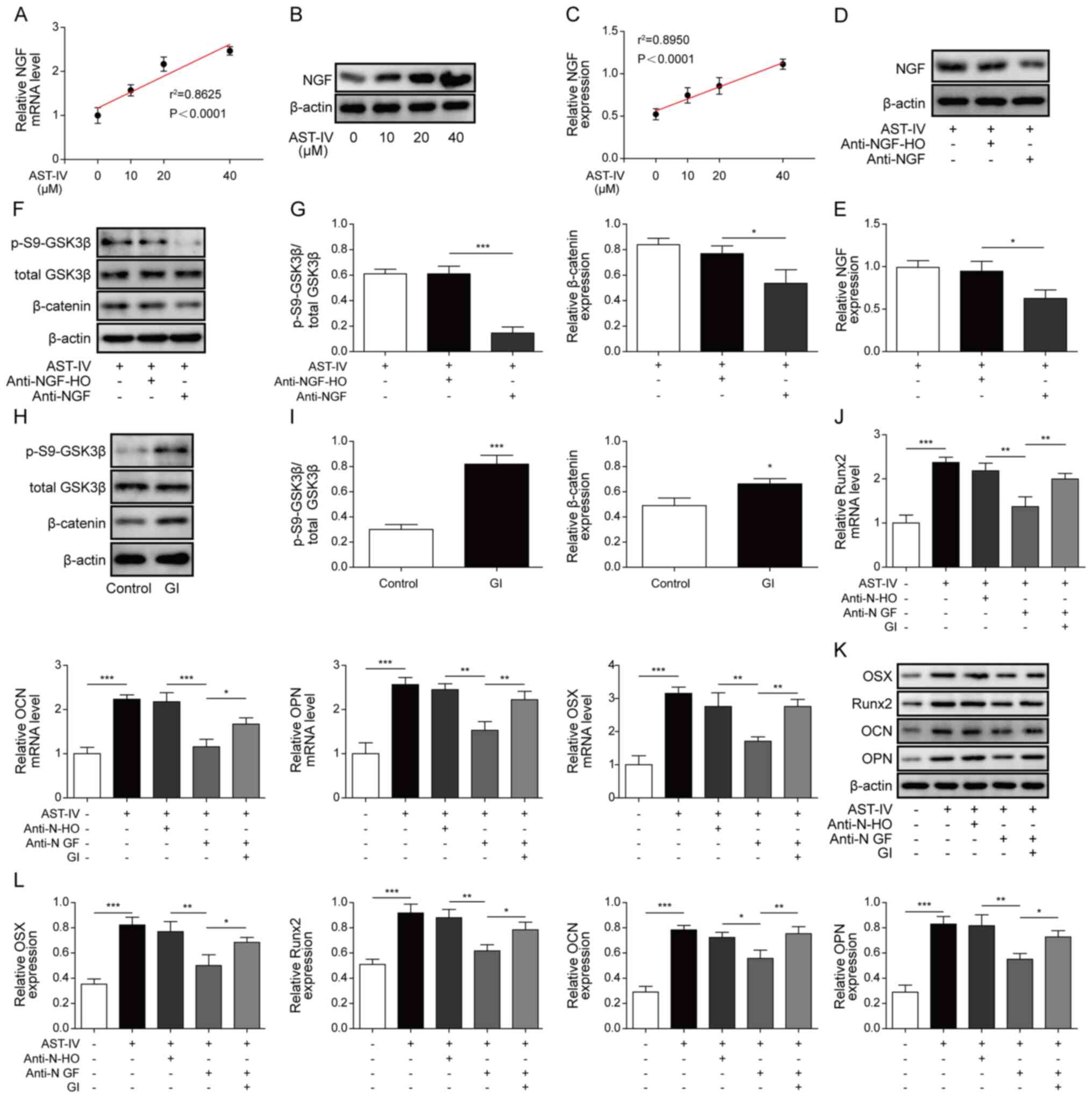 | Figure 4.AST-IV-mediated osteogenic
differentiation is dependent on NGF expression levels. (A)
Expression levels of NGF mRNA in BMSCs following incubation with 0,
10, 20 or 40 µM AST-IV. (B) NGF protein expression levels and (C)
linear association between BMSCs incubated with 0, 10, 20 or 40 µM
AST-IV. (D) Determination and (E) quantification of NGF protein
expression levels in BMSCs following treatment with AST-IV and
anti-NGF antibody or anti-NGF-H0 (negative control). (F) Western
blotting and (G) relative expression levels of p-S9-GSK3β and total
GSK3β in BMSCs following treatment with AST-IV, anti-NGF antibody
and anti-NGF-H0. β-catenin was used as a control. (H) Western
blotting and (I) relative protein expression levels of p-S9-GSK3β
and total GSK3β in GI-treated BMSCs. β-catenin was used as the
control. (J) Runx2, OCN, OPN and OSX mRNA levels in AST-IV-cultured
BMSCs following GI and anti-NGF treatment. Runx2, OCN, OPN and OSX
protein levels were (K) detected and (L) quantified in
AST-IV-cultured BMSCs following GI and anti-NGF treatment. n=3;
*P<0.05, **P<0.01 and ***P<0.001. AST-IV,
astragaloside-IV; NGF, neuronal growth factor; BMSCs, bone
marrow-derived mesenchymal stem cells; GSK3β, glycogen synthase
kinase 3β; GI, GSK3β inhibitor. |
Discussion
Osteoporosis, or bone mass loss, is one of the most
prevalent diseases that affects the elderly population (age, ≥50
years) globally, and it can cause painful fractures (21). Ageing, as well as low body weight,
low levels of sex hormones, menopause and smoking, is a risk factor
for osteoporosis (22). A decline
in osteogenic differentiation capacity of BMSCs is one of the
central mechanisms underlying osteoporosis (23). Osteogenic differentiation can
replenish bone loss in patients with osteoporosis. However, the
cellular and molecular causes underlying decreased osteogenic
differentiation capacity of BMSCs in patients with osteoporosis
have not yet been fully elucidated.
Certain natural medicines from plants, such as
extracts from astragalus, have proven helpful in BMSC osteoblastic
differentiation (14,15). Cheng et al reported that
AST-I promotes osteoblast differentiation via the Wnt/β-catenin
pathway (14). Kong et al
demonstrated that AST-II induces osteogenic activity of osteoblasts
(15). To the best of our
knowledge, however, the functions of AST-IV in osteogenesis have
not previously been elucidated. Therefore, the present study
investigated the role of AST-IV in BMSC osteoblastic
differentiation. The experimental results shown that AST-IV
increased osteogenesis of BMSCs in vitro. The
NGF/GSK3β/β-catenin signalling pathway was found to be elevated by
AST-IV.
GSK3β/β-catenin has been reported to be a key
regulator of osteogenesis (24–27).
Pan et al (28) demonstrated
that conditionally knocking out the transcription factor Yap in
mouse osteoblast lineage cells resulted in trabecular bone loss.
However, β-catenin overexpression in Yap-deficient BMSCs diminished
the osteogenesis deficit. Wang et al (29) revealed that adiponectin facilitated
BMSC osteogenesis, and that the Wnt/β-catenin pathway was involved
in the osteogenic effect of adiponectin. Therefore, the
GSK3β/β-catenin signalling pathway is key in promoting osteogenic
differentiation. To the best of our knowledge, the present study is
the first to demonstrate that AST-IV induces phosphorylation of
GSK3β (Ser-9); this is known to enhance the activity of β-catenin,
which promotes BMSC osteogenic differentiation (30,31).
In the present study, overexpression of GSK3β resulted in
downregulated β-catenin protein and slowed BMSC
differentiation.
NGF is important not only for the development and
maintenance of sympathetic and sensory neurons but also for
promoting mineralization. This capability makes it a potential
target for bone and tissue regeneration. Evidence has indicated
that NGF notably contributes to osteogenic differentiation, and it
has been assessed both in vitro and in vivo in
different types of cells (32,33).
Jin et al (30) investigated
the effect of biphasic calcium phosphates (BCP) combined with NGF
on the growth of osteoblasts in vitro and the combined
therapeutic effect on the repair of calvarial defects in
vivo; their results confirmed that the effect of
neuro-osteological interactions via combinatorial treatment with
NGF and BCP can promoted osteogenesis and bone formation (30). Another study by Jiang et al
(31) demonstrated the involvement
of NGF signalling in calcification of human articular chondrocytes
and the importance of NGF signalling in articular cartilage
homeostasis. It has also been reported by Cui et al
(34) that exogenous treatment with
NGF increased ALP levels, as well as ARS and calcium mineral
deposition. Yao et al (35)
reported that local injection of exogenous nerve growth factor
increased bone mass, amount of bone trabecula, and bone maturity
in vivo. Thus, evidence indicates that NGF is involved in
osteogenic differentiation of BMSCs. To the best of our knowledge,
the present study is the first to demonstrate that AST-IV increases
BMSC osteogenesis, which may be a result of upregulated NGF
expression levels in BMSCs treated with AST-IV.
The present study demonstrated that both
GSK3β/β-catenin signalling and NGF participate in AST-IV-induced
osteogenesis and that β-catenin expression levels are negatively
affected by GSK3β and positively affected by NGF. A regulatory loop
for AST-IV, containing NGF, GSK3β and β-catenin, during BMSC
differentiation was identified. AST-IV is upstream of the
signalling process that inhibits the function of GSK3β via
phosphorylating the Ser9 site; phosphorylated GSK3β induced
increased expression levels of β-catenin, resulting in
differentiation of BMSCs.
Runx2 and OSX are specific osteoblast transcription
factors involved in regulating osteoblast-associated genes.
Previous research has demonstrated that OSX may serve a key role in
epigenetic regulation of differentiation from MSCs to osteoblasts
(36–38). The direct regulatory mechanisms of
Runx2 and OSX in regulation of BMSC osteoblastic differentiation by
AST-IV require further investigation. In the present study, Runx2
and OSX were increased during AST-IV-induced BMSC osteoblastic
differentiation via the NGF/GSK3β/β-catenin axis. The results not
only provided an understanding of the molecular pathways involved
in regulating NGF-mediated osteogenic differentiation of BMSCs, but
also identified potential targets in the treatment of osteoporosis.
However, further investigation is required to assess the clinical
effectiveness and safety of AST-IV.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
NYS designed the study. NYS and BD guaranteed the
integrity of the study. XLL participated in the conceptualization
of the study. XHW, BD and NYS performed the experiments. XHW and JG
acquired the data. XHW, NYS and JG conducted the data analysis. JG
performed the statistical analysis. NYS prepared and revised the
manuscript. XLL and BD reviewed and edited the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Grob GN: From aging to pathology: The case
of osteoporosis. J Hist Med Allied Sci. 66:1–39. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kondo T, Endo I and Matsumoto T: The
pathology, diagnosis and therapy on osteoporosis. Nihon Rinsho.
72:1774–1779. 2014.(In Japanese). PubMed/NCBI
|
|
3
|
Hu H, Li Z, Lu M, Yun X, Li W, Liu C and
Guo A: Osteoactivin inhibits dexamethasone-induced osteoporosis
through up-regulating integrin β1 and activate ERK pathway. Biomed
Pharmacother. 105:66–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM
and Luo SQ: Oxidative stress inhibits osteoblastic differentiation
of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun.
314:197–207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu X, Hou W, He L, Han F, Lu M, Lu X,
Duan K, Guo T and Weng J: AMOT130/YAP pathway in topography-induced
BMSC osteoblastic differentiation. Colloids Surf B Biointerfaces.
182:1103322019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu H, Wang M, Zhao C, Li R, Yang J, Pei
G, Ye T, Zuo X, Liu L, Chong Lee Shin OL, et al: GAG and collagen
II attenuate glucocorticoid-induced osteoporosis by regulating
NF-κB and MAPK signaling. Am J Transl Res. 10:1762–1772.
2018.PubMed/NCBI
|
|
7
|
Cui Q, Xing J, Yu M, Wang Y, Xu J, Gu Y,
Nan X, Ma W, Liu H and Zhao H: Mmu-miR-185 depletion promotes
osteogenic differentiation and suppresses bone loss in osteoporosis
through the Bgn-mediated BMP/Smad pathway. Cell Death Dis.
10:1722019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haghighizadeh E, Shahrezaee M, Sharifzadeh
SR and Momeni M: Transforming growth factor-β3 relation with
osteoporosis and osteoporotic fractures. J Res Med Sci. 24:462019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu B, Xue F, Zhang C and Li G: Ginkgolide
B promotes osteoblast differentiation via activation of canonical
Wnt signalling and alleviates osteoporosis through a bone anabolic
way. J Cell Mol Med. 23:5782–5793. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Wang H, Yin T, Liu Y, Zhou W, Fan
X, Wu L, Song C, Shao J and Yang T: TMEM18 inhibits osteogenic
differentiation of rat bone marrow-derived mesenchymal stem cells
by inactivating β-catenin. Exp Cell Res. 383:1114912019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao R, Li Y, Lin Z, Wan J, Xu C, Zeng Y
and Zhu Y: miR-199b-5p modulates BMSC osteogenesis via suppressing
GSK-3β/β-catenin signaling pathway. Biochem Biophys Res Commun.
477:749–754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta A, Chatree S, Buo AM, Moorer MC and
Stains JP: Connexin43 enhances Wnt and PGE2-dependent activation of
β-catenin in osteoblasts. Pflugers Arch. 471:1235–1243. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma J, Zhang ZL, Hu XT, Wang XT and Chen
AM: Metformin promotes differentiation of human bone marrow derived
mesenchymal stem cells into osteoblast via GSK3β inhibition. Eur
Rev Med Pharmacol Sci. 22:7962–7968. 2018.PubMed/NCBI
|
|
14
|
Cheng X, Wei B, Sun L, Hu X, Liang J and
Chen Y: Astragaloside I stimulates osteoblast differentiation
through the Wnt/β-catenin signaling pathway. Phytother Res.
30:1680–1688. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kong XH, Niu YB, Song XM, Zhao DD, Wang J,
Wu XL, Zhang R and Mei QB: Astragaloside II induces osteogenic
activities of osteoblasts through the bone morphogenetic
protein-2/MAPK and Smad1/5/8 pathways. Int J Mol Med. 29:1090–1098.
2012.PubMed/NCBI
|
|
16
|
Lian X, Shen CC, Sun HJ and Zeng YJ:
Cytological mechanism of astragaloside IV in promoting repair of
bone defects. J Biol Regul Homeost Agents. 33:511–516.
2019.PubMed/NCBI
|
|
17
|
Li M, Wang W, Geng L, Qin Y, Dong W, Zhang
X, Qin A and Zhang M: Inhibition of RANKL-induced
osteoclastogenesis through the suppression of the ERK signaling
pathway by astragaloside IV and attenuation of
titanium-particle-induced osteolysis. Int J Mol Med. 36:1335–1344.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin YY, Li WP, Gong HL, Zhu FF, Li WZ and
Wu GC: Protective effect of astragaloside on focal cerebral
ischemia/reperfusion injury in rats. Am J Chin Med. 38:517–527.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan Z, Kang T, Zhang X, Tong Y and Chen S:
Nerve growth factor prevents arsenic-induced toxicity in PC12 cells
through the AKT/GSK-3β/NFAT pathway. J Cell Physiol. 234:4726–4738.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ketschek A, Jones S, Spillane M, Korobova
F, Svitkina T and Gallo G: Nerve growth factor promotes
reorganization of the axonal microtubule array at sites of axon
collateral branching. Dev Neurobiol. 75:1441–1461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou S, Huang G and Chen G: Synthesis and
biological activities of drugs for the treatment of osteoporosis.
Eur J Med Chem. 197:1123132020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koromani F, Trajanoska K, Rivadeneira F
and Oei L: Recent advances in the genetics of fractures in
osteoporosis. Front Endocrinol (Lausanne). 10:3372019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ağaçayak KS, Güven S, Koparal M, Güneş N,
Atalay Y and Atilgan S: Long-term effects of antihypertensive
medications on bone mineral density in men older than 55 years.
Clin Interv Aging. 9:509–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu H, Zhao C, Zhang P, Liu Y, Jiang Y, Wu
E, Xue H, Liu C and Li Z: miR-26b modulates OA induced BMSC
osteogenesis through regulating GSK3β/β-catenin pathway. Exp Mol
Pathol. 107:158–164. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou Y, Li J, Zhou K, Liao X, Zhou X and
Shen K: The methylation of Notch1 promoter mediates the
osteogenesis differentiation in human aortic valve interstitial
cells through Wnt/β-catenin signaling. J Cell Physiol.
234:20366–20376. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Luo E, Bi R, Ye B, Hu J and Zou S:
Wnt/β-catenin signaling is required for distraction osteogenesis in
rats. Connect Tissue Res. 59:45–54. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng T, Niu J, Pi B, Lu Y, Wang J, Zhang
W, Li B, Yang H and Zhu X: Osteogenesis enhancement of silk
fibroin/α-TCP cement by N-acetyl cysteine through Wnt/β-catenin
signaling pathway in vivo and vitro. J Mech Behav Biomed Mater.
101:1034512019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan JX, Xiong L, Zhao K, Zeng P, Wang B,
Tang FL, Sun D, Guo HH, Yang X, Cui S, et al: YAP promotes
osteogenesis and suppresses adipogenic differentiation by
regulating β-catenin signaling. Bone Res. 6:182018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Zhang X, Shao J, Liu H, Liu X and
Luo E: Adiponectin regulates BMSC osteogenic differentiation and
osteogenesis through the Wnt/β-catenin pathway. Scientific reports.
7:36522017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin P, Yin F, Huang L, Zheng L, Zhao J and
Zhang X: Guangxi cobra venom-derived NGF promotes the osteogenic
and therapeutic effects of porous BCP ceramic. Exp Mol Med.
49:e3122017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang Y and Tuan RS: Role of NGF-TrkA
signaling in calcification of articular chondrocytes. FASEB J.
33:10231–10239. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yada M, Yamaguchi K and Tsuji T: NGF
stimulates differentiation of osteoblastic MC3T3-E1 cells. Biochem
Biophys Res Commun. 205:1187–1193. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tomlinson RE, Li Z, Li Z, Minichiello L,
Riddle RC, Venkatesan A and Clemens TL: NGF-TrkA signaling in
sensory nerves is required for skeletal adaptation to mechanical
loads in mice. Proc Natl Acad Sci USA. 114:E3632–E3641. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cui GS, Zeng JY, Zhang J and Lu R: Effect
of nerve growth factor on osteogenic potential of type 2 diabetic
mice bone marrow stromal cell in vitro. Zhonghua Kou Qiang Yi Xue
Za Zhi. 53:97–102. 2018.(In Chinese). PubMed/NCBI
|
|
35
|
Yao Y, Du Y, Gu X, Guang MK, Huang B and
Gong P: Local injection of exogenous nerve growth factor improves
early bone maturation of implants. Hua Xi Kou Qiang Yi Xue Za Zhi.
36:128–132. 2018.(In Chinese). PubMed/NCBI
|
|
36
|
Tae JY, Lee H, Lee H, Ko Y and Park JB:
Osteogenic potential of cell spheroids composed of varying ratios
of gingiva-derived and bone marrow stem cells using concave
microwells. Exp Ther Med. 16:2287–2294. 2018.PubMed/NCBI
|
|
37
|
He S, Yang S, Zhang Y, Li X, Gao D, Zhong
Y, Cao L, Ma H, Liu Y, Li G, et al: LncRNA ODIR1 inhibits
osteogenic differentiation of hUC-MSCs through the
FBXO25/H2BK120ub/H3K4me3/OSX axis. Cell Death Dis. 10:9472019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Almalki SG and Agrawal DK: Key
transcription factors in the differentiation of mesenchymal stem
cells. Differentiation. 92:41–51. 2016. View Article : Google Scholar : PubMed/NCBI
|