Introduction
Obesity has been a serious health problem worldwide
for decades and it is now considered as a potential trigger of
other metabolic disorders, including cardiovascular diseases,
diabetes and cancer (1,2). The accumulation of adipose tissue is
the most common cause of obesity (3). It has been reported that 30% of
adipose tissue is derived from preadipocytes that undergo
adipogenesis and develop into mature adipocytes (4). Adipogenesis is a multifactorial
process that is regulated by various elements, including microRNAs
(miRNAs/miRs), transcription factors, epigenetic regulators and
diverse signaling pathways, such as PPARγ/MAPK, PI3K/Akt and
Wnt/β-catenin (5). In addition, the
development of obesity is known to be accompanied by low-grade
inflammation (6). In this context,
adipose tissue functions as an endocrine organ, secreting a variety
of inflammation-related adipocytokines, including IL-6, TNF-α and
IL-8 (2). TNF-α is highly induced
in adipose tissue compared with in other tissues and its expression
has been discovered to affect adipocyte metabolism, including
glucose consumption, lipolysis and adipocyte differentiation
(7).
miRNAs are a class of small non-coding RNAs of 20–24
nucleotides in length, which negatively regulate the expression of
target proteins. Therefore, miRNAs are involved in a variety of
biological events, including stem cell differentiation, cell
proliferation and death, neurogenesis, hematopoiesis and immune
responses (8,9). In adipocytes, miRNAs function as
regulators of differentiation by targeting adipocyte-related
factors, such as peroxisome proliferator-activated receptor
(PPAR)-γ, which was reported to be downregulated by miR-27b, miR-31
and miR-138, resulting in the inhibition of the adipogenic process
(9–12). The dysfunction or abnormal
expression of miRNAs has been associated with the development of
cancer (13,14), cardiovascular diseases (15), diabetes and obesity (16,17).
Therefore, the potential association of miRNA dysregulation and the
onset of obesity has rapidly become a topic of interest. For
example, Ortega et al (18)
compared the miRNA expression profiles in pre- and mature
adipocytes of obese and non-obese people, and observed significant
differences in the expression of 71 distinct miRNAs between the two
groups.
miR-424 in an intragenic miRNA, and a member of the
miR-16 family, which clusters with miR-503 on chromosome Xq26.3
(19). Previous studies have
reported the functional relationship between miR-424 and several
types of disease and biological processes, including cancer
(20–23), cell differentiation (24), diabetes (25), angiogenesis (26), vascular diseases (27) and inflammatory diseases (28,29).
The role of miR-424 in adipogenesis has been studied in recent
years, and its expression has been associated with waist-to-hip
ratio and to body fat mass related parameters (30,31).
Functionally, the expression of miR-424 was identified to be
regulated by PPAR-γ, an important transcription factor during
adipogenesis (32). Interestingly,
in non-obese women, those with higher fat droplet measurements had
upregulated expression levels of miR-424 (30). These reports provided novel
information about the role of miR-424 in obesity. However, to the
best of our knowledge, the molecular mechanisms of this miRNA
during adipogenesis are yet to be determined.
A previous study revealed that miR-424 expression
levels were closely associated with fat deposition in women
(30); hence, in the present study,
it was questioned whether a similar expression pattern would be
observed in children. In the current study, the expression levels
of miR-424 in the adipose tissue of obese and non-obese children
were compared. To further elucidate the mechanisms of miR-424, the
changes in miR-424 expression levels during adipogenesis were also
analyzed. Furthermore, the association between miR-424 and the
adipocytokine TNF-α was investigated, and the results demonstrated
a negative regulatory effect of TNF-α on miR-424. Finally,
TargetScan, PicTar and microRNA.org
softwares were used to predict the target genes of miR-424. The
putative downstream miR-424 signaling pathways were analyzed and an
association between miR-424 and signaling pathways closely
associated with adipogenesis, including the Wnt signaling pathway,
were identified.
Materials and methods
Study participants
A total of 40 male pediatric patients (age, 6–12
years) undergoing surgery for abdominal disorders were
prospectively chosen to obtain abdominal fat biopsies in The
Affiliated Hospital of Nantong University between August 2019 and
September 2019. The following exclusion criteria were used:
Presence of malignancy, an endocrine disorder or severe systemic
illness. Subjects considered obese were chosen according to the
Working Group on Obesity in China (WGOC) in 2003 [body mass index
(BMI) above the age- and sex-appropriate 95th percentile] (33). Written informed consent was obtained
from the parents or legal guardians of all participants. The
methods and experiments were approved by the Ethics Committee of
The Affiliated Hospital of Nantong University (approval no.
2019-K050; Nantong, China).
Cell culture
Human visceral preadipocytes (HPA-V cells) were
obtained from ScienCell Research Laboratories, Inc. Preadipocyte
medium (PAM) containing 1% preadipocyte growth supplement, 1%
penicillin/streptomycin solution and 5% FBS was also obtained from
ScienCell Research Laboratories, Inc. (cat. no. 7211).
Preadipocytes were cultured in a humidified atmosphere at 37°C with
5% CO2. Serum-free PAM (containing 100 nM dexamethasone,
0.5 mM 3-isobutyl-1-methylxanthine, 50 nM insulin and 100 mM
rosiglitazone) was used to induce the differentiation of confluent
human preadipocytes at day 0, followed by replacement of the medium
every 2 days for the next 4 days in the 37°C incubator.
Subsequently, the medium was replaced with serum-free PAM
(containing 50 nM insulin), which was then replaced every 2 days
until lipid droplets started accumulating in the cells (day 15).
Cells were collected at different time periods (days 0, 1, 4, 7, 10
and 15) during the cell culture period during the adipogenesis.
Human preadipocytes were cultured at 37°C overnight
in serum-free PAM media. Following the incubation, the cells were
treated with 10 ng/ml TNF-α (Sigma-Aldrich; Merck KGaA) or the same
volume of PBS for 0, 12, 24 and 48 h at 37°C, as previously
described (34). 293T cells
(American Type Culture Collection) were cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) in a humidified atmosphere at 37°C with 5%
CO2.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the human adipocytes
and tissues using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
DNA was removed by DNaseI digestion (Takara, Bio, Inc.). Total RNA
was then reverse transcribed into cDNA using the Reverse
Transcriptase kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with 200 ng total RNA as the template. qPCR was subsequently
performed using SYBRGreen kits (Vazyme Biotech Co., Ltd.) on an
Applied Biosystems 7500 Sequence Detection system (Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
The following thermocycling conditions were used for qPCR: Initial
denaturation of 95°C for 10 min; followed by 40 cycles of 95°C for
15 sec and 60°C for 1. miRNA expression levels were quantified
using the 2−ΔΔCq method (35) and normalized to the internal
reference gene U6. miR-424 and U6 snRNA were designed and
synthesized by Guangzhou RiboBio Co., Ltd. (36,37).
Fluorescence reporter constructs and
dual luciferase reporter assay
The potential TNF-α binding sites were predicted
using Genomatix software (www.genomatix.de). Luciferase wild-type (WT) and
mutant (mut) miR-424 reporter plasmids, pro-miR-424-WT and
pro-miR-424-mut, respectively, were synthesized and inserted into
the luciferase reporter cloning vector pEZX-FR01 (GeneCopoeia,
Inc.) by Shanghai Generay Biotech Co., Ltd. Both plasmids contained
the 1,500 bp proximal promoter sequences of miR-424 (chrX:
134546712-134548211). The pro-miR-424-mut reporter plasmid had a
mutated fragment of 5′-TTATTTTAGGAAGGA-3′ at chrX:
134546762-134546776 to replace the original sequence
5′-GGCGGGGCTTCCTTC-3′.
Briefly, 293T cells were seeded in six-well plates
(5×105/well) and incubated at 37°C for 24 h before
transfection. Following the incubation, 250 ng/well pro-miR-424-WT
and pro-miR-424-mut plasmids were co-transfected with 25 ng/well of
Renilla luciferase vector (pRLTK; Invitrogen; Thermo Fisher
Scientific, Inc.), using Lipofectamine® 2000 (Thermo
Fisher Scientific, Inc.). Following 24 h of incubation at 37°C, 10
ng/ml TNF-α was added to the cells, together with culture medium
replacement for an additional 24 h of incubation. Finally, the
relative luciferase activity was measured using a Dual Luciferase
Reporter Assay system (cat. no. E1901; Promega Corporation),
according to the manufacturer's protocol. Luciferase activity was
normalized to Renilla luciferase activity.
miRNA target prediction and functional
annotation
TargetScan (www.targetscan.org/vert_72; version 7.0), PicTar
(pictar.mdc-berlin.de) and microRNA.org programs (www.microrna.org) were used to predict the target
genes of miR-424, using the default parameters. miRNA targets were
obtained by manually selecting the intersecting elements identified
in the miR-424 target prediction program. All target genes were
annotated using the Kyoto Encyclopedia of Genes and Genomes (KEGG;
www.genome.jp/kegg/pathway.html) and Gene Ontology
(GO) databases (geneontology.org). GO functional term enrichment
analysis, which organizes genes into hierarchical categories and
uncovers the gene regulatory network on the basis of biological
processes was used to analyze the main functions of the miR-424
target genes (38,39). Specifically, a two-sided Fisher's
exact test and a χ2 test were used to classify the GO
category. Multiple-test correction was performed by calculating the
false discovery rate (FDR) (40).
The FDR was defined as FDR=1-(Nk)/T, where Nk
was the number of Fisher's test P-values <χ2 test
P-values and T was the theoretical frequency. P-values were
computed for the GOs of all the miR-424 target genes. Enrichment
provides a measure of the significance of the function: As the
enrichment increases, the corresponding function is more specific,
which helps to identify GO terms with more concrete function
description in the experiment. Within the significant category, the
enrichment (Re) was given by the equation: Re=(nf/n)/(Nf/N) where
nf was the number of flagged genes within the particular category,
n is the total number of genes within the same category, Nf is the
number of flagged genes in the entire microarray and N is the total
number of genes in the microarray (41,42).
Signaling pathway enrichment analysis was performed
for the differentially expressed genes, using KEGG (43), Biocarta (44) and Reactome databases (45). Significant signaling pathways were
selected on the basis of the Fisher's exact test and χ2
test, and the threshold of significance was defined by the P-value
(P<0.01) and FDR (q<0.05). The enrichment Re was calculated
as above (46–48).
Statistical analysis
All experiments were performed independently ≥3
times and the data are presented as the mean ± SEM. Statistical
analyses were performed using GraphPad Prism software (version 5.0;
GraphPad Software, Inc.). One-way ANOVA followed by Tukey's post
hoc test was used to compare data of ≥3 groups, whereas a paired
Student's t-test was used to compare the statistical differences
between two groups. P≤0.05 was considered to indicate a
statistically significant difference.
Results
Differential miR-424 expression levels
between obese and non-obese individuals
Concerning the clinicopathological data of the study
participants (Table SI), there was
no differences identified between age, whereas subjects in the
obese group exhibited significantly increased BMIs compared with
subjects in the non-obese group. To determine the association
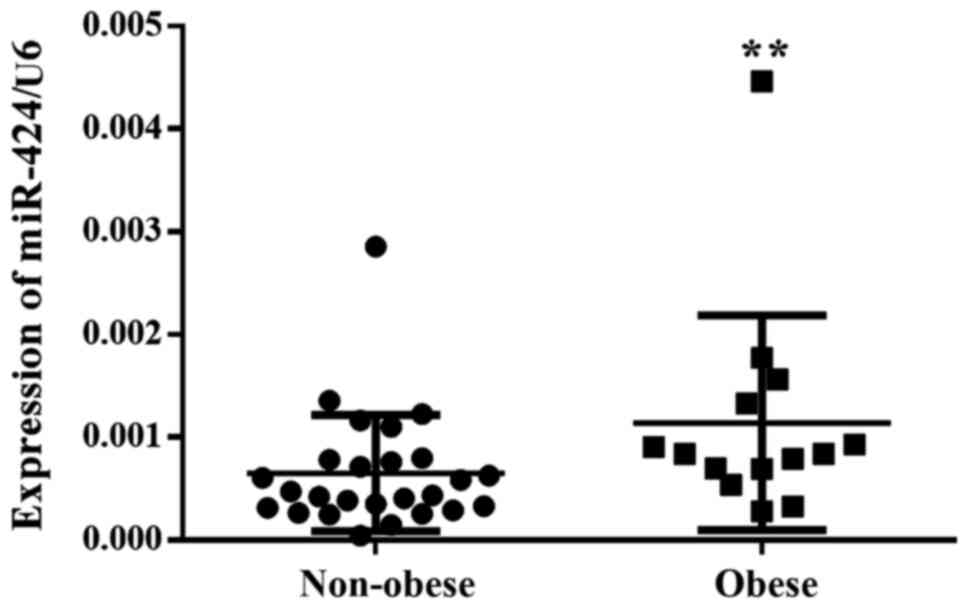
between miR-424 and obesity in children, miR-424 expression levels
were analyzed in the abdominal fat biopsies from patients
undergoing surgery for abdominal disorders. The results revealed
that the miR-424 expression levels were significantly upregulated
in the biopsies from obese patients compared with non-obese
patients, suggesting a positive association between miR-424
expression levels and obesity in children (Fig. 1).
miR-424 expression levels are
downregulated during the maturation of human preadipocytes
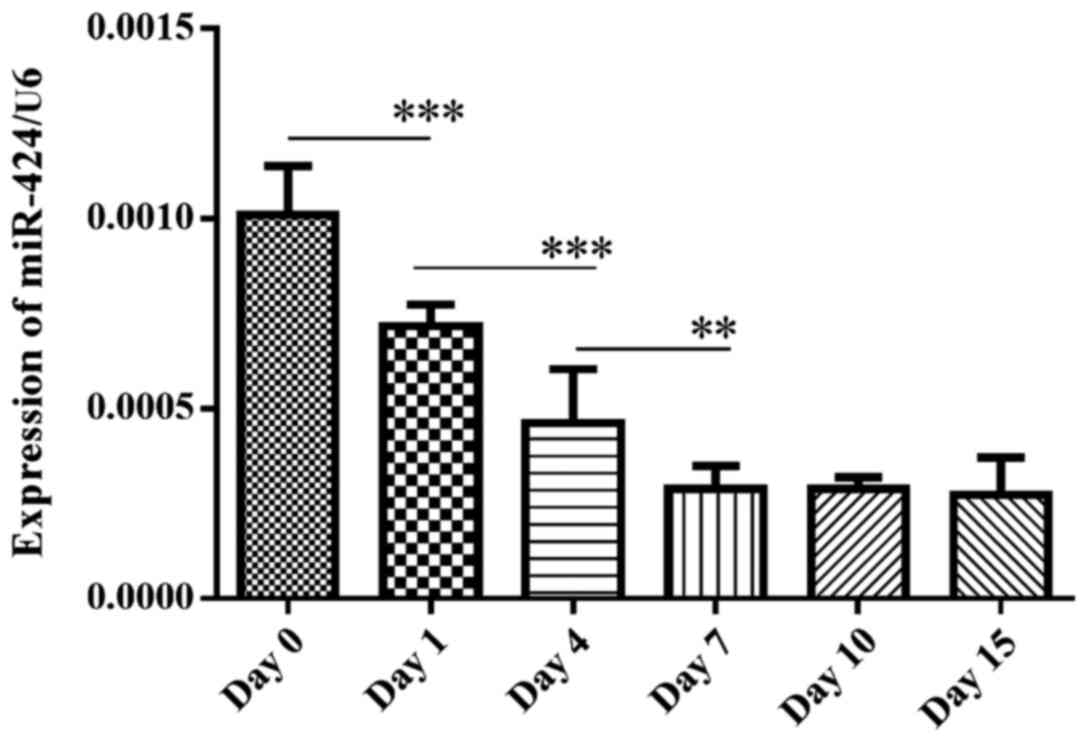
The variation in miR-424 expression levels during
different stages of human preadipocyte maturation was investigated.
As shown in Fig. 2, miR-424
expression levels were the highest in the preadipocytes, and then
continuously decreased with the maturation progression and reached
the lowest point between days 7–15. According to the literature
review (49,50), it was predicted that at day 10 after
induction, >80% of cells exhibited a typical adipocyte
phenotype, suggesting that miR-424 expression levels may be
downregulated during the differentiation of human preadipocytes
into adipocytes.
miR-424 expression levels are
downregulated by TNF-α in human adipocytes
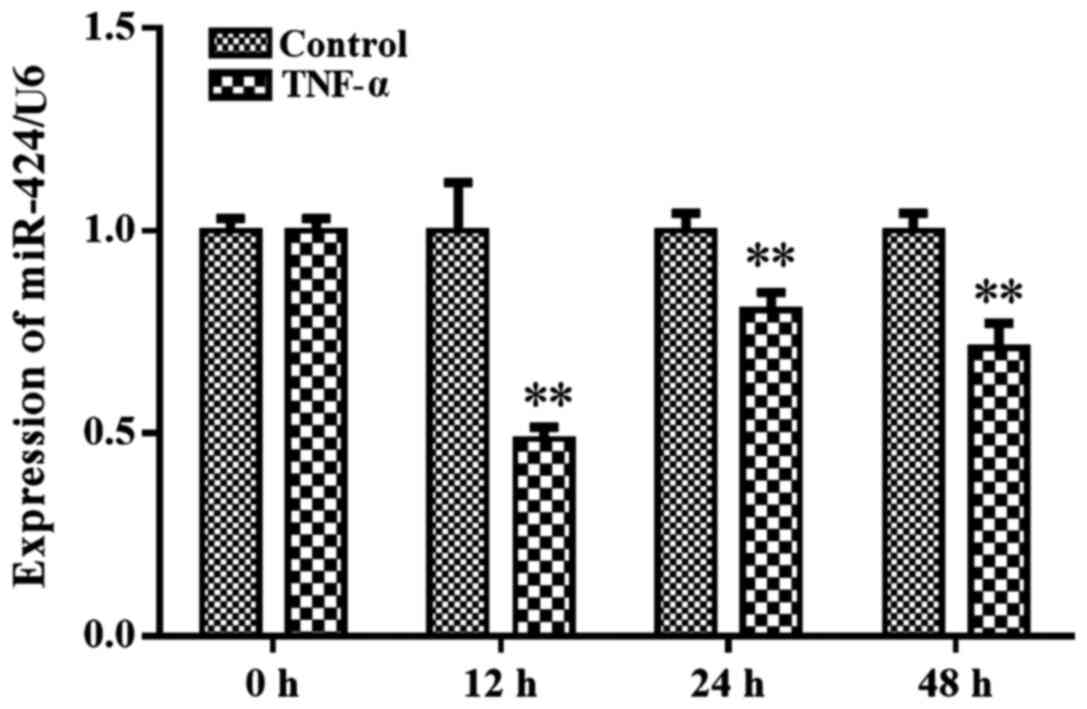
To determine whether proinflammatory cytokines were
involved in regulating miR-424 expression levels, the effects of
IL-6 and TNF-α on the expression levels of miR-424 in human
adipocytes were investigated. Mature adipocytes were treated with
10 ng/ml TNF-α and the miR-424 expression levels were analyzed at
different time points (12, 24 and 48 h) following normalization to
U6 expression levels. While IL-6 had no significant effects on
miR-424 expression levels (data not shown), TNF-α treatment
significantly downregulated the expression levels of miR-424 at 12,
24 and 48 h compared with the control cells (Fig. 3). The lowest expression levels of
miR-424 were observed at 12 h post TNF-α treatment, with ~2.17-fold
lower expression levels compared with the control group. These
results suggested that TNF-α may downregulate miR-424 expression
levels in adipocytes.
TNF-α downregulates miR-424 expression
levels by reducing its promoter activity
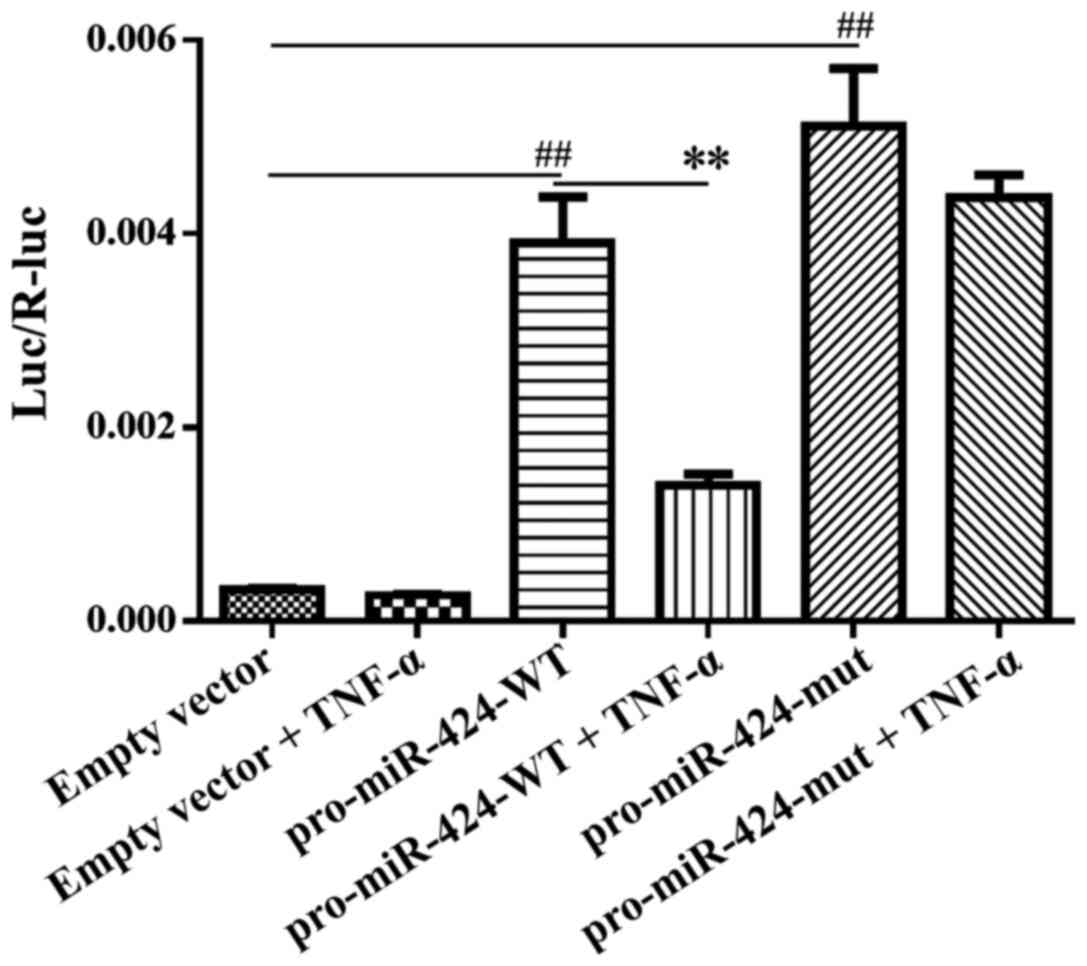
To support the aforementioned findings, dual
luciferase reporter assays were performed using 293T cells. The 1.5
kB upstream sequence of the miR-424 precursor (chrX:
134546712-134548211) was selected as the candidate promoter
fragment, synthesized and inserted into the pEZX-FR01 vector to
generate a pro-miR-424-WT plasmid. The results revealed that
pro-miR-424-WT had promoter activity, which drove the expression of
the downstream reporter gene when compared with the vector alone
(Fig. 4).
It was hypothesized that TNF-α may bind to the
promoter region of miR-424, therefore, the potential TNF-α binding
sites were predicted using Genomatix software. One of the predicted
binding sites, located at chrX: 134546762-134546776, was selected
for experimental validation and then mutated to generate the
pro-miR-424-mut plasmid. To analyze and compare the effects of
TNF-α on pro-miR-424-WT and pro-miR-424-mut, 293T cells were used.
TNF-α treatment lead to a decrease in the relative luciferase
activities of both miR-424 promoters; however, only the
pro-miR-424-WT showed a significant reduction in relative
luciferase activity following TNF-α treatment compared with the
pro-miR-424-WT alone (Fig. 4),
indicating that the effect of TNF-α may be attenuated by a mutation
at the promoter binding site. Overall, these results suggested that
TNF-α may inhibit miR-424 transcription by binding to specific
sites in its promoter region.
GO functional term and signaling
pathway enrichment analyses of miR-424 target genes
To study the biological implications of miR-424,
target genes of miR-424 were predicted through GO functional term
enrichment analysis. Three areas, including biological process,
molecular function and cellular component are primarily covered in
GO annotation, which provides controlled annotations to describe
genes and their products in a given organism (51). As the present study aimed to
identify the function of miR-424, biological process was assessed.
The GO analysis results identified that the target genes of miR-424
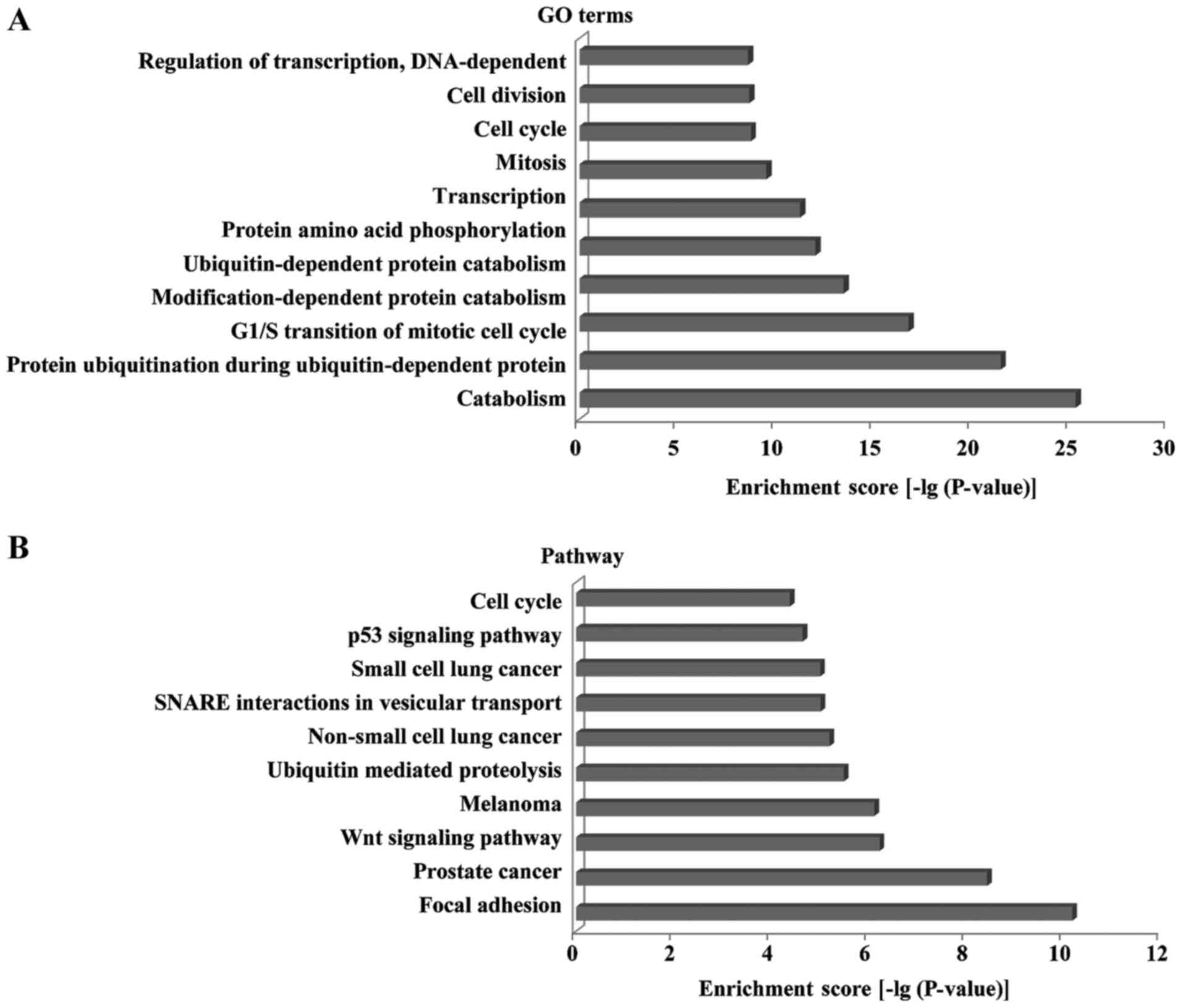
were classified into diverse categories, among which the most
frequent GO term was ‘Catabolism’ followed by ‘protein
ubiquitination during ubiquitin-dependent protein’ and ‘G1/S
transition of mitotic cell cycle’ (Fig.
5A). Moreover, signaling pathway enrichment analysis predicted
that the miR-424 target genes were involved in diverse processes,
including ‘Wnt signaling pathway’, ‘p53 signaling pathway’,
‘Ubiquitin mediated proteolysis’, ‘SNARE interactions in vesicular
transport’, ‘Focal adhesion’ and ‘Cell cycle’ (Fig. 5B). These results further implied
that miR-424 may target genes in prostate cancer, melanoma and lung
cancer (small-cell and non-small cell).
Discussion
Obesity has become a serious public health problem,
with the number of affected individuals increasing worldwide. Since
1980, the number of cases of obesity has doubled in >70
countries. In 2015, there were 100 million and 600 million cases of
obesity reported in children and adults, respectively (52). The dysregulation of preadipocyte
differentiation is a main cause of obesity (53). Therefore, it is important to study
the regulatory mechanisms of preadipocyte differentiation. miRNAs
have been discovered to have an important role in the regulation of
inflammatory responses by adipocytes, and are thus associated with
the development of obesity (34,54).
miR-424, an inflammation-related miRNA implicated in diverse
cellular events and diseases, has been studied for its potential as
a clinical and diagnostic biomarker of cancer (55), heart failure (56) and diabetes (57,58).
In the context of obesity, miR-424 was identified to negatively
regulate adipocyte differentiation (59). However, the mechanistic details on
the miR-424-mediated inhibition of adipocyte differentiation remain
unclear.
In the present study, the differential expression
levels of miR-424 in abdominal fat biopsies of obese and non-obese
children were analyzed. BMI is the gold standard measurement of
obesity; however, children cannot be identified as obese by only
calculating BMI. According to the WGOC in 2003, the criteria for
determining obesity in children is a BMI > age- and
sex-appropriate 95th percentile (33). For example, 6-year-old children with
a BMI of >18.1 are categorized as obese, while 11-year-old
children with the same BMI are recognized as non-obese, and are
diagnosed as obese when the BMI is >22.7 (60). Thus, BMI is not suitable for the
diagnosis of obesity in children and is not useful for detecting
the relevance between BMI and miRNA expression, the present study
revealed that the expression levels of miR-424 in the adipose
tissue were positively associated with obesity. Our previous data
using a miRNA microarray demonstrated that the expression levels of
miR-424 were upregulated in mature adipocytes compared with in
preadipocytes, both in human adipose-derived mesenchymal stem cells
and human stromal vascular cells (61). However, in subsequent PCR analysis,
miR-424 expression levels were observed to continuously decline
during preadipocyte differentiation, maintaining an low, but
steady, level in mature adipocytes (59). Aiming to find an appropriate
explanation for these opposing results, Gene Expression Omnibus
(GEO) datasets related to miR-424 and obesity were searched.
Interestingly, one of the profiles (ID: 30947926; www.ncbi.nlm.nih.gov/geoprofiles/?term=30947926)
revealed that the expression levels of miR-424 were downregulated
during preadipocyte differentiation in subcutaneous and mesenteric
adipose cells (62,63). Conversely, in omental cells, miR-424
expression levels were upregulated, suggesting that miR-424
expression may be tissue or cell-dependent. Therefore, it was
hypothesized that the results of the present study may be caused by
environmental factors in the patient abdominal tissues, which are
absent from cultured adipocyte cells (30). Another possibility is that miR-424
may be downregulated during adipocyte differentiation due to its
negative roles in adipogenesis. However, when an elevated number of
adipocytes accumulate, there may be a feedback effect, which in
turn would upregulate miR-424 to exert its inhibitory functions,
thereby restricting the further development of adipose tissues
(64).
Proinflammatory cytokines are essential factors in
regulating adipocyte maturation (65). TNF-α, a secreted inflammatory
factor, was discovered to be positively associated with obesity,
both in adults and children (66,67)
and has been shown to affect adipogenesis by regulating gene
expression (7). Our previous
studies revealed that several miRNAs are positively regulated by
TNF-α during preadipocyte differentiation, including miR-1908 and
miR-146b (34,54,58,68).
In the present study, it was observed that miR-424 expression
levels were inhibited by TNF-α treatment in mature adipocytes,
suggesting that miR-424 may have opposing functions to TNF-α,
miR-1908 and miR-146b. This was further confirmed through dual
luciferase reporter assays, in which TNF-α significantly inhibited
the transcription of a reporter gene via binding to the miR-424
promoter.
In biological systems, miRNAs normally exert their
cellular functions by regulating the expression of target proteins
(69). Aiming to further understand
the function of miR-424 in adipogenesis, its target genes were
predicted and their functions were bioinformatically analyzed. The
target genes were found to be enriched in several processes, among
which focal adhesion, the Wnt signaling pathway and ubiquitin
mediated proteolysis have all been previously associated with
adipogenesis (70–72). These results improved the current
understanding of the mechanisms through which miR-424 may regulate
adipogenesis.
In conclusion, the results of the present study
revealed that miR-424 expression levels were upregulated in
abdominal fat biopsies from obese children. In cell culture
experiments, miR-424 expression levels were discovered to be
significantly downregulated during preadipocyte differentiation.
Furthermore, TNF-α treatment was discovered to downregulate miR-424
expression through binding to its promoter region. The prediction
of miR-424 target genes involved in adipogenesis revealed signaling
pathways and biological processes that may regulate preadipocyte
differentiation. However, additional studies on miR-424 regulatory
mechanisms are required to provide a deeper understanding of the
function of miR-424 in adipocyte differentiation and its potential
association with obesity onset. The present study provided novel
insights into the association between adipogenesis and miR-424
expression levels, opening the possibility of miR-424 being
characterized in the future as a candidate biomarker for the
diagnosis of obesity and related diseases.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Chen-Bo Ji
(Nanjing Maternity and Child Health Care Hospital, Nanjing, China)
for the assistance with the experiments and valuable discussion. We
also thank Dr Xu-chu Duan (Nantong University, Nantong, China) for
his assistance with writing the manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81671359 and
81500649), Jiangsu Provincial Medical Innovation Team (grant no.
CXTDA2017001) and the 333 High Level Talents Training Project of
Jiangsu Province.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XRG, XC and LJZ conceived and designed the study.
QZX performed the experiments. ZYF analyzed the data and drafted
the manuscript. XRG and XC reviewed and edited the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
parents or legal guardians of all participants, and the study
protocol was approved by the ethics committees and the
institutional review board of The Affiliated Hospital of Nantong
University (approval no. 2019-K050; Nantong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Somasundar P, McFadden DW, Hileman SM and
Vona-Davis L: Leptin is a growth factor in cancer. J Surg Res.
116:337–349. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Trayhurn P and Wood IS: Adipokines:
Inflammation and the pleiotropic role of white adipose tissue. Br J
Nutr. 92:347–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gérard C and Brown KA: Obesity and breast
cancer-role of estrogens and the molecular underpinnings of
aromatase regulation in breast adipose tissue. Mol Cell Endocrinol.
466:15–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sarantopoulos CN, Banyard DA, Ziegler ME,
Sun B, Shaterian A and Widgerow AD: Elucidating the preadipocyte
and its role in adipocyte formation: A comprehensive review. Stem
Cell Rev Rep. 14:27–42. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ambele MA, Dhanraj P, Giles R and Pepper
MS: Adipogenesis: A complex interplay of multiple molecular
determinants and pathways. Int J Mol Sci. 21:42832020. View Article : Google Scholar
|
|
6
|
Barat P, Meiffred MC, Brossaud J, Fuchs D,
Corcuff JB, Thibault H and Capuron L: Inflammatory, endocrine and
metabolic correlates of fatigue in obese children.
Psychoneuroendocrinology. 74:158–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cawthorn WP and Sethi JK: TNF-alpha and
adipocyte biology. FEBS Lett. 582:117–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ambros V: The functions of animal
microRNAs. Nat. 431:350–355. 2004. View Article : Google Scholar
|
|
9
|
Hamam D, Ali D, Kassem M, Aldahmash A and
Alajez NM: microRNAs as regulators of adipogenic differentiation of
mesenchymal stem cells. Stem Cells Dev. 24:417–425. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karbiener M, Fischer C, Nowitsch S,
Opriessnig P, Papak C, Ailhaud G, Dani C, Amri EZ and Scheideler M:
MicroRNA miR-27b impairs human adipocyte differentiation and
targets PPARgamma. Biochem Biophys Res Commun. 390:247–251. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun F, Wang J, Pan Q, Yu Y, Zhang Y, Wan
Y, Wang J, Li X and Hong A: Characterization of function and
regulation of miR-24-1 and miR-31. Biochem Biophys Res Commun.
380:660–665. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Z, Bian C, Zhou H, Huang S, Wang S,
Liao L and Zhao RC: MicroRNA hsa-miR-138 inhibits adipogenic
differentiation of human adipose tissue-derived mesenchymal stem
cells through adenovirus EID-1. Stem Cells Dev. 20:259–267. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nature reviews. Cancer. 6:857–866.
2006.PubMed/NCBI
|
|
14
|
Alajez NM: Cancer stem cells. From
characterization to therapeutic implications. Saudi Med J.
32:1229–1234. 2011.PubMed/NCBI
|
|
15
|
Quiat D and Olson EN: MicroRNAs in
cardiovascular disease: From pathogenesis to prevention and
treatment. J Clin Invest. 123:11–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao L, Lin EJ, Cahill MC, Wang C, Liu X
and During MJ: Molecular therapy of obesity and diabetes by a
physiological autoregulatory approach. Nat Med. 15:447–454. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hilton C, Neville MJ and Karpe F:
MicroRNAs in adipose tissue: Their role in adipogenesis and
obesity. Int J Obes (Lond). 37:325–332. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ortega FJ, Moreno-Navarrete JM, Pardo G,
Sabater M, Hummel M, Ferrer A, Rodriguez-Hermosa JI, Ruiz B, Ricart
W, Peral B and Fernández-Real JM: miRNA expression profile of human
subcutaneous adipose and during adipocyte differentiation. PLoS
One. 5:e90222010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Caporali A and Emanueli C: MicroRNA-503
and the extended microRNA-16 family in angiogenesis. Trends
Cardiovasc Med. 21:162–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu L, Ding GF, He C, Sun L, Jiang Y and
Zhu L: MicroRNA-424 is down-regulated in hepatocellular carcinoma
and suppresses cell migration and invasion through c-Myb. PLoS One.
9:e916612014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao H, Liu X, Chen S, Xia W and Chen X:
Decreased expression of serum miR-424 correlates with poor
prognosis of patients with hepatocellular carcinoma. Int J Clin Exp
Pathol. 8:14830–14835. 2015.PubMed/NCBI
|
|
22
|
Wen J, Hu Y, Liu Q, Ling Y, Zhang S, Luo
K, Xie X, Fu J and Yang H: miR-424 coordinates multilayered
regulation of cell cycle progression to promote esophageal squamous
cell carcinoma cell proliferation. EBioMedicine. 37:110–124. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu L, Yang F, Lin B, Chen X, Yin S, Zhang
F, Xie H, Zhou L and Zheng S: MicroRNA-424 expression predicts
tumor recurrence in patients with hepatocellular carcinoma
following liver transplantation. Oncol Lett. 15:9126–9132.
2018.PubMed/NCBI
|
|
24
|
Rosa A, Ballarino M, Sorrentino A,
Sthandier O, De Angelis FG, Marchioni M, Masella B, Guarini A,
Fatica A, Peschle C and Bozzoni I: The interplay between the master
transcription factor PU.1 and miR-424 regulates human
monocyte/macrophage differentiation. Proc Natl Acad Sci USA.
104:19849–19854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barutta F, Tricarico M, Corbelli A,
Annaratone L, Pinach S, Grimaldi S, Bruno G, Cimino D, Taverna D,
Deregibus MC, et al: Urinary exosomal microRNAs in incipient
diabetic nephropathy. PLoS One. 8:e737982013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vimalraj S, Saravanan S, Raghunandhakumar
S and Anuradha D: Melatonin regulates tumor angiogenesis via
miR-424-5p/VEGFA signaling pathway in osteosarcoma. Life Sci.
256:1180112020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ghosh G, Subramanian IV, Adhikari N, Zhang
X, Joshi HP, Basi D, Chandrashekhar YS, Hall JL, Roy S, Zeng Y and
Ramakrishnan S: Hypoxia-induced microRNA-424 expression in human
endothelial cells regulates HIF-α isoforms and promotes
angiogenesis. J Clin Invest. 120:4141–4154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Staszel T, Zapala B, Polus A,
Sadakierska-Chudy A, Kieć-Wilk B, Stępień E, Wybrańska I, Chojnacka
M and Dembińska-Kieć A: Role of microRNAs in endothelial cell
pathophysiology. Pol Arch Med Wewn. 121:361–366. 2011.PubMed/NCBI
|
|
29
|
de Gonzalo-Calvo D, Dávalos A, Montero A,
García-González A, Tyshkovska I, González-Medina A, Soares SMA,
Martínez-Camblor P, Casas-Agustench P, Rabadán M, et al:
Circulating inflammatory miRNA signature in response to different
doses of aerobic exercise. J Appl Physiol (1985). 119:124–134.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gasparotto AS, Borges DO, Sassi MGM,
Milani A, Rech DL, Terres M, Ely PB, Ramos MJ, Meihnardt NG and
Mattevi VS: Differential expression of miRNAs related to
angiogenesis and adipogenesis in subcutaneous fat of obese and
nonobese women. Mol Biol Rep. 46:965–973. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu L, Fan Q, Zhang F, Guo X, Liang X, Du
Y, Li P, Wen Y, Hao J, Wang W and He A: A genomewide integrative
analysis of GWAS and eQTLs data identifies multiple genes and gene
sets associated with obesity. Biomed Res Int.
2018:38485602018.PubMed/NCBI
|
|
32
|
Lee A, Papangeli I, Park Y, Jeong HN, Choi
J, Kang H, Jo HN, Kim J and Chun HJ: A PPARgamma-dependent
miR-424/503-CD40 axis regulates inflammation mediated angiogenesis.
Sci Rep. 7:25282017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ji CY; Working Group on Obesity in China,
: Report on childhood obesity in China (1)-body mass index
reference for screening overweight and obesity in Chinese
school-age children. Biomed Environ Sci. 18:390–400.
2005.PubMed/NCBI
|
|
34
|
Shi C, Zhu L, Chen X, Gu N, Chen L, Zhu L,
Yang L, Pang L, Guo X, Ji C and Zhang C: IL-6 and TNF-α induced
obesity-related inflammatory response through transcriptional
regulation of miR-146b. J Interferon Cytokine Res. 34:342–348.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pilli T, Cantara S, Marzocchi C, Cardinale
S, Santini C, Cevenini G and Pacini F: Diagnostic value of
circulating microRNA-95 and −190 in the differential diagnosis of
thyroid nodules: A validation study in 1000 consecutive patients.
Thyroid. 27:1053–1057. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cong Z, Diao Y, Xu Y, Li X, Jiang Z, Shao
C, Ji S, Shen Y, De W and Qiang Y: Long non-coding RNA linc00665
promotes lung adenocarcinoma progression and functions as ceRNA to
regulate AKR1B10-ERK signaling by sponging miR-98. Cell Death Dis.
10:842019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu X: Up-regulation of miR-20a by HPV16
E6 exerts growth-promoting effects by targeting PDCD6 in cervical
carcinoma cells. Biomed Pharmacother. 102:996–1002. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gene Ontology Consortium: The gene
ontology (GO) project in 2006. Nucleic Acids Res. 34:D322–D326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dupuy D, Bertin N, Hidalgo CA, Venkatesan
K, Tu D, Lee D, Rosenberg J, Svrzikapa N, Blanc A, Carnec A, et al:
Genome-scale analysis of in vivo spatiotemporal promoter activity
in Caenorhabditis elegans. Nat Biotechnol. 25:663–668. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schlitt T, Palin K, Rung J, Dietmann S,
Lappe M, Ukkonen E and Brazma A: From gene networks to gene
function. Genome Res. 13:2568–2576. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gu S, Su P, Yan J, Zhang X, An X, Gao J,
Xin R and Liu Y: Comparison of gene expression profiles and related
pathways in chronic thromboembolic pulmonary hypertension. Int J
Mol Med. 33:277–300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rhodin K, Divaris K, North KE, Barros SP,
Moss K, Beck JD and Offenbacher S: Chronic periodontitis
genome-wide association studies: Gene-centric and gene set
enrichment analyses. J Dent Res. 93:882–890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ignatieva EV, Afonnikov DA, Saik OV,
Rogaev EI and Kolchanov NA: A compendium of human genes regulating
feeding behavior and body weight, its functional characterization
and identification of GWAS genes involved in brain-specific PPI
network. BMC Genet. 17 (Suppl 3):1582016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Amemiya T, Gromiha MM, Horimoto K and
Fukui K: Drug repositioning for dengue haemorrhagic fever by
integrating multiple omics analyses. Sci Rep. 9:5232019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kanehisa M, Goto S, Kawashima S, Okuno Y
and Hattori M: The KEGG resource for deciphering the genome.
Nucleic Acids Res. 32:D277–D280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yi M, Horton JD, Cohen JC, Hobbs HH and
Stephens RM: WholePathwayScope: A comprehensive pathway-based
analysis tool for high-throughput data. BMC Bioinformatics.
7:302006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Draghici S, Khatri P, Tarca AL, Amin K,
Done A, Voichita C, Georgescu C and Romero R: A systems biology
approach for pathway level analysis. Genome Res. 17:1537–1545.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hiragun A, Sato M and Mitsui H:
Establishment of a clonal cell line that differentiates into
adipose cells in vitro. In vitro. 16:685–693. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shimizu K, Sakai M, Ando M, Chiji H,
Kawada T, Mineo H and Taira T: Newly developed primary culture of
rat visceral adipocytes and their in vitro characteristics. Cell
Biol Int. 30:381–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Babbi G, Martelli PL and Casadio R:
PhenPath: A tool for characterizing biological functions underlying
different phenotypes. BMC Genomics. 20 (Suppl 8):5482019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
GBD 2015 Obesity Collaborators, ; Afshin
A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L,
Mokdad AH, Moradi-Lakeh M, et al: Health effects of overweight and
obesity in 195 countries over 25 years. N Engl J Med. 377:13–27.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Longo M, Raciti GA, Zatterale F, Parrillo
L, Desiderio A, Spinelli R, Hammarstedt A, Hedjazifar S, Hoffmann
JM, Nigro C, et al: Epigenetic modifications of the Zfp/ZNF423 gene
control murine adipogenic commitment and are dysregulated in human
hypertrophic obesity. Diabetologia. 61:369–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kuang Q, Li J, You L, Shi C, Ji C, Guo X,
Xu M and Ni Y: Identification and characterization of NF-kappaB
binding sites in human miR-1908 promoter. Biomed Pharmacother.
74:158–163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fang Y, Liang X, Xu J and Cai X: miR-424
targets AKT3 and PSAT1 and has a tumor-suppressive role in human
colorectal cancer. Cancer Manag Res. 10:6537–6547. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Marques FZ, Vizi D, Khammy O, Mariani JA
and Kaye DM: The transcardiac gradient of cardio-microRNAs in the
failing heart. Eur J Heart Fail. 18:1000–1008. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kong Q, Guo X, Guo Z and Su T: Urinary
exosome miR-424 and miR-218 as biomarkers for type 1 diabetes in
children. Clin Lab. 65:2019. View Article : Google Scholar
|
|
58
|
Sayed AS, Xia K, Li F, Deng X, Salma U, Li
T, Deng H, Yang D, Haoyang Z, Yang T and Peng J: The diagnostic
value of circulating microRNAs for middle-aged (40-60-year-old)
coronary artery disease patients. Clinics (Sao Paulo). 70:257–263.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Huang S, Qi-Lin XU, Wang SH, Han Q and
Zhao CH: miR-424 negatively regulates adipogenic differentiation of
human adipose tissue-derived mesenchymal stem cells. Basic Clin
Med. 2012.
|
|
60
|
Lei YT, Ma J, Hu PJ, Dong B, Zhang B and
Song Y: The status of spermarche, menarche and corresponding
relationships with nutritional status among students of 13 ethnic
minorities in southwest China in 2014. Zhonghua Yu Fang Yi Xue Za
Zhi. 53:492–496. 2019.(In Chinese). PubMed/NCBI
|
|
61
|
Shi C, Huang F, Gu X, Zhang M, Wen J, Wang
X, You L, Cui X, Ji C and Guo X: Adipogenic miRNA and
meta-signature miRNAs involved in human adipocyte differentiation
and obesity. Oncotarget. 7:40830–40845. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tchkonia T, Lenburg M, Thomou T, Giorgadze
N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan
J, Karagiannides I, et al: Identification of depot-specific human
fat cell progenitors through distinct expression profiles and
developmental gene patterns. Am J Physiol Endocrinol Metab.
292:E298–E307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Divoux A, Sandor K, Bojcsuk D, Talukder A,
Li X, Balint BL, Osborne TF and Smith SR: Differential open
chromatin profile and transcriptomic signature define
depot-specific human subcutaneous preadipocytes: Primary outcomes.
Clin Epigenetics. 10:1482018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Glantschnig C, Koenen M, Gil-Lozano M,
Karbiener M, Pickrahn I, Williams-Dautovich J, Patel R, Cummins CL,
Giroud M, Hartleben G, et al: A miR-29a-driven negative feedback
loop regulates peripheral glucocorticoid receptor signaling. FASEB
J. 33:5924–5941. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jiang N, Li Y, Shu T and Wang J: Cytokines
and inflammation in adipogenesis: An updated review. Front Med.
13:314–329. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
El-Haggar SM and Mostafa TM: Adipokines
and biochemical changes in Egyptian obese subjects: Possible
variation with sex and degree of obesity. Endocrine. 48:878–885.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chang CJ, Jian DY, Lin MW, Zhao JZ, Ho LT
and Juan CC: Evidence in obese children: Contribution of
hyperlipidemia, obesity-inflammation, and insulin sensitivity. PLoS
One. 10:e01259352015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Jiang X, Yang L, Pang L, Chen L, Guo X, Ji
C, Shi C and Ni Y: Expression of obesityrelated miR1908 in human
adipocytes is regulated by adipokines, free fatty acids and
hormones. Mol Med Rep. 10:1164–1169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Daniel R, Wu Q, Williams V, Clark G,
Guruli G and Zehner Z: A panel of microRNAs as diagnostic
biomarkers for the identification of prostate cancer. Int J Mol
Sci. 18:12812017. View Article : Google Scholar
|
|
70
|
Hyväri L, Ojansivu M, Juntunen M,
Kartasalo K, Miettinen S and Vanhatupa S: Focal adhesion kinase and
ROCK signaling are switch-like regulators of human adipose stem
cell differentiation towards osteogenic and adipogenic lineages.
Stem Cells Int. 2018:21906572018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kennell JA and MacDougald OA: Wnt
signaling inhibits adipogenesis through beta-catenin-dependent and
-independent mechanisms. J Biol Chem. 280:24004–24010. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
van Loon NM, Ottenhoff R, Kooijman S,
Moeton M, Scheij S, Roscam Abbing RLP, Gijbels MJJ, Levels JHM,
Sorrentino V, Berbée JFP, et al: Inactivation of the E3 ubiquitin
ligase IDOL attenuates diet-induced obesity and metabolic
dysfunction in mice. Arterioscler Thromb Vasc Biol. 38:1785–1795.
2018. View Article : Google Scholar : PubMed/NCBI
|