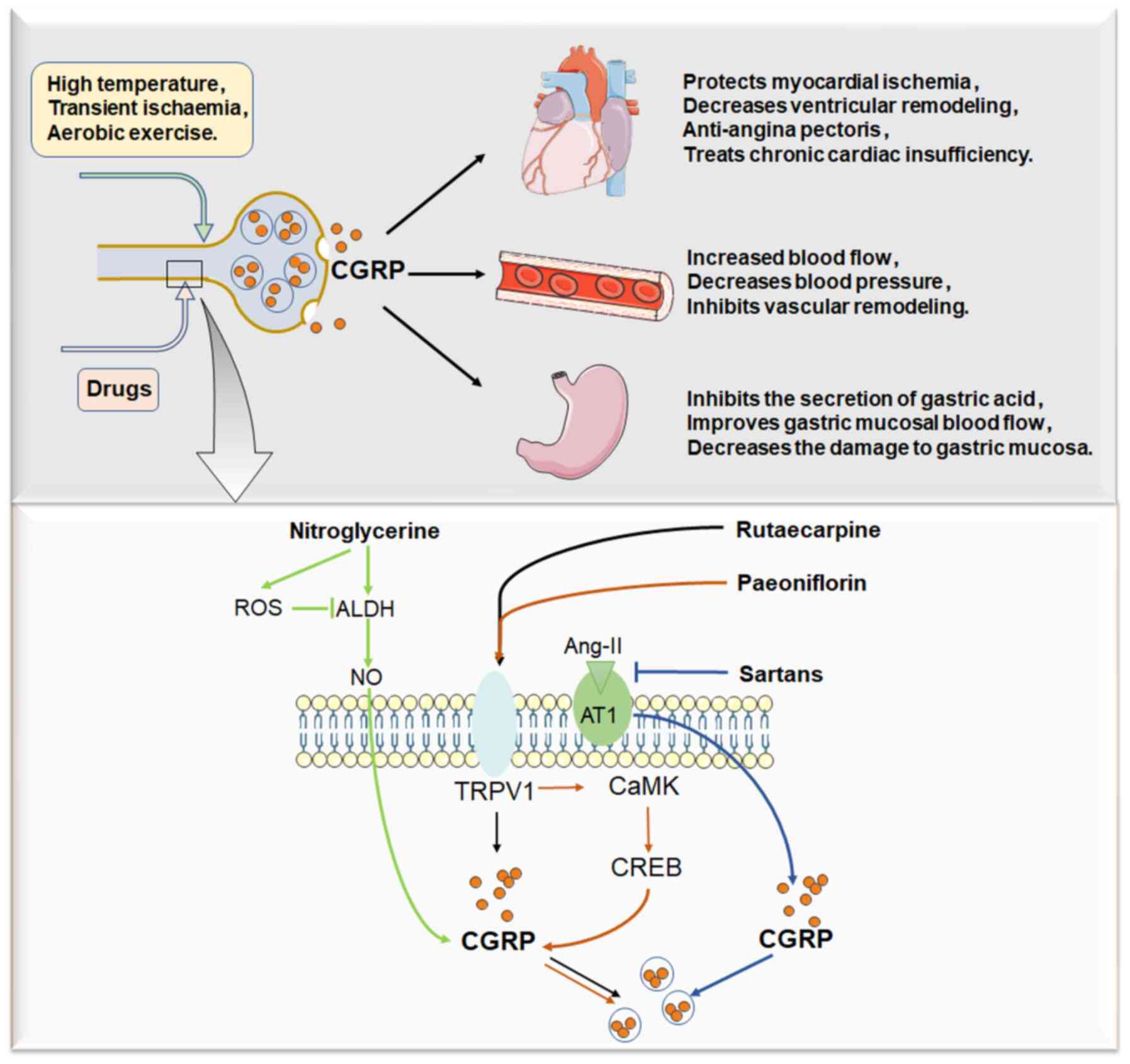
Calcitonin gene-related peptide (CGRP) is the first
neuropeptide synthesized by gene recombination and biosynthesis
that consists of 37 amino acids and is classified into two
subtypes, α-CGRP and β-CGRP, which are encoded by the
calcitonin/α-CGRP and β-CGRP genes, respectively (1,2). CGRP
is a neurotransmitter of the capsaicin-sensitive sensory nerve,
which is synthesized in neuronal cells and stored at the nerve
endings (1). The transient receptor
potential channel vanilloid type 1 (TRPV1), also known as vanilloid
receptor subtype 1 (VR1), is a critical receptor that regulates the
synthesis and release of CGRP (3).
Given that the binding site of TRPV1 is located in the inner side
of the cell membrane, its endogenous ligand anandamide is only
active following intracellular transport (4). CGRP has exhibited extensive and
complex biological activity. For example, in addition to its
involvement in neuralgia and migraine, CGRP can regulate vascular
tone, maintain the balance of circulation, attenuate ischaemic
injury and inhibit cardiac fibroblast proliferation, as well as
cardiac remodelling (5).
Furthermore, CGRP plays a positive role in protecting the gastric
mucosa in the gastrointestinal system (6,7). It
has been reported that CGRP participates in the pathophysiological
processes of several cardiovascular diseases, including
hypertension, myocardial infarction, heart failure and pulmonary
hypertension (5). Exogenous CGRP
has been proven to be effective in the treatment of hypertension,
pulmonary hypertension, acute lung injury, cerebral/cardiac
ischaemia-reperfusion injury and chronic heart failure, suggesting
that promoting endogenous synthesis and release of CGRP may be a
novel direction of drug research and development (8–11). The
present review summarizes the existing studies focusing on
CGRP-mediated pharmacological effects of drugs, as presented in
Table I.
Nitroglycerine, a classic anti-angina drug that is
extensively applied in clinical settings, can be used for treating
and alleviating chronic cardiac insufficiency (12). Previous studies have demonstrated
that CGRP is involved in nitroglycerine treatment of myocardial
ischaemia and chronic cardiac insufficiency (13,14).
Nitroglycerine decreases cardiac blood volume,
ventricular wall tension and myocardial oxygen consumption by
dilating venules, which also dilates the coronary artery and
increases the blood supply of the myocardial ischaemic area to
attenuate angina pectoris (15).
Nitroglycerine produces nitric oxide (NO) under the catalysis of
aldehyde dehydrogenase (ALDH), and NO mediates the vasodilation of
nitroglycerine (16). Previous
studies have confirmed the key role of CGRP in mediating the
inhibitory effect of nitroglycerine on angina. The results of the
vessel ring ex vivo experiment demonstrated that the
vasodilation effect of nitroglycerine can be reversed by CGRP
receptor antagonists and capsaicin (17–19).
In vivo, pre-treatment with capsaicin can also neutralize
the hypotensive effect of nitroglycerine as it can deplete CGRP
(20). An experiment was designed
to assess whether the vasodilation effect associated with
nitroglycerine was mediated by CGRP. Based on the polymorphisms of
the ALDH gene, 40 unrelated male healthy volunteers with a mean age
of 28 years (25 to 32 years) were enrolled, screened and
stochastically divided into two groups according to their ALDH
genotype, as follows: The ALDH2*1/*1 group (nine individuals,
normal wild-type homozygote, ALDH 504 is glutamic acid, with high
enzyme activity) and the ALDH2*2 group (nine individuals, carrying
a mutant allele, ALDH 504 is lysine, resulting in reduced enzyme
activity). Following treatment with nitroglycerine, the ALDH2*1/*1
group exhibited a distinct blood pressure drop and a significant
increase in the concentration of CGRP in the blood, while the
ALDH2*2 group displayed less changes in these two parameters
(21). An additional study
suggested that increased CGRP expression ultimately accelerates the
antithrombotic effect of nitroglycerine (22). Collectively, these findings support
the hypothesis that nitroglycerine releases NO to increase the
concentration of CGRP, and subsequently plays an antagonistic role
in the occurrence and development of angina (23).
Ischaemia-reperfusion injury is regarded as an
aggravated injury in an ischaemic heart resulting from reperfusion
following a period of ischaemia (24). Ischaemic preconditioning refers to
the phenomenon by which transient ischaemia, prior to heart
ischaemia, partially alleviates cardiac damage for 5–30 min
(25). The protection of ischaemic
preconditioning is mediated by endogenous active substances
expressed due to ischaemic stimulation, such as adenosine and CGRP,
which are also considered endogenous myocardial protective
substances since they are released by the heart (26). It has been reported that transient
cardiac ischaemia increases CGRP expression, while pretreatment
with either a CGRP receptor blocker or capsaicin can offset cardiac
defence mediated by ischaemic preconditioning, including the
improvement of cardiac function, reduction in the myocardial
infarction area and decreased creatine kinase release (27). The protective effect of CGRP on
ischaemic myocardium may be associated with the repair of injured
cardiomyocytes, the suppression of active oxygen species induced by
hypoxia/reoxygenation and the balance of the mitochondrial membrane
potential (28). CGRP induced by
aerobic exercise has also been demonstrated to be protective in
ischaemic myocardium (29).
Considering that TRPV1 is temperature-sensitive, previous studies
assessed whether the protection of ischaemic myocardium induced by
high-temperature pre-adaptation was potentially associated with the
induction of CGRP release. The experimental results demonstrated
that both a CGRP receptor blocker and capsaicin can counteract the
ischaemic myocardial protection associated with high-temperature
pre-adaptation (19). Given that
nitroglycerine can release GCRP, the hypothesis that pretreatment
with nitroglycerine can imitate ischaemic preconditioning and serve
a protective role in the ischaemic heart was investigated. These
processes have only been demonstrated in vivo and in
vitro (30). The results
demonstrated that CGRP serum levels are significantly decreased in
diabetic rats with acute myocardial ischaemia-reperfusion, and
exogenous CGRP can recover myocardial cell damage from high glucose
and ischaemia-reperfusion (31).
Paeoniflorin, a pinane monoterpene glucoside extracted from the
root of paeonia lactiflora pall, has been demonstrated to provide
heart protection in diabetic mice by activating the
TRPV1/calmodulin-dependent protein kinase/cAMP response element
binding protein/CGRP signaling pathway (32).
Chronic cardiac insufficiency, also known as chronic
heart failure, is a complicated syndrome induced by either
ventricular filling or ejection capacity insufficiency, which stems
from organic causes or functional cardiac diseases. Given that the
pathological process of chronic cardiac insufficiency is partly
associated with haemodynamic changes, drugs can improve cardiac
function by dilating blood vessels (arteries and veins) and
affecting haemodynamics (33).
Nitrate esters currently play a significant role in the treatment
of chronic cardiac insufficiency. By dilating the venules,
nitroglycerine decreases the cardiac blood volume and ventricular
wall tension, and strengthens the left ventricular compliance,
myocardial contractility and cardiac output, ultimately improving
cardiac function (17,34). A previous study demonstrated that
nitroglycerine can be used in the treatment of chronic cardiac
insufficiency by stimulating the CGRP signaling pathway. According
to the gene type, patients were divided into two groups, as
follows: The ALDH2*1/*1 group and the ALDH2*2 group. Following
treatment with nitroglycerine, the experimental data indicated that
the levels of CGRP in the ALDH2*1/*1 homozygote group were
significantly increased, whereas the patients in this group
exhibited apparent improvements in both blood pressure and left
ventricular ejection fraction compared with those in the ALDH2*2
mutant group (34).
The underlying molecular mechanism of the tolerance
to nitroglycerine following its continuous application remains
unclear. Previous studies have concluded that nitroglycerine
tolerance is associated with thiol (-SH) consumption of tissue
cells, since the application of drugs containing -SH, such as
captopril or N-acetylcysteine, can partially reverse the tolerance
to nitroglycerine (35). Previous
studies have reported that the tolerance may be associated with
nitroglycerine-induced oxidative stress (generation of active
oxygen species), which restrains the activity of ALDH2, the release
of NO and eventually attenuates the vasomotor effect (36). In addition, CGRP mediates the
vasodilation effect of nitroglycerine, resulting in tolerance from
a secondary decrease in CGRP caused by NO reduction (37). Previous in vivo and in
vitro experiments have demonstrated that when nitroglycerine is
tolerated, the levels of CGRP and the vasodilation effect decrease
simultaneously, whereas pretreatment with drugs containing -SH can
markedly increase the levels of NO and CGRP, partially reversing
the tolerance (23,36,37). A
recent study demonstrated that nitroglycerine tolerance may
intensify the anti-ischaemic effect and cardiovascular mortality in
rats, whereas exposure to a CGRP antagonist (CGRP8-37)
may improve this condition (38).
It is widely accepted that the occurrence and
development of hypertension is strongly associated with peripheral
vascular resistance. CGRP induces vascular relaxation by decreasing
the peripheral vascular resistance. Thus, suppressing CGRP
synthesis and release can promote the development of hypertension
(39). A previous study
demonstrated that α-CGRP knockout animals exhibit higher basal
blood pressure (40). In addition,
both phenol-induced hypertension rats and spontaneous hypertension
rats display lower levels of CGRP in the blood (4,41).
Clinical studies have reported that CGRP levels in the blood of
patients with essential hypertension and pregnancy hypertension are
significantly lower than those in healthy subjects (4). Recently, it has been demonstrated that
endogenous α-CGRP induced by physical activity can protect cardiac
function during treatment of mice with chronic hypertension
(42).
Released CGRP is regulated by several endogenous
active substances, including NO, bradykinin, neuropeptide Y and
angiotensin II (Ang II). Ang II inhibits CGRP release by activating
anterior membrane angiotensin receptor 1 (AT1) (43). Sartans are AT1 receptor blockers,
which can prevent Ang II from contracting blood vessels leading to
the stimulation of aldosterone secretion. In addition, they can
decrease peripheral vascular resistance and lower blood pressure
(44). Thus, it was hypothesized
that sartans can promote CGRP release by blocking the AT1 receptor
since Ang II can activate the AT1 receptor and suppress the release
of CGRP.
Previous studies have confirmed that while sartans
decrease the blood pressure of spontaneously hypertensive rats,
CGRP expression is significantly increased in the blood, heart,
kidney and dorsal root ganglion (45,46).
In addition, a clinical trial demonstrated that following treatment
with the Ang II type 1 receptor blocker olmesartan, the systolic
and diastolic blood pressure levels of patients with hypertension
were normalized, and CGRP levels also increased (47). Taken together, these findings
support the hypothesis that the molecular mechanism of blood
pressure reduction of sartans may also be associated with the CGRP
pathway.
Pulmonary hypertension is a chronic respiratory
disease characterized by persistent pulmonary hypertension caused
by pulmonary capillary occlusion. Despite that the pathogenesis of
pulmonary hypertension remains unclear, it is universally accepted
that the vascular remodeling caused by smooth muscle cell
proliferation and migration is the main contributing factor to the
development of this disease (56).
CGRP expression in the blood has been decrease in both mature and
newborn rats suffering from pulmonary hypertension (57). It has been reported that continuous
hypoxia for 3 weeks can cause persistent pulmonary hypertension in
neonatal rats, with a decrease in plasma CGRP expression (58). In addition, targeted knockout of the
CGRP receptor gene has been demonstrated to aggravate
hypoxia-induced pulmonary hypertension (59). CGRP suppresses the extracellular
signaling-regulated kinase 1/2 pathway, which contributes to the
inhibition of vascular remodeling through a vasodilation effect,
and the control of the proliferation of vascular smooth muscle
cells (5). A previous study
demonstrated that exogenous CGRP can inhibit the proliferation of
smooth muscle cells induced by hypoxia in a concentration-dependent
manner (60). Adenoviral
transfection of the CGRP gene significantly decreases the pulmonary
vascular resistance of rats with hypoxia-induced pulmonary
hypertension, improving pulmonary vascular remodelling (53). It has also been reported that
transfection of CGRP into endothelial progenitor cells mitigates
pulmonary hypertension and vascular remodelling (57). Given that the release of CGRP
alleviates pulmonary hypertension, rutaecarpine may also relieve
pulmonary hypertension due to its activating effect on CGRP. It has
been demonstrated that rutaecarpine inhibits the pulmonary
hypertension induced by monocrotaline or hypoxia in rats by
elevating CGRP levels in the blood (51). A recent study discovered that
rutaecarpine inhibits the Notch1/eIF3a signaling pathway in order
to improve the synthesis and release of CGRP, alleviating pulmonary
fibrosis and the epithelial-to-mesenchymal transition process
(61).
As a compensatory response to the increase in
ventricular pressure, cardiac remodeling is usually accompanied by
pathological changes, including cardiac hypertrophy and cardiac
fibroblast proliferation. Cardiac fibroblasts account for 60–70% of
the total number of cardiac cells and play an important role in
cardiac remodeling. Under pathological conditions, cardiac
fibroblasts proliferate and differentiate into myofibroblasts
(62). However, this process acts
in an autocrine manner and several active substances, such as Ang
II, endothelin and inflammatory factors can further promote the
proliferation of cardiac fibroblasts (63). CGRP has been demonstrated to inhibit
the proliferation of cardiac fibroblasts and cardiac remodelling
(51). In addition, exogenous CGRP
has been reported to inhibit the senescence of cardiac fibroblasts
and the development of cardiac fibrosis by increasing the
expression levels of klotho (64).
A previous study demonstrated that pressure loading (coarctation of
the aortic arch) aggravates the left ventricular hypertrophy and
fibrosis of calcitonin/α-CGRP gene knockout mice (65). TRPV1 gene knockout also exacerbates
cardiac remodeling in mice (66).
Another study demonstrated that rutaecarpine alleviates left
ventricular remodeling induced by isoproterenol by activating CGRP,
which regulates the expression of apoptosis-related genes,
including Bax and Bcl2 (62). To
investigate whether the potential molecular mechanism of
rutaecarpine-mediated inhibition of cardiac remodeling acts via the
CGRP signaling pathway, an experiment was designed to construct a
right ventricular remodeling model in rats with pulmonary
hypertension induced by monocrotaline and hypoxia. The results
demonstrated that rutaecarpine affects the eIF3a/p27 signaling
pathway by promoting CGRP release, eventually suppressing cardiac
remodelling (51).
Aforementioned studies have focused on neuronal
CGRP, which acts as the transmitter of sensory nerves (Fig. 1) (39,43).
However, it has been reported that CGRP also exists in non-nerve
tissue cells, such as endothelial cells, endothelial progenitor
cells, lymphocytes, bronchial epithelial cells and adipocytes, and
plays an important role in regulating local tissues (86). The α- and β-subtypes of CGRP have
been observed in endothelial cells and are regulated by TRPV1 to
maintain physiological function of endothelial cells (87). A recent study suggested that cardiac
fibroblasts synthesize and secrete CGRP, which suppresses their
activation in an autocrine manner (88). Drugs such as clonidine, which is an
α-receptor agonist regulating CGRP through a non-nerve pathway,
have been demonstrated to influence the secretion of CGRP in
endothelial cells by affecting its synthesis and secretion in local
tissues (89). In addition,
rutaecarpine delays cell senescence by promoting the expression and
secretion of CGRP in endothelial progenitor cells (90). Thus, whether drugs can affect CGRP
synthesis and secretion of other non-nerve cells remains to be
investigated.
As the pivotal receptor affecting the release of
CGRP, the TRP receptor contains several subtypes, including TRPC,
TRPV, TRPM, TRPML, TRPP, TRPA and TRPN (91). Previous studies that assessed the
regulation of CGRP synthesis and release focused on TRPV1, which is
considered a significant target for drug discovery (54,91).
Transient receptor potential ankyrin 1 (TRPA1) is localized in
primary sensory dorsal root ganglion neurons. It was confirmed that
H2S induced NO production and the subsequent activation of the
neuroendocrine HNO-TRPA1-CGRP signaling pathway is the main cause
of vasodilatory effects (92). A
previous study demonstrated that cinnamaldehyde activates TRPA1 and
inhibits hypoxia-induced cardiac fibrosis through a molecular
mechanism that involves the upregulation of cardiac
fibroblast-derived CGRP . Collectively, these studies suggest that
TRPA1 may also be considered a novel target for drug discovery.
The data reported in the present review demonstrates
that CGRP participates in the regulation of the function of
multiple organs. It also mediates the pharmacological effects of
marketed drugs, such as nitroglycerine and sartans. These studies
not only improve our understanding on the therapeutic effects of
marketed drugs, but also provide novel targets for drug discovery.
Thus, targeting endogenous CGRP synthesis and release may be
considered a novel direction for drug research and development.
Not applicable.
The present study was supported by grants from the
National Natural Scientific Foundation of China (grant nos.
81703518 and 81973406), the Hunan Provincial Natural Scientific
Foundation (grant nos. 2019JJ50849 and 2020JJ4823), the Scientific
Research Project of Hunan Provincial Health and Family Planning
Commission (grant no. B20180253) and the Fundamental Research Funds
for the Central Universities of Central South University (grant no.
2020zzts822).
Not applicable.
WJM and YCY contributed equally in classifying the
literature and drafting the manuscript. BKZ and YJL collected and
analyzed the literature. WQL designed the framework of this article
and critically revised the manuscript. All authors have read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Rosenfeld MG, Mermod JJ, Amara SG, Swanson
LW, Sawchenko PE, Rivier J, Vale WW and Evans RM: Production of a
novel neuropeptide encoded by the calcitonin gene via
tissue-specific RNA processing. Nature. 304:129–135. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wimalawansa SJ, Morris HR, Etienne A,
Blench I, Panico M and MacIntyre I: Isolation, purification and
characterization of beta-hCGRP from human spinal cord. Biochem
Biophys Res Commun. 167:993–1000. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng J and Li YJ: The vanilloid receptor
TRPV1: Role in cardiovascular and gastrointestinal protection. Eur
J Pharmacol. 627:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li D, Chen BM, Peng J, Zhang YS, Li XH,
Yuan Q, Hu CP, Deng HW and Li YJ: Role of anandamide transporter in
regulating calcitonin gene-related peptide production and blood
pressure in hypertension. J Hypertens. 27:1224–1232. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li XW, Hu CP, Wu WH, Zhang WF, Zou XZ and
Li YJ: Inhibitory effect of calcitonin gene-related peptide on
hypoxia-induced rat pulmonary artery smooth muscle cells
proliferation: Role of ERK1/2 and p27. Eur J Pharmacol.
679:117–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Evangelista S: Role of calcitonin
gene-related Peptide in gastric mucosal defence and healing. Curr
Pharm Des. 15:3571–3576. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng G, Wang Q, Xu X, Liu Z, Li Z and Liu
G: The protective effects of calcitonin gene-related peptide on
gastric mucosa injury of gastric ischemia reperfusion in rats.
Immunopharmacol Immunotoxicol. 33:84–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sukhishvili E, Bekaia G and Kvachadze I:
Effect of exogenous calcitonin gene-related Peptide on systemic
arterial blood pressure in pregnant and non-pregnant rats. Georgian
Med News. 71–75. 2011.(In Russian). PubMed/NCBI
|
|
9
|
Yang W, Xv M, Yang WC, Wang N, Zhang XZ
and Li WZ: Exogenous α-calcitonin gene-related peptide attenuates
lipopolysaccharide-induced acute lung injury in rats. Mol Med Rep.
12:2181–2188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang SI, Yuan Y, Jiao S, Luo QI and Yu J:
Calcitonin gene-related peptide protects rats from cerebral
ischemia/reperfusion injury via a mechanism of action in the MAPK
pathway. Biomed Rep. 4:699–703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chai W, Mehrotra S, Jan Danser AH and
Schoemaker RG: The role of calcitonin gene-related peptide (CGRP)
in ischemic preconditioning in isolated rat hearts. Eur J
Pharmacol. 531:246–253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scardi S, Pandullo C, Pivotti F, Ceschia G
and Pollavini G: Hemodynamic and anti-angina effects of transdermal
nitroglycerin after acute and chronic administration. Additive
effect of sublingual isosorbide dinitrate. G Ital Cardiol.
16:895–903. 1986.(In Italian). PubMed/NCBI
|
|
13
|
Li YJ and Du YH: CGRP-mediated
cardiovascular effect of nitroglycerin. Med Hypotheses. 60:693–698.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou ZH, Peng J, Ye F, Li NS, Deng HW and
Li YJ: Delayed cardioprotection induced by nitroglycerin is
mediated by alpha-calcitonin gene-related peptide. Naunyn
Schmiedebergs Arch Pharmacol. 365:253–259. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Glyceryl trinitrate and angina. Br Med J.
4:2521969. View Article : Google Scholar
|
|
16
|
Munzel T, Daiber A and Mulsch A:
Explaining the phenomenon of nitrate tolerance. Circ Res.
97:618–628. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Booth BP, Tabrizi-Fard MA and Fung H:
Calcitonin gene-related peptide-dependent vascular relaxation of
rat aorta. An additional mechanism for nitroglycerin. Biochem
Pharmacol. 59:1603–1609. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei EP, Moskowitz MA, Boccalini P and
Kontos HA: Calcitonin gene-related peptide mediates nitroglycerin
and sodium nitroprusside-induced vasodilation in feline cerebral
arterioles. Circ Res. 70:1313–1319. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song QJ, Li YJ and Deng HW: Early and
delayed cardioprotection by heat stress is mediated by calcitonin
gene-related peptide. Naunyn Schmiedebergs Arch Pharmacol.
359:477–483. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou ZH, Deng HW and Li YJ: The depressor
effect of nitroglycerin is mediated by calcitonin gene-related
peptide. Life Sci. 69:1313–1320. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo R, Chen XP, Guo X, Chen L, Li D, Peng
J and Li YJ: Evidence for involvement of calcitonin gene-related
peptide in nitroglycerin response and association with
mitochondrial aldehyde dehydrogenase-2 (ALDH2) Glu504Lys
polymorphism. J Am Coll Cardiol. 52:953–960. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Booth BP, Nolan TD and Fung HL:
Nitroglycerin-inhibited whole blood aggregation is partially
mediated by calcitonin gene-related peptide-a neurogenic mechanism.
Br J Pharmacol. 122:577–583. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu R, Li XH and Li YJ:
Nitroglycerin-induced myocardial protection and tolerance: Role for
CGRP. Trends Pharmacol Sci. 35:369–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Binder A, Ali A, Chawla R, Aziz HA, Abbate
A and Jovin IS: Myocardial protection from ischemia-reperfusion
injury post coronary revascularization. Expert Rev Cardiovasc Ther.
13:1045–1057. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou FW, Li YJ, Lu R and Deng HW:
Protection of calcitonin gene-related peptide-mediated
preconditioning against coronary endothelial dysfunction induced by
reperfusion in the isolated rat heart. Life Sci. 64:1091–1097.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Zhang M, Yang C, Dun Y, Zhang Y and
Hao Y: Nitroglycerin protects small intestine from
ischemia-reperfusion injury via NO-cGMP pathway and upregulation of
alpha-CGRP. J Gastrointest Surg. 13:478–485. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li YJ, Song QJ and Xiao J: Calcitonin
gene-related peptide: An endogenous mediator of preconditioning.
Acta Pharmacol Sin. 21:865–869. 2000.PubMed/NCBI
|
|
28
|
Guo Z, Liu N, Chen L, Zhao X and Li MR:
Independent roles of CGRP in cardioprotection and hemodynamic
regulation in ischemic postconditioning. Eur J Pharmacol.
828:18–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Zhang L, Jia L, Liu J, Liu K, Feng
Q and Wang Q: Calcitonin gene-related peptide in aerobic exercise
induces collateral circulation development in rat ischemia
myocardium. Biomed Pharmacother. 82:561–567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu CP, Li YJ and Deng HW: The
cardioprotective effects of nitroglycerin-induced preconditioning
are mediated by calcitonin gene-related peptide. Eur J Pharmacol.
369:189–194. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li TP, Guo Z, Liu CJ, Sun T, Chen L and
Zhao X: Association of down-regulation of calcitonin gene-related
peptide and substance P with increase of myocardial vulnerability
in diabetic neuropathic rats. Peptides. 96:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han F, Zhou D, Yin X, Sun Z, Han J, Ye L,
Zhao W, Zhang Y, Wang Z and Zheng L: Paeoniflorin protects diabetic
mice against myocardial ischemic injury via the transient receptor
potential vanilloid 1/calcitonin gene-related peptide pathway. Cell
Biosci. 6:372016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kosarev MM, Obrezan AG, Strel'nikov AA and
Gur'ianov AV: Modern principles of diagnostics of chronic cardiac
insufficiency. Klin Med (Mosk). 89:8–13. 2011.(In Russian).
PubMed/NCBI
|
|
34
|
Peng LM, Chen XP, Sun J, Guo YJ, Li L, Mo
L, Xie W, Li YJ, Yang TL and Li CC: Influence of ALDH2 Glu504Lys
polymorphism on nitroglycerin response in chronic heart failure and
involvement of calcitonin gene related peptide (CGRP). Int J Clin
Pharmacol Ther. 50:701–711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou ZH, Jiang JL, Peng J, Deng HW and Li
YJ: Reversal of tolerance to nitroglycerin with N-acetylcysteine or
captopril: A role of calcitonin gene-related peptide. Eur J
Pharmacol. 439:129–134. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen YR, Nie SD, Shan W, Jiang DJ, Shi RZ,
Zhou Z, Guo R, Zhang Z and Li YJ: Decrease in endogenous CGRP
release in nitroglycerin tolerance: Role of ALDH-2. Eur J
Pharmacol. 571:44–50. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou ZH, Deng HW and Li YJ: Involvement of
calcitonin gene-related peptide in the development of tolerance to
nitroglycerin in the rat. Eur J Pharmacol. 427:137–141. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kezeli T, Rukhadze T, Gongadze N, Sukoyan
G, Dolidze N, Chipashvili M and Mirziashvili M: Effect of
calcitonin gene-related peptide antagonist on the cardiovascular
events, mortality, and prostaglandin E2 production by
nitrate-induced tolerant rats with acute myocardial infarction.
EPMA J. 7:62016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Márquez-Rodas I, Longo F, Rothlin RP and
Balfagón G: Pathophysiology and therapeutic possibilities of
calcitonin gene- related peptide in hypertension. J Physiol
Biochem. 62:45–56. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li J, Zhao H, Supowit SC, DiPette DJ and
Wang DH: Activation of the renin-angiotensin system in
alpha-calcitonin gene-related peptide/calcitonin gene knockout
mice. J Hypertens. 22:1345–1349. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Deng PY, Ye F, Cai WJ, Deng HW and Li YJ:
Role of calcitonin gene-related peptide in the phenol-induced
neurogenic hypertension in rats. Regul Pept. 119:155–161. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Skaria T, Mitchell KJ, Vogel O, Walchli T,
Gassmann M and Vogel J: Blood pressure normalization-independent
cardioprotective effects of endogenous, physical activity-induced
αCGRP (α calcitonin gene-related peptide) in chronically
hypertensive mice. Circ Res. 125:1124–1140. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Russell FA, King R, Smillie SJ, Kodji X
and Brain SD: Calcitonin gene-related peptide: Physiology and
pathophysiology. Physiol Rev. 94:1099–1142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hobara N, Gessei-Tsutsumi N, Goda M,
Takayama F, Akiyama S, Kurosaki Y and Kawasaki H: Long-term
inhibition of angiotensin prevents reduction of periarterial
innervation of calcitonin gene-related peptide (CGRP)-containing
nerves in spontaneously hypertensive rats. Hypertens Res.
28:465–474. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Harada N, Shimozawa N and Okajima K: AT(1)
receptor blockers increase insulin-like growth factor-I production
by stimulating sensory neurons in spontaneously hypertensive rats.
Transl Res. 154:142–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shi RZ, Hu CP, Luo D, Li D, Pan W, Li SX,
Yang TL, Li YJ and Zhang GG: Decreased anandamide transporter
activity and calcitonin gene-related peptide production in
spontaneously hypertensive rats: Role of angiotensin II. Eur J
Pharmacol. 680:81–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ravarotto V, Pagnin E, Maiolino G,
Fragasso A, Carraro G, Rossi B and Calò LA: The blocking of
angiotensin II type 1 receptor and RhoA/Rho kinase activity in
hypertensive patients: Effect of olmesartan medoxomil and
implication with cardiovascular-renal remodeling. J Renin
Angiotensin Aldosterone Syst. 16:1245–1250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jia S and Hu C: Pharmacological effects of
rutaecarpine as a cardiovascular protective agent. Molecules.
15:1873–1881. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang L, Hu CP, Deng PY, Shen SS, Zhu HQ,
Ding JS, Tan GS and Li YJ: The protective effects of rutaecarpine
on gastric mucosa injury in rats. Planta Med. 71:416–419. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hu CP, Li NS, Xiao L, Deng HW and Li YJ:
Involvement of capsaicin-sensitive sensory nerves in
cardioprotection of rutaecarpine in rats. Regul Pept. 114:45–49.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li WQ, Li XH, Du J, Zhang W, Li D, Xiong
XM and Li YJ: Rutaecarpine attenuates hypoxia-induced right
ventricular remodeling in rats. Naunyn Schmiedebergs Arch
Pharmacol. 389:757–767. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang Y, Chen Q, Jia S, He L, Wang A, Li D,
Li Y and Li X: Involvement of TRPV1 in the expression and release
of calcitonin gene-related peptide induced by rutaecarpine. Mol Med
Rep. 17:5168–5174. 2018.PubMed/NCBI
|
|
53
|
Bivalacqua TJ, Hyman AL, Kadowitz PJ,
Paolocci N, Kass DA and Champion HC: Role of calcitonin
gene-related peptide (CGRP) in chronic hypoxia-induced pulmonary
hypertension in the mouse. Influence of gene transfer in vivo.
Regul Pept. 108:129–133. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Deng PY and Li YJ: Calcitonin gene-related
peptide and hypertension. Peptides. 26:1676–1685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Deng PY, Ye F, Cai WJ, Tan GS, Hu CP, Deng
HW and Li YJ: Stimulation of calcitonin gene-related peptide
synthesis and release: Mechanisms for a novel antihypertensive
drug, rutaecarpine. J Hypertens. 22:1819–1829. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gumusel B, Hao Q, Hyman AL, Kadowitz PJ,
Champion HC, Chang JK, Mehta JL and Lippton H: Analysis of
responses to adrenomedullin-(13–52) in the pulmonary vascular bed
of rats. Am J Physiol. 274:H1255–H1263. 1998.PubMed/NCBI
|
|
57
|
Zhao Q, Liu Z, Wang Z, Yang C, Liu J and
Lu J: Effect of prepro-calcitonin gene-related peptide-expressing
endothelial progenitor cells on pulmonary hypertension. Ann Thorac
Surg. 84:544–552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Keith IM, Tjen-A-Looi S, Kraiczi H and
Ekman R: Three-week neonatal hypoxia reduces blood CGRP and causes
persistent pulmonary hypertension in rats. Am J Physiol Heart Circ
Physiol. 279:H1571–H1578. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Qing X and Keith IM: Targeted blocking of
gene expression for CGRP receptors elevates pulmonary artery
pressure in hypoxic rats. Am J Physiol Lung Cell Mol Physiol.
285:L86–L96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Qin XP, Ye F, Hu CP, Liao DF, Deng HW and
Li YJ: Effect of calcitonin gene-related peptide on angiotensin
II-induced proliferation of rat vascular smooth muscle cells. Eur J
Pharmacol. 488:45–49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gao YX, Jiang LL, Zhang Q, Zuo DZ and Li
XW: Rutaecarpine protects against bleomycin-induced pulmonary
fibrosis through inhibiting Notch1/eIF3a signaling pathway in rats.
Zhongguo Zhong Yao Za Zhi. 43:3530–3538. 2018.(In Chinese).
PubMed/NCBI
|
|
62
|
Li JZ, Peng J, Xiao L, Zhang YS, Liao MC,
Li XH, Hu CP, Deng HW and Li YJ: Reversal of isoprenaline-induced
cardiac remodeling by rutaecarpine via stimulation of calcitonin
gene-related peptide production. Can J Physiol Pharmacol.
88:949–959. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ma ZG, Yuan YP, Wu HM, Zhang X and Tang
QZ: Cardiac fibrosis: New insights into the pathogenesis. Int J
Biol Sci. 14:1645–1657. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li WQ, Tan SL, Li XH, Sun TL, Li D, Du J,
Wei SS, Li YJ and Zhang BK: Calcitonin gene-related peptide
inhibits the cardiac fibroblasts senescence in cardiac fibrosis via
up-regulating klotho expression. Eur J Pharmacol. 843:96–103. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li J, Carnevale KA, Dipette DJ and Supowit
SC: Renal protective effects of α-calcitonin gene-related peptide
in deoxycorticosterone-salt hypertension. Am J Physiol Renal
Physiol. 304:F1000–F1008. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Huang W, Rubinstein J, Prieto AR, Thang LV
and Wang DH: Transient receptor potential vanilloid gene deletion
exacerbates inflammation and atypical cardiac remodeling after
myocardial infarction. Hypertension. 53:243–250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Warzecha Z, Dembinski A, Ceranowicz P,
Dembinski M, Cieszkowski J, Kownacki P and Konturek PC: Role of
sensory nerves in gastroprotective effect of anandamide in rats. J
Physiol Pharmacol. 62:207–217. 2011.PubMed/NCBI
|
|
68
|
Young RL, Cooper NJ and Blackshaw LA:
Chemical coding and central projections of gastric vagal afferent
neurons. Neurogastroenterol Motil. 20:708–718. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Tache Y, Pappas T, Lauffenburger M, Goto
Y, Walsh JH and Debas H: Calcitonin gene-related peptide: Potent
peripheral inhibitor of gastric acid secretion in rats and dogs.
Gastroenterology. 87:344–349. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Holzer P and Guth PH: Neuropeptide control
of rat gastric mucosal blood flow. Increase by calcitonin
gene-related peptide and vasoactive intestinal polypeptide, but not
substance P and neurokinin A. Circ Res. 68:100–105. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Peskar BM, Ehrlich K and Peskar BA: Role
of ATP-sensitive potassium channels in prostaglandin-mediated
gastroprotection in the rat. J Pharmacol Exp Ther. 301:969–974.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kinoshita Y, Inui T and Chiba T:
Calcitonin gene-related peptide: A neurotransmitter involved in
capsaicin-sensitive afferent nerve-mediated gastric mucosal
protection. J Clin Gastroenterol. 17 (Suppl 1):S27–S32. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hayashi H, Ohno T, Nishiyama K, Boku K,
Katori M and Majima M: Transient prevention of ethanol-induced
gastric lesion by capsaicin due to release of endogenous calcitonin
gene-related peptide in rats. Jpn J Pharmacol. 86:351–354. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ohno T, Hattori Y, Komine R, Ae T,
Mizuguchi S, Arai K, Saeki T, Suzuki T, Hosono K, Hayashi I, et al:
Roles of calcitonin gene-related peptide in maintenance of gastric
mucosal integrity and in enhancement of ulcer healing and
angiogenesis. Gastroenterology. 134:215–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Shimozawa N, Okajima K, Harada N, Arai M,
Ishida Y, Shimada S, Kurihara H and Nakagata N: Contribution of
sensory neurons to sex difference in the development of
stress-induced gastric mucosal injury in mice. Gastroenterology.
131:1826–1834. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhao Z, Gong S, Wang S and Ma C: Effect
and mechanism of evodiamine against ethanol-induced gastric ulcer
in mice by suppressing Rho/NF-κB pathway. Int Immunopharmacol.
28:588–595. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li NS, Luo XJ, Dai Z, Liu B, Zhang YS,
Yang ZC and Peng J: Beneficial effects of capsiate on
ethanol-induced mucosal injury in rats are related to stimulation
of calcitonin gene-related Peptide release. Planta Med. 78:24–30.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Luo XJ, Li NS, Zhang YS, Liu B, Yang ZC,
Li YJ, Dong XR and Peng J: Vanillyl nonanoate protects rat gastric
mucosa from ethanol-induced injury through a mechanism involving
calcitonin gene-related peptide. Eur J Pharmacol. 666:211–217.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu YZ, Zhou Y, Li D, Wang L, Hu GY, Peng
J and Li YJ: Reduction of asymmetric dimethylarginine in the
protective effects of rutaecarpine on gastric mucosal injury. Can J
Physiol Pharmacol. 86:675–681. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Luo XJ, Peng J and Li YJ: Recent advances
in the study on capsaicinoids and capsinoids. Eur J Pharmacol.
650:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Czekaj R, Majka J, Ptak-Belowska A,
Szlachcic A, Targosz A, Magierowska K, Strzalka M, Magierowski M
and Brzozowski T: Role of curcumin in protection of gastric mucosa
against stress-induced gastric mucosal damage. Involvement of
hypoacidity, vasoactive mediators and sensory neuropeptides. J
Physiol Pharmacol. 67:261–275. 2016.PubMed/NCBI
|
|
82
|
Czekaj R, Majka J, Magierowska K,
Sliwowski Z, Magierowski M, Pajdo R, Ptak-Belowska A, Surmiak M,
Kwiecien S and Brzozowski T: Mechanisms of curcumin-induced
gastroprotection against ethanol-induced gastric mucosal lesions. J
Gastroenterol. 53:618–630. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Luo DN, Li FJ and Zou YY: Therapeutic
effects of rutaecarpine on dextran sodium sulfate-induced
experimental colitis in mice. Zhonghua Yi Xue Za Zhi. 98:533–538.
2018.(In Chinese). PubMed/NCBI
|
|
84
|
Satyanarayana MN: Capsaicin and gastric
ulcers. Crit Rev Food Sci Nutr. 46:275–328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yan L, Li QF, Rong YT, Chen YH, Huang ZH,
Wang ZZ and Peng J: The protective effects of rutaecarpine on acute
pancreatitis. Oncol Lett. 15:3121–3126. 2018.PubMed/NCBI
|
|
86
|
Hu R, Li YJ and Li XH: An overview of
non-neural sources of calcitonin gene-related peptide. Curr Med
Chem. 23:763–773. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Luo D, Zhang YW, Peng WJ, Peng J, Chen QQ,
Li D, Deng HW and Li YJ: Transient receptor potential vanilloid
1-mediated expression and secretion of endothelial cell-derived
calcitonin gene-related peptide. Regul Pept. 150:66–72. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li W, Zhang Z, Li X, Cai J, Li D, Du J,
Zhang B, Xiang D, Li N and Li Y: CGRP derived from cardiac
fibroblasts is an endogenous suppressor of cardiac fibrosis.
Cardiovasc Res. 116:1335–1348. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhang YM, Peng J, Hu CP, Jiang QT, Jiang
GL and Li YJ: Clonidine induces calcitonin gene-related peptide
expression via nitric oxide pathway in endothelial cells. Peptides.
30:1746–1752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhou Z, Peng J, Wang CJ, Li D, Li TT, Hu
CP, Chen XP and Li YJ: Accelerated senescence of endothelial
progenitor cells in hypertension is related to the reduction of
calcitonin gene-related peptide. J Hypertens. 28:931–939. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Randhawa PK and Jaggi AS: TRPV1 channels
in cardiovascular system: A double edged sword? Int J Cardiol.
228:103–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Eberhardt M, Dux M, Namer B, Miljkovic J,
Cordasic N, Will C, Kichko TI, de la Roche J, Fischer M, Suárez SA,
et al: H2S and NO cooperatively regulate vascular tone by
activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat
Commun. 5:43812014. View Article : Google Scholar : PubMed/NCBI
|