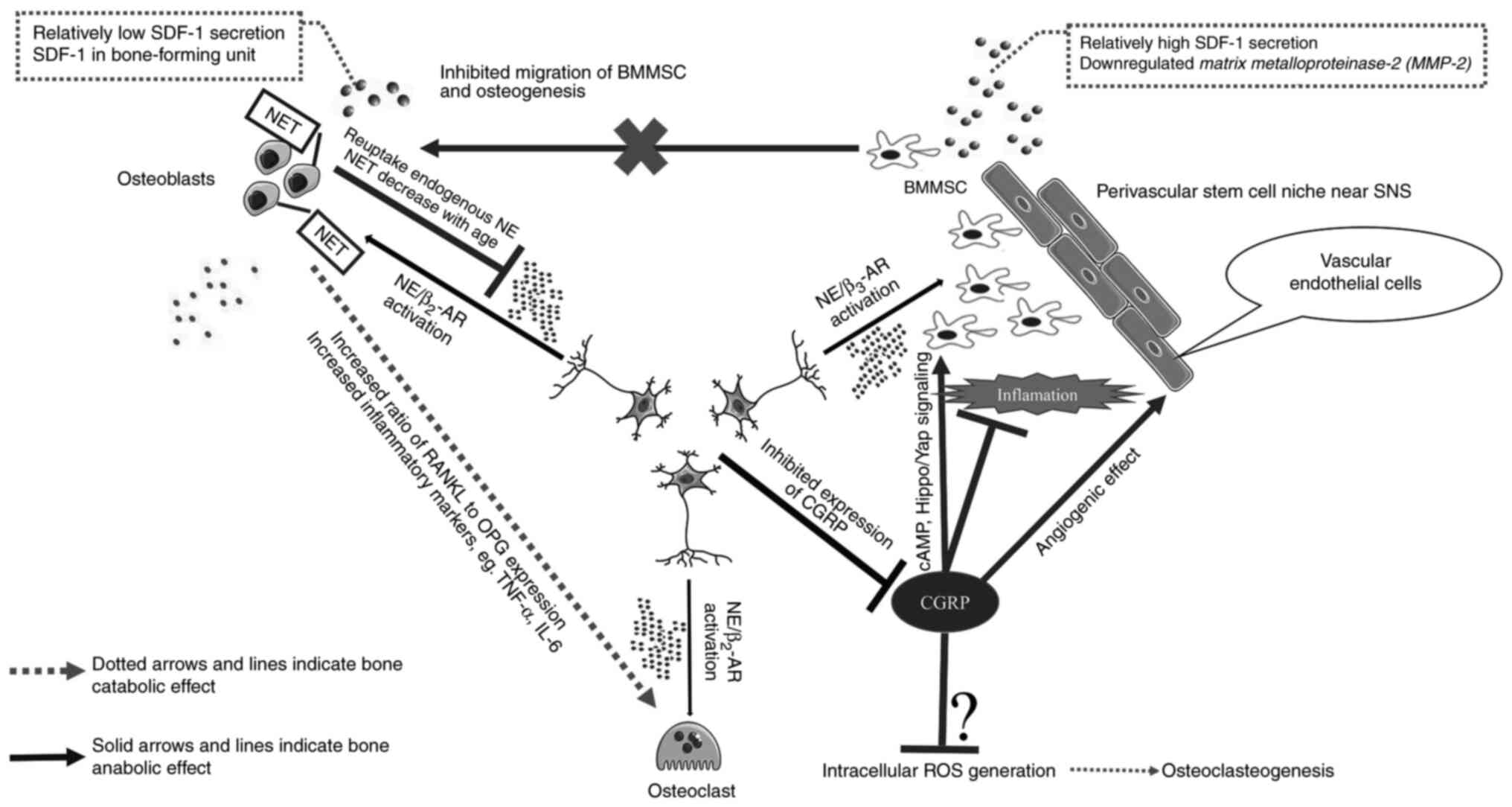
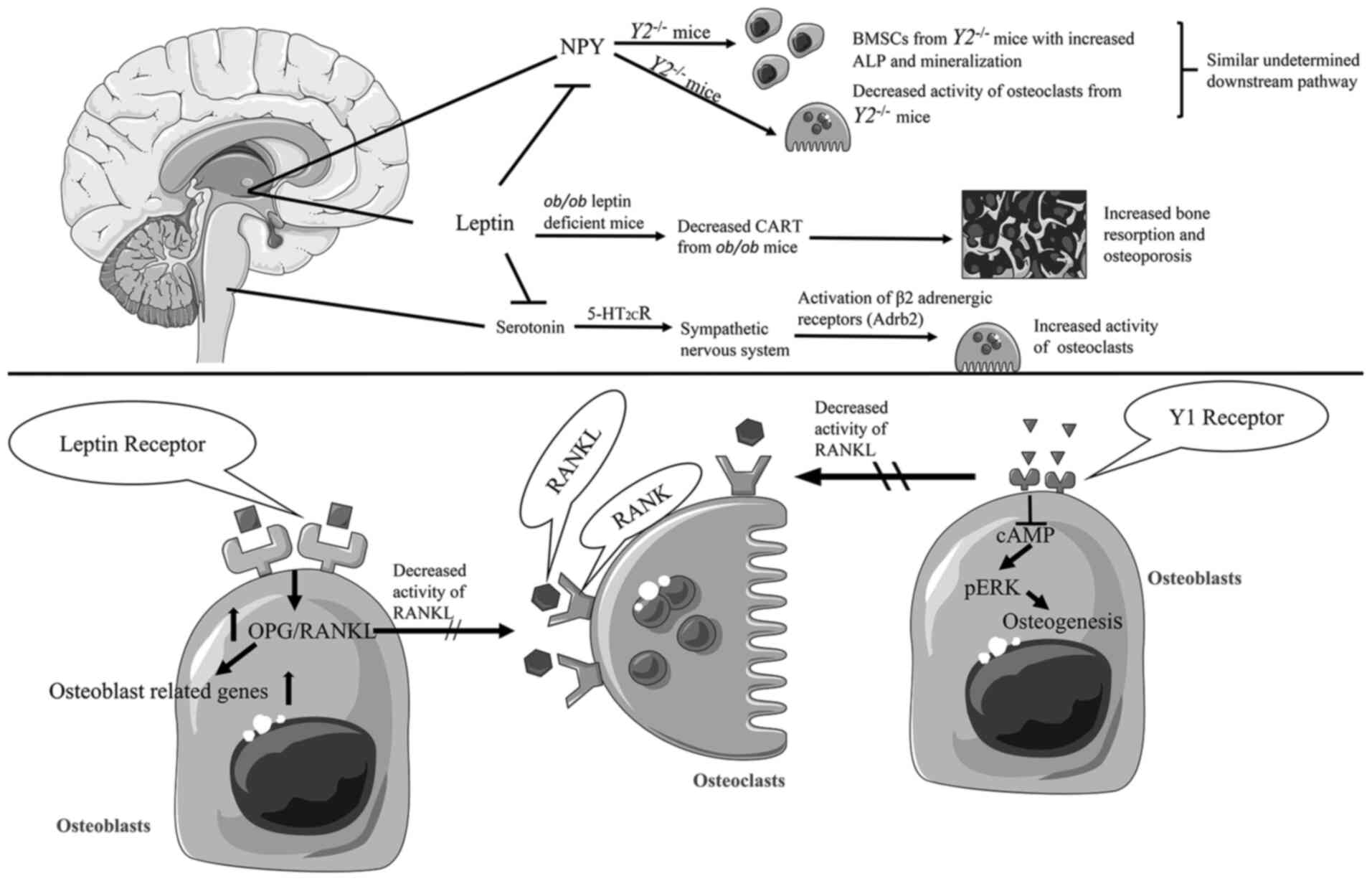
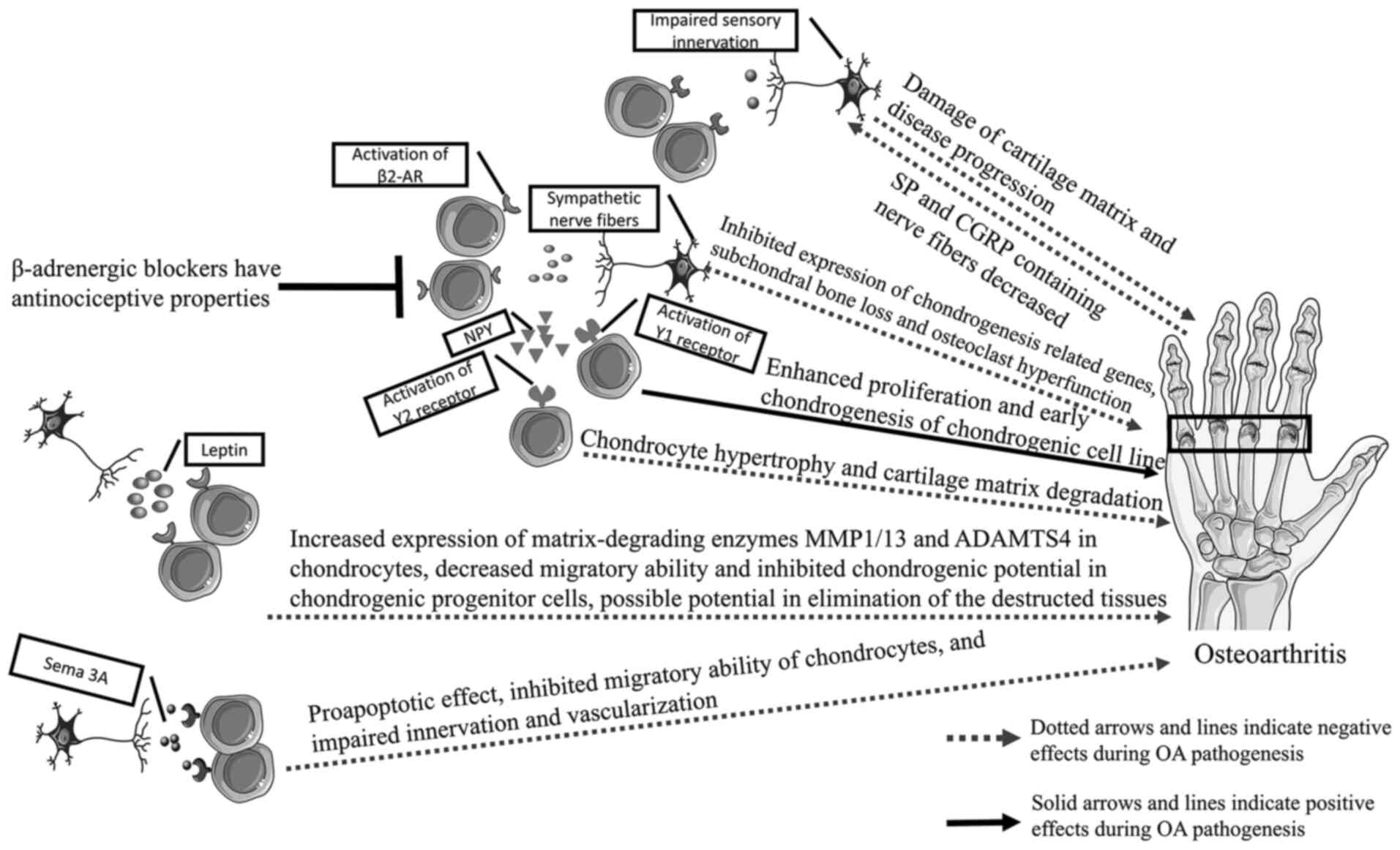
|
1
|
Florencio-Silva R, Sasso GR, Sasso-Cerri
E, Simões MJ and Cerri PS: Biology of bone tissue: Structure,
function, and factors that influence bone cells. Biomed Res Int.
2015:4217462015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mertens C, Decker C, Engel M, Sander A,
Hoffmann J and Freier K: Early bone resorption of free
microvascular reanastomized bone grafts for mandibular
reconstruction-a comparison of iliac crest and fibula grafts. J
Craniomaxillofac Surg. 42:e217–e223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan JJ, Mu TW, Qin JJ, Bi L and Pei GX:
Different effects of implanting sensory nerve or blood vessel on
the vascularization, neurotization, and osteogenesis of
tissue-engineered bone in vivo. Biomed Res Int. 2014:4125702014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang L, Wei JH, Yang X, Yang ZH, Sun MY,
Cheng XB, Xu LQ, Lei DL and Zhang CP: Preventing early-stage graft
bone resorption by simultaneous innervation: Innervated iliac bone
flap for mandibular reconstruction. Plast Reconstr Surg.
139:e1152–e1161. 2017. View Article : Google Scholar
|
|
5
|
Brady RD, Grills BL, Church JE, Walsh NC,
McDonald AC, Agoston DV, Sun M, O'Brien TJ, Shultz SR and McDonald
SJ: Closed head experimental traumatic brain injury increases size
and bone volume of callus in mice with concomitant tibial fracture.
Sci Rep. 6:344912016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martin CD, Jimenez-Andrade JM, Ghilardi JR
and Mantyh PW: Organization of a unique net-like meshwork of CGRP+
sensory fibers in the mouse periosteum: Implications for the
generation and maintenance of bone fracture pain. Neurosci Lett.
427:148–152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsuo K, Ji S, Miya A, Yoda M, Hamada Y,
Tanaka T, Takao-Kawabata R, Kawaai K, Kuroda Y and Shibata S:
Innervation of the tibial epiphysis through the intercondylar
foramen. Bone. 120:297–304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niedermair T, Kuhn V, Doranehgard F,
Stange R, Wieskötter B, Beckmann J, Salmen P, Springorum HR, Straub
RH, Zimmer A, et al: Absence of substance P and the sympathetic
nervous system impact on bone structure and chondrocyte
differentiation in an adult model of endochondral ossification.
Matrix Biol. 38:22–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horsnell H and Baldock PA: Osteoblastic
actions of the neuropeptide Y system to regulate bone and energy
homeostasis. Curr Osteop Rep. 14:26–31. 2016. View Article : Google Scholar
|
|
10
|
Zheng XF, Zhao ED, He JY, Zhang YH, Jiang
SD and Jiang LS: Inhibition of substance P signaling aggravates the
bone loss in ovariectomy-induced osteoporosis. Prog Biophys Mol
Biol. 122:112–121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang T, Guo Y, Yuan Y, Xin N, Zhang Q, Guo
Q and Gong P: Deficiency of α Calcitonin-gene-related peptide
impairs peri-implant angiogenesis and osseointegration via
suppressive vasodilative activity. Biochem Biophys Res Commun.
498:139–145. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu S, Mei G, Wang Z, Zou ZL, Liu S, Pei
GX, Bi L and Jin D: Neuropeptide substance P improves osteoblastic
and angiogenic differentiation capacity of bone marrow stem cells
in vitro. Biomed Res Int. 2014:5960232014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Benschop RJ, Collins EC, Darling RJ, Allan
BW, Leung D, Conner EM, Nelson J, Gaynor B, Xu J, Wang XF, et al:
Development of a novel antibody to calcitonin gene-related peptide
for the treatment of osteoarthritis-related pain. Osteoarthritis
Cartilage. 22:578–585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duan JX, Zhou Y, Zhou AY, Guan XX, Liu T,
Yang HH, Xie H and Chen P: Calcitonin gene-related peptide exerts
anti-inflammatory property through regulating murine macrophages
polarization in vitro. Mol Immunol. 91:105–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lim JE, Chung E and Son Y: A neuropeptide,
Substance-P, directly induces tissue-repairing M2 like macrophages
by activating the PI3K/Akt/mTOR pathway even in the presence of
IFNγ. Sci Rep. 7:94172017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiao K, Niu LN, Li QH, Ren GT, Zhao CM,
Liu YD, Tay FR and Wang MQ: β2-Adrenergic signal transduction plays
a detrimental role in subchondral bone loss of temporomandibular
joint in osteoarthritis. Sci Rep. 5:125932015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen R, Hao Z, Chen X, Fu Q and Ma Y:
Neuropeptide Y enhances proliferation and chondrogenic
differentiation of ATDC5 cells. Neuropeptides. 80:1020222020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu W, Zhu C, Xu W, Jiang L and Jiang S:
Neuropeptide Y1 receptor regulates glucocorticoid-induced
inhibition of osteoblast differentiation in murine MC3T3-E1 cells
via ERK signaling. Int J Mol Sci. 17:21502016. View Article : Google Scholar
|
|
19
|
Kondo H, Takeuchi S and Togari A:
β-Adrenergic signaling stimulates osteoclastogenesis via reactive
oxygen species. Am J Physiol Endocrinol Metab. 304:E507–E515. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukuda T, Takeda S, Xu R, Ochi H, Sunamura
S, Sato T, Shibata S, Yoshida Y, Gu Z, Kimura A, et al: Sema3A
regulates bone-mass accrual through sensory innervations. Nature.
497:490–493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okubo M, Kimura T, Fujita Y, Mochizuki S,
Niki Y, Enomoto H, Suda Y, Toyama Y and Okada Y: Semaphorin 3A is
expressed in human osteoarthritic cartilage and antagonizes
vascular endothelial growth factor 165-promoted chondrocyte
migration: An implication for chondrocyte cloning. Arthritis Rheum.
63:3000–3009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baldock PA, Lee NJ, Driessler F, Lin S,
Allison S, Stehrer B, Lin EJ, Zhang L, Enriquez RF, Wong IP, et al:
Neuropeptide Y knockout mice reveal a central role of NPY in the
coordination of bone mass to body weight. PLoS One. 4:e84152009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baldock PA, Allison S, McDonald MM,
Sainsbury A, Enriquez RF, Little DG, Eisman JA, Gardiner EM and
Herzog H: Hypothalamic regulation of cortical bone mass: Opposing
activity of Y2 receptor and leptin pathways. J Bone Miner Res.
21:1600–1607. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong IP, Nguyen AD, Khor EC, Enriquez RF,
Eisman JA, Sainsbury A, Herzog H and Baldock PA: Neuropeptide Y is
a critical modulator of leptin's regulation of cortical bone. J
Bone Miner Res. 28:886–898. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Johnston AP, Yuzwa SA, Carr MJ, Mahmud N,
Storer MA, Krause MP, Jones K, Paul S, Kaplan DR and Miller FD:
Dedifferentiated schwann cell precursors Secreting paracrine
factors are required for regeneration of the mammalian digit tip.
Cell Stem Cell. 19:433–448. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jones RE, Salhotra A, Robertson KS, Ransom
RC, Foster DS, Shah HN, Quarto N, Wan DC and Longaker MT: Skeletal
stem cell-schwann cell circuitry in mandibular repair. Cell Rep.
28:2757–2766.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cai XX, Luo E and Yuan Q: Interaction
between Schwann cells and osteoblasts in vitro. Int J Oral Sci.
2:74–81. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Castañeda-Corral G, Jimenez-Andrade JM,
Bloom AP, Taylor RN, Mantyh WG, Kaczmarska MJ, Ghilardi JR and
Mantyh PW: The majority of myelinated and unmyelinated sensory
nerve fibers that innervate bone express the tropomyosin receptor
kinase A. Neuroscience. 178:196–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu ZX, Barker JS, Batchelor TP and Dey RD:
Interleukin (IL)-1 regulates ozone-enhanced tracheal smooth muscle
responsiveness by increasing substance P (SP) production in
intrinsic airway neurons of ferret. Respir Physiol Neurobiol.
164:300–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li FX, Xu F, Lin X, Wu F, Zhong JY, Wang
Y, Guo B, Zheng MH, Shan SK and Yuan LQ: The role of substance P in
the regulation of bone and cartilage metabolic activity. Front
Endocrinol (Lausanne). 11:772020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Opolka A, Straub RH, Pasoldt A, Grifka J
and Grässel S: Substance P and norepinephrine modulate murine
chondrocyte proliferation and apoptosis. Arthritis Rheum.
64:729–739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oliva F, Tarantino U and Maffulli N:
Immunohistochemical localization of calcitonin gene-related peptide
and substance P in the rat knee cartilage at birth. Physiol Res.
54:549–556. 2005.PubMed/NCBI
|
|
33
|
Wang L, Zhao R, Shi X, Wei T, Halloran BP,
Clark DJ, Jacobs CR and Kingery WS: Substance P stimulates bone
marrow stromal cell osteogenic activity, osteoclast
differentiation, and resorption activity in vitro. Bone.
45:309–320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mei G, Zou Z, Fu S, Xia L, Zhou J, Zhang
Y, Tuo Y, Wang Z and Jin D: Substance P activates the Wnt signal
transduction pathway and enhances the differentiation of mouse
preosteoblastic MC3T3-E1 cells. Int J Mol Sci. 15:6224–6240. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tuzmen C and Campbell PG: Crosstalk
between neuropeptides SP and CGRP in regulation of BMP2-induced
bone differentiation. Connect Tissue Res. 59 (Supp1):S81–S90. 2018.
View Article : Google Scholar
|
|
36
|
Matsui S, Yamane T, Kobayashi-Hattori K
and Oishi Y: Calcitonin gene-related peptide regulates
mitogen-activated protein kinase pathway to decrease transforming
growth factor β1-induced hepatic plasminogen activator inhibitor-1
mRNA expression in HepG2 cells. Biosci Biotechnol Biochem.
78:787–790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Goto T, Nakao K, Gunjigake KK, Kido MA,
Kobayashi S and Tanaka T: Substance P stimulates late-stage rat
osteoblastic bone formation through neurokinin-1 receptors.
Neuropeptides. 41:25–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hong HS, Lee J, Lee E, Kwon YS, Lee E, Ahn
W, Jiang MH, Kim JC and Son Y: A new role of substance P as an
injury-inducible messenger for mobilization of CD29(+) stromal-like
cells. Nat Med. 15:425–435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Um J, Jung N, Chin S, Cho Y, Choi S and
Park KS: Substance P enhances EPC mobilization for accelerated
wound healing. Wound Repair Regen. 24:402–410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dubon MJ and Park KS: The mechanisms of
substance P-mediated migration of bone marrow-derived mesenchymal
stem cell-like ST2 cells. Int J Mol Med. 37:1105–1111. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sohn SJ: Substance P upregulates
osteoclastogenesis by activating nuclear factor kappa B in
osteoclast precursors. Acta Otolaryngol. 125:130–133. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fukuda A, Goto T, Kuroishi KN, Gunjigake
KK, Kataoka S, Kobayashi S and Yamaguchi K: Hemokinin-1
competitively inhibits substance P-induced stimulation of
osteoclast formation and function. Neuropeptides. 47:251–259. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sang X, Wang Z, Shi P, Li Y and Cheng L:
CGRP accelerates the pathogenesis of neurological heterotopic
ossification following spinal cord injury. Artif Cells Nanomed
Biotechnol. 47:2569–2574. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang L, Shi X, Zhao R, Halloran BP, Clark
DJ, Jacobs CR and Kingery WS: Calcitonin-gene-related peptide
stimulates stromal cell osteogenic differentiation and inhibits
RANKL induced NF-kappaB activation, osteoclastogenesis and bone
resorption. Bone. 46:1369–1379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
He H, Chai J, Zhang S, Ding L, Yan P, Du W
and Yang Z: CGRP may regulate bone metabolism through stimulating
osteoblast differentiation and inhibiting osteoclast formation. Mol
Med Rep. 13:3977–3984. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang Q, Guo Y, Yu H, Tang Y, Yuan Y,
Jiang Y, Chen H, Gong P and Xiang L: Receptor activity-modifying
protein 1 regulates the phenotypic expression of BMSCs via the
Hippo/Yap pathway. J Cell Physiol. 234:13969–13976. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mishima T, Ito Y, Hosono K, Tamura Y,
Uchida Y, Hirata M, Suzsuki T, Amano H, Kato S, Kurihara Y, et al:
Calcitonin gene-related peptide facilitates revascularization
during hindlimb ischemia in mice. Am J Physiol Heart Circ Physiol.
300:H431–H439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang S, Li J, Jiang H, Gao Y, Cheng P,
Cao T, Li D, Wang J, Song Y, Liu B, et al: Dorsal root ganglion
maintains stemness of bone marrow mesenchymal stem cells by
enhancing autophagy through the AMPK/mTOR pathway in a coculture
system. Stem Cells Int. 2018:84789532018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhou Z, Hu CP, Wang CJ, Li TT, Peng J and
Li YJ: Calcitonin gene-related peptide inhibits angiotensin
II-induced endothelial progenitor cells senescence through
up-regulation of klotho expression. Atherosclerosis. 213:92–101.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Blackwell KA, Raisz LG and Pilbeam CC:
Prostaglandins in bone: Bad cop, good cop? Trends Endocrinol Metab.
21:294–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Guo C, Yuan L, Wang JG, Wang F, Yang XK,
Zhang FH, Song JL, Ma XY, Cheng Q and Song GH: Lipopolysaccharide
(LPS) induces the apoptosis and inhibits osteoblast differentiation
through JNK pathway in MC3T3-E1 cells. Inflammation. 37:621–631.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou Y, Zhang H, Zhang G, He Y, Zhang P,
Sun Z, Gao Y and Tan Y: Calcitonin generelated peptide reduces
Porphyromonas gingivalis LPSinduced TNFα release and apoptosis in
osteoblasts. Mol Med Rep. 17:3246–3254. 2018.PubMed/NCBI
|
|
53
|
Loi F, Córdova LA, Zhang R, Pajarinen J,
Lin TH, Goodman SB and Yao Z: The effects of immunomodulation by
macrophage subsets on osteogenesis in vitro. Stem Cell Res Ther.
7:152016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hong HS and Son Y: Substance P ameliorates
collagen II-induced arthritis in mice via suppression of the
inflammatory response. Biochem Biophys Res Commun. 453:179–184.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu X, Liu H, Xiong Y, Yang L, Wang C,
Zhang R and Zhu X: Postmenopausal osteoporosis is associated with
the regulation of SP, CGRP, VIP, and NPY. Biomed Pharmacother.
104:742–750. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Webster JM, Fenton CG, Langen R and Hardy
RS: Exploring the interface between inflammatory and therapeutic
glucocorticoid induced bone and muscle loss. Int J Mol Sci.
20:57682019. View Article : Google Scholar
|
|
57
|
Chen H, Hu B, Lv X, Zhu S, Zhen G, Wan M,
Jain A, Gao B, Chai Y, Yang M, et al: Prostaglandin E2 mediates
sensory nerve regulation of bone homeostasis. Nat Commun.
10:1812019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Grässel SG: The role of peripheral nerve
fibers and their neurotransmitters in cartilage and bone physiology
and pathophysiology. Arthritis Res Ther. 16:4852014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jikko A, Murakami H, Yan W, Nakashima K,
Ohya Y, Satakeda H, Noshiro M, Kawamoto T, Nakamura S, Okada Y, et
al: Effects of cyclic adenosine 3′,5′-monophosphate on chondrocyte
terminal differentiation and cartilage-matrix calcification.
Endocrinology. 137:122–128. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Buma P, Verschuren C, Versleyen D, Van der
Kraan P and Oestreicher AB: Calcitonin gene-related peptide,
substance P and GAP-43/B-50 immunoreactivity in the normal and
arthrotic knee joint of the mouse. Histochemistry. 98:327–339.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Salo PT, Hogervorst T, Seerattan RA,
Rucker D and Bray RC: Selective joint denervation promotes knee
osteoarthritis in the aging rat. J Orthop Res. 20:1256–1264. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Eitner A, Pester J, Nietzsche S, Hofmann
GO and Schaible HG: The innervation of synovium of human
osteoarthritic joints in comparison with normal rat and sheep
synovium. Osteoarthritis Cartilage. 21:1383–1391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Howard MR, Millward-Sadler SJ, Vasilliou
AS, Salter DM and Quinn JP: Mechanical stimulation induces
preprotachykinin gene expression in osteoarthritic chondrocytes
which is correlated with modulation of the transcription factor
neuron restrictive silence factor. Neuropeptides. 42:681–686. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Muschter D, Fleischhauer L, Taheri S,
Schilling AF, Clausen-Schaumann H and Grässel S: Sensory
neuropeptides are required for bone and cartilage homeostasis in a
murine destabilization-induced osteoarthritis model. Bone.
133:1151812020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kim SJ, Kim JE, Kim SH, Kim SJ, Jeon SJ,
Kim SH and Jung Y: Therapeutic effects of neuropeptide substance P
coupled with self-assembled peptide nanofibers on the progression
of osteoarthritis in a rat model. Biomaterials. 74:119–130. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Nishiura T and Abe K: Alpha1-adrenergic
receptor stimulation induces the expression of receptor activator
of nuclear factor kappaB ligand gene via protein kinase C and
extracellular signal-regulated kinase pathways in MC3T3-E1
osteoblast-like cells. Arch Oral Biol. 52:778–785. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhu Y, Ma Y and Elefteriou F: Cortical
bone is an extraneuronal site of norepinephrine uptake in adult
mice. Bone Rep. 9:188–198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Mitchell J, Lai LP, Peralta F, Xu Y and
Sugamori K: β2-adrenergic receptors inhibit the expression of
collagen type II in growth plate chondrocytes by stimulating the
AP-1 factor Jun-B. Am J Physiol Endocrinol Metab. 300:E633–E639.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liang H, Zeng Y, Feng Y, Wu H, Gong P and
Yao Q: Selective β2-adrenoreceptor signaling regulates
osteoclastogenesis via modulating RANKL production and
neuropeptides expression in osteocytic MLO-Y4 cells. J Cell
Biochem. Nov 1–2018.doi: 10.1002/jcb.27998. Online ahead of
print.
|
|
70
|
Fonseca TL, Jorgetti V, Costa CC, Capelo
LP, Covarrubias AE, Moulatlet AC, Teixeira MB, Hesse E, Morethson
P, Beber EH, et al: Double disruption of α2A- and α2C-adrenoceptors
results in sympathetic hyperactivity and high-bone-mass phenotype.
J Bone Miner Res. 26:591–603. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Grässel S and Muschter D: Peripheral nerve
fibers and their neurotransmitters in osteoarthritis pathology. Int
J Mol Sci. 18:9312017. View Article : Google Scholar
|
|
72
|
Hamrick MW and Ferrari SL: Leptin and the
sympathetic connection of fat to bone. Osteoporos Int. 19:905–912.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Pierroz DD, Bonnet N, Bianchi EN, Bouxsein
ML, Baldock PA, Rizzoli R and Ferrari SL: Deletion of β-adrenergic
receptor 1, 2, or both leads to different bone phenotypes and
response to mechanical stimulation. J Bone Miner Res. 27:1252–1262.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Khosla S, Drake MT, Volkman TL, Thicke BS,
Achenbach SJ, Atkinson EJ, Joyner MJ, Rosen CJ, Monroe DG and Farr
JN: Sympathetic β1-adrenergic signaling contributes to regulation
of human bone metabolism. J Clin Invest. 128:4832–4842. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Arai M, Sato T, Takeuchi S, Goto S and
Togari A: Dose effects of butoxamine, a selective β2-adrenoceptor
antagonist, on bone metabolism in spontaneously hypertensive rat.
Eur J Pharmacol. 701:7–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Farr JN, Charkoudian N, Barnes JN, Monroe
DG, McCready LK, Atkinson EJ, Amin S, Melton LJ III, Joyner MJ and
Khosla S: Relationship of sympathetic activity to bone
microstructure, turnover, and plasma osteopontin levels in women. J
Clin Endocrinol Metab. 97:4219–4227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ma Y, Krueger JJ, Redmon SN, Uppuganti S,
Nyman JS, Hahn MK and Elefteriou F: Extracellular norepinephrine
clearance by the norepinephrine transporter is required for
skeletal homeostasis. J Biol Chem. 288:30105–30113. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Bajayo A, Bar A, Denes A, Bachar M, Kram
V, Attar-Namdar M, Zallone A, Kovács KJ, Yirmiya R and Bab I:
Skeletal parasympathetic innervation communicates central IL-1
signals regulating bone mass accrual. Proc Natl Acad Sci USA.
109:15455–15460. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Du Z, Wang L, Zhao Y, Cao J, Wang T, Liu
P, Zhang Y, Yang X, Cheng X, Liu B and Lei D: Sympathetic
denervation-induced MSC mobilization in distraction osteogenesis
associates with inhibition of MSC migration and osteogenesis by
norepinephrine/adrb3. PLoS One. 9:e1059762014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Méndez-Ferrer S, Michurina TV, Ferraro F,
Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A,
Enikolopov GN and Frenette PS: Mesenchymal and haematopoietic stem
cells form a unique bone marrow niche. Nature. 466:829–834. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wu B, Wang L, Yang X, Mao M, Ye C, Liu P,
Yang Z, Yang X, Lei D and Zhang C: Norepinephrine inhibits
mesenchymal stem cell chemotaxis migration by increasing stromal
cell-derived factor-1 secretion by vascular endothelial cells via
NE/abrd3/JNK pathway. Exp Cell Res. 349:214–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Matic I, Matthews BG, Kizivat T, Igwe JC,
Marijanovic I, Ruohonen ST, Savontaus E, Adams DJ and Kalajzic I:
Bone-specific overexpression of NPY modulates osteogenesis. J
Musculoskelet Neuronal Interact. 12:209–218. 2012.PubMed/NCBI
|
|
83
|
Parker RM and Herzog H: Regional
distribution of Y-receptor subtype mRNAs in rat brain. Eur J
Neurosci. 11:1431–1448. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lee NJ, Doyle KL, Sainsbury A, Enriquez
RF, Hort YJ, Riepler SJ, Baldock PA and Herzog H: Critical role for
Y1 receptors in mesenchymal progenitor cell differentiation and
osteoblast activity. J Bone Miner Res. 25:1736–1747. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Driessler F and Baldock PA: Hypothalamic
regulation of bone. J Mol Endocrinol. 45:175–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Sousa DM, Baldock PA, Enriquez RF, Zhang
L, Sainsbury A, Lamghari M and Herzog H: Neuropeptide Y Y1 receptor
antagonism increases bone mass in mice. Bone. 51:8–16. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Baldock PA, Allison SJ, Lundberg P, Lee
NJ, Slack K, Lin EJ, Enriquez RF, McDonald MM, Zhang L, During MJ,
et al: Novel role of Y1 receptors in the coordinated regulation of
bone and energy homeostasis. J Biol Chem. 282:19092–19102. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lee NJ, Nguyen AD, Enriquez RF, Doyle KL,
Sainsbury A, Baldock PA and Herzog H: Osteoblast specific Y1
receptor deletion enhances bone mass. Bone. 48:461–467. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lundberg P, Allison SJ, Lee NJ, Baldock
PA, Brouard N, Rost S, Enriquez RF, Sainsbury A, Lamghari M,
Simmons P, et al: Greater bone formation of Y2 knockout mice is
associated with increased osteoprogenitor numbers and altered Y1
receptor expression. J Biol Chem. 282:19082–19091. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Shi YC, Lin S, Castillo L, Aljanova A,
Enriquez RF, Nguyen AD, Baldock PA, Zhang L, Bijker MS, Macia L, et
al: Peripheral-specific y2 receptor knockdown protects mice from
high-fat diet-induced obesity. Obesity (Silver Spring).
19:2137–2148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Khor EC, Yulyaningsih E, Driessler F,
Kovaĉić N, Wee NKY, Kulkarni RN, Lee NJ, Enriquez RF, Xu J, Zhang
L, et al: The y6 receptor suppresses bone resorption and stimulates
bone formation in mice via a suprachiasmatic nucleus relay. Bone.
84:139–147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Jiang ZQ, Zhou YL, Chen X, Li LY, Liang
SY, Lin S and Shu MQ: Different effects of neuropeptide Y on
proliferation of vascular smooth muscle cells via regulation of
Geminin. Mol Cell Biochem. 433:205–211. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Alasvand M, Rashidi B, Javanmard SH,
Akhavan MM and Khazaei M: Effect of blocking of neuropeptide Y Y2
receptor on tumor angiogenesis and progression in normal and
diet-induced obese C57BL/6 mice. Glob J Health Sci. 7:69–78. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Fetissov SO, Xu ZQ, Byrne LC, Hassani H,
Ernfors P and Hökfelt T: Neuropeptide y targets in the
hypothalamus: Nitric oxide synthesizing neurones express Y1
receptor. J Neuroendocrinol. 15:754–760. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lai LP and Mitchell J: Beta2-adrenergic
receptors expressed on murine chondrocytes stimulate cellular
growth and inhibit the expression of Indian hedgehog and collagen
type X. J Cell Biochem. 104:545–553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Martin LJ, Piltonen MH, Gauthier J,
Convertino M, Acland EL, Dokholyan NV, Mogil JS, Diatchenko L and
Maixner W: Differences in the antinociceptive effects and binding
properties of propranolol and bupranolol enantiomers. J Pain.
16:1321–1333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Light KC, Bragdon EE, Grewen KM, Brownley
KA, Girdler SS and Maixner W: Adrenergic dysregulation and pain
with and without acute beta-blockade in women with fibromyalgia and
temporomandibular disorder. J Pain. 10:542–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Valdes AM, Abhishek A, Muir K, Zhang W,
Maciewicz RA and Doherty M: Association of beta-blocker use with
less prevalent joint pain and lower opioid requirement in people
with osteoarthritis. Arthritis Care Res (Hoboken). 69:1076–1081.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kang X, Qian Z, Liu J, Feng D, Li H, Zhang
Z, Jin X, Ma Z, Xu M, Li F, et al: Neuropeptide Y acts directly on
cartilage homeostasis and exacerbates progression of osteoarthritis
through NPY2R. J Bone Miner Res. 35:1375–1384. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang L, Zhang L, Pan H, Peng S, Lv M and
Lu WW: Levels of neuropeptide Y in synovial fluid relate to pain in
patients with knee osteoarthritis. BMC Musculoskelet Disord.
15:3192014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Kuo LE, Kitlinska JB, Tilan JU, Li L,
Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R,
et al: Neuropeptide Y acts directly in the periphery on fat tissue
and mediates stress-induced obesity and metabolic syndrome. Nat
Med. 13:803–811. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
102
|
Worzfeld T and Offermanns S: Semaphorins
and plexins as therapeutic targets. Nat Rev Drug Discov.
13:603–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Gomez C, Burt-Pichat B, Mallein-Gerin F,
Merle B, Delmas PD, Skerry TM, Vico L, Malaval L and Chenu C:
Expression of Semaphorin-3A and its receptors in endochondral
ossification: Potential role in skeletal development and
innervation. Dev Dyn. 234:393–403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Li Y, Yang L, He S and Hu J: The effect of
semaphorin 3A on fracture healing in osteoporotic rats. J Orthop
Sci. 20:1114–1121. 2015.PubMed/NCBI
|
|
105
|
Hayashi M, Nakashima T, Taniguchi M,
Kodama T, Kumanogoh A and Takayanagi H: Osteoprotection by
semaphorin 3A. Nature. 485:69–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kular J, Tickner J, Chim SM and Xu J: An
overview of the regulation of bone remodelling at the cellular
level. Clin Biochem. 45:863–873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Sun J, Wei X, Wang Z, Liu Y, Lu J, Lu Y,
Cui M, Zhang X and Li F: Inflammatory milieu cultivated Sema3A
signaling promotes chondrocyte apoptosis in knee osteoarthritis. J
Cell Biochem. 119:2891–2899. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Dénes A, Boldogkoi Z, Uhereczky G, Hornyák
A, Rusvai M, Palkovits M and Kovács KJ: Central autonomic control
of the bone marrow: Multisynaptic tract tracing by recombinant
pseudorabies virus. Neuroscience. 134:947–963. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Sharan K and Yadav VK: Hypothalamic
control of bone metabolism. Best Pract Res Clin Endocrinol Metab.
28:713–723. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Spiegelman BM and Flier JS: Obesity and
the regulation of energy balance. Cell. 104:531–543. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Camerino C, Zayzafoon M, Rymaszewski M,
Heiny J, Rios M and Hauschka PV: Central depletion of brain-derived
neurotrophic factor in mice results in high bone mass and metabolic
phenotype. Endocrinology. 153:5394–5405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Elefteriou F, Ahn JD, Takeda S, Starbuck
M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, et al:
Leptin regulation of bone resorption by the sympathetic nervous
system and CART. Nature. 434:514–520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Yadav VK, Oury F, Suda N, Liu ZW, Gao XB,
Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, et
al: A serotonin-dependent mechanism explains the leptin regulation
of bone mass, appetite, and energy expenditure. Cell. 138:976–989.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Mercer JG, Hoggard N, Williams LM,
Lawrence CB, Hannah LT, Morgan PJ and Trayhurn P: Coexpression of
leptin receptor and preproneuropeptide Y mRNA in arcuate nucleus of
mouse hypothalamus. J Neuroendocrinol. 8:733–735. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Hamrick MW, Della-Fera MA, Choi YH,
Pennington C, Hartzell D and Baile CA: Leptin treatment induces
loss of bone marrow adipocytes and increases bone formation in
leptin-deficient ob/ob mice. J Bone Miner Res. 20:994–1001. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Lamghari M, Tavares L, Camboa N and
Barbosa MA: Leptin effect on RANKL and OPG expression in MC3T3-E1
osteoblasts. J Cell Biochem. 98:1123–1129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Turner RT, Kalra SP, Wong CP, Philbrick
KA, Lindenmaier LB, Boghossian S and Iwaniec UT: Peripheral leptin
regulates bone formation. J Bone Miner Res. 28:22–34. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Hamrick MW, Pennington C, Newton D, Xie D
and Isales C: Leptin deficiency produces contrasting phenotypes in
bones of the limb and spine. Bone. 34:376–383. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ealey KN, Fonseca D, Archer MC and Ward
WE: Bone abnormalities in adolescent leptin-deficient mice. Regul
Pept. 136:9–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yu B, Jiang K, Chen B, Wang H, Li X and
Liu Z: Leptin differentially regulates chondrogenesis in mouse
vertebral and tibial growth plates. BMC Musculoskelet Disord.
18:2352017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Yan M, Zhang J, Yang H and Sun Y: The role
of leptin in osteoarthritis. Medicine (Baltimore). 97:e02572018.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Sandell LJ: Obesity and osteoarthritis: Is
leptin the link? Arthritis Rheum. 60:2858–2860. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yaykasli KO, Hatipoglu OF, Yaykasli E,
Yildirim K, Kaya E, Ozsahin M, Uslu M and Gunduz E: Leptin induces
ADAMTS-4, ADAMTS-5, and ADAMTS-9 genes expression by
mitogen-activated protein kinases and NF-ĸB signaling pathways in
human chondrocytes. Cell Biol Int. 39:104–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Su YP, Chen CN, Huang KC, Chang HI, Lee
KC, Lo CM and Chang SF: Leptin induces MMP1/13 and ADAMTS 4
expressions through bone morphogenetic protein-2 autocrine effect
in human chondrocytes. J Cell Biochem. 119:3716–3724. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Chang SF, Hsieh RZ, Huang KC, Chang CA,
Chiu FY, Kuo HC, Chen CN and Su YP: Upregulation of bone
morphogenetic protein-2 synthesis and consequent collagen II
expression in leptin-stimulated human chondrocytes. PLoS One.
10:e01442522015. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Zhao X, Dong Y, Zhang J, Li D, Hu G, Yao
J, Li Y, Huang P, Zhang M, Zhang J, et al: Leptin changes
differentiation fate and induces senescence in chondrogenic
progenitor cells. Cell Death Dis. 7:e21882016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Liu Q, Lei L, Yu T, Jiang T and Kang Y:
Effect of brain-derived neurotrophic factor on the neurogenesis and
osteogenesis in bone engineering. Tissue Eng Part A. 24:1283–1292.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Foudah D, Redondo J, Caldara C, Carini F,
Tredici G and Miloso M: Human mesenchymal stem cells express
neuronal markers after osteogenic and adipogenic differentiation.
Cell Mol Biol Lett. 18:163–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Parfejevs V, Debbache J, Shakhova O,
Schaefer SM, Glausch M, Wegner M, Suter U, Riekstina U, Werner S
and Sommer L: Injury-activated glial cells promote wound healing of
the adult skin in mice. Nat Commun. 9:2362018. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhang Z, Zhang Y, Zhou Z, Shi H, Qiu X,
Xiong J and Chen Y: BDNF regulates the expression and secretion of
VEGF from osteoblasts via the TrkB/ERK1/2 signaling pathway during
fracture healing. Mol Med Rep. 15:1362–1367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Kilian O, Hartmann S, Dongowski N, Karnati
S, Baumgart-Vogt E, Härtel FV, Noll T, Schnettler R and Lips KS:
BDNF and its TrkB receptor in human fracture healing. Ann Anat.
196:286–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Su YW, Chung R, Ruan CS, Chim SM, Kuek V,
Dwivedi PP, Hassanshahi M, Chen KM, Xie Y, Chen L, et al:
Neurotrophin-3 induces BMP-2 and VEGF activities and promotes the
bony repair of injured growth plate cartilage and bone in rats. J
Bone Miner Res. 31:1258–1274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Walsh DA, McWilliams DF, Turley MJ, Dixon
MR, Fransès RE, Mapp PI and Wilson D: Angiogenesis and nerve growth
factor at the osteochondral junction in rheumatoid arthritis and
osteoarthritis. Rheumatology (Oxford). 49:1852–1861. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Takeda K, Shiba H, Mizuno N, Hasegawa N,
Mouri Y, Hirachi A, Yoshino H, Kawaguchi H and Kurihara H:
Brain-derived neurotrophic factor enhances periodontal tissue
regeneration. Tissue Eng. 11:1618–1629. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhang J, Wang L, Cao H, Chen N, Yan B, Ao
X, Zhao H, Chu J, Huang M and Zhang Z: Neurotrophin-3 acts on the
endothelial-mesenchymal transition of heterotopic ossification in
rats. J Cell Mol Med. 23:2595–2609. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Shen L, Zeng W, Wu YX, Hou CL, Chen W,
Yang MC, Li L, Zhang YF and Zhu CH: Neurotrophin-3 accelerates
wound healing in diabetic mice by promoting a paracrine response in
mesenchymal stem cells. Cell Transplant. 22:1011–1021. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Caporali A and Emanueli C: Cardiovascular
actions of neurotrophins. Physiol Rev. 89:279–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Itoyama T, Yoshida S, Tomokiyo A, Hasegawa
D, Hamano S, Sugii H, Ono T, Fujino S and Maeda H: Possible
function of GDNF and Schwann cells in wound healing of periodontal
tissue. J Periodontal Res. Jun 20–2020.doi: 10.1111/jre.12774.
(Online Ahead of Print). View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Yajima S, Lammers CH, Lee SH, Hara Y,
Mizuno K and Mouradian MM: Cloning and characterization of murine
glial cell-derived neurotrophic factor inducible transcription
factor (MGIF). J Neurosci. 17:8657–8666. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Chen Z, Li W, Wang H, Wan C, Luo D, Deng
S, Chen H and Chen S: Klf10 regulates odontoblast differentiation
and mineralization via promoting expression of dentin matrix
protein 1 and dentin sialophosphoprotein genes. Cell Tissue Res.
363:385–398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Subramaniam M, Pitel KS, Withers SG,
Drissi H and Hawse JR: TIEG1 enhances Osterix expression and
mediates its induction by TGFβ and BMP2 in osteoblasts. Biochem
Biophys Res Commun. 470:528–533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Gale Z, Cooper PR and Scheven BA: Glial
cell line-derived neurotrophic factor influences proliferation of
osteoblastic cells. Cytokine. 57:276–281. 2012. View Article : Google Scholar : PubMed/NCBI
|