Introduction
Bronchopulmonary dysplasia (BPD) is the most common
chronic lung disease in premature infants worldwide, and the
predominant pathological features are alveolar dysplasia and
pulmonary vascular development disorders (1,2). In
total, ~10,000 infants are diagnosed with BPD annually in the
United States (3). Infants with BPD
require respiratory support in early life and for long-term
pulmonary dysfunction, including persistent airway obstruction and
distal lung development (1,4). Furthermore, infants with BPD often
show delays in the development of the nervous system (5). Given that there are no effective
preventive and therapeutic measures, BPD remains one of the most
challenging issues in neonatology.
A previous study suggested that premature lung
exposure to hyperoxia induces lung epithelial cell damage, which
triggers pathological changes in alveolar dysplasia (6). In addition, the apoptosis of lung
epithelial cells is a key factor in the pathological process of
alveolar developmental arrest (7).
The two main apoptosis pathways are the death receptor pathway and
the mitochondrial pathway (8,9).
Previous studies have demonstrated that endoplasmic reticulum
stress (ERS)-related apoptosis is a noncanonical apoptosis pathway
involved in several lung diseases, including chronic obstructive
pulmonary disease (COPD), interstitial lung disease and lung cancer
(10–13). The ERS-mediated apoptosis pathway
has been investigated; however, only a few studies have assessed
its role in BPD (14). A previous
study identified that the ER is significantly enlarged in alveolar
type II epithelial (AEII) cells of BPD rats (15), suggesting that functional changes in
the ER may be involved in the development of BPD; however, the
specific molecular mechanism requires further investigation.
Previous studies have demonstrated that protein
kinase RNA-like endoplasmic reticulum kinase (PERK),
inositol-requiring enzyme 1α (IRE1α) and activating transcription
factor 6 (ATF6) can initiate the ERS-mediated apoptosis signaling
pathway, and thereby induce apoptosis in injured cells (16,17).
The IRE1α pathway, which is the most conserved and important
pathway in the unfolded protein response (UPR), serves a key role
within several pathological processes and determines cell fate
(18–21). For example, an increasing amount of
evidence has demonstrated that IRE1α plays a key role in the
initiation, growth, metastasis and angiogenesis of cancer cells.
The X-box binding protein (XBP1) branch of IRE1 provides tumor
cells with the ability to adapt to unfavorable microenvironmental
conditions and promote their survival and growth (18). In addition, IRE1 is also involved in
important physiological processes, such as lipid metabolism, cell
differentiation, inflammation and energy regulation.
IRE1α-knockdown 3T3L1 cells were found to have notable defects in
adipogenesis (19). However,
studies on the role of the IRE1α pathway in BPD are limited.
Thus, the present study established a rat BPD model
to verify the presence of ERS activation in BPD, and to determine
the role of the IRE1α/X-box binding proteins (XBP1s)/c-Jun
N-terminal kinase (JNK) signaling pathway components: IRE1α,
p-IRE1α, XBP1s, JNK and p-JNK. Changes in gene and protein
expression, and the occurrence of apoptosis were monitored to
determine the role of the ERS signaling pathway in the pathogenesis
of BPD and to provide an experimental basis for the clinical
prevention and treatment of BPD.
Materials and methods
Animal models
A total of 40 Sprague-Dawley (SD) rats (32 females
and 8 males, aged 4 weeks, 220–250 g) were purchased from the
Experimental Animal Center of China Medical University and all
animal experiments were approved by the Animal Ethics committee of
China Medical University (Shenyang, China; approval no.
2019PS308K). The rats were housed in a standard pathogen-free
environment (SPF level), at a temperature of 22±2°C, and relative
humidity 60–70%. The rats were fed ordinary feed and sterilized
water. The light/dark cycle was 12 h light/12 h dark. The rats were
mated at a female/male ratio of 4:1, and gave birth naturally at 22
days of pregnancy. The newborn SD rats (together with the mother
rats) were randomly divided into 2 groups within 12 h after birth,
model group (n=40) and control group (n=40). According to the
method we established in a previous study (22), the model group newborn rats were
placed in a glass oxygen box to maintain the oxygen concentration
at 80–85%. Soda lime was used to absorb CO2 in the
oxygen box to keep the concentration <0.5%. The silica gel
absorbed water to maintain the oxygen level. The temperature of the
box was 25–27°C, the humidity was 60–70%, and the oxygen
concentration was monitored with a digital oxygen meter every day.
The control rats inhaled fresh air. All additional conditions and
control factors were the same in both groups. The oxygen box was
opened for 30 min every 24 h to replenish food and water and to
change the bedding, and maternal rats in the model group and the
control group were exchanged to prevent oxygen poisoning.
Collection of lung samples
A total of 8 pups from each group were randomly
selected at 1, 3, 7 and 14 days after the start of the experiment
and anesthetized by sevoflurane inhalation. Lung tissues were
removed under aseptic conditions and the right middle lobe was
fixed with 4% paraformaldehyde (PFA; Beyotime Institute of
Biotechnology), for >24 h at room temperature, for hematoxylin
and eosin (H&E) staining, immunohistochemistry (IHC) and
terminal deoxynucleotidyl transferase dUTP nick-end labeling
(TUNEL). The remaining lung tissue samples were stored at −80°C for
protein and mRNA analysis.
Lung histology and morphometric
analysis
Lung tissues were fixed with 4% PFA for 24–48 h at
room temperature, dehydrated in a graded alcohol series. Then, the
tissues were embedded in paraffin at room temperature.
Paraffin-embedded tissue samples were cut into 4-µm-thick sections
and conventional dewaxing and rehydration was performed.
Morphological changes were observed by H&E staining for 10 min
at room temperature. For each group of animals 6 slices at
different time points were randomly selected, and 10 visual fields
in each slice were randomly selected to observe pathological
changes under the high power lens of a light microscope (Nikon C1
System; Nikon Corporation) at magnification ×200. The radial
alveolar count (RAC) and mean alveolar diameter (MAD) are simple
and relatively accurate measures of lung development, larger RAC
values indicate more complete alveolar development and larger MAD
values indicate larger alveoli and less mature alveolar
development. The RAC was measured using the Emery and Mithal method
(23,24), whereas the MAD was measured using
Image-Pro Plus 6.0 software (Media Cybernetics, Inc.).
Transmission electron microscopy
(TEM)
Fresh lung tissues were sampled within 1–3 min, the
size of the sampled tissue was 1 m3, as thin as
possible. Immediately after removing the tissue, fresh lung tissues
were fixed with 2.5% glutaraldehyde (Beijing Solarbio Science &
Technology Co., Ltd.) for 2 h at room temperature, and then with 1%
succinic acid, dehydrated with acetone and placed in epoxy resin
prior to being impregnated and embedded with epoxy resin for 72 h.
Then, the ultra-thin sections were produced and double-stained with
uranyl acetate and lead nitrate for 30 min. The ultrastructural
changes in alveolar epithelial type II (AEII) cells and organelles
were observed by TEM (H-600; Hitachi, Ltd.).
TUNEL staining
TUNEL staining was performed to detect apoptosis in
lung tissue. Tissue sections were routinely dewaxed, rehydrated in
an ethanol gradient and then washed with PBS. Following which, 3%
hydrogen peroxide (H202) was added and
sections were incubated at 37°C for 20 min to eliminate endogenous
peroxidase activity. Then, the sections were treated with
proteinase K at 37°C for 8 min, and washed with PBS three times for
5 min. The sections were then incubated at 37°C for 1 h in a
humidified chamber with 50 µl of the TUNEL mixture (Roche
Diagnostics) per sample, according to the manufacturer's protocol.
Subsequently, sections were then stained with DAPI (Beyotime
Institute of Biotechnology) at room temperature for 5 min for
nuclear staining. For each group of animals six slices were
randomly selected to observe the TUNEL-positive cells under the
high power lens of a light microscope (Nikon C1 System; Nikon
Corporation) at magnification ×200. The percentage of apoptotic
cells was determined as the percentage of the total cells positive
for TUNEL.
IHC
The paraffin-embedded lung tissue sections were
baked in an oven at 60°C for 30 min. After routine dewaxing and
gradient alcohol rehydration at room temperature, they were
thoroughly washed with PBS. Then, 3% H202 was
added and sections were incubated at 37°C for 20 min to eliminate
endogenous peroxidase activity. The sections were subjected to
heat-mediated antigen retrieval for 8 min using citric acid, and
then cooled to room temperature, and washed with PBS. Goat serum
(50 µl; OriGene Technologies, Inc.) was used for blocking at 37°C
for 30 min, and then sections were incubated with primary
antibodies against: Glucose regulated protein 78,000 (GRP78;
1:5,000; cat. no. ab21685) phosphorylated (p)-IRE1α (1:500; cat.
no. ab48187) and p-JNK (1:300; cat. no. ab124956; all from Abcam)
overnight at 4°C. Following this, sections were washed four times
with PBS, and then incubated with goat anti-mouse/anti-rabbit IgG
and horseradish peroxidase-labeled streptavidin (cat. no. SP-9001;
OriGene Technologies, Inc.) at 37°C for 20 min. Subsequently, DAB
(OriGene Technologies, Inc.) development was performed under an
optical microscope (Nikon C1 System; Nikon Corporation), and the
color development was stopped when brown particles appeared in the
nucleus or cytoplasm. For the negative control, the primary
antibodies (GRP78, p-IRE1α and p-JNK) were replaced with PBS; all
other steps were performed as aforementioned.
Western blot analysis
Total protein was extracted from lung tissues using
RIP (Beyotime Institute of Biotechnology), quantified using a BCA
protein assay kit (Beyotime Institute of Biotechnology), and then
diluted with loading buffer and boiled for 5 min. Protein samples
(50 µg/lane) were separated by 10 or 12% SDS-PAGE and subsequently
transferred onto a PVDF membrane (EMD Millipore) at 100 V for 30
min. Subsequently, the PVDF membrane was blocked with non-fat milk
diluted with 5% TBST at room temperature for 1 h. Membranes were
incubated with primary antibodies against: GRP78 (1:2,000; cat. no.
ab21685; Abcam), p-IRE1α (1:2,000; cat. no. ab48187; Abcam), IRE1α
(1:1,000; cat. no. 3294; Cell Signaling Technology, Inc.), XBP1-s
(1:1,000; cat. no. 27901S; Cell Signaling Technology, Inc.), p-JNK
(1:2,000; cat. no. ab124956; Abcam), JNK (1:2,000; cat. no.
ab179461; Abcam) and β-actin (1:4,000; cat. no. 60008-1-Ig;
ProteinTech Group, Inc.) overnight at 4°C. Following the primary
incubation, membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (1:5,000; goat
anti-mouse and anti-rabbit IgG-HRP; cat. nos. SA00001-1 and
SA00001-2, respectively; Wuhan Sanying Biotechnology) at room
temperature for 2 h. Proteins bands were detected using the Super
ECL Plus (Thermo Fisher Scientific, Inc.) and luminescence analysis
was performed with a chemiluminescence imaging system (C300; Azure
Biosystems, Inc.). The bands were standardized according to
β-actin.
Reverse transcription-quantitative
(RT-q)PCR
RNA was extracted using TRIzol® reagent
(Takara Biotechnology Co., Ltd.). According to the manufacturer's
protocols, 1 ml TRIzol was added to 50 mg lung tissues and then the
tissues were cut into pieces and mixed thoroughly. Chloroform (200
µl) was added to the tissues for 5 min at room temperature,
followed by centrifugation (12,000 × g) at 4°C for 15 min to get
the supernatant. Then, isopropanol was added at room temperature
for 10 min and centrifuged (12,000 × g) at 4°C for 10 min. Finally,
75% ethanol was added three times at room temperature for 5 min.
The mRNA was reverse transcribed into cDNA using a Prime Script™ RT
reagent kit (Takara Biotechnology Co., Ltd.) to remove genomic DNA
for 15 min at room temperature, with the following conditions: 37°C
for 15 min, and 85°C for 5 sec. The primers (Table I) were designed and synthesized by
Sangon Biotech Co., Ltd., and qPCR was performed using a 7500
Real-Time PCR System (Applied Biosystems, Thermo Fisher Scientific,
Inc.) and a PCR amplification kit (Takara Biotechnology Co., Ltd.)
in a reaction volume of 20 µl. The thermocycling conditions were as
follows: 95°C for 30 sec, 95°C for 5 sec, 60°C for 34 sec, 95°C for
15 sec, 60°C for 1 min, 95°C for 30 sec, and 60°C for 15 sec, for a
total of 40 cycles. Relative expression levels were calculated
using the 2−ΔΔCq method and normalized to β-actin
(25).
 | Table I.Primer sequences used for
quantitative PCR. |
Table I.
Primer sequences used for
quantitative PCR.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| GRP78 |
CCGTAACAATCAAGGTCTACGA |
AAGGTGACTTCAATCTGGGGTA |
| XBP1-s |
GCACCTCTAAGCTCTTCA |
CCTCATATCCACAGTCACT |
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc.). Unpaired Student's t-test was used to
compare difference between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
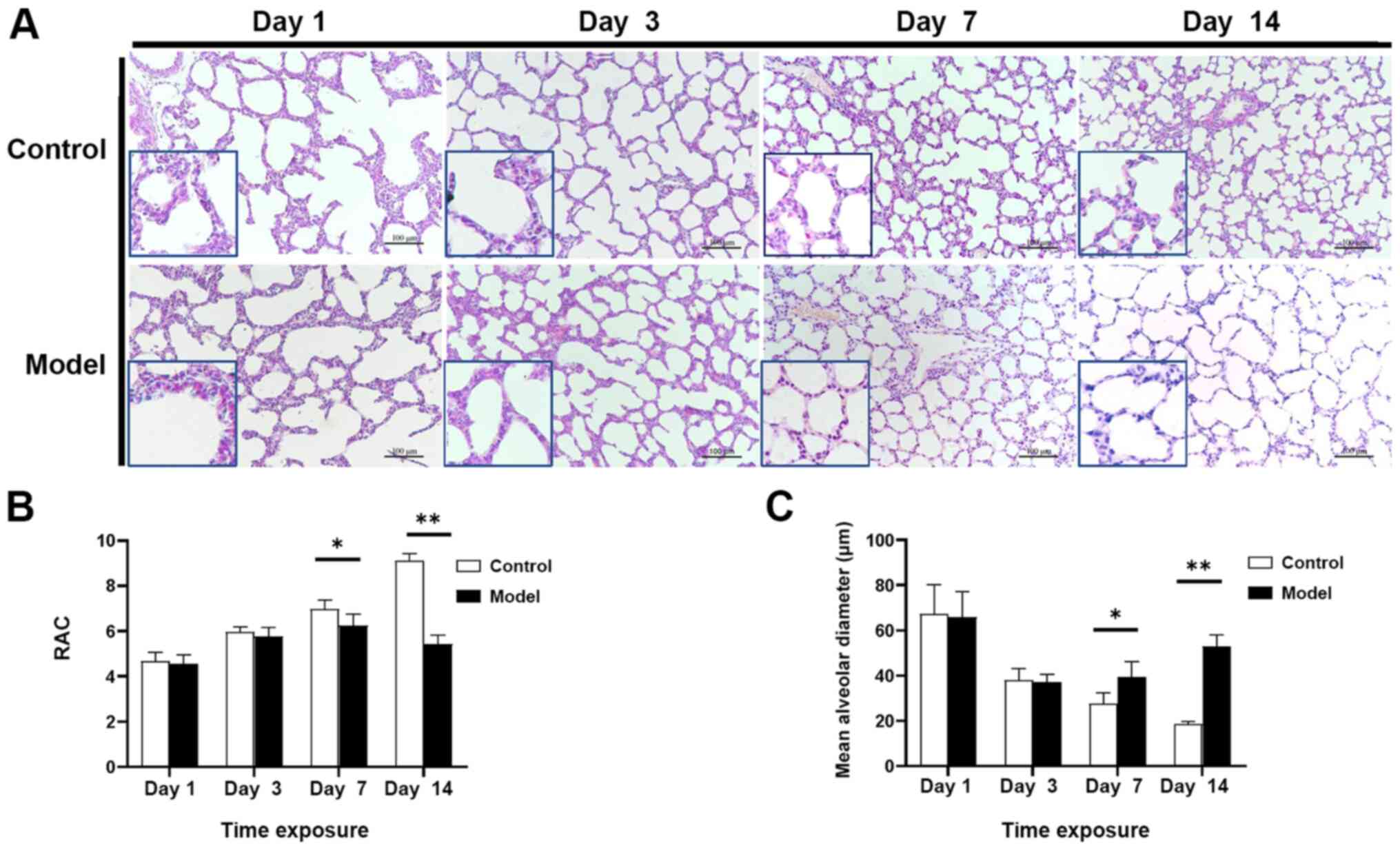
Morphological changes in lung tissue
and differences in lung development
The H&E staining results demonstrated that lung
tissues in the control group gradually improved with the age of the
rats; the alveolar number gradually increased, the alveolar
interval became thinner and the irregular alveolar morphology
gradually disappeared as the alveoli adopted a uniform size. At day
14, alveolarization was complete. Alveolar development in the model
group was significantly delayed compared with the control group,
with a significant reduction in the number of alveoli, an increased
alveolar diameter, an irregular alveolar shape, simple alveolar
structure and unclear alveolar ridges and stimulation intervals
(Fig. 1A).
Compared with the control group, the RAC in the
model group was significantly decreased starting at day 7
(P<0.05) and this difference was more pronounced at day 14
(P<0.01). These findings suggested that the model group
experienced a block in alveolar development starting at day 7 and
development was significantly delayed compared with the control
group. Another indicator of alveolar development is MAD, which was
significantly greater in the model group compared with the control
group at day 7 (P<0.05), with a more pronounced increase at day
14, indicating that the alveoli in the model group gradually became
larger compared with those in the control group (P<0.01;
Fig. 1B and C).
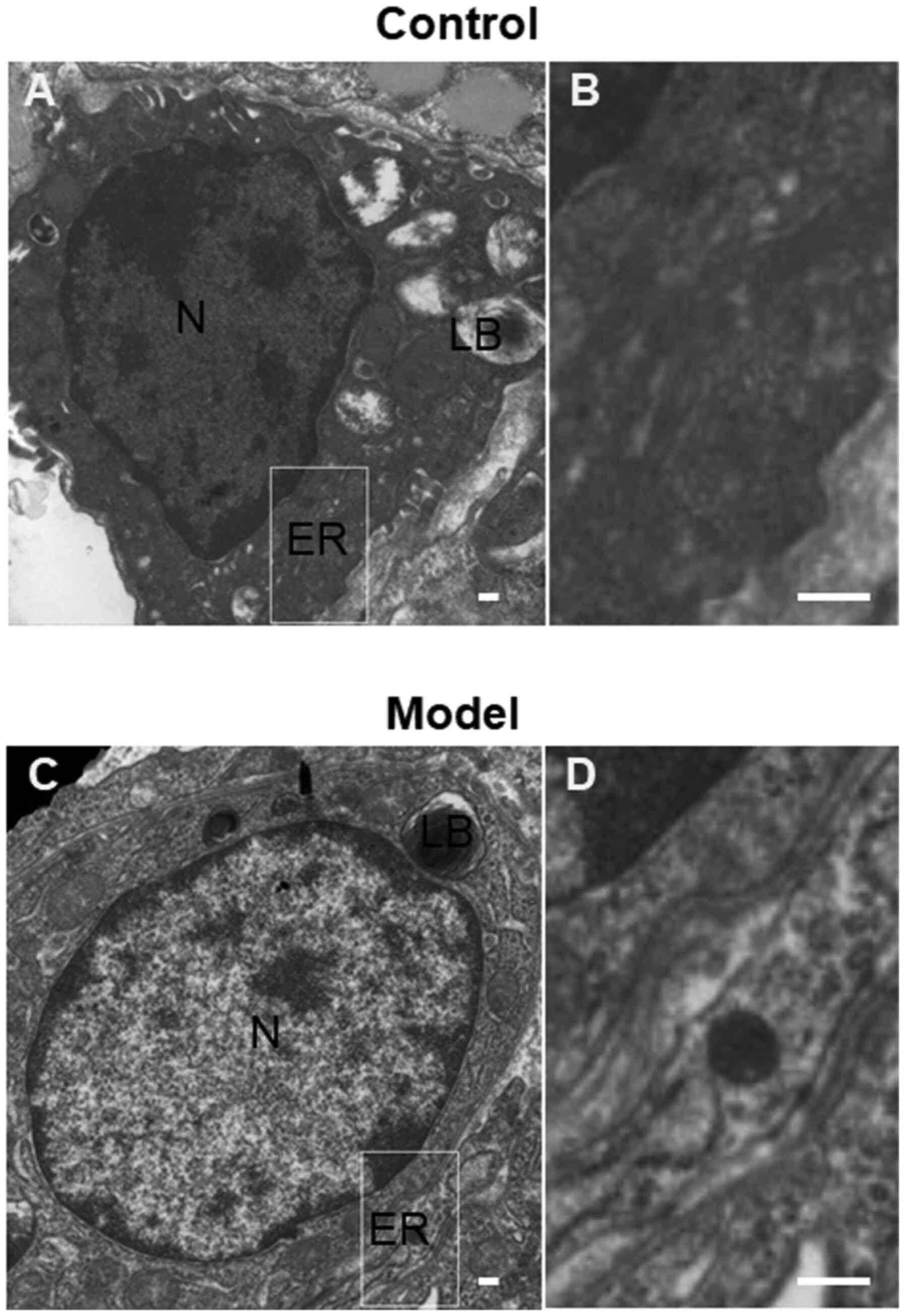
Ultrastructural changes in AEII cells
in lung tissue
TEM demonstrated that the AEII cells in the control
group were cubic in form, with microvilli at the top and
characteristic lamellar bodies in the cytoplasm. The organelles,
including the ER and mitochondria, were normal. In the AEII cells
in the model group, chromatin accumulation at the nuclear periphery
was observed and the microvilli at the top of the cell were sparse
and detached. The cytoplasmic lamellar bodies were destroyed and
vacuolization, mitochondrial swelling, ER expansion and
degranulation were observed (Fig.
2).
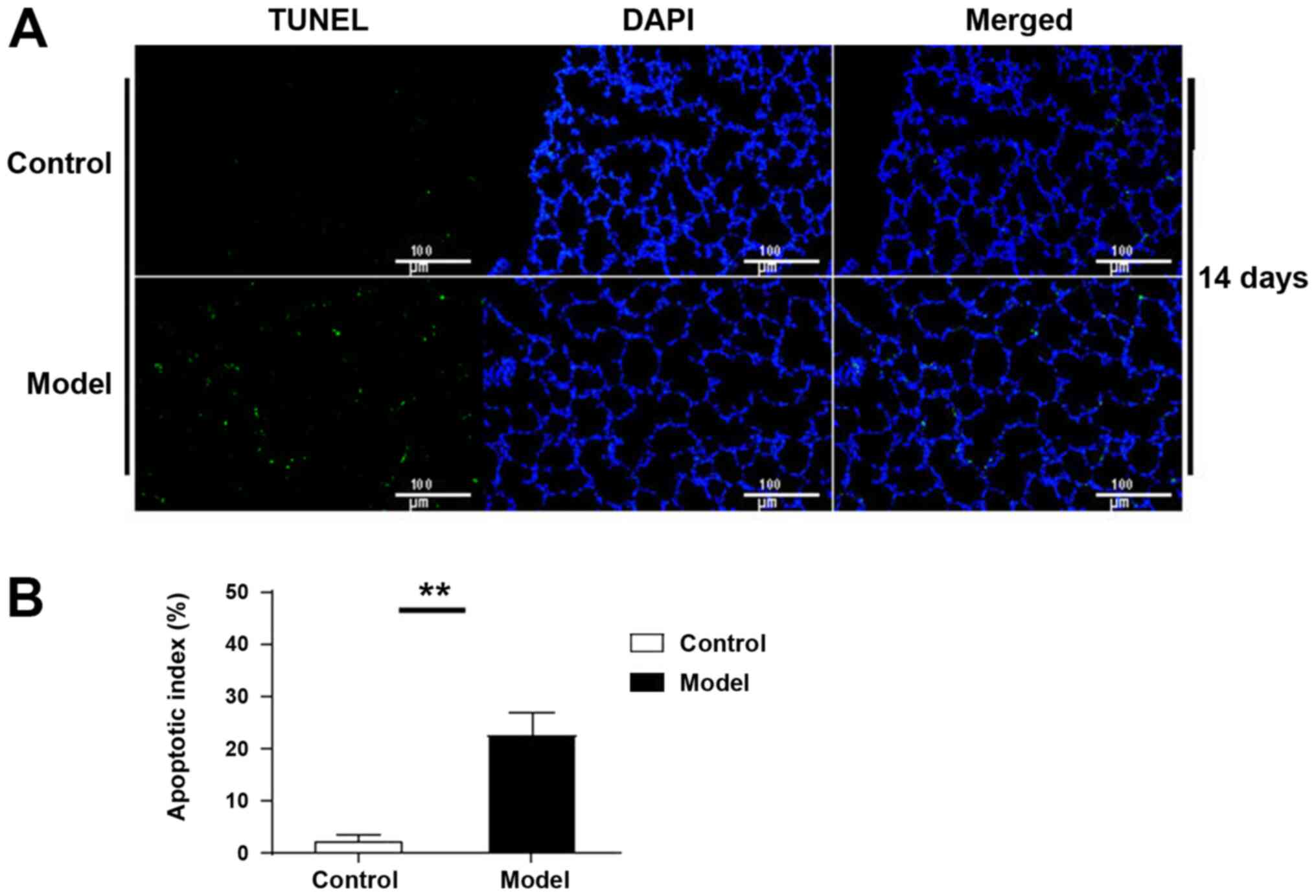
Detection of apoptosis in lung
tissue
TUNEL-positive cells were observed in lung tissue at
day 14 in the control group, while the number of apoptotic cells in
the model group was significantly increased (P<0.01; Fig. 3).
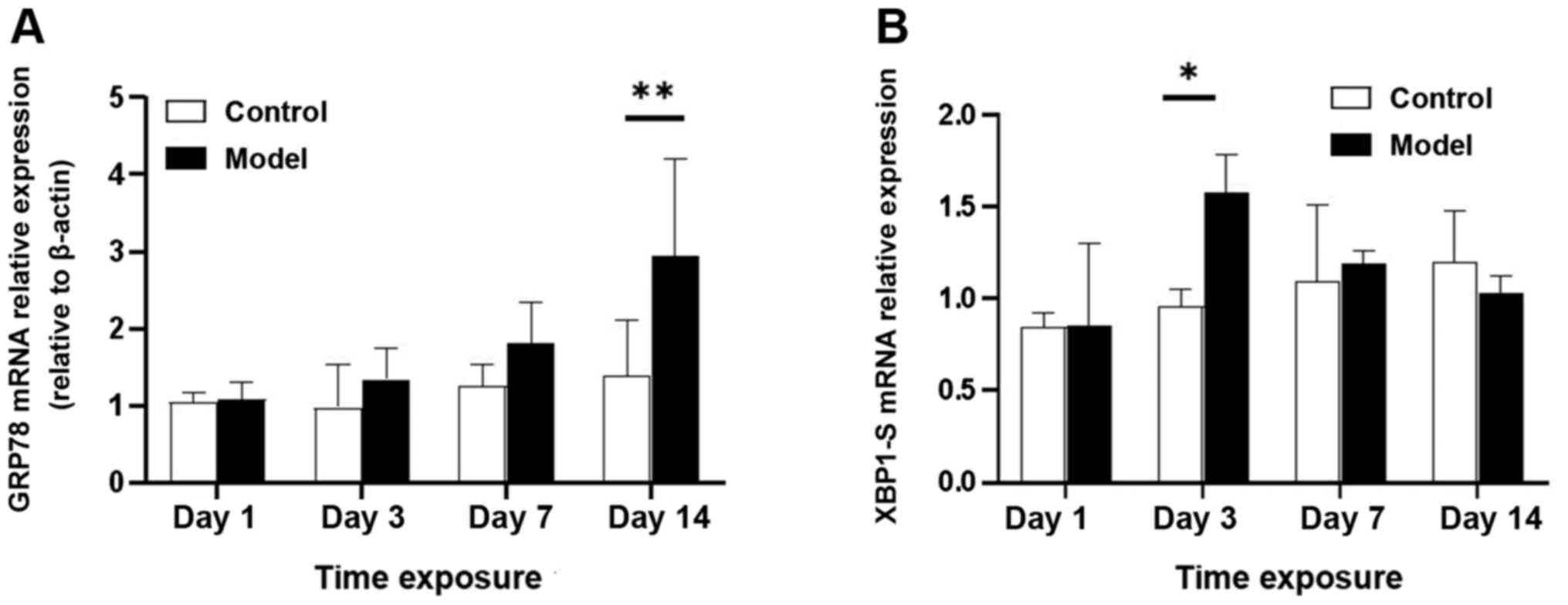
RT-qPCR analysis of GRP78 and XBP1-s
mRNA levels in lung tissue
RT-qPCR analysis demonstrated that GRP78 mRNA levels
were upregulated by exposure to hyperoxia (Fig. 4A). Compared with the control group,
the model group exhibited GRP78 upregulation at day 3, with a
significant increase on day 14 (P<0.05); GRP78 protein levels
demonstrated a similar change (Fig.
5A). Consistent with this finding, XBP1-s mRNA expression
peaked on day 3 (P<0.05; Fig.
4B) and then began to decline prior to reaching the lowest
level on day 14.
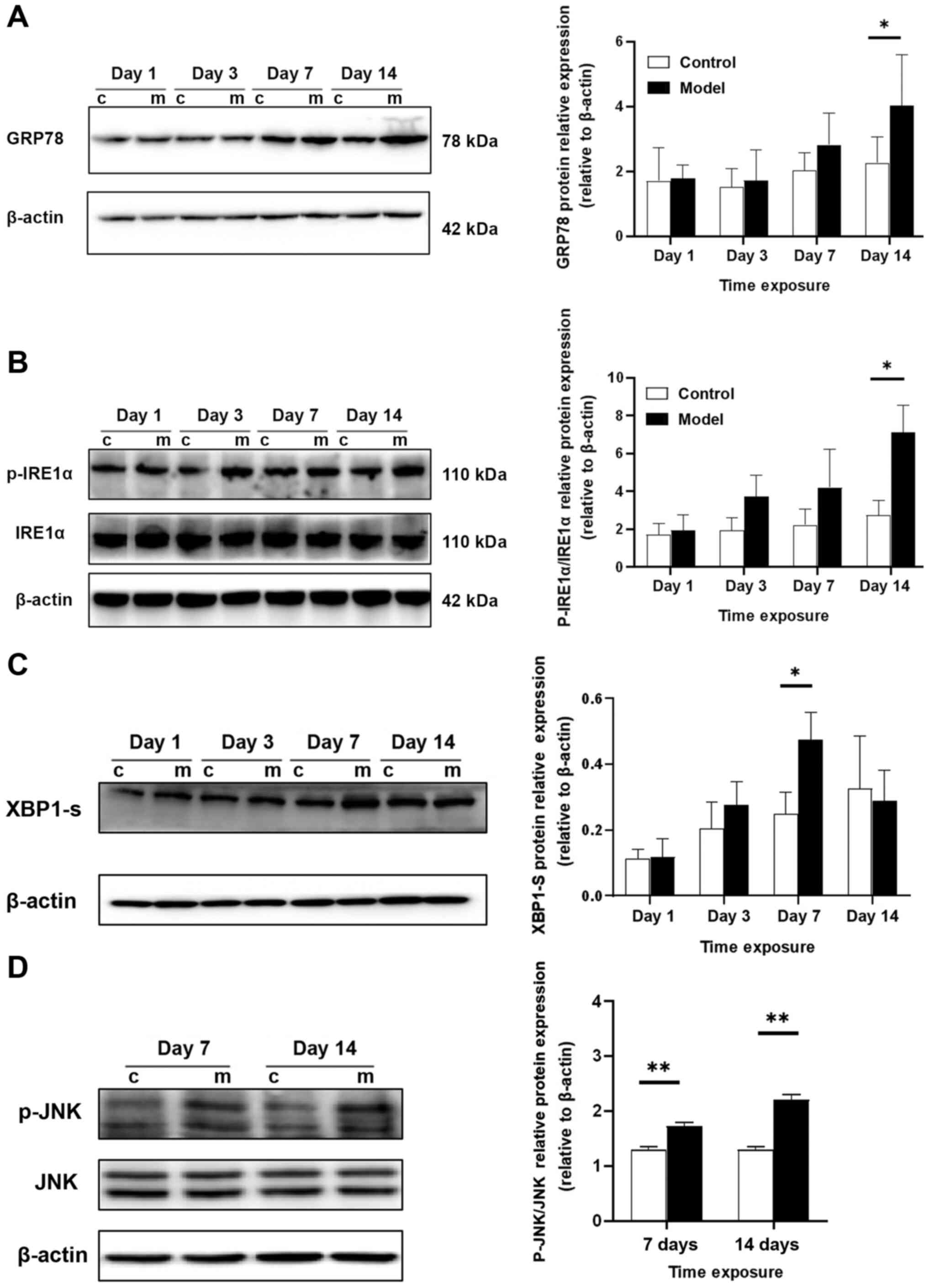 | Figure 5.Western blot analysis of GRP78,
p-IRE1α, IRE1α, XBP1-s, p-JNK and JNK protein expression levels in
lung tissue. (A) GRP78 protein levels gradually increased with
hyperoxia exposure and were markedly increased on day 14. (B)
Protein levels of p-IRE1α and IRE1α. The p-IRE1α/IRE1α ratio
progressively increased over time; it was upregulated in the
treatment group beginning at day 3 and was markedly increased at
day 14. (C) XBP1s protein expression peaked at day 7 and then began
to decrease, reaching the lowest level at day 14. (D) Protein
levels of p-JNK and JNK. The p-JNK/JNK ratio increased beginning at
day 7 and was significantly increased at day 14. Relative protein
expression was normalized to β-actin expression. *P<0.05,
**P<0.01. GRP78, glucose regulated protein 78,000; p-,
phosphorylated; IRE1α, inositol requiring kinase enzyme 1 α; XBP1s,
X-box binding protein 1. |
Western blot analysis of GRP78,
p-IRE1α, IRE1α, XBP1-s, p-JNK and JNK protein expression levels in
lung tissue
Western blot analysis demonstrated that GRP78
protein expression was upregulated with hyperoxia exposure; GRP78
expression increased beginning on day 3 and was significantly
increased on day 14 (P<0.05). The p-IRE1α/IRE1α ratio gradually
increased over time; it increased starting at day 3 in the
experimental group and peaked at day 14 (P<0.05); the expression
trend was consistent with that of GRP78. Changes in XBP1-s protein
expression were relatively delayed, with the highest expression at
day 7 (P<0.05) and decreased expression at day 14. The p-JNK/JNK
ratio was increased on day 7 (P<0.01) and this increase was more
pronounced on day 14 (P<0.01; Fig.
5A-D).
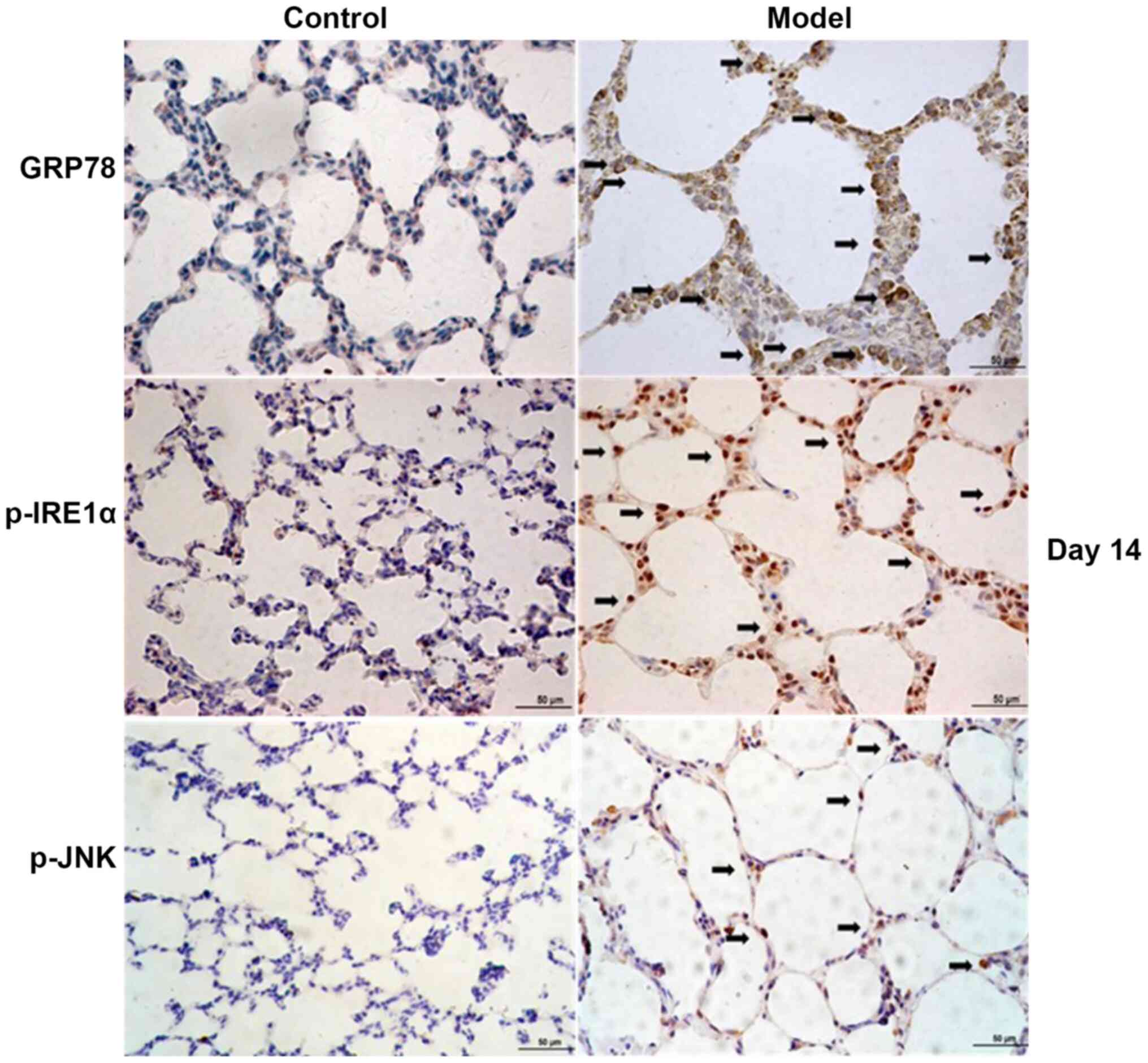
Immunohistochemistry analysis of the
localization and expression of GRP78, p-IRE1α, XBP1 and p-JNK
Immunohistochemistry analysis revealed that in the
model group, GRP78 protein was mainly localized in the cytoplasm of
AEII cells, closely surrounding the nucleus, consistent with ER
localization. p-IRE1α, p-JNK and XBP1 were mainly localized in the
nucleus of AEII cells (Fig. 6).
Discussion
Obstruction of alveolar development is a
pathological characteristic in preterm infants with BPD (1,2). In
the present study, upon exposure to hyperoxia, the number of
alveoli gradually decreased, the alveoli became larger, the
alveolar structure was simplified and alveolar development was
blocked. The RAC is an important indicator of the degree of
alveolarization (24). Compared
with the control group, the RAC in the model group was
significantly decreased at day 7 of hyperoxia exposure and further
decreased with increasing age. RAC analysis also demonstrated the
stagnation of alveolar development in the model group. These
pathological changes are consistent with the pathological features
of alveolar development in BPD.
When ERS occurs, the UPR survival pathway is
initiated. Three UPR signaling pathways are mediated by ATF6, PERK
and IRE1, and a series of cascading pathways are activated to
increase protein processing capacity and protein folding ability by
reducing protein translation. The increased degradation of
misfolded proteins relieves the pressure on the ER and promotes
cell survival (16,17). Previous studies have demonstrated
that ERS serves an important role in lung diseases, including COPD,
pulmonary fibrosis, acute lung injury and lung cancer (10–13).
GPR78 is significantly upregulated by ERS only and is extensively
used as a marker of ERS (26,27).
In the present study, the ultrastructure of AEII cells in the lung
tissue of the model group demonstrated substantial hyperplasia and
expansion, suggesting a dysfunctional ER structure and a
significant increase in GRP78 gene and protein expression. Taken
together, these results indicated that the activation of ERS was
involved in the development of BPD, which was consistent with the
reports of Lu et al (14)
and Teng et al (28).
Among the three signaling pathways that mediate the
UPR, the IRE1α pathway is the most sensitive and conservative
(29,30). IRE1α is a kinase/endonuclease
(RNase) with multiple activities. Upon ERS activation, IRE1α is
immediately activated by oligomerization and autophosphorylation.
When activated, the RNase domain of IRE1α excises the 26-nucleotide
intron from the XBP1-u mRNA and cleaves the exon by RtcB ligation
to generate the active transcription factor XBP1-s, an important
protective factor in cells. Upon activation, XBP1-s immediately
enters the nucleus and upregulates its target genes, including
those that encode ER chaperone and ER-associated degradation
components, thereby enhancing the folding and degradation of
proteins and maintaining ER homeostasis. However, with prolonged
overactivation of ERS, XBP1-s activation is gradually inhibited and
the apoptosis pathway is progressively activated (). Previous
studies have demonstrated that when ERS is overactivated, IRE1α
activates JNK by interacting with tumor necrosis factor (TNF)
receptor-associated factor 2 (TRAF2) (29–32).
Activated JNK can induce apoptosis through the death receptor
pathway or the mitochondrial pathway (32).
Previous studies have demonstrated that the
protective effects of XBP1 are important in a number of diseases
and targeting these protective effects has been proposed as a
strategy for the treatment of these diseases (33–37).
Akiyama et al (33) reported
that XBP1 can alleviate glucose dysfunction by improving insulin
sensitivity and stimulating insulin secretion. Furthermore,
Casas-Tinto et al (34)
identified that the spliced form of XBP1 attenuates neuronal damage
in cell culture models of Alzheimer's disease. In addition, a
previous study demonstrated that XBP1 is critical for the
differentiation of dendritic cells (37). These previous studies proposed that
targeting these protective effects could be a strategy for the
treatment of these diseases.
In the present study, western blotting and
immunohistochemistry analyses demonstrated that IRE1α
phosphorylation increased with hyperoxia exposure, which was
consistent with the trend in GRP78 levels, indicating that IRE1α is
rapidly activated following ERS activation. XBP1-s mRNA levels
peaked on day 3 (P<0.05) and then began to decline, reaching the
lowest level on day 14. Changes in XBP1-s protein levels were
relatively delayed, peaking at day 7 and then declining. These
findings indicated that the IRE1α/XBP1 pathway is activated at the
onset of ERS. However, over time, XBP1-s activation was gradually
inhibited when ERS was overactivated. In addition, the changes in
XBP1-s mRNA and protein levels in the control group demonstrated
that XBP1-s expression increased with increasing age, suggesting
that this protein may be associated with lung development. This
finding further indicated that excessive ERS led to the inhibition
of XBP1-s and may be responsible for the obstruction of alveolar
development in BPD.
When ERS was overactivated, in addition to
downstream XBP1 inhibition and IRE1α/JNK pathway activation, the
induction of apoptosis was observed. Previous studies have
demonstrated that JNK-induced apoptosis is an important cause of
several diseases, including cancer, liver dysfunction, metabolic
disorders and neurodegenerative diseases (38–41).
The apoptosis of lung epithelial cells is an important mechanism of
BPD (1). It has been demonstrated
that the apoptosis-related gene, Fas/FasL initiates
apoptosis in caspase-dependent AT-II cells and that the signal is
transduced by Bcl-2/Bax; these events are key in BPD-related
alveolar dysplasia (42). In
addition, the present study demonstrated that when BPD developed
and the change in RAC became evident, ERS was overactivated, XBP1-s
began to decrease and JNK activation gradually increased; these
differences were more pronounced at day 14. Furthermore, TUNEL
experiments confirmed that apoptosis was significantly increased at
day 14. These findings suggest that the apoptosis pathway is
activated when ERS is overactivated.
Taken together, the present study demonstrated that
ERS was present during the development and progression of BPD, and
that ERS overactivation inhibited the protective IRE1α/XBP1-s
pathway and activated the proapoptotic IRE1α/JNK pathway to induce
the apoptosis of lung epithelial cells, which may lead to BPD. The
key factors in the pathogenesis of alveolar dysplasia have been
elucidated; however, further research is required to clarify the
exact mechanism.
Acknowledgements
The authors would like to thank Dr Liu Dongyan
(Laboratory Research Center, Shengjing Hospital of China Medical
University, Shenyang, China) for her technical assistance.
Funding
The present study was supported by the National
Natural Science Foundation of china (grant no. 81571479).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XT designed the study and contributed to manuscript
writing. ML collected the rat lung tissue and contributed to
establishing the bronchopulmonary dysphasia rat model. NL and WH
contributed to analysis and interpretation of data. JF made
contributions to the conception and design of the project. XX
contributed to the design of the study and revised the work
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Ethics committee of China Medical University (Shenyang, China;
approval no. 2019PS308K).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kalikkot Thekkeveedu R, Guaman MC and
Shivanna B: Bronchopulmonary dysplasia: A review of pathogenesis
and pathophysiology. Respir Med. 132:170–177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Voynow JA: ‘New’ bronchopulmonary
dysplasia and chronic lung disease. Paediatr Respir Rev. 24:17–18.
2017.PubMed/NCBI
|
|
3
|
Stoll BJ, Hansen NI, Bell EF, Shankaran S,
Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, et
al: Neonatal outcomes of extremely preterm infants from the NICHD
neonatal research network. Pediatrics. 126:443–456. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hwang JS and Rehan VK: Recent advances in
bronchopulmonary dysplasia: Pathophysiology, prevention, and
treatment. Lung. 196:129–138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DeMauro SB: The impact of bronchopulmonary
dysplasia on childhood outcomes. Clin Perinatol. 45:439–452. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bhandari V: Hyperoxia-derived lung damage
in preterm infants. Semin Fetal Neonatal Med. 15:223–229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pagano A and Barazzone-Argiroffo C:
Alveolar cell death in hyperoxia-induced lung injury. Ann N Y Acad
Sci. 1010:405–416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cavalcante GC, Schaan AP, Cabral GF,
Santana-da-Silva MN, Pinto P, Vidal AF and Ribeiro-Dos-Santos A: A
cell's fate: An overview of the molecular biology and genetics of
apoptosis. Int J Mol Sci. 20:41332019. View Article : Google Scholar
|
|
9
|
Sayers TJ: Targeting the extrinsic
apoptosis signaling pathway for cancer therapy. Cancer Immunol
Immunother. 60:1173–1180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marciniak SJ: Endoplasmic reticulum stress
in lung disease. Eur Respir Rev. 26:1700182017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei J, Rahman S, Ayaub EA, Dickhout JG and
Ask K: Protein misfolding and endoplasmic reticulum stress in
chronic lung disease. Chest. 143:1098–1105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Zhang Y, Liu X and Wang J, Li B,
Liu Y and Wang J: Alantolactone enhances gemcitabine sensitivity of
lung cancer cells through the reactive oxygen species-mediated
endoplasmic reticulum stress and Akt/GSK3β pathway. Int J Mol Med.
44:1026–1038. 2019.PubMed/NCBI
|
|
13
|
Naiel S, Tat V, Padwal M, Vierhout M,
Mekhael O, Yousof T, Ayoub A, Abed S, Dvorkin-Gheva A and Ask K:
Protein misfolding and endoplasmic reticulum stress in chronic lung
disease: Will cell-specific targeting be the key to the cure?
Chest. 157:1207–1220. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu HY, Zhang J, Wang QX, Tang W and Zhang
LJ: Activation of the endoplasmic reticulum stress pathway
involving CHOP in the lungs of rats with hyperoxia-induced
bronchopulmonary dysplasia. Mol Med Rep. 12:4494–4500. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li M, Pan B, Shi Y, Fu J and Xue X:
Increased expression of CHOP and LC3B in newborn rats with
bronchopulmonary dysplasia. Int J Mol Med. 42:1653–1665.
2018.PubMed/NCBI
|
|
16
|
Iurlaro R and Muñoz-Pinedo C: Cell death
induced by endoplasmic reticulum stress. FEBS J. 283:2640–2652.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gong J, Wang XZ, Wang T, Chen JJ, Xie XY,
Hu H, Yu F, Liu HL, Jiang XY and Fan HD: Molecular signal networks
and regulating mechanisms of the unfolded protein response. J
Zhejiang Univ Sci B. 18:1–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin C, Jin Z, Chen NZ, Lu M, Liu CB, Hu WL
and Zheng CG: Activation of IRE1α-XBP1 pathway induces cell
proliferation and invasion in colorectal carcinoma. Biochem Biophys
Res Commun. 470:75–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sha H, He Y, Chen H, Wang C, Zenno A, Shi
H, Yang X, Zhang X and Qi L: The IRE1alpha-XBP1 pathway of the
unfolded protein response is required for adipogenesis. Cell Metab.
9:556–564. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang D, Niwa M and Koong AC: Targeting
the IRE1α-XBP1 branch of the unfolded protein response in human
diseases. Semin Cancer Biol. 33:48–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu J, He GT, Zhang WJ, Xu J and Huang QB:
IRE1α signaling pathways involved in mammalian cell fate
determination. Cell Physiol Biochem. 38:847–858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou A, Fu J, Yang H, Zhu Y, Pan Y, Xu S
and Xue X: Hyperoxia stimulates the transdifferentiation of type II
alveolar epithelial cells in newborn rats. Am J Physiol Lung Cell
Mol Physiol. 308:L861–L872. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cooney T and Thurlbeck W: The radial
alveolar count method of Emery and Mithal: A reappraisal
1-postnatal lung growth. Thorax. 37:572–579. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Herring MJ, Putney LF, Wyatt G, Finkbeiner
WE and Hyde DM: Growth of alveoli during postnatal development in
humans based on stereological estimation. Am J Physiol Lung Cell
Mol Physiol. 307:L338–L344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang M, Ye R, Barron E, Baumeister P, Mao
C, Luo S, Fu Y, Luo B, Dubeau L, Hinton DR and Lee AS: Essential
role of the unfolded protein response regulator GRP78/BiP in
protection from neuronal apoptosis. Cell Death Differ. 17:488–498.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Liu R, Ni M, Gill P and Lee AS:
Cell surface relocalization of the endoplasmic reticulum chaperone
and unfolded protein response regulator GRP78/BiP. J Biol Chem.
285:15065–15075. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Teng R, Jing X, Michalkiewicz T, Afolayan
A, Wu T and Konduri G: Attenuation of endoplasmic reticulum stress
by caffeine ameliorates hyperoxia-induced lung injury. Am J Physiol
Lung Cell Mol Physiol. 312:L586–L598. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y and Brandizzi F: IRE1: ER stress
sensor and cell fate executor. Trends Cell Biol. 23:547–555. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adams CJ, Kopp MC, Larburu N, Nowak PR and
Ali MMU: Structure and molecular mechanism of ER stress signaling
by the unfolded protein response signal activator IRE1. Front Mol
Biosci. 6:112019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hetz C, Martinon F, Rodriguez D and
Glimcher LH: The unfolded protein response: Integrating stress
signals through the stress sensor IRE1α. Physiol Rev. 91:1219–1243.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Verma G and Datta M: The critical role of
JNK in the ER-mitochondrial crosstalk during apoptotic cell death.
J Cell Physiol. 227:1791–1795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akiyama M, Liew CW, Lu S, Hu J, Martinez
R, Hambro B, Kennedy RT and Kulkarni RN: X-box binding protein 1 is
essential for insulin regulation of pancreatic α-cell function.
Diabetes. 62:2439–2449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Casas-Tinto S, Zhang Y, Sanchez-Garcia J,
Gomez-Velazquez M, Rincon-Limas DE and Fernandez-Funez P: The ER
stress factor XBP1s prevents amyloid-beta neurotoxicity. Hum Mol
Genet. 20:2144–2160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tashiro E: Screening and identification of
inhibitors of endoplasmic reticulum stress-induced activation of
the IRE1α-XBP1 branch. J Antibiot (Tokyo). 72:899–905. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen L, Li Q, She T, Li H, Yue Y, Gao S,
Yan T, Liu S, Ma J and Wang Y: IRE1α-XBP1 signaling pathway, a
potential therapeutic target in multiple myeloma. Leuk Res.
49:7–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hasegawa D, Calvo V, Avivar-Valderas A,
Lade A, Chou HI, Lee YA, Farias EF, Aguirre-Ghiso JA and Friedman
SL: Epithelial Xbp1 is required for cellular proliferation and
differentiation during mammary gland development. Mol Cell Biol.
35:1543–1556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mehan S, Meena H, Sharma D and Sankhla R:
JNK: A stress-activated protein kinase therapeutic strategies and
involvement in Alzheimer's and various neurodegenerative
abnormalities. J Mol Neurosci. 43:376–390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Seki E, Brenner D and Karin M: A liver
full of JNK: Signaling in regulation of cell function and disease
pathogenesis, and clinical approaches. Gastroenterology.
143:307–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feng J, Lu S, Ou B, Liu Q, Dai J, Ji C,
Zhou H, Huang H and Ma Y: The role of JNk signaling pathway in
obesity-driven insulin resistance. Diabetes Metab Syndr Obes.
13:1399–1406. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu Q, Wu W, Jacevic V, Franca TCC, Wang X
and Kuca K: Selective inhibitors for JNK signalling: A potential
targeted therapy in cancer. J Enzyme Inhib Med Chem. 35:574–583.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
De Paepe ME, Gundavarapu S, Tantravahi U,
Pepperell JR, Haley SA, Luks FI and Mao Q: Fas-ligand-induced
apoptosis of respiratory epithelial cells causes disruption of
postcanalicular alveolar development. Am J Pathol. 173:42–56. 2008.
View Article : Google Scholar : PubMed/NCBI
|