Introduction
In recent years, due to the environmental pollution,
as well as increasing living and working pressure, premature
ovarian insufficiency (POI) and female infertility have become a
global issue (1,2). POI is defined as the cessation of
ovarian function, which usually occurs before the age of 40 and is
characterized by reduced estrogen levels and elevated gonadotropin
levels (3). It has been previously
reported that the incidence of POI in females ≤40 years of age is
~1% whereas incidence for females aged <30 years is 1 (4,5). In
addition, among female patients with POI, 10–28% would experience
primary amenorrhea whilst 4–18% would exhibit secondary amenorrhea
(4,5).
The pathogenesis of POI remains poorly understood
(6). At present, follicle donation
and hormone replacement therapy (HRT) are the primary treatment
methods for POI (7). In particular
HRT can also relieve the symptoms of POI. However, the clinical
application of HRT is limited due to the increased risk of
endometrial cancer, breast cancer, vaginal bleeding, liver and
kidney function impairment and vascular embolism (8). Therefore, it would be of great value
to identify novel agents that confer little to no side effects but
can significantly improve the symptoms in a similar manner to HRT.
The ginsenoside Rg1 is a natural estrogen that has various reported
pharmacological anti-oxidation and anti-aging effects (9,10).
Previous studies (11–13) demonstrated that unlike synthetic
estrogen, Rg1 would not increase the risk of breast cancer and
endometrial cancer (14–16). Mitochondrial oxidative stress can be
treated by enhancing endogenous antioxidants (17,18).
However, it remains unknown whether senescence of the reproductive
system can be delayed by Rg1 by enhancing the anti-oxidation
pathway in a D-galactose (D-gal)-induced rodent aging model.
D-gal is an agent that has been widely recognized to
induce aging and has been previously used for the establishment of
animal models of aging in various organs (19,20).
To date, ~400 articles have been reported using this model. It has
also been reported that female individuals (fish, fruit flies, rats
and mice) with galactosemia would eventually develop POI (21). The mechanisms underlying POI would
likely be revealed by investigating individuals with galactosemia
(22). Continuous injection of
D-gal elevates galactose levels, leading to the further
accumulation of free radicals and subsequent oxidative damage,
resulting in the acceleration of the aging process of cells
(20). The mechanism of this type
of aging process is similar to that of natural aging (23). Additionally, a D-gal-induced aging
model is easy to establish (24).
Therefore, a D-gal-induced aging model can be regarded to be an
adequate model for the investigation into the mechanisms underlying
ovary aging.
The p53-p21-serine/threonine kinase (STK) pathway is
an important signal transduction pathway involved in the aging
process (25). This signaling
pathway is associated with the p53-mediated DNA stress damage and
repairing (26). The P53
gene is a tumor suppressor gene that encodes the p53 protein and
serves a decisive role in repairing stress-induced DNA damage and
the maintenance of gene and genome stability (27). P53 expression is low in
normal cells, but can be activated when cells are under stress in
conditions such as DNA repair and apoptosis (28). In turn, the p53-encoded product can
regulate Bax expression, which may mediate p53-dependent apoptosis.
In addition, p53 can indirectly induce apoptosis by downregulating
Bcl2 expression (29). Therefore,
apoptosis and aging are processes that are mutually associated. The
detection of STK, p21, p53, Bcl2 and Bax of the apoptosis signaling
pathway may be useful in understanding the possible mechanisms of
D-gal-induced uterine aging.
In the present study, an aging model was established
in mice by D-gal treatment, following which the effects of Rg1 on
the histopathology of the ovary and uterus were investigated. The
weights of the uterus and ovary, and the expression of oxidative
stress and inflammatory biomarkers in the uterus and ovary were
then measured and analyzed.
Materials and methods
Study animals
In total, 100 C57BL/6 female mice (SPF grade),
6–8-weeks old, weighing 20±2 g (ranging from 18 to 20 g) were
obtained from the Medical and Laboratory Animal Center of Chongqing
(Chongqing, China). These animals were housed in standard
conditions, in a 12/12 h dark/light cycle, at 18–20°C, with free
access to food and water. All animal experimental procedures were
approved by the Institutional Animal Care and Use Committee of
Chongqing Medical University. All surgeries were performed under
sodium pentobarbital anesthesia. Reduced heart rate and decreased
respiration rate were used as the main humane endpoints to
determine when animals should be euthanized. ‘Guidelines for
euthanasia of experimental animals’ were followed to minimize
suffering and distress of animals (30).
The mice were subsequently divided into the
following four groups randomly (n=25 mice per group): (i) The D-gal
group, where the mice were subcutaneously (s.c.) injected with
D-gal (200 mg/kg/d for 42 days) and intraperitoneally (i.p.)
injected with an equivalent volume (2 ml) of saline (daily from day
15 onwards for 28 days); ii) the Rg1 group, where the mice were
first s.c. injected with an equivalent volume (2 ml) of saline
(daily for 14 days), followed by an i.p. injection of 20 mg/kg/d
ginsenoside Rg1 daily for 28 days (cat. no. RSZD-121106; purity,
98.3%; dissolved in ddH2O; Xi'an Haoxuan Biological
Technology Co., Ltd.); iii) the Rg1 + D-gal group, where the mice
were first s.c. injected with only D-gal (200 mg/kg/d daily for 42
days), followed by a combination treatment with i.p. Rg1 injection
(20 mg/kg/d, daily), for another 28 days); and iv) the saline group
(negative control), where the mice received equivalent volume of
saline (s.c. and i.p.) at the same time points.
After 42 days of injection, the mice were
anaesthetized with 2–3% isoflurane in a specialized chamber
(31,32). When the limbs of the mice were
paralyzed and their breathing slowed down, they were taken out of
the inducing chamber. The corneal and pain reflexes of mice were
then examined. If both corneal and pain reflexes were absent, the
mice were considered to be under full anesthesia. Once
anaesthetized, their eyeballs were quickly removed and whole blood
samples were collected, following which the mice were sacrificed by
hemorrhagic shock. Animal death was confirmed by observing cardiac
and respiratory arrest for 3–5 min before the ovaries and uterus
tissues were removed and weighed.
Microscopy preparation
For light microscopy, specimens were fixed in 10%
neutral formalin (at room temperature for 24 h) and embedded with
paraffin. The specimens were sectioned (3–4 µm), which were then
subjected to hematoxylin and eosin (H&E), Gomori's trichrome
and van Gieson elastic staining.
For electron microscopy, specimens were promptly
fixed in 4% glutaraldehyde with 0.1 M cacodylate buffer (at 4°C for
>2 h) and fixed in 1.0% osmium tetroxide (at 4°C for 1 h). After
dehydration using an ascending ethanol gradient followed by
propylene oxide, the specimens were embedded in Epon 812 (Beijing
XinWangWeiTuo Technology Co., Ltd.). After sectioning, the
specimens were cut into ultra-thin sections using a Porter-Blum
MT-2 ultramicrotome and stained with uranyl acetate and lead
citrate (at 60°C for 24 h). These sections were observed under a
Hitachi HU-11D electron scanning microscope.
H&E staining
On the last day of drug administration (day 42),
animals were sacrificed under anesthesia and the uterus and ovary
tissues were removed from each animal. The tissues were fixed with
4% paraformaldehyde at 4°C overnight, dehydrated using an ascending
ethanol gradient followed by xylene and embedded in paraffin. The
embedded tissues were then cut into 5-µm thick continuous sections,
which were stained with H&E (at 4°C for 24 h). The number of
ovarian follicles was analyzed with a light microscope using the
Image-Pro Plus 6.0 software (Media Cybernetics, Inc.).
Chemical colorimetric analysis
On day 42, ovary and uterus tissues were collected
and lysed with RIPA lysis buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology) for 30 min on ice. After centrifugation
at 10,000 × g at 4°C for 10 min, the supernatant was collected. The
activities or contents of oxidation-associated biomarkers
superoxide dismutase (T-SOD; cat. no. A001-3), malondialdehyde
(MDA; cat. no. A003-1) and glutathione peroxidase (GSH-px; cat. no.
A005) were measured using corresponding chemical colorimetric assay
kits (Nanjing Jiancheng Bioengineering Institute).
ELISA
The levels of interleukin (IL)-6 (cat. no. EK0411),
tumor necrosis factor (TNF)-α (cat. no. EK0527) and IL-1β (cat. no.
EK0394; all purchased from Wuhan Boster Biological Technology,
Ltd.) in tissue lysates of the ovary and uterus were measured using
corresponding ELISA kits in accordance with the manufacturer's
protocols. In addition, the contents of estradiol 2 (E2; cat. no.
EK7003), follicle-stimulating hormone (FSH; cat. no. EK0302) and
anti-mullerian hormone (AMH; cat. no. EK1037) were also detected
using the corresponding kits (Wuhan Boster Biological Technology,
Ltd.) according to the manufacturer's protocols.
Reverse transcription-quantitative PCR
(RT-qPCR)
Ovary and uterus tissues were first harvested before
total RNA was extracted using the TRIzol® reagent
(Biolab Biotechnology Co., Ltd.). ReverTra Ace-α™ first strand cDNA
synthesis kit (Toyobo Life Science) was used for reverse
transcription (denaturation at 94°C and annealing at 55°C). qPCR
was subsequently performed using the CFX96 Real-Time PCR detection
kit (Biolab Biotechnology Co., Ltd.) was used with the following
conditions: Initial denaturation at 94°C for 5 min, followed by 35
cycles of denaturation at 94°C for 20 sec, annealing at 55°C for 30
sec, and extension at 72°C for 30 sec. The primer sequences were as
follows: p21 forward, 5′-AGTGTGCCGTTGTCTCTTCG-3′ and reverse,
5′-ACACCAGAGTGCAAGACAGC-3′; p53 forward, 5′-AGAGACCGCCGTACAGAAGA-3′
and reverse, 5′-CTGTAGCATGGGCATCCTTT-3′; STK forward,
5′-GTCGCAGGTTCTTGGTCACT-3′ and reverse, 5′-CGAATCTGCACCGTAGTTGA-3′;
Bax forward, 5′-AAACTGGTGCTCAAGGCCCT-3′ and reverse,
5′-AGCAGCCGCTCACGGAG-3′; Bcl-2 forward, 5′-AGCGACGAGAGAAGTCATCC-3′
and reverse, 5′-CTGTAGCATGGGCATCCTTT-3′; and GAPDH forward,
5′-GCAAAGTGGAGATTGTTGCC-3′ and reverse, 5′-CCGTATTCATTGTCATACCA-3′.
The 2−ΔΔCq method was used to calculate relative
expression levels of target genes (33).
Statistical analysis
Data were presented as the mean ± SD. Statistical
analysis was performed with SPSS 17.0 software (SPSS, Inc.).
One-way ANOVA was performed for group comparisons followed by
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
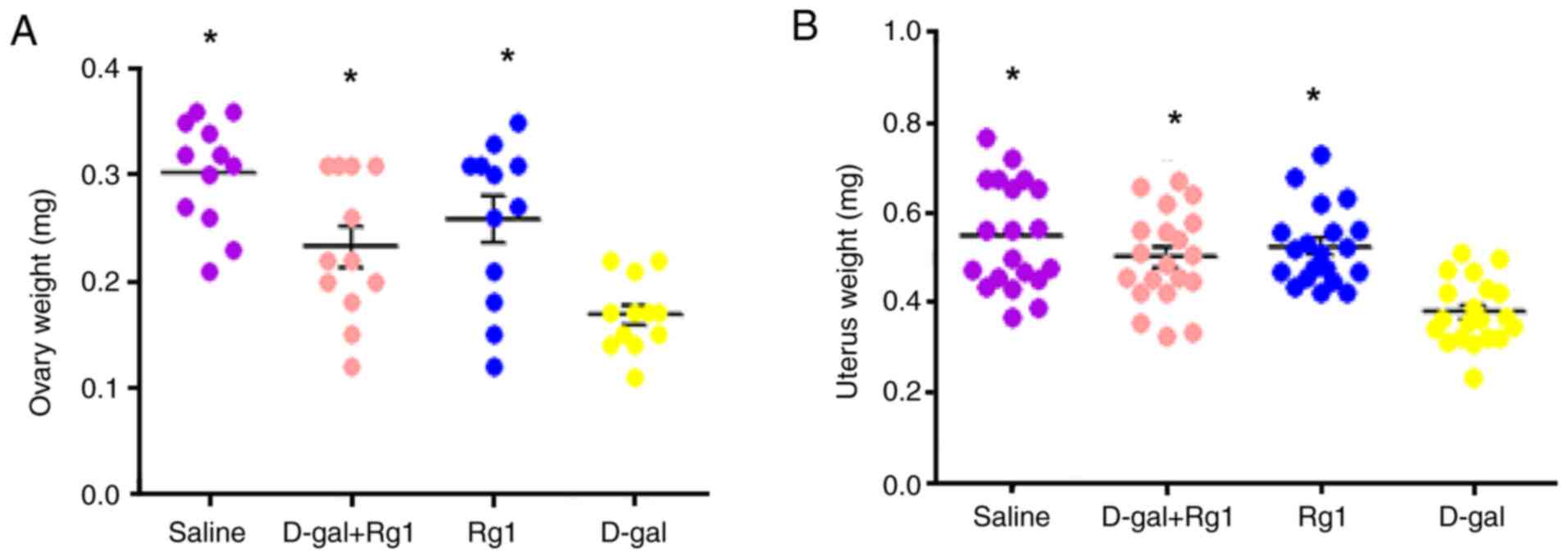
Rg1 increases the weight coefficient
of ovary and uterus
To investigate the effects of Rg1 on the mouse POI
model, the weights of ovary and uterus were analyzed after D-gal
and/or Rg1 administration. D-gal treatment significantly reduced
the ovary and uterus weight compared with that in the saline group
(Fig. 1). In addition, the weights
of these two organs in the Rg1 + D-gal group exhibited increases
compared with those in the D-gal group, suggesting that the
inhibitory effects of D-gal were reversed by the injection of Rg1.
However, no significant difference was found between the Rg1 and
saline groups (Fig. 1). Therefore,
these data suggest that Rg1 can improve the general condition of
POI model mice.
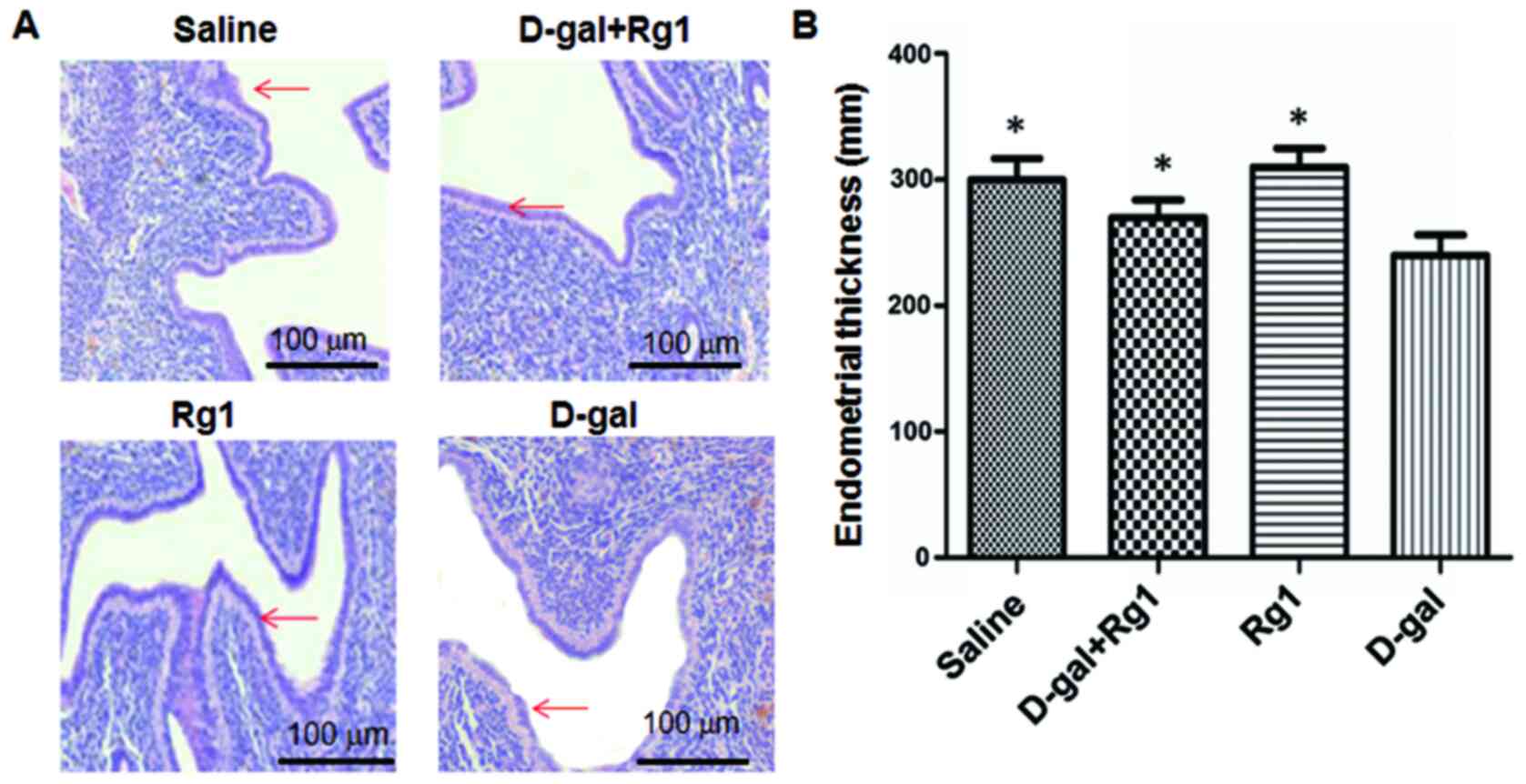
Rg1 improves pathological damages in
the ovary and uterus
Pathological changes in the ovary and uterus were
evaluated by the H&E staining (Fig.
2) and electron microscopy (Fig.
3). As shown in Fig. 2A, the
pathological changes in uterine muscles induced by D-gal were
improved by Rg1 treatment. Statistically, the endometrial thickness
of the Rg1 group was significantly higher than the D-gal group
(P<0.05; Fig. 2B). Electron
microscopy showed that large quantities of myeloid bodies could be
observed in the granule cells with nuclear brittle fissure, nuclear
dissolution and nucleation in the granule cells in uterus tissues
from the D-gal group (Fig. 3).
Additionally, there was necrotic tissue in the ovarian stroma in
the D-gal group. However, none were observed in tissues from the
Rg1, Rg1 + D-gal or saline groups. There were also large numbers of
myeloid bodies and fibrosis in the uterine muscle from the D-gal
group, which were found to be improved in that of Rg1, Rg1 + D-gal
and saline groups. These results suggest that Rg1 can alleviate
pathological damage in the ovary and uterus of mice following D-gal
induced POI.
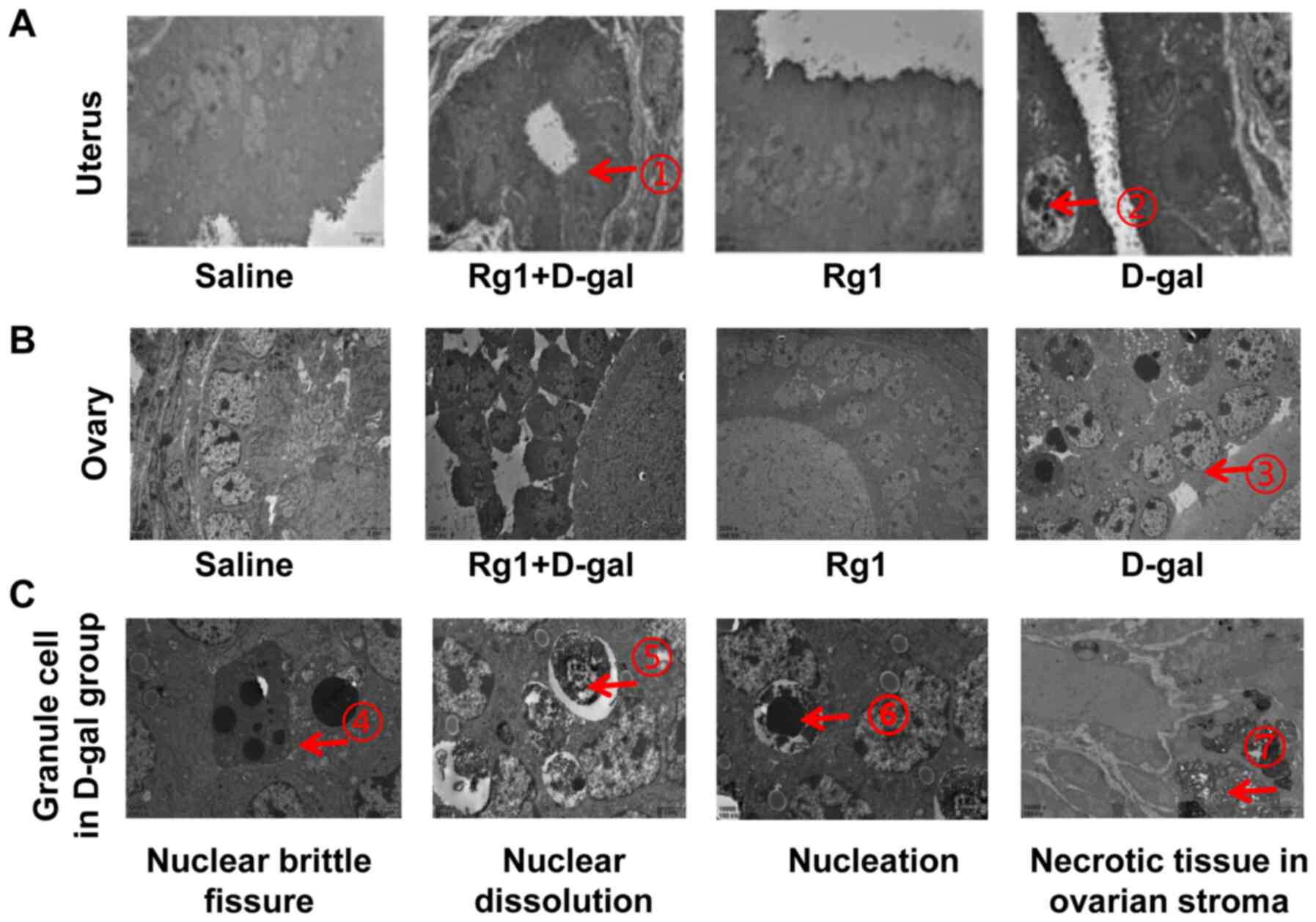 | Figure 3.Pathological changes in the uterus
and ovary as evaluated using electron microscopy. (A) In the
uterus, the endometrial glands were found in the Rg1 + D-gal group
as indicated by arrow 1 and 2 shows the myeloid bodies in the
granule cells. (B) There were also large amounts of myeloid bodies
in the granule cells of the ovary, as indicated by red arrow 3. (C)
In the granule cells of the D-gal group, arrow 4 indicates nuclear
brittle fissure, arrow 5 shows nuclear dissolution and arrow 6
indicates nucleation. However, none of these features could be
observed in the Rg1, Rg1 + D-gal or saline groups. Furthermore,
there were large numbers of myeloid bodies and larger extent of
fibrosis in the uterine muscle in the D-gal group, as indicated by
arrow 7, which were improved in the uterine muscle of Rg1, Rg1 +
D-gal and saline groups. Magnification, ×4. Rg1, ginsenoside Rg1;
D-gal, D-galactose. |
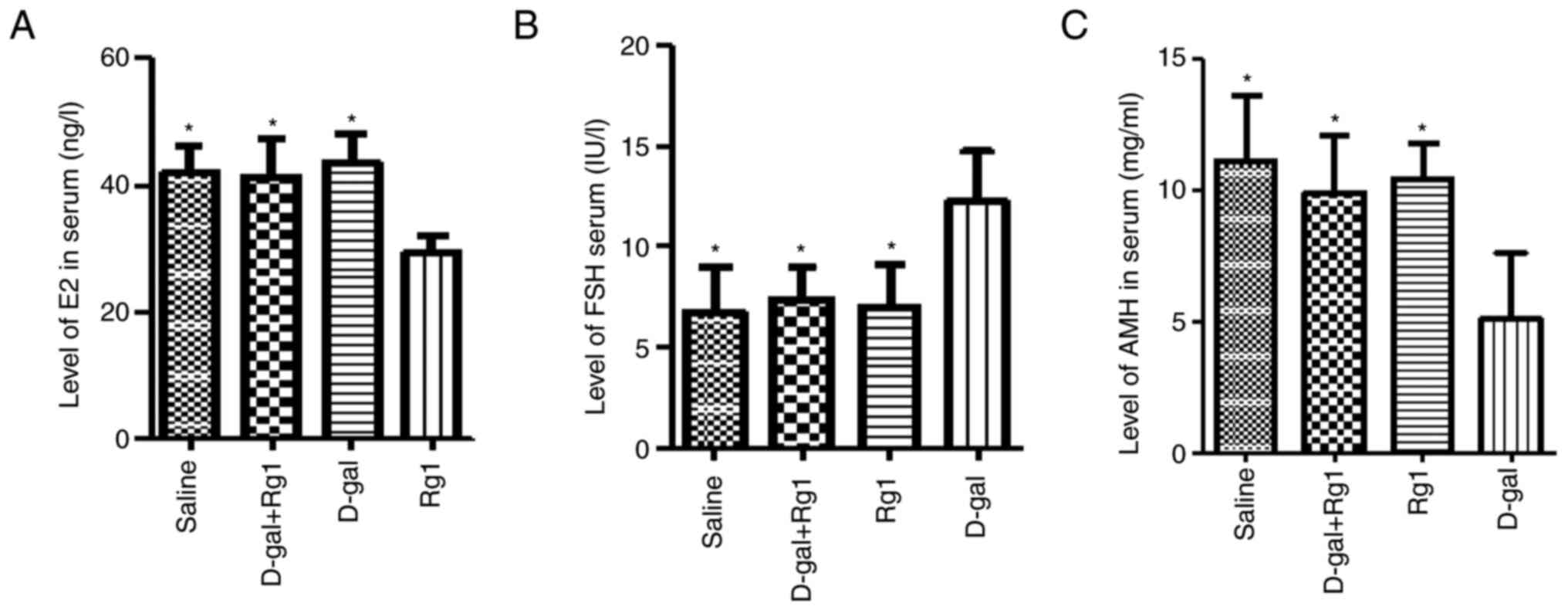
Rg1 treatment upregulates the levels
of E2, FSH and AMH in serum
Hormone levels after Rg1 treatment in the POI models
were next investigated. On day 42 of administration, there were
significantly lower AMH and E2 levels and significantly higher FSH
levels in the D-gal group compared with those in the other three
groups (Fig. 4). By contrast, Rg1 +
D-gal group exhibited comparable levels of these hormones compared
with those in the saline and Rg1 groups (Fig. 4). This observation suggests that Rg1
serves an anti-aging role in this D-gal-induced POI mouse
model.
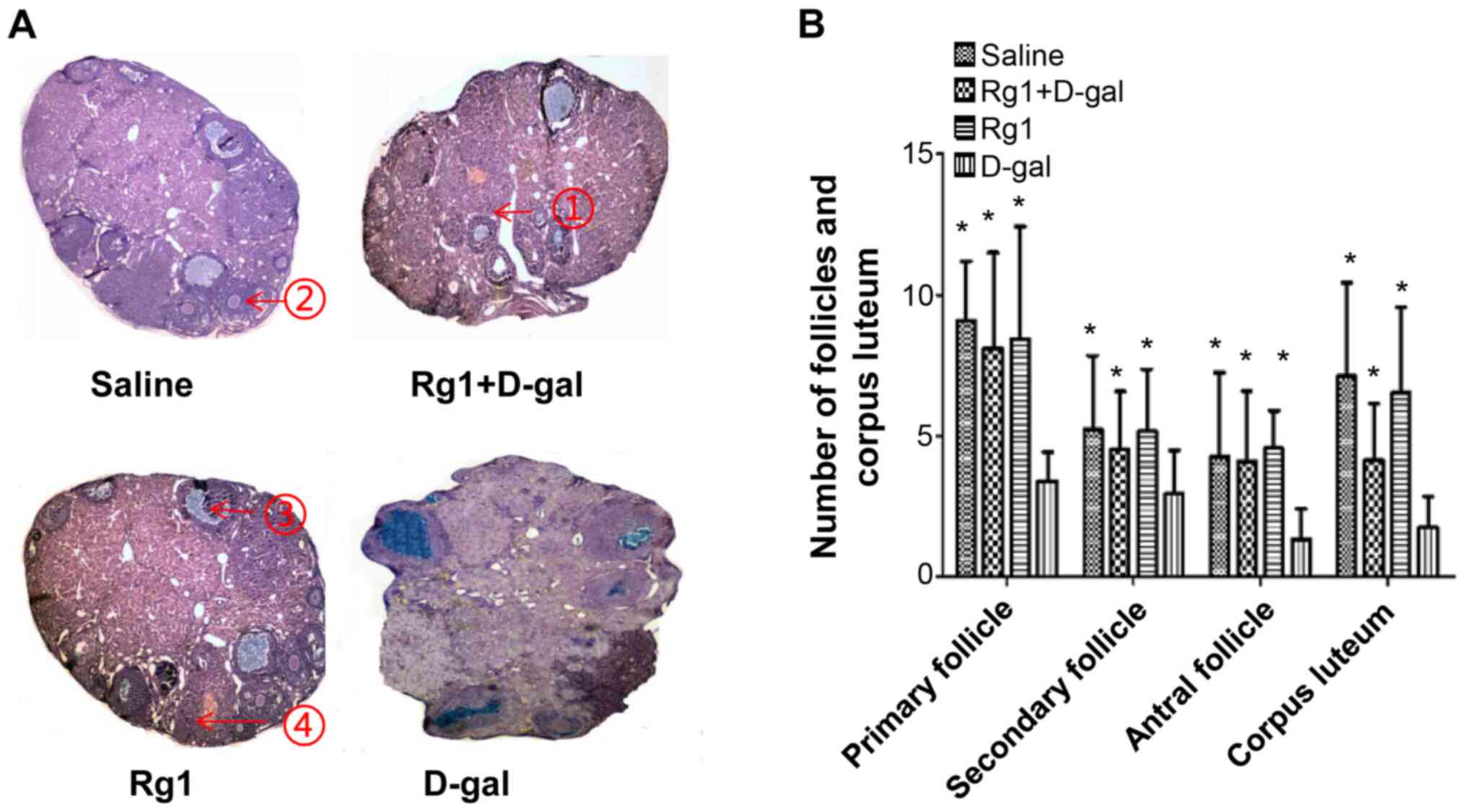
Rg1 improves the ovarian damage
induced by D-gal
The role of Rg1 on ovarian follicle maturation was
examined further using H&E staining. The numbers of primary,
secondary, sinus follicles and corpus luteum (CL) in the Rg1 +
D-gal group were higher compared with those in the D-gal group
(Fig. 5). At day 42 after D-gal
administration, Rg1, Rg1 + D-gal and saline groups exhibited
follicles of different stages and CL. However, a distinct ovary
architecture was observed in the D-gal group, where the ovary mass
was reduced and there were no small follicles, CL or antral
follicles (Fig. 5A). In addition,
ovaries from the D-gal + Rg1 group exhibited significantly more CL,
antral follicles and growing follicles compared with those in the
D-gal group (Fig. 5B). Ovaries from
the saline, Rg1 and Rg1 + D-gal groups also demonstrated similar
follicle numbers without significant differences among each other
(Fig. 5B). These results suggest
that Rg1 can improve the ovarian damages induced by D-gal treatment
in POI mice.
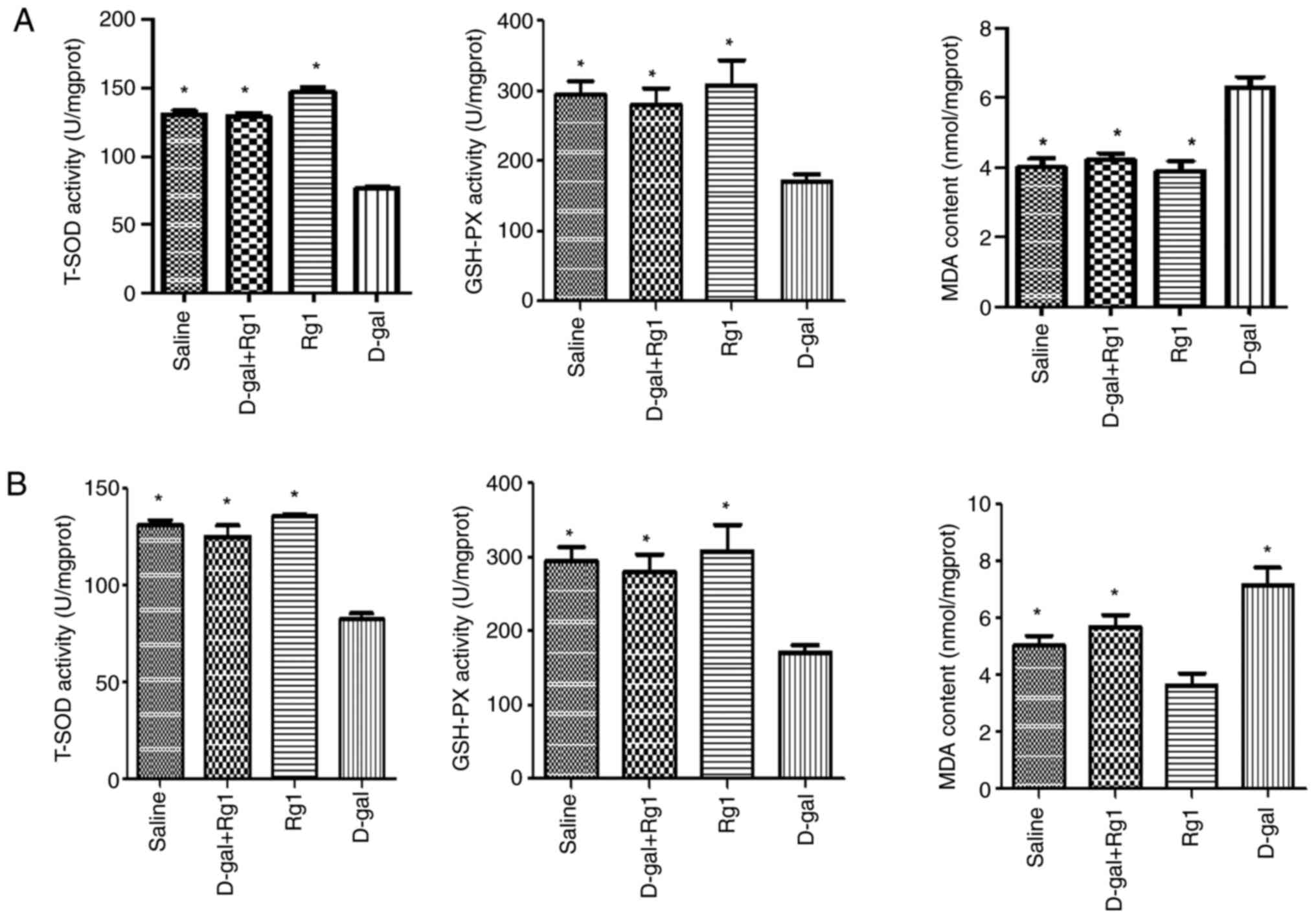
Anti-oxidative effects of Rg1 on ovary
and uterus of POI mice
The anti-oxidative effects of Rg1 were next
evaluated by measuring GSH-Px activity, MDA content and T-SOD
activity in the ovary and uterus. Compared with those in the saline
group, tissues from the D-gal group had significantly reduced
GSH-Px and T-SOD activities, but significantly increased MDA
content (Fig. 6). However, these
effects aforementioned were found to be significantly reversed by
Rg1 (Fig. 6). These results suggest
that Rg1 exerts anti-oxidative effects by enhancing the activities
of endogenous anti-oxidative defense enzymes.
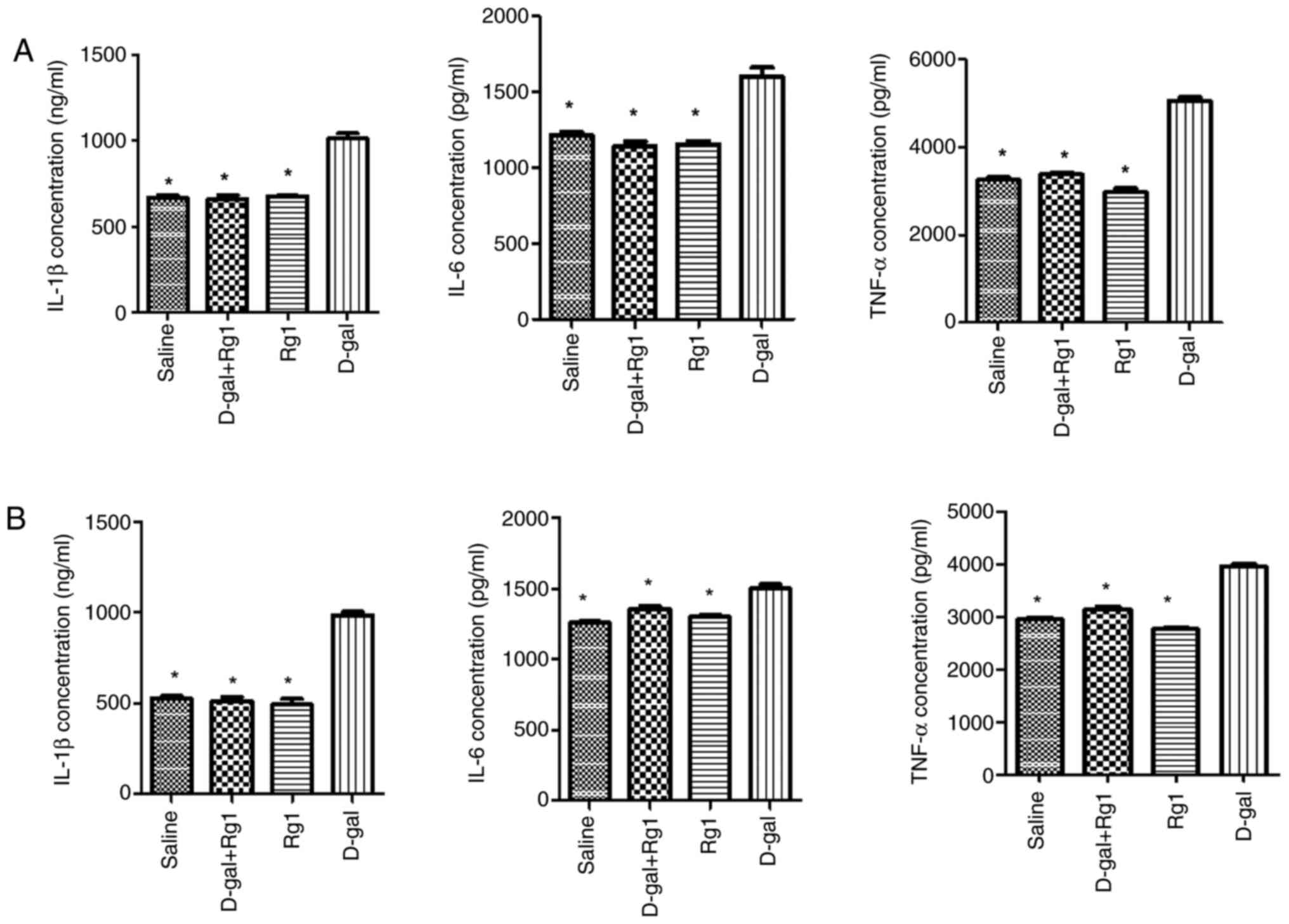
Rg1 decreases pro-inflammatory
cytokines levels in POI mice
Chronic inflammation in the ovary and uterus is
associated with POI (4). Therefore,
TNF-α, IL-6 and IL-1β levels in the ovary and uterus tissues were
subsequently measured using ELISA. Tissues from the D-gal + Rg1
group exhibited significantly reduced TNF-α, IL-6 and IL-1β levels
compared with those in the D-gal group (Fig. 7). This suggests that Rg1 treatment
alleviates inflammation in D-gal-induced aging in the ovary and
uterus.
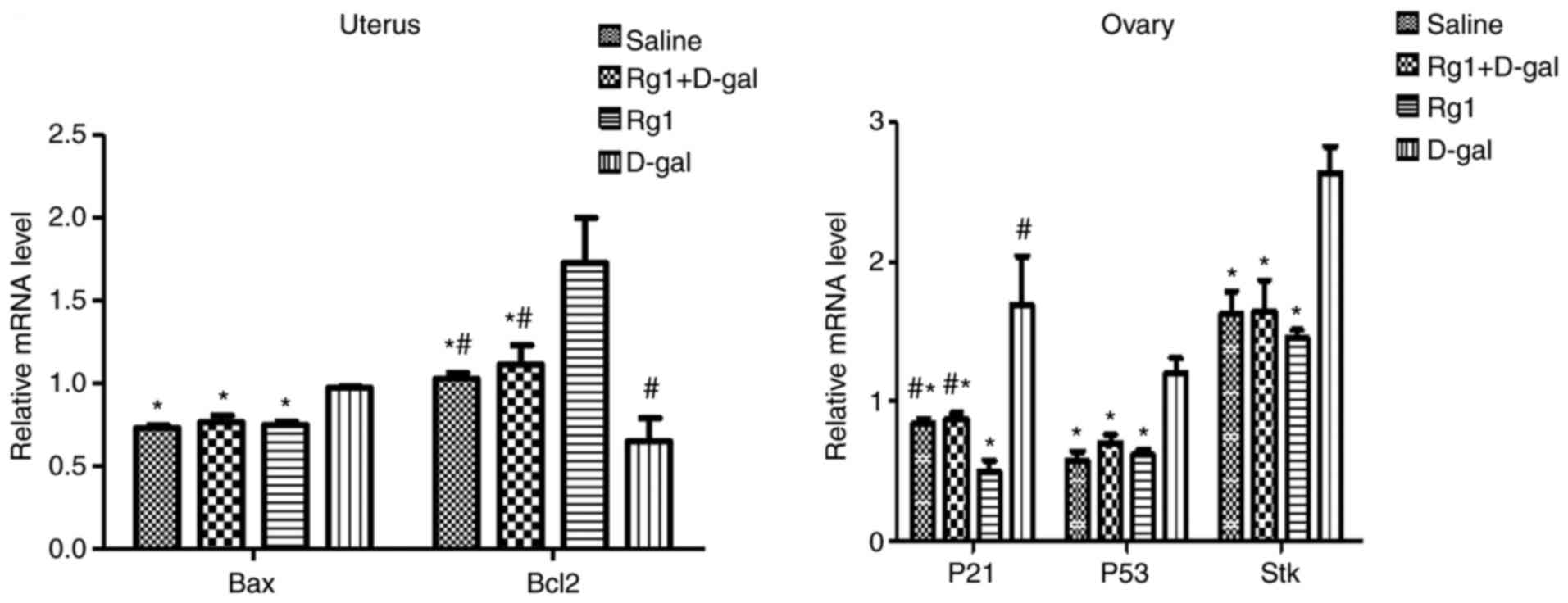
Rg1 affects aging-related signaling
pathways in ovary and uterus
To determine whether Rg1 could affect the signaling
pathways related to aging in the uterus (Bax-Bcl-2) and ovary
(p53-p21-STK) (34,35), RT-qPCR was performed. The relative
mRNA expression levels of Bax in the uterus, and p53, p21 and STK
in the ovary tissues of the D-gal group were significantly higher
compared with those in the Rg1 group (Fig. 8). By contrast, the relative mRNA
expression levels of Bcl-2 in the uterus of the D-gal group were
significantly lower compared with those in the Rg1 group (Fig. 8). These results suggest that Rg1 can
reverse the changes in the relative mRNA expression of Bax, p53,
p21, STK and Bcl-2 induced by D-gal in POI mouse models.
Discussion
Aging is the phenomenon of degenerative changes in
the structure and function of human cells and tissues, which is an
inevitable biological process (36). The main manifestations of this
process include organ weight loss, cell atrophy, cytoplasmic
pigmentation, reduced functional metabolism, weakened adaptive
capacity and low disease resistance (37). Investigation on aging has become a
research hotspot (38). POI
represents a spectrum of disorders in the female reproductive
system that leads to ovarian dysfunction (39). Its clinical criteria primarily
include: i) Secondary amenorrhea for ≥4 consecutive months; ii)
elevated FSH level (FSH >25 U/l, for two times, with the time
interval >4 weeks); and iii) low estradiol levels before the age
of 40 years (40). POI can result
in senile dementia, osteoporosis and climacteric syndrome in female
individuals, seriously affecting their health (41). It eventually develops into premature
ovarian failure (FSH >40 U/l) and the decreased estrogen levels,
which is accompanied by varying degrees of perimenopausal symptoms
and represents the end stage of POI (42). However, its pathogenesis remains
unclear.
Ginsenoside Rg1 can be isolated from Panax ginseng
and has been used in traditional Chinese medicine for >2,000
years. Rg1 has been documented to confer a range of effects on
aging rats and mice, including increasing mass index of uterus and
ovary, amelioration of perimenopausal syndromes, recovery of
estrous cycle, and promotion of hormone secretion (43,44).
In addition, Rg1 also exhibits antioxidant and
proliferation-promoting activities (43). Except for ovary and uterus, D-gal
administration has been previously demonstrated to cause
inflammatory damage to the liver, lungs and spleen (43). D-gal aging model was previously
established (45). In the present
study, this model was used to investigate the potential anti-aging
effects of Rg-1 on POI. Following treatment with D-gal, high
quantities of myeloid bodies, nuclear brittle fissure, nuclear
dissolution and nucleation were observed in the granule cells. This
was also coupled with the observation of necrotic tissue in the
ovarian stroma. In addition, myeloid bodies and fibrosis were
observed in the uterine muscle of the D-gal group after
administration of D-gal for 42 days, which was attenuated by Rg1
treatment. By contrast, D-gal treatment induced serious and
persistent damage in the ovaries. Additionally, D-gal significantly
decreased the secretion of sex hormones and the numbers of ovarian
follicles. These results suggest that D-gal treatment induced ovary
failure and reduced ovary function in female mice. However, Rg1
administration increased the weights of ovary and uterus in
D-gal-treated mice, demonstrating potential functional recovery in
the ovary and uterus caused by Rg1. Rg1 was also found to delay
ovary granular cell senescence (Fig.
3) and prevented endometrium destruction by mediating
anti-oxidant and anti-inflammatory effects. AMH can be used as one
of the biomarkers for assessing ovary function (46), whereas follicle counts can be
applied to reflect ovary reservation (47). In addition, endocrine function can
be reflected mainly by measuring hormone levels, including FSH, LH
and E2 (48).
Based on the provocative tests, dysfunctional gonads
may be observed in 75–96% of patients with galactosemia, which may
result in hypergonadotropic hypogonadism (43). This can be explained by previous
findings that galactose can attenuate FSH bioactivity and exert
direct toxic effects (49). In
addition, FSH can be used to indirectly reflect hypothalamic
response and ovary function (50).
AMH is secreted by primary granulosa cells through the preovulatory
follicles during the late preantral stage (51) that can be used to reflect ovary
function during classic galactosemia (52). It was found in the present study
that in mice treated with D-gal, serum FSH levels were
significantly increased, whilst the serum AMH and E2 levels were
significantly reduced in a manner that could be reversed by Rg1.
Therefore, this suggests that Rg1 can delay senescence induced by
D-gal.
Macroscopic ovary morphology parallels that of FSH
levels (53). Qin et al
(54) previously demonstrated that
the number of oocytes was significantly decreased in female rats
after exposure to high levels of galactose during gestation. Thakur
et al (55) demonstrated
that galactitol levels may affect follicular maturation and
ovulation. In the present study, D-gal treatment decreased follicle
numbers, which was reversed by Rg1. Serum FSH levels tend to be
higher during follicle development failure in patients with POI
patients, suggesting that FSH receptor function may be either
attenuated or impaired on granule cells, causing insensitivity to
FSH stimulation and resulting in inhibition of follicle growth
(56).
Although the mechanism of aging is complex, the free
oxygen radical theory has gained increasing attention (57). Under physiological conditions,
oxygen free radicals produced in the body are in maintained in
balance by the free radical scavenging system (58). When this balance is broken, the
activities of antioxidant enzymes (SOD) is reduced (59). Excessive generation of oxygen free
radicals would result in oxidative stress damage, leading to the
increased production of the lipid peroxide MDA and eventually cell
membrane damage, decreased tissue and organ function and subsequent
aging of the body (60). Therefore,
eliminating excess oxygen free radicals may serve to be an
important strategy to delay aging. Accumulation of galactitol
during galactosemia impairs free radical scavenging activities by
interfering with glutathione reductase (23). Therefore, antioxidant therapy may
minimize ovary and uterus damage in patients with galactosemia. In
the present study, Rg1 treatment increased T-SOD and GSH-Px
activities whilst decreasing MDA content. This suggests that Rg1
can protect the ovary against D-gal-induced oxidative stress,
possibly by savaging free radicals and activating antioxidant
enzymes. Notably, T-SOD activities were found to be increased in
the mice treated only with Rg1 compared with the D-gal group, in
line with previous studies (61–63),
further confirming the antioxidative effects of Rg1. Oocyte
apoptosis can be promoted by a number of factors, including Fas
ligands, pro-inflammatory cytokines, gonadal GnRH-like proteins and
androgens (64). In the present
study, Rg1 treatment inhibited the expression of pro-inflammatory
cytokines TNF-α, IL-6 and IL-1β, suggesting that it exerted
anti-inflammatory and oocyte-promoting effects.
p53 regulates p21 by modulating the expression of
the downstream gene P21, which is a cyclin-dependent kinase
inhibitor (24). The p21 protein
can in turn bind to the Cyclin-cdk complex, thereby inhibiting its
protein kinase activity towards the retinoblastoma protein
(65). Dephosphorylated Rb remains
bound to the transcriptional regulator E2F, preventing it from
activation, causing G1 arrest and cell senescence
(66). A previous study has shown
that the intrinsic death pathway mediated by p53 can induce
follicular atresia (67), where p21
could be transcriptionally activated by this accumulation of the
p53 protein (68,69). The present study found that D-gal
increased STK, p21 and p53 levels in a manner that was reversed by
Rg1, suggesting that Rg1 can delay ovary aging by downregulating
the expression of senescence markers. The pro-apoptotic protein Bax
and anti-apoptotic protein Bcl-2 are common markers of apoptosis
(70). Hussein et al
(71) previously found that the
Bcl-2 family member-mediated intrinsic pathway was the main
mechanism underlying uterine cellular apoptosis. Bcl2-Bax operates
antagonistically, which work by forming homodimers or heterodimers
(72). When this balance in their
expression is disrupted, they can form Bcl2-Bcl2, Bax-Bax
homodimers or even Bcl2-Bax heterodimers. If Bax levels are high,
the levels of Bax-Bax homodimers are increased, whilst Bcl2
activity would be decreased to accelerate apoptosis (73). By contrast, high levels of Bcl2
would lead to the formation of Bcl2-Bax heterodimers, which are
more stable compared with homodimers and can inhibit apoptosis
(74). The Bcl2/Bax ratio serves
key roles in ovarian granulocyte apoptosis (75). The present study revealed that D-gal
increased Bax expression whilst decreasing that of Bcl-2 to promote
apoptosis but Rg1 treatment inhibited apoptosis by upregulating Bax
and whilst downregulating Bcl-2, which delayed ovarian aging caused
by D-gal.
In conclusion, Rg1 was found to improve ovary and
uterus pathological damage in POI mouse models by promoting their
antioxidant and anti-inflammatory capacities. The underlying
molecular mechanism(s) may be associated with the activation of the
p21-p53-STK signaling pathway in the ovary and Bax-bcl2 in the
uterus.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China Grants (grant no. X2271), the
Guizhou Provincial Administration of traditional Chinese Medicine
and Science and Technology Research of traditional Chinese Medicine
(grant no. QZYY-2019-093) and the Science and Technology Project
Jointly Supported by the Zunyi City and The First People's Hospital
of Zunyi (grant no. 187; 2018).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZX, LH and XL designed the study. LH provided the
theoretical knowledge of POI, collected the experimental data,
performed the H&E staining, and the electron microscopy
experiments. XW assisted in the completion of electron microscopy
experiments and ELISA. DC performed the PCR assay, and collected
the experimental data. LH, ZX and XL interpreted the data. DC
searched the literature. LH prepared the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experimental procedures were performed in
accordance with the Institutional Animal Care and Use Committee of
Chongqing Medical University, which was approved by the
Institutional Animal Care and Use Committee of Chongqing Medical
University (Chongqing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
D-gal
|
D-galactose
|
|
POI
|
premature ovarian insufficiency
|
References
|
1
|
Rossetti R, Ferrari I, Bonomi M and
Persani L: Genetics of primary ovarian insufficiency. Clin Genet.
91:183–198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laven JS: Primary ovarian insufficiency.
Semin Reprod Med. 34:230–234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qin C, Chen Y, Lin Q, Yao J, Wu W and Xie
J: The significance of polymorphism and expression of oestrogen
metabolism-related genes in Chinese women with premature ovarian
insufficiency. Reprod Biomed Online. 35:609–615. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang Y, Hu C, Ye H, Luo R, Fu X, Li X,
Huang J, Chen W and Zheng Y: Inflamm-aging: A new mechanism
affecting premature ovarian insufficiency. J Immunol Res.
2019:80698982019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
European Society for Human Reproduction
and Embryology (ESHRE) Guideline Group on POI, ; Webber L, Davies
M, Anderson R, Bartlett J, Braat D, Cartwright B, Cifkova R, de
Muinck Keizer-Schrama S, Hogervorst E, et al: ESHRE guideline:
Management of women with premature ovarian insufficiency. Hum
Reprod. 31:926–937. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soman M, Huang LC, Cai WH, Xu JB, Chen JY,
He RK, Ruan HC, Xu XR, Qian ZD and Zhu XM: Serum androgen profiles
in women with premature ovarian insufficiency: A systematic review
and meta-analysis. Menopause. 26:78–93. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vujović S, Ivovic M, Tančić-Gajić M,
Marina L, Ljubic A, Dragojević-Dikić S and Genazzani AR:
Endometrium receptivity in premature ovarian insufficiency-how to
improve fertility rate and predict diseases? Gynecol Endocrinol.
34:1011–1015. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patel H, Arruarana V, Yao L, Cui X and Ray
E: Effects of hormones and hormone therapy on breast tissue in
transgender patients: A concise review. Endocrine. 68:6–15. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu C, Wang Y, Liu H, Mu H, Lu Y, Zhang J
and Huang J: Oral administration of Ginsenoside Rg1 prevents
cardiac toxicity induced by doxorubicin in mice through
anti-apoptosis. Oncotarget. 8:83792–83801. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu J, Mu X, Zeng J, Xu C, Liu J, Zhang M,
Li C, Chen J, Li T and Wang Y: Ginsenoside Rg1 prevents cognitive
impairment and hippocampus senescence in a rat model of
D-galactose-induced aging. PLoS One. 9:e1012912014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang N, Zhou B, Wang Y, Li Y, Liu J, Li P,
Yang N, Zhou B, Wang Y, et al: Research progress of the biological
activity of the ginsenoside Rg. Chinese J Traditional Chinese Med.
33:1463–1465. 2018.(In Chinese).
|
|
12
|
Tang F, Lu M, Yu L, Wang Q, Mei M, Xu C,
Han R, Hu J, Wang H and Zhang Y: Inhibition of TNF-α-mediated NF-κB
activation by ginsenoside Rg1 contributes the attenuation of
cardiac hypertrophy induced by abdominal aorta coarctation. J
Cardiovasc Pharmacol. 68:257–264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee HY, Park SH, Chae SW, Soung NK, Oh MJ,
Kim JS, Kim YO and Chae HJ: Aqueous ginseng extract has a
preventive role in RANKL-induced osteoclast differentiation and
estrogen deficiency-induced osteoporosis. J Functional Foods.
13:192–203. 2015. View Article : Google Scholar
|
|
14
|
Cui Y, Shu XO, Gao YT, Cai H, Tao MH and
Zheng W: Association of ginseng use with survival and quality of
life among breast cancer patients. Am J Epidemiol. 163:645–653.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ryan J, Scali J, Carriere I, Ritchie K and
Ancelin ML: Hormonal treatment, mild cognitive impairment and
Alzheimer's disease. Int Psychogeriatr. 20:47–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao E and Mu Q: Phytoestrogen biological
actions on Mammalian reproductive system and cancer growth. Sci
Pharm. 79:1–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng Y, Shen LH and Zhang JT:
Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and
its mechanism of action. Acta Pharmacol Sin. 26:143–149. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Cai D, Yao X, Zhang Y, Chen L, Jing
P, Wang L and Wang Y: Protective effect of ginsenoside Rg1 on
hematopoietic stem/progenitor cells through attenuating oxidative
stress and the Wnt/β-Catenin signaling pathway in a mouse model of
d-Galactose-induced aging. Int J Mol Sci. 17:8492016. View Article : Google Scholar
|
|
19
|
He L: Establishment of D-gal induced
premature ovarian failure animal model. Proc Clin Med. 25:762–764.
2016.(In Chinese).
|
|
20
|
Huang JL, Yu C, Su M, Yang SM, Zhang F,
Chen YY, Liu JY, Jiang YF, Zhong ZG, et al: Probucol, a
“non-statin” cholesterol-lowering drug, ameliorates D-galactose
induced cognitive deficits by alleviating oxidative stress via
Keap1/Nrf2 signaling pathway in mice. Aging (Albany NY).
11:8542–8555. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thakur M, Shaeib F, Khan SN, Kohan-Ghadr
HR, Jeelani R, Aldhaheri SR, Gonik B and Abu-Soud HM: Galactose and
its metabolites deteriorate metaphase II mouse oocyte quality and
subsequent embryo development by disrupting the spindle structure.
Sci Rep. 7:2312017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Banerjee S, Chakraborty P, Saha P,
Bandyopadhyay SA, Banerjee S and Kabir SN: Ovotoxic effects of
galactose involve attenuation of follicle-stimulating hormone
bioactivity and up-regulation of granulosa cell p53 expression.
PLoS One. 7:e307092012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sozen B, Ozekinci M, Erman M, Gunduz T,
Demir N and Akouri R: Dehydroepiandrosterone supplementation
attenuates ovarian ageing in a galactose-induced primary ovarian
insufficiency rat model. J Assist Reprod Genet. 36:2181–2189. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim EM, Jung CH, Kim J, Hwang SG, Park JK
and Um HD: The p53/p21 complex regulates cancer cell invasion and
apoptosis by targeting Bcl-2 family proteins. Cancer Res.
77:3092–3100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zińczuk J, Zaręba K, Guzińska-Ustymowicz
K, Kędra B, Kemona A and Pryczynicz A: p16, p21, and p53 proteins
play an important role in development of pancreatic intraepithelial
neoplastic. Ir J Med Sci. 187:629–637. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ibnat N, Kamaruzman NI, Ashaie M and
Chowdhury EH: Transfection with p21 and p53 tumor suppressor
plasmids suppressed breast tumor growth in syngeneic mouse model.
Gene. 701:32–40. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fujino T, Yokokawa R, Oshima T and
Hayakawa M: SIRT1 knockdown up-regulates p53 and p21/Cip1
expression in renal adenocarcinoma cells but not in normal
renal-derived cells in a deacetylase-independent manner. J Toxicol
Sci. 43:711–715. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dmitrieva AI, Serebryakova VA, Rakitin SS,
Kudyakov LA, Novitskii VV, Yankovich KI and Sevostyanova NV: Study
of the association of polymorphisms of p53 and p21 with the risk of
development of stomach cancer. Bull Exp Biol Med. 164:95–98. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramadan MA, Shawkey AE, Rabeh MA and
Abdellatif AO: Expression of P53, BAX, and BCL-2 in human malignant
melanoma and squamous cell carcinoma cells after tea tree oil
treatment in vitro. Cytotechnology. 71:461–473. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cicero L, Fazzotta S, Palumbo VD, Cassata
G and Lo Monte AI: Anesthesia protocols in laboratory animals used
for scientific purposes. Acta Biomed. 89:337–342. 2018.PubMed/NCBI
|
|
31
|
Boivin GP, Bottomley MA, Schiml PA, Goss L
and Grobe N: Physiologic, behavioral, and histologic responses to
various euthanasia methods in C57BL/6NTac male mice. J Am Assoc Lab
Anim Sci. 56:69–78. 2017.PubMed/NCBI
|
|
32
|
Wang Q, Yin J, Wang S, Cui D, Lin H, Ge M,
Dai Z, Xie L, Si J, Ma K, et al: Effects of activin A and its
downstream ERK1/2 in oxygen and glucose deprivation after
isoflurane-induced postconditioning. Biomed Pharmacother.
84:535–543. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saat N, Risvanli A, Dogan H, Onalan E,
Akpolat N, Seker I and Sahna E: Effect of melatonin on torsion and
reperfusion induced pathogenesis of rat uterus. Biotech Histochem.
94:533–539. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen MJ, Chou CH, Shun CT, Mao TL, Wen WF,
Chen CD, Chen SU, Yang YS and Ho HN: Iron suppresses ovarian
granulosa cell proliferation and arrests cell cycle through
regulating p38 mitogen-activated protein kinase/p53/p21 pathway.
Biol Reprod. 97:438–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
da Costa JP, Vitorino R, Silva GM, Vogel
C, Duarte AC and Rocha-Santos T: A synopsis on aging-theories,
mechanisms and future prospects. Ageing Res Rev. 29:90–112. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Flatt T and Partridge L: Horizons in the
evolution of aging. BMC Biol. 16:932018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun C, Fan S, Wang X, Lu J, Zhang Z, Wu D,
Shan Q and Zheng Y: Purple sweet potato color inhibits endothelial
premature senescence by blocking the NLRP3 inflammasome. J Nutr
Biochem. 26:1029–1040. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sullivan SD, Sarrel PM and Nelson LM:
Hormone replacement therapy in young women with primary ovarian
insufficiency and early menopause. Fertil Steril. 106:1588–1599.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Riegle van West K, Stinear C and Buck R:
The effects of poi on physical and cognitive function in healthy
older adults. J Aging Phys Act. Nov 8–2018.(Online ahead of print).
PubMed/NCBI
|
|
41
|
Sundaresan NR, Saxena VK, Sastry KV,
Nagarajan K, Jain P, Singh R, Anish D, Ravindra PV, Saxena M and
Ahmed KA: Cytokines and chemokines in postovulatory follicle
regression of domestic chicken (Gallus gallus domesticus).
Dev Comp Immunol. 32:253–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He L, Ling L, Wei T, Wang Y and Xiong Z:
Ginsenoside Rg1 improves fertility and reduces ovarian pathological
damages in premature ovarian failure model of mice. Exp Biol Med
(Maywood). 242:683–691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mou Z, Huang Q, Chu SF, Zhang MJ, Hu JF,
Chen NH and Zhang JT: Antidepressive effects of ginsenoside Rg1 via
regulation of HPA and HPG axis. Biomed Pharmacother. 92:962–971.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mo J, Zhou Y, Yang R, Zhang P, He B, Yang
J, Li S, Shen Z and Chen P: Ginsenoside Rg1 ameliorates palmitic
acid-induced insulin resistance in HepG2 cells in association with
modulating Akt and JNK activity. Pharmacol Rep. 71:1160–1167. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yan Z, Dai Y, Fu H, Zheng Y, Bao D, Yin Y,
Chen Q, Nie X, Hao Q, Hou D and Cui Y: Curcumin exerts a protective
effect against premature ovarian failure in mice. J Mol Endocrinol.
60:261–271. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mullen RD, Ontiveros AE, Moses MM and
Behringer RR: AMH and AMHR2 mutations: A spectrum of reproductive
phenotypes across vertebrate species. Dev Biol. 455:1–9. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Roness H and Meirow D: Fertility
preservation: Follicle reserve loss in ovarian tissue
transplantation. Reproduction. 158:F35–F44. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Warren L, Murawski M, Wilk K, Zieba DA and
Bartlewski PM: Suitability of antral follicle counts and
computer-assisted analysis of ultrasonographic and magnetic
resonance images for estimating follicular reserve in porcine,
ovine and bovine ovaries ex situ. Exp Biol Med (Maywood).
240:576–584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Song D, Zhong Y, Qian C, Zou Q, Ou J, Shi
Y, Gao L, Wang G, Liu Z, Li H, et al: Human umbilical cord
mesenchymal stem cells therapy in cyclophosphamide-induced
premature ovarian failure rat model. Biomed Res Int.
2016:25175142016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu Y, Li YJ and Wang L: Changes of
expression of p53-p21 in premature ovarian failure in fluorosis
female mice. Chongqing Med. 13:1712–1715. 2018.
|
|
51
|
Podfigurna-Stopa A, Czyzyk A, Grymowicz M,
Smolarczyk R, Katulski K, Czajkowski K and Meczekalski B: Premature
ovarian insufficiency: The context of long-term effects. J
Endocrinol Invest. 39:983–990. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
He L: Preliminary study on animal model of
ovarian premature failure induced by d-galactose. Clin Med
Practice. 10:762–764. 2016.
|
|
53
|
Ali DE, Shah M, Ali A, Malik MO, Rehman F,
Badshah H, Ehtesham E and Vitale SG: Treatment with metformin and
combination of metformin plus pioglitazone on serum levels of IL-6
and IL-8 in polycystic ovary syndrome: A randomized clinical trial.
Horm Metab Res. 51:714–722. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Qin C, Yuan Z, Yao J, Zhu W, Wu W and Xie
J: AMH and AMHR2 genetic variants in Chinese women with primary
ovarian insufficiency and normal age at natural menopause. Reprod
Biomed Online. 3:311–318. 2014. View Article : Google Scholar
|
|
55
|
Thakur M, Feldman G and Puscheck EE:
Primary ovarian insufficiency in classic galactosemia: Current
understanding and future research opportunities. J Assist Reprod
Genet. 35:3–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang W, Wang Y, Wu J, Yang L and Liu Z:
Protective effects of electroacupuncture pretreatment on ovarian in
rats with premature ovarian insufficiency. Zhongguo Zhen Jiu.
38:405–411. 2018.(In Chinese). PubMed/NCBI
|
|
57
|
Yuan S, Wen J, Cheng J, Shen W, Zhou S,
Yan W, Shen L, Luo A and Wang S: Age-associated up-regulation of
EGR1 promotes granulosa cell apoptosis during follicle atresia in
mice through the NF-κB pathway. Cell Cycle. 15:2895–2905. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang F, Pei R, Zhang Z, Liao J, Yu W, Qiao
N, Han Q, Li Y, Hu L, Guo J, et al: Copper induces oxidative stress
and apoptosis through mitochondria-mediated pathway in chicken
hepatocytes. Toxicol In Vitro. 54:310–316. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Falfushynska HI, Gnatyshyna LL, Deneha HV,
Osadchuk OY and Stoliar OB: Manifestations of oxidative stress and
molecular damages in ovarian cancer tissue. Ukr Biochem J.
87:93–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang L, Kong XJ, Wang ZQ, Xu FS and Zhu
YT: A study on neuroprotective effects of curcumin on the diabetic
rat brain. J Nutr Health Aging. 20:835–840. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Biswas P, Mukhopadhyay A, Kabir SN and
Mukhopadhyay PK: High-protein diet ameliorates arsenic-induced
oxidative stress and antagonizes uterine apoptosis in rats. Biol
Trace Elem Res. 192:222–233. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xie W, Zhou P, Sun Y, Meng X, Dai Z, Sun G
and Sun X: Protective effects and target network analysis of
ginsenoside Rg1 in cerebral ischemia and reperfusion injury: A
comprehensive overview of experimental studies. Cells. 7:2702018.
View Article : Google Scholar
|
|
63
|
Fan C, Song Q, Wang P, Li Y, Yang M and Yu
SY: Neuroprotective effects of ginsenoside-Rg1 against
depression-like behaviors via suppressing glial activation,
synaptic deficits, and neuronal apoptosis in rats. Front Immunol.
9:28892018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yang MZ, Li SG, et al: Effect of
ginsenosideRg3 on ageing of vascular smooth muscle cells and itsm
echanism. Heart BrainVessel Dis. 17:1079–1082. 2015.
|
|
65
|
Chen M, Zhang H, Zhang G, Zhong A, Ma Q,
Kai J, Tong Y, Xie S, Wang Y, Zheng H, et al: Targeting TPX2
suppresses proliferation and promotes apoptosis via repression of
the PI3k/AKT/P21 signaling pathway and activation of p53 pathway in
breast cancer. Biochem Biophys Res Commun. 507:74–82. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Uxa S, Bernhart SH, Mages CFS, Fischer M,
Kohler R, Hoffmann S, Stadler PF, Engeland K and Müller GA: DREAM
and RB cooperate to induce gene repression and cell-cycle arrest in
response to p53 activation. Nucleic Acids Res. 47:9087–9103. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ma M, Chen XY, Li B and Li XT: Melatonin
protects premature ovarian insufficiency induced by tripterygium
glycosides: Role of SIRT1. Am J Transl Res. 9:1580–1602.
2017.PubMed/NCBI
|
|
68
|
Hussein MR: Apoptosis in the ovary:
Molecular mechanisms. Hum Reprod Update. 11:162–177. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
el-Deiry WS, Tokino T, Velculescu VE, Levy
DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW and
Vogelstein B: WAF1, a potential mediator of p53 tumor suppression.
Cell. 75:817–825. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hussein MR, Haemel AK and Wood GS:
Apoptosis and melanoma: Molecular mechanisms. J Pathol.
199:275–288. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hormozi M, Ghoreishi S and Baharvand P:
Astaxanthin induces apoptosis and increases activity of antioxidant
enzymes in LS-180 cells. Artif Cells Nanomed Biotechnol.
47:891–895. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Vartak SV, Iyer D, Santhoshkumar TR,
Sharma S, Mishra A, Goldsmith G, Srivastava M, Srivastava S, Karki
SS, Surolia A, et al: Novel BCL2 inhibitor, Disarib induces
apoptosis by disruption of BCL2-BAK interaction. Biochem Pharmacol.
131:16–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Rahmani M, Nkwocha J, Hawkins E, Pei X,
Parker RE, Kmieciak M, Leverson JD, Sampath D, Ferreira-Gonzalez A
and Grant S: Cotargeting BCL-2 and PI3K induces BAX-dependent
mitochondrial apoptosis in AML cells. Cancer Res. 78:3075–3086.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mousa AM, Al-Fadhli AS, Rao MS and
Kilarkaje N: Gestational lead exposure induces developmental
abnormalities and up-regulates apoptosis of fetal cerebellar cells
in rats. Drug Chem Toxicol. 38:73–83. 2015. View Article : Google Scholar : PubMed/NCBI
|