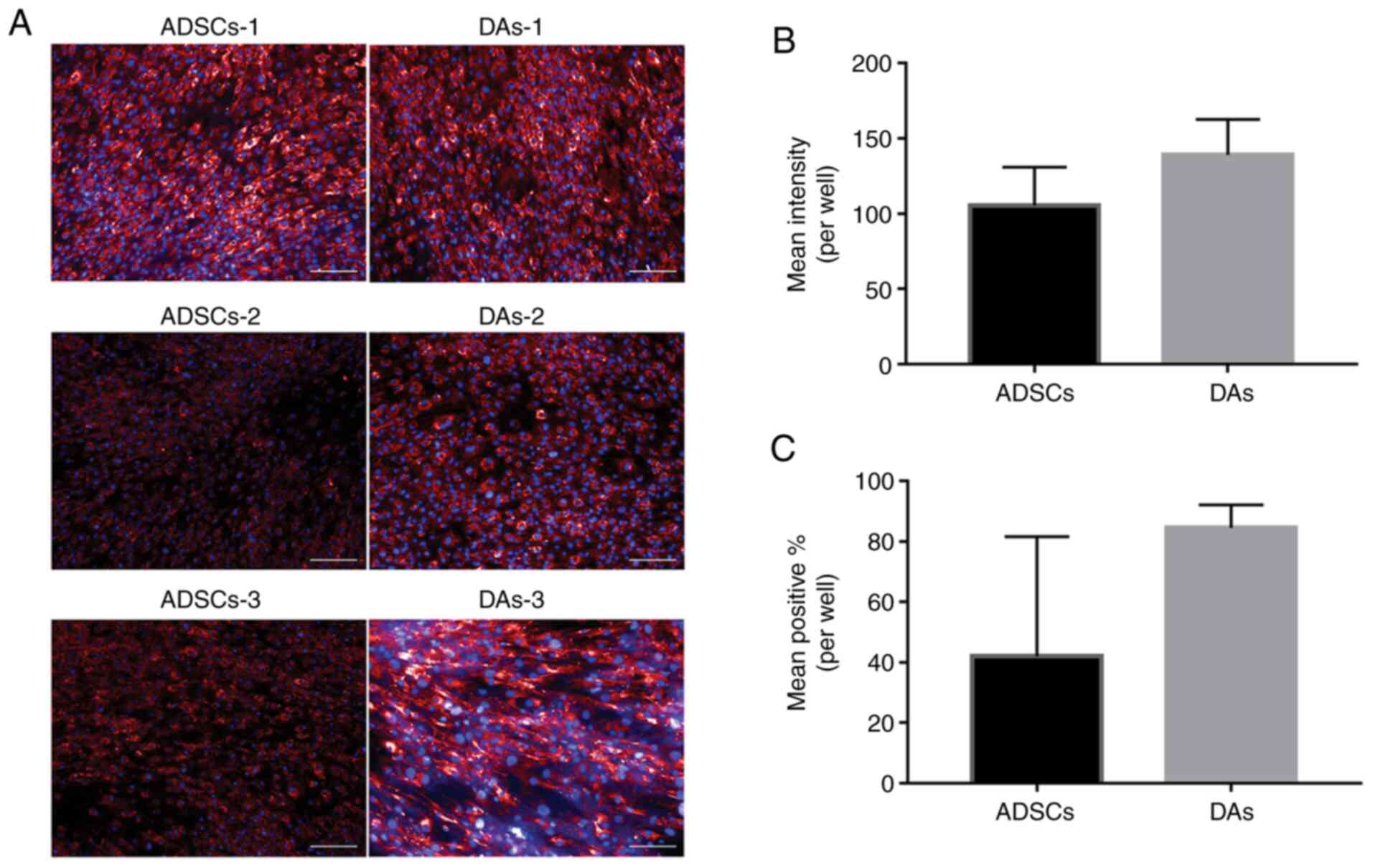
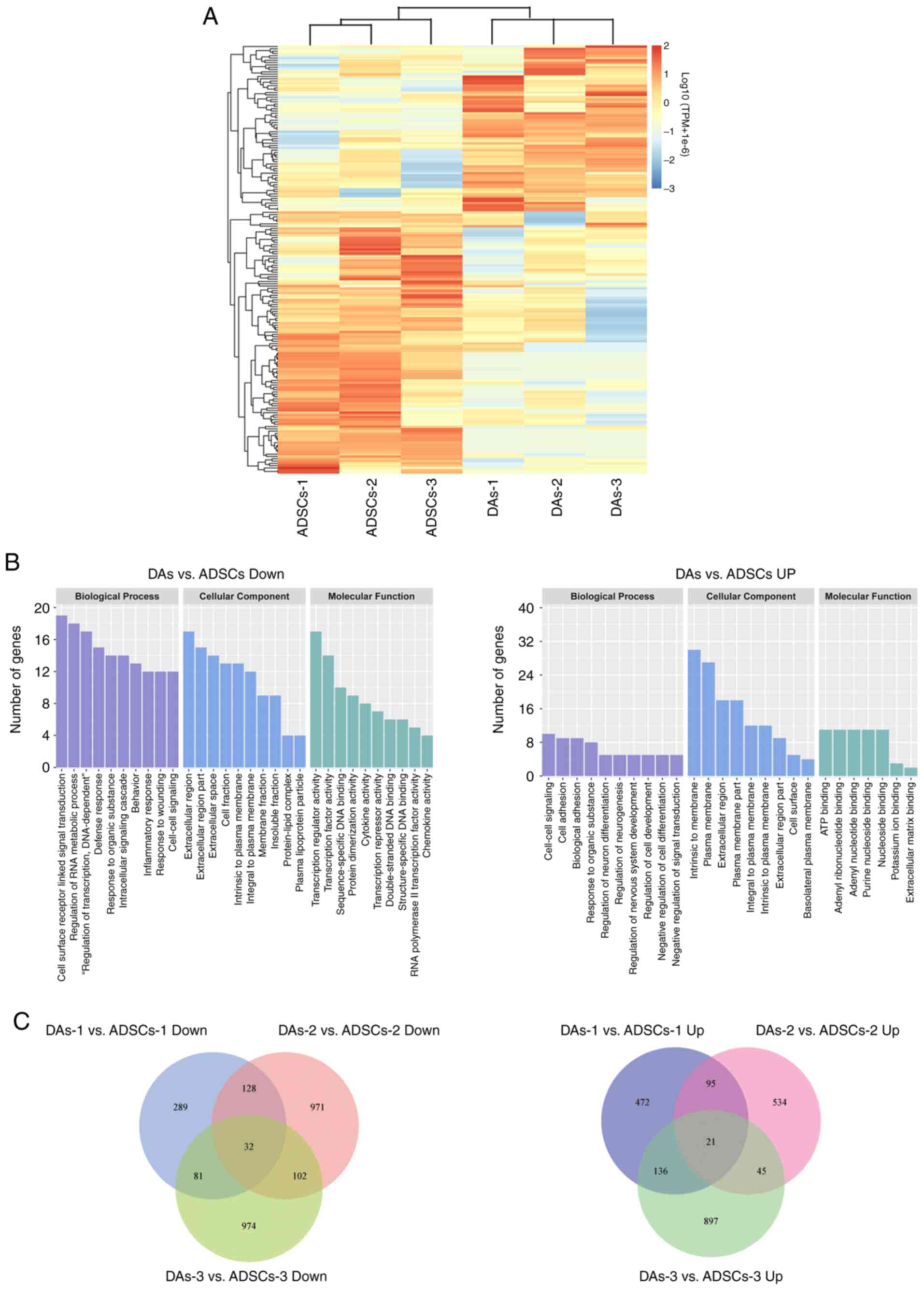
|
1
|
Wu S, Coombs DM and Gurunian R:
Liposuction: Concepts, safety, and techniques in body-contouring
surgery. Cleve Clin J Med. 87:367–375. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Svalgaard JD, Juul S, Vester-Glovinski PV,
Haastrup EK, Ballesteros OR, Lynggaard CD, Jensen AK,
Fischer-Nielsen A, Herly M and Munthe-Fog L: Lipoaspirate storage
time and temperature: Effects on stromal vascular fraction quality
and cell composition. Cells Tissues Organs. 209:54–63. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choudhery MS, Badowski M, Muise A, Pierce
J and Harris DT: Donor age negatively impacts adipose
tissue-derived mesenchymal stem cell expansion and differentiation.
J Transl Med. 12:82014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kornicka K, Marycz K, Tomaszewski KA,
Marędziak M and Śmieszek A: The effect of age on osteogenic and
adipogenic differentiation potential of human adipose derived
stromal stem cells (hASCs) and the impact of stress factors in the
course of the differentiation process. Oxid Med Cell Longev.
2015:3091692015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nordberg RC, Zhang J, Griffith EH, Frank
MW, Starly B and Loboa EG: Electrical cell-substrate impedance
spectroscopy can monitor age-grouped human adipose stem cell
variability during osteogenic differentiation. Stem Cells Transl
Med. 6:502–511. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu M, Kohan E, Bradley J, Hedrick M,
Benhaim P and Zuk P: The effect of age on osteogenic, adipogenic
and proliferative potential of female adipose-derived stem cells. J
Tissue Eng Regen Med. 3:290–301. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sasahara Y, Kubota Y, Kosaka K, Adachi N,
Yamaji Y, Nagano H, Akita S, Kuroda M, Tanaka T, Bujo H, et al:
Adipose-derived stem cells and ceiling culture-derived
preadipocytes cultured from subcutaneous fat tissue differ in their
epigenetic characteristics and osteogenic potential. Plast Reconstr
Surg. 144:644–655. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zuk PA, Zhu M, Ashjian P, De Ugarte DA,
Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick
MH: Human adipose tissue is a source of multipotent stem cells. Mol
Biol Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kunze KN, Burnett RA, Wright-Chisem J,
Frank RM and Chahla J: Adipose-derived mesenchymal stem cell
treatments and available formulations. Curr Rev Musculoskelet Med.
13:264–280. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsubosaka M, Matsumoto T, Sobajima S,
Matsushita T, Iwaguro H and Kuroda R: The influence of
adipose-derived stromal vascular fraction cells on the treatment of
knee osteoarthritis. BMC Musculoskelet Disord. 21:2072020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramakrishnan VM and Boyd NL: The adipose
stromal vascular fraction as a complex cellular source for tissue
engineering applications. Tissue Eng Part B Rev. 24:289–299. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heo JS, Choi Y, Kim HS and Kim HO:
Comparison of molecular profiles of human mesenchymal stem cells
derived from bone marrow, umbilical cord blood, placenta and
adipose tissue. Int J Mol Med. 37:115–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naderi N, Combellack EJ, Griffin M,
Sedaghati T, Javed M, Findlay MW, Wallace CG, Mosahebi A, Butler
PE, Seifalian AM, et al: The regenerative role of adipose-derived
stem cells (ADSC) in plastic and reconstructive surgery. Int Wound
J. 14:112–124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sugihara H, Yonemitsu N, Miyabara S and
Yun K: Primary cultures of unilocular fat cells: Characteristics of
growth in vitro and changes in differentiation properties.
Differentiation. 31:42–49. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jumabay M and Boström KI: Dedifferentiated
fat cells: A cell source for regenerative medicine. World J Stem
Cells. 7:1202–1214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kishimoto N, Momota Y, Hashimoto Y,
Tatsumi S, Ando K, Omasa T and Kotani J: The osteoblastic
differentiation ability of human dedifferentiated fat cells is
higher than that of adipose stem cells from the buccal fat pad.
Clin Oral Investig. 18:1893–1901. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watson JE, Patel NA, Carter G, Moor A,
Patel R, Ghansah T, Mathur A, Murr MM, Bickford P, Gould LJ, et al:
Comparison of markers and functional attributes of human
adipose-derived stem cells and dedifferentiated adipocyte cells
from subcutaneous fat of an obese diabetic donor. Adv Wound Care
(New Rochelle). 3:219–228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saler M, Caliogna L, Botta L, Benazzo F,
Riva F and Gastaldi G: hASC and DFAT, multipotent stem cells for
regenerative medicine: A comparison of their potential
differentiation in vitro. Int J Mol Sci. 18:182017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reumann MK, Linnemann C, Aspera-Werz RH,
Arnold S, Held M, Seeliger C, Nussler AK and Ehnert S: Donor site
location is critical for proliferation, stem cell capacity, and
osteogenic differentiation of adipose mesenchymal stem/stromal
cells: Implications for bone tissue engineering. Int J Mol Sci.
19:18682018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bi H, Li H, Zhang C, Mao Y, Nie F, Xing Y,
Sha W, Wang X, Irwin DM and Tan H: Stromal vascular fraction
promotes migration of fibroblasts and angiogenesis through
regulation of extracellular matrix in the skin wound healing
process. Stem Cell Res Ther. 10:3022019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tansriratanawong K, Tabei I, Ishikawa H,
Ohyama A, Toyomura J and Sato S: Characterization and comparative
DNA methylation profiling of four adipogenic genes in
adipose-derived stem cells and dedifferentiated fat cells from
aging subjects. Hum Cell. 33:974–989. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shukla L, Yuan Y, Shayan R, Greening DW
and Karnezis T: Fat therapeutics: The clinical capacity of
adipose-derived stem cells and exosomes for human disease and
tissue regeneration. Front Pharmacol. 11:1582020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baer PC: Adipose-derived mesenchymal
stromal/stem cells: An update on their phenotype in vivo and in
vitro. World J Stem Cells. 6:256–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brooks AES, Iminitoff M, Williams E,
Damani T, Jackson-Patel V, Fan V, James J, Dunbar PR, Feisst V and
Sheppard HM: Ex vivo human adipose tissue derived mesenchymal
stromal cells (ASC) are a heterogeneous population that demonstrate
rapid culture-induced changes. Front Pharmacol. 10:16952020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kilinc MO, Santidrian A, Minev I, Toth R,
Draganov D, Nguyen D, Lander E, Berman M, Minev B and Szalay AA:
The ratio of ADSCs to HSC-progenitors in adipose tissue derived SVF
may provide the key to predict the outcome of stem-cell therapy.
Clin Transl Med. 7:52018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bourin P, Bunnell BA, Casteilla L,
Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K and
Gimble JM: Stromal cells from the adipose tissue-derived stromal
vascular fraction and culture expanded adipose tissue-derived
stromal/stem cells: A joint statement of the International
Federation for Adipose Therapeutics and Science (IFATS) and the
International Society for Cellular Therapy (ISCT). Cytotherapy.
15:641–648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maurizi G, Poloni A, Mattiucci D, Santi S,
Maurizi A, Izzi V, Giuliani A, Mancini S, Zingaretti MC, Perugini
J, et al: Human white adipocytes convert into ‘rainbow’ adipocytes
in vitro. J Cell Physiol. 232:2887–2899. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shen JF, Sugawara A, Yamashita J, Ogura H
and Sato S: Dedifferentiated fat cells: An alternative source of
adult multipotent cells from the adipose tissues. Int J Oral Sci.
3:117–124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bougaret L, Delort L, Billard H, Lequeux
C, Goncalves-Mendes N, Mojallal A, Damour O, Vasson MP and
Caldefie-Chezet F: Supernatants of adipocytes from obese versus
normal weight women and breast cancer cells: In vitro impact on
angiogenesis. J Cell Physiol. 232:1808–1816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Torres-Torrillas M, Rubio M, Damia E,
Cuervo B, Del Romero A, Peláez P, Chicharro D, Miguel L and Sopena
JJ: Adipose-derived mesenchymal stem cells: A promising tool in the
treatment of musculoskeletal diseases. Int J Mol Sci. 20:202019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kazama T, Fujie M, Endo T and Kano K:
Mature adipocyte-derived dedifferentiated fat cells can
transdifferentiate into skeletal myocytes in vitro. Biochem Biophys
Res Commun. 377:780–785. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matsumoto T, Kano K, Kondo D, Fukuda N,
Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M, et al:
Mature adipocyte-derived dedifferentiated fat cells exhibit
multilineage potential. J Cell Physiol. 215:210–222. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Oki Y, Watanabe S, Endo T and Kano K:
Mature adipocyte-derived dedifferentiated fat cells can
trans-differentiate into osteoblasts in vitro and in vivo only by
all-trans retinoic acid. Cell Struct Funct. 33:211–222. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Diekman BO, Estes BT and Guilak F: The
effects of BMP6 overexpression on adipose stem cell chondrogenesis:
Interactions with dexamethasone and exogenous growth factors. J
Biomed Mater Res A. 93:994–1003. 2010.PubMed/NCBI
|
|
36
|
Jeon HJ, Yoon KA, An ES, Kang TW, Sim YB,
Ahn J, Choi EK, Lee S, Seo KW, Kim YB, et al: Therapeutic effects
of human umbilical cord blood-derived mesenchymal stem cells
combined with cartilage acellular matrix mediated via bone
morphogenic protein 6 in a rabbit model of articular cruciate
ligament transection. Stem Cell Rev Rep. 16:596–611. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sung SE, Hwang M, Kim AY, Lee EM, Lee EJ,
Hwang SK, Kim SY, Kim HK and Jeong KS: MyoD overexpressed equine
adipose-derived stem cells enhanced myogenic differentiation
potential. Cell Transplant. 25:2017–2026. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Zuo X, Yang B, Li Z, Xue Y, Zhou
Y, Huang J, Zhao X, Zhou J, Yan Y, et al: MicroRNA directly
enhances mitochondrial translation during muscle differentiation.
Cell. 158:607–619. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Perrini S, Ficarella R, Picardi E,
Cignarelli A, Barbaro M, Nigro P, Peschechera A, Palumbo O, Carella
M, De Fazio M, et al: Differences in gene expression and cytokine
release profiles highlight the heterogeneity of distinct subsets of
adipose tissue-derived stem cells in the subcutaneous and visceral
adipose tissue in humans. PLoS One. 8:e578922013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets-update. Nucleic Acids Res. 41(D1): D991–D995.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Conley SM, Hickson LJ, Kellogg TA,
McKenzie T, Heimbach JK, Taner T, Tang H, Jordan KL, Saadiq IM,
Woollard JR, et al: Human obesity induces dysfunction and early
senescence in adipose tissue-derived mesenchymal stromal/stem
cells. Front Cell Dev Biol. 8:1972020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Prieto González EA: Heterogeneity in
adipose stem cells. Adv Exp Med Biol. 1123:119–150. 2019.
View Article : Google Scholar : PubMed/NCBI
|